Highlights
-
•
There is a lack of prospective level I evidence for the use of PBT for most adult cancers including oropharyngeal squamous cell carcinoma (OPSCC).
-
•
TORPEdO is the UK’s first PBT clinical trial and aims to determine the benefits of PBT for OPSCC.
-
•
Training and support has been provided before and during the trial to reduce variations of contouring and radiotherapy planning.
-
•
There is a strong translational component within TORPEdO. Imaging and physics data along with blood, tissue collection will inform future studies in refining patient selection for IMPT.
1. Introduction/rationale
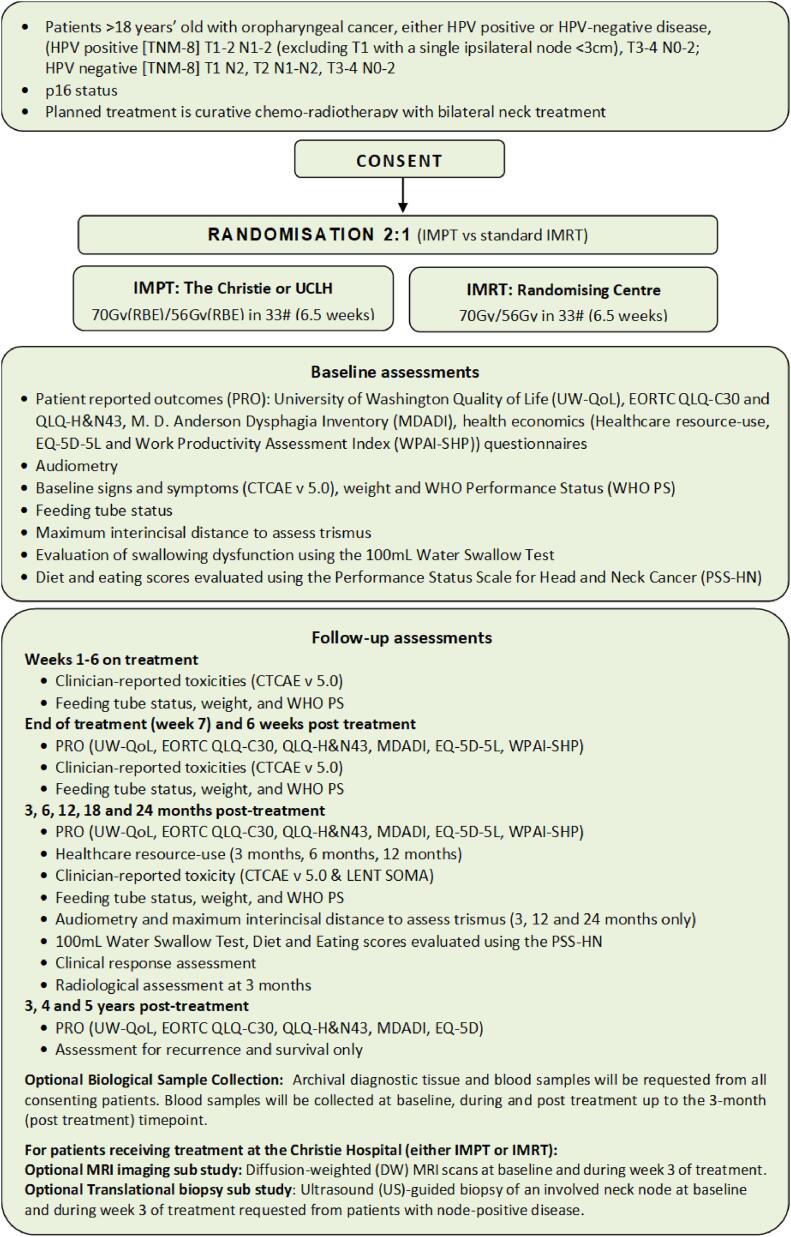
In 2020, there were an estimated 98,412 cases of oropharyngeal cancer worldwide [1]. Numbers of oropharyngeal squamous cell carcinoma (OPSCC) are rising rapidly due to an increase in HPV-related disease. For locally advanced OPSCC, concurrent chemo-intensity modulated radiotherapy (IMRT) is a standard of care [2]. The treatment of OPSCC with concurrent chemo-radiotherapy is associated with severe acute and late side-effects, which can have a profound detrimental impact on long-term quality-of-life (QoL) [3].Fig. 1.
Fig. 1.
Trial schema.
There is a need to improve treatment related toxicities and health related (HR)-QoL for patients treated with chemo-radiotherapy for OPSCC. Technological advances in radiotherapy delivery, including use of proton beam therapy (PBT), aim to increase sparing of normal tissues with improved functional outcomes and HR-QoL for patients [4], [5].
In the UK, two state-of-the-art PBT facilities are operational; the first (at The Christie NHS Foundation Trust, Manchester) began treating patients in December 2018, and the second (at University College London Hospital, UCLH) in December 2021. Each centre expects to treat up to 750 patients per annum with up to 40 % of capacity for those treated as part of clinical trials.
There is a lack of prospective level I evidence for the use of PBT for most adult cancers [6], including OPSCC. The use of normal tissue complication probability (NTCP) models to select patients for PBT was not considered sufficient justification for the use of PBT in the UK. It was agreed that a high-quality clinical trial of PBT to determine and quantify the benefits of PBT for patients with OPSCC was required to justify the: (i) inconvenience to patients and families who need to travel and stay away from home for a centralised service, (ii) increased treatment costs [7] and (iii) greater resource intensiveness (to account for treatment planning and delivery uncertainties) [8]. The trial also affords valuable opportunity to prospectively evaluate the NTCP models for patient selection, contributing to our understanding of which patients may benefit most from the use of PBT. TORPEdO is the UK’s first PBT clinical trial and aims to determine the benefits of PBT for OPSCC.
2. Design
TORPEdO is a phase III multi-centre open-label randomised controlled trial to assess whether intensity modulated proton therapy (IMPT) compared IMRT reduces treatment-related toxicities in patients with locally advanced oropharyngeal squamous cell carcinoma. The trial is registered [ISRCTN: 16424014].
2.1. Study objectives
2.1.1. Primary objective
To assess whether IMPT compared with IMRT reduces treatment-related toxicities in patients with locally advanced oropharyngeal squamous cell carcinoma.
2.1.2. Secondary objectives
-
•
To validate a biomarker (normal tissue complication probability (NTCP)-model) as a predictor of benefit from IMPT versus IMRT.
-
•
To estimate the cost-effectiveness of IMPT versus IMRT for OPSCC in the UK
-
•
Define methodology and processes for future UK-led IMPT trials.
2.1.3. Exploratory objectives
-
•
To perform diffusion-weighted magnetic resonance imaging (DW-MRI) at baseline and week three of treatment to determine threshold changes in apparent diffusion co-efficient (ADC) in organs at risk (OARs) that could predict the occurrence of late toxicities.
-
•
To collect biopsy samples at baseline and week three of treatment to evaluate per-treatment changes in the tumour micro-environment for IMRT and IMPT.
-
•
To offer those who decline participation in TORPEdO an opportunity to enrol in a recruitment factors study, to understand barriers to enrolment in UK proton trials.
-
•
To collect samples and treatment planning data for future linked translational research studies.
2.2. Patient population
All patients provide written informed consent to participate. Inclusion and exclusion criteria are as follows:
2.2.1. Inclusion criteria
-
•
Histologically confirmed oropharyngeal squamous cell carcinoma
-
•
Human papillomavirus (HPV) positive [TNM version 8] T1-2 N1-2 (excluding T1 with a single ipsilateral node <3 cm), T3-4 N0-2
-
•
HPV negative [TNM version 8] T1 N2, T2 N1-N2, T3-4 N0-2
-
•
Local multi-disciplinary team decision for concurrent chemoradiotherapy with bilateral neck treatment
-
•
Age ≥ 18 years
-
•
WHO performance status 0–1
-
•
Adequate renal function, glomerular filtration rate (GFR) ≥ 60 ml/min calculated using Cockcroft-Gault formula
-
•
Adequate cognitive ability (in the opinion of the local principal investigator (PI) or delegated co-investigator) to complete patient reported outcome (PRO) assessments
-
•
Willingness to comply with the protocol, including travel to the proton centre for IMPT treatment
2.2.2. Exclusion criteria
-
•
Feeding tube insertion required for nutrition (for example due to dysphagia, trismus or low weight/body mass index) prior to treatment [Note: patients who have a feeding tube inserted as part of a planned prophylactic feeding tube approach remain eligible for the study.]
-
•
N3 disease
-
•
Upfront neck dissection
-
•
Use of induction chemotherapy
-
•
Contra-indication to the use of cisplatin for cycle 1 concurrent chemotherapy.
-
•
Previous head and neck radiotherapy
-
•
Major surgery within 6 months of trial entry
-
•
Permanent pacemaker or implantable cardioverter defibrillator
-
•
Any invasive malignancy within previous 2 years (other than non-melanomatous skin carcinoma or cervical carcinoma in situ)
-
•
Previous or concurrent illness (e.g., active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, active peptic ulcer disease or gastritis), which in the investigator’s opinion would interfere with completion of therapy, trial assessments or follow up
-
•
Pregnancy, lactating women or women of childbearing potential unwilling or unable to use adequate non-hormonal contraception (male patients should also use contraception if sexually active)
-
•
Pre-existing speech or swallowing problems unrelated to the diagnosis of cancer, which in the local principal investigator’s or delegated co-investigator’s opinion would interfere with completion of therapy, trial assessments or follow up
2.3. Treatment allocation
Treatment allocation is by minimisation with a random element to account for imbalances between IMPT and IMRT groups ensuring comparability of clinically important pre-specified prognostic factors (balancing factors) between treatment groups. Balancing factors are; randomising centre, bilateral neck nodes, site of disease, p16 status, smoking status and T-stage.
2.4. Treatment description
Consenting patients are randomised in a 2:1 ratio to IMPT at a proton centre vs standard IMRT at the recruiting centre. Radiotherapy doses ((when accounting for the greater relative biological effectiveness of PBT) are the same in both treatment groups; a therapeutic dose of 70 Gy (relative biological effective [RBE] equivalent) and an elective dose of 56 Gy (RBE equivalent), both delivered in 33 once-daily fractions over 6.5 weeks using a simultaneous integrated boost technique. An RBE of 1.1 is applied for PBT and 1.0 for IMRT.
Details of the schedule of assessments and follow-up are shown in Table 1.
Table 1.
Schedule of assessments.
| Treatment Period For IMPT patients ALL assessments during treatment period will be at Proton Beam centre |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASSESSMENT/VISIT | Screening (Pre randomisation) | Pre-trt (After randomisation) | RT week 1 | RT week 2 | RT week 3 | RT week 4 | RT week 5 | RT week6 | RT week 7 | Week 6 post RT | 3 months post RT | 6 months post RT | 12 months post RT | 18 months post RT | 24 months post RT | Annually at yr 3,4 & 5 post RT |
| Histological confirmation (p16 by IHC and TMN 8 staging) | X | |||||||||||||||
| Physical examination (Including height) | X | |||||||||||||||
| Patient weight + WHO PS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Radiological assessment (MRI/PET-CT/CT thorax) | X1 | X2 | ||||||||||||||
| ECG/BSA (concurrent chemotherapy assessments) optional as per local hospital policy | X | |||||||||||||||
| Dental assessment (where possible)*either time point acceptable | X* | X* | ||||||||||||||
| Blood test (FBC, electrolytes, liver function tests) | X | X | X | X | X | X | X | X | ||||||||
| Calculated renal function (Cockcroft Gault Formula/see page 6) | X | |||||||||||||||
| NM isotope GFR (optional, per local policy) *either time point is acceptable | X* | X* | ||||||||||||||
| 5-point customised thermoplastic shell and radiotherapy planning CT scan | X | |||||||||||||||
| Repeat radiotherapy planning CT scan | X4 | |||||||||||||||
| Radiotherapy planning re-plan | X5 | |||||||||||||||
| Adverse events & toxicities: CTCAE (+LENT SOMA 3-24mths) | X | X | X | X | X | X | X | X | X | X6 | X6 | X6 | X6 | X6 | ||
| Clinical response assessment 7 | X | X | X | X | X | X | X | |||||||||
| Audiometry | X3 | X | X | X | ||||||||||||
| Maximum interincisal opening MIO to assess for trismus *either time point is acceptable | X* | X* | X | X | X | |||||||||||
| Feeding tube status | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| PRO questionnaires8 (UW-QOL, EORTC QLQ-C30, QLQ H&N 43, MDADI, EQ-5D-5L, Healthcare resource use9 and WPAI-SHP10) | X | X | X | X | X | X | X | X | X | |||||||
| Swallowing function assessments (100 ml Water Swallow Test + PSS-HN) | X | X | X | X | X | X | ||||||||||
| Translational sub-study (optional) | ||||||||||||||||
| EDTA blood (10 ml) for genomics12 | X11 | |||||||||||||||
| Streck bloods(40 ml pre-trt; 30 ml thereafter)for liquid biopsies12 | X11 | X | X | X | ||||||||||||
| EDTA bloods (30 ml) for immune markers12 | X11 | X | X | X | ||||||||||||
| EDTA bloods (10 ml) for proteomics | X11,13 | X | X | X | X | X | X | X | X | |||||||
| Diagnostic FFPE block request for transcriptomics | X | |||||||||||||||
| DW-MRI sub-study (optional)14 | X | X | ||||||||||||||
| Biopsy sub-study (optional)14 | X | X | ||||||||||||||
1Baseline: MRI neck + CT thorax preferred, however CT neck + CT thorax is acceptable. 2At 3 months, PET-CT is preferred, however MRI neck + CT Thorax acceptable (may be performed earlier if clinically indicated). 3baseline audiometry should ideally be conducted at randomising centre. 4Repeat planning CT scan to be ideally performed on Wednesday of week three. 5Re-plan where needed, to start on new plan Monday of week five. 6LENT SOMA as well as CTCAE assessments. 7Additional tests will be requested if clinically indicated as in standard practice. 8PRO booklets will be given to the patient in clinic at screening, end of treatment, 6 weeks post RT, and then sent to the patient’s home address for completion at 3, 6, 12, 18, 24, 36, 48, 60 months. 9Healthcare resource use questionnaire only completed at screening, and 3, 6, and 12 months post RT. 10 WPAI-SHP questionnaires not completed years 3–5. 11Samples will be taken as described in the Sample Collection Manual 12 Samples to be collected and sent the same day on Mon-Weds only, and not two days prior to a bank holiday. 13Two proteomic samples taken pre-treatment. 14Patients recruited by The Christie only.
2.5. Radiotherapy quality Assurance (RT QA)
A comprehensive RT QA programme for the TORPEdO trial has been designed and implemented by the National Radiotherapy Trials Quality Assurance (RTTQA) Group including pre-trial and on-trial components. The QA processes for the TORPEdO trial has been streamlined, where possible, with previous head and neck trial QA based on both centre and PI trial participation.
For pre-trial QA centres must complete the following prior to site activation: 1) Facility questionnaire, 2) Benchmark outlining cases, 3) Benchmark planning cases 4) Dosimetry audit (subject to prior RTTQA accreditation).
On trial QA includes: Independent prospective case review of all contouring cases and the first plan from each centre, and retrospective case review of all other plans. Digital Imaging and Communications in Medicine (DICOM) data is collected for all patients. Treatment plan QA is supported by professionals with experience from an independent European proton centre. Radiotherapy planning and delivery guidelines are provided in appendix 2.
2.6. Associated research
2.6.1. Health economics study
A within-trial economic component has been integrated into the study to estimate the cost-effectiveness of IMPT from the perspective of the UK NHS, patients and society. Healthcare resource use will be collected from hospital electronic patient records as well as via patient or family member self-reporting using a patient resource-use questionnaire developed specifically for the study. Patient resource use from the date of randomisation to the end of 1-year follow-up will be used to define cumulative healthcare costs alongside within trial responses to the EQ-5D-5L questionnaire for the within-trial economic component.
2.6.2. Imaging and physics based translational study
The collection of all pre-accrual (benchmarking) and during-accrual (recruited patients) DICOM-RT and associated non-DICOM data, will underpin future studies to validate and develop improved NTCP models, refining patient selection for IMPT and explore the following hypotheses:
-
•
Correlation of image-based data mining DICOM data and per-voxel RT doses with continuous toxicity variables (e.g., trismus, audiometry, lymphopenia) will improve dose constraints for normal tissue sub-structures.
-
•
Data from weekly cone beam CT images and the repeat verification CT scan will increase understanding of how often to adapt IMPT and IMRT plans and allow development of meaningful thresholds and a clinical traffic light protocol for adaptation.
2.6.3. Biological sub studies
These sub-studies are co-ordinated by the Manchester Cancer Research Centre at the University of Manchester. TORPEdO provides an opportunity to collect samples and data for translational research to inform the design of future biomarker-driven trials aimed at the optimal selection of IMPT vs IMRT based on not only NTCP models but also tumour and normal tissue genomics. Due to differences in the more complex nature of the DNA damage produced by protons vs photons, the following hypotheses are made:
-
•
Patients with tumours exhibiting a defective DNA damage response profile associated with defects in homologous recombination will benefit more from protons compared to photons.
-
•
There will be some non-overlapping genetic variants that increase the risk of toxicity to protons compared to photons.
-
•
Protons will generate an enhanced immune response compared with photons
-
•
Protons will generate differential DNA methylation patterns compared with photons due to differences in DNA damage and normal tissue irradiation.
2.7. Safety reporting
Serious Adverse Events (SAEs) are reported after the commencement of radiotherapy and within 30 days of the last radiotherapy fraction.
2.8. Endpoints
The co-primary endpoints measured at 12 months after completion of chemoradiotherapy are:
-
•
University of Washington Quality of Life Questionnaire version 4 (UW-QoL v4.0) physical composite score;and
-
•
gastrostomy dependence or Common Terminology Criteria for Adverse Events (CTCAE) grade 3 wt loss (i.e. ≥ 20 % weight loss from baseline)
A patient-reported outcome measure combining a number of relevant physical toxicity endpoints was chosen as a highly relevant way to test the ability of proton therapy to reduce late toxicity and improve quality of life for patients. This choice was supported by patient focus groups, clinicians and commissioners, as a valid comparator, which, if satisfied would lead to a change in practice.
Gastrostomy dependence is a highly relevant endpoint as long-term dependence has a profound negative impact on quality of life for patients [9]. It is a surrogate for severe swallowing dysfunction and other functional impairments such as problems with chewing, taste disturbance or oral dryness. The potential for physician-bias in tube removal is mitigated by the composite inclusion of grade 3 wt loss, where premature tube removal would likely translate into increased weight loss.
Secondary endpoints include longitudinal pattern of health-related quality of life (assessed using the following questionnaires: UW-QoL, EORTC QLQ-C30, EORTC QLQ-H&N43 and M.D. Anderson Dysphagia Inventory (MDADI)), tube feeding status, weight loss >10 % from baseline at any timepoint after 6 months post-treatment, acute and late severe toxicity (assessed using CTCAE v5.0), clinician-rated swallowing function assess using the 100 ml Water Swallow Test [10] and, Performance Status Scale for Head and Neck Cancer (PSS-HN) [11] (normalcy of diet, place of eating and understandability of speech subscales), hearing loss, maximum interincisal opening (MIO) to assess trismus, resection rates, loco-regional tumour control, overall survival and cost-effectiveness.
2.8. Statistical considerations
2.8.1. Hypotheses
The intention of the trial is show superiority of IMPT over IMRT in terms of the patient reported and clinician reported co-primary outcome measures. However, demonstration of a significant treatment effect on either of the co-primary endpoints will be considered sufficient to support a conclusion of effectiveness. The use of 2:1 randomisation aligns with NHS England’s preference to increase access to proton therapy for patients within clinical trials and increases power for secondary and exploratory endpoints relating to IMPT.
The sample size requirements are driven by the clinician reported composite endpoint; thus, this is considered first.
2.8.2. Sample size/power considerations for clinician reported co-primary endpoint
The clinician reported co-primary endpoint is a composite binary endpoint where an “event” is.
-
•
gastrostomy dependence or,
-
•
CTCAE grade 3 wt loss (≥20 % from baseline) at 12 months after completion of chemoradiotherapy.
This endpoint was used in a prospective cohort study [12] including 150 patients treated 1:2 with IMPT or IMRT, matched by patient, tumour and treatment factors. In this study, gastrostomy-tube dependence, or grade 3 wt loss after treatment favoured IMPT with rates of 25 % for IMRT and 8 % for IMPT with an odds ratio of 0.23 (95 % CI: 0.07–0.73; p-value = 0.01). The event rate in the IMRT group is consistent with rates of feeding tube dependence alone at 12 months after treatment reported in a systematic review (range 6.9 % to 29.0 %) [13] and with data published from The Christie (16 %) [14].
The event rate in the IMRT control group is thus assumed to be 25 %. The target treatment effect was selected based on an odds ratio of 0.23 translating to a 7 % event rate in the IMPT group. Using a chi-squared based comparison of proportions, with 2:1 randomisation (IMPT: IMRT), 80 % power, and 2-sided 2.5 % significance, 165 participants (110 IMPT: 55 IMRT) are required to detect a difference of 25 % (IMRT) versus 7 % (IMPT) in the proportion of patients with feeding tube dependence or grade 3 wt loss, at 12 months.
An inflation of 10 % has been applied to allow for non-evaluability (e.g., due to drop-out due to disease recurrence or death before 12 months, non-compliance with the 12-month questionnaire or requirement for tube feeding prior to commencement of chemo-radiotherapy). This gives a target sample size of 183 participants (122 IMPT: 61 IMRT).
2.8.3. Sample size/power considerations for patient reported co-primary endpoint
The patient reported co-primary endpoint is the UW-QoL physical composite score. In a previous cohort of head and neck cancer patients treated with IMRT, mean UW-QoL physical composite score 12 months after treatment completion was 71.2 (SD = 14.05), compared to 89.3 at baseline [15]. An 8-point improvement represents a ‘moderate’ and clinically important increase and is consistent with: 1) an NTCP model-based estimate of the mean difference of 13 points at 12 months [16], [17] and 2) the change in EORTC QLQ-C30 Physical Function of 9 points at 12 months seen between patients treated with volumetric modulated arc therapy (VMAT) and with protons [18], [19]. With 156 participants (104 IMPT: 52 IMRT) an 8-point improvement in the mean UW-QoL physical composite scale (assuming equal SD of 14.05 in each group) can be detected at the two-sided 2.5 % significance level with 86 % power (two-sample t-test).
In a contemporary ICR-CTSU managed head and neck cancer radiotherapy trial (DARS) [20] where HR-QoL questionnaires are centrally administered, return rates at 12 months post-treatment are approximately 85 %. Applying a 15 % inflation for non-evaluability gives the target sample size of 183 participants (122 IMPT: 61 IMRT).
2.8.4. Interim analysis and stopping rules
Recruitment will be closely monitored by the Trial Management Group (TMG) with escalation to the independent Trial Steering Committee (TSC) should it fall below 50 % of target.
An Independent Data Monitoring Committee (IDMC) will review the accumulating data at least annually in confidence.
An initial review of the statistical assumptions underlying the power calculations for each of the co-primary endpoints will be undertaken after 25 control arm patients have been followed up for 12 months.
No formal stopping rule based on the primary endpoint is proposed although a pre-planned review will take place when 12-month primary endpoint data are available for half the target sample size (estimated month 32 from start of recruitment). There is no suggestion that IMPT will be less effective than IMRT and given the pattern of recurrence seen in this disease setting, and the relatively short accrual period of the trial, any early stopping rule based on recurrence rates is likely to be based on limited information. To monitor recurrence rates, the number of loco-regional recurrences out of the number of patients who have started trial treatment at that point will be tabulated by treatment group along with a p-value from Fisher’s exact test. These data will be sent to the IDMC, along with further details on the site of loco-regional recurrences reported and the IDMC will use this information along with any other emerging data to advise on early stopping of the trial.
2.9. Planned timeline
TORPEdO recruited its first patient on 25th February 2020. There was a three-month hiatus to recruitment from March to May 2020 due to the first wave of the COVID-19 pandemic. As of 5th October 2022, the trial is open in 16 centres and is expected to complete recruitment by October 2023.
Ethics approval
TORPEdO was approved by the North West – Greater Manchester West Research Ethics Committee (19/NW/0700).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. EH acknowledges support from a Cancer Research UK Network Accelerator Award Grant (A21993) to the Advanced Radiotherapy Network (ART-NET) consortium and Cancer Research UK Clinical Trials Unit core funding to ICR-CTSU (C1491/A15955).
We thank Professor Adrian Crellin (NHS England National Clinical Lead Proton Beam Therapy), Dr Ed Smith (Clinical Lead, Proton Beam Therapy, Christie), Dr Yen-Ching Chang Clinical Lead, Proton Beam Therapy, UCLH) and members of the TORPEdO TSC and IDMC for their continued support. We also thank Hakan Nyström (Skandion clinic, Uppsala, Sweden), Petra Witt Nyström (University Hospital of Uppsala, Sweden) and members of the patient focus groups (Manchester, Sheffield, Leeds) for their support and expertise in trial development.
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Competing interests [last 36 months]
EH declares funding from Cancer Research UK for the central coordination of the TORPEdO trial. EH also declares grants from Varian Medical Systems Inc., Astra Zeneca, Janssen-Cilag, Bayer, Roche Products LTD, Merck Sharp & Dohme received by the Institute of Cancer Research. CW declares funding from Cancer Research UK for the provision of translational study materials in the TORPEdO trial. DT declares consulting fees from Merck, Sharp & Dohme and is Deputy Chair of the NCRI CTRad Proton Beam Therapy Strategy Group. CS declares Chairmanship of the Chairman NCRI CTRad Proton Beam Therapy Strategy Group. HL declares consulting fees/honoraria from IBA proton therapy, Chair of the Safety Monitoring Committee (UPGRADE-trial), Member of the Global Advisory Committee (IBA), Member of the RayCare Clinical Advisory Board (RaySearch), Chair of Netherlands Society for Radiation Oncology (NVRO) and research department support from IBA, RaySearch, Elekta, Mirada, Siemens. RB declares consulting fees from Rutherford Cancer Centres, payment/honoraria from British Dental Assoc/Rutherford Cancer Centres/BAOMS, Chair of Medical Advisory Group for Rutherford Cancer Centre South Wales and member of All Wales PBT Reference Group.
Funding
TORPEdO is funded by Cancer Research UK (CRUK/18/010; A27172). Funding is also received from The Taylor Family Foundation (CRUK/C19941/A30286).
The trial is sponsored by The Institute of Cancer Research and centrally managed by The Institute of Cancer Research Clinical Trials and Statistics Unit (ICR-CTSU). TORPEdO is supported by the National Institute for Health Research funded National Radiotherapy Trials Quality Assurance Group (RTTQA).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.11.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R., Laversanne M., Soerjomataram I., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Mehanna H, Evans M, Beasley M, Chatterjee S, Dilkes M, et al. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016 May;130(S2):S90-S96. [DOI] [PMC free article] [PubMed]
- 3.Nutting C.M., Morden J.P., Harrington K.J., Urbano T.G., Bhide S.A. at al Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011 Feb;12(2):127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price J., Hall E., West C., Thomson D. TORPEdO - A Phase III Trial of Intensity-modulated Proton Beam Therapy Versus Intensity-modulated Radiotherapy for Multi-toxicity Reduction in Oropharyngeal Cancer. Clin Oncol (R Coll Radiol) 2020 Feb;32(2):84–88. doi: 10.1016/j.clon.2019.09.052. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Research Institute Clinical and Translational Radiotherapy Research Working Group (CTRad) Proton Beam Clinical Trial Strategy Group. Proton Beam Therapy – the Challenges of Delivering High-quality Evidence of Clinical Benefit. Clin Oncol (R Coll Radiol). 2018 May;30(5):280-284. [DOI] [PubMed]
- 6.Ofuya M., McParland L., Murray L., Brown S., Sebag-Montefiore D., et al. Systematic review of methodology used in clinical studies evaluating the benefits of proton beam therapy. Clin Transl Radiat Oncol. 2019 Jul;12(19):17–26. doi: 10.1016/j.ctro.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zietman A.L. Too Big to Fail? The Current Status of Proton Therapy in the USA. Clin Oncol (R Coll Radiol) 2018 May;30(5):271–273. doi: 10.1016/j.clon.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Crellin A. The Road Map for National Health Service Proton Beam Therapy. Clin Oncol (R Coll Radiol) 2018 May;30(5):277–279. doi: 10.1016/j.clon.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Langendijk J.A., Doornaert P., Verdonck-de Leeuw I.M., Leemans C.R., Aaronson N.K., et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008 Aug 1;26(22):3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 10.Patterson J.M., Hildreth A., McColl E., Carding P.N., Hamilton D., et al. The clinical application of the 100mL water swallow test in head and neck cancer. Oral Oncol. 2011 Mar;47(3):180–184. doi: 10.1016/j.oraloncology.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 11.List M.A., Ritter-Sterr C., Lansky S.B. A performance status scale for head and neck cancer patients. Cancer. 1990 Aug 1;66(3):564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard P., Garden A.S., Gunn G.B., Rosenthal D.I., Morrison W.H., et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer - A case matched analysis. Radiother Oncol. 2016 Jul;120(1):48–55. doi: 10.1016/j.radonc.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wopken K., Bijl H.P., Langendijk J.A. Prognostic factors for tube feeding dependence after curative (chemo-) radiation in head and neck cancer: A systematic review of literature. Radiother Oncol. 2018 Jan;126(1):56–67. doi: 10.1016/j.radonc.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Mehta S, Kent Y, McPartlin A, Garcez K, Sykes A et al. Feeding tube dependency at 12 months post treatment in oropharyngeal cancer patient treated with radical intent- a single institute experience. Eur J Surg Oncol. 2018; Volume 44(Supplement 1): S45-S46.
- 15.Roe J.W., Drinnan M.J., Carding P.N., Harrington K.J., Nutting C.M. Patient-reported outcomes following parotid-sparing intensity-modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncol. 2014 Dec;50(12):1182–1187. doi: 10.1016/j.oraloncology.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Langendijk J.A., Lambin P., De Ruysscher D., Widder J., Bos M., et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013 Jun;107(3):267–273. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Rwigema JC, Langendijk JA, van der Laan HP, Lukens JN, Swisher-McClure SD, et al. Proton Therapy Improves Toxicity for Oropharyngeal Cancer: An Outcomes and Predictive Model-based Approach, International Journal of Radiation Oncology*Biology*Physics, Volume 99, Issue 2, Supplement, 2017, Pages S135-S136. [DOI] [PubMed]
- 18.Sharma S., Zhou O., Thompson R.F., Lukens J.N., Lin A., et al. A Comparative Study of Patient-Reported Xerostomia and Dysgeusia in Oropharyngeal Squamous Cell Carcinoma (OPSCC) Treated With Volumetric Modulated Arc Therapy (VMAT) or Proton Pencil Beam Scanning (PBS) International Journal of Radiation Oncology*Biology*Physics, Volume 93, Issue. 2015;3(Supplement):S69–S70. [Google Scholar]
- 19.Sharma S, Zhou O, Thompson RF, Lukens JN, Lin A, et al. Correlation of Dosimetric Parameters to Patient-Reported Taste Changes After Radiation for Oropharyngeal Squamous Cell Carcinoma, International Journal of Radiation Oncology*Biology*Physics, Volume 96, Issue 2, Supplement, 2016, Page E354.
- 20.Nutting C, Rooney K, Foran B, Pettit L, Beasley M, et al. Results of a randomized phase III study of dysphagia-optimized intensity modulated radiotherapy (Do-IMRT) versus standard IMRT (S-IMRT) in head and neck cancer. Journal of Clinical Oncology 2020 38:15_suppl, 6508-6508.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.