Abstract
Pasteurella haemolytica, the causative agent of shipping fever pneumonia in cattle, produces a leukotoxin (LKT) which lyses ruminant leukocytes with high efficiency but is reputed to not affect leukocytes from nonruminant species. In this study, we tested the supposition that LKT binding correlates positively with susceptibility to intoxication of susceptible isolated bovine lymphocytes and lymphoma tissue culture cells (BL3 cells) and negatively with reputed nonsusceptible equine, porcine, and canine lymphocytes and human lymphoid tissue culture cells (Raji cells). Bovine lymphocytes and BL3 cells were highly susceptible to LKT intoxication, exhibiting both substantial increase in intracellular Ca2+ concentration and marked leukolysis. Exposure of reputed LKT-nonsusceptible porcine lymphocytes and Raji cells to LKT caused a slightly increased intracellular Ca2+ concentration but no leukolysis. No LKT effect was detected for equine and canine lymphocytes. LKT bound to lymphoid cells from all species tested. Intact 102-kDa LKT was recovered from exposed isolated lymphoid cell membranes. Pro-LKT acylation was not required for LKT binding to BL3 cells. LKT binding was rapid, with maximal binding occurring by 3 min, and was proportional to the LKT concentration in the range 0.04 to 4.0 μg/ml. For this LKT concentration range, BL3 cells bound more LKT than did porcine lymphocytes or Raji cells, suggesting that LKT binds to BL3 cells with higher affinity than to porcine lymphocytes or Raji cells. Above 4.0 μg/ml, LKT demonstrated saturable binding to BL3 cells. Neutralizing anti-LKT monoclonal antibody (MAb) MM601 diminished LKT binding to BL3 by 36% while decreasing leukolysis by 81%. In contrast, MM601 did not diminish LKT binding to Raji cells. Pretreatment of target cells with 120 μg of protease K per ml diminished LKT binding to BL3 cells by 75%, with only a 25% decrease in leukolysis. However, pretreatment with 150 μg of protease K per ml abolished the remaining 25% of LKT binding and 75% leukolysis. Therefore, P. haemolytica LKT binds rapidly to susceptible and to reputed nonsusceptible lymphoid cells. LKT binding resulting in species-specific leukolysis was characterized by high affinity, inhibition by MAb MM601, and relative resistance to protease K pretreatment of lymphoid cells. Two types of LKT binding to lymphoid cells are proposed. High-affinity binding leads to efficient leukolysis. In some lymphoid cells from reputed LKT-nonsusceptible species, low-affinity LKT binding may cause a low-efficiency increase in the intracellular Ca2+ concentration without leading to leukolysis.
The gram-negative bacterial “repeats-in-toxin” (RTX) family of pore-forming cytolysins is composed of several medically important toxins with extensive genetic sequence homology and similarities in gene arrangement, mechanism of pro-toxin activation, toxin secretion, and mechanism of target cell intoxication (24). Most of the RTX toxins intoxicate a wide range of cell types from a variety of host species. These general cytolysins are termed hemolysins and, as exemplified by Escherichia coli alpha-hemolysin, can cause increased membrane permeability in erythrocytes, a variety of nucleated cells, and artificial membranes (2, 13). Two RTX toxins, Pasteurella haemolytica leukotoxin (LKT) and Actinobacillus actinomycetemcomitans leukotoxin (LTX), are host species-specific leukolytic toxins (21, 23). LKT is specific for ruminant leukocytes and platelets, and ruminants are the only species commonly infected by P. haemolytica (6). Likewise, LTX is specific for primate leukocytes, and its parent bacterium, A. actinomycetemcomitans, causes periodontitis in human beings but does not commonly infect other species (22).
The leukocyte target cell specificity of the RTX leukotoxins has been hypothesized to be mediated by a specific receptor mechanism. This hypothesis is supported by the identification of β2-integrins as receptors for the RTX leukotoxins (11, 12). Based on the receptor hypothesis, it is logical to predict that RTX leukotoxins bind to leukocytes from susceptible species but not to leukocytes from nonsusceptible species. This supposition is supported by the observation that LKT bound to susceptible bovine leukocytes but not to nonsusceptible porcine and human leukocytes in a flow cytometric assay to assess LKT binding (3). However, another study detected binding of LTX to nonsusceptible cells (20).
We tested the supposition that LKT binding correlates positively with susceptibility to LKT intoxication by comparing LKT binding to bovine and human lymphoid tissue culture cells and isolated peripheral blood lymphocytes from cattle, horses, pigs, and dogs. A whole-cell enzyme-linked immunosorbent assay (ELISA) was used to assess LKT binding, and LKT-induced increased intracellular Ca2+ concentration and leakage of the cytoplasmic enzyme lactate dehydrogenase (LDH) were used to assess susceptibility to LKT intoxication.
(A portion of this research was submitted by Yude Sun in partial fulfillment of the requirements for the Ph.D. degree.)
MATERIALS AND METHODS
Preparation of LKT and LKT mutant CCS.
Concentrated culture supernatants (CCS) containing active LKT (LKT) and CCS containing no LKT protein [LKT(−)] were prepared from a wild-type strain (Ph89010807N) of P. haemolytica and an isogenic strain (11–36 LKT−) with an allelic lktCA replacement mutation, respectively (14). Another P. haemolytica wild-type strain, SH1217, and an isogenic strain, SH1562, containing a nonpolar insertion in the lktC gene (8) were kindly provided by Sarah Highlander, Department of Microbiology and Immunology, Baylor College of Medicine, Houston, Tex., and were used as the sources of LKT and inactive pro-LKT preparations, respectively. CCS were prepared by inoculating 1.0 liter of RPMI 1640 medium containing NaHCO3 at 2.2 g/liter to an optical density at 600 nm (OD600) = 0.25 with the wild-type or mutant P. haemolytica prepared by growth overnight on 5% bovine blood agar followed by growth in brain heart infusion medium to late logarithmic phase. The cultures in RPMI 1640 medium were grown at 37°C and 120 oscillations/min for approximately 2.5 h to an OD600 of 0.9 to 1.0. All subsequent steps were conducted at 4°C. The bacteria were removed by centrifugation, and culture supernatants were concentrated and partially purified by 0 to 60% ammonium sulfate precipitation (361 g of ammonium sulfate/liter). The precipitates were collected by centrifugation and resuspended at 0.5 mg of protein/ml in 50 mM sodium phosphate–100 mM sodium chloride buffer (pH 7.0) (phosphate-NaCl), dialyzed against the same buffer, and stored at −135°C.
Tissue culture lymphoid cells.
Bovine BL3 lymphoma (CRL8037) and human Raji lymphocytic leukemia (CCL86) cells were obtained from and cultured as described by the American Type Culture Collection (Rockville, Md.).
Isolation of peripheral blood lymphocytes.
Blood was obtained by jugular venipuncture from healthy animals maintained as blood donors and anticoagulated by the addition of 15 U of sodium heparin per ml. Lymphocytes were isolated by a method modified from that of Reeves and Renshaw (18). The heparinized blood in 50-ml conical centrifuge tubes was centrifuged at 600 × g for 30 min at 25°C, and the plasma was aspirated to a level 10 to 15 mm above the buffy coat interface with the plasma. The buffy coat was carefully transferred to another centrifuge tube with as small an amount of the erythrocyte column as possible and diluted (1:3, vol/vol) with Ca2+- and Mg2+-free Hanks balanced salt solution without phenol red (HBSS − Ca − Mg). The diluted buffy coat (30 to 35 ml) was carefully layered onto 15 ml of Ficoll-Paque (Sigma Chemical Co., St. Louis, Mo.) and centrifuged at 500 × g for 20 min with brake in the off position. After centrifugation, lymphocytes and monocytes in the white interface between the plasma and the Ficoll-Paque were transferred to another conical centrifuge tube, washed twice with 30 ml of HBSS − Ca − Mg, and resuspended at 5 × 106 cells/ml in RPMI 1640 medium containing 10% fetal bovine serum. This final cell suspension was free of erythrocytes and contained approximately 90% lymphocytes (mean, 89.9%; range, 72 to 99%) and 5% monocytes (mean, 4.5%; range, 0 to 9%). To remove monocytes, 5 ml of cell suspension was transferred to a 50-mm tissue culture-treated Nunclon petri dish (Nalge Nunc International, Milwaukee, Wis.) and incubated at 37°C for 2 h. Following the incubation, the medium was removed and nonadherent cells were collected by centrifugation. These cells were 99% lymphocytes and were >95% viable as determined by the trypan blue exclusion assay.
Assay of intracellular Ca2+ concentration.
Phosphate-buffered saline (PBS)-washed cells were loaded with the fluorescent Ca2+ indicator Fluo3 (Molecular Probes Inc., Eugene, Oreg.) by incubating cells in 5 μM acetoxymethyl ester of Fluo3 (in dimethyl sulfoxide containing 0.14% pluronic acid) in the dark for 30 min at 25°C with constant mixing on a cell rotator (7). The Fluo3-loaded cells were collected by centrifugation at 200 × g for 15 min at 4°C, washed with 10 ml of PBS, resuspended in 3 ml of HBSS − Ca − Mg, and then enumerated with a hemocytometer. The increase in intracellular Ca2+ concentration was measured by using 2 × 106 Fluo3-loaded cells in 0.25 ml of HBSS − Ca − Mg containing 1 mM CaCl2 and 1:250 anti-fluorescein antibody (Molecular Probes Inc.) exposed at 37°C for 5 min to 4.0 μg of CCS LKT or LKT(−) per ml, 4 μM 4-bromo-A23187 (Sigma Chemical Co.), or PBS. Fluorescence intensity (490-nm excitation, 523-nm emission) was measured in a fluorescence plate reader (Cytofluor 2300 fluorescence measurement system; Millipore Corp., Bedford, Mass.).
Assay of leukolysis.
Following measurement of Fluo3 fluorescence intensity as described in the preceding section, the incubation was continued for a total of 2 h, the cells were collected by centrifugation at 700 × g for 5 min, and leukolysis was determined by measuring the leakage of the intracellular enzyme LDH. Following transfer of 100 μl of incubation supernatant to wells of a 96-well flat-bottom microtiter plate and warming to 37°C, 100 μl of LDH assay reagent (LDH-L 50) (Sigma Chemical Co.) prewarmed to 37°C was added to all wells and the change in absorbance at 340 nm was measured in a thermally controlled kinetic microtiter plate reader (ThermoMax; Molecular Devices, Palo Alto, Calif.) for 2 min at 37°C. Data were reported as the change in milliunits per minute. Maximal LDH leakage was determined by exposing cells to 0.1% Triton X-100.
Whole-cell ELISA for LKT binding.
Lymphoid tissue culture cells and isolated blood lymphocytes (3 × 106/ml) were exposed to various concentrations of CCS LKT, LKT(−), pro-LKT, or PBS at 25°C for 0.5 to 20 min. The exposure was terminated by 10-fold dilution in PBS at 4°C. The cells were washed with PBS at 4°C, and cells from each exposure were transferred to the wells of a dot blot apparatus (MilliBlot-D system; Millipore Corp.). The cells were applied to the nitrocellulose membrane well bottoms by vacuum. The wells were washed with PBS, blocked by incubation with 3% gelatin–PBS for 1 h, and washed twice with PBS–0.15% Tween 20. Bound LKT was detected by incubating the nitrocellulose membrane with 1:500 anti-LKT monoclonal antibody (MAb) C6 (14) followed by biotinylated goat anti-mouse immunoglobulin G and streptavidin-alkaline phosphatase with appropriate incubation and washing steps. The nitrocellulose membrane was developed with 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (fast BCIP/NBT; Sigma Chemical Co.). The immunodot blots were scanned into tagged image files by using a flatbed scanner (HP ScanJet IIcx; Hewlett-Packard Co., Boise, Idaho), and the density of dots was measured (SigmaScan; Jandal Scientific Software, San Rafael, Calif.). The density values of dot spots were expressed as relative scanning density (white = 0; black = 256). The negative controls included for each blot were no cells or cells blotted with an irrelevant MAb, MOPC-21 (Sigma Chemical Co.), in place of MAb C6. The positive control for determining the relative scanning density = 256 was LKT spotted directly onto an unblocked nitrocellulose well bottom not containing cells followed by blocking and by reaction with MAb C6, secondary antibody, and streptavidin-alkaline phosphatase.
Membrane isolation.
Cytoplasmic membranes were prepared from 109 PBS-washed tissue culture cells suspended in 50 ml of 50 mM 3-(N-morpholino)propanesulfonate (MOPS)–100 mM NaCl buffer (pH 7.0) (MOPS-NaCl) by gently breaking cells by five passes of a Potter-Elvenhjem tissue grinder with 0.1-mm clearance. Unbroken cells and nuclei were removed by centrifugation at 700 × g for 15 min, and the plasma membranes were collected by centrifugation at 25,000 × g for 45 min, washed twice with distilled water, and resuspended in MOPS-NaCl buffer at 2 mg of protein/ml (bicinchoninic acid microprotein assay; Pierce Chemical Co., Rockford, Ill.), and stored at −135°C. The isolated membranes for BL3 and Raji cells had similar phospholipid-to-protein ratios of 0.76 and 0.70 mg of phospholipid per mg of protein, respectively (25).
Identification of intact 102-kDa LKT associated with LKT-exposed isolated lymphoid cell membranes.
To further evaluate LKT binding, 0 to 8.0 μg of CCS LKT or LKT(−) or an equivalent volume of PBS was incubated with isolated lymphoid cell membranes (50 μg of protein) in a total volume of 0.25 ml for 0 to 20 min at 25°C. The incubation was terminated by dilution in 2.5 ml of MOPS-NaCl at 4°C. Unbound LKT was removed from the membranes by two washes with 2.5 ml of MOPS-NaCl at 4°C. Washed membranes were dissolved in 50 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiled for 1.5 min, and a 15-μl portion was subjected to SDS-PAGE (10% polyacrylamide) and blotted onto nitrocellulose membranes. A no-membrane control was run in which the assay setup was identical to that containing membranes but following incubation, instead of the termination and washing steps, a 25-μl aliquot of the assay mixture was removed and mixed with 25 μl of SDS-PAGE sample buffer, the sample was processed as above, and a 15-μl portion was subjected to electrophoresis and blotting. Following blocking with 1% gelatin, the nitrocellulose membranes were incubated with 1:500 anti-LKT MAb C6 followed by biotinylated goat anti-mouse immunoglobulin G and streptavidin-alkaline phosphatase (with appropriate incubation and wash steps). Bands were developed with 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium.
Effect of preincubation of LKT with neutralizing anti-LKT MAb MM601 on LKT binding to and leukolysis of lymphoid cells.
Mouse ascitic fluid containing murine neutralizing anti-LKT MAb MM601 was kindly provided by S. Srikumaran, Department of Veterinary Sciences, University of Nebraska, Lincoln, Nebr. CCS LKT (40 μg/ml), LKT(−), or PBS was preincubated with 1:25-diluted MAb MM601 (10) or irrelevant MAb MOPC-21 for 30 min at 4°C. BL3 cells or Raji cells (3 × 106/ml) were subsequently exposed to the preincubated LKT, LKT(−), or PBS for 3 min at 25°C. The amount of LKT bound was measured by the whole-cell ELISA as described in a previous section. The degree of neutralization of the preincubation of LKT with MAb MM601 was determined by exposing BL3 cells to the preincubated LKT, LKT(−), or PBS for 120 min at 37°C and then measuring LDH leakage as described in a previous section.
Effect of pretreatment of lymphoid cells with protease K on LKT binding and leukolysis.
BL3 or Raji cells (3 × 106 cells/ml) were pretreated with 20 to 200 μg of protease K (Sigma Chemical Co.) at 25°C for 10 min and then washed twice with PBS. The cells were resuspended in RPMI 1640 at 3 × 106 cells/ml for assessment of the effect of cell surface proteolysis on LKT binding and leukolysis as described in previous sections.
Experimental reproducibility and statistical analysis.
Experiments measuring intracellular Ca2+ concentration, LDH leakage, and LKT whole-cell ELISA binding were conducted in triplicate or quadruplet as indicated, and a statistical analysis program (SigmaStat, Jandel Scientific) was used to calculate standard deviations and assess statistical differences and pairwise comparisons. P < 0.05 was considered significant. Identification of LKT binding to isolated target cell membranes was determined by single exposures and analysis. All experiments were repeated to assess reproducibility.
RESULTS
Susceptibility of lymphoid cells to LKT-mediated intoxication.
Bovine and human lymphoid tissue culture cells and bovine, equine, porcine, and canine peripheral blood lymphocytes were tested for susceptibility to LKT intoxication by assessing increased intracellular Ca2+ concentration and leakage of LDH in these cells exposed to CCS from a wild-type P. haemolytica strain producing LKT and its LKT(−) isogenic strain. The Ca2+ ionophore A23187 was used as a positive control for increased intracellular Ca2+ concentration, and the LKT or LKT(−) intracellular Ca2+ data was normalized to that of the positive control and expressed as a percentage of the A23187 fluorescence intensity by using a formula in which the fluorescence intensity induced by the Ca2+ ionophore A23187 was defined as 100% and that induced by the negative-control PBS exposure was defined as 0%. The increase in the intracellular Ca2+ concentration in bovine lymphocytes and BL3 lymphoma cells on exposure to LKT was comparable to the increase caused by A23187 (Table 1). A low but significant increase in intracellular Ca2+ concentration was observed for porcine lymphocytes and human Raji cells exposed to LKT compared to that for cells exposed to LKT(−).
TABLE 1.
Susceptibility to and binding of LKT to lymphoid cells from various species
| Lymphoid cell linea | %
A23187 Fluo3 fluorescence
intensityb
|
% Specific LDH
leakagec
|
LKT binding
indexd
|
|||
|---|---|---|---|---|---|---|
| LKT | LKT(−) | LKT | LKT(−) | LKT | LKT(−) | |
| BL3 | 145 ± 8e* | 8.0 ± 4.2e* | 64.8 ± 6.2e* | 0.0 ± 0.4e* | 1.00 | 0.05 |
| Raji | 9.0 ± 2.6f* | 5.2 ± 1.8f* | 0.2 ± 2.0f | 0.0 ± 0.5e | 1.54 | 0.01 |
| BL | 72.7 ± 4.8g* | 1.1 ± 0.8g* | 84.1 ± 10.5e* | 8.9 ± 2.3f* | 1.45 | 0.08 |
| EL | −0.3 ± 1.1h | −0.3 ± 1.2g | 1.0 ± 0.9f | 0.5 ± 1.2g | 0.88 | −0.01 |
| PL | 8.2 ± 1.9f* | −1.1 ± 1.1g* | 2.1 ± 1.5f | 1.7 ± 1.5g | 1.25 | 0.00 |
| CL | −0.1 ± 0.9h | −0.1 ± 1.3g | 0.5 ± 1.2f | 2.4 ± 1.8g | 1.19 | 0.03 |
BL, EL, PL, and CL are bovine, equine, porcine, and canine lymphocytes, respectively.
Percent A23187 Fluo3 fluorescence intensity was calculated by using the following formula: percent A23187 Fluo3 fluorescence intensity = [(Fluo3 fluorescence intensity of cell exposed to LKT or LKT(−) − Fluo3 fluorescence intensity of cell exposed to PBS)/(Fluo3 fluorescence intensity of cell exposed to A23187 − Fluo3 fluorescence intensity of cell exposed to PBS)] × 100. Values are means ± standard deviations (n = 4). Means in each column with different superscripts differ. The asterisks indicate that means differ between LKT and LKT(−) for lymphoid cells from a particular species.
Percent specific LDH leakage was calculated by using the following formula: percent specific LDH leakage = [(milliunits of LDH leaked from cells exposed to LKT or LKT(−) − milliunits of LDH leaked from cells exposed to PBS)/(milliunits of LDH leaked from cells exposed to 0.1% Triton X-100 − milliunits of LDH leaked from cells exposed to PBS)] × 100. Values are means ± standard deviations (n = 4). Means in each column with different superscripts differ (P < 0.05). The asterisks indicate that means differ between LKT and LKT(−) for lymphoid cells from a particular species.
LKT binding index was calculated by using the following formula: ELISA dot blot scanning density for a particular lymphoid cell type/ELISA dot blot scanning density for BL3 cells. Values are means (n = 3).
LKT induced high levels of LDH leakage in bovine lymphocytes and BL3 cells (Table 1). Bovine lymphocytes exposed to LKT(−) had low LDH leakage, but it was significantly higher than for other cells exposed to LKT(−). We conclude that only bovine-origin lymphoid cells are susceptible to LKT-mediated cell lysis but that some non-bovine-origin lymphoid cells may be susceptible to slight LKT-induced increases in intracellular Ca2+ concentration.
Binding of LKT to lymphoid cells.
LKT binding to lymphoid cells was quantified by using a whole-cell ELISA with a dot blot format and murine anti-LKT MAb C6. Deletion of the primary antibody, secondary antibody, or streptavidin alkaline phosphatase conjugate or replacement of the anti-LKT MAb C6 with irrelevant mouse MAb resulted in background levels of scanned density for these dot blots. For comparison of LKT binding to lymphoid cells from various species, LKT binding was expressed as a binding index by using a formula (Table 1) in which the scanning density for LKT and the PBS negative control for BL3 cells were assigned values of 1.00 and 0.00, respectively. LKT was observed to bind to lymphoid cells from all species tested (Table 1).
Recovery of the intact 102-kDa LKT from LKT-exposed isolated lymphoid cell membranes.
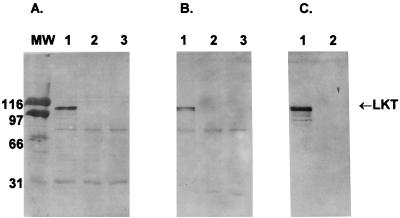
LKT binding to lymphoid cells was further assessed by exposing isolated lymphoid cell membranes to LKT and identifying bound LKT by Western blot analysis. LKT was identified in both BL3 and Raji cell membranes (Fig. 1). No 102-kDa LKT band was observed when the isolated BL3 or Raji cell membranes were incubated with LKT(−). Exclusion of membranes or LKT from the assay, exclusion of any of the immunologic assay components, or substitution of an irrelevant MAb for MAb C6 resulted in no detectable 102-kDa band. A band present at ≈70 kDa in all lanes of Fig. 1A and B is believed to be membrane-associated alkaline phosphatase. This band developed when akaline phosphatase substrate was added in the absence of preincubation of the nitrocellulose membrane with streptavidin-alkaline phosphatase.
FIG. 1.
(A and B) Intact 102-kDa LKT identified as the C6 immunoreactive component of washed LKT-exposed isolated membranes for both LKT-susceptible BL3 cells (A) and reputed LKT-nonsusceptible Raji cells (B). Isolated membranes from BL3 or Raji cells (250 μg of protein/ml) exposed for 20 min at 25°C to 4 μg of LKT per ml in lane 1, 4 μg of LKT(−) mutant per ml in lane 2, or PBS in lane 3 were diluted 1:10 in MOPS-NaCl and washed twice in the same buffer to remove unbound LKT, and the washed membrane pellet was subjected to SDS-PAGE and Western blotting with anti-LKT MAb C6. (C) Controls were identical exposure mixtures lacking added isolated target cell membranes and processed without the termination dilution or washing steps. MW, molecular weight (in thousands).
Pro-LKT binding to BL3 cells.
The necessity of LKT acylation for binding was tested by comparing LKT binding to BL3 cells with that for nonacylated pro-LKT by using the whole-cell ELISA. Although the scanning density for pro-LKT binding to BL3 cells was slightly lower than that for active acylated LKT (68 ± 2 and 73 ± 5, respectively [mean ± standard deviation of three determinations]) this difference was not significant. It is possible that affinity of MAb anti-LKT C6 is slightly lower for pro-LKT than for LKT, accounting for the apparent difference in pro-LKT versus LKT binding. The scanning density for cells with no LKT was 51 ± 4, which is significantly different from the densities in the presence of LKT or pro-LKT (P < 0.05).
Time and concentration dependence of LKT binding.
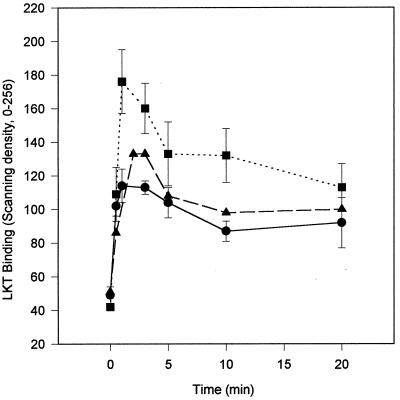
Using the whole-cell ELISA binding or the Western blot assay to examine the time dependence of LKT binding demonstrated that binding was rapid (Fig. 2) and was similar to that of the time of onset of the initial step of LKT intoxication reported previously (4). The amount of LKT bound was maximal by 3 min and then declined to a lower plateau by 10 min of exposure. This pattern was observed for LKT binding to both BL3 cells and Raji cells as well as to isolated BL3 cell membranes.
FIG. 2.
LKT (40 μg/ml) bound rapidly to 3 × 106 BL3 cells per ml (●), 3 × 106 Raji cells per ml (■), or 250 μg of BL3 cell membranes per ml (▴) at 25°C. The amounts of LKT bound for BL3 and Raji cells were determined by whole-cell ELISA, and the amount bound for BL3 cell membranes was determined by the western blot assay. Values for LKT binding to BL3 and Raji cells are means and standard deviations (n = 3) expressed as the relative scanning density. Values for LKT binding to BL3 cell membranes are the relative scanning density for the 102-kDa band on the Western blot.
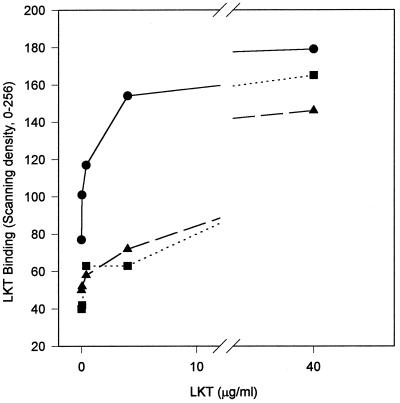
The dependence of LKT binding on LKT concentration was examined over the range 0.04 to 40 μg of LKT/ml (Fig. 3). The 50% lytic dose for the LKT preparations used was 0.4 μg of LKT/ml; therefore, the concentration range used for binding experiments spanned the sublytic to supralytic range for LKT. For experiments with BL3 cells and either the whole-cell ELISA or Western blot binding assay, the amount of LKT bound was proportional to the LKT concentration over the range 0.04 to 4.0 μg of LKT/ml but the amount of LKT bound plateaued at LKT concentrations of >4.0 μg/ml. For all LKT concentrations of >0.04 μg/ml, the supernatant fraction of the binding-assay mixture contained unbound LKT. This pattern of binding suggests saturable binding, which is a feature of specific receptor-mediated binding.
FIG. 3.
LKT (●) or LKT(−) (▴) binding to 3 × 106 BL3 cells per ml, determined by whole-cell ELISA, or LKT (■) binding to 250 μg of isolated BL3 cell membranes per ml, determined by Western blot analysis, is proportional to the LKT concentration for the range from 0.04 to 4.0 μg/ml in a 3-min exposure at 25°C. Values for LKT and LKT(−) binding to BL3 cells are means (n = 3) expressed as the relative scanning density. Values for LKT binding to BL3 cell membranes are the relative scanning density for the 102-kDa band on the Western blot.
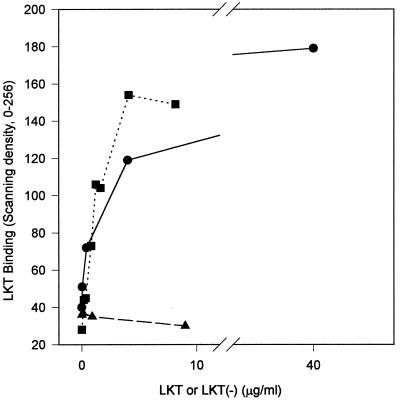
LKT binding to isolated bovine lymphocytes was similar to binding to BL3 cells (Fig. 4); however, LKT binding to minimally susceptible isolated pig lymphocytes or Raji cells exhibited markedly lower binding at 4.0 μg of LKT/ml but similar binding to 40.0 μg of LKT/ml (Fig. 4). This data suggests that LKT binding to minimally susceptible cells was of lower affinity than that to BL3 cells or isolated bovine lymphocytes.
FIG. 4.
Isolated bovine lymphocytes (●) (3 × 106/ml) bound more LKT following a 3-min exposure at 25°C for LKT concentrations ranging from 0.04 to 4.0 μg/ml than did the same number of isolated porcine lymphocytes (▴) or Raji cells (■) in the whole-cell ELISA. Values for LKT binding are means (n = 3) expressed as the relative scanning density.
Effect of pre-incubation of LKT with neutralizing anti-LKT MAb MM601 on LKT binding to lymphoid cells.
Preincubation of LKT with neutralizing anti-LKT MAb MM601 at concentrations sufficient to decrease LKT-induced leukolysis by 81% reduced LKT binding to BL3 cells by 36% (Table 2). Preincubation of LKT with MAb MM601 did not diminish LKT binding to Raji cells.
TABLE 2.
Effect of preincubation of LKT with neutralizing MAb MM601 on LKT binding to susceptible BL3 and minimally susceptible Raji cells
| Pre-incubation component(s) | LKT binding (scanning
density)a to:
|
|
|---|---|---|
| BL3 cells | Raji cells | |
| PBS | 36 ± 4b | 46 ± 2e |
| LKT(−) | 35 ± 3b | 46 ± 2e |
| LKT | 98 ± 4c | 119 ± 5f |
| LKT + anti-LKT MAb MM601 | 76 ± 6d | 124 ± 7f |
| LKT + irrelevant MAb MOPC21 | 93 ± 5c | ND |
A total of 3 × 106 BL3 or Raji cells/ml were exposed to 40.0 μg of LKT per ml for 3 min at 25°C. Values are means ± standard deviations (n = 3). Means in each column with different superscript differ (P < 0.05). ND, not done.
Effect of pretreatment of lymphoid cells with protease K on LKT binding and LDH leakage.
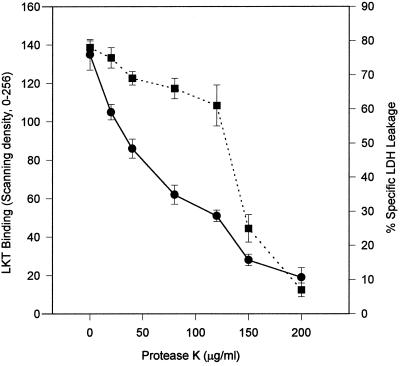
Diminution of ligand binding to target cells pretreated with proteases has been used to implicate specific protein receptors in ligand binding. Pretreatment of BL3 cells with protease K resulted in diminution of both LKT binding and leukolysis (Fig. 5). However, the pattern of the diminution of LKT binding by protease pretreatment was different from that of the diminution of LKT-induced leukolysis. LKT binding to BL3 cells appeared to be more sensitive to protease K pretreatment than did leukolysis, suggesting the possibility that a small portion of bound LKT either participates in or is necessary for leukolysis. Similar protease K pretreatment of Raji cells also diminished LKT binding to these cells.
FIG. 5.
Pretreatment of 3 × 106 BL3 cells per ml with 20 to 200 μg of protease K per ml at 25°C for 10 min followed by washing to remove protease K resulted in diminished LKT binding (●) and LDH leakage (■) during subsequent exposure at 25°C to 40.0 μg of LKT per ml for 3 min for LKT binding and 2 h for LDH leakage. Values for LKT binding and LDH leakage are means and standard deviations (n = 3) expressed as the relative scanning density and percent specific LDH leakage, respectively.
DISCUSSION
Based on the findings presented herein, the supposition that susceptibility to LKT-induced intoxication is correlated positively with LKT binding is shown to be false. LKT was found to bind to cells which are highly susceptible to LKT (bovine lymphocytes and lymphoma cells) as well as to cells which are either minimally susceptible (porcine lymphocytes and human Raji cells) or nonsusceptible (equine and canine lymphocytes) to LKT. However, particular characteristics of LKT binding to lymphoid cells, i.e., high-affinity binding, inhibition of binding by neutralizing anti-LKT MAb MM601, and relative resistance of LKT binding and leukolysis to target cell protease pretreatment, did correlate with a high susceptibility to LKT-induced intoxication.
A rough approximation of the Kd for LKT binding to BL3 cells can be made from the half-maximal binding concentration of LKT with BL3 cells of 1 μg/ml (Fig. 3), which corresponds to an estimated Kd of 10 nM. A Kd in the nanomolar range supports high-affinity binding of LKT to BL3 cells and isolated bovine lymphocytes and is compatible with a specific receptor mechanism of LKT binding. The CD18 component of β2-integrin has been identified as a bovine-specific, leukocyte-specific receptor for LKT (12). Ligand-blotting experiments demonstrated that LKT specifically recognizes bovine CD18 but does not recognize human CD18 from Raji cells. As reported herein, pro-LKT acylation is not required for LKT binding to BL3 cells and, similarly, LKT recognition of bovine CD18 does not require pro-LKT acylation (12).
Preincubation of LKT with neutralizing anti-LKT MAb MM601 inhibits most LKT intoxication phenomena. The concentration of MAb MM601 used in our experiments was sufficient to inhibit 81% of the LKT-induced leukolysis of BL3 cells; however, this concentration of MM601 decreased LKT binding by only 36%. This suggests that some, and possibly the majority, of LKT binding to BL3 cells involves another binding mechanism in addition to specific binding of LKT to bovine CD18. We propose that LKT binding to CD18 leads to leukolysis, whereas the proposed second type of LKT binding does not induce or is inefficient at inducing leukolysis, and that inhibition of LKT binding by MAb MM601 occurs by blocking LKT binding to CD18. In this proposed scheme, LKT binding to CD18 results in LKT insertion and leukolysis of the target cell. In contrast, the LKT binding to BL3 cells, which was not inhibited by MM601, is envisioned not to occur via CD18 but is an adsorptive type of binding not resulting in efficient LKT insertion and leukolysis (16, 17).
Further evidence for this dual-binding model was observed in experiments involving pretreatment of BL3 cells with protease K. The different sensitivities of LKT binding and LKT-induced leukolysis of BL3 cells to pretreatment with protease K suggest two types of LKT binding. Pretreatment of BL3 cells with 120 μg of protease K per ml resulted in the loss of 75% of LKT binding but only a 25% decrease in LKT-induced leukolysis. We suggest that this more protease-sensitive LKT binding is the reversible, non-CD18, nonleukolytic LKT adsorptive binding. Increasing the pretreatment concentration of protease K from 120 to 150 μg/ml further reduced LKT binding by 25%, but this final 25% reduction in LKT binding was associated with loss of 75% of the leukolytic activity. This finding is compatible with 25% of the total LKT binding being mediated by CD18, resulting in LKT insertion and hence in leukolysis.
Previous studies of target cell susceptibility to LKT have relied on cytolysis as an indicator of susceptibility. However, cytolysis requires relatively high toxin doses and is the terminal event in a complex intoxication pathway (5). Increased intracellular Ca2+ concentration is an early intoxication event and may be more sensitive than cytolysis for detection of susceptibility to LKT intoxication. Based on the increased intracellular Ca2+ concentration, some nonruminant lymphoid cells are susceptible to LKT intoxication. However, in these cells, the extent or duration of increased intracellular Ca2+ concentration may not be sufficient to trigger subsequent leukolytic events, and therefore leukolysis is not observed as a feature of LKT intoxication in these cells. At sublytic LKT concentrations, even LKT-susceptible cells exhibit increased intracellular Ca2+ concentration, but leukolysis is not observed as a consequence of this low-level increase in intracellular Ca2+ concentration (7).
It may not be surprising that LKT causes low-efficiency intoxication of some nonruminant leukocytes, because LKT has low-efficiency non-species-specific hemolytic activity (14). P. haemolytica derives its species name from its weak beta-hemolytic phenotype (15). CCS from wild-type strains causes low-efficiency non-species-specific hemolysis (≈8% of the leukolytic activity), but this hemolytic activity and the beta-hemolytic phenotype are lost for a strain with a lktCA gene allelic replacement deletion mutation (14). Likewise, a mutant strain created by a nonpolar insertion in the lktC gene, causing production of inactive pro-LKT, is also associated with a loss of the beta-hemolytic phenotype, indicating that LKT has hemolytic activity (8).
Erythrocytes are reputed to lack the leukocyte-specific β2-integrin which serves as an LKT receptor; therefore, LKT hemolysis must involve an alternative target cell binding mechanism. This hypothesis is compatible with earlier work of Welch and colleagues (9, 19), in which binding of E. coli alpha-hemolysin to erythrocytes was found to involve different toxin domains from those involved in alpha-hemolysin binding to leukocytes. We propose that LKT, like alpha-hemolysin, has erythrocyte binding domains but that unlike alpha-hemolysin, target cell intoxication mediated by the putative LKT erythrocyte binding domains are inefficient compared to the leukolytic CD18 binding domains. We further propose that the LKT erythrocyte binding domains are involved in LKT binding to nonruminant lymphoid cells. The alpha-hemolysin erythrocyte binding domains are proposed to bind via “nonspecific” interactions with membrane phospholipids (16). However, Bauer and Welch (1) have questioned whether RTX toxins interact nonspecifically with target cells, as had been widely supposed. These workers found that alpha-hemolysin bound saturably to erythrocytes, which is not characteristic of nonspecific phospholipid binding. Likewise, our observations that LKT binding to Raji cells was diminished by protease K pretreatment of these target cells suggest a specific protein receptor rather than “nonspecific” phospholipid binding of LKT to Raji cells.
Brown et al. (3) observed specific LKT binding to bovine but not to procine or human leukocytes by using a flow cytometric assay. This binding was nearly completely inhibited by preincubation of LKT with MAb MM601 or pretreatment of target cells with 500 μg of protease K per ml. We suggest that this flow cytometric assay detected the high-affinity CD18-mediated specific LKT binding but did not detect the low-affinity adsorptive LKT binding. It is possible that the gate settings for the flow cytometric assay “screened” out the low-affinity LKT binding, while the whole-cell ELISA assay may have detected even relatively weak binding.
We propose that LKT binds to ruminant leukocytes by the β2-integrin CD18 binding, resulting in high-efficiency intoxication leading to leukolysis, and by low-affinity binding, resulting in low-efficiency intoxication. For ruminant leukocytes, the intoxication mediated by β2-integrin binding far overshadows the proposed low-affinity binding. In contrast, for nonruminant leukocytes, LKT is unable to recognize the nonruminant β2-integrins present on these cells (12) and therefore binds only by the low-affinity binding, leading to either no or low-efficiency intoxication.
ACKNOWLEDGMENTS
Yude Sun was supported by a Graduate Research Assistantship from the Office of Research, College of Veterinary Medicine, Oklahoma State University. This research was supported by the U.S. Department of Agriculture through National Research Initiative Competitive Grants Program grants 94-37204-0450 and 95-37204-2134 and a Hatch grant to the Oklahoma Agricultural Experiment Station (Project OKL02249).
REFERENCES
- 1.Bauer M E, Welch R A. Association of RTX toxins with erythrocytes. Infect Immun. 1996;64:4665–4672. doi: 10.1128/iai.64.11.4665-4672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhakdi S, Mackman N, Nicaud J M, Holland I B. Escherichia colihemolysin may damage target cells by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown J F, Leite F, Czuprynski C J. Binding of Pasteurella haemolyticaleukotoxin to bovine leukocytes. Infect Immun. 1997;65:3719–3724. doi: 10.1128/iai.65.9.3719-3724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinkenbeard K D, Mosier D A, Timko A L, Confer A W. Effects of Pasteurella haemolyticaleukotoxin on cultured bovine lymphoma cells. Am J Vet Res. 1989;50:271–275. [PubMed] [Google Scholar]
- 5.Clinkenbeard K D, Mosier D A, Confer A W. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella haemolyticaleukotoxin. Infect Immun. 1989;57:420–425. doi: 10.1128/iai.57.2.420-425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinkenbeard K D, Upton M L. Lysis of bovine platelets by Pasteurella haemolyticaleukotoxin. Am J Vet Res. 1991;52:453–457. [PubMed] [Google Scholar]
- 7.Cudd L A, Clarke C R, Clinkenbeard K D, Shelton M, Clinkenbeard P, Murphy G. Role of intracellular calcium in Pasteurella haemolytica leukotoxin-induced bovine neutrophils leukotriene B4production. FEMS Microbiol Lett. 1999;172:123–129. doi: 10.1111/j.1574-6968.1999.tb13459.x. [DOI] [PubMed] [Google Scholar]
- 8.Fedorova N D, Highlander S K. Generation of targeted nonpolar gene insertions and operon fusions in Pasteurella haemolyticaand creation of a strain that produces and secretes inactive leukotoxin. Infect Immun. 1997;65:2593–2598. doi: 10.1128/iai.65.7.2593-2598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forestier C, Welch R A. Identification of RTX toxin target cell-specific domains by use of hybrid genes. Infect Immun. 1991;59:4212–4220. doi: 10.1128/iai.59.11.4212-4220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentry M J, Srikumaran S. Neutralizing monoclonal antibodies to Pasteurella haemolyticaleukotoxin affinity-purify the toxin from crude culture supernatants. Microb Pathog. 1991;10:411–417. doi: 10.1016/0882-4010(91)90086-p. [DOI] [PubMed] [Google Scholar]
- 11.Lally E T, Kieba I R, Sato A, Green C L, Rosenbloom J, Korostoff J, Wang J F, Shenker B J, Ortlepp S, Robinson M K, Billings P C. RTX toxins recognize a beta2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Clinkenbeard K D, Ritchey J W. Bovine CD18 identified as a receptor for the RTX toxin Pasteurella haemolyticaleukotoxin. Vet Microbiol. 1999;67:93–99. doi: 10.1016/s0378-1135(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 13.Menestrina G, Ropele M, Serra M D, Pederzolli C, Hugo F, Pellet S, Welch R A. Binding of antibodies to functional epitopes on the pore formed by Escherichia colihemolysin in cell and model membranes. Biochim Biophys Acta. 1995;1238:72–80. doi: 10.1016/0005-2736(95)00113-h. [DOI] [PubMed] [Google Scholar]
- 14.Murphy G L, Whitworth L C, Clinkenbeard K D, Clinkenbeard P A. Hemolytic activity of the Pasteurella haemolyticaleukotoxin. Infect Immun. 1995;63:3209–3212. doi: 10.1128/iai.63.8.3209-3212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newsom I E, Cross F. Some bipolar organisms found in pneumonia in sheep. J Am Vet Med Assoc. 1932;80:711–719. [Google Scholar]
- 16.Ostolaza H, Bartolome B, Ortiz de Zarate I, de la Cruz F, Goñi F M. Release of lipid vesicle contents by the bacterial protein toxin alpha-haemolysin. Biochim Biophys Acta. 1993;1147:81–88. doi: 10.1016/0005-2736(93)90318-t. [DOI] [PubMed] [Google Scholar]
- 17.Ostolaza H, Goñi F M. Interaction of the bacterial protein toxin alpha-haemolysin with model membranes: protein binding does not always lead to lytic activity. FEBS Lett. 1995;371:303–306. doi: 10.1016/0014-5793(95)00927-2. [DOI] [PubMed] [Google Scholar]
- 18.Reeves J H, Renslaw H W. Surface membrane markers on bovine peripheral blood lymphocytes. Am J Vet Res. 1987;39:917–923. [PubMed] [Google Scholar]
- 19.Rowe G E, Pellett S, Welch R A. Analysis of toxinogenic functions associated with RTX repeats region and monoclonal antibody D12 epitope of Escherichia colihemolysin. Infect Immun. 1994;62:579–588. doi: 10.1128/iai.62.2.579-588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato N, Takahashi K, Ohta H, Kurihara H, Fukui K, Murayama Y, Taniguchi S. Effects of Ca2+ on the binding of Actinobacillus actinomycetemcomitansleukotoxin and the cytotoxicity to promyelocytic leukemia HL-60 cells. Biochem Mol Biol Int. 1993;29:899–905. [PubMed] [Google Scholar]
- 21.Shewen P E, Wilkie B N. Cytotoxin of Pasteurella haemolyticaacting on bovine leukocytes. Infect Immun. 1982;35:911–914. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taichman N S, Simpson D L, Sakurada S, Cranfield M, DiRenzo J, Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitansleukotoxin in primates. Oral Microbiol Immunol. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai C C, Shenker B J, DiRienzo J M, Malamud D, Taichman N S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitanswith polymyxin B. Infect Immun. 1984;43:700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch R A, Bauer M E, Kent A D, Leeds J A, Moayeri M, Regassa L B, Swenson D L. Battling against host phagocytes: the wherefore of the RTX family of toxins? Infect Agents Dis. 1995;4:254–272. [PubMed] [Google Scholar]
- 25.Zhou X, Arthur G. Improved procedures for the determination of lipid phosphorus by malachite green. J Lipid Res. 1992;33:1233–1236. [PubMed] [Google Scholar]