Abstract
Mucosal delivery of vaccines is dependent on the identification of safe and effective adjuvants that can enhance the immunogenicity of protein antigens administered by nasal or oral routes. In this study we demonstrate that two mutants of Escherichia coli heat-labile toxin (LT), LTK63, which lacks ADP-ribosylating activity, and LTR72, which has partial enzyme activity, act as potent mucosal adjuvants for the nasal delivery of an acellular pertussis (Pa) vaccine. Both LTK63 and LTR72 enhanced antigen-specific serum immunoglobulin G (IgG), secretory IgA, and local and systemic T-cell responses. Furthermore, using the murine respiratory challenge model for infection with Bordetella pertussis, we demonstrated that a nasally delivered diphtheria, tetanus, and acellular pertussis (DTPa) combination vaccine formulated with LTK63 as an adjuvant conferred a high level of protection, equivalent to that generated with a parenterally delivered DTPa vaccine formulated with alum. This study also provides significant new information on the roles of the binding and enzyme components of LT in the modulation of Th1 and Th2 responses. LTK63, which lacks enzyme activity, promoted T-cell responses with a mixed Th1–Th2 profile, but LTR72, which retains partial enzyme activity, and the wild-type toxin, especially at low dose, induced a more polarized Th2-type response and very high IgA and IgG antibody titers. Our findings suggest that the nontoxic AB complex has broad adjuvant activity for T-cell responses and that the ADP-ribosyltransferase activity of the A subunit also appears to modulate cytokine production, but its effect on T-cell subtypes, as well as enhancing, may be selectively suppressive.
The majority of pathogenic microorganisms initiate infection at the mucosal surfaces of the lung and the gastrointestinal tract. Vaccines that activate mucosal immunity may be able to stop colonization by pathogens before they gain entry, offering an effective defense against a wide variety of diseases. Patient compliance with vaccination programs and vaccination in developing countries would also benefit, as sterile needles would not be required. However, most purified protein antigens are poorly immunogenic when ingested or inhaled (1). Despite the many practical and immunological advantages, the development of effective mucosal vaccines has been hindered by the lack of appropriate delivery systems and adjuvants to enhance immune responses following oral or nasal immunization (1, 36).
The heat-labile toxin of Escherichia coli (LT) (11), cholera toxin (CT) (20), and pertussis toxin (PT) (29, 30) have been shown to act as powerful mucosal adjuvants. LT and CT are each composed of two subunits, a monomeric enzymatically active A subunit that ADP-ribosylates GTP-binding proteins and a pentameric nontoxic B subunit that binds GM1 gangliosides at the surfaces of eukaryotic cells. The clinical use of these toxins as adjuvants has not been possible due to their toxicity; therefore, nontoxic mutants have been generated by site-directed mutagenesis. The mechanism of adjuvant action of LT has long been controversial (37). Some reports have indicated that the B subunit alone can act as an adjuvant (7), while others indicate that the adjuvant action is associated with the ADP-ribosyltransferase activity of the A subunit (17). Studies with nontoxic mutants of LT and PT, without ADP-ribosylating activity, suggest that adjuvanticity is derived from the independent contribution of the nontoxic AB complex and the enzymatic activity of the A subunit (11, 13, 29, 30).
Bordetella pertussis is the causative agent of whooping cough, a disease that still causes high levels of morbidity and mortality in children. A highly effective whole-cell pertussis vaccine (Pw) has been available since the 1940s. However, vaccine uptake was severely reduced in some countries due to concern over its safety (6). This led to the development of acellular pertussis vaccines (Pa) composed of purified proteins, which are putative protective antigens of the bacteria, such as PT in a genetically or chemically detoxified form, filamentous hemagglutinin (FHA), pertactin (PRN), and agglutinogens 2 and 3 (14, 15, 27). The results of clinical trials have shown that certain Pa are effective in preventing severe pertussis in children; however, a clear immunological correlate of protection could not be determined (14, 15). We have established a reliable animal model, in which we have shown that the rate of B. pertussis clearance following respiratory challenge of immunized mice correlated with pertussis vaccine efficacy in children (21).
The rationale of the present study was to examine the capacity of mutants of LT to enhance immune responses and protection with a nasally delivered pertussis vaccine. A number of groups have already reported that LT, LT mutants, or the purified B subunit of LT (LT-B) can enhance immune responses with mucosally delivered antigens (7–11, 13, 17, 34). However, there have been a limited number of studies demonstrating protection against mucosal infection in established challenge models. The use of an established respiratory challenge model for B. pertussis has allowed us to address this objective and significantly to compare protection to that achieved with the equivalent parenterally delivered alum-adsorbed vaccine, with known potency in clinical trials. Furthermore, access to mutants of LT which either lack ADP-ribosylating activity or have partial enzyme activity provided ideal tools for examining the influence of the enzyme and binding domains on adjuvant activity, in particular on the induction of Th1 and Th2 cells. There is still some controversy about the mechanism of action and about the adjuvant effect of LT for Th1 and Th2 responses. Therefore, we addressed the hypothesis that the enzyme and the binding domain may have distinct influences on the induction of Th1 and Th2 cells.
MATERIALS AND METHODS
Mice.
Female BALB/c mice were obtained from Harlan UK Ltd. (Bicester Oxon, United Kingdom) and were housed according to the regulations of the Irish Department of Health. All mice were 6 to 8 weeks old at the initiation of each of the experiments.
Bacterial antigens and adjuvants.
Heat-killed B. pertussis was prepared by incubation of cells at 80°C for 30 min. Purified FHA, PRN, and recombinant PT (rPT) (PT-9K/129G), tetanus toxoid (TT), and cross-reacting material (CRM-197) of diphtheria toxin (DT) were prepared by Chiron Corporation, Siena, Italy, as described previously (25–27). The mutants of E. coli LT, LTK63 and LTR72, were created by site-directed mutagenesis as previously described (11, 13).
Immunizations.
Groups of BALB/c mice were immunized at 0 and 4 weeks with a two-component Pa, which consisted of FHA (2.5 μg) and rPT (5.0 μg) alone or with either LTK63 or LTR72 (1.0 or 10 μg) as an adjuvant. Mice were immunized either with the vaccine dose resuspended in 25 μl and applied to the external nares with a micropipette or following light halothane anesthesia with the vaccine dose resuspended in 50 μl and applied to the external nares with a micropipette. In experiments to directly compare nasal and systemic delivery of a combined diphtheria, tetanus, and acellular pertussis (DTPa) vaccine, FHA (2.5 μg), PT-9K/129G (5 μg), PRN (2.5 μg), TT (10 μg), and the DT mutant CRM-197 (10 μg) were formulated either with alum (300 μg) or with LTK63 (10 μg). The alum-adsorbed vaccine was given in a volume of 300 μl by the intramuscular (i.m.) route and the LTK63-formulated vaccine was given in a 40-μl volume by the intranasal (i.n.) route.
B. pertussis respiratory challenge.
B. pertussis W28 phase I was grown under agitation conditions at 37°C in Stainer-Scholte liquid medium. Bacteria from a 48-h culture were resuspended at a concentration of approximately 2 × 1010 cells/ml in physiological saline containing 1% casein. The challenge inoculum was administered to mice over a period of 15 min by means of a nebulizer; mice then remained in the chamber for a further 15 min. Groups of four mice were sacrificed at 0, 3, 7, 10, and 14 days, and the numbers of viable B. pertussis organisms in the lungs were assessed. Lungs were removed aseptically from the infected mice and homogenized in 1 ml of sterile physiological saline with 1% casein on ice. Aliquots of 100 μl of undiluted or serially diluted homogenate from individual lungs were spotted in triplicate onto Bordet-Genou agar plates, and the number of colonies was assessed after 5 days of incubation. Results are reported as the mean number of viable B. pertussis organisms for individual lungs from four mice per time point per experimental group.
T-cell responses.
Mice were immunized at 0 and 4 weeks, and at week 6, the spleen, superior cervical lymph nodes, and posterior mediastinal (thoracic) lymph nodes were removed and immune responses were evaluated. Spleen cells from individual mice or pooled lymph node cells (2 × 106 cells/ml) from naïve or immunized mice were cultured in triplicate in 8% fetal calf serum-supplemented RPMI at 37°C with heat-killed bacteria (106 or 107 cells/ml), heat-inactivated rPT (1 to 5 μg/ml), or FHA (1 to 5 μg/ml) or with phorbal myristate acetate (PMA; Sigma) and anti-mouse CD3 (Pharmingen) or medium only as positive and negative controls, respectively. In experiments with DTPa formulations, responses were also tested against PRN, TT, or CRM-197 (1 to 15 μg/ml). Supernatants were removed after 72 h; the concentrations of gamma interferon (IFN-γ), interleukin-4 (IL-4) and IL-5 were determined by immunoassay; and T-cell proliferation was assessed after 4 days of culture by [3H]thymidine uptake as described previously (21). Results are expressed as mean counts per minute or mean cytokine concentration for the optimum concentration of antigen in assays performed in triplicate on individual spleen cells or pooled lymph node cells from four to five mice.
Antibody assays.
Levels of antigen-specific immunoglobulin G (IgG) in the sera of immunized and control mice were determined by enzyme-linked immunosorbent assay (ELISA). Purified antigens (FHA, PT, PRN, TT, and DT; 1.0 μg/ml) were used to coat the ELISA plates. The plates were blocked with milk protein; then serially diluted serum samples were added, and the bound antibody was detected by anti-mouse IgG (Fc-specific) alkaline phosphatase conjugate (Sigma). Antigen-specific IgA in lungs was detected by ELISA. Lungs were homogenized in RPMI–8% fetal calf serum containing the protease inhibitor phenylmethylsulfonyl fluoride (Sigma) at 0.1 mM. ELISA plates were coated with antigen as for the IgG assay, and serially diluted lung homogenate was added. The bound antibody was detected with sheep anti-mouse IgA (Sigma), followed by donkey anti-sheep IgG alkaline phosphatase conjugate (Sigma). Results are expressed as end point titers, calculated by regression of the straight part of a curve of optical density versus serum or lung homogenate dilution to a cutoff of 2 standard deviations above background control values for serum or lung homogenates from naïve mice.
RESULTS
LT mutants enhance local and systemic T-cell and antibody responses to nasally delivered B. pertussis antigens.
Formulation of a two-component Pa, comprising rPT and FHA, with either the LTR72 or the LTK63 mutant as an adjuvant significantly enhanced local and systemic T-cell responses following nasal immunization. Strong T-cell proliferation and cytokine production were detected in spleen cells and in thoracic and cervical lymph node cells in response to killed B. pertussis, PT, or FHA in tests conducted 2 weeks after two immunizations (Fig. 1 through 3; also data not shown). In contrast, B. pertussis-specific T-cell responses in spleens and local lymph nodes from mice intranasally immunized with rPT and FHA were significantly weaker or, in some cases, undetectable. However, strong responses to the polyclonal stimulus, PMA–anti-CD3, indicated that these T cells were capable of responding in vitro.
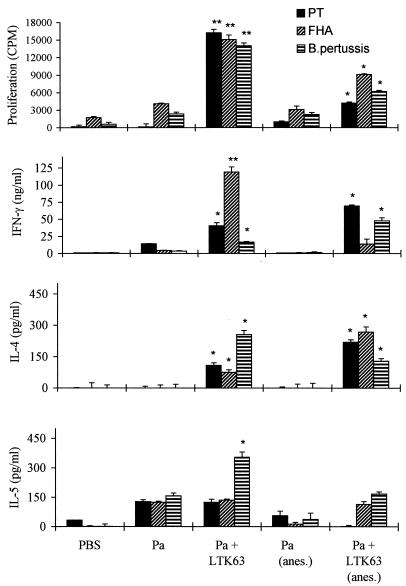
FIG. 1.
LTK63 enhances proliferation and Th1 and Th2 responses to nasally delivered Pa. BALB/c mice were immunized twice (weeks 0 and 4) i.n. with a two-component Pa consisting of rPT and FHA with or without LTK63 (10 μg) and with or without light anesthesia (anes.). At 6 weeks, spleen cells were isolated and stimulated in vitro with PT, FHA, or killed B. pertussis. After 72 h, supernatants were removed and IFN-γ, IL-4, and IL-5 concentrations were measured by immunoassay. Proliferation was assessed after 4 days. Results are mean cytokine concentrations or counts per minute for proliferation assays on spleen cells from four or five mice per experimental group. Error bars, standard deviations. ∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus Pa alone (by Student's t test).
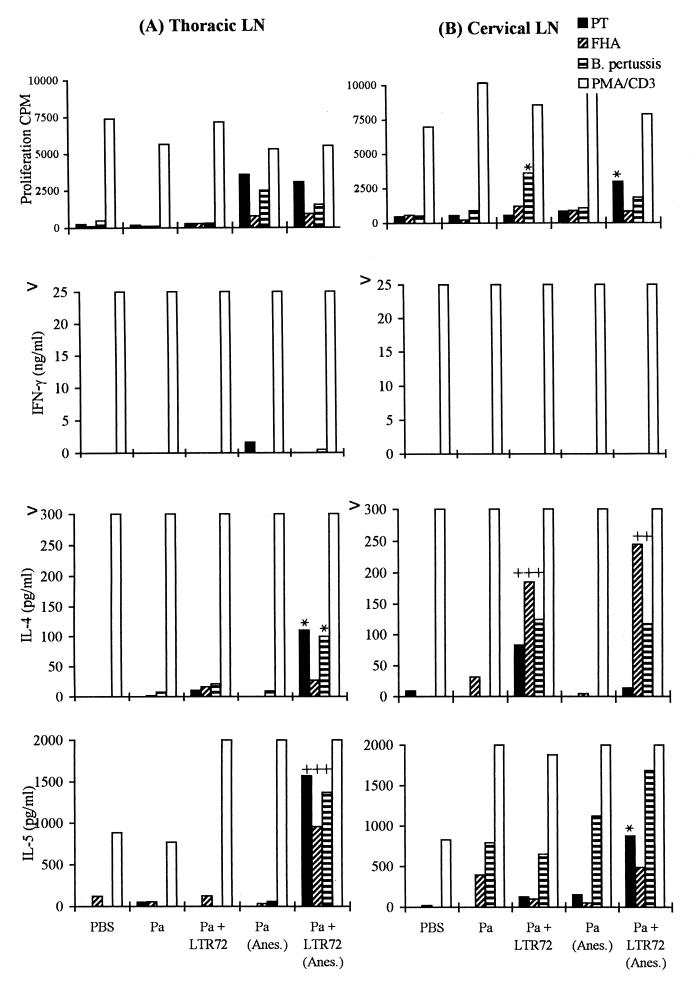
FIG. 3.
LTR72 enhances local lymph node Th2-type responses to nasally delivered Pa. BALB/c mice were immunized twice (weeks 0 and 4) i.n. with a two-component Pa consisting of rPT and FHA with or without LTR72 (1 μg) and with or without light anesthesia (Anes.). At 6 weeks, thoracic lymph node (LN) cells (A) and superficial cervical LN cells (B) were prepared, and proliferation and cytokine assays were performed as described in the legend to Fig. 1. ∗ and +, P < 0.05 and P < 0.01, respectively, versus Pa alone (by Student's t test).
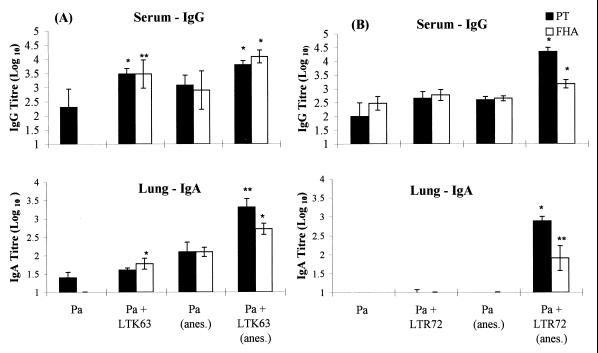
Both the LTK63 and LTR72 mutants also enhanced local and systemic antibody production following intranasal delivery of Pa (Fig. 4). Immunization with rPT–FHA in solution generated weak and inconsistent anti-PT and anti-FHA serum IgG and lung IgA responses. The antibody responses generated by immunization with Pa without adjuvant were quite variable between experiments and between mice in the same experiments; this is in agreement with the findings of other studies on mucosally delivered soluble antigens. In contrast, formulation of Pa with LTR72 or LTK63 resulted in consistently strong serum IgG responses specific for PT and FHA and also significantly enhanced IgA responses, especially when the vaccine was delivered under anesthesia.
FIG. 4.
Intranasal immunization with Pa formulated with LTK63 (A) or LTR72 (B) as an adjuvant enhances serum IgG and lung IgA responses. Mice were immunized as described in the legends to Fig. 1 and 2. Two weeks after immunization, serum and lung homogenates were prepared and anti-FHA and anti-PT IgG and IgA levels were determined by ELISA. ∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus Pa alone (by Student's t test).
Effects of enzyme activity and toxin dose on the enhancing effects of LT mutants on Th1 and Th2 responses.
The cytokine profiles obtained following in vitro stimulation of spleen cells and local lymph node cells from mice immunized with LTK63 or LTR72 showed that the ADP-ribosylation activity of the toxins plays an important role in the modulation of the immune response. Immunization with Pa formulated with LTK63, the nontoxic mutant, which is devoid of any enzyme activity, enhanced the production of IL-4, IL-5, and IFN-γ by spleen cells (Fig. 1) and lymph node cells (data not shown) in response to specific antigen stimulation in vitro. This mixed Th1–Th2- or Th0-type profile has been found by at least six independent experiments with B. pertussis and other antigens. In contrast, 1.0 μg of LTR72, which retains partial enzyme activity, appeared to selectively enhance Th2 cells, with both spleen (Fig. 2) and local lymph node cells (Fig. 3) producing high levels of antigen-specific IL-5 and moderate levels of IL-4, but no detectable IFN-γ.
FIG. 2.
LTR72 enhances systemic Th2-type responses to nasally delivered Pa. BALB/c mice were immunized twice (weeks 0 and 4) i.n. with a two-component Pa consisting of rPT and FHA with or without LTR72 (1 μg) and with or without light anesthesia (Anes.). At 6 weeks, spleen cells were isolated and proliferation and cytokine assays were performed as described in the legend to Fig. 1. ∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus Pa alone (by Student's t test).
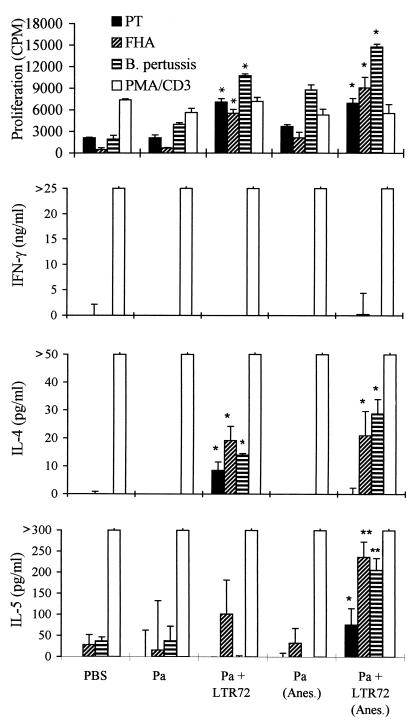
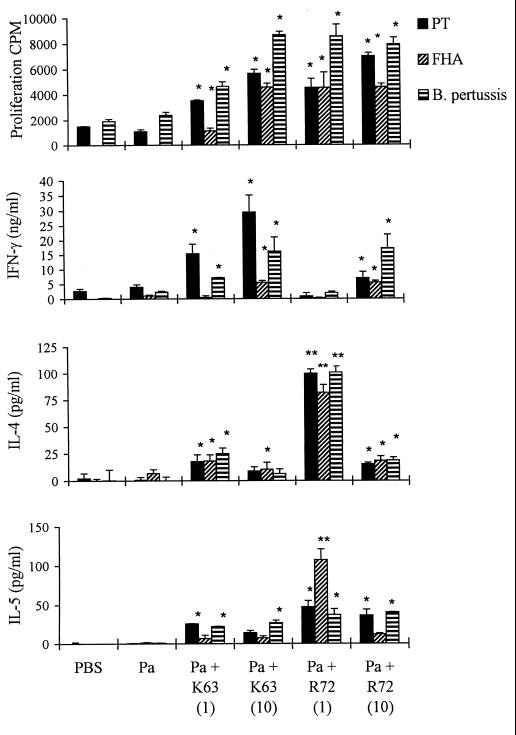
In the experiments for which results are shown in Fig. 1 through 3, we chose 10 and 1.0 μg of LTK63 and LTR72, respectively, on the basis of previous studies that had compared the adjuvanticities of LTK63, LTR72, and wild-type LT for intranasal delivery of a model protein antigen, ovalbumin (13). Although increasing adjuvant action was reported with increasing doses of the mutants in the range of 2.5 ng to 10 μg per mouse, LTR72 was more effective than LTK63 at equivalent doses. Furthermore, the adjuvanticity of active LT peaked at 1 μg and was less effective at 10 μg (13). In experiments that directly compared the adjuvanticities of the toxins in vivo, BALB/c mice were immunized with rPT–FHA formulated with 1 or 10 μg of LTK63 or LTR72 as an adjuvant, and the resulting immune responses were assessed. Intranasal immunization with Pa composed of rPT and FHA in solution generated weak T-cell responses, whereas addition of 1 μg of LTK63 to the formulation enhanced proliferation, as well as IFN-γ and IL-5 production, by spleen cells (Fig. 5) and lymph node cells (data not shown) in response to FHA or killed B. pertussis. Increasing the dose to 10 μg of LTK63 resulted in modest further enhancements of proliferation and IFN-γ production (Fig. 5). Consistent with the findings of previous experiments, 1.0 μg of LTR72 selectively augmented Th2 responses, with elevated levels of antigen-induced IL-4 and IL-5 production compared with those observed with Pa alone. Wild-type LT (1.0 μg) also selectively enhanced IL-4 and IL-5 production, but the effect was not as dramatic as that observed with LTR72 (data not shown). Furthermore, the mice that received the Pa with 1.0 μg of LTR72 as an adjuvant had significantly higher anti-FHA and anti-PT IgG and IgA antibody titers than those immunized with Pa formulated with LTK63 or wild-type LT (data not shown). Increasing the dose of LTR72 from 1.0 to 10 μg resulted in enhancement of IFN-γ levels and lower levels of IL-4 and IL-5 (Fig. 5). Thus, the enzyme activity and the dose of the toxin appear to affect the cytokine profile of the antigen-specific T cells induced. It appears that the trace amounts of ADP-ribosylation activity present in low doses of LTR72 are sufficient to modulate the cytokine profile to Th2 and act as a potent adjuvant for antibody responses. Conversely, the adjuvant effect of LTK63, which is mediated by the binding effect of the AB complex, is pushed more toward the Th1 subtype. Furthermore, at higher doses of LTR72, the AB binding activity may outweigh the enzyme activity, resulting in enhancement of Th1 as well as Th2 cell induction.
FIG. 5.
Effect of toxin dose on the adjuvant effect of LTK63 (K63) and LTR72 (R72). BALB/c mice were immunized twice (weeks 0 and 4) i.n. with a two-component Pa consisting of rPT (5.0 μg), FHA (2.5 μg), and either LTK63 (1 or 10 μg) or LTR72 (1 or 10 μg) under light anesthesia. At 6 weeks, spleen cells were isolated, and proliferation and cytokines were assayed as described in the legend to Fig. 1. Results are mean values for four or five mice per experimental group. Error bars, standard deviations. ∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus Pa alone (by Student's t test).
Influence of anesthesia and delivery volume on the immune responses generated by intranasal immunization.
There is evidence to suggest that the volume of the dose administered and the use of anesthesia can affect the distribution of a drug or vaccine following intranasal administration in rodents (24, 35). The use of anesthesia, especially with higher volumes, may favor antigen penetration into the lungs, whereas more of the antigen is retained in the nasal tract following immunization with a lower volume in the absence of anesthesia. We assessed the immune responses induced by Pa, alone and with each of the LT mutants as an adjuvant, delivered intranasally by using these two different protocols. Delivery of the two-component Pa alone with or without anesthesia generated weak T-cell proliferation and low levels of IL-4, IL-5, or IFN-γ production; the immunogenicity of the soluble antigens was particularly poor with the higher volume of vaccine delivered under anesthesia (Fig. 1 through 3). Addition of LTR72 or LTK63 enhanced the T-cell responses, but this was influenced by the mode of immunization. The most significant enhancements were observed for IFN-γ production in response to FHA with LTK63 administered in the lower volume without anesthesia and for IL-5 production in response to PT or FHA with LTR72 administered in a higher volume with anesthesia (Fig. 1 through 3). In contrast, IL-4, IL-5, and IFN-γ levels were not enhanced to the same extent with LTR72 administered without anesthesia. Examining the responses in the different local lymph nodes (Fig. 3) provides indirect evidence that the delivery protocol was directing the antigens to different areas of the respiratory tract. Intranasal immunization with the lower volume without anesthesia activated a Th2 response in the superior cervical lymph nodes, whereas immunization with the higher volume under anesthesia, which we believe allowed penetration into the lungs, primed responses in both the superior cervical and thoracic nodes.
Intranasal delivery of the vaccine following light anesthesia results in antibody titers higher than those observed in the absence of anesthesia (Fig. 4). The higher IgA titers observed may be explained by our sampling of the IgA in lung homogenates rather than nasal washes (logistics limited our ability to perform nasal washes). However, we also observed higher serum IgG levels in the mice immunized under anesthesia.
Protection against B. pertussis respiratory infection with mucosally delivered Pa formulated with LTK63 or LTR72.
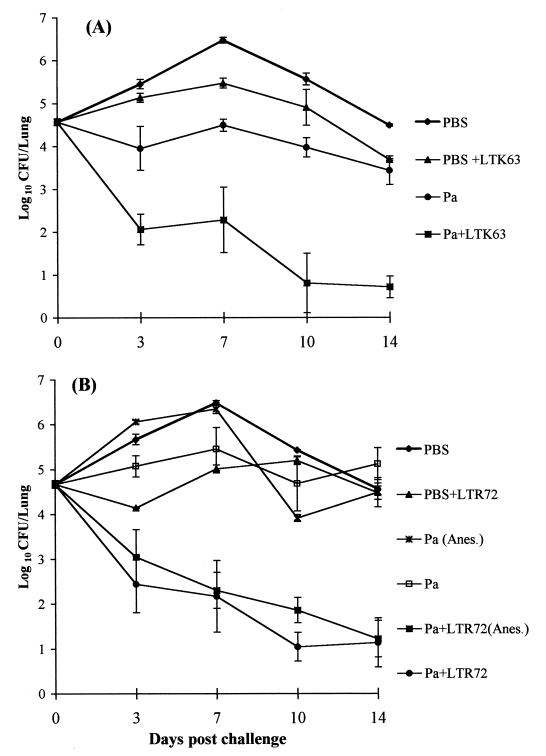
Mills et al. (21) have shown that the protection of immunized mice in a respiratory challenge model correlates with the estimated pertussis vaccine efficacy in clinical trials. We used this model to assess the protective efficacy of the nasally delivered Pa formulated with LTK63 or LTR72. The results given in Fig. 6 show that a nasally delivered formulation composed of FHA (2.5 μg) and rPT (5 μg) with LTK63 (10 μg) or LTR72 (1 μg) as an adjuvant provided levels of protection significantly greater than those achieved with the soluble antigens alone. Furthermore, the levels of protection surpassed those previously observed with a conventional parenterally delivered two-component Pa containing FHA (25 μg) and PTd (25 μg) adsorbed to alum (21), suggesting that the formulation described above would have superior clinical efficacy. Nasal delivery of Pa with LTR72 in 25 μl without an anesthetic conferred marginally, but not significantly, better protection than the same vaccine administered in 50 μl under light anesthesia. Interestingly, nasal immunization with the LT mutants alone appeared to confer some protection against B. pertussis respiratory challenge. The CFU counts were almost 10-fold lower at 7, 10, and 14 days after the challenge of mice immunized with LTK63 in phosphate-buffered saline (PBS) compared with PBS alone. Furthermore, immunization with LTR72 alone resulted in significantly lower CFU counts at 3 and 7 days (but not at 10 and 14 days) after challenge.
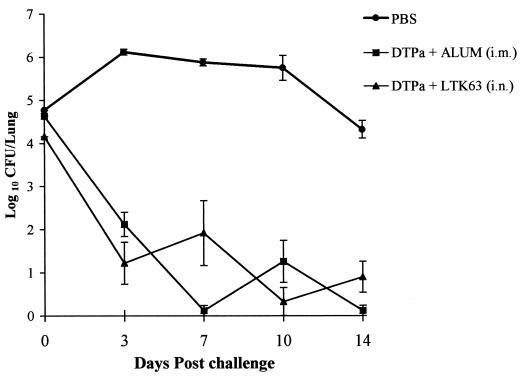
FIG. 6.
Kinetics of B. pertussis clearance from the lungs after respiratory challenge of immunized mice. Groups of 20 BALB/c mice were immunized twice (at weeks 0 and 4) i.n. with a two-component Pa consisting of rPT and FHA with or without LTK63 (10 μg) or with the adjuvant alone (LTK63 in PBS) or PBS alone (A) or with a two-component Pa consisting of rPT and FHA with or without LTR72 (1 μg) and with or without light anesthesia (Anes.) or with the adjuvant alone (LTR72 in PBS) or PBS alone (B). Mice were challenged 2 weeks after the second immunization by aerosol inoculation with B. pertussis. Results are presented as mean lung CFU counts assessed on four individual mice per experimental group at each time point. Error bars, standard errors.
Efficacy of mucosally delivered DTPa vaccine with LTK63 equals that of a conventional parenterally delivered DTPa vaccine adsorbed to alum.
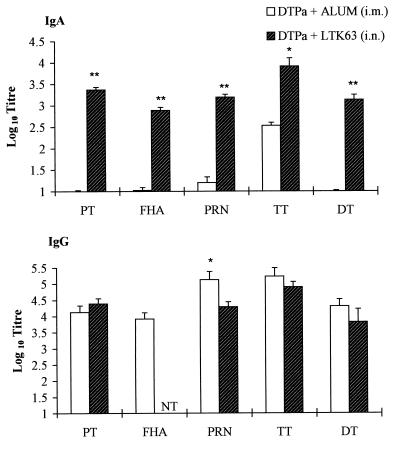
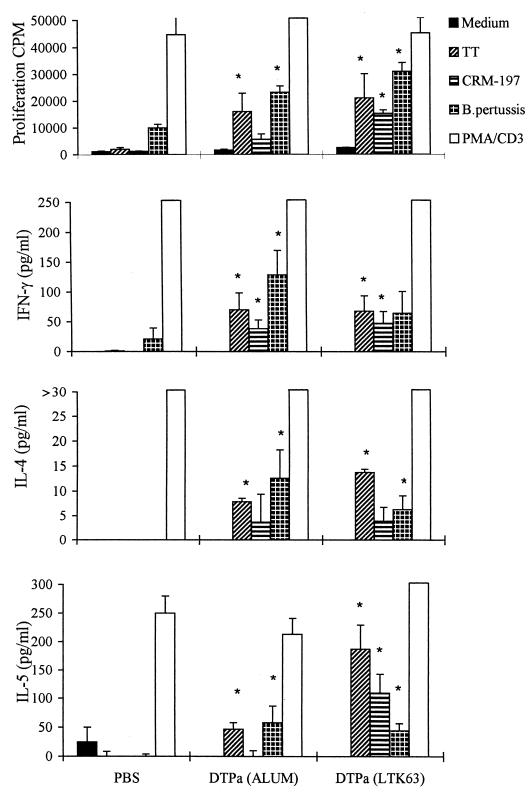
Pertussis vaccines are routinely administered to children as parenterally delivered DTP combination vaccines adsorbed to alum. Therefore, we directly compared a DTPa combination vaccine given by the nasal route with LTK63 as an adjuvant with the same antigens adsorbed to alum and injected by the i.m. route. The pertussis components included rPT (5 μg), FHA (2.5 μg), and PRN (2.5 μg), the third pertussis antigen present in many Pa. TT (10 μg) and CRM-197 (10 μg) were used for the tetanus and diphtheria components, respectively. As already shown in Fig. 1 and 4 for the pertussis antigens, intranasally delivered DTPa with LTK63 enhanced cellular and humoral immune responses to tetanus and diphtheria as well as pertussis antigens (Fig. 7 and 8). The levels of serum IgG specific for PT, PRN, TT, and DT observed after intranasal immunization with DTPa with LTK63 as an adjuvant were equivalent to those observed after i.m. immunization with DTPa adsorbed to alum; however, the mucosal immunization had the advantage of also enhancing local IgA responses (Fig. 7). Furthermore, B. pertussis-, TT-, and DT-specific T-cell proliferation and IL-4, IL-5, and IFN-γ production were detected in spleen cells from mice immunized by either route (Fig. 8).
FIG. 7.
Intranasal delivery of DTPa with LTK63 generates potent IgG and IgA responses against diphtheria, tetanus, and pertussis antigens. BALB/c mice were immunized i.n. with DTPa containing rPT (5 μg), FHA (2.5 μg), PRN (2.5 μg), TT (10 μg), CRM-197 (10 μg), and LTK63 (10 μg) in a 40-μl volume following light halothane anesthesia or with the same DTPa vaccine adsorbed to alum and injected by the i.m. route. Serum IgG and lung IgA levels against PT, FHA, PRN, TT, and DT were assessed 2 weeks after the second immunization. ∗ and ∗∗, P < 0.05 and P < 0.01, respectively, versus Pa alone (by Student's t test).
FIG. 8.
Comparative T-cell responses generated following nasal delivery of DTPa with LTK63 and parenteral delivery of DTPa adsorbed to alum. Mice were immunized as described in the legend to Fig. 7, and T-cell responses were assessed against TT, CRM-197, and killed B. pertussis as described in the legend to Fig. 1. ∗, P < 0.05 versus Pa alone (by Student's t test).
The level of protection against B. pertussis challenge conferred by the i.n. delivered DTPa and LTK63 was not significantly different from that observed with the alum-adsorbed vaccine given by the i.m. route (Fig. 9). The bacterial clearance curve shows that although the kinetics of clearance were different with the two vaccine delivery methods, they were equally protective.
FIG. 9.
A mucosally delivered DTPa vaccine with LTK63 as an adjuvant confers protection against B. pertussis infection equivalent to that of a parenterally delivered DTPa formulation adsorbed to alum. Groups of 20 BALB/c mice were immunized as described in the legend to Fig. 7. Control mice were immunized with alum only by the i.m. route. The kinetics of B. pertussis clearance from the lungs after respiratory challenge of immunized mice was assessed as described in the legend to Fig. 6.
DISCUSSION
This study demonstrates that E. coli LT mutants are capable of enhancing local and systemic T-cell and secretory and circulating antibody responses induced by antigens from B. pertussis delivered by the nasal route. We describe for the first time a mucosally delivered combined DTPa vaccine formulation that is capable of generating a level of protection against B. pertussis infection which is equivalent to that observed with the same antigens adsorbed to alum administered by a parenteral route. This study also provides significant new information on the adjuvant effect of LT on the induction of T-cell responses. Our findings demonstrate that the absence of ADP-ribosyltransferase activity, instead of diminishing the adjuvant action of LT, actually broadens its effect. The nontoxic mutant LTK63 was found to be capable of enhancing both Th1 and Th2 subtypes of T cells, whereas the wild-type toxin and the LTR72 mutant, which has partial enzyme activity, selectively enhanced Th2 cells, especially at low doses.
There have been a number of previous reports of mucosal delivery of pertussis antigens using a variety of delivery systems (4, 5, 16, 32). However, the level of protection documented either was not compared with that conferred by the conventional vaccine or did not approach that observed with alum-adsorbed antigens given by parenteral route. The highly significant correlation between B. pertussis clearance in immunized mice and vaccine efficacy in children in the murine respiratory challenge model (21) has provided us with an excellent system for testing the efficacy of experimental mucosal vaccine formulations and for predicting how they might perform in clinical trials. The two-component Pa composed of the B. pertussis antigens FHA and rPT with either LTK63 or LTR72 as an adjuvant conferred a high level of protection against B. pertussis respiratory challenge. By extrapolation from our correlation curve, the potency index achieved with FHA and rPT delivered nasally with LT mutants surpassed that observed with a two-component Pa composed of FHA and chemically detoxified PT (21). Furthermore, we have described for the first time a mucosally delivered DTPa combination vaccine which was as effective as the equivalent alum-adsorbed delivered by a parenteral route.
The nontoxic mutant, LTK63, and the partially active mutant, LTR72, were capable of enhancing the protective efficacy of the nasally delivered two-component Pa to an equivalent level. However, it is likely that the protection induced by the two formulations involved a different combination of immune effector mechanisms. It is now well established that protection against B. pertussis requires a combination of cellular and humoral immunity (21). It has been demonstrated that Th1 cells can transfer immunity to naïve immunosuppressed mice (22, 28). Furthermore, reduced bacterial clearance or disseminating atypical disease was observed following B. pertussis infection of IFN-γ defective or IFN-γ receptor-defective mice (2, 19). However, B cells and antibody production have also been found to be of crucial importance in protection against B. pertussis; B-cell-deficient mice with disruptions of the μ chain gene developed a chronic infection and failed to clear the bacteria from the lungs and could not be immunized with Pa or Pw (19, 21). Natural infection or immunization with Pw selectively induces Th1 cells and confers a higher level of protection than parenterally delivered Pa (22, 28). Nevertheless, the Pa can also confer a reasonable level of protection, and these vaccines induce Th2 cells in mice (28) and a mixed Th1–Th2 response in children (31). The present study demonstrates that the protection conferred with intranasally delivered Pa with LTK63 as an adjuvant relies more heavily on cellular immunity. In contrast, the vaccine formulated with LTR72 enhanced Th2 and antibody responses; lung IgA and circulating IgG levels were increased and IL-4- and IL-5-producing cells were activated in the local lymph nodes and in the periphery. While nasally delivered Pa with LTK63 and LTR72 protect to a level equivalent to that observed with parenterally delivered Pa, the protection observed does not equal that reported for parenterally delivered Pw, which induces a strongly Th1-biased immune response. This is consistent with the lack of complete polarization to Th1 with either of the LT mutants.
There is some controversy on the roles of the binding and enzyme components of LT in its adjuvant activity. It has been reported that the induction of antibody responses against the B subunit of LT required the presence of the A subunit and hence ADP-ribosyltransferase activity (8). Furthermore, Lycke et al. (17) failed to see any adjuvant action in LT mutants without enzyme activity, but they used the oral route of delivery, which may require the added potency of enzyme activity for an adjuvant effect to be observed. In contrast, studies by a number of independent groups have demonstrated adjuvant effects following nasal delivery of LT mutants with reduced or defective ADP-ribosyltransferase activity (7, 9, 10, 11, 13). The results from the present study confirm that the ADP-ribosyltransferase activity of LT is not essential for the adjuvant action of LT, but more significantly, they demonstrate that the A subunit does have immunomodulatory activity which is distinct from that of the binding domain. Our findings are in agreement with a report by Giuliani et al. (13) suggesting that the adjuvant activity of LT is derived from independent contributions of the nontoxic AB complex and the enzyme activity, but in addition our study demonstrates that the receptor binding and enzyme activities have distinct modulatory effects on T-cell subtype induction in vivo.
We observed augmentation of both Th1 and Th2 responses by the nontoxic mutant LTK63 but more Th2-biased responses with the wild type and with the partially active mutant, LTR72. Nasal immunization with Pa or DTPa vaccine in the presence of the enzymatically inactive mutant LTK63 enhanced the production of IFN-γ, IL-4, and IL-5 by antigen-stimulated spleen and lymph node cells. In contrast, immunization with Pa in the presence of 1.0 μg of LT or LTR72 selectively enhanced IL-4- and IL-5-secreting T cells. The enhancing effect of LT on type 2 T cells is consistent with that observed with mucosally delivered CT (20). In addition, we have previously reported that a mutant of PT lacking ADP-ribosylation, PT/9K-129G, enhanced Th1 responses (29a, 30). However, the wild-type PT enhanced both Th1 and Th2 cells in vivo (30). Furthermore, it has been reported that oral delivery of TT with LT induced T cells with mixed Th1- and Th2-type responses in the spleen and Peyer's patches (34). In the present study we observed highly polarized Th2 responses with low doses of LTR72 but some enhancement of IFN-γ production when the dose of toxin was increased from 1 to 10 μg per mouse. Furthermore, increasing the dose of adjuvant also appeared to augment the Th1 component of the mixed Th1–Th2 profile observed with LTK63. It appears that the binding domain enhances IFN-γ production and that this effect is more pronounced at higher doses of the toxin, whereas the enzyme activity selectively enhances Th2 responses and/or suppresses Th1 cells. However, the latter effect may be evident only at lower doses of LTR72, when the enhancing effect of the AB binding domain on IFN-γ production is diminished.
There are a number of possible mechanisms that may explain the differential effects of LT, LTR72, and LTK63 on Th1 and Th2 responses. The ADP-ribosyltransferase activity of LT and LTR72 results in increased cyclic AMP (cAMP) levels, and it has been reported that cAMP accumulation up-regulates IL-4 and IL-5 production by activated CD4+ T cells while down-regulating IL-2 production. It is likely that Th1 and Th2 cells differ in their sensitivities to an increase in cAMP. T-cell receptor-mediated proliferation in Th2 cells is not affected by CT, whereas Th1 cells are inhibited (23). Accumulation of cAMP in antigen-presenting cells has been shown to correlate with an increase in prostaglandin E2 synthesis, which in turn has been shown to have a negative effect on the production of IL-2 and IFN-γ, while upregulating IL-5 production (33).
An alternative, but not exclusive, explanation for the distinct effects of LT and LT mutants on the induction of Th cell subtypes is provided by our recent studies on the effect of LT on the production of cytokines that regulate the induction of Th1 and Th2 cells. We have preliminary evidence that LT and LTR72 suppress IL-12 production in a dose-dependent fashion, whereas LTK63 enhances IL-12 and IFN-γ production both in vivo and in vitro (29a). This is consistent with a recent report demonstrating that CT and LT, but not CT-B, can inhibit IL-12 production and IL-12 receptor β1 and β2 chain expression (3). We have previously reported that IL-12 production by macrophages is crucial for the development of the protective Th1 response to B. pertussis infection or immunization (18). The purified antigen components of the Pa failed to elicit IL-12 production by macrophages, resulting in a Th2-type response, and addition of exogenous IL-12 as an adjuvant switched the response to Th1–Th2 and enhanced protection (18). This is consistent with our finding that nasal delivery of LTR72 during B. pertussis infection suppressed Th1 responses and exacerbated the infection, whereas LTK63 enhanced Th1 responses and augmented the rate of bacterial clearance (29a).
We also demonstrated that manipulating delivery of the vaccine, targeting the antigens to the upper or lower respiratory tract, influenced the resulting immune responses. The use of anesthetic and a relatively high delivery volume, which allows penetration into the lungs (24, 35), resulted in higher local IgA and Th2 responses in thoracic and cervical lymph nodes. In contrast, a lower volume without anesthesia, which would have confined the vaccine to the nasal cavity, resulted in stronger Th1 responses, especially in the superior cervical lymph nodes, but weaker antibody responses. Antigens delivered to the nasal tract may be taken up by resident antigen-presenting cells, and these pass through the nasal epithelium layer to the superior cervical lymph nodes, where an immune response is initiated (12). If the antigen penetrates further into the lung (e.g., following anesthesia), then the thoracic nodes are also activated. The bias toward Th2 responses in the gut and lungs may reflect the natural host mechanism of limiting inflammatory responses at mucosal surfaces, where a continuous array of antigens are ingested or inhaled. This is consistent with the observation that the more Th2-biasing mutant LTR72 had a more pronounced adjuvant effect on the immune responses to antigen delivered into the lungs. Furthermore, the effect on T-cell response was most dramatic in the thoracic lymph nodes, which drain the lungs. Thus, it appears that targeting of the toxins to different immunological sites, the binding to distinct receptors or their activation/inhibition of distinct G proteins, and the dose administered may all influence the adjuvant effect for Th1 and Th2 cells.
The efficacy of LTK63 and LTR72 as mucosal adjuvants demonstrated in the present study has made the possibility of nasally administered vaccines realizable and also provides a basis for use of nasally delivered LT mutants in immunotherapeutic intervention aimed at modulating T-cell responses in vivo. By using LTK63, with a broad stimulatory effect on T-cell responses, or low-dose LTR72, which activates the induction of Th2 cells and suppresses type-1 responses, it should be possible to design mucosally delivered therapies or vaccines that modulate the most appropriate immune response to provide protection against a variety of infectious and immune-mediated diseases.
REFERENCES
- 1.Almeida A J, Alpar H O. Nasal delivery of vaccines. J Drug Targeting. 1996;3:455–467. doi: 10.3109/10611869609015965. [DOI] [PubMed] [Google Scholar]
- 2.Barbic J, Leef M F, Burns D L, Shahin R D. Role of gamma interferon in natural clearance of Bordetella pertussisrespiratory infection. Infect Immun. 1997;65:4904–4908. doi: 10.1128/iai.65.12.4904-4908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun M C, He J, Wu C Y, Kelsall B. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor β1 and β2 chain expression. J Exp Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill E S, O'Hagan D T, Illum L, Barnard A, Mills K H G, Redhead K. Immune responses and protection against Bordetella pertussisinfection after intranasal immunization of mice with filamentous haemagglutinin in solution or incorporated in biodegradable microparticles. Vaccine. 1995;13:455–462. doi: 10.1016/0264-410x(94)00008-b. [DOI] [PubMed] [Google Scholar]
- 5.Cahill E S, O'Hagan D T, Illum L, Redhead K. Mice are protected against Bordetella pertussisinfection by intra-nasal immunization with filamentous haemagglutinin. FEMS Microbiol Lett. 1993;107:211–216. doi: 10.1111/j.1574-6968.1993.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:RR1–RR25. [PubMed] [Google Scholar]
- 7.deHaan L, Feil L K, Verweij W R, Holtrop M, Hol W G J, Agsteribbe E, Wilschut J. Mutational analysis of the role of ADP-ribosylation activity and GM1-binding activity in the adjuvant properties of the Escherichia coliheat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur J Immunol. 1998;28:1243–1250. doi: 10.1002/(SICI)1521-4141(199804)28:04<1243::AID-IMMU1243>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.deHaan L, Holtrop M, Verweij W R, Agsteribbe E, Wilschut J. Mucosal immunogenicity of the Escherichia coliheat-labile enterotoxin: role of the A-subunit. Vaccine. 1996;14:260–266. doi: 10.1016/0264-410x(95)00235-s. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson B L, Clements J D. Dissociation of Escherichia coliheat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douce G, Turcotte C, Cropley L, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coliheat labile toxin lacking ADP-ribosyltransferase activity act as non-toxic mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douce G, Giuliani M M, Giannelli V, Pizza M, Rappuoli R, Dougan G. Mucosal immunogenicity of genetically detoxified derivatives of heat labile toxin from Escherichia coli. Vaccine. 1998;16:1065–1073. doi: 10.1016/s0264-410x(98)80100-x. [DOI] [PubMed] [Google Scholar]
- 12.Frieke Kuper C, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 13.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coliheat-labile toxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco D, Salmaso S, Mastrantonio P, Guiliano M, Tozzi A G, Anemona A, Giori Delgi Atti M L, Giammanco A, Panel P, Blackwelder W C, Klein D L, Wassilak S G F the Progetto Pertosase Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med. 1996;334:341–347. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson L, Hallander H O, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular and a whole cell pertussis vaccine. N Engl J Med. 1996;334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 16.Jones D H, McBride B W, Thornton C, O'Hagan D T, Robinson A, Farrar G H. Orally administered microencapsulated Bordetella pertussis fimbriae protect mice from B. pertussisrespiratory infection. Infect Immun. 1996;64:489–494. doi: 10.1128/iai.64.2.489-494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coliheat labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 18.Mahon B P, Ryan M S, Griffin F, Mills K H G. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussisand enhances the efficacy of an acellular pertussis vaccine by promoting the induction of Th1 cells. Infect Immun. 1996;64:5295–5301. doi: 10.1128/iai.64.12.5295-5301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahon B P, Sheahan B J, Griffin F, Murphy G, Mills K H G. Atypical disease after Bordetella pertussisrespiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee J R. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 21.Mills K H G, Ryan M, Ryan E, Mahon B P. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun. 1998;66:594–602. doi: 10.1128/iai.66.2.594-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz E, Zubiaga A M, Merrow M, Sauter N P, Huber B T. Cholera toxin discriminated between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizov R, Takahashi M, Hirshman C A, Croxton T. Halothane inhibition of ion transport of the tracheal epithelium. A possible mechanism for the anesthetic-induced impairment of mucociliary clearance. Anesthesiology. 1992;76:985–989. doi: 10.1097/00000542-199206000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Podda A, Nencioni L, Marsili I, Peppoloni S, Volpini G, Donati D, DiTommaso A, DeMagistris M T, Rappuoli R. Phase I clinical trial of an acellular pertussis vaccine composed of genetically detoxified pertussis toxin combined with FHA and 69 kDa. Vaccine. 1991;9:741–745. doi: 10.1016/0264-410x(91)90290-m. [DOI] [PubMed] [Google Scholar]
- 26.Porro M, Saletti M, Nencioni L, Tagliaferri L, Marsili I. Immunogenic correlation between cross-reacting material (CRM-197) produced by a mutant of Corynebacterium diphtheriaeand diphtheria toxoid. J Infect Dis. 1980;142:716–724. doi: 10.1093/infdis/142.5.716. [DOI] [PubMed] [Google Scholar]
- 27.Rappuoli R, Pizza M, Podda A, De Magistris M T, Nencioni L. Towards third generation whooping cough vaccines. Trends Biotechnol. 1991;9:232–238. doi: 10.1016/0167-7799(91)90076-t. [DOI] [PubMed] [Google Scholar]
- 28.Redhead K, Watkins J, Barnard A, Mills K H G. Effective immunization against Bordetella pertussisinfection in mice is dependent on the induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley L, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Ryan, E., and K. H. G. Mills. Unpublished data.
- 30.Ryan M, McCarthy L, Rappuoli R, Mahon B P, Mills K H G. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the costimulatory molecules B7-1, B7-2 and CD28. Int Immunol. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 31.Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Oymar K, Miller E, Storsaeter J, Mills K H. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahin R D, Leef M, Eldridge J, Hudson M, Gilley R. Adjuvanticity and protective immunity elicited by Bordetella pertussis antigens encapsulated in poly(dl-lactide-Co-glycolide) microspheres. Infect Immun. 1995;63:1195–1200. doi: 10.1128/iai.63.4.1195-1200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snijdewint F G M, Kalinski P, Wierenga E A, Bos J D, Kapsenberg M L. Prostaglandin-E2differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- 34.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa L, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia colilabile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 35.Thompson A H, McRoberts J G, Crowe S R, London L, London S D. Optimal induction of upper respiratory tract immunity to reovirus 1/L by combined upper and lower respiratory tract inoculation. Vaccine. 1999;17:1404–1415. doi: 10.1016/s0264-410x(98)00382-x. [DOI] [PubMed] [Google Scholar]
- 36.Walker R I. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–399. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 37.Williams N A, Hirst T R, Nashar T O. Immune modulation by cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]