Abstract
There is an increasing demand for functional foods to attend the consumers preference for products with health benefits. Peach (Prunus persica), from Rosaceae family, is a worldwide well-known fruit, and its processing generates large amounts of by-products, consisting of peel, stone (seed shell + seed), and pomace, which represent about 10% of the annual global production, an equivalent of 2.4 million tons. Some studies have already evaluated the bioactive compounds from peach by-products, although, the few available reviews do not consider peach by-products as valuable materials for product design methodology. Thereby, a novelty of this review is related to the use of these mostly unexplored by-products as alternative sources of valuable components, encouraging the circular bioeconomy approach by designing new food products. Besides, this review presents recent peach production data, compiles briefly the extraction methods for the recovery of lipids, proteins, phenolics, and fiber from peach by-products, and also shows in vivo study reports on anti-inflammatory, anti-obesity, and anti-cerebral ischemia activities associated with peach components and by-product. Therefore, different proposals to recover bioactive fractions from peach by-products are provided, for further studies on food-product design.
Keywords: Design thinking, Circular economy, Bioactive ingredients, Sustainable processes
Introduction
The search for sustainable industrial processes has guided the production of chemicals and fuels from biomass-based economy (Arora et al., 2018). However, millions of ton of by-products from food processing industry are currently generated worldwide, being disposed of in landfills, used in composting or for animal feed (Rico et al., 2020). The industry is dealing with the increasing interest in naturalness as current and future trends in global trades. The companies, aware of the consumers demands, are continuously looking for process innovations to maintain the natural characteristics of the products. Therefore, understanding the concept of product design is highly relevant for the development of food products, warranting naturalness attributes. Firstly, the highest challenge for companies is understanding the customer’s necessities and capture their attention, transforming the consumer’s ideas into chemical and physical parameters of the final product, improving its appeal (Taifouris et al., 2020). Therefore, in order to attend these demands, the companies must follow modern and innovative technologies to provide competitive and sustainable products, resulting in optimized food production with less water and energy consume, and also better use of edible and non-edible parts of food raw materials and supplies. Consequently, the companies need to improve the design, optimization, and development of different formulations and techniques, before introducing new food products to the market (Granato et al., 2020).
Besides, it is essential to change the form of production, converting the linear economy into a sustainable circular bioeconomy. The philosophy of the dominant economic model of “take, make and dispose” must be urgently transformed into a sustainable model (Maina et al., 2017). For that, the concept of upcycled foods, which is the use of food ingredients or processed food materials, that would, otherwise, be discarded, must be properly understood (Goodman-Smith et al., 2021). Applying the concepts of circular economy and biorefinery is decisive to recover high-added value molecules since the growing demand for processed foods increases the generation of residues or by-products from the processed foods (Banerjee et al., 2018, 2019). Mostly, these biomasses are not fully exploited, with insufficient knowledge about their nutritional and economic values (Banerjee et al., 2018). Some fruit residues have already been studied considering the biorefinery concept, such as pineapple, orange, mango, and banana (Arora et al., 2018; Banerjee et al., 2019; Naranjo et al., 2014; Torre et al., 2019). However, no similar data have been found for peach by-products. Therefore, a search of Scopus database (http://www.scopus.com) was performed using a different set of Booleans concerning peach and its residues or by-products from studies published from 2010 to 2021. The search considered the title, abstract, and keywords of the published works, and the results are presented in Fig. 1.
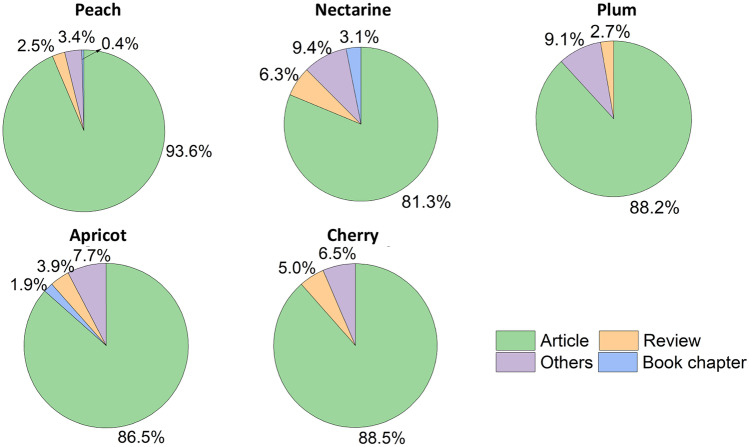
Fig. 1.
Publications about peach, nectarine, cherry, apricot and plum from 2010 to 2022 according to SCOPUS Database Platform (www.scopus.com). Source: the author
Regarding documents, the first search included the terms “peach” AND Prunus AND residue OR waste OR “by-product", showing 236 documents, represented by research articles (93.6%), book chapters (0.4%), review articles (2.5%), book chapter (0.4%) and other (3.4%). In order to expand the search and comparing with other fruits of the same genus such as nectarine, plum, apricot, and cherry, and using the same Booleans (Fig. 1), the results showed the peach fruit represented highest quantity of article (93.6%), followed cherry (88.5%), and plum (88.2%). These searches demonstrate the growing interest in peach by-products, although, there is still a lack of reviews to support the researches in this area. Those studies found from this search are related to wood residues from the peach plantation and the removal of pharmaceutical compounds using systems with different stone residues. Therefore, the present review deals with the contribution of peach by-products bringing a combined product design approach to the biorefinery concept considering its use as biomass, and as a potential source of bioactive compounds, highlighting in vivo studies.
Food production suffered major transformations in recent years, mostly stimulated by the increasing demand for sustainably processed foods, which must be considered safe, fresh, natural and with high-nutritional value (Granato et al., 2020). Due to the COVID-19 pandemic, the search for natural components with bioactivities such as anti-inflammatory has deeply increased because they may play a crucial role in many diseases, including viral infections. This is explained by the use of products enriched with bioactive compounds, which can contribute to human health and the immune system (Benvenutti et al., 2021).
Although there are few literature data related to the extraction of bioactive compounds from peach residues and by-products associated with biological activities, additional information on how to improve the use of these residues is still in need. In this context, this review focuses on peach industrial by-products, production data, chemical composition, and biorefinery and product design approaches for the recovery and use of these compounds.
Peach Fruit and Its Industrial Processing
Customers widely appreciate peach fruits (Prunus persica) for their taste, texture, juiciness, and nutritional value. According to genomic and phenotypic evidence, some reports accredit peach origin to southwest China (Patra & Baek, 2016). Besides, this fruit belongs to the Rosaceae family (Nowicka & Wojdyło, 2019), and nowadays it is estimated that there are currently more than 400 cultivars worldwide (Li & Wang, 2020). Therefore, peach is one of the most variable fruit species, with diverse germplasms, and different shapes, sizes, seeds, peel, and pulp colors (red, white, or yellow flesh). The fruit also presents a climacteric peak, where the respiration and evolution of ethylene show a drastic increase, compared to non-climacteric fruits. Therefore, because it is highly perishable, cold storage after harvesting is an important ally in reducing respiration rates and maturity associated to metabolic changes during senescence (Minas et al., 2018). The peach fruit is divided into three parts. The first is the pulp or mesocarp, corresponding to 75.2% of the fruit weight. The pulp is juicy, yellow and usually has an acid and sweet taste, varying widely, and with pH on average from 3.50 to 4.00 (Featherstone, 2015). The second part is the peel (exocarp), which represents 22.5% of the fruit. The last part is the stone, the endocarp, which is formed by the seed (inside), covered by a hard shell, denominated seed shell or kernel shell. The seed represents 5 to 12.5% of the fruit weight, depending on the peach specie (De França Sousa et al., 2018; Nowicka & Wojdyło, 2019). In general, the stones consist an average of 6% of seed and 94% of seed shell (Uysal et al., 2014). According to the Food and Agriculture Organization Corporate Statistical Database (FAOSTAT, 2020), the world production of peaches and nectarines in 2020 was over 24.5 million tones. Peaches and nectarines are very similar, and belong to the same specie (Prunus persica) and family (Rosaceae). For that reason, the data are presented combined (Carrasco et al., 2013). In 2020 production of the five continents were Asia 73.6%, Europe 14.9%, Americas 7%, Africa 4.3%, and Oceania 0.3%, where China, Spain, Italy, Türkiye, Greece, Iran, and the USA are the world leader countries in peach production (FAOSTAT, 2020). In 2020, Brazil occupied the 15th world position, with peaches and nectarines production of 201,880 ton.
Besides the economic importance, peaches have several nutritional benefits due to their high content of vitamin A, potassium and organic acids, sugars, and minerals, conferring interesting nutritional value (Manzoor et al., 2012). This fruit is highly consumed and destined for different food products such as jellies, sweets, and tea, among others. Besides, peaches are also used in formulations of various cosmetic products (Vásquez-Villanueva et al., 2015). Canned peaches in syrup cover 93% of the total processed peach products, while peach jam covers 6% and peach juice only 1% (Kamenidou et al., 2002).
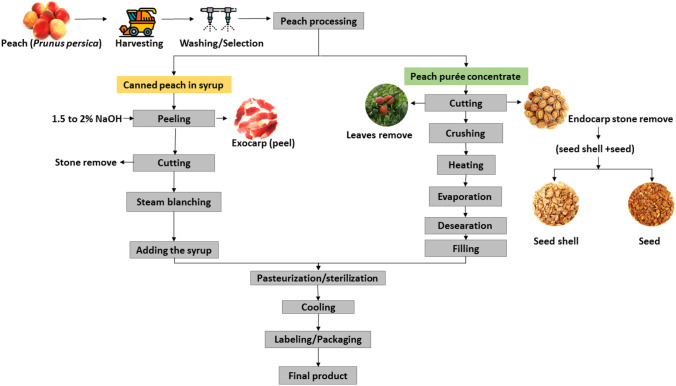
The peach industrial process depends on the final product, with the most popular represented by peach in syrup (canned or glass vessels) and concentrated peach purée, where the last is used as an ingredient for formulations such as baby food, juice, jams, pulp and yogurt (Aktağ & Gökmen, 2021). Figure 2 shows a flowchart of peach processing for these featured products and their respective generated by-products.
Fig. 2.
Processing steps of peach (purée concentrate and canned peach in syrup) and their by-products obtained. Source: The author
Basically, the processing of peaches in syrup consists of harvesting, selection, peel chemical removal (sodium hydroxide solutions from 1.5 to 2% concentration, near boiling temperature) (Featherstone, 2015), cutting stone removal and steam blanching. It is followed by filling the syrup at the glass or canned packaging, for final pasteurization. Peach purée concentrate is obtained by washing/selecting, removing the leaves and the stones (formed by seed and seed shell) (Fig. 2), crushing, heating (90–95 °C), evaporation (60–75 °C), deaeration, filling and sterilization (105–120 °C), and purée concentration (Aktağ & Gökmen, 2021), which is ready for packaging and commercialization.
The main residues generated from peach processing are peel, pomace, and stone. According to Plazzotta et al. (2020), around 15 million metric ton of peach are processed worldwide each year to produce juices, with approximately 10% of discard, depending on the fruit’s ripeness. Consequently, based on the annual global production and considering 10% of residues or by-products (Plazzotta et al., 2020), above 2.4 million ton of peach by-products are globally generated each year. Besides, considering the Brazilian production in 2020, about 20 thousand ton of peach by-products are generated each year in Brazil. The increase in fruit harvesting and processing, and the lack of proper handling methods and infrastructure, also increase the generation of by-products, which are poorly explored worldwide. Studies demonstrate that peach by-products contain oils (seed), anthocyanins (pomace), pectin (peel and pomace), and proteins (seed) (Faravash & Ashtiani, 2008; Pagán et al., 2001; Vásquez-Villanueva et al., 2015; Wu et al., 2011).
Normally, peach processing by-products are disposed in landfills, used as animal feed, burned, or used in steam production. Therefore, considering the possibility to improve the use of these by-products, new strategies have been developed for the recovery of relevant substances from these biomasses, such as bioactive compounds, vitamins, pectin, and phenolics, which could be recovered for further use in pharmaceutical, food, and cosmetic industry. Consequently, the following topics discuss relevant aspects for better use of peach by-products, and are valuable for applying the product design methodology to obtain derived products from the peach processing chain.
Chemical Composition
Considering that peach by-products are represented mostly by peel, pomace, and stone (seed + seed shell), it is important to investigate the components from these constituents to evaluate their potential uses.
Different studies have shown that fruit peels present several beneficial substances, such as flavonoids, hydroxycinnamic acids, flavanols, anthocyanins, and carotenoids, which are highly accumulated in the peel compared to the pulp of various fruits (Michailidis et al., 2021). Patra and Baek (2016) evaluated the peach peel composition and detected components such as chlorogenic acid, catechin, epicatechin, rutin, and cyanidin-3-glycoside. Additionally, Saidani et al. (2017) detected the following phenolic compounds from peach peel: Flavonols (quercetin-3-galactoside; quercetin-3-O-glucoside plus quercetin-3-o-rutinoside; kaempferol-3-O-glucoside), Hydroxycinnamic acid (neochlorogenic acid; p-coumaroylquinic acid; chlorogenic acid; 4-caffeoylquinic acid; caffeoylquinic acid derivative) and anthocyanin (cyanidin-3-O-glucoside). The content of flavonoids from peach peal ranged from 39.3 to 245 mg equivalent of catechins/100 g of fresh weight (fw) while for peach pulp these values ranged from 8.18 to 112 catechins/100 g of fresh weight, considering nine different peach cultivars. Besides, Saidani et al. (2017) indicated chlorogenic acid as the main hydroxycinnamic acid from peach peel (from 6.74 to 31.2 mg/100 fw), followed by neochlorogenic acid (1.02–7.98 mg/100 fw) and anthocyanins (from 0.24 to 17.6 mg cianidine-3-glycoside/100 g fw). These compounds were more representative from peach peal compared to peach pulp. In addition, Redondo et al. (2021) detected high amounts of quercetin from peach peel (7.1 mg 100/g fw), compared to other fruits such as plum (5.8 mg 100/g fw) and apricot (6.4 mg 100/g fw).
Nowicka and Wojdyło (2019) evaluated seeds composition of 20 different peach varieties. The authors detected (+)-catechin content ranging from 49.49 to 250.79 mg/100 g dry matter (dm). The flavan-3-ols dimers, procyanidin B1 and procyanidin B2, were detected in most seeds, with average content of 150.65 and 28.12 mg/100 g dm, respectively. The group of hydroxycinnamic acids was the second main polyphenolic group detected in peach seeds, after flavan-3-ols. These phenolic acids ranged from 130.94 mg to 2275.95 mg/100 g. In addition, the main carotenoids found were β-carotene and xanthophylls (mono- or di-hydroxylated carotenoids), zeaxanthin, β-cryptoxanthin, and violaxanthin (Lara et al., 2020).
The peach pomace is a by-product from the juice processing and contains the fruit peel and pulp. Several studies have shown that peach pomace is rich in pectin (Faravash & Ashtiani, 2007), while peach pulp has phenolic compounds and anthocyanins, with contents varying with peach species (Cevallos-Casals et al., 2006). Caffeic acid, protocatechuic acid, and gallic acid were also detected in peach pomace (El Darra et al., 2018). For instance, the methanolic extract presented from 100 to 449 mg of chlorogenic acid equivalent (CGA)/100 g of fresh pulp weight, while the main identified anthocyanin, the cyanidin 3-glycoside, varied from 6 to 37 mg of cyanidin 3-glucoside equivalents/100 g fresh pulp weight. Liu et al. (2018), evaluated the antioxidant potential from peel and pulp of four varieties of peach from China. Methanol/acetone extracts showed that peels have higher phenolic content, from 45.5 to 64.8%, compared to the pulp. Abidi et al. (2015) evaluated the aroma and phenolic compounds from nectarine Prunus persica (L. Batsch) genotypes, with results indicating this fruit, from the same family as peach, as a good source of phenolic compounds, especially ascorbic acid. The authors identified more than 60 volatile compounds, including 10 carboxylic acids, 10 aldehydes, 5 alcohols, 3 esters, 12 ketones, 8 lactones, and 12 terpenoids.
Finally, the lignocellulosic composition of peach stone consists mainly of lignin, cellulose, and hemicellulose, forming complex bonds on cell walls, where the lignin provides physical protection around the sugar fraction (Buratti et al., 2018). Uysal et al. (2014) indicated peach stone (seed shell + seed) is composed of 46% cellulose, 14% hemicellulose, and 33% lignin. For Instance, Hong et al. (2021) characterized the phenolic acids from the ethanolic extract (70%, 20 mL) from peach seed shell, identifying the main Hydroxycinnamic acid components as p-Coumaric acid 4-O-glucoside, 3-Caffeoylquinic acid, 3-Feruloylquinic acid, 3-p-Coumaroylquinic acid, m-Coumaric acid, Hydroxycaffeic acid. The second group was Hydroxybenzoic acids, formed by: Ellagic acid acetyl-xyloside, Protocatechuic acid 4-O-glucoside, 2-Hydroxybenzoic acid, 2,3-Dihydroxybenzoic acid, 3-O-Methylgallic acid. The third group was the Hydroxyphenylpropanoic acids: 3-Hydroxy-3- (3-hydroxyphenyl) propionic acid. The fourth group was Hydroxyphenylpropanoic acids: Dihydrocaffeic acid 3-O -glucuronide and 3-Hydroxy-3-(3-hydroxyphenyl) propionic acid. The main anthocyanin detected was Cyanidin 3-O- (2-O- (6-O- (E) -caffeoyl-D glucoside) -D-glucoside) -5-O-D-glucoside. These results indicate the high-value of the components present in the peach seed shell, which suggest possible uses in food, nutraceutical, and pharmaceutical industries.
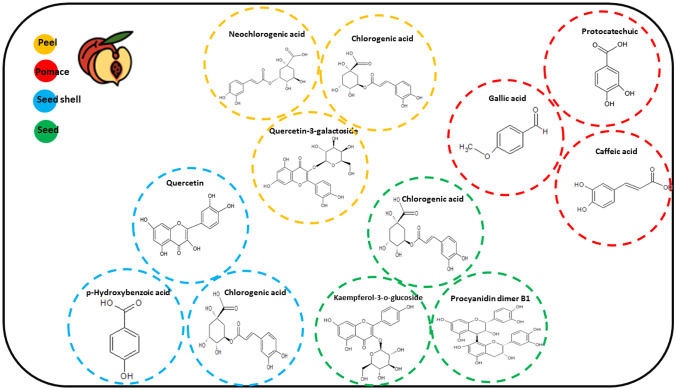
The main phenolic compounds detected from the peach by-products (peel, pomace and seed and seed shell) are highlighted in Fig. 3, based on the works by El Darra et al. (2018); Hong et al. (2021); Nowicka and Wojdyło (2019); Saidani et al. (2017). The main groups of detected compounds were hydroxycinnamic acids, flavonols, hydroxybenzoic acids, and procyanidins, which were detected by various researchers on peach by-products.
Fig. 3.
The main compounds and their respective chemical composition structures of the by-products (pomace, peel, and seed). Source: the author
Besides, some studies have evaluated that peach seeds are also rich in fatty acids and protein. For instance, Hao et al. (2019) used gas chromatography–mass spectrometry (GC–MS) to define the fatty acid composition from peach seed oil, indicating 86% of unsaturated fatty acids, within the total fatty acids content. The main unsaturated fatty acids were oleic (55.2%) and linoleic (30.8%) acids, while the main saturated fatty acids were palmitic (7.97%), stearic (2.37%), and α-linolenic (0.11%) acids. Peach seed extracts were obtained by Shukla and Kant (2020) using Soxhlet with different solvents, petroleum ether, chloroform, ethyl acetate, ethanol, and water. The results provided 29.36% of protein and 7.48% of crude fat from the dry seed. In addition to the high protein and fat contents, peach seeds are also rich in phenolic compounds (González-García et al., 2016; Sánchez-Vicente et al., 2009).
Peach by-products are considerable sources of nutrients such as lipids, proteins, fibers, and carbohydrates, and the centesimal composition for peach by-products (pomace, peel and stone) is summarized in Table 1. For instance, the oil and protein content from peach seeds represents 48.4% and 26.7%, respectively (Gettens, 2016; Lazos, 1991; Rahma & El-Aal, 1988) while the fiber content from the peach peel represents 57% (Pla et al., 2012; Yangilar, 2016), which makes seed and peel as interesting by-products. Besides, the most recent and relevant works about the chemical composition of peach by-products are summarized in Table 2. It shows the highest relevance of peach peel as a research subject, compared to other peach by-products, with most compounds evaluated by various chromatography methods.
Table 1.
Average centesimal composition of peach by-products, in percentage (%) of dry samples
| Fruit by-product | Carbohydrate | Fiber | Protein | Lipids | Ashes | Reference |
|---|---|---|---|---|---|---|
| Pomace | 13.06 | 38.85 | 5.32 | 0.37 | 2.13 | Pagán and Ibarz (1999), Pagán et al. (2001), Sarkar and Choudhury (2014) |
| Peel | 31.00 | 57.48 | 9.01 | 2.88 | 4.30 | Pla et al. (2012), Yangilar (2016) |
| Seed | 12.91 | 4.0 | 26.77 | 48.41 | 3.82 | Gettens (2016), Lazos (1991), Rahma and El-Aal (1988) |
Table 2.
Summarizes chemical composition data from peach by-products and the identification methods used
| Source | Work proposal | Identification technique * | Chemical composition | References |
|---|---|---|---|---|
| Identify anthocyanins from peach peel; the regulatory role of 1-methylcyclopropene and ethylene on anthocyanin accumulation, and the mechanism of ethylene-mediated inhibition of anthocyanin biosynthesis in the fruit | HPLC-TOF–MS | Cyanidin 3-Glucoside, Cyanidin 3-Galactoside, Cyanidin 3-Rutinoside, Delphinidin-3-Glucoside, Petunidin-3-Galactoside and Petunidin-3-Glucoside | Zhang et al. (2022) | |
| Identify and quantify the phenolics profile | UPLC-MS | Quercetin-3-Galactoside (FO1), Quercetin-3-O-Glucoside Plus Quercetin-3-O-Rutinoside (FO2) And Kaempferol-3-O-Glucoside | Saidani et al. (2017) | |
| Determine the polyphenols profile and antioxidant capacity from peach peel and pulp, of 6 different peach cultivars and one nectarine cultivar | HPLC–DAD | Flavonols (Quercetin-3-Rutinoside, Quercetin-3-Glucoside, Quercetin-3-Rhamnoside and Kaempferol-3-Rutinoside) | Stojanovic et al. (2016) | |
| Peel | Report the phenolic and vitamin C composition, in vitro antioxidant potencies and metal chelating activity of pulp and peel for five peach cultivars | HPLC–DAD | Hydroxybenzoic Acid (Protocatechuic Acid), Two Hydroxycinnamates (Chrologenic Acid, Neo-Chlorogenic Acid), One Flavan 3-Ols ((+)-Catechin) And One Flavonol (Quercetin-3-Rutinoside) | Liu et al. (2015) |
| Determine a time course of UV-B-stimulated transcription of genes involved in UVR8 signaling, in phenolics biosynthesis and their transcriptional regulators; phenolics quantification from peach peel | UHPLC-ESI-QTOF-MS | Cyanidin (2-(3,4-Dihydroxyphenyl) Chromenyl-3,5,7-Triol; Anthocyanins), (+)–Catechin (Flavanols), Luteolin (3 ′, 4 ′, 5,7-Tetrahydroxyflavone; Resveratrol (3,4 ′, 5-Trihydroxy-Trans-Stilbene; Stilbenes), 5-Pentadecylresorcinol (Alkylphenols), Hydroxycinnamic Acids, Sesamine (Furofuran Lignans) and Matairesinol (Dibenzylbutyrolactone and Dihydroxydibenzylbutane Lignans | Santin et al. (2019) | |
| Seeds | Recover phenolic compounds from the stone from peach (Prunus persica L.), nectarine (Prunus nucipersicaL.), plum (Prunus domesticaL.) and apricot (Prunus armeniaca L.) |
HPLC–PDA; LC-ESI-QTOF-MS/MS |
Gallic acid, Protocatechuic acid, p-Hydroxybenzoic acid, Chlorogenic acid, Caffeic acid, Catechin, Epicatethin, Epicatechin gallate, Quercetin and Kaempferol | Hong et al. (2021) |
| Separate components from Prunus persica kernel for possible development of anti-inflammatory, analgesic, and antipyretic medicinal agents from natural resources | LC–ESI–MS/MS | Amigdalin, Prunasin, Apigenin O-pentoside, Methylated flavonoid haxoside and Naringenin O-hexoside | Elshamy et al. (2019) | |
| Identify the peptides from peach seeds and their relation with the protective effect of genotypes in which they were identified | RP-HPLC-ESI-Q-TOF | Peptides contained high amounts of hydrophobic amino acids and imidazole-containing amino acids | Hernández-Corroto et al. (2018) | |
| Evaluate the oxidative stability, thermal behavior, antioxidant activity, phenolic content, and physicochemical properties of Prunus persica kernel oil | GC | Palmitic acid, Palmitoleic acid, Stearic acid, Oleic acid and Linoleic acid | Sodeifian and Sajadian (2021) | |
| Peptides in fraction PSH-3 kilodaltons (kDa) from peach seeds | LC-Q-TOF–MS/MS | Peptide isoleucine–tyrosine–serine–proline–histidine (IYSPH) | Vásquez-Villanueva et al. (2019) | |
| Pomace | To assess the binding capacity of the soluble peach fiber (SPF) as influenced by the microfluidization pretreatment and cellulase hydrolysis | HPAEC-PAD | Lactose, glucose, xylose,mannose, and fructose, rhamnose and anrabinose | Xu et al. (2015) |
*HPLC-TOF–MS high-performance liquid chromatography coupled mass spectrometry time-of-flight, UPLC–MS ultrahigh-pressure liquid chromatography coupled mass spectrometry, HPLC DAD high-performance liquid chromatography diode array detection, UHPLC/QTOF ultrahigh-pressure liquid chromatography coupled quadrupole-time-of-flight, UHPLC-ESI-QTOF-MS ultrahigh-pressure liquid chromatography coupled to a quadrupole-time-of-flight high-resolution mass spectrometer via an electrospray ionization system, HPLC–PDA LC-ESI-QTOF-MS/MS high-performance liquid chromatography photodiode array and liquid chromatography electrospray ionization quadrupole-time-of-flight mass/mass spectrometry, LC–ESI–MS/MS liquid chromatography electrospray ionization mass/mass spectrometry, RP-HPLC-ESI-Q-TOF reversed-phase high-performance liquid chromatography coupled to electrospray-ionization quadrupole-time-of-flight mass spectrometry, CG gas chromatography, LC-Q-TOF–MS/MS liquid chromatography quadrupole-time-of-flight mass/mass spectrometry, HPAEC-PAD high performance anion exchange chromatography coupled with a pulse amperometric detector. The data from Table 2 comprises recent publications from 2015-2021
Recovery of Valuable Components from Peach By-Products
Extraction methods are the most common operations used to obtain natural extracts from raw materials, for further use as a food ingredient (Zhang et al., 2018). Several extraction techniques can be used, from classical, or conventional procedures, to emergent or alternative methods. Maceration and Soxhlet (SOX) are within the conventional and well established most used methods. Otherwise, pressurized liquid extraction (PLE), supercritical fluid extraction (SFE), microwave-assisted extraction (MAE), and ultrasound-assisted extraction (UAE) are within the alternative procedures (Rudke et al., 2020). Nowadays, various studies reported the use of these emergent methods due to their advantages over conventional techniques, such as high yields or selectivity, and use of solvents generally recognized as safe (GRAS). Besides, these alternative methods are fast, have low energy and solvent consumption, and can be very selective towards target compounds. Then, these combined advantages can designate these methods as ecological processes with less environmental impact than traditional procedures (Martins et al., 2020). Various studies involving different extractions methods for peach by-products are analyzed as follows.
Plazzotta et al. (2020) studied the pomace from peach juice processing. Microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE), both methods using ethanol: water (70:30) as solvent at 80 °C, were applied for the recovery of antioxidant compounds from the peach juice pomace. The optimization of the processes indicates the best extraction conditions as 540 W during 50 s for MAE, and using 23% amplitude during 120 s for UAE. The extract samples recovered from the frozen peach residues provided higher flavonoids, anthocyanins, and vitamin C, compared to extracts from dry residues. Plazzotta et al. (2021) used two extraction methods with ethanol (70%) to obtain unpurified bioactive compounds for peach pomace, a conventional thermal extraction, at 50 °C for up to 90 min, and the pulsed electric field (PEF) technology with an energy input of 0.0014 to 2.88 kJ/kg. The processing time required for PEF was much lower, in the order of microseconds (μs), compared to conventional thermal extraction (average time of 40 min, reaching similar extraction efficiency than PEF). The bioactive fraction recovered by PEF from peach pomace reached total flavonoid content (TFC) of 86 mg QE/100 dm, total phenolics content (TPC) of 386 mg GAE/100 g dm, and DPPH value of 80 mg TE/100 g dm. Otherwise, the conventional thermal treatment, set at 50 °C, provided maximum values for TPC, TFC and DPPH of 524 ± 27 mg GAE/100 g dm, 108 ± 1 mg QE/100 g dm and 143 ± 7 mg TE/100 g dm, respectively. Although, the traditional extraction method shows samples with the highest values of TPC, TFC, and DPPPH, the PEF method is a promising technology because it has lower energy costs than traditional methods, and recovers valuable compounds.
Adil et al. (2007) recovered polyphenols from peach pomace by subcritical extraction with CO2 added with ethanol. The effect of the process parameters, pressure (20–60 MPa), temperature (40–60 °C), ethanol concentration (14–20% by weight), and extraction time (10–40 min) were studied. The results indicate that the increase in all parameters significantly contributes to the recovery of total phenolics content (TPC), and DPPH performance to evaluate the antioxidant activity. The optimum conditions were 51 MPa, 52.3 °C, 20% ethanol content and 40 min of extraction. TPC values were up to 0.26 mg of gallic acid equivalent/g of dried sample, with AE of 1.5 mg of DPPH /mg dried sample. Faravash and Ashtiani (2008) recovered pectin from peach pomace in an acid medium (with HCL at 60 °C) followed by precipitation with ethanol (96%). The experiments were carried out using different HCl volumes (35, 65, 105, and 130 mL), acid washing times of 30, 60, and 180 min and ethanol:extract ratios of 0.5, 1, and 1.5. The maximum pectin recovery yield was 9.94 ± 0.2%, obtained using 65 mL of HCl, the extraction yield of 1.5, and the acid washing time of 120 min.
Mezzomo et al. (2010) recovered the oily fraction from peach seed (also called peach almond) by different methods: Soxhlet with different solvents (n-hexane, dichloromethane, ethyl acetate and ethanol), hydrodistillation, and ethanol maceration followed by partitioning with the solvents n-hexane, dichloromethane, ethyl acetate and ethanol. The Supercritical Fluid Extraction (SFE) was conducted, to compare with the traditional low-pressure methods, at the conditions of 30, 40 and 50 °C and at 100, 200 and 300 bar, carried out with CO2 and ethanol as co-solvent in concentrations of 2% and 5% (w/w). The Soxhlet with ethanol provided higher yield values compared to SFE. The main fatty acids detected from the oily fraction were oleic and linoleic acids, particularly for the samples recovered by Soxhlet with ethanol: water mixture (50:50 v/v) and SFE with 5% ethanol at 50 °C/300 bar. The authors concluded that peach seeds are an important source of edible oil with high content of unsaturated fatty acids that have health benefits.
Peach seeds were used by González-García et al. (2016) for the recovery of protein. The seeds were first defatted with hexane and washed with MeOH/H2O and acetone/H2O. The extraction, using a native buffer and a denatured buffer as solvents, was conducted by applying sonication for 10 min, followed by gentle shaking overnight. The recovered protein was precipitated using acetone at 1:2 ratio (extract:acetone), and stored in freezer overnight. The method of Combinatorial Peptide Ligand Libraries (CPLLs) aids the reduction of the dynamic range of protein concentration, contributing to identify higher number of proteins compared to conventional methods. The results from CPLLs enable the identification of 97 unique genetic products from peach seeds. In addition, 14 bioactive peptides were also detected within the protein sequence from peach seeds. The results from this work suggest that the bioactive peptides recovered from peach seeds are valuable and worth investigating, as proteins are important for human nutrition and present high added value.
Several extraction methods have been studied to obtain different valuable fractions from peach by-products. These procedures are summarized in Table 3, which contains the extraction methods applied to peach by-products, the solvents and operating conditions used, and the main results obtained. The data from Table 3 represent the most recent publications on this subject. However, these available works have not explored the concept of biorefinery applied to peach by-products, nor discussed the food design approach to value the recovered fractions from peach by-products or residues. These relevant aspects, addressed to improve the peach processing chain, are presented in the next sections.
Table 3.
Summarized data from extraction procedures applied for peach by-products: methods, solvents, operational conditions; main results
| Source | Extraction | Solvent | Extraction parameters | Compound | References |
|---|---|---|---|---|---|
| Maceration | Methanol and acetone | One gram of sample and 20 mL of acidic methanol–water (50:50, v/v, pH 2) at room temperature/1 h. 20 mL of 70% aqueous acetone added to the residue, followed by stirring, shaking and centrifugation | Phenolic compounds, flavonoid and L-ascorbic acid and antioxidant assay | Liu et al. (2015) | |
| Ultrasound | HCl concentrations (0, 0.1, 1 or 2%) | 30 mL of 80% (v/v) acetone solution/60 min | Polyphenolic compounds | Stojanovic et al. (2016) | |
| Ultrasound | HCl concentration 0.01% | Independent variables: potency range (10–50%), sample mass (0.1–0.5 g) and aqueous ethanol (EtOH) concentration (20–80%, v/v) and fixed 15 min | Total phenolic content, anthocyanin, p-hydroxybenzoic acid and p-coumaric acid | Kurtulbaş and Şahin (2022) | |
| Peel | Homogenizer-assisted extraction | Deep eutectic solvent (lactic acid/glycerol mixture (1/1) | Independent variables: water (10, 30, and 50%, v/v), time (30, 60, and 90 s), speed (6000, 8000, and 10,000 rpm) | Total phenolic content and chlorogenic acid | Şahin and Mehmet (2022) |
| Microwave assisted extraction | HCl 0.01% (v/v) | Independent variables: Power (350–500 W), time (30–90 s) and aqueous ethanol (EtOH) concentration (20–80%, v/v) | Total phenolics content, total anthocyanins content, p-hydroxybenzoic acid and p-coumaric acid) | Kurtulbaş et al. (2022) | |
| Maceration | With 5 mL of 5% HPO3; 10 mL MeOH/H2O/formic acid (60:38:2 v/v/v); 10 mL water | Adding 5 mL of 5%; (60:38:2 v/v/v); 10 mL | Ascorbic acid, total phenolics content, flavonoids, polyphenols and antioxidant capacity and sugar | Saidani et al. (2017) | |
| Maceration | 1 mL + 1 mL 95% EtOH centrifuged 4500 RPM 10 min diluted with 50% EtOH |
Extracted at 25 °C by ethanol (absolute, ≥ 99.5%) (400, 1500 and 250 mL) (3 × 72 h) |
Total phenol, flavonoids and carotenoids | Loizzo et al. (2015) | |
| Extraction Soxhlet | Petroleum ether, chloroform, ethyl acetate, ethanol, and water | 60 cycles of siphoning, completed with each solvent and extraction continued until siphon tube became colorless | Alkaloids, carbohydrate, glycosides, inulin, protein, steroids/triterpenoids, fixed oils and fats, flavonoids, phenolic, compound and tannins, gums and mucilage | Shukla and Kant (2020) | |
| Seed | Sonication (10 min) | MeOH/H2O (80:20), acetone/H2O (80:20) | Extract:acetone ratio of 1:2 and centrifuged (30 min, 13,400 rpm) | Bioactive peptides | González-García et al. (2016) |
| Ultrasound | 5 mL of extraction buffer (100 mM Tris–HCl (pH 7.5) | 1 min at 30% of amplitude, after centrifugation at 4000 g/10 min. Supernatant collected, added to 10 mL of cold acetone, and kept in the fridge for 15 min | Peptides | Vásquez-Villanueva et al. (2015) | |
| Pomace | Microwave (MAE), ultrasound assisted extraction (UAE) | Ethanol:water (70:30) | Optimal MAE (540 W, 50 s) and UAE (23%, 120 s) | Polyphenols, flavonoids, anthocyanins, and vitamin C | Plazzotta et al. (2020) |
| Pulsed electric fields (PEF), Conventional thermal treatment (CTT) | Ethanol:water (70:30) | PEF at 50 °C and 90 min and PP dispersions were thus treated at 0.8–10 kV/cm, using 4–30 monopolar pulses of 4 μs | Bioactive extracts | Plazzotta et al. (2021) |
The data from Table 3 comprises recent publications from 2015-2021
Biorefinery Concept for the Processing of Peach By-Products
The biorefinery concept arises from the need to reduce the existing dependence on fossil-based resources to cover the rising demand for energy, fuels, chemicals, polymers, and oil (Torre et al., 2019). Biomass and food by-products are also considered a potential source of high-added value chemicals such as fermentable sugar, furfural, citric acid, pectin, proteins, lipids, phenolic compounds, among other substances (Maciel-Silva et al., 2019; Ortiz-Sanchez et al., 2021). The food by-products are abundant, renewable and inexpensive bio-residues, or biomasses, which can be fully processed to obtain valuable bioactive compounds, as an interesting alternative from economic and environmental aspects (Gullón et al., 2020). Besides, the adequate processes for the recovery of these valuable compounds, allied with the appropriate pre-treatments to modify the cellulose, lignin, and hemicellulose, converting the lignocellulosic biomasses, are of upmost relevance, contributing to efficient conversion of underestimated by-products, such as peach residues (Manara et al., 2014). These methods can be applied, individually or combined, in a biorefinery approach, preferably represented by ecological and sustainable processes to attend the Sustainable Development Goals (SDG) of the United Nations (UN). The growing interest in this area expands the application of new technologies, enabling the development of innovative products and businesses, expanding the portfolio of the existing companies, and contributing to achieving new markets.
Newest approaches have been showing the use of a deep eutectic solvent (DES) as a relevant alternative for the recovery of valuable phytochemicals from food products (Benvenutti et al., 2019). Şahin and Bilgin (2022) used a lactic acid/glycerol mixture (1/1) and the addition of water (10, 30 and 50% v/v) to recover bioactive compounds from the peach peel. The results showed that with the use of DES with the addition of 50% water, at the optimized condition of 90 s and 8400 rpm, the recovered extract presented a polyphenol content of 10.68 mg-GAE/g-dried and chlorogenic acid 2.69 mg/g-dried. Skiba and Vorobyova (2022) used a green solvent named NADES (natural deep eutectic solvent) composed of lactic acid, glucose, and water (5:1:3) to obtain phenolic compounds from peach pomace, and the main identified compounds were chlorogenic acid (1.04 mg/g extract), gallic acid (1.04 mg/g extract), and ferulic acid (0.115 mg/g extract). This extract was compared with plasma-assisted extraction with water, which provided higher antioxidant activity in terms of 50% inhibition concentration (IC50 of 1.05 mg/mL) compared to the extract recovered by NADES (IC50 of 1.3 mg/mL). Mármol et al. (2021) evaluated the extraction of bioactive compounds from peach pomace using ethanol: water (w/w) ratio (70:30) as a solvent, and followed by homogenization at 1600 rpm for 2 min. The results showed that the most representative phenolic component recovered was the ellagic acid (28.26 mg/100 g).
These works indicate that peach presents interesting phytochemical compounds that were detected in peach peel and pomace. Using green solvents at different extraction methods is an alternative to promote the valorization of these biomasses, which is in line with the biorefinery concept. Nevertheless, the optimization of a selected extraction procedure, preferably performed on a laboratory scale, is high relevance for the industrial processing of agro-industrial by-products (Martins et al., 2020).
In Brazil, there is a huge generation of residual biomasses, mostly agro-industrial residues or by-products, which has not yet received much attention as a direct energy source, such as biodiesel. Biodiesel is a fuel obtained from vegetable oils, animal fats, or residual vegetable oils (Longati et al., 2020). According to Ribeiro et al. (2018), the highest concentration of biodiesel processing plants in Brazil is located in the South region, and the most used raw materials are soybean oil (70%), beef fat (17%), and used frying oil (1%). These examples show the limited use of other biomasses, although several studies have been indicating other abundant and renewable plant-based biomasses as valuable alternatives for the application of the integrated biorefinery concept. Among these commodities and important fruits, we can mention: soy, orange, corn, tamarind and mango (Longati et al., 2020; Ma et al., 2021; Martins et al., 2020; Monteiro et al., 2021; Ortiz-Sanchez et al., 2021). As already mentioned, because peach is an important worldwide commodity, some researchers have already proposed the use of peach residues as a source of high-value products. However, there is a lack of studies related to the use of integrated paths, in a biorefinery concept, for the valorization of this residue. Therefore, besides biofuel production, the biorefinery concept involves biomass conversion to produce biofuels, energy, and also valuable chemicals (Cherubini, 2010). Consequently, following the concept of circular bioeconomy (principles related to reuse, reduction and recycling), the process integration, allows the recovery of different products from the original biomass and also improves sustainability. Yet, industrial units still have a considerable challenge in combining these two concepts.
Bioactivities Associated to Peach By-Products
Most literature studies about peach by-products are related to the bioactive compounds and antioxidant activity associated with peel, pomace, seeds, and seed shell. To justify the significance of these peach by-products, as sources of biological active components, the most recent and relevant studies concerning this subject are discussed below, highlighting biological activities such as antimicrobial, antioxidant, antidiabetic, anti-obesity, anti-inflammatory, and anti-cerebral ischemia.
Antioxidant Activity
Hong et al. (2021) evaluated the antioxidant activity and the phenolics content of the ethanolic extract (70%, 20 mL) from the shell of the peach seed. The results show the total phenolics content (TPC) of 0.47 (mg gallic acid equivalents (GAE)/g), total flavonoids content (TFC) of 0.18 (mg quercetin equivalents (QE)/g), total tannins content (TTC) of 0.07 (mg catechin equivalents (CE)/g), while the antioxidant activity by 2,2′-Diphenyl-1-picrylhydrazyl antioxidant assay (DPPH) was 0.98 (mg ascorbic acid equivalents (AAE)/ g), while by ferric reducing-antioxidant power (FRAP) assay was 0.54 (mg AAE/g), and by 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay was 0.43 (mg AAE/g), with total antioxidant capacity (TAC) of 0.27 (mg AAE/g), estimated by phosphomolybdate method. According to Hong et al. (2021), these results show higher radical scavenging capacity compared to other seed shells such as nectarine (0.23 mg AAE/g), plum (0.21 mg AAE/g), and apricot (0.25 mg AAE/g).
Another relevant study, by Nowicka and Wojdyło (2019), shows that the total polyphenols content of peach seeds, from 20 different cultivars, ranged from 3.8 to 12.7 g/100 dry matter, while the cyanogenic glycoside content ranged from 17.4 to 245.7 mg/100 of dry matter. The main identified polyphenols were from Flavan-3-ols group, (+)-catechin, (−)-epicatechin, (−)-epicatechin gallate; and from Polymeric procyanidins group (B1, B2 and procyanidin A2). The main phenolic acids were Hydroxycinnamic acids (chlorogenic acid, neochlorogenic acid, 3-O-p-coumaroyluinic acid and cis-5-p-coumaroylquinic acid, 2-O-caffeoyl-L-malate, phenylpropanoid o-diphenol phaselic acid) and Hydroxybenzoic acids (ellagic acid, dilactone of hexahydroxydiphenic acid). The peach seeds were also characterized by high antioxidative potential and the ability to inhibit enzymes linked to obesity, type 2 diabetes and Alzheimer’s disease. Generally, confirmed that peach seed could be a valuable source of polyphenols used by the pharmaceutical industry.
Redondo et al. (2021) detected and quantified the polyphenols and evaluated the antioxidant activity from peach pulp, peel and seeds, from fruits in commercial maturity. The evaluated extracts were recovered by methanol: water solution (80:20; v:v). The results from peel extract showed the presence of quercetin 6.2 mg/100 g, chlorogenic acid 3.9 mg/100 g, and neochlorogenic acid 7.1 mg/100 g, while from seed extract, the detected and quantified compounds were quercetin (5.5 mg/100 g), chlorogenic acid (4.0 mg/100 g), neochlorogenic acid (4.9 mg/100 g), and ferulic acid (5.7 mg/100 g). As for the pulp, the results detected were quercetin (5.4 mg/100 g), chlorogenic acid (1.2 mg/100 g), and neochlorogenic acid (4.8 mg/100 g). These values suggest that the peach seeds present relevant content of phenolic compounds compared to other fruit parts, and the antioxidant potential provided by the seed extracts confirm this behavior. For instance, the results by FRAP method provided the highest antioxidant capacity for peach seed (3.3 mmol Trolox/100 FW), while peach peel was 2.2 mmol Trolox/100 FW and pulp 0.2 mmol Trolox/100 FW. However, it is essential to note that the values vary with the conditions used to recover the extracts, such as type of solvent, extraction technique, fruit maturity, and harvest conditions (soil, geographical aspects and plant variety). The study by Redondo et al. (2021) reveals the potential of peach by-products as a source of bioactive compounds with different potential uses, such as in pharmaceutical, cosmetic, food, or animal feed formulations. It is also important to highlight that the peach pomace contains pulp and peel. El Darra et al. (2018) recovered polyphenols from peach pomace by solid–liquid ratio 1:10 (w:v) extraction using β-cyclodextrin and ethanol as solvent, and identified the components gallic acid, caffeic acid, and protocatechin from the recovered extract. The mixture of β cyclodextrin and ethanol selectively enhanced the extraction of gallic and caffeic acids with yields 220 and 328 μg/g dry matter, respectively, while ethanol was more selective to protocatechinic (4899 μg/g dry matter).
Mokrani and Madani (2016) investigated the effect of the following extraction parameters (solid/liquid) of peach fruit (Prunus persica L): solvent type (ethanol, methanol, acetone and water), time (30–450 min), and temperature (25–70 °C). The recovered extracts were evaluated in terms of total phenolics content (TPC), total flavonoids content (TFC), and antioxidant potential. Optimized extraction condition provided a peach extract with high values of TPC, TFC, DPPH radical scavenging activity and FRAP activity, referent of 363 mg GAE/100 g, 81 mg QE/100 g, 48% inhibition percentage and 317 mg AAE/100 g, respectively. With these results, and comparing with data presented by Hong et al. (2021), Nowicka and Wojdyło (2019) and by Redondo et al. (2021), it is possible to highlight that peach by-products have relevant bioactive compounds, comparable to the edible part of the fruit. Thus, further studies should be carried out to enhance the use of these by-products as a source of relevant bioactive compounds, and add value to these biomasses.
Antimicrobial Activity
Patra and Baek (2016) evaluated the biological synthesis of gold nanoparticles (AuNPs) generated from peach peel aqueous extract. The extract showed strong antibacterial synergistic activity when combined with the positive control substances kanamycin (inhibition zone from 9.38 to 20.45 mm) and rifampicin (inhibition zone from 9.52 to 25.23 mm), and also synergistic anticandidal activity when combined with amphotericin B (inhibition zone from 10.09 to 15.47 mm) for five pathogenic Candida species. In addition, the extract presented strong antioxidant potential by elimination of 1,1-diphenyl-2-picrilhidroxil radical, nitric oxide, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical. Although the significant antimicrobial potential detected for peach peel, there is a lack of information related to this important bioactivity associated with peach by-products, which should be further investigated.
Antidiabetic and Anti-obesity
Kan et al. (2020) investigated the influence of peach peel phenolic extract in preventing lipid accumulation and intestinal microbiota composition of mice with high-fat diet-induced obesity. Extracts were obtained with methanol by ultrasound technique (400 W, 40 °C) for 3 h. The recovered extracts showed that peach peel significantly inhibited lipid accumulation in mice fed with a high-fat diet. Meanwhile, treatment with peach peel phenolic extract regulated the expression levels of mRNA involved in lipid metabolism. Furthermore, the results showed that the extract regulated the intestinal microbiota composition, increasing the relative abundance of Lactobacillus, Bacteroides, Lachnospiraceae, Prevotellaceae Alloprevotella, Akkermansia, Roseburia, and Ruminococcus. This effect might be attributed to the phenolic content and composition of the peach peel extracts, showing the relevance of this by-product to provide a functional food additive to improve lipid metabolic abnormalities associated with obesity. Based on what has been discussed so far, few studies have evaluated the potential of by-products activities. Thus, some studies are still focused on the edible part of the fruit. For instance, Nowicka et al. (2018) provided important insights on properties of the extract from the pulp of Prunus persica L. Batsch, obtained by sonication with methanol:H2O (80:20%, v/v) + 1% HCl at 20 °C for 15 min. Results from in vitro analysis show the potential to inhibit enzymes relevant for the management of type 2 diabetes (α-amylase, α-glucosidase) and obesity (pancreatic lipase). The yellow peach extract, with high carotenoids content (varieties Harrow Diamond and Harrow Beauty), showed high inhibitory activity towards porcine pancreatic lipase.
Sharma et al. (2018) evaluated the antidiabetic activity of extract from leaves of Prunus persica. The extract prepared by percolation with 90% ethanol at room temperature was concentrated under reduced pressure at 50–55 °C and used at in vivo assays with adult male rats. The results show that 200 mg extract/kg provided significant anti-hyperglycemic activity. Improvement in rat body weight and lipid profile was also observed by streptozotocin induced diabetic rat model. The extract good performance suggests its possible use as a natural drug candidate for diabetes mellitus treatment.
Song et al. (2019) investigated the anti-obesity effect of extracts from flowers of Prunus persica L. Batsch, obtained with distilled water at 100 °C for 2 h under reflux. The results from in vivo assays using peach flower extract show a significant reduction in body weight, abdominal fat mass, serum glucose, alanine transaminase and aspartate aminotransferase levels, and liver and spleen weights from mice. Besides, the extracts also reduced the levels of glucose, cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and leptin, while the levels of AST/ALT and adiponectin/leptin ratios and adiponectin were increased. The results suggest an anti-obesity effect of the extract in obese mice, which may improve the hepatic lipid metabolism by reducing lipogenesis and increasing fatty acid oxidation.
Wang et al. (2017) evaluated the antidiabetic activity potential of peach gum polysaccharides, from peach tree trunks and fruits. The peach gum exudate was dissolved in boiling water at a ratio of 1:100 (w/v), and filtered to obtain the supernatant solution. The concentrated solution was precipitated overnight in four folds of ethanol, for purification, and the resulting polysaccharides were dried. The peach gum polysaccharide reduced significantly (42.76%) the postprandial blood glucose in streptozotocin-induced diabetic mice. Histology and immunohistochemistry results confirmed that the polysaccharide, consisting of arabinose, xylose, and galactose, partially restored the pancreatic islets in diabetic mice, and enhanced the expression of pancreatic duodenal homeobox-1, insulin and hexokinase-1. The results may suggest the peach gum-derived polysaccharide as a relevant non-insulin therapy for diabetes treatment, probably due to the presence of the detected compounds.
Anti-inflammatory Potential
Elshamy et al. (2019) prepared seed extract from nectarine (Prunus persica,variety nucipersica) by maceration with methanol 70%, a fruit from the same family as peach. The acute anti-inflammatory effect of the extract was evaluated by a carrageenan-induced rat hind paw edema test. The nectarine seed extract was administrated in rats at doses of 50 and 100 mg/kg reducing significantly the paw edema by 11 and 47%, respectively. The results suggest that alcoholic nectarine seed extract is a safe natural anti-inflammatory, providing analgesic activity through central and peripheral mechanisms. Furthermore, the authors evaluated the content of total polyphenols (55.91 ± 5.78 mg/g) and flavonoids (29.89 ± 0.55 mg/g), indicating that the extract is a promising source of these constituents, which are possibly related to the anti-inflammatory effect.
Another interesting study concerning the edible part of the fruit was conducted by Kim et al. (2017) which prepared white-flesh peach extract (WFPE) using ethanol 80% for 48 h at room temperature. The study evaluated the effect of the extract on the excretion of nicotine and 1-hydroxypyrene metabolites in smokers and chronic nicotine-induced tissue damage in mice. The WFPE inhibited nitrotyrosine expression and inflammatory responses in liver, kidney, and lung tissues of nicotine-treated mice. The results showed that WFPE increased the metabolism of toxic smoke components of tobacco in smokers and protected normal tissues against nicotine toxicity in mice. Although this research has been conducted for peach edible parts, and no anti-inflammatory studies were conducted for peach by-products, the peach flesh is also part of the pomace, therefore, some of these activities could be also associated to the pomace (by-products), and should be further investigated to confirm this active behavior.
Seo et al. (2020) evaluated the anti-inflammatory potential of the methanolic extract from P. persica on lipopolysaccharide stimulated glial cells. Glial cells are immune cells from the central nervous system, with a key role in numerous neurodegenerative diseases, including Alzheimer’s, Parkinson’s and Huntington’s disease. We used different parts of the peach fruit (leaves, fruits, and twigs) to obtain the methanolic extracts, prepare by sonication (1,500 W, 40 kHz) frequency for 15 min at 2 h intervals for 3 days. The results indicated that the combined methanolic extracts reduced the transcription of several pro-inflammatory enzymes (nitric oxide synthase and cyclooxygenase-2) and cytokines (tumor necrosis factor-α, interleukin (IL)-1β and IL-6) in lipopolysaccharide-stimulated cells. Furthermore, the extract inhibited the activation of nuclear factors and several mitogen-activated protein kinases necessary for the transcription of the pro-inflammatory mediator. Therefore, it can be used as a potential therapeutic agent for neurodegenerative diseases and neurotoxicity by suppressing glial cell activation. The evaluated extract presented chlorogenic acid, catechin, quercetin, and quercetin-3-O-glycoside, and the anti-inflammatory effect on PPB on BV2 cells may be due to the presence of phenolics and flavonols.
Cerebral Ischemia
Li et al. (2020) studied aqueous extract mixtures from peach seeds and rhubarb (Rheum palmatum L) rhizome, and its effect on cerebral ischemia/reperfusion injury (I/R), using different mixture proportions (peach seeds: rhubarb) (m/v), 1:0, 2:3, 1:1, 3:2 and 0:1. The results demonstrate that the extracts effectively reduced the neurobehavioral defect scores, the areas of cerebral infarction caused by cerebral ischemia and pathological brain tissue injury, and inhibited abnormal increases in inflammatory factors. Also, the extracts alleviated the I/R damage by altering the uncontrolled expression of the adenosine protein (ADORA2A). The extract that provided the best regulatory effect was obtained from a 1:1 ratio of peach seed: dried rhubarb root.
Other Applications
Different applications from peach by-products have been studied and presented in the literature. For instance, applications in pharmaceuticals, cosmetics, food products, and energy industries were discussed. Fratebianchi et al. (2017) used peach pomace as a source of carbon and energy to produce polygalacturonase in submerged cultures using Aspergillus Sojae. Papaioannou and Liakopoulou-Kyriakides (2012) studied peach peels as carbon source for Blakeslea trispora culture for carotenoid production. The results indicate 76% of β-carotene production, and this high performance suggests the use of this residue as substrate culture for carotenoids production on industrial scale.
Li et al. (2018) evaluated the chemical characteristics of peach seed shells, the sugar yields, and the impact of Deep Eutectic Solvent (DES), formed by choline chloride 1:2: lactic acid, as biomass (seed shell) pre-treatment, affecting physical and chemical properties and lignin extraction. The results from this biomass, compared to typical woody and herbaceous biomass, exhibit significantly higher bulk density and hardness. Enzymatic saccharification of peach seed shells treated with DES provided a high glucose yield (above 90%). DES pretreatment removed 70.2% of lignin from peach seed shell, while the pretreatment with dilute acid and alkali recovered 22.2% and 48.7% of lignin. Besides, the enzymatic hydrolysis of lignin using Cellic® CTec2 and HTec2 recovered up to 90% sugar yield from the sample pretreated with DES, while the sample pretreated with alkali provided 57.3% sugar yield, and pretreated with dilute acid provided 49.7% sugar yield. Therefore, this biomass is interesting for the conversion of biofuels and chemicals, especially using DES pretreatment, which provides a high influence on lignin fractionation.
Uysal et al. (2014) produced activated carbon from peach stone (seed + seed shell) with zinc chloride activation. The first step was carried out at different temperatures, 300 °C and 400 °C, to obtain the bio-oil. The second step consisted of activating the pre-carbonized coal after impregnation with zinc chloride at 500 °C to 700 °C. The results showed that the pre-carbonization temperature had a significant effect on the surface area of the activated carbon, and that the produced bio-oil had fungicidal activity against the Coriolus versicolor. In addition, the adsorption capacities of activated carbons for phenol and methylene blue were 51.6–64.9 mg/g and 104.2–121.9 mg/g, respectively.
Product Design: Functional Food Product
Functional foods are natural or industrially processed food products that, when regularly consumed at satisfactory levels in a diversified diet, have positive health effects beyond basic nutrition (Granato et al., 2020). The Functional Food Center (FFC) from USA (Texas, USA) is committed to leading research on functional foods as well as bringing the functional foods to the mainstream worldwide food markets, and introducing a new approach for the definition of functional foods (Martirosyan & Singh, 2015). This new concept describes functional foods as products containing biologically active compounds in effective and non-toxic quantities, derived from natural or processed food sources, and clinically tested, based on specific biomarkers, providing health benefits for prevention, management or treatment of chronic diseases or their symptoms (Alongi & Anese, 2021). Therefore, functional foods must be regulated by the Food and Drug Administration (FDA) before approval, and are suitable if they are constituted by compounds Generally Recognized as Safe (GRAS).
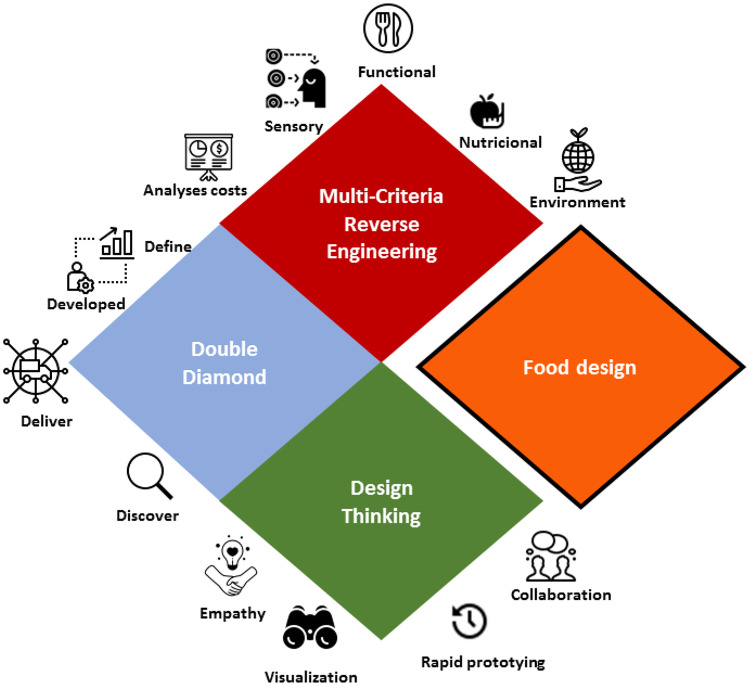
Given the above assumptions about functional foods, the increase in various types of chronic diseases has motivated the food industry to develop food products that help consumers to prevent these diseases and provide other health benefits (Alongi & Anese, 2021). According to Micheli et al. (2019), the design thinking concept could be utilized in different areas including public policy, education, healthcare, politics, and social and economic development. This concept has emerged in sustainability literature to generate positive economic, social, and environmental impacts, in association with business transition, food innovation, and applied business innovation (Massari et al., 2021). Meanwhile, the use of design thinking, double diamond, and multi-criteria reverse engineering methodologies (Fig. 4) is fundamental to reach the consumer’s new needs, and also to elucidate the industrial challenges for the use of by-products as a source of bioactive and functional compounds. Therefore, the health benefits from peach by-products (discussed previously) may suggest its industrial use to provide functional foods. Although, the long way that has to be crossed to produce these new products can be facilitated by the product design methodology, as discussed below.
Fig. 4.
Methodologies design thinking, double diamond, and multi-criteria reverse engineering approach in food design. Source: the author
The design thinking method is based on solving problems and has become an important approach for food design. According to Olsen (2015), this methodology can contribute to food innovation using empathy, visualization, rapid prototyping, and collaboration (Fig. 4). According to Tkaczewska et al. (2021), the method to create products must be economically and technologically viable, following the aspects: (I) observation and synthesis, (II) visualization and rapid prototyping, and (III) revising and refining. For Batat and Addis (2021), the design thinking concept is a high contribution to food innovation, when designing sustainable food products and services that respond to the needs of consumers.
Another methodology proposed for food design is Multi-Criteria Reverse Engineering (MCRE). This technique simultaneously considers several criteria of the product's properties, increasing the complexity of the problem. For instance, MCRE enables the selection of adequate process conditions for designing or redesigning food, food processes, and food-related systems (Thomopoulos et al., 2019). According to Dima et al. (2020), the MCRE as a design method for functional foods considers the following aspects: to develop a nutritional, sensorial, and functional evaluation of the food product, attending the consumer’s needs; to select target bioactive components, its nutraceuticals distribution systems, encapsulation technique, food matrix, or the excipient; to choose the packaging method, analyze costs, impacts on the environment; to analyze the perception of the food product, and, finally, to test the bioavailability of nutraceuticals incorporated into the food product (Fig. 4); therefore, combining several specific criteria for functional food design.
The Double Diamond model, another product design methodology also known as 4D (Sijtsema et al., 2020), consists of four steps: discover, define, develop, and deliver (Fig. 4). The first step (Discover) is essential to establish new opportunities, markets and processes, and is performed with the aid of literature data. The “Define” step is fundamental to select insights to demarcate and redefine the study topic, accompanied by the definition of the adequate technologies, ingredients, and packages. The third step is to “Develop” the design refine it, and teste it with multidisciplinary teams, which involves developing samples/concepts with different processing, ingredients, packaging, target groups, and sensory research. The last step, “Deliver”, is the final prototype (food design), packaging and distribution, which are tested, produced and launched.
These models can help the product design by combining different tools that can be a solution to obtain innovative products aligned with sustainability, reducing agro-industrial by-products or improving their use and value. Therefore, based on what has been discussed so far, peach by-products (pomace, peel, seed shell, and seed) are relevant biomasses with high application potential (components with valuable bioactivities associated). For instance, the recovered products could be use in food, pharmaceutical, and cosmetic formulations. Besides, the use of these bioactive compounds implies in several processing steps, such as recovery, purification, isolation, identification and protection. Moreover, it is necessary to evaluate the production chain and the different sectors, such as logistics, transport, and packaging, to direct their by-products for further processing. For instance, by-product processing consists of drying, milling, extraction, fractionation, and others, which provide different products that must be evaluated in terms of quality and processing yield (efficiency). Therefore, the “product design” may provide the adequate feasibility for the definition of the “new process”.
Because of the strong relevance of the 17 SDG from ONU, there is a growing search for greener processes and protocols to obtain bioactive substances from industrial food by-product. To achieve these objectives, it is still necessary to study the extraction methods capable of reducing solvent and energy consumption and, also increasing the overall eco-sustainability of the food life cycle.
Some existing business models can serve as inspiration. For instance, the company Rubian Extracts, a Brazilian startup, recently developed natural extracts by supercritical CO2 technology, recovering biocompounds from by-products. Combining biorefinery and circular economy concepts is the key for the sustainable recovery of by-products, for application as food ingredient, with functional properties. Therefore, governmental policies may incentive partnerships between private companies, and researchers as a fast solution for food-product design, seeking benefits for society.
Conclusion and Perspectives
Peach processing by-products (pomace, seeds, and seed shells) are valuable renewable biomasses due to their content in lipids, proteins, phenolic compounds, fibers, and relevant bioactive phytochemicals including neochlorogenic acid, gallic acid, caffeic acid, and procyanidin, which are associated to bioactivities such as anti-inflammatory, anti-obesity, and anti-cerebral ischemia. The recovery of these valuable phytochemicals from peach by-products by using emerging green technologies (Pulsed electric field, supercritical fluid extraction, microwave-assisted extraction, and ultrasound-assisted extraction) may contribute to the development of new products for food or cosmetic industries. The valorization of peach by-products represents an opportunity to develop food products by combining the biorefinery concept with methodologies of design thinking, double diamond, and multi-criteria reverse engineering. These methodologies may improve the peach processing chain by optimizing the use of underestimated by-products, contributing to a sustainable industry. Therefore, peach by-products are promising sources of several molecules of high value, which contribute to rise the interest in these biomasses.
Author Contribution
Carla Roana Monteiro Rudke (carla.roana@posgrad.ufsc.br) conceived and structured the review. Acácio Antonio Ferreira Zielinski (acacio.zielinski@ufsc.br) and Sandra Regina Salvador Ferreira (s.ferreira@ufsc.br) contributed to the conception of the work, supervision, revising, and editing the manuscript.
Funding
The authors wish to thank the Brazilian founding agency CAPES (Coordination for the Improvement of Higher-Level Personnel, Brazil), for the financial support and fellowship, projects CAPES/PROEX-1624/2018, and CAPES/PRINT (Project 88887.511819/2020-00).
Data Availability
All data sharing and material are results from research and properly referenced along with the manuscript.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carla Roana Monteiro Rudke, Email: carla.roana@posgrad.ufsc.br.
Acácio Antônio Ferreira Zielinski, Email: acacio.zielinski@ufsc.br.
Sandra Regina Salvador Ferreira, Email: s.ferreira@ufsc.br.
References
- Abidi W, Zeballos JL, Granell A, Gogorcena Y. Bioactive compounds, sugar content and volatiles compounds in nectarine (Prunus persica (L.) Batsch) fruits. Journal of New Sciences. 2015;1:830–835. [Google Scholar]
- Adil IH, Çetin HI, Yener ME, Bayindirli A. Subcritical (carbon dioxide + ethanol) extraction of polyphenols from apple and peach pomaces, and determination of the antioxidant activities of the extracts. Journal of Supercritical Fluids. 2007;43(1):55–63. doi: 10.1016/j.supflu.2007.04.012. [DOI] [Google Scholar]
- Aktağ IG, Gökmen V. Investigations on the formation of α-dicarbonyl compounds and 5-hydroxymethylfurfural in apple juice, orange juice and peach puree under industrial processing conditions. European Food Research and Technology. 2021;247:797–805. doi: 10.1007/s00217-020-03663-0. [DOI] [Google Scholar]
- Alongi M, Anese M. Re-thinking functional food development through a holistic approach. Journal of Functional Foods. 2021;81:104466. doi: 10.1016/J.JFF.2021.104466. [DOI] [Google Scholar]
- Arora A, Banerjee J, Vijayaraghavan R, MacFarlane D, Patti AF. Process design and techno-economic analysis of an integrated mango processing waste biorefinery. Industrial Crops and Products. 2018;116:24–34. doi: 10.1016/j.indcrop.2018.02.061. [DOI] [Google Scholar]
- Banerjee S, Patti AF, Ranganathan V, Arora A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food and Bioproducts Processing. 2019;117:38–50. doi: 10.1016/j.fbp.2019.06.012. [DOI] [Google Scholar]
- Banerjee, S., Ranganathan, V., Patti, A., & Arora, A. (2018). Valorisation of pineapple wastes for food and therapeutic applications. Trends in Food Science and Technology, 82, 60–70. 10.1016/j.tifs.2018.09.024
- Batat W, Addis M. Designing food experiences for well-being: A framework advancing design thinking research from a customer experience perspective. European Journal of Marketing. 2021;55(9):2392–2413. doi: 10.1108/EJM-12-2020-0893. [DOI] [Google Scholar]
- Benvenutti L, Zielinski AAF, Ferreira SRS. Which is the best food emerging solvent: IL, DES or NADES? Trends in Food Science & Technology. 2019;90:133–146. doi: 10.1016/J.TIFS.2019.06.003. [DOI] [Google Scholar]
- Benvenutti, L., Zielinski, A. A. F., & Ferreira, S. R. S. (2021). Jaboticaba (Myrtaceae cauliflora) fruit and its by-products: Alternative sources for new foods and functional components. Trends in Food Science and Technology, 112, 118–136. 10.1016/j.tifs.2021.03.044
- Buratti C, Foschini D, Barbanera M, Fantozzi F. Fermentable sugars production from peach tree prunings: Response surface model optimization of NaOH alkaline pretreatment. Biomass and Bioenergy. 2018;112:128–137. doi: 10.1016/j.biombioe.2017.12.032. [DOI] [Google Scholar]
- Carrasco B, Meisel L, Gebauer M, Garcia-Gonzales R, Silva H. Breeding in peach, cherry and plum: From a tissue culture, genetic, transcriptomic and genomic perspective. Biological Research. 2013;46(3):219–230. doi: 10.4067/S0716-97602013000300001. [DOI] [PubMed] [Google Scholar]
- Cevallos-Casals BA, Byrne D, Okie WR, Cisneros-Zevallos L. Selecting new peach and plum genotypes rich in phenolic compounds and enhanced functional properties. Food Chemistry. 2006;96(2):273–280. doi: 10.1016/j.foodchem.2005.02.032. [DOI] [Google Scholar]
- Cherubini F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Conversion and Management. 2010;51(7):1412–1421. doi: 10.1016/J.ENCONMAN.2010.01.015. [DOI] [Google Scholar]
- De França Sousa S, Batista da Silva F, Calou de Araújo A, Palmeira Gomes J. Determinação das propriedades físicas e físico-químicas de pêssegos cultivar Rubimel. Revista Brasileira De Tecnologia Agroindustrial. 2018;12(2):2627–2644. doi: 10.3895/rbta.v12n2.7166. [DOI] [Google Scholar]
- Dima, C., Assadpour, E., Dima, S., & Jafari, S. M. (2020). Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Current Opinion in Food Science, 33, 21–29. 10.1016/j.cofs.2019.11.006
- El Darra N, Rajha HN, Debs E, Saleh F, El-Ghazzawi I, Louka N, Maroun RG. Comparative study between ethanolic and beta-cyclodextrin assisted extraction of polyphenols from peach pomace. International Journal of Food Science. 2018;2018:1–9. doi: 10.1155/2018/9491681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshamy AI, Abdallah HMI, El Gendy AENG, El-Kashak W, Muscatello B, De Leo M, Pistelli L. Evaluation of Anti-inflammatory, antinociceptive, and antipyretic activities of Prunus persica var nucipersica (Nectarine) Kernel. Planta Medica. 2019;85(11–12):1016–1023. doi: 10.1055/a-0955-5876. [DOI] [PubMed] [Google Scholar]
- FAOSTAT. (2020). Crops- peaches and nectarines. https://www.fao.org/faostat/en/#data/QCL
- Faravash RS, Ashtiani FZ. The effect of pH, ethanol volume and acid washing time on the yield of pectin extraction from peach pomace. International Journal of Food Science and Technology. 2007;42(10):1177–1187. doi: 10.1111/j.1365-2621.2006.01324.x. [DOI] [Google Scholar]
- Faravash RS, Ashtiani FZ. The influence of acid volume, ethanol-to-extract ratio and acid-washing time on the yield of pectic substances extraction from peach pomace. Food Hydrocolloids. 2008;22(1):196–202. doi: 10.1016/j.foodhyd.2007.04.003. [DOI] [Google Scholar]
- Featherstone S. A complete course in canning and related processes. 14. Woodhead Publishing; 2015. [Google Scholar]
- Fratebianchi D, Crespo JM, Tari C, Cavalitto S. Control of agitation rate and aeration for enhanced polygalacturonase production in submerged fermentation by Aspergillus sojae using agro-industrial wastes. Journal of Chemical Technology and Biotechnology. 2017;92(2):305–310. doi: 10.1002/jctb.5006. [DOI] [Google Scholar]
- Gettens, C. S. (2016). Propriedades funcionais, nutricionais e atividade antimicrobiana de subprodutos agroindustriais de pêssego e sua aplicação em cookies. Dissertação de Mestrado, Programa de Pós-Graduação em Nutrição e Alimentos, Faculdade de Nutrição, Universidade Federal de Pelotas, Rio Grande do Sul, Brasil. https://wp.ufpel.edu.br/ppgna/files/2016/02/Cristina-Gettens.pdf
- González-García E, Marina ML, García MC, Righetti PG, Fasoli E. Identification of plum and peach seed proteins by nLC-MS/MS via combinatorial peptide ligand libraries. Journal of Proteomics. 2016;148:105–112. doi: 10.1016/j.jprot.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Goodman-Smith F, Bhatt S, Moore R, Mirosa M, Ye H, Deutsch J, et al. Sustainability communication retail potential for upcycled foods: evidence from New Zealand. Sustainability. 2021;13(5):2624. doi: 10.3390/su13052624. [DOI] [Google Scholar]
- Granato D, Barba FJ, Bursać Kovačević D, Lorenzo JM, Cruz AG, Putnik P. Annual review of food science and technology functional foods: Product development, technological trends, efficacy testing, and safety. Annual Review of Food Science and Technology. 2020;11:93–118. doi: 10.1146/annurev-food-032519. [DOI] [PubMed] [Google Scholar]
- Gullón P, Gullón B, Muñiz-Mouro A, Lú-Chau TA, Eibes G. Valorization of horse chestnut burs to produce simultaneously valuable compounds under a green integrated biorefinery approach. Science of the Total Environment. 2020;730:139143. doi: 10.1016/j.scitotenv.2020.139143. [DOI] [PubMed] [Google Scholar]
- Hao E, Pang G, Du Z, Lai YH, Chen JR, Xie J, et al. Peach kernel oil downregulates expression of tissue factor and reduces atherosclerosis in ApoE knockout mice. International Journal of Molecular Sciences. 2019;20(2):1–13. doi: 10.3390/ijms20020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Corroto E, Marina ML, García MC. Multiple protective effect of peptides released from Olea europaea and Prunus persica seeds against oxidative damage and cancer cell proliferation. Food Research International. 2018;106:458–467. doi: 10.1016/J.FOODRES.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Hong Y, Wang Z, Barrow CJ, Dunshea FR, Suleria HAR, Angelo SD. High-throughput screening and characterization of phenolic compounds in stone fruits waste by LC-ESI-QTOF-MS/MS and their potential antioxidant activities. Antioxidants. 2021;10(2):234. doi: 10.3390/antiox10020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenidou I, Tzimitra-Kalogianni I, Zotos Y, Mattas K. Household purchasing and consumption behaviour towards processed peach products. New Medit: Mediterranean Journal of Economics, Agriculture and Environment. 2002;1(1):45. [Google Scholar]
- Kan J, Chen C, Huo T, Xie W, Hui Y, Liu J, Jin CH. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. Journal of Functional Foods. 2020;72:104082. doi: 10.1016/j.jff.2020.104082. [DOI] [Google Scholar]
- Kim H-J, Park K-K, Chung W-Y, Lee SK, Kim K-R. Protective effect of white-fleshed peach (Prunus persica (L.) Batsch) on chronic nicotine-induced toxicity. Journal of Cancer Prevention. 2017;22(1):22–32. doi: 10.15430/jcp.2017.22.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtulbaş E, Şahin S. Phytochemical properties in the waste by-products of peach: Optimization and storage stability studies. Biomass Conversion and Biorefinery. 2022;1:3. doi: 10.1007/s13399-022-03150-4. [DOI] [Google Scholar]
- Kurtulbaş E, Sevgen S, Samli R, Şahin S. Microwave-assisted extraction of bioactive components from peach waste: Describing the bioactivity degradation by polynomial regression. Biomass Conversion Biorefinery. 2022;1:3. doi: 10.1007/s13399-022-02909-z. [DOI] [Google Scholar]
- Lara MV, Bonghi C, Famiani F, Vizzotto G, Walker RP, Drincovich MF. Stone fruit as biofactories of phytochemicals with potential roles in human nutrition and health. Frontiers in Plant Science. 2020;11:4–21. doi: 10.3389/fpls.2020.562252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazos ES. Composition and oil characteristics of apricot, peach and cherry kernel. Grasas y Aceites. 1991;42(2):127–131. doi: 10.3989/gya.1991.v42.i2.1260. [DOI] [Google Scholar]
- Li L-L, Liu Y-R, Sun C, Yan Y-G, Tang Z-S, Sun J, et al. Taoren-dahuang herb pair reduces eicosanoid metabolite shifts by regulating ADORA2A degradation activity in ischaemia/reperfusion injury rats. Journal of Ethnopharmacology. 2020;260:113014. doi: 10.1016/J.JEP.2020.113014. [DOI] [PubMed] [Google Scholar]
- Li W, Amos K, Li M, Pu Y, DeBolt S, Ragauskas AJ, Shi J. Fractionation and characterization of lignin streams from unique high-lignin content endocarp feedstocks. Biotechnology for Biofuels. 2018;11(1):1–14. doi: 10.1186/s13068-018-1305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang L. Genetic resources, breeding programs in China, and Gene mining of peach: A review. Horticultural Plant Journal. 2020;6(4):205–215. doi: 10.1016/j.hpj.2020.06.001. [DOI] [Google Scholar]
- Liu H, Cao J, Jiang W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT - Food Science and Technology. 2015;63(2):1042–1048. doi: 10.1016/j.lwt.2015.04.052. [DOI] [Google Scholar]
- Liu H, Jiang W, Cao J, Ma L. Evaluation of antioxidant properties of extractable and nonextractable polyphenols in peel and flesh tissue of different peach varieties. Journal of Food Processing and Preservation. 2018;42(6):1–9. doi: 10.1111/jfpp.13624. [DOI] [Google Scholar]
- Loizzo MR, Pacetti D, Lucci P, Núñez O, Menichini F, Frega NG, Tundis R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive compounds and antioxidant activity of pulp, peel and seed ethanolic extracts. Plant Foods for Human Nutrition. 2015;70(3):331–337. doi: 10.1007/s11130-015-0498-1. [DOI] [PubMed] [Google Scholar]
- Longati AA, Batista G, Cruz AJG. Current Opinion in Green and Sustainable Chemistry. Elsevier B.V.; 2020. Brazilian integrated sugarcane-soybean biorefinery: Trends and opportunities. [Google Scholar]
- Ma Q, Li Z, Guo L, Zhai H, Ren H. Formation of high carbohydrate and acylation condensed lignin from formic acid-acetic acid-H2O biorefinery of corn stalk rind. Industrial Crops and Products. 2021;161:113165. doi: 10.1016/j.indcrop.2020.113165. [DOI] [Google Scholar]
- Maciel-Silva FW, Mussatto SI, Forster-Carneiro T. Integration of subcritical water pretreatment and anaerobic digestion technologies for valorization of açai processing industries residues. Journal of Cleaner Production. 2019;228:1131–1142. doi: 10.1016/j.jclepro.2019.04.362. [DOI] [Google Scholar]
- Maina S, Kachrimanidou V, Koutinas A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Current Opinion in Green and Sustainable Chemistry. 2017;8:18–23. doi: 10.1016/J.COGSC.2017.07.007. [DOI] [Google Scholar]
- Manara P, Zabaniotou A, Vanderghem C, Richel A. Catalysis Today. Elsevier; 2014. Lignin extraction from Mediterranean agro-wastes: Impact of pretreatment conditions on lignin chemical structure and thermal degradation behavior; pp. 25–34. [Google Scholar]
- Manzoor M, Anwar F, Mahmood Z, Rashid U, Ashraf M. molecules Variation in minerals, phenolics and antioxidant activity of peel and pulp of different varieties of peach (Prunus persica L.) fruit from Pakistan. Molecules. 2012;17:6491–6506. doi: 10.3390/molecules17066491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mármol I, Quero J, Ibarz R, Ferreira-Santos P, Teixeira JA, Rocha CMR, et al. Valorization of agro-food by-products and their potential therapeutic applications. Food and Bioproducts Processing. 2021;128:247–258. doi: 10.1016/J.FBP.2021.06.003. [DOI] [Google Scholar]
- Martins, C. M., Ferro, D. M., de Brito, E. S., & Ferreira, S. R. S. (2020). Industrial relevance of Tamarindus indica L. by-products as source of valuable active metabolites. Innovative Food Science and Emerging Technologies, 66, 102518. 10.1016/j.ifset.2020.102518
- Martirosyan DM, Singh J. A new definition of functional food by FFC: What makes a new definition unique? Functional Foods in Health and Disease. 2015;5(6):209–223. doi: 10.31989/ffhd.v5i6.183. [DOI] [Google Scholar]
- Massari S, Principato L, Antonelli M, Pratesi CA. Learning from and designing after pandemics. CEASE: A design thinking approach to maintaining food consumer behaviour and achieving zero waste. Socio-Economic Planning Sciences. 2021;82:101143. doi: 10.1016/j.seps.2021.101143. [DOI] [Google Scholar]
- Mezzomo N, Mileo BR, Friedrich MT, Martínez J, Ferreira SRS. Supercritical fluid extraction of peach (Prunus persica) almond oil: Process yield and extract composition. Bioresource Technology. 2010;101(14):5622–5632. doi: 10.1016/j.biortech.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Michailidis M, Karagiannis E, Nasiopoulou E, Skodra C, Molassiotis A, Tanou G. Peach, apple, and pear fruit quality: To peel or not to peel? Horticulturae. 2021;7(4):1–15. doi: 10.3390/horticulturae7040085. [DOI] [Google Scholar]
- Micheli P, Wilner SJS, Hussain Bhatti S, Mura M, Beverland MB. Doing design thinking: Conceptual review, synthesis, and research agenda. Journal of Product Innovation Management. 2019;36(2):124–148. doi: 10.1111/jpim.12466. [DOI] [Google Scholar]
- Minas, I. S., Tanou, G., & Molassiotis, A. (2018). Environmental and orchard bases of peach fruit quality. Scientia Horticulturae, 235, 307–322. 10.1016/j.scienta.2018.01.028
- Mokrani A, Madani K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Separation and Purification Technology. 2016;162:68–76. doi: 10.1016/j.seppur.2016.01.043. [DOI] [Google Scholar]
- Monteiro CRM, Ávila PF, Pereira MAF, Pereira GN, Bordignon SE, Zanella E, et al. Hydrothermal treatment on depolymerization of hemicellulose of mango seed shell for the production of xylooligosaccharides. Carbohydrate Polymers. 2021;253:117274. doi: 10.1016/j.carbpol.2020.117274. [DOI] [PubMed] [Google Scholar]
- Naranjo JM, Cardona CA, Higuita JC. Use of residual banana for polyhydroxybutyrate (PHB) production: Case of study in an integrated biorefinery. Waste Management. 2014;34(12):2634–2640. doi: 10.1016/j.wasman.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Nowicka P, Wojdyło A. Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. European Food Research and Technology. 2019;245:1123–1136. doi: 10.1007/s00217-018-3214-1. [DOI] [Google Scholar]
- Nowicka P, Wojdyło A, Laskowski P. Inhibitory potential against digestive enzymes linked to obesity and type 2 diabetes and content of bioactive compounds in 20 cultivars of the peach fruit grown in Poland. Plant Food for HUman Nutrition. 2018;73:314–320. doi: 10.1007/s11130-018-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NV. Design thinking and food innovation. Trends in Food Science & Technology. 2015;41(2):182–187. doi: 10.1016/J.TIFS.2014.10.001. [DOI] [Google Scholar]
- Ortiz-Sanchez M, Solarte-Toro JC, Orrego-Alzate CE, Acosta-Medina CD, Cardona-Alzate CA. Integral use of orange peel waste through the biorefinery concept: An experimental, technical, energy, and economic assessment. Biomass Conversion and Biorefinery. 2021;11:645–659. doi: 10.1007/s13399-020-00627-y. [DOI] [Google Scholar]
- Pagán J, Ibarz A. Extraction and rheological properties of pectin from fresh peach pomace. Journal of Food Engineering. 1999;39(2):193–201. doi: 10.1016/S0260-8774(98)00163-0. [DOI] [Google Scholar]
- Pagán J, Ibarz A, Llorca M, Pagán A, Barbosa-Cánovas GV. Extraction and characterization of pectin from stored peach pomace. Food Research International. 2001;34(7):605–612. doi: 10.1016/S0963-9969(01)00078-3. [DOI] [Google Scholar]
- Papaioannou EH, Liakopoulou-Kyriakides M. Agro-food wastes utilization by Blakeslea trispora for carotenoids production. Acta Biochimica Polonica. 2012;59(1):151–153. doi: 10.18388/abp.2012_2194. [DOI] [PubMed] [Google Scholar]
- Patra JK, Baek KH. Comparative study of proteasome inhibitory, synergistic antibacterial, synergistic anticandidal, and antioxidant activities of gold nanoparticles biosynthesized using fruit waste materials. International Journal of Nanomedicine. 2016;11:4691–4705. doi: 10.2147/IJN.S108920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla MFDE, González P, Sette P, Portillo F, Rojas AM, Gerschenson LN. Effect of processing on physico-chemical characteristics of dietary fibre concentrates obtained from peach (Prunus persica L.) peel and pulp. Food Research International. 2012;49(1):184–192. doi: 10.1016/j.foodres.2012.07.060. [DOI] [Google Scholar]
- Plazzotta S, Ibarz R, Manzocco L, Martín-Belloso O. Optimizing the antioxidant biocompound recovery from peach waste extraction assisted by ultrasounds or microwaves. Ultrasonics Sonochemistry. 2020;63:104954. doi: 10.1016/j.ultsonch.2019.104954. [DOI] [PubMed] [Google Scholar]
- Plazzotta S, Ibarz R, Manzocco L, Martín-Belloso O. Modelling the recovery of biocompounds from peach waste assisted by pulsed electric fields or thermal treatment. Journal of Food Engineering. 2021;290:110196. doi: 10.1016/j.jfoodeng.2020.110196. [DOI] [Google Scholar]
- Rahma EH, El-Aal MHA. Chemical characterization of peach kernel oil and protein: Functional properties, in vitro digestibility and amino acids profile of the flour. Food Chemistry. 1988;28(1):31–43. doi: 10.1016/0308-8146(88)90004-0. [DOI] [Google Scholar]
- Redondo D, Gimeno D, Calvo H, Venturini ME, Oria R, Arias E. Antioxidant activity and phenol content in different tissues of stone fruits at thinning and at commercial maturity stages. Waste and Biomass Valorization. 2021;12:1861–1875. doi: 10.1007/s12649-020-01133-y. [DOI] [Google Scholar]
- Ribeiro ECB, Moreira AC, Ferreira LMDF, da Silva César A. Biodiesel and social inclusion: An analysis of institutional pressures between biodiesel plants and family farmers in southern Brazil. Journal of Cleaner Production. 2018;204:726–734. doi: 10.1016/j.jclepro.2018.09.085. [DOI] [Google Scholar]
- Rico, X., Gullón, B., Alonso, J. L., & Yáñez, R. (2020). Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Research International, 132, 109086. 10.1016/j.foodres.2020.109086 [DOI] [PubMed]
- Rudke AR, de Andrade CJ, Ferreira SRS. Trends in Food Science and Technology. Elsevier Ltd; 2020. Kappaphycus alvarezii macroalgae: An unexplored and valuable biomass for green biorefinery conversion. [Google Scholar]
- Şahin, S., & Bilgin, M. (2022). Valorization of peach (Prunus persica L.) waste into speciality products via green methods. Biomass Conversion and Biorefinery,1(3), 123–132. 10.1007/s13399-021-01947-3
- Saidani F, Giménez R, Aubert C, Chalot G, Betrán JA, Gogorcena Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. Journal of Food Composition and Analysis. 2017;62:126–133. doi: 10.1016/J.JFCA.2017.04.015. [DOI] [Google Scholar]
- Sánchez-Vicente Y, Cabañas A, Renuncio JA, Pando C. Supercritical fluid extraction of peach (Prunus persica) seed oil using carbon dioxide and ethanol. Journal of Supercritical Fluids. 2009;49(2):167–173. doi: 10.1016/j.supflu.2009.01.001. [DOI] [Google Scholar]
- Santin M, Lucini L, Castagna A, Rocchetti G, Hauser MT, Ranieri A. Comparative “phenol-omics” and gene expression analyses in peach (Prunus persica) skin in response to different postharvest UV-B treatments. Plant Physiology and Biochemistry. 2019;135:511–519. doi: 10.1016/J.PLAPHY.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Sarkar, P., & Choudhury, G. S. (2014). Peach pomace processing using twin screw extrusion. Journal of Microbiology, Biotechnology and Food Sciences, 3(4), 279–285. https://www.jmbfs.org/wp-content/uploads/2014/01/jmbfs_0381_sarkar.pdf
- Seo KH, Choi SY, Jin Y, Son H, Kang YS, Jung SH, et al. Anti-inflammatory role of Prunus persica L. Batsch methanol extract on lipopolysaccharide-stimulated glial cells. Molecular Medicine Reports. 2020;21:2030–2040. doi: 10.3892/mmr.2020.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Kumar S, Sharma M, Upadhyay N, Kumar S, Ahmed Z, Mahindroo N. Anti-diabetic, anti-oxidant and anti-adipogenic potential of quercetin rich ethyl acetate fraction of prunus persica. Pharmacognosy Journal. 2018;10(3):463–469. doi: 10.5530/pj.2018.3.76. [DOI] [Google Scholar]
- Shukla RK, Kant R. Assessment of phytochemical screening by fourier transform infrared spectroscopic analysis of peach (Prunus persica) seed biomass from Uttarakhand region of India. Journal of Applied and Natural Science. 2020;12(4):519–524. doi: 10.31018/jans.v12i4.2330. [DOI] [Google Scholar]
- Sijtsema SJ, Fogliano V, Hageman M. Tool to Support citizen participation and multidisciplinarity in food innovation: Circular food design. Frontiers in Sustainable Food Systems. 2020;4:582193. doi: 10.3389/fsufs.2020.582193. [DOI] [Google Scholar]
- Skiba M, Vorobyova V. Green synthesis and characterization of silver nanoparticles using Prunus persica L. (peach pomace) with natural deep eutectic solvent and plasma-liquid process Graphical abstract Keywords Natural deep eutectic solvent · Silver nanoparticle · Peach pomace. Chemical Papers. 2022;76:5789–5806. doi: 10.1007/s11696-022-02274-1. [DOI] [Google Scholar]
- Sodeifian G, Sajadian SA. Antioxidant capacity, physicochemical properties, thermal behavior, and oxidative stability of nectarine (Prunus persica var. nucipersica) kernel oil. Journal of Food Processing and Preservation. 2021;45(2):1–11. doi: 10.1111/jfpp.15198. [DOI] [Google Scholar]
- Song J, Kim Y-S, Kim L, Park HJ, Lee D, Kim H. Anti-obesity effects of the flower of Prunus persica in high-fat diet-induced obese mice. Nutrients. 2019;11(9):2176. doi: 10.3390/nu11092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic BT, Mitic SS, Stojanovic GS, Mitic MN, Kostic DA, Paunovic DD, Arsic BB. Phenolic profile and antioxidant activity of pulp and peel from peach and nectarine fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2016;44(1):175–182. doi: 10.15835/nbha44110192. [DOI] [Google Scholar]
- Taifouris, M., Martín, M., Martínez, A., & Esquejo, N. (2020). Challenges in the design of formulated products: Multiscale process and product design. Current Opinion in Chemical Engineering, 27, 1–9. 10.1016/j.coche.2019.10.001
- Thomopoulos R, Baudrit C, Boukhelifa N, Boutrou R, Buche P, Guichard E, et al. Multi-criteria reverse engineering for food: Genesis and ongoing advances. Food Engineering Reviews. 2019;11:44–60. doi: 10.1007/s12393-018-9186-x. [DOI] [Google Scholar]
- Tkaczewska J, Kulawik P, Morawska-tota M, Zaj M, Guzik P, Paj P, Duli R. Protocol for designing new functional food with the addition of food industry by-products, using design thinking techniques – A case study of a snack with antioxidant properties for physically active people. Foods. 2021;10(4):694. doi: 10.3390/foods10040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre IDL, Martin-Dominguez V, Acedos MG, Esteban J, Santos VE, Ladero M. Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. Applied Microbiology and Biotechnology. 2019;103:5975–5991. doi: 10.1007/s00253-019-09929-2. [DOI] [PubMed] [Google Scholar]
- Uysal T, Duman G, Onal Y, Yasa I, Yanik J. Production of activated carbon and fungicidal oil from peach stone by two-stage process. Journal of Analytical and Applied Pyrolysis. 2014;108:47–55. doi: 10.1016/j.jaap.2014.05.017. [DOI] [Google Scholar]
- Vásquez-Villanueva R, Marina ML, García MC. Revalorization of a peach (Prunus persica (L.) Batsch) byproduct: Extraction and characterization of ACE-inhibitory peptides from peach stones. Journal of Functional Foods. 2015;18:137–146. doi: 10.1016/j.jff.2015.06.056. [DOI] [Google Scholar]
- Vásquez-Villanueva R, Orellana JM, Marina ML, García MC. Isolation and characterization of angiotensin converting enzyme inhibitory peptides from peach seed hydrolysates: In vivo assessment of antihypertensive activity. Journal of Agricultural and Food Chemistry. 2019;67(37):10313–10320. doi: 10.1021/acs.jafc.9b02213. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lin D, Wang X, Zhu W, Ye J, Li G, et al. The impact of a novel peach gum-derived polysaccharide on postprandial blood glucose control in streptozotocin-induced diabetic mice. International Journal of Biological Macromolecules. 2017;98:379–386. doi: 10.1016/j.ijbiomac.2017.01.085. [DOI] [PubMed] [Google Scholar]
- Wu H, Shi J, Xue S, Kakuda Y, Wang D, Jiang Y, et al. Essential oil extracted from peach (Prunus persica) kernel and its physicochemical and antioxidant properties. LWT - Food Science and Technology. 2011;44(10):2032–2039. doi: 10.1016/j.lwt.2011.05.012. [DOI] [Google Scholar]
- Xu H, Jiao Q, Yuan F, Gao Y. In vitro binding capacities and physicochemical properties of soluble fiber prepared by microfluidization pretreatment and cellulase hydrolysis of peach pomace. LWT - Food Science and Technology. 2015;63(1):677–684. doi: 10.1016/J.LWT.2015.03.033. [DOI] [Google Scholar]
- Yangilar F. Production and evaluation of mineral and nutrient contents, chemical composition, and sensory properties of ice creams fortified with laboratory-prepared peach fibre. Food and Nutrition Research. 2016;60:1–9. doi: 10.3402/fnr.v60.31882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Medicine (United Kingdom) 2018;13(1):1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ling J, Zhou H, Tian M, Huang W, Luo S, et al. 1-Methylcyclopropene counteracts ethylene inhibition of anthocyanin accumulation in peach skin after harvest. Postharvest Biology and Technology. 2022;183:111737. doi: 10.1016/J.POSTHARVBIO.2021.111737. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sharing and material are results from research and properly referenced along with the manuscript.