Abstract
Abstract
The spread of chikungunya virus (CHIKV) is reaching pandemic levels, and vaccines and antivirals to control CHIKV infection have yet to be approved. Virus-like particles (VLPs), a self-assembled native multi-subunit protein structure, could potentially be used as an antigen for serological detection and vaccine development. In the current study, we describe the production of novel CHIKV VLPs from mosquitoes using a Baculovirus/Mosquito (BacMos) system in a simple Biosafety Level‐2 laboratory. Substantial envelope and capsid protein secretions were detected in culture medium. Co-fractionation of CHIKV E2, E1, and capsid proteins via sucrose gradient ultracentrifugation provided evidence of VLP formation. Transmission electron microscopy and dynamic light scattering analysis revealed the formation of VLPs in the form of spherical particles with a diameter of roughly 40 nm in transduced cells and culture medium. VLP-based IgM capture ELISA in CHIKV patient sera revealed native epitopes on the VLPs. These non-purified VLPs were shown to act as an antigen in CHIKV-specific IgM capture ELISA. The immunization of CHIKV-VLPs alone in mice induced a balance CHIKV-specific IgG2a/IgG1 antibodies and neutralized antibody responses. The study provides support for the hypothesis that mosquito cell-derived CHIKV VLPs could serve as a novel antigen for serological detection and the development of vaccines against CHIKV infection.
Key points
• CHIKV VLPs secreted from BacMos-CHIKV 26S-transduced mosquito cell.
• This CHIKV VLPs potentially serve as an alternative capture antigen for MAC-ELISA.
• Unadjuvanted CHIK VLPs induce CHIKV-specific IgG and NT responses in mice.
Keywords: Chikungunya virus, Virus-like particles, Baculovirus, Mosquito cell
Introduction
Chikungunya virus (CHIKV) is mosquito-borne arbovirus virus in the genus alphavirus of the family Togaviridae transmitted by infected Aedes mosquitoes. CHIKV infection causes fever, rash, myalgia, and persistent incapacitating arthralgia in humans. No antiviral treatments or vaccines to control CHIKV infection have been approved. Analgesics and/or nonsteroidal anti-inflammatory drugs alone or conjugation with steroid are effective treatments for CHIKV infection (Rosario et al. 2015). CHIKV is an enveloped virus with a positive-stranded RNA genome, comprising two open reading frames (ORFs) embedded between non-translated regions, 5′-UTR and 3′-UTR. The first ORF located at the 5′-end encodes a polyprotein precursor of nonstructural proteins (nsP1, nsP2, nsP3, and nsP4) for viral replication and transcription. The second ORF encodes the polyprotein of the structural proteins (C, E3, E2, and E1) for viral assembly. The C-pE2-6 k-E1 polyprotein is encoded by 26S subgenomic RNA. Co- and post-translational cleavage produces mature structural alphavirus proteins during viral replication (Liljeström and Garoff 1991). Autoproteolytic serine proteinase liberates the capsid from the N-terminus of the nascent polyprotein (Melancon and Garoff 1987). Envelope polyproteins transported into the endoplasmic reticulum (ER) lumen via an N-terminal signal sequences, and processed into heterodimeric pE2/ E1 anchoring on the plasma membrane via C-terminal transmembrane domains (Strauss and Strauss 1994). During transport, and probably just before its arrival at the cell surface, pE2 is cleaved by host furin into E2 and E3, thereby initiating the maturation process (Ozden et al. 2008; Yap et al. 2017; Zhang et al. 2003). Virus budding occurs at the cell membrane, where the nucleocapsid is enveloped by glycoproteins E1/E2 on the lipid membrane, whereupon the release of E3 triggers viral maturation. Heterodimeric E2/E1 display on the viral membrane of mature virion is respectively responsible for viral attachment and membrane fusion (Li et al. 2010).
The lack of approved vaccines and antivirals means that vector control is currently the best approach to controlling CHIKV outbreaks. An effective vaccination regime is crucial to limiting CHIKV infection (Erasmus et al. 2016; Goyal et al. 2018). Virus-like particles (VLPs), protein-based nanoparticles derived from viruses, are configurative and antigenic to their corresponding native viruses [54], which makes them highly effective as subunit vaccines and diagnostic antigens (Charlton Hume et al. 2019; Tariq et al. 2022; Theillet et al. 2019).
CHIKV VLP production begins with the expression of 26S cDNA (C-E3-E2-6 K-E1) from a DNA expression plasmid transfected into mammalian cells (Akahata et al. 2010; Chen et al. 2019; Noranate et al. 2014; Urakami et al. 2017). CHIKV VLPs have also been produced using yeast (Saraswat et al. 2016) and baculovirus (Metz et al. 2013; Wagner et al. 2014) expression systems. CHIKV VLPs mimicking the nanoparticle configuration of viruses have been shown to induce protective antibody responses in mice and non-human primates (Akahata et al. 2010; Metz et al. 2013; Saraswat et al. 2016) induce the expression of cross-protective neutralizing antibodies (NAbs) against all CHIKV genotypes in humans (Goo et al. 2016). In phase 1 and 2 clinical trials, CHIKV VLP vaccine candidates demonstrated sufficient safety, tolerability, and immunogenicity (Chang et al. 2014; Chen et al. 2020). Nonetheless, mammalian cell-derived CHIKV VLPs must be administered at least twice to attain 100% seroconversion and induce the production of neutralizing antibodies at meaningful levels. A tedious transfection procedure (Chen et al. 2019) and the potentially cytotoxic effects of VLPs further impede the production of alphavirus VLPs from mammalian cells (Barry et al. 2010; Joe et al. 1998). The inherent safety, scalability, and high yield of the baculovirus-Lepidoptera expression system (BES) could provide benefits over other expression systems (Metz et al. 2013). VLPs have been generated from mammalian (a host of CHIKV) and Lepidoptera (insect) cell lines. Production at elevated pH levels has also been shown to promote the production of CHIKV VLPs in mammalian and Lepidoptera cells (Akahata and Nabel 2012; Wagner et al. 2014). Mosquito cell (another host cell of CHIKV) may also provide a platform by which to elucidate the folding of arboviral glycoproteins to yield authentic VLPs. Compared to the acidic milieu of BES (pH 6.4), it appears that a neutral milieu may provide superior conditions for CHIKV VLP production. Baculovirus is capable of efficient gene transduction into mosquito cells (Naik et al. 2018), and Baculovirus/Mosquito (BacMos) systems have been used to generate recombinant proteins from mosquito cells (Chang et al. 2020; Lin et al. 2021a, 2021b). In the current study, we demonstrated the use of a BacMos system to express the full-length CHIKV 26S cDNA assembly within VLPs in mosquito cells. Mosquito-derived CHIKV VLPs exhibited similarities to native CHIKV virions in terms of morphology, antigenicity, and immunogenicity. These CHIKV VLPs could potentially serve as antigens for diagnostic purposes and vaccine development.
Materials and methods
Cells and viruses
AP-61 cells (from Aedes pseudoscutellaris) were cultured in Leibovitz’s L-15 medium (Thermo Fisher, Waltham, MA, USA) with 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher, Waltham, MA, USA) and Antibiotic–Antimycotic (Gibco, Thermo Fisher, Waltham, MA, USA) at 28 °C. C6/36 mosquito cells (ATCC® CRL-1660™) (from Aedes albopictus) were cultured in RPMI 1640 Medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS and Antibiotic–Antimycotic at 28 °C under 5% CO2. Vero cells were cultured in Dulbecco’s Modified Eagles’ Medium (DMEM) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS, glutamine (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), pyruvate (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), and penicillin /streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C under 5% CO2. BHK-21 cells were cultured in RPMI 1640 Medium containing 10% FBS at 37 °C under 5% CO2. Baculovirus propagation and viral titrations were performed using SF-21 cells. Viral titers of CHIKVs propagated in C6/36 cells were determined using BHK-21 cells.
Generation of the recombinant baculovirus
pBac-IR-GFP-hr1pag1-JEV-prM-E, a transfer vector designed to construct the recombinant baculovirus of BacMos-JEV prM-E for JE VLP production, has been described previously (Chang et al. 2020). The transfer vector (pBac-IR-GFP-hr1pag1-CHIKV 26S) for BacMos-CHIKV 26S was constructed by replacing JE prME gene (BglII–NotI) of pBac-IR-GFP-hr1pag1-JEV-prM-E with synthetic 3.7-kb BglII-NotI 26S cDNA from CHIKV strain LR2006_OPY1 (GenBank: KT449801). Production of the recombinant baculovirus (BacMos-CHIKV 26S) (Fig. 1a) was conducted in accordance with previously described protocols (Naik et al. 2018).
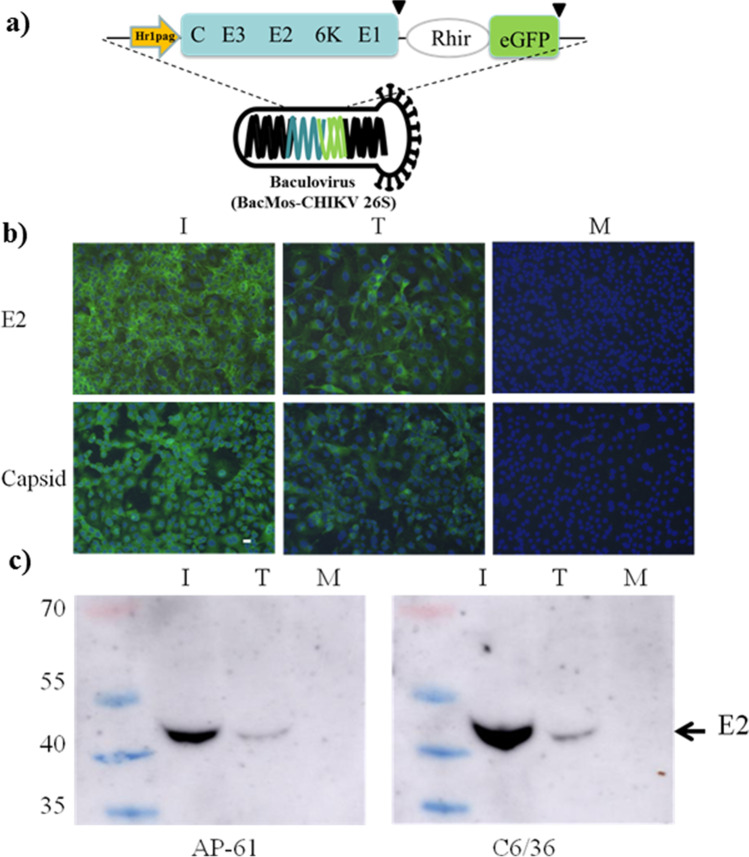
Fig. 1.
Expression of CHIKV structural proteins in BacMos-CHIKV 26S-transduced mosquito cells. a Schematic illustration showing that recombinant baculovirus (BacMos-CHIKV 26S) harbored the hr1pag1–CHIKV 26S cDNA and the Rhir-eGFP gene. The bi-cistronic baculovirus transfer vector containing the Rhir IRES was located between the hr1pag1–CHIKV 26S cDNA and the eGFP gene. Abbreviations: hr1pag1, a mosquito promoter; CHIKV 26S cDNA, encoded polyprotein of 26S (C-E3-E2-6 K-E1) of CHIKV strain LR2006_OPY1; Rhir, an IRES (internal ribosome entry site); eGFP, a green fluorescent protein gene; and ▼, translational stop codon. b Immunofluorescence results showing the expression of CHIKV structural proteins on transduced AP61 cells. Infected (I, left panels), transduced (T, middle panels), or untreated AP-61 cells (M) stained with either mAb anti-CHIKV E2 (upper panels) or mAb anti-CHIKV capsid (lower panels), and Hoechst 33342. Scale bars, 30 μm. c Secretion of CHIKV E2 glycoprotein from BacMos-CHIKV 26S-transduced AP-61 (left panel) and C6/36 (right panel) cells. Culture supernatant samples from CHIKV-infected (I) BacMos-CHIKV 26S-transduced (T) or mock (M) cells were subjected to WB analysis for CHIKV E2 mAb (5C5). CHIKV E2 protein is indicated by arrows on the right
Immunofluorescence microscopy
AP-61 cells were either transduced using BacMos-CHIKV-26S at an MOI of 10 or infected with CHIKV (Indonesia/0706aTw/2007/FJ807897) at an MOI of 0.1. After three days, the transduced cells and untreated cells (Mock) were fixed and stained using either anti-CHIKV E2 mAb (5C5 from Taiwan CDC) or anti-CHIKV capsid mAb (19B02, NativeAntigen) (1:100) for 1 h. The stained cells were subsequently washed three times with PBS and then incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:400; Invitrogen, A11029) and Hoechst 33,342 (10 µM) for 1 h. After a final wash with PBS, images of the cells were captured using an inverted fluorescence microscope (Olympus Model IX71, Tokyo, Japan).
WB analysis
Samples were resolved in Laemmli sample buffer (with or without β-mercaptoethanol) and separated by SDS-PAGE (10%) before being electro-transferred onto a PVDF membrane. The membrane was blocked and then incubated with anti-E2 mAb (5C5) (1:1000), anti-E2 mAb (Chk265, Absolute Antibody) (1:1000), or rabbit anti-E1 serum (Kuo et al. 2011) (1:1000). The membrane was washed and then incubated with a 1:5000 dilution of horseradish peroxidase (HRP)-conjugated secondary antibodies. Following a second round of washing, the membrane was incubated with ECL substrate, and the resulting signals were captured using the Amersham Image 600 (GE Healthcare).
VLP production and purification
The production of CHIKV VLPs involved plating 1 × 107AP-61 cells in a T175 flask followed by incubation for 24 h (h). The cells were transduced with baculovirus at an indicated MOI of 20. Following a 12-h incubation period, the cells were washed once using PBS. Culture medium was harvested and refreshed at 2, 4, 6, and 8 dpt. Culture supernatant was passed through a 0.45-μm filter to remove debris, after which the VLPs were concentrated using a MidiKros Module Tangential Flow Filtration (TFF) System (750,000 molecular weight cut-off, Spectrum Repligen, Rancho Dominguez, CA, USA). The concentrated VLPs were subsequently loaded onto a 20% sucrose cushion and centrifuged at 300,00 rpm (45Ti rotor; Beckman) at 4 °C for 4 h. The resulting pellets were resuspended in NTE buffer (50 mM Tris–HCl pH 7.4, 100 mM NaCl, 0.1 mM EDTA) and subjected to a sucrose gradient (25% and 55%). Following another (12 h) round of centrifugation at 350,00 rpm (SW41 rotor; Beckman) at 4 °C, 10 fractions were collected using Pipetman P1000 (from top to bottom) for WB analysis (anti-E2 Chk265 mAb, rabbit anti-CHIKV E1 serum, or anti-capsid 19B02 mAb) and transmission electron microscopy (TEM) assays. The VLPs were quantified by comparing the Coomassie blue-stained band densities of E1/E2 proteins to the band densities of known quantities of proteins.
EM observation
Transduced or infected C6/36 cells were washed with PBS and fixed with 2.5% glutaraldehyde and 2% osmium tetroxide in the dark for 60 min, followed by 1% uranyl acetate staining for 60 min, before being dehydrated in ethanol. The material was embedded in resin. Ultrathin sections were contrasted with uranyl acetate and observed via TEM analysis. Partially purified VLPs were deposited onto a copper grid for negative staining using 2% uranyl acetate. TEM observation was performed using a Hitachi HT7700 at an accelerating voltage of 100 kV.
Dynamic light scattering
Partially purified VLPs were characterized using a Malvern Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). One milliliter of each sample was analyzed in a disposable solvent-resistant micro cuvette (ZEN0040). The measurement was performed at 25 °C, and each sample underwent two series of 15 runs. The size distribution of CHIKV VLPs was obtained using the Zetasizer software suite.
MAC-ELISA
MAC‐ELISA was conducted in accordance with the protocol (Chang et al. 2020) with slight modifications. Briefly, goat anti-human IgM antibodies were incubated in a 100-fold dilution of human sera in a 96‐well plate. Following this, 100 μl of undiluted or diluted CHIKV VLPs (the culture supernatant of transduced AP-61 cells) or UV-inactive CHIKV 9,500,004 strain (1.67 × 106 pfu/ml) was added to each well. After washing with PBS, 4000-fold dilutions of AP-labeled mouse anti-CHIKV mAb (5C5) were added to the wells. After a final PBS wash, p-nitrophenyl-phosphate (Sigma) was added before measuring the optical density (OD) at 405 nm using a Dynatech MR700 microplate reader.
Mice immunization
BALB/c mice (n = 3) were subcutaneously (SC) immunized with three doses of 0.25 μg or 1 μg VLPs at 8, 11, and 13 weeks of age. Another group of mice was SC-inoculated with PBS to serve as negative controls. Blood was collected from mice at 10, 14, and 15 weeks of age. Following coagulation, the blood was centrifuged, and the sera were harvested, aliquoted, and stored at − 80 °C until use.
Evaluation of IgG, IgG1, and IgG2a responses using ELISA assays
IgG responses were measured in triplicate by coating 96-well plates with purified recombinant CHIKV E2 protein (Kuo et al. 2011) (2 μg/ml per well) in 100 μl of carbonate-bicarbonate buffer (NaHCO3, 18.2 mM Na2CO3, pH 9.6). The wells were then incubated at 4 °C overnight. After blocking, 100 μl of twofold serial dilutions of serum sample (from 1:100 to 1: 12,800) was added to each well and incubated at 37 °C for 1 h. Following this, 100 μl of diluted horseradish peroxidase (HRP)-conjugated anti-mouse IgG (KPL), IgG1 (Zymed), or IgG2a (Invitrogen) antibodies were added to each well, and color was generated by adding a substrate solution of SureBlue TMB peroxidase. Absorbance at 450 nm was measured using a microplate reader. The endpoint titer was defined as the highest serum dilution at which the OD450 value ≥ 0.2. The data are shown as the means ± SEM.
Pseudotyped neutralization test
Synthetic 3.7-kb 26S cDNA from CHIKV strain LR2006_OPY1 (GenBank: KT449801) was sub-cloned into a pack2 vector to construct pack2-CMV-HHB-26S-poly A. To package CHIKV-26S pseudovirus, 1 × 106 Lenti-X 293 T cells (Takara)/well (6-well plate) in lentivirus packing medium (Invitrogen) had been transfected with 1.7 μg pLV-EF1a-Intron-Luciferase-IRES-Bsd, 1 μg pack1, and 0.8 μg pack2-CMV-HHB-26S-poly A using Lipofectamine 3000 reagent. Cell media were changed at 6-h post-transfection. At 24-h and 52-h post-transfection, the supernatant containing pseudovirus material was harvested and filtered using a 0.45-µm syringe filter. The pseudovirus stock was stored at − 80 °C. Sera were first heat-inactivated over a period of 30 min at 56 °C and diluted via twofold serial dilution (1:100 to 1:6400) using DMEM with 5% FBS, before undergoing incubation with 3 × 105 relative light unit (RLU) of the pseudovirus (positive control) or growth medium (negative control) at 37 °C for 1 h. The mixtures were then dispensed into 6 × 104 Vero cells/well (96-well plate). After incubation for 16 h, the culture medium was refreshed (100 µL/well) and incubation was continued at 37 °C for another 48 h. Cells were lysed using Bright-Glo reagent (Promega) for 3 min. The lysate was then transferred to a white plate to measure the luminescence using an LB 960 Luminometer (Berthold). The ID50 (half-maximal inhibitory dilution) of neutralizing antibody titers was calculated using GraphPad Prism as the reciprocal of the dilution. The RLUs were reduced by 50% compared to pseudovirus-alone control wells.
Microneutralization assay
Heat-inactivated sera were serially diluted in MEM (1∶10 to 1∶1,280) and incubated with an equal volume of CHIKV (100 TCID50) Taiwan autochthonous strain (GenBank: MN871956.1) of East/Central/South African (ECSA) genotype (Chen et al. 2021) at 37 °C. After 60 min, the virus-serum mixture was added to a monolayer of BHK-21 cells (10,000 cells (for patient and mouse samples) per well) in a 96-well flat-bottom plate and incubated in a 5% CO2 incubator at 37 °C for 4 days. The highest titer at which no cytopathogenic effect was observed was recorded as the nAb titer. After an incubation period of four days, infected cells were fixed and stained using a 0.1% crystal violet solution. Neutralizing titers (FRμNT90) were determined by 90% protection against cytopathic effects.
Results
Expression and secretion of CHIKV structural proteins in mosquito cells
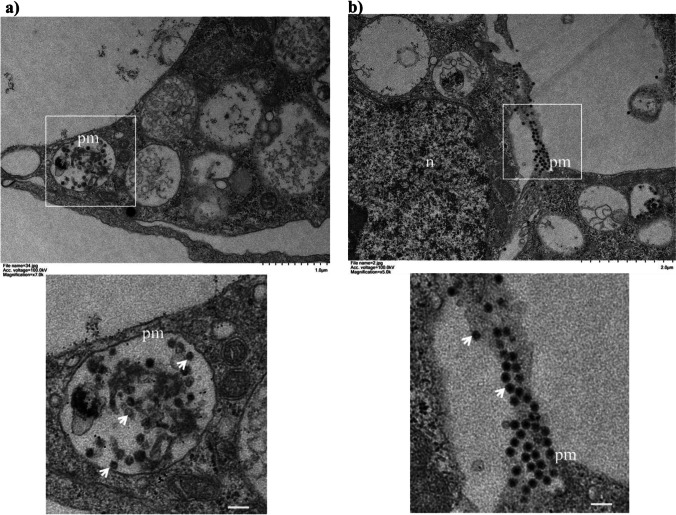
IFA staining using E2 or capsid-specific antibodies was performed to determine whether a baculovirus (BacMos-CHIKV 26S) vector could induce the expression of CHIKV structural proteins in transduced mosquito cells (Fig. 1a). Results (Fig. 1b) revealed the expression of E2 and capsid on infected and transduced cells, and these mock cells were non-reactive to specific antibody binding. To test for the secretion of CHIKV glycoprotein from transduced cells, the culture supernatant was subjected to western blot (WB) analysis using an anti-E2 monoclonal antibody (mAb) (5C5). Results (Fig. 1c) revealed a protein band migrating to the 50 kDa (E2) gel position from infected and transduced cells, but not from the mock control. These results indicate the production and secretion of CHIKV structural proteins in transduced mosquito cells. To further characterize the secretion of CHIKV E2 structural proteins, culture supernatants from two mosquito cell lines (AP-61 and C6/36) at 2 and 5 days post-transduction (dpt) were subject to WB analysis with a neutralizing monoclonal antibody (mAb) (Chk265). Results (Fig. 2a) revealed a dose-dependent secretion of E2 in AP-61 cells only. The yield of E2 from AP-61 cells exceeded that from C6/36 cells. WB results (Fig. 2b) revealed that E2 secretion in both cells continued from 1 to 8 dpt with a peak at 5 dpt in AP-61 cells. Our initial work focused on the production of E2 (VLP) at 1 to 8 dpt from the culture supernatant of transduced AP-61 cells with a multiplicity of infection (MOI) of 20, wherein the medium was refreshed at intervals of one day. We investigated CHIKV VLP formation in transduced C6/36 cells and CHIKV-infected cells using TEM analysis. Our results revealed the presence of spherical particles with an average diameter of 40 nm in the lumen of transduced cells (Fig. 3a). Note that in infected cells, the diameter of typical CHIKV virions is 60 nm (Fig. 3b). Taken together, these results demonstrate the expression, intracellular assembly, and potentially secretion of CHIKV VLPs in BacMos-CHIKV 26S-transduced mosquito cells.
Fig. 2.
Characterization of CHIKV E2 secretion by AP-61 and C6/36 mosquito cell lines using an NT mAb (Chk265). a Viral dose titration. Both cell lines were transduced using BacMos-CHIKV 26S at an indicated MOI of 1, 5, 10, and 20. Culture supernatants harvested at 2 dpt (upper panel) and 5 dpt (lower panel) were subjected to WB analysis. Untreated (mock) cells served as a negative control. b Time course. Both cell lines were transduced using BacMos-CHIKV 26S at an indicated MOI of 10. Culture supernatants harvested daily between 0 and 8 dpt (with refreshed medium) were subjected to WB analysis. CHIKV E2 protein is indicated by arrows on the right. Protein sizes (kDa) are indicated by markers (M) on the left
Fig. 3.
TEM image of CHIKV VLPs in the lumen of BacMos-CHIKV 26S-transduced C6/36 cells. a BacMos-CHIKV 26S-transduced C6/36 cell and b CHIKV-infected C6/36 cell were examined using TEM analysis. Boxed areas in the upper panels represent the same region as that shown in the bottom panels. VLPs and CHIKV virions (lower panels) are indicated by white arrows. pm, plasma membrane; n, nucleus. Scale bars, 100 nm
CHIKV VLP characterization
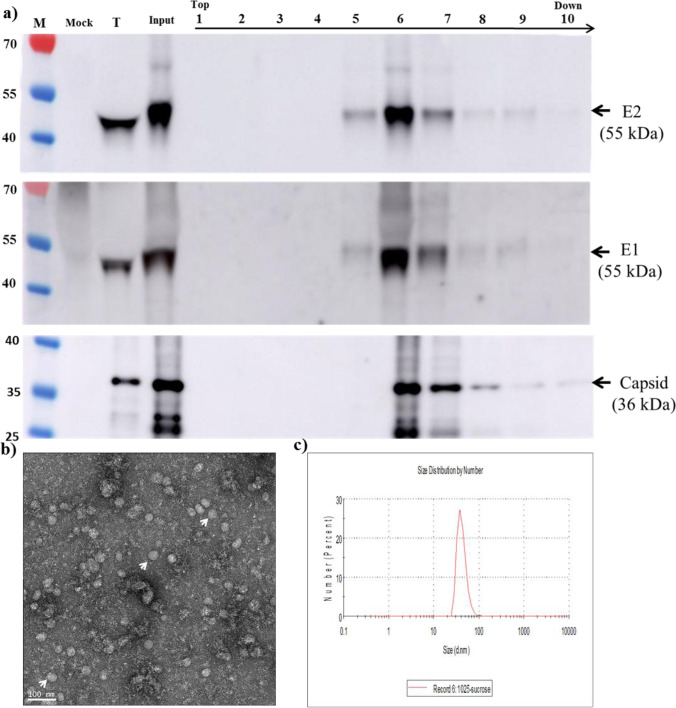
To confirm the secretion of CHIKV VLPs, culture supernatant from transduced cells was subjected to ultracentrifugation using two-layer sucrose gradient banding. WB analysis (Fig. 4a) revealed the co-fractionation of CHIKV E2, E1, and capsid proteins across fractions 6–9. Electron microscopy results of purified fraction 6, which was rich in CHIKV proteins (Fig. 4b), revealed the presence of CHIKV VLPs appearing as spherical particles with an average diameter of 40 nm, similar to intracellular VLPs (Fig. 3a). These measurements were confirmed by dynamic light scattering analysis (Fig. 4c), Taken together, these results indicate the secretion of CHIKV VLPs from BacMos-CHIKV 26S-transduced mosquito cells.
Fig. 4.
VLP characterization a Co-fractionation of CHIKV structural proteins via sucrose gradient banding. The sucrose density gradient after ultracentrifugation was used to isolate CHIKV VLPs. Fractions between 1 (top) and 10 (bottom) after sucrose gradient banding were subjected to WB analysis using anti-CHIKV E2 (upper panel), anti-CHIKV E1 (middle panel) or anti-CHIKV capsid (lower panel). CHIKV E2, E1, and capsid proteins indicated by arrows on the right. Protein sizes (kDa) of markers (M) shown on the left; Mock, culture supernatant from mock cells; T, culture supernatant from BacMos-CHIKV 26S-transduced cells; and Input, resuspension after cushion down; b electron micrograph of CHIKV VLPs. Partially purified CHIKV VLPs were examined using TEM. VLPs are indicated by white arrows; c dynamic light scattering. The size distribution of CHIKV VLPs was determined using dynamic light scattering
Antigenicity of CHIKV VLPs
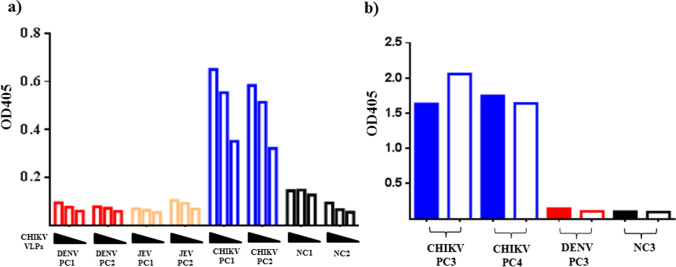
To characterize the antigenicity of CHIKV VLPs (i.e., as capture antigens), culture supernatant (non-purified VLPs) from transduced cells was subjected to CHIKV IgM antibody-capture enzyme-linked immunosorbent assays (MAC-ELISA). The following were also subjected to MAC-ELISA: sera from two Dengue Virus (DENV)-IgM-positive patients, two Japanese encephalitis virus (JEV)-IgM-positive patients, two CHIKV-IgM-positive patients, and two normal patients. Our results in Fig. 5a show the dose-dependent signal of VLP in the sera of the two CHIKV-IgM-positive patients (OD = 0.3 to 0.7). To determine whether non-purified VLPs could be used as an alternative to inactive CHIKV virus (standard antigen) for MAC-ELISA, further analysis was performed using the sera from two other CHIKV-IgM-positive patients, one DENV-IgM-positive patient, and one normal patient. Our results in Fig. 5b revealed specific signals in the sera of the two CHIKV-IgM-positive patients when the virus (OD = 1.6 and 1.7) or VLPs (OD = 2.0 and 1.6) were used as capture antigens. The signals generated by non-purified VLPs and the virus in the sera of CHIKV patients were OD = 2.057 and 1.64 stronger than the signals generated in the control sera of DENV patients (OD = 0.107) and normal individual (OD = 0.096) (Fig. 5b). These results indicate that mosquito cell-derived CHIKV VLPs display specific virion-like epitopes, which could serve as an alternative capture antigen for CHIKV MAC-ELISA.
Fig. 5.
Native epitope analysis of non-purified CHIKV VLPs in human sera using MAC-ELISA: a Serial diluted (1, 1:2, and 1:4) culture supernatant of baculovirus transduced AP-61 cells underwent MAC-ELISA with two sera of DENV infection (DENV PC1 and DENV PC2; red), two sera of JEV infection (JEV PC1 and JEV PC2; orange), two sera of CHIKV infection (CHIKV PC1 and CHIKV PC2; blue), and two normal sera (NC1 and NC2; black); b CHIKV VLPs (1 × culture supernatant of BacMos-CHIKV-26S-transduced-AP-61 cells; open bar) and inactive CHIKV virus (solid bar) were subjected to MAC-ELISA with two other sera of CHIKV infection (CHIKV PC3 and CHIKV PC4; blue), one other serum of DENV infection (DENV PC3; red), and one other normal serum (NC3, black)
Immunogenicity of CHIKV VLPs
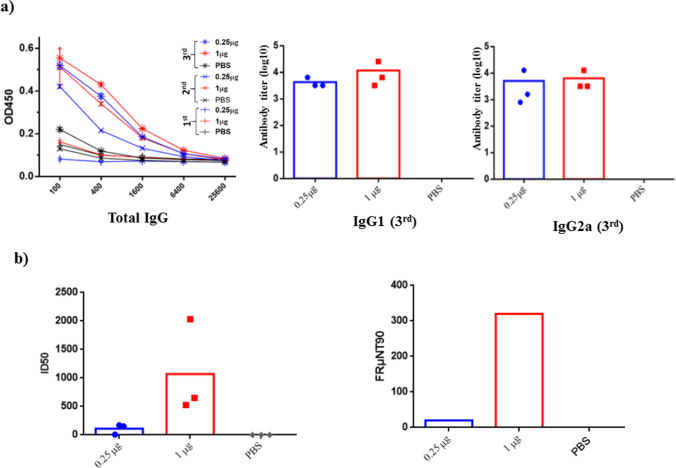
The immunogenic properties of CHIKV VLPs were investigated by immunizing mice with three doses of VLPs (0.25 or 1 μg per mouse) in the absence of an adjuvant at intervals of two weeks. After the second and third immunizations, we observed significant increases in the titers of E2-specific total IgG in both VLP groups (Fig. 6a). After the third immunization, we observed robust titers of total IgG, IgG1, and IgG2a antibodies in both VLP groups (0.25 and 1 μg). Ratios of IgG2a/IgG1 were calculated using the end-point dilution titers of each mouse. IgG2a/IgG1 ratios of two group mice are 0.125, 0.5, and 4 (average of 2) for 0.25 μg dose as well as 0.5, 1, and 0.5 (average of 1) for 1 μg dose, indicating a balanced Th1/Th2 immune response induced by CHIKV VLP immunization. Neutralizing (NT) activity was characterized by subjecting individual sera to pseudovirus-based neutralization assays (Fig. 6b). Following the third immunization, notable NT activity was detected in the sera of both VLP-containing groups. However, the average ID50 titer (1,070) from 1 μg CHIK-VLPs-immunized mice was significantly higher than the average ID50 titer (110) from 0.25 μg CHIK-VLPs-immunized mice. NT activity was verified by subjecting pooled sera to CHIKV-based neutralization assays (Fig. 5b). As with the ID50 titers, FRμNT90 results (Fig. 6b) revealed that immunization with VLPs induced potent NT responses against isolated CHIKV strains in a clinical setting. Note that the average FRμNT90 titer (320) from 1 μg CHIK-VLPs-immunized mice was higher than the average FRμNT90 titer (20) from 0.25 μg CHIK-VLPs-immunized mice. Taken together, it appears that immunization using CHIK VLPs alone can induce a balance CHIKV-specific IgG2a/IgG1 antibodies and NT responses in mice.
Fig. 6.
Immunogenicity of CHIKV VLPs. BALB/c mice (3 mice/group) received three doses of 0.25 µg VLPs (blue), 1 µg VLPs (red), or PBS (black). a CHIKV E2-specific IgG responses. CHIKV E2-specific antibody responses were determined by ELISA. Total IgG titration after 1st (│), 2nd (╳), and 3rd (*) immunization (left panel). Error bars indicate the mean SEM of concentrations from pooled sera. Subtype IgG1 (middle panel) and IgG2a (right panel) titrations after 3rd immunization.; b NT activity after 3rd immunization. Individual ID50 titers were determined using CHIKV-26S pseudotyped virus (left panel). FRμNT90 titer of pooled sera (right panel)
Discussion
Arboviruses, such as chikungunya, pose a public health threat associated with significant economic losses (Cheng et al. 2016; Pang et al. 2016). The dual-host infectious cycle involved in CHIKV transmission suggests that specific viral glycoprotein signatures may be involved in mutual infections (Hafer et al. 2009; He et al. 2010). For example, the carbohydrates in insect cells are less complex than those in mammalian cells (Hsieh and Robbins 1984). Researchers have also identified different N-glycosylation patterns in the CHIKV VLPs from HEK293 and SfBasic insect cell lines (Lancaster et al. 2016). Mosquito-derived CHIKV VLPs bearing signatures specific to early CHIKV infection in humans could potentially be used as an antigen for diagnostic purposes and/or in vaccine development. Native antigen preparations could form the basis of counter-measures against CHIKV infection. BacMos systems are non-replicating viral-vectored transient gene expression systems, which have been used to produce Flaviviridae VLPs (Chang et al. 2020; Lin et al. 2021a). In the current study, we used a BacMos system to produce VLPs for CHIKV, which is another arbovirus in the family Togaviridae. Our IFA results (Fig. 1) revealed the expression of viral capsid and glycoproteins in transduced mosquito cells. E2 glycoprotein secretion was detected in the culture medium of transduced cells. Our results demonstrate the efficacy of BacMos in promoting the expression and secretion of CHIKV E2 glycoproteins in two mosquito cell lines (C6/36 and AP-61) (Figs. 1c and 2). Transient E2 secretion was detected in transduced AP-61 cells at 1–8 dpt with a peak at 4–7 dpt. Interestingly, the yield of CHIKV VLPs from transduced AP-61 cells exceeded that from transduced C6/36 cells (Fig. 2). Using dynamic light scattering and TEM analysis, we also observed VLP particles with an average diameter of 40 nm within the transduced cells (Fig. 3a, b, c), which is significantly smaller than that of standard CHIKV VLPs of 65–70 nm (Metz et al. 2013), 65 nm (Akahata et al. 2010), or 50 to 60 nm (Noranate et al. 2014). CHIKV VLP secretion was confirmed by co-fractionation of CHIKV E2, E1, and capsid proteins following sucrose gradient banding (Fig. 4a). These results indicate that the expression of CHIKV 26S subgenomic cDNA (C-E3-E2-6 k-E1 polyprotein) in mosquito cells is sufficient for VLP formation.
MAC-ELISA is the preferred approach in the serological diagnosis of CHIKV infection (Johnson et al. 2016). Anti-CHIKV IgM is elicited within a week after the onset of symptoms and may persist for up to 35 months (Chelluboina et al. 2019; Costa et al. 2021). MAC-ELISA is robust to antigen impurities and false-positive reactions caused by extraneous antibodies, such as rheumatoid factors (Dittmar et al. 1979). MAC-ELISA based on whole virus antigens raises the issue of biosafety (Martin et al. 2000), such that the CHIKV virus would have to be inactivated via chemical treatment prior to use (Perrin and Morgeaux 1995). Unfortunately, inactive agents can be detrimental to the native structure of viral envelope proteins, thereby compromising the sensitivity of MAC-ELISA analysis (Fan et al. 2017). The identification of safe surrogate antigens is crucial to the serological diagnosis of CHIKV. Recombinant proteins are non-infectious and scalable; however, they have seldom been used as an alternative to inactivated whole virus particle in MAC-ELISA analysis (Cho et al. 2008; Priya et al. 2014; Yap et al. 2010). Another approach involves virus-mimicking particles for the detection of CHIKV IgM (Theillet et al. 2019). In the current study, MAC-ELISA signals that were indicative of non-purified CHIKV VLPs were comparable to those indicative of CHIKV (Fig. 5). Note that we did not observe serological cross-reactivity with JEV and DENV infections. These results suggest that the identified VLPs possess intrinsic antigenic properties of the virions, which makes them a viable alternative to virions in the detection of CHIKV IgM. Moreover, non-purified CHIKV VLPs produced in a simple Biosafety Level‐2 laboratory would not require chemical inactivation, thereby reducing the risk of altering native epitopes. It is also possible that VLPs produced in mosquito cells (which is similar to the natural production cycle of the virus) mimic native CHIKV more closely than do VLPs derived from Lepidoptera cell lines (Sf21). Essentially, mosquito cell-derived CHIKV VLPs appear to provide a safe alternative to virions for MAC-ELISA.
Immunogenicity assays of CHIKV VLPs in mice revealed an immune response characterized by high titers of total IgG as well as subtype IgG1 and IgG2a against CHIKV-E2 (Fig. 6a). These results revealed a Th1/Th2 response in CHIKV VLP-immunized mice. CHIKV VLPs alone were sufficient to induce a balanced IgG1/ IgG2b (c) response, which is in line with previous reports (Arévalo et al. 2019; Zhao et al. 2021). Pseudotyped-virus NT assays using sera collected 2 weeks after the final immunization revealed that even administering VLPs in low doses (0.25 and 1 μg) was sufficient to elicit potent ID50 titers in mice (Fig. 6b). The FRμNT90 titers were as follows: 0.25 μg (20) and 1 μg (320). Triple vaccinations of nonadjuvant VLPs (0.25 μg) induced potent FRμNT90 titers (20) against isolated strains. Note that mice administered 0.25 μg VLPs presented low levels of neutralizing antibodies (FRμNT90 = 20) (Fig. 6b). In vivo RRV assays revealed that a μNT titer cutoff of 10 should be sufficient to protect mice from CHIKV infection (Wressnigg et al. 2015). Note that a sequence from the CHIKV OPY1 strain was used to produce the desired CHIKV VLP; however, a clinical CHIKV isolate (GenBank: MN871956.1) was used to evaluate FRμNT90. Viral glycoproteins of MN871956.1 differed from the OPY1 strain (GenBank: KT449801) in terms of five amino acids (S531G, A590V, E1021K, A1036V, and V1127I). Our data revealed that CHIKV VLPs with adjuvant CpG did not enhance NT responses in mice (data not shown). The mosquito cell-derived CHIKV VLP vaccine alone conferred the most pronounced effect at doses as low as 1 μg per dose. CHIKV VLPs immunization of mice resulted in a balance CHIKV specific IgG2a/IgG1 response and the induction of potent neutralizing antibodies (FRμNT90 titers with values of 20 and 320) against clinical isolates. The associated dominant Th1 (IgG2a) response that improved CD8 + T cell immune surveillance could enhance the clearance of CHIKV infection and prevent persistent infection in joint-associated tissue (Davenport et al. 2020). Nonetheless, vaccination with CHIKV VLPs was shown to confer cross-protective NAbs against all CHIKV genotypes in humans (Goo et al. 2016).
This study was subject to a number of limitations, which should be considered in the interpretation of our results. To begin with, the vaccine strategy involving CHIKV VLP without adjuvant therapy proved effective in only a limited number of BALB/c mice (N = 3). In the future, we will first assess the effectiveness of this vaccine strategy in terms of joint footpad swelling and muscle pathology in C57BL/6 mice (Hallengärd et al. 2014). Second, we will use ELISpot to evaluate the T cell-mediated response in splenocytes, intracellular staining, and/or cytokine secretions. Further research will be required to determine the role of cellular and humoral immunities. Finally, the dose sparing with and without adjuvants will be evaluated to reduce the amount of CHIKV VLPs needed to elicit a robust and persistent immune response in animal models.
This paper describes an effective and straightforward approach to the production of CHIKV VLPs. Mosquito cell-derived VLPs produced using the BacMos system provide a scalable approach to manufacturing, without the risk of working with infectious agents. Above data also demonstrated that the VLPs maintained their major native epitopes, which were recognized by an NT mAb (Chk265) and CHIKV patient sera. Importantly, immunizing mice with VLPs alone induced a balance CHIKV-specific IgG2a/IgG1antibodies and a notable NT response against clinical CHIKV isolates. CHIKV VLPs derived from this novel insect-based system are a promising alternative approach to vaccine development and antigen detection. The fact that these VLPs are not infectious means that there is no need for chemical inactivation, which might otherwise compromise antigen or vaccine efficacy. Furthermore, that these VLPs are in the form of nano-particulate nature and retain a variety of native epitopes may even confer efficacy and safety to this vaccine without the use of an adjuvant. Taken together, it appears that these CHIKV VLPs exhibit high antigenicity and immunogenicity. Our results also provide a platform by which to study CHIKV assembly in mosquito cells and the production of VLPs related to other alphaviruses.
Author contribution
Conceptualization, S.-C.K.; data curation, S.-K.T., Y.-L.H., and P.-Y.S.; investigation, S.-K.T., Y.-L.H., S.-F.C., S.-H.H., and S.-C.K.; methodology, T.- Y. W., D.-J.C., Y.-L.H., P.-Y.S., H.-T.L., H.-C.L., C.-H.W., and C.-C. H.; project administration, S.-C.K.; supervision, Y.-L.H., C.-C.L., and S.-C.K.; validation, S.-C.K.; writing—original draft, S.-C.K. All authors have read and agreed to the published version of the manuscript.
Funding
Partial financial support was received from the NHRI (NHRI-110A1-MRCO-08212101 & NHRI-11A1-MRCO-08222201), MOST 109–2327-B-016–002 and MOST 110–2740-B-016–001, Medical Affairs Bureau (MND-MAB-110–075 & MND-MAB-D-111081), and National Defense Medical Center (IPM-110-G7 & 111-P-12) of the Republic of China.
Data availability
The datasets supporting the conclusions of this article are included within the article.
Declarations
Ethical approval
The Institutional Animal Care approved the animal study protocol and Use Committee (Institute of Preventive Medicine, National Defense Medical Center, Taiwan) is to ensure compliance with 3Rs (Replacement, Reduction and Refinement) spirit. (Project License: 109–20 in 2021). This study involving clinical human serum samples reviewed and approved by the Taiwan Centers for Disease Control Institutional Review Board (IRB 109105).
Conflict of interest
The authors declare no competing interests.
Footnotes
Shan-Ko Tsai and Yu-Lin Hsu are co-first author.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shan-Ko Tsai and Yu-Lin Hsu contributed equally to this work.
References
- Akahata W, Nabel GJ. A specific domain of the Chikungunya virus E2 protein regulates particle formation in human cells: implications for alphavirus vaccine design. J Virol. 2012;86(16):8879–8883. doi: 10.1128/jvi.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16(3):334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo MT, Huang Y, Jones CA, Ross TM. Vaccination with a chikungunya virus-like particle vaccine exacerbates disease in aged mice. PLoS Negl Trop Dis. 2019;13(4):e0007316. doi: 10.1371/journal.pntd.0007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G, Fragkoudis R, Ferguson MC, Lulla A, Merits A, Kohl A, Fazakerley JK. Semliki forest virus-induced endoplasmic reticulum stress accelerates apoptotic death of mammalian cells. J Virol. 2010;84(14):7369–7377. doi: 10.1128/jvi.02310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Dowd KA, Mendoza FH, Saunders JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME, Bailer RT, Koup RA, Schwartz RM, Akahata W, Nabel GJ, Mascola JR, Pierson TC, Graham BS, Ledgerwood JE. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. The Lancet. 2014;384(9959):2046–2052. doi: 10.1016/s0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- Chang YH, Chiao DJ, Hsu YL, Lin CC, Wu HL, Shu PY, Chang SF, Chang JH, Kuo SC (2020) Mosquito cell-derived Japanese encephalitis virus-like particles induce specific humoral and cellular immune responses in mice. Viruses 12(3). 10.3390/v12030336 [DOI] [PMC free article] [PubMed]
- Charlton Hume HK, Vidigal J, Carrondo MJT, Middelberg APJ, Roldão A, Lua LHL. Synthetic biology for bioengineering virus-like particle vaccines. Biotechnol Bioeng. 2019;116(4):919–935. doi: 10.1002/bit.26890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelluboina S, Robin S, Aswathyraj S, Arunkumar G. Persistence of antibody response in chikungunya. Virusdisease. 2019;30(3):469–473. doi: 10.1007/s13337-019-00534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Demirji J, Ivleva VB, Horwitz J, Schwartz R, Arnold F. The transient expression of CHIKV VLP in large stirred tank bioreactors. Cytotechnology. 2019;71(6):1079–1093. doi: 10.1007/s10616-019-00346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GL, Coates EE, Plummer SH, Carter CA, Berkowitz N, Conan-Cibotti M, Cox JH, Beck A, O’Callahan M, Andrews C, Gordon IJ, Larkin B, Lampley R, Kaltovich F, Gall J, Carlton K, Mendy J, Haney D, May J, Bray A, Bailer RT, Dowd KA, Brockett B, Gordon D, Koup RA, Schwartz R, Mascola JR, Graham BS, Pierson TC, Donastorg Y, Rosario N, Pape JW, Hoen B, Cabié A, Diaz C, Ledgerwood JE. Effect of a chikungunya virus-like particle vaccine on safety and tolerability outcomes: a randomized clinical trial. JAMA. 2020;323(14):1369–1377. doi: 10.1001/jama.2020.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY, Huang AS, Yang CF, Hsu TC, Wang TC, Su CL, Chang MC, Peng SH, Shu PY. Chikungunya infection: first autochthonous cases in Taiwan. J Formos Med Assoc. 2021;120(7):1526–1530. doi: 10.1016/j.jfma.2020.10.032. [DOI] [PubMed] [Google Scholar]
- Cheng G, Liu Y, Wang P, Xiao X. Mosquito defense strategies against viral infection. Trends Parasitol. 2016;32(3):177–186. doi: 10.1016/j.pt.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B, Jeon BY, Kim J, Noh J, Kim J, Park M, Park S. Expression and evaluation of Chikungunya virus E1 and E2 envelope proteins for serodiagnosis of Chikungunya virus infection. Yonsei Med J. 2008;49(5):828–835. doi: 10.3349/ymj.2008.49.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D, Coêlho M, Gouveia P, Bezerra LA, Marques CDL, Duarte A, Valente LM, Magalhães V. Long-term persistence of serum-specific anti-chikungunya IgM antibody - a case series of Brazilian patients. Rev Soc Bras Med Trop. 2021;54:e0855. doi: 10.1590/0037-8682-0855-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport BJ, Bullock C, McCarthy MK, Hawman DW, Murphy KM, Kedl RM, Diamond MS, Morrison TE (2020) Chikungunya virus evades antiviral CD8(+) T cell responses to establish persistent infection in joint-associated tissues. J Virol 94(9). 10.1128/jvi.02036-19 [DOI] [PMC free article] [PubMed]
- Dittmar D, Cleary TJ, Castro A. Immunoglobulin G- and M-specific enzyme-linked immunosorbent assay for detection of dengue antibodies. J Clin Microbiol. 1979;9(4):498–502. doi: 10.1128/jcm.9.4.498-502.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus JH, Rossi SL, Weaver SC. Development of vaccines for chikungunya fever. J Infect Dis. 2016;214(suppl 5):S488–s496. doi: 10.1093/infdis/jiw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Ye X, Ku Z, Kong L, Liu Q, Xu C, Cong Y, Huang Z (2017) Beta-propiolactone inactivation of coxsackievirus a16 induces structural alteration and surface modification of viral capsids. J Virol 91(8). 10.1128/jvi.00038-17 [DOI] [PMC free article] [PubMed]
- Goo L, Dowd KA, Lin TY, Mascola JR, Graham BS, Ledgerwood JE, Pierson TC. A Virus-like particle vaccine elicits broad neutralizing antibody responses in humans to all chikungunya virus genotypes. J Infect Dis. 2016;214(10):1487–1491. doi: 10.1093/infdis/jiw431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Chauhan A, Goyal V, Jaiswal N, Singh S, Singh M. Recent development in the strategies projected for chikungunya vaccine in humans. Drug Des Devel Ther. 2018;12:4195–4206. doi: 10.2147/dddt.s181574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafer A, Whittlesey R, Brown DT, Hernandez R. Differential incorporation of cholesterol by Sindbis virus grown in mammalian or insect cells. J Virol. 2009;83(18):9113–9121. doi: 10.1128/jvi.00755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallengärd D, Kakoulidou M, Lulla A, Kümmerer BM, Johansson DX, Mutso M, Lulla V, Fazakerley JK, Roques P, Le Grand R, Merits A. Liljeström P (2014) Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J Virol. 2014;88(5):2858–2866. doi: 10.1128/jvi.03453-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Piper A, Meilleur F, Myles DA, Hernandez R, Brown DT, Heller WT. The structure of Sindbis virus produced from vertebrate and invertebrate hosts as determined by small-angle neutron scattering. J Virol. 2010;84(10):5270–5276. doi: 10.1128/jvi.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P, Robbins PW. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984;259(4):2375–82. doi: 10.1016/S0021-9258(17)43362-X. [DOI] [PubMed] [Google Scholar]
- Joe AK, Foo HH, Kleeman L, Levine B. The transmembrane domains of Sindbis virus envelope glycoproteins induce cell death. J Virol. 1998;72(5):3935–3943. doi: 10.1128/jvi.72.5.3935-3943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BW, Russell BJ, Goodman CH. Laboratory diagnosis of chikungunya virus infections and commercial sources for diagnostic assays. J Infect Dis. 2016;214(suppl 5):S471–s474. doi: 10.1093/infdis/jiw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SC, Chen YJ, Wang YM, Kuo MD, Jinn TR, Chen WS, Chang YC, Tung KL, Wu TY, Lo SJ. Cell-based analysis of Chikungunya virus membrane fusion using baculovirus-expression vectors. J Virol Methods. 2011;175(2):206–215. doi: 10.1016/j.jviromet.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Lancaster C, Pristatsky P, Hoang VM, Casimiro DR, Schwartz RM, Rustandi R, Ha S. Characterization of N-glycosylation profiles from mammalian and insect cell derived chikungunya VLP. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1032:218–223. doi: 10.1016/j.jchromb.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Li L, Jose J, Xiang Y, Kuhn RJ, Rossmann MG. Structural changes of envelope proteins during alphavirus fusion. Nature. 2010;468(7324):705–708. doi: 10.1038/nature09546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P, Garoff H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol. 1991;65(1):147–154. doi: 10.1128/jvi.65.1.147-154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-T, Chiao D-J, Kuo S-C. Production of mosquito cell-derived Zika virus-like particles using BacMos system. J Med Sci. 2021;41(3):134–139. doi: 10.4103/jmedsci.jmedsci_106_20. [DOI] [Google Scholar]
- Lin HT, Chen CC, Chiao DJ, Chang TY, Chen XA, Young JJ, Kuo SC. Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice. Int J Biol Macromol. 2021;193(Pt B):1885–1897. doi: 10.1016/j.ijbiomac.2021.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38(5):1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon P, Garoff H. Processing of the Semliki Forest virus structural polyprotein: role of the capsid protease. J Virol. 1987;61(5):1301–1309. doi: 10.1128/jvi.61.5.1301-1309.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz SW, Gardner J, Geertsema C, Le TT, Goh L, Vlak JM, Suhrbier A, Pijlman GP. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl Trop Dis. 2013;7(3):e2124. doi: 10.1371/journal.pntd.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik NG, Lo YW, Wu TY, Lin CC, Kuo SC, Chao YC. Baculovirus as an efficient vector for gene delivery into mosquitoes. Sci Rep. 2018;8(1):17778. doi: 10.1038/s41598-018-35463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noranate N, Takeda N, Chetanachan P, Sittisaman P, A-nuegoonpipat A, Anantapreecha S. Characterization of chikungunya virus-like particles. PLoS One. 2014;9(9):e108169. doi: 10.1371/journal.pone.0108169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden S, Lucas-Hourani M, Ceccaldi PE, Basak A, Valentine M, Benjannet S, Hamelin J, Jacob Y, Mamchaoui K, Mouly V, Desprès P, Gessain A, Butler-Browne G, Chrétien M, Tangy F, Vidalain PO, Seidah NG. Inhibition of Chikungunya virus infection in cultured human muscle cells by furin inhibitors: impairment of the maturation of the E2 surface glycoprotein. J Biol Chem. 2008;283(32):21899–21908. doi: 10.1074/jbc.M802444200. [DOI] [PubMed] [Google Scholar]
- Pang X, Xiao X, Liu Y, Zhang R, Liu J, Liu Q, Wang P, Cheng G. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat Microbiol. 2016;1:16023. doi: 10.1038/nmicrobiol.2016.23. [DOI] [PubMed] [Google Scholar]
- Perrin P, Morgeaux S. Inactivation of DNA by beta-propiolactone. Biologicals: J Int Assoc Biol Stand. 1995;23(3):207–211. doi: 10.1006/biol.1995.0034. [DOI] [PubMed] [Google Scholar]
- Priya R, Khan M, Rao MK, Parida M. Cloning, expression and evaluation of diagnostic potential of recombinant capsid protein based IgM ELISA for chikungunya virus. J Virol Methods. 2014;203:15–22. doi: 10.1016/j.jviromet.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Rosario V, Munoz-Louis R, Valdez T, Adames S, Medrano J, Paulino I, Paula J, Alba-Fériz R. Chikungunya infection in the general population and in patients with rheumatoid arthritis on biological therapy. Clin Rheumatol. 2015;34(7):1285–1287. doi: 10.1007/s10067-015-2979-x. [DOI] [PubMed] [Google Scholar]
- Saraswat S, Athmaram TN, Parida M, Agarwal A, Saha A, Dash PK. Expression and characterization of yeast derived chikungunya virus like particles (CHIK-VLPs) and its evaluation as a potential vaccine candidate. PLoS Negl Trop Dis. 2016;10(7):e0004782. doi: 10.1371/journal.pntd.0004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq H, Batool S, Asif S, Ali M, Abbasi BH (2022) Virus-Like particles: revolutionary platforms for developing vaccines against emerging infectious diseases. Front Microbiol 12(4137). 10.3389/fmicb.2021.790121 [DOI] [PMC free article] [PubMed]
- Theillet G, Martinez J, Steinbrugger C, Lavillette D, Coutard B, Papageorgiou N, Dalbon P, Leparc-Goffart I, Bedin F. Comparative study of chikungunya virus-like particles and pseudotyped-particles used for serological detection of specific immunoglobulin M. Virology. 2019;529:195–204. doi: 10.1016/j.virol.2019.01.027. [DOI] [PubMed] [Google Scholar]
- Urakami A, Sakurai A, Ishikawa M, Yap ML, Flores-Garcia Y, Haseda Y, Aoshi T, Zavala FP, Rossmann MG, Kuno S, Ueno R, Akahata W (2017) Development of a novel virus-like particle vaccine platform that mimics the immature form of alphavirus. Clin Vaccine Immunol 24(7). 10.1128/cvi.00090-17 [DOI] [PMC free article] [PubMed]
- Wagner JM, Pajerowski JD, Daniels CL, McHugh PM, Flynn JA, Balliet JW, Casimiro DR, Subramanian S. Enhanced production of Chikungunya virus-like particles using a high-pH adapted spodoptera frugiperda insect cell line. PLoS ONE. 2014;9(4):e94401. doi: 10.1371/journal.pone.0094401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wressnigg N, van der Velden MV, Portsmouth D, Draxler W, O’Rourke M, Richmond P, Hall S, McBride WJ, Redfern A, Aaskov J, Barrett PN, Aichinger G. An inactivated Ross River virus vaccine is well tolerated and immunogenic in an adult population in a randomized phase 3 trial. Clin Vaccine Immunol. 2015;22(3):267–273. doi: 10.1128/cvi.00546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap G, Pok KY, Lai YL, Hapuarachchi HC, Chow A, Leo YS, Tan LK, Ng LC. Evaluation of Chikungunya diagnostic assays: differences in sensitivity of serology assays in two independent outbreaks. PLoS Negl Trop Dis. 2010;4(7):e753. doi: 10.1371/journal.pntd.0000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap ML, Klose T, Urakami A, Hasan SS, Akahata W, Rossmann MG. Structural studies of Chikungunya virus maturation. Proc Natl Acad Sci U S A. 2017;114(52):13703–13707. doi: 10.1073/pnas.1713166114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fugère M, Day R, Kielian M. Furin processing and proteolytic activation of Semliki Forest virus. J Virol. 2003;77(5):2981–2989. doi: 10.1128/jvi.77.5.2981-2989.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Deng Y, Niu P, Song J, Wang W, Du Y, Huang B, Wang W, Zhang L, Zhao P, Tan W. Co-Immunization with CHIKV VLP and DNA Vaccines induces a promising humoral response in mice. Front Immunol. 2021;12:655743. doi: 10.3389/fimmu.2021.655743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.