Abstract
Background
Anti-tumour necrosis factor (anti-TNF) agents are associated with increased infection risk among elderly inflammatory bowel disease (IBD) patients, and thus, alternative biologics may be preferable. However, little comparative data exist on the safety and efficacy of vedolizumab and ustekinumab in elderly IBD patients.
Aims
To compare the safety and effectiveness of ustekinumab and vedolizumab in elderly Crohn’s disease patients.
Methods
Patients ≥ 60 years old who commenced ustekinumab or vedolizumab for Crohn’s disease (CD) were included. Primary outcome was serious infections, defined as requiring hospitalisation. Efficacy was assessed by treatment persistence and clinical response rates. We appropriately adjusted for confounders using propensity score-matched analysis weighted by the inverse predicted probability of treatment weighing and performed a logistic regression analysis to assess factors associated with serious infections and treatment persistence.
Results
Eighty-three patients commencing ustekinumab and 42 commencing vedolizumab therapy were included. In a propensity adjusted cohort, the rate of serious infection and treatment persistence were comparable between ustekinumab and vedolizumab. There was a significant reduction in HBI at 6 and 12 months compared to baseline in both groups. Male gender was positively associated with serious infection risk at 12 months, and penetrating disease behaviour was positively associated with 12-month treatment persistence. Baseline HBI score was negatively associated with 12-month treatment persistence. Cox regression analysis showed no overall difference in treatment discontinuation-free and serious infection-free survival by 12 months.
Conclusions
We observed comparable safety and effectiveness for ustekinumab and vedolizumab in treating elderly CD patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-022-07770-8.
Keywords: Crohn’s disease, Elderly, Vedolizumab, Ustekinumab, Safety, Effectiveness
Introduction
The incidence and prevalence of inflammatory bowel disease (IBD) are increasing worldwide with a prevalence rate exceeding 0.3% in North America and Europe [1]. At the same time, the population is also ageing with an estimated 1 in 4 Europeans aged 60 or over [2]. Thus, the proportion of elderly IBD patients, defined as those over the age of 60, is set to increase. It is estimated that up to 25%–30% of the IBD population are aged 60 or older, of which half are typically diagnosed over the age of 60, referred to as elderly or late-onset IBD [3]. Furthermore, the incidence of elderly onset IBD is also increasing—for instance in a population-based cohort study, the incidence increased from 11.71 per 100,000 persons in 1991 to 23.66 per 100,000 persons in 2010 [3].
The management of elderly IBD patients pose unique challenges for a number of reasons. Firstly, age-related decline in immune function may lead to increased risk of infections and malignancies with immunomodulatory therapy [4]. In keeping with this, older patients are at greater risk of opportunistic infections compared to younger IBD patients. Furthermore, the higher prevalence of co-morbidities and frailty among elderly patients increases the risk of infections and drug interactions. Finally, a limited number of elderly patients are enrolled on to clinical trials due to age restrictions for inclusion, and hence, much of the limited evidence for safety and efficacy of medications among elderly IBD patients are derived from real-world cohort studies. As a consequence, there is often reluctance among IBD clinicians to prescribe immunomodulatory and biological therapy to elderly IBD patients, with fewer elderly patients receiving such therapies [5].
Current evidence suggests that many of the commonly used treatments for IBD are associated with worse outcomes among elderly patients. For instance, in a large cohort of elderly IBD patients, current steroid use and steroid exposure within the previous 90 days were associated with an increased risk of serious infections [6]. Steroid use also worsens pre-existing conditions such as diabetes and heart failure in the elderly [7]. The use of immunomodulatory therapy is also associated with an increased risk of infections [8] and malignancies among the elderly. In the pivotal French study (CESAME), the risk of lymphoma [9] and urothelial cancers [10] were all increased among older patients treated with thiopurines. Various cohort studies have reported an increased risk of infections and mortality with anti-tumour necrosis factor (TNF) agents in the elderly [11, 12]. In particular, the risk of opportunistic infections in the elderly is higher with a combination of anti-TNF agents and immunosuppressants [13]. Thus, newer alternative biologics which can be prescribed as monotherapy may be preferred among the elderly.
The evidence for efficacy and safety outcomes with the newer non-anti-TNF biologics among the elderly is limited. The α4β7 antibody, vedolizumab, which blocks gut lymphocyte trafficking is generally perceived to be safer among elderly patients due to its gut selectivity. In the registrational GEMINI trials, efficacy and safety appeared similar across all age groups. Patients over the age of 55 who were on vedolizumab had the lowest rate of severe infections and side effect-related hospitalisation [14]. There were also no differences in malignancy or deaths between different age groups. Subsequent studies so far have shown mixed results though with some studies suggesting similar efficacy outcomes to younger IBD patients [15] whereas other studies suggest a higher infection risk among the elderly [16] with rates similar to anti-TNF agents [17].
There are limited data on the efficacy and safety of the p-40 antibody ustekinumab, which targets interleukins-12 and 23 signalling. A Dutch prospective multi-centre cohort study enrolled 203 and 207 vedolizumab- and ustekinumab-treated IBD patients, respectively, and showed that co-morbidities rather than age were associated with adverse safety outcomes with either agent [18]. However, this study was limited by the inclusion of only a small number of elderly IBD patients (36 vedolizumab and 27 ustekinumab). A further recent study included 39 elderly (> 65 years) Crohn’s disease patients treated with ustekinumab and reported a similar safety profile but lower efficacy compared to younger patients [19]. To the contrary, a recently published Spanish cohort in abstract form showed similar efficacy and serious infection rates among elderly compared to a standard adult IBD cohort but with a higher rate of de novo neoplasms among the elderly [20]. A pooled analysis of data from clinical trials across all indications showed comparable efficacy and safety among elderly patients compared to a standard cohort [21], but it is important to note the limitations of clinical trial data due to restricted inclusion of patients with significant co-morbidities. Furthermore, there are no comparative data to date on the safety and effectiveness of ustekinumab and vedolizumab in the elderly to inform positioning of these therapies. We sought to compare the safety and efficacy of ustekinumab and vedolizumab among elderly CD patients.
Materials and Methods
We conducted a multi-centre study of elderly (> 60 years old) CD patients treated with vedolizumab and ustekinumab across 4 hospitals in the North of England. All patients were treated with a standard induction regime for each of the respective agents. Maintenance dosing was 8 weekly for vedolizumab and 8 or 12 weekly for ustekinumab after completion of induction therapy. In the United Kingdom, ustekinumab is licenced for 8- or 12-weekly administration and the frequency of treatment is determined by the treating clinician. Dose optimisation during the maintenance phase were at the discretion of the treating clinician. Steroid taper was at the choice of the treating clinician but budesonide 9 mg tapered over 12 weeks and prednisolone 40 mg once daily with step-wise reduction over eight weeks is standard practice in the study centres. We collected baseline clinical information including concomitant immunomodulator and steroid therapy, body mass index, disease extent and duration, prior anti-TNF therapy and surgery, smoking status, and Harvey-Bradshaw Index. Follow-up data included Harvey-Bradshaw Index, C-reactive protein and faecal calprotectin (where available) at months 2, 4, 6 and 12 after initiation. Data were included if collected within two weeks of each specified time point (4 weeks at the 12-month time point). Due to the differing administration schedules for ustekinumab and vedolizumab, we allowed an interval of ± 2 weeks at each time point. Harvey–Bradshaw Index was routinely assessed whenever patients attended for vedolizumab infusion. For patients receiving ustekinumab, Harvey-Bradshaw Index was monitored by inflammatory bowel disease specialist nurses in some study sites as part of drug response monitoring. We also recorded details of dose escalation, adverse events and discontinuation of biological therapy if they occurred and need for surgery. Dose optimisation of vedolizumab or ustekinumab was based on clinical grounds of suboptimal response combined with objective markers of active disease. Follow-up was curtailed at 12 months as the number of patients treated with ustekinumab beyond this period was limited. Clinical remission was defined as a Harvey–Bradshaw Index of < 5 and clinical response was defined as a reduction in Harvey–Bradshaw Index of ≥ 3 points from the baseline value [22]. We consulted the STROBE statement checklist for observational studies.
Outcomes of Interest
The primary outcome measure was the occurrence of serious infections defined as those that required hospitalisation. Incident malignancies which occurred after treatment commencement and recurrent malignancies were recorded. We assessed effectiveness primarily by treatment persistence and used rates of clinical response and steroid-free treatment persistence as further measures of treatment efficacy. All patients who had at least one induction dose of ustekinumab or vedolizumab were included to calculate response and remission rates even if they had discontinued therapy. Finally, we assessed the association of clinical variables with the risk of serious infection and treatment persistence. We only assessed outcomes at 12 months as there were only few patients with follow-up data beyond this period.
Statistical Analysis
Categorical variables are summarised as frequency (%) and continuous variables as median (interquartile range, IQR) or mean (standard deviation, SD). We conducted univariate analysis using independent sample t tests, Mann–Whitney U test and Chi-square test for continuous parametric, continuous non-parametric and categorical data, respectively. Where counts were small, Fisher’s exact test was used to compare categorical data. In order to reduce the effects of treatment selection bias and confounding factors, we initially performed a multiple logistic regression model using Firth logistic regression. We subsequently performed a propensity score-matched analysis by inverse probability of treatment weighing (IPTW) [23]. The denominators of the stabilised weights were adjusted for baseline confounders (predictors of treatment and outcome), namely Charlson co-morbidity index, perianal disease, disease behaviour, disease severity, smoking, steroid use at the time of infection and disease duration. The confounders for the propensity score matching were identified on the basis of baseline co-variate differences and factors commonly known to impact on treatment outcome. The numerators of the stabilised weights were equal to the probability of receiving the patient’s treatment without considering covariates. The weighted remission/response/steroid-free remission models included only the treatment variable. Missing baseline data were handled using multiple imputation by chain equations with 5 imputed datasets as described previously [24]. Primary and secondary outcome measures were not imputed. Finally, we assessed 12-month discontinuation-free and serious infection-free survival analysis using Cox regression analysis. A P value of < 0.05 was considered significant.
All analyses were carried out using R v. 1.2.504 software (R Core Team 2020, R Foundation for statistical computing, Vienna, Austria) [25]
Ethical Standards
The project used anonymized, routinely collected data extracted by clinical teams as part of local quality improvement activities at the participating centres and analysed for the purpose of local audit of compliance with relevant guidance from the National Institute for Health and Care Excellence and to generate benchmarking data for clinical outcome and safety achieved for different agents at the participating centres. Each site registered the biologics audit with their respective institutional audit department and received approval. As routinely collected data, they are exempt from the need for ethics committee approval in the United Kingdom and the need to take written informed consent. All data were fully anonymised before pooled analysis.
Results
Cohort
We included 83 patients treated with ustekinumab and 42 patients treated with vedolizumab after excluding patients < 60 years of age. We excluded patients with insufficient baseline data. The baseline characteristics of the included subjects are summarised in Table 1. Approximately two thirds of patients (66.7% and 66.3% for vedolizumab and ustekinumab, respectively) in either group were exposed to prior anti-TNF agents and 20% of patients had been treated with two anti-TNF agents. Ustekinumab and vedolizumab-treated patients were followed up for a median of 12 (IQR 2.75) and 12 (IQR 0) months, respectively. Median treatment duration was 12 (IQR 5) and 12 (IQR 2) months for ustekinumab- and vedolizumab-treated patients respectively. Thirty two patients (39%) in the ustekinumab group were treated with 12-weekly injections and the remainder were initiated on 8-weekly injections.
Table 1.
Baseline characteristics of patients treated with ustekinumab or vedolizumab who received at least induction dosing of vedolizumab or ustekinumab for Crohn’ disease
| Variable | Vedolizumab (N = 42) | Ustekinumab (N = 83) | P value |
|---|---|---|---|
| Age, mean (SD) | 68.8 (6.85) | 67.6 (6.20) | 0.332 |
| Sex, male N (%) | 13 (31.0) | 33 (39.8) | 0.442 |
| BMI kg/m2, mean (SD) | 25.0 (5.70) | 26.5 (5.92) | 0.164 |
| Smoking status: N (%) | 0.218 | ||
| Current | 3 (7.14) | 16 (19.3) | |
| Ex-smoker | 21 (50.0) | 37 (44.6) | |
| Never smoked | 17 (40.5) | 29 (34.9) | |
| Charlson co-morbidity index, mean (SD) | 4.76 (2.34) | 4.19 (1.70) | 0.245 |
| Disease extent: N (%) | 0.681 | ||
| L1—ileal | 13 (31.0) | 29 (34.9) | |
| L2—colonic | 7 (16.7) | 13 (15.7) | |
| L3—ileocolonic | 20 (47.6) | 35 (42.2) | |
| L4—isolated upper disease | 1 (2.38) | 6 (7.23) | |
| Age at diagnosis: N (%) | 0.362 | ||
| A1—< 17 | 1 (2.38) | 0 | |
| A2—18–39 | 7 (16.7) | 13 (15.7) | |
| A3—40+ | 34 (81.0) | 70 (84.3) | |
| Behaviour: N (%) | 0.832 | ||
| B1—non-stricturing, non-penetrating | 21 (50.0) | 39 (47.0) | |
| B2—stricturing | 14 (33.3) | 33 (39.8) | |
| B3—penetrating | 6 (14.3) | 11 (13.3) | |
| Perianal disease: N (%) | 2 (4.76) | 8 (9.64) | 0.493 |
| Previous resections: N (%) | 17 (40.5) | 42 (50.6) | 0.378 |
| HBI at initiation, median (IQR) | 6 (3) | 7 (5) | 0.034 |
| Concomitant immunomodulator at initiation: N (%) | 3 (7.14) | 13 (15.7) | 0.288 |
| Thiopurines | 2 (4.76) | 10 (12.0) | 0.325 |
| Methotrexate | 1 (2.38) | 3 (3.61) | 1.00 |
| Previous anti-TNF exposure: N (%) | 28 (66.7) | 55 (66.3) | 0.998 |
| One | 20 (47.6) | 39 (47.0) | |
| Two | 8 (19.0) | 16 (19.3) | |
| None | 14 (33.3) | 28 (33.7) | |
| 1st anti-TNF: N (%) | |||
| Adalimumab | 11 (39.3) | 20 (36.4) | 0.984 |
| Infliximab | 17 (60.7) | 35 (63.6) | 0.984 |
| Previous anti-TNF primary non-response: N (%) | 0.106 | ||
| One | 8 (19.0) | 13 (15.7) | |
| Two | 2 (4.8) | 0 (0.0) | |
| None | 32 (76.2) | 70 (84.3) | |
| Previous ustekinumab exposure: N (%) | 3 (7.14) | ||
| Ustekinumab primary non-response: N (%) | 0 | ||
| Previous vedolizumab exposure: N (%) | 15 (18.1) | ||
| Previous vedolizumab primary non-response: N (%) | 11 (73.3) | ||
| Steroids at baseline: N (%) | 10 (23.8) | 31 (37.3) | 0.186 |
| Budesonide | 5 (11.9) | 14 (16.9) | 1.00 |
| Prednisolone | 5 (11.9) | 17 (20.5) | 1.00 |
| Dosing schedule: N (%) | |||
| 4 weekly | 20 (47.6) | ||
| 6 weekly | |||
| 8 weekly | 22 (52.4) | 50 (60.2) | |
| 12 weekly | 33 (39.8) | ||
| Not recorded |
Significance of bold is highlighted
P values calculated from two sample t tests, Mann Whitney U tests and chi-square tests for normally distributed continuous variables, non-normally distributed variables and categorical variables, respectively
SD standard deviation, BMI body mass index, TNF tumour necrosis factor, HBI Harvey-Bradshaw Index
Comparative Safety
By 12 months, 10.0% of ustekinumab-treated patients and 13.8% of vedolizumab-treated patients had developed a serious infection. The list of serious infections and non-serious infections are summarised in Table 2. The overall serious infection rate was 110.6/1000 patient-years for vedolizumab and 75.5/1000 patient-years for ustekinumab. Of the patients who developed a serious infection, 25% on vedolizumab and 20% on ustekinumab were on systemic steroids within 4 weeks of developing the infection.
Table 2.
List of serious and non-serious infections in ustekinumab- and vedolizumab-treated patients by 12 months
| Infections | Treatment group | |
|---|---|---|
| Vedolizumab | Ustekinumab | |
| Serious infections: N (%) | ||
| Line infection | 1 (2.4) | 1 (1.2) |
| Pneumonia | 1 (2.4) | 3 (3.6) |
| Urinary tract with sepsis | 0 | 1 (1.2) |
| Soft tissue | 1 (2.4) | 0 |
| Pelvic sepsis | 1 (2.4) | 0 |
| Non-serious infections: N (%) | ||
| Pneumonia | 1 (2.4) | 1 (1.2) |
| Upper respiratory tract | 11 (26.2) | 7 (8.4) |
| Flu-like/coryzal | 0 | 3 (3.6) |
| Urinary tract | 3 (7.1) | 5 (6.0) |
| Gastrointestinal | 1 (2.4) | 2 (2.4) |
| Minor cellulitis | 1 (2.4) | 1 (1.2) |
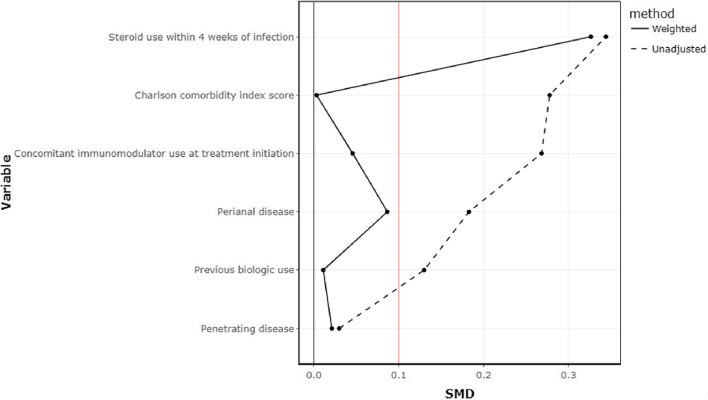
We next performed a propensity weighted cohort appropriately adjusting for predictors of serious infection. The co-variate balance after IPTW matching is shown in Fig. 1 and supplementary table 1. In a propensity score adjusted logistic regression analysis, the rates of serious infection at 6 months (OR 1.61, 95% CI 0.209–12.4, P = 0.644) and 12 months (OR 1.24, 95% CI 0.293–5.26, P = 0.766) were not significantly different between vedolizumab-treated and ustekinumab-treated patients (Table 3).
Fig. 1.
Examination of co-variate balance for risk of serious infection before and after inverse probability of treatment weighing
Table 3.
Comparative safety of ustekinumab and vedolizumab
| Outcome | Cohort | OR [95% CI] | P value |
|---|---|---|---|
| Development of severe or life-threatening infection by 6 months | Unweighted | 1.86 [0.216–16.0] | 0.543 |
| Weighted | 1.61 [0.209–12.4] | 0.644 | |
| Development of severe or life-threatening infection by 12 months | Unweighted | 1.44 [0.330–5.93] | 0.610 |
| Weighted | 1.24 [0.293–5.26] | 0.766 | |
| Development of non-severe infection by 6 months | Unweighted | 1.17 [0.400–3.20] | 0.769 |
| Weighted | 0.954 [0.332–2.74] | 0.929 | |
| Development of non-severe infection by 12 months | Unweighted | 1.74 [0.660–4.54] | 0.259 |
| Weighted | 1.40 [0.520–3.78] | 0.500 |
Both unweighted and inverse probability of treatment-weighted rates are presented
Two patients in each group developed a malignancy following initiation. In the ustekinumab group, one patient was diagnosed with metastatic pancreatic cancer 30 months after initiation and another with non-metastatic melanoma 13 months after initiation. In the vedolizumab group, one patient developed a sarcoma within 24 months and another patient developed lung cancer 11 months after treatment initiation. No infusion reactions or cutaneous lesions were reported. Two patients on ustekinumab reported new onset arthralgia.
Predictors of Serious Infection
We next examined the effect of baseline clinical variables on serious infection risk. Univariate analysis showed that male sex, higher Charlson co-morbidity index, and previous anti-TNF primary non-response were associated with the development of serious infection by 12 months (P < 0.1) (Supplementary Table 2). In a logistic regression model, only male gender was associated with a risk of serious infection in ustekinumab- and vedolizumab-treated patients (OR 8.8, 95% CI 1.29–154, P = 0.0249) whereas treatment group did not have a significant association with serious infection risk (Table 4).
Table 4.
Firth logistic regression of variables associated with severe infection risk at 12 months in patients who received vedolizumab or ustekinumab
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Treatment group (ustekinumab vs vedolizumab) | 0.708 | 0.0711–5.86 | 0.743 |
| Sex (male) | 8.84 | 1.29–154 | 0.0249 |
| Charlson co-morbidity index | 1.53 | 0.933–3.25 | 0.101 |
| Steroid use within 4 weeks of infection | 4.44 | 0.292–110 | 0.282 |
| Previous anti-TNF primary non-response | 1.20 | 0.122–11.3 | 0.868 |
Significance of bold is highlighted
TNF tumour necrosis factor
Comparative Effectiveness
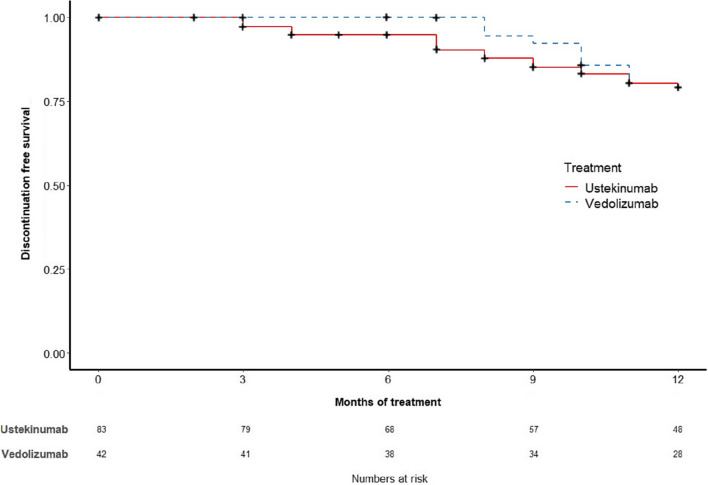
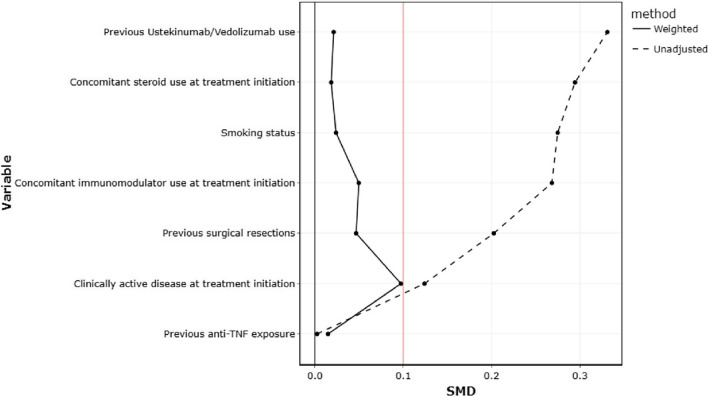
Treatment persistence rates were not significantly different between ustekinumab and vedolizumab (Fig. 2). Specifically, the treatment persistence rates in the ustekinumab and vedolizumab groups, respectively, were 98.8% vs 97.6% at 2 months (P = 1.00), 84.8% vs 88.1% at 6 months (P = 0.431), and 68.1% vs 80.0% at 12 months (P = 0.374, supplementary table 3). Similarly, there were no significant differences in both unweighted and weighted clinical remission rates at 6 and 12 months between ustekinumab and vedolizumab (Supplementary table 3). Reasons for discontinuation are summarised in Table 5. We next performed propensity matching using IPTW appropriately adjusting for confounders. The co-variate balance after IPTW matching is shown in Fig. 3 and Supplementary Table 1. The unweighted and weighted rates for treatment persistence, steroid-free treatment persistence and clinical remission were not significantly different between ustekinumab and vedolizumab-treated patients at 6 and 12 months on logistic regression analysis (Table 6). Both vedolizumab and ustekinumab were associated with significant reductions in Harvey–Bradshaw Index from baseline to 12 months (Fig. 4A and B). Three patients from both treatment groups underwent intestinal resection during follow-up.
Fig. 2.
Inverse probability treatment-weighted Kaplan–Meier curve of treatment persistence with ustekinumab and vedolizumab (crosses represent censors)
Table 5.
Reasons for treatment discontinuation by 12 months
| Reasons for treatment discontinuation: N (%) | Treatment group | |
|---|---|---|
| Vedolizumab | Ustekinumab | |
| Patient request | 0 | 5 (6.0) |
| Adverse event | 2 (4.8) | 4 (4.8) |
| Primary non-response | 3 (7.1) | 8 (9.6) |
| Secondary non-response | 0 | 3 (3.6) |
| Malignancy | 0 | 1 (1.2) |
| Other | 2 (4.8) | 1 (1.2) |
Fig. 3.
Examination of co-variate balance for treatment persistence before and after inverse probability of treatment weighing
Table 6.
Comparative effectiveness of ustekinumab and vedolizumab. Both unweighted and inverse probability of treatment-weighted rates are presented
| Outcome | Cohort | OR [95% CI] | P value |
|---|---|---|---|
| Treatment persistence at 6 months | Unweighted | 1.33 [0.453–4.43] | 0.621 |
| Weighted | 1.02 [0.303–3.42] | 0.976 | |
| Treatment persistence at 12 months | Unweighted | 1.87 [0.735–5.24] | 0.205 |
| Weighted | 1.23 [0.436–3.45] | 0.696 | |
| Steroid-free treatment persistence at 6 months | Unweighted | 1.93 [0.776–5.30] | 0.175 |
| Weighted | 1.43 [0.512–3.98] | 0.493 | |
| Steroid-free treatment persistence at 12 months | Unweighted | 1.86 [0.774–4.75] | 0.177 |
| Weighted | 1.34 [0.511–3.50] | 0.550 | |
| Clinical remission at 6 months | Unweighted | 2.30 [0.911–6.06] | 0.0835 |
| Weighted | 2.15 [0.787–5.85] | 0.134 | |
| Clinical remission at 12 months | Unweighted | 1.28 [0.481–3.50] | 0.623 |
| Weighted | 1.10 [0.390–3.09] | 0.858 |
Fig. 4.
A, B Harvey–Bradshaw Index trend in elderly patients treated for Crohn’s disease with vedolizumab (A) and ustekinumab (B) at baseline, 6 and 12 months. Horizontal line represents median and boxes represent interquartile range
Predictors of Efficacy
We also examined the effect of baseline clinical variables on treatment persistence. Univariate analysis showed that smoking status, disease behaviour, baseline Harvey–Bradshaw index score, previous anti-TNF primary non-response and baseline steroid use affected treatment persistence rates at 12 months (P < 0.1) (Supplementary Table 5). In a logistic regression model, penetrating disease (OR 6.76, 95% CI 1.08–115, p=0.0403) and baseline Harvey–Bradshaw index score (OR 0.837, 95% CI 0.702–0.983, p=0.0297) were significantly associated with treatment persistence rates at 12 months (Table 7).
Table 7.
Firth logistic regression of variables associated with treatment persistence at 12 months in patients who received vedolizumab or ustekinumab
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Treatment group (ustekinumab vs vedolizumab) | 0.979 | 0.265–3.69 | 0.974 |
| Current smoker | 1.05 | 0.175–7.51 | 0.956 |
| Ex-smoker | 1.10 | 0.302–4.05 | 0.884 |
| Stricturing disease | 0.479 | 0.128–1.70 | 0.254 |
| Penetrating disease | 6.76 | 1.08–115 | 0.0403 |
| HBI at initiation | 0.837 | 0.702–0.983 | 0.0297 |
| Previous anti-TNF primary non-response | 0.899 | 0.298–2.83 | 0.850 |
| Steroids at baseline | 0.420 | 0.127–1.35 | 0.143 |
Significance of bold is highlighted
TNF = tumour necrosis factor, HBI = Harvey-Bradshaw Index
Cox Regression Analysis
Unweighted and weighted Cox regression analysis demonstrated that there was no difference in overall treatment discontinuation-free survival (hazard ratio 1.01, 95% CI 0.741–1.37) and serious infection-free survival (hazard ratio 1.26, 95% CI 0.336–4.75) between ustekinumab- and vedolizumab-treated patients by 12 months (Table 8).
Table 8.
Cox regression analysis of 12-month discontinuation-free survival and severe infection-free survival of patients who received vedolizumab or ustekinumab. Both unweighted and inverse probability of treatment-weighted rates are presented
| Outcome | Cohort | Hazard ratio [95% CI] | P value |
|---|---|---|---|
| Discontinuation-free survival by 12 months | Unweighted | 0.986 [0.648–1.50] | 0.948 |
| Weighted | 1.01 [0.741–1.37] | 0.960 | |
| Severe infection-free survival by 12 months | Unweighted | 1.47 [0.396–5.49] | 0.563 |
| Weighted | 1.26 [0.336–4.75] | 0.731 |
Discussion
In this multi-centre retrospective study, we found comparable safety and effectiveness for ustekinumab and vedolizumab over a 12-month period in elderly Crohn’s disease patients, adjusting for potential confounding using inverse probability weighing.
The frequency of serious infection noted in our study is numerically higher than figures from previously reported studies of elderly patients treated with vedolizumab. In a large Medicare study, the incidence rate for an infection related hospitalisation was 30/1000 patient-years [26], approximately 3–4-fold lower than that noted in our cohort. A similar magnitude of severe infection risk (38.5/1000 patient-years) was observed among 213 vedolizumab-treated patients in a Veterans Affairs study [27]. However, the proportion of patients who developed a serious infection is broadly consistent with that reported in the literature. In a previous cohort study of 103 vedolizumab-treated patients, 17% of patients developed a serious infection (13.8% in our cohort) [17]. In a Spanish cohort of 212 ustekinumab-treated elderly patients, a serious infection rate of 7% was reported [20] which is similar to our cohort. The magnitude of risk also appears broadly comparable to that reported for anti-TNF agents among elderly patients. For instance, de Jong et al. reported serious infection rates of 61.2/1000 patient-years among elderly patients treated with anti-TNF agents [28]. An earlier cohort study of 95 elderly anti-TNF-treated patients reported a serious infection rate of 11% [11] which is similar to the proportions noted for both ustekinumab and vedolizumab in our study. These figures are also consistent with a recent meta-analysis which reported a serious infection rate of 7% among elderly patients treated with biological agents [29]. The slight variation in infection risk noted among the studies could largely be explained by differences in co-morbidities, frailty and concomitant steroid therapy between the patient populations. It is now well recognised that co-morbidities [18] and frailty [30] rather than age dictate infection risk with biological therapy. Although we did not measure frailty in our study, there is a strong correlation between burden of co-morbidities and frailty [30]. In our cohort, we noted a trend for an association between co-morbidities and infection risk although this was not statistically significant. It is likely that our study was under-powered to detect an association. Interestingly, there was no significant difference in the incidence of serious infections between patients treated with 8-weekly or 12-weekly ustekinumab.
The overall efficacy figures for ustekinumab in our study are slightly lower compared to other real-world cohort studies in elderly Crohn’s disease patients. For instance, a single-centre cohort study from the USA reported response rates in excess of 90% in an elderly cohort of 39 patients but importantly remission was only achieved in 28% of patients [19]. Similarly, in the recently published Spanish cohort of 212 elderly patients, the week 54 response rate was 70% [20]. These differences are likely explained by differences in patient populations—for instance, in our cohort we had a lower proportion of patients with penetrating phenotype (13% vs 44% in the US study) and a greater proportion of smokers. Interestingly, both disease phenotype [31] and smoking [32] have been reported to influence response to biological therapy. It is also likely that the lower efficacy figures may reflect the reported lower efficacy among elderly patients with both anti-TNF agents [28] and ustekinumab [19]. The reasons for this apparent decrease in efficacy among elderly are unclear and may simply reflect differences in disease severity and phenotype in various cohorts. Our efficacy outcomes for vedolizumab are broadly consistent with observations from other cohorts of elderly patients. The remission and steroid-free remission rates in our cohort are broadly similar to figures reported in a large bi-national cohort [16] and a nationwide cohort [33] of elderly Crohn’s disease patients treated with vedolizumab. Similarly, treatment persistence rates at 12 months in our cohort is similar to that reported in a cohort of 108 elderly IBD patients [34].
There are no published data on the comparative safety and effectiveness of ustekinumab and vedolizumab in elderly IBD patients. Previous comparative studies among younger anti-TNF-exposed patients showed no difference in safety but there were some differences in effectiveness between the two agents although findings are inconsistent. Although an intitial cohort study reported superior effectiveness of ustekinumab over vedolizumab in anti-TNF refractory CD patients [35], a subsequent larger study did not demonstrate a difference between the two agents [36]. There have been a few studies which have compared the effectiveness and safety of vedolizumab against anti-TNF agents among the elderly with varying results. For instance, in the initially published multi-centre retrospective cohort study, the risk of serious infection was similar between the two agents [17]. A subsequent larger study using the Medicare claims database of 1152 anti-TNF and 480 vedolizumab-treated elderly patients showed a lower risk of infection related hospitalisation among vedolizumab-treated patients [26]. The discrepant findings are likely related to differences in patient population in terms of co-morbidities and frailty. These inconsistencies underline the need for further comparative data among the elderly to inform appropriate positioning of biological therapies in this cohort.
In our cohort, male sex was associated with an increased risk of serious infections whereas penetrating phenotype and clinically active disease were associated with treatment persistence. In keeping with our findings, several studies including sub-group analyses from randomised trials and observational studies suggest disease activity to be an independent predictor of poor response to both vedolizumab [37] and ustekinumab [31]. We were unable to confirm associations with biochemical markers of disease activity (e.g. elevated C-reactive protein) as these were not consistently recorded in our cohort. Higher treatment persistence rates in patients with a penetrating phenotype have been previously reported by a previous French cohort study which showed higher remission rates for both ustekinumab and vedolizumab [38]. Our finding of an increased risk of serious infection in men, although intriguing and not previously reported in association with biological therapy, is not entirely unexpected. Men are at increased risk of infections in general and several factors including protective effects of oestradiol, lifestyle factors and a higher burden of co-morbidities have been postulated to explain this difference [39].
Our study has some limitations. The study is retrospective and non-randomised and does not account for inherent treatment selection bias. We attempted to adjust for potential confounding between treatment groups by applying inverse probability weighing to provide unbiased treatment effect estimates, but the validity of this analysis relies on the assumption that all confounders have been accounted for. Baseline characteristics were well matched between the two groups and, therefore, justify the comparison but the study is somewhat limited by the small number of patients. Due to limitations on endoscopy imposed by the Covid-19 pandemic, data on mucosal healing were unavailable. Furthermore, due to restrictions on laboratory services during the pandemic, non-essential serviecs were suspended. Only 8 patients in the vedolizumab group and 14 patients in the ustekinumab group had complete non-invasive biomarker data (C-reactive protein and faecal calprotectin) which precluded meaningful analysis. The duration of follow-up is also limited and ideally studies with longer follow-up duration are required to confirm outcomes over a prolonged period. Due to the retrospective nature of the study, it is possible that the rates of clinical effectiveness and adverse events may have been over- or under-estimated.
In summary, we report that ustekinumab and vedolizumab were comparable in effectiveness and safety at both early time points and up to 1 year of treatment in an elderly cohort of Crohn’s disease patients. Further prospective studies are required to confirm our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Author’s contribution
GGG, JF, EL, GB, and VR were involved in data collection and drafting of the manuscript. GGG and SS were involved in data analysis. JKL, PJS, CS and PKF were involved in drafting and final revision of the manuscript. SS was involved in study design, data collection, analysis, drafting and final revision of the manuscript. Guarantor of the article Dr Sreedhar Subramanian.
Funding
This research was not directly funded.
Declarations
Conflict of interest
GGG, JF, EL, GB and VR report no conflicts of interest. JKL has received speaker fees from Dr. Falk pharmaceuticals. SS has received speaker fees from Abbvie, Dr. Falk pharmaceuticals, Takeda, Janssen, Celltrion, Bristol-Myers Squibb and received educational grants from Abbvie, Takeda and Janssen and is an advisory board member for Abbvie, Dr. Falk pharmaceuticals, Vifor pharmaceuticals, Janssen, Takeda and Celltrion. SS is an associate editor for AP&T. PKF received a Shire Innovation Fund award and has received funded conference travel from Shire and Tillotts. PJS has received speaker fees from Takeda, Janssen, Celltrion, Abbvie, Amgen, Dr. Falk pharmaceuticals, Tillotts Pharma and has been an advisory board member for Abbvie, Celltrion and Janssen. EL has received speaker fees from Janssen and a research grant from Galapagos. CPS has received unrestricted research grants from Abbvie and Janssen, has provided consultancy to Arena, Galapagos, Dr. Falk, Fresenius Kabi, Abbvie, Janssen and Takeda, and had speaker arrangements with Janssen, Dr. Falk, Abbvie, Pfizer and Takeda. All authors approve the final version of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Rechel B, Grundy E, Robine JM, et al. Ageing in the European Union. Lancet. 2013;381:1312–1322. doi: 10.1016/S0140-6736(12)62087-X. [DOI] [PubMed] [Google Scholar]
- 3.Jeuring SF, van den Heuvel TR, Zeegers MP, et al. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age-an increasing distinct entity? Inflamm Bowel Dis. 2016;22:1425–1434. doi: 10.1097/MIB.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 4.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Shi HY, Tang W, et al. systematic review and meta-analysis: phenotype and clinical outcomes of older-onset inflammatory bowel disease. J Crohns Colitis. 2016;10:1224–1236. doi: 10.1093/ecco-jcc/jjw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brassard P, Bitton A, Suissa A, Sinyavskaya L, Patenaude V, Suissa S. Oral corticosteroids and the risk of serious infections in patients with elderly-onset inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1795–1802; quiz 1803. [DOI] [PubMed]
- 7.Akerkar GA, Peppercorn MA, Hamel MB, Parker RA. Corticosteroid-associated complications in elderly Crohn's disease patients. Am J Gastroenterol. 1997;92:461–464. [PubMed] [Google Scholar]
- 8.Calafat M, Manosa M, Canete F, et al. Increased risk of thiopurine-related adverse events in elderly patients with IBD. Aliment Pharmacol Ther. 2019;50:780–788. doi: 10.1111/apt.15458. [DOI] [PubMed] [Google Scholar]
- 9.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 10.Bourrier A, Carrat F, Colombel JF, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. 2016;43:252–261. doi: 10.1111/apt.13466. [DOI] [PubMed] [Google Scholar]
- 11.Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30–35. doi: 10.1016/j.cgh.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53:849–853. doi: 10.1136/gut.2003.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzetti R, Zullo A, Ridola L, et al. Higher risk of tuberculosis reactivation when anti-TNF is combined with immunosuppressive agents: a systematic review of randomized controlled trials. Ann Med. 2014;46:547–554. doi: 10.3109/07853890.2014.941919. [DOI] [PubMed] [Google Scholar]
- 14.Yajnik V, Khan N, Dubinsky M, et al. Efficacy and safety of vedolizumab in ulcerative colitis and crohn's disease patients stratified by age. Adv Ther. 2017;34:542–559. doi: 10.1007/s12325-016-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shashi P, Gopalakrishnan D, Parikh MP, Shen B, Kochhar G. Efficacy and safety of vedolizumab in elderly patients with inflammatory bowel disease: a matched case-control study. Gastroenterol Rep (Oxf). 2020;8:306–311. doi: 10.1093/gastro/goz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen NA, Plevris N, Kopylov U, et al. Vedolizumab is effective and safe in elderly inflammatory bowel disease patients: a binational, multicenter, retrospective cohort study. United European Gastroenterol J. 2020;8:1076–1085. doi: 10.1177/2050640620951400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adar T, Faleck D, Sasidharan S, et al. Comparative safety and effectiveness of tumor necrosis factor alpha antagonists and vedolizumab in elderly IBD patients: a multicentre study. Aliment Pharmacol Ther. 2019;49:873–879. doi: 10.1111/apt.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asscher VER, Biemans VBC, Pierik MJ, et al. Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab- and ustekinumab-treated patients with inflammatory bowel disease-a prospective multicentre cohort study. Aliment Pharmacol Ther. 2020;52:1366–1376. doi: 10.1111/apt.16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg R, Aggarwal M, Butler R, et al. Real-world effectiveness and safety of ustekinumab in elderly crohn's disease patients. Dig Dis Sci. 2021 doi: 10.1007/s10620-021-07117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casas Deza D, Lamuela Calvo LJ, Arbonés Mainar JM, et al. Effectiveness and safety of ustekinumab in elderly patients: real world evidence from ENEIDA registry. J Crohns Colitis. 2021;15:S298–S299. doi: 10.1093/ecco-jcc/jjab076.388. [DOI] [PubMed] [Google Scholar]
- 21.Abreu MT, Ott E, Gasink C, et al. S885 safety of ustekinumab in older IBD patients (≥60 Years): pooled safety analysis through 5 years in CD and 2 years in UC and all approved indications. Off J Am Coll Gastroenterol (ACG). 2021;116:S416. doi: 10.14309/01.ajg.0000777072.98694.77. [DOI] [Google Scholar]
- 22.Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn's disease activity and Harvey–Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363. doi: 10.1016/j.cgh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 25.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2021.
- 26.Kochar B, Pate V, Kappelman MD, et al. Vedolizumab Is associated with a lower risk of serious infections than anti-tumor necrosis factor agents in older adults. Clin Gastroenterol Hepatol. 2022;20(1299–1305):e1295. doi: 10.1016/j.cgh.2021.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan N, Pernes T, Weiss A, et al. Incidence of infections and malignancy among elderly male patients with IBD exposed to vedolizumab, prednisone, and 5-ASA medications: a nationwide retrospective cohort study. Adv Ther. 2021;38:2586–2598. doi: 10.1007/s12325-021-01713-x. [DOI] [PubMed] [Google Scholar]
- 28.de Jong ME, Smits LJT, van Ruijven B, et al. Increased discontinuation rates of anti-TNF therapy in elderly inflammatory bowel disease patients. J Crohns Colitis. 2020;14:888–895. doi: 10.1093/ecco-jcc/jjaa012. [DOI] [PubMed] [Google Scholar]
- 29.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Bonovas S. Systematic review with meta-analysis: biologics and risk of infection or cancer in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:820–830. doi: 10.1111/apt.15692. [DOI] [PubMed] [Google Scholar]
- 30.Kochar B, Cai W, Cagan A, Ananthakrishnan AN. Pretreatment frailty is independently associated with increased risk of infections after immunosuppression in patients with inflammatory bowel diseases. Gastroenterology. 2020;158(2104–2111):e2102. doi: 10.1053/j.gastro.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Ma C, Fedorak RN, Kaplan GG, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn's disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther. 2017;45:1232–1243. doi: 10.1111/apt.14016. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Kuenzig ME, Ricciuto A, et al. Smoking may reduce the effectiveness of anti-TNF therapies to induce clinical response and remission in Crohn's disease: a systematic review and meta-analysis. J Crohns Colitis. 2021;15:74–87. doi: 10.1093/ecco-jcc/jjaa139. [DOI] [PubMed] [Google Scholar]
- 33.Khan N, Pernes T, Weiss A, et al. Efficacy of vedolizumab in a nationwide cohort of elderly inflammatory bowel disease patients. Inflamm Bowel Dis. 2021;28(5):734–744. doi: 10.1093/ibd/izab163. [DOI] [PubMed] [Google Scholar]
- 34.Pabla BS, Alex Wiles C, Slaughter JC, et al. Safety and efficacy of vedolizumab versus tumor necrosis factor alpha antagonists in an elderly IBD population: a single institution retrospective experience. Dig Dis Sci. 2021;67(7):3129–3137. doi: 10.1007/s10620-021-07129-5. [DOI] [PubMed] [Google Scholar]
- 35.Townsend T, Razanskaite V, Dodd S, et al. Comparative effectiveness of ustekinumab or vedolizumab after one year in 130 patients with anti-TNF-refractory Crohn's disease. Aliment Pharmacol Ther. 2020;52:1341–1352. doi: 10.1111/apt.16057. [DOI] [PubMed] [Google Scholar]
- 36.Lenti MV, Dolby V, Clark T, et al. A propensity score-matched, real-world comparison of ustekinumab vs vedolizumab as a second-line treatment for Crohn's disease. The Cross Pennine study II. Aliment Pharmacol Ther. 2022;55:856–866. doi: 10.1111/apt.16742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14(1593–1601):e1592. doi: 10.1016/j.cgh.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Alric H, Amiot A, Kirchgesner J, et al. The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn's disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther. 2020;51:948–957. doi: 10.1111/apt.15706. [DOI] [PubMed] [Google Scholar]
- 39.Gay L, Melenotte C, Lakbar I, et al. Sexual dimorphism and gender in infectious diseases. Front Immunol. 2021;12:698121. doi: 10.3389/fimmu.2021.698121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.