Abstract
The clinical use of anti-EGFR antibody-based cancer therapy has been limited by antibody-EGFR binding in normal tissues, so developing pH-dependent anti-EGFR antibodies that selectively bind with EGFR in tumors—by taking advantage of the acidity of tumor microenvironment relative to normal tissues—may overcome these limitations. Here, we generated pH-dependent anti-EGFR antibodies with cross-species reactivity for human and mouse EGFR, and we demonstrate that pH-dependent antibodies exhibit tumor-selective binding by binding strongly to EGFR under acidic conditions (pH 6.5) but binding weakly under neutral (pH 7.4) conditions. Based on screening a non-immune human antibody library and antibody affinity maturation, we initially generated antibodies with cross-species reactivity for human and mouse EGFR. A structure model was subsequently constructed and interrogated for hotspots affecting pH-dependent binding, which supported development of a cross-reactive pH-dependent anti-EGFR antibody, G532. Compared with its non-pH-dependent antibody variant, G532 exhibits improved tumor selectivity, tumor penetration, and antitumor activity. Thus, beyond showing that pH-dependent anti-EGFR antibodies can overcome multiple limitations with antibody-based cancer therapies targeting EGFR, our study illustrates a structure-guided antibody-antigen binding pH-dependency engineering strategy to enhance antibody tumor selectivity and tumor penetration, which can inform the future development of antibody-based cancer therapies targeting other ubiquitously expressed molecules.
Keywords: EGFR antibody, pH-dependent, cross-reactivity, tumor selectivity, tumor penetration
Graphical abstract

EGFR expression in normal tissues hinders the utility of anti-EGFR antibodies. Here, we developed an anti-EGFR antibody with pH-dependent tumor selectivity. The pH-dependent anti-EGFR antibody exhibits improved tumor selectivity, tumor penetration, and antitumor activity over its non-pH-dependent variant. Our study thus illustrates a strategy to enhance antibody tumor selectivity and tumor penetration.
Introduction
The human epidermal growth factor receptor (hEGFR) is a transmembrane glycoprotein with an amino-terminal 621 amino acid (Leu25-Ser645) extracellular region (ECR) comprising four domains (I–IV).1,2 EGFR can be activated by at least seven different growth factors (EGF, transforming growth factor-a [TGFa], betacellulin [BTC], heparin binding EGF-like growth factor [HB-EGF], epiregulin [EREG], epigen [EPGN], and amphiregulin [AREG])—with varying receptor-binding affinities—to regulate cell proliferation and differentiation.3,4 EGF is a high-affinity ligand of EGFR: it binds with EGFR domains I and III to stabilize EGFR dimerization, which promotes activation of receptor tyrosine kinases.2,4 EGFR plays a causal role in the development and maintenance of many types of cancer and is a well-validated oncology target.5,6 Therapeutic anti-EGFR antibodies, including Cetuximab, Panitumumab, and Necitumumab, which bind to domain III of human EGFR, are in use for the clinical treatment of solid tumors including colorectal cancer and non-small-cell lung cancer.7,8,9,10,11 In addition, many investigations based on anti-EGFR antibodies (and related antibody-based therapies) for cancer therapy are ongoing.12,13,14,15
Four mechanisms of action (MOA) have been reported to contribute to the antitumor activity of anti-EGFR antibodies: inhibiting ligand binding to block oncogenic signaling; triggering EGFR internalization and degradation to down-regulate oncogenic signaling; antibody-dependent cell-mediated cytotoxicity; and indirectly activating tumor-reactive T cells for tumor regression by increasing dendritic cell (DC) cross-presentation.16,17,18,19,20,21,22 However, given that EGFR is expressed in normal tissues of diverse origins (e.g., epithelial, mesenchymal, and neuronal), and considering that EGFR is known to affect normal cellular processes including proliferation, differentiation, and development,23,24 there are problems with on-target/off-tumor toxicity that have limited the clinical utility of anti-EGFR antibodies.25,26,27,28 Thus, obtaining tumor-selective anti-EGFR antibodies should substantially improve the efficacy of these therapies in cancer treatment.
Generally, three strategies are used to acquire therapeutic antibodies with tumor selectivity: modulating antibody binding affinity, developing antibodies targeting tumor-specific antigens, or developing antibodies that selectively bind with their targets in the tumor microenvironment.29,30,31,32,33 Antibodies with high affinity would recognize tumors with lower target expression as well as normal tissues, so developing antibodies with intermediate affinity can enhance antibody selectivity toward tumors with highly up-regulated antigens.32,33 Nimotuzumab is an anti-EGFR antibody with ∼10-fold lower binding affinity than Cetuximab.34 In contrast to Cetuximab’s binding to normal cells with low EGFR expression levels, Nimotuzumab selectively binds with tumor cells with high EGFR levels, so it follows that Nimotuzumab treatment exhibits relatively low on-target/off-tumor toxicity and is suitable for patients with high EGFR expression or gene amplification.34,35
EGFRvIII is a common ECR truncation mutant of EGFR found with glioblastoma multiforme (GBM), head and neck cancer, and breast cancer; it has an in-frame deletion of 267 amino acids from EGFR ECR domains I and II.36 EGFRvIII is tumor specific, and there is no evidence that it occurs in normal tissues.36 Illustrating the identification of a tumor-selective anti-EGFR antibody (DH8.3) by targeting tumor-specific antigens, an antibody with higher binding affinity for EGFRvIII than wild-type (WT) EGFR showed tumor selectivity in glioblastoma expressing EGFRvIII29,37; note that antibody-specific targeting EGFRvIII cannot exhibit tumor selectivity in cancer types expressing WT EGFR but not EGFRvIII (e.g., colorectal cancer and pancreatic cancer).36 Another strategy to acquire tumor-selective antibodies frequently takes advantage of the acidic extracellular pH in the tumor microenvironment resulting from the Warburg effect,38,39,40,41 and investigators have successfully developed tumor-selective pH-dependent antibodies that bind strongly to their antigens under acidic conditions in solid tumors (pH of solid tumors ranges 6.5 and 6.9, and can be as low as 6.0) but bind weakly (or not at all) under neutral (pH 7.2–7.5) conditions in normal tissues.30,42
Mouse tumor models are widely used during the preclinical development of antitumor therapies.43 While many anti-EGFR antibodies can elicit potent antitumor activity in preclinical mouse tumor models, most therapeutic anti-EGFR antibodies can recognize human EGFR but not mouse EGFR (mEGFR); thus, the on-target/off-tumor effects and other problems resulting from antibody binding with EGFR in normal tissues cannot be predicted based on experimental programs using mouse tumor models. Humanized mice provide a solution for evaluating the performance of therapeutic antibodies in vivo.44,45 However, EGFR humanized mice are difficult to obtain, owing to the essential function of mouse EGFR binding with its diverse ligands, and EGFR deficiency is known to lead to multiple abnormalities in mice.6,46 Thus, developing anti-EGFR antibodies with adequate cross-reactive binding with hEGFR and mEGFR should greatly aid evaluations (e.g., pharmacokinetics, safety, and therapeutic efficacy) of anti-EGFR antibodies in mouse tumor models.
Here, we aimed to develop pH-dependent anti-EGFR antibodies to overcome limitations with anti-EGFR antibodies owing to EGFR expression in normal tissues. We initially generated antibodies with cross-species reactivity for human and mouse EGFR by screening a non-immune human antibody library and through antibody affinity maturation. We then generated a structure model of an anti-EGFR antibody in complex with EGFR, and interrogated it for residues affecting pH-dependent binding, which supported development of a cross-reactive pH-dependent anti-EGFR antibody, G532, as well as a non-pH-dependent variant of G532 (G532Ctrl). We also experimentally confirmed that the pH-dependent binding of G532 improves its tumor selectivity, tumor penetration, and antitumor activity over G532Ctrl. Thus, our study shows how use of pH-dependent antibodies for cancer therapies targeting EGFR can improve antibody tumor selectivity and tumor penetration. Our study also practically demonstrates a structure-guided antibody-antigen binding pH-dependency engineering strategy that can be applied for developing pH-dependent antibodies targeting other tumor-associated antigens that are expressed in normal tissues.
Results
Selection of a pH-dependent anti-EGFR antibody interacting with EGFR H370 and H433 from a non-immune human antibody library
To identify pH-dependent human antibodies against EGFR, we produced a recombinant protein containing the extracellular region (ECR) of hEGFR (residues Leu25-Lys642) and used it to select pH-dependent antibodies from our non-immune phage display human scFv antibody library (library size: 1.1 × 1010).47 To screen for pH-dependent antibodies, the non-immune phage display library was incubated with the hEGFR ECR in a pH 6.0 solution, washed with pH 6.0 washing buffer, and then eluted using pH 7.4 buffer (Figure S1). Single clones were then randomly picked and screened for binding to the hEGFR ECR at pH 6.0 and pH 7.4 using ELISA. A total of 31 phage-scFv antibodies with unique sequences were thusly selected, and both their binding affinity and pH-dependency were evaluated (Figure S2, Table S1), antibodies with poor binding pH-dependency (e.g., 11C04) or poor binding affinity at pH 6.0 (e.g., 11C05) were excluded from further evaluation. At last, 14C07 and 11A10 were selected for their relatively high binding affinity and pH-dependency, and both were converted into the full-length human immunoglobulin (Ig)G1 format (Figure S2).
We next compared the binding affinity of 14C07, 11A10, and the Food and Drug Administration (FDA)-approved therapeutic anti-EGFR mAb Cetuximab for hEGFR at pH 6.0 and pH 7.4, and found that both 14C07 and 11A10 exhibited potent pH-dependent binding, with dramatically higher binding affinity at pH 6.0 than at pH 7.4 (Figure 1A). In contrast, the binding affinity of Cetuximab was quite similar at the two pH values (Figure 1A). We then compared the ligand-blocking ability of 14C07, 11A10, and Cetuximab in assays with EGFR and one of its ligands (EGF) at pH 6.0 with ELISA. Both 14C07 and Cetuximab could block EGFR-EGF binding at pH 6.0, doing so in a dose-dependent manner (Figure 1B). In contrast, 11A10 did not block EGFR-EGF binding (Figure 1B). Given that binding affinity of 14C07 and Cetuximab is comparable at pH 6.0 (assessed with ELISA), the dramatic difference detected for Cetuximab and 14C07 in blocking EGF-EGFR binding strongly suggests that Cetuximab and 14C07 do not have identical binding epitopes (Figures 1A and 1B). Considering that inhibition of ligand binding to therapeutically block an oncogenic signal is one of the known MOAs with the anti-EGFR antibodies used in clinical treatment,6 we chose 14C07 as the lead antibody for further investigation.
Figure 1.
Characterization of pH-dependent anti-human EGFR antibodies selected from a phage display non-immune human scFv library
(A) Cetuximab, 11A10, and 14C07 binding with hEGFR ECR at pH 6.0, and pH 7.4, analyzed with ELISA. Different antibodies in human IgG1 form at the indicated concentrations were added to 96-well plates coated with hEGFR ECR, followed by extensive washing. Then antibody-hEGFR binding was detected with an HRP-conjugated anti-hFc secondary antibody. The EC50 values are shown. (B) Ligand-binding blocking activity of Cetuximab, 11A10, and 14C07 for EGF binding to hEGFR at pH 6.0, analyzed with ELISA. EGF-mFc was mixed with different antibodies in human IgG1 form at the indicated concentrations, and added to 96-well plates coated with hEGFR ECR, followed by extensive washing. Then EGF-hEGFR binding was measured using an HRP-conjugated anti-mFc secondary antibody. The IC50 values are shown. n.m., not measurable. (C) Cetuximab and 14C07 (in human IgG1 form) binding with WT hEGFR ECR, and its two variants containing H370A or H433A mutation at pH 6.5 and pH 7.4. The binding assay was performed similarly as in Figure 1A. The EC50 values and binding pH-dependency (EC50 ratio [pH 7.4/pH 6.5]) are shown in the table at the right. n.m., not measurable. (D) Binding cross-reactivity of 14C07 and G5V2 to human and mouse EGFR at pH 6.5 and pH 7.4. 14C07 and G5V2 (in human IgG1 form) at the indicated concentrations were added to 96-well plates coated with hEGFR ECR or mEGFR ECR. Then antibody binding with hEGFR or mEGFR was measured with an HRP-conjugated anti-hFc secondary antibody. The EC50 values and binding pH-dependency (EC50 ratio [pH 7.4/pH 6.5]) are shown in the table at the right.
Histidine (H) has been repeatedly demonstrated to contribute to the binding between pH-dependent antibodies and their antigens.30,48 A previously reported crystal structure of EGFR/EGF complex revealed that two histidines (H370 and H433) in EGFR’s domain III (residues Lys335-Lys538) participate in the EGFR-EGF interaction.2 This motivated our speculation that EGFR H370 and H433 may participate in EGFR-14C07 binding. Pursuing this, we produced two hEGFR ECR variants containing H370A or H433A mutations, and assayed the binding of both Cetuximab and 14C07 with WT hEGFR ECR and the mutant variants at both pH 6.5 and pH 7.4. 14C07 binding with EGFR was greatly impaired by both H370A and H433A mutations, whereas Cetuximab binding with EGFR was impaired by the H433A mutation but not the H370A mutation (Figure 1C). Also note that the pH-dependency of binding between 14C07 and EGFR ECR H370A was more than 2-fold weaker than the pH-dependency between 14C07 and WT EGFR ECR (Figure 1C). These findings provide four insights into 14C07’s epitope on the hEGFR ECR: (1) The epitopes of 14C07 and Cetuximab are not identical; (2) 14C07 can block EGF-EGFR binding, and residues H370 and H433 in EGFR’s domain III participate in EGFR-14C07 binding, indicating that the binding epitope of 14C07 is located on EGFR domain III; (3) EGFR H370 affects both the binding affinity and the pH-dependency of 14C07-EGFR binding; and (4) H433A abolishes 14C07-EGFR binding, indicating that H433 may exert a stronger influence on 14C07-EGFR pH-dependent binding than H370. Considering H370 and H433 residues are conserved between hEGFR and mEGFR, we speculated that 14C07 may exhibit cross-reactive, pH-dependent binding to both hEGFR and mEGFR. Assessing 14C07 binding with hEGFR and mEGFR showed that 14C07 bound with both hEGFR and mEGFR, consistently doing so in a dose-dependent and pH-dependent manner (Figure 1D). Distinct from 14C07, our analysis showed that Cetuximab elicits only weak cross-reactive binding with mEGFR (Figure S3).
Antibody affinity maturation of 14C07 generates an anti-EGFR antibody with cross-reactivity with both human and mouse EGFR
To generate pH-dependent anti-EGFR antibodies with higher binding affinity than 14C07, we first used point mutation scanning to select permissive amino acid sites in six CDRs of 14C07 (Figure S4), after which we constructed sub-libraries of 14C07 containing random mutations of these selected permissive amino acid sites. Selection of 14C07 sub-libraries was then performed based on improved binding affinity over 14C07 at pH 6.5 (Figure S5). However, we failed to generate antibodies with significantly improved binding affinity and pH-dependency over 14C07. But we serendipitously obtained an anti-EGFR antibody G5V2 with improved cross-reactivity (i.e., with improved binding to both human and mouse EGFR over 14C07): G5V2’s binding affinity for hEGFR and mEGFR at pH 6.5 (half maximal effective concentration [EC50] ratio, mEGFR/hEGFR = 1.233) is much closer than 14C07’s binding affinity for hEGFR and mEGFR at pH 6.5 (EC50 ratio, mEGFR/hEGFR = 8.729) (Figure 1D, Table S2). The difference in affinity between the pH 7.4 and pH 6.5 conditions was much less pronounced for G5V2 as compared with 14C07 (Figure 1D). Thus, adopting a traditional antibody engineering strategy—screening libraries containing CDR random mutations based on alanine scanning—failed to generate an antibody with adequate binding affinity and binding pH-dependency. We therefore adopted a rational approach for the further development of the highly cross-reactive G5V2.
A G5V2-hEGFR structure model enables identification of a hotspot increasing the pH-dependency of G5V2-hEGFR binding
To support the rational engineering of G5V2, we initially adopted a structural approach and sought candidate hotspots affecting pH-dependent binding between G5V2 and EGFR. We generated a G5V2 scFv structure model using ABodyBuilder.49 We then manually docked a complex structure model of G5V2-hEGFR (Figure 2A) with the help of a previously reported crystal structure of a Cetuximab/EGFR complex (PDB: 1YY9),2,50 as guided by the following four considerations: (1) previous results suggesting the binding epitope of the parental antibody of G5V2 (14C07) is located on EGFR domain III (Figures 1B and 1C) and that both G5V2 and Cetuximab exhibit comparable binding affinity with EGFR domain III and the EGFR ECR (Figure S6), suggesting that the binding epitope of G5V2 and Cetuximab is located on EGFR domain III; (2) 14C07 can block EGFR-EGF binding, indicating that its variant G5V2 interacts with EGFR at the EGFR-EGF binding interface; (3) 14C07-EGFR binding was abolished by the H433A mutation and was impaired by the H370A mutation (Figure 1C), indicating that both H433 and H370 may be positioned at the binding interface between hEGFR and G5V2 (a 14C07 variant); (4) G5V2 exhibits comparable binding affinity with human and mouse EGFR, indicating that the G5V2-EGFR binding interface conserves highly similar or identical residues between mouse and human EGFR (Figure 2B).
Figure 2.
A G5V2-hEGFR structure model reveals a hotspot determining the pH-dependency of G5V2-hEGFR binding
(A) A complex structure model of G5V2-hEGFR. A G5V2 scFv structure model was first generated with ABodyBuilder. Then a complex structure model of G5V2-hEGFR was manually docked using PyMOL. Carbon atoms are colored in cyan for the VL of G5V2, green for the VH of G5V2, and gray for the hEGFR ECR. Ribbon representation of G5V2 scFv and hEGFR ECR in orthogonal views (left). A detailed view of the G5V2-hEGFR interface revealing the locations of G5V2 CDRs and hEGFR residues H370, H433, R377, L406, Q435, and K489 (right). (B) A detailed view of the G5V2-hEGFR complex model revealing the locations of amino acids on hEGFR domain III that are distinct from mouse EGFR (colored in orange). (C) G5V2 binding at pH 7.4 with the WT hEGFR ECR and its variants containing multiple mutations analyzed with ELISA. (D) The G5V2-hEGFR complex model indicated that the G5V2 LCDR1 Y32 residue is positioned in close proximity with hEGFR H433, and that the G5V2 LCDR2 D52D53 residues face hEGFR H370. (E) Binding activity of the phage display Fab form of WT G5V2, and its variants (G5V2 Y32E, G5V2 Y32D, G5V2 Y32H) to hEGFR ECR at pH 6.5 and pH 7.4. Multiple phage-Fab antibodies at the indicated concentrations were added to coated hEGFR ECR, followed by extensive washing. Then antibody-hEGFR binding was analyzed with an HRP-conjugated anti-M13 secondary antibody. The EC50 values, and binding pH-dependency (EC50 ratio [pH 7.4/pH 6.5]) are shown in the table. n.m., not measurable.
Supporting the reliability of the conformational epitope indicated by the G5V2-EGFR structure model, ELISA-based binding analysis showed that alanine substitution of five amino acids—positioned at the binding interface revealed by the G5V2-hEGFR complex structure model (R377A, L406A, H433A, Q435A, and K489A)—significantly reduced the binding of G5V2 to hEGFR (Figures 2A and 2C).
Previous studies have shown that protonation of ionizable residues like histidine can contribute to pH-dependent binding,30,42,51 so we hypothesized that increasing the extent of interactions between H and acidic amino acids (aspartate [D] or glutamate [E]) may promote pH-dependent binding between G5V2 and EGFR. ELISA assessment of G5V2 binding with WT hEGFR ECR and the mutant variants containing H433A or H370A mutations showed that G5V2-hEGFR binding was impaired by the H433A mutation but not the H370A mutation (Figure 2C). Consistent with this result, examining the G5V2-hEGFR complex structure model indicated a close contact between G5V2 LCDR1 Y32 and hEGFR H433, yet relatively weak contact between G5V2 LCDR2 D52D53 and hEGFR H370 (Figure 2D). These results support the targeting of H433 for engineering of pH-dependency. Collectively, we speculate that introducing a new H-D/E interaction between hEGFR H433 and G5V2 LCDR1 Y32 might enhance the pH-dependency of G5V2.
We then generated three G5V2 variants containing LCDR1 Y32E, Y32D, or Y32H mutations in phage display Fab antibody form, and compared the binding of these antibodies with hEGFR ECR at pH 6.5 and pH 7.4. The Y32H mutation did not significantly affect binding pH-dependency or affinity (Figure 2E). In sharp contrast, the Y32E and Y32D mutations dramatically increased binding pH-dependency (Figure 2E). Thus, our result indicates that the close interaction of G5V2 LCDR1 Y32-EGFR H433 suggested by the G5V2-EGFR structure model is a hotspot determining G5V2-EGFR pH-dependent binding, and by introducing an H-D/E interaction between G5V2 LCDR1 Y32 and EGFR H433, we greatly increased the pH-dependency of G5V2-EGFR binding.
Generating a pH-dependent anti-EGFR antibody, G532, based on structure-guided engineering of G5V2
G5V2 Y32E was then selected for further engineering owing to its ∼13-fold higher pH-dependent binding over WT G5V2 and its ∼3-fold higher binding affinity over G5V2 Y32D (Figure 2E). Note that the Y32E mutation decreased the binding affinity of G5V2 at pH 6.5 by ∼8-fold (Figure 2E), suggesting that the antitumor activity of G5V2 Y32E is likely weaker than G5V2, owing to its lower binding affinity than G5V2. Given our goal of generating a pH-dependent anti-EGFR antibody with improved binding affinity while maintaining adequate pH-dependency to support selective binding in the acidic tumor microenvironment, we next focused on HCDR2, which our structural analysis indicated had no obvious impact on pH-dependency (Figure 2A). We then constructed and screened a sub-library of G5V2 Y32E HCDR2 random mutations (Figure S5). Ultimately, a candidate pH-dependent anti-EGFR antibody (G532) was selected from the sub-library based on its adequate binding affinity at pH 6.5 and its obvious pH-dependent binding with hEGFR ECR (EC50 ratio, pH 7.4/pH 6.5 = 8.083) (Figures 3A and 3B).
Figure 3.
Generation and characterization of a pH-dependent cross-reactive anti-EGFR antibody (G532) from structure-guided engineering of G5V2
(A) Schematic depicting the process of structure-guided engineering of G5V2. The schematic diagram illustrates relationships between indicated antibodies (left), and sequence alignment of HCDR2, LCDR1, and LCDR2 of the indicated antibodies (right) are shown. (B) G5V2, G532, G532V, and G532Ctrl (in human IgG1 form) binding with hEGFR ECR at pH 6.5 and pH 7.4. The binding assay was performed similarly as in Figure 1A. The EC50 values and binding pH-dependency (EC50 ratio [pH 7.4/pH 6.5]) are shown in the table at the right. (C and D) FACS analysis of G532 and G532Ctrl (in human IgG1 form) binding with A-431 cells (C), and CT26-hEGFR cells (D) at pH 6.5 and pH 7.4. The cells were incubated with the indicated antibodies prior to staining with FITC-conjugated anti-human IgG secondary antibody. Antibody binding to cells was then assessed based on the fluorescence intensity for FITC. Representative histograms showing antibody-cell binding at the antibody concentration of 0.823 nM (left); binding curves of median fluorescence intensity (MFI) versus antibody concentration (right) are shown. (E) Ligand-binding blocking activity of Control IgG, G532, and G532Ctrl (in human IgG1 form) for EGF binding to hEGFR at pH 6.5 and pH 7.4. The assay was performed similarly as in Figure 1B. The half maximal inhibitory concentration (IC50) values are shown in the table at the right. n.m., not measurable.
To assess the specific impact of H-D/E interactions on the observed pH-dependent binding, we disrupted two H-D/E interactions between G532 and EGFR (G532 LCDR1 E32 and EGFR H433; G532 LCDR2 D52D53 and EGFR H370) indicated by the G5V2-hEGFR complex structure model, and generated the G532 variant G532V, which had ∼5-fold lower binding pH-dependency with hEGFR ECR than G532 (Figures 3A and 3B). Notably, G532V retained weak binding pH-dependency (EC50 ratio, pH 7.4/pH 6.5 = 1.636) (Figure 3B). We then mutated amino acids positioned close to G532V LCDR1 H32 into amino acids with smaller sidechains, aiming to generate a G532 variant with abolished binding selectivity at pH6.5. Finally, introducing a LCDR1 K31N mutation into G532V generated a non-pH-dependent G532 variant, G532Ctrl, with no binding selectivity at pH 6.5 (EC50 ratio, pH 7.4/pH 6.5 = 0.759) (Figures 3A and 3B).
We next used SPR to assess binding affinity of G532, and G532Ctrl binding with hEGFR and with mEGFR. Despite that G532 and its variants exhibited weak binding affinity, G532 exhibited potent binding selectivity for both hEGFR and mEGFR at pH 6.5 than pH 7.4; in contrast, G532Ctrl exhibited similar binding affinity for both hEGFR and mEGFR at pH 6.5 and pH 7.4 (Table 1 and Figure S7). We then compared binding affinity and selectivity of G532, and G532Ctrl in fluorescence-activated cell sorting (FACS) with cell lines (A-431 cell line and a mouse colon carcinoma cell line expressing hEGFR [CT26-hEGFR] [Figure S8]) expressing full-length EGFR. G532 exhibited obvious pH-dependent binding with both A-431 and CT26-hEGFR cells; in contrast, G532Ctrl exhibited similar binding affinity for both tested cell lines at pH 6.5 and pH 7.4 (Figures 3C and 3D, Table S3). We further compared the ligand-blocking ability of G532 and G532Ctrl at pH 6.5 and pH 7.4 with ELISA: G532 could block EGFR-EGF binding at pH 6.5 but not at pH 7.4, in contrast, G532Ctrl could block EGFR-EGF binding at both pH 6.5 and pH 7.4 (Figure 3E). This result indicates that G532 exhibits pH-dependent ligand-blocking ability.
Table 1.
SPR analysis of the binding affinity and pH-dependency of G532 and G532Ctrl
| Human EGFR |
Mouse EGFR |
|||||
|---|---|---|---|---|---|---|
| KD (M) pH 6.5 |
KD (M) pH 7.4 |
KD ratio (pH 7.4/6.5) |
KD (M) pH 6.5 |
KD (M) pH 7.4 |
KD ratio (pH 7.4/6.5) |
|
| G532 | 2.94E-07 | 3.90E-06 | 13.262 | 5.47E-07 | 1.81E-06 | 3.309 |
| G532Ctrl | 4.06E-07 | 2.43E-07 | 0.599 | 1.27E-06 | 7.43E-07 | 0.586 |
The dissociation constant (KD) values were calculated with a steady-state affinity model using Biacore T200 evaluation software.
Collectively, our structure-guided engineering successfully generated G532, a pH-dependent anti-EGFR antibody with potent pH-dependent ligand-blocking ability. In addition, we generated a non-pH-dependent variant of G532Ctrl, which can be used for further characterization of the therapeutic utility of G532 in mice tumor models.
The binding pH-dependency of G532 promotes tumor penetration, tumor selectivity, and antitumor activity
Both G532 and its variant G532Ctrl exhibit strong binding cross-reactivity with human and mouse EGFR (Table 1 and Figure S7), thus enabling us to assess their binding selectivity between normal tissues and tumors in mouse models. We first monitored the distributions of G532 and G532Ctrl in mouse tumors. We treated CB-17 SCID mice bearing A-431 tumors with G532, G532Ctrl, or Control IgG; 48 h later, the A-431 tumors and mice livers were collected for immunofluorescence staining against human IgG to detect antibody accumulation. In repeated experiments, tumors from mice treated with G532 showed stronger, and more uniform human IgG fluorescence signals than did tumors from mice treated with G532Ctrl (Figures 4A–4C and S9), indicating that G532 achieves superior tumor penetration compared with G532Ctrl. We speculate that two factors may have contributed to this finding: (1) The higher binding affinity of G532Ctrl over G532 at pH 7.4 would suggest that a higher proportion of the G532Ctrl population would engage in binding to EGFR in normal tissues than G532. Subsequently, less G532Ctrl would be available to reach tumor sites than G532 after intraperitoneal injection. (2) Previous research has shown that the pH in tumors tends to decrease with increasing distance from capillaries.52 Thus, G532’s superior tumor penetration may relate to its increasing binding selectivity at acidic pH along with increasing distance from capillaries in tumors.
Figure 4.
Characterization of tumor selectivity and tumor penetration of G532 and G532Ctrl
(A) Immunofluorescence images of whole-tumor specimens showing penetration of Control IgG, G532, or G532Ctrl into A-431 tumors. CB17-SCID mice bearing A-431 tumors were treated with Control IgG, G532, or G532Ctrl (10 mg/kg, in human IgG1 form) by intraperitoneal injection, and then killed 48 h later to collect A-431 tumors. Frozen sections of A-431 tumors were prepared, and fixed in pH 6.5 4% PFA before staining with Alexa Fluor 633-conjugated anti-human IgG secondary antibody. The penetration of indicated antibodies was then assessed based on the fluorescence intensity of Alexa Fluor 633. Each image uses its individual scale bar. (B–D) The accumulation of G532 and G532Ctrl in A-431 tumors and mouse livers was analyzed by immunofluorescence staining. CB17-SCID mice bearing A-431 tumors were treated with Control IgG, G532, or G532Ctrl (10 mg/kg, in human IgG1 form) by intraperitoneal injection, and then killed 48 h later to collect mice livers and A-431 tumors. Frozen sections of mice livers and A-431 tumors were prepared, and fixed in pH 6.5 4% PFA before staining. The sections were then incubated with Rat anti-mouse CD31 prior to staining with FITC-conjugated anti-human IgG secondary antibody and Alexa Fluor 647-conjugated anti-Rat IgG secondary antibody. The Alexa Fluor 647 signal indicates tumor vasculature. Antibody enrichment was assessed based on the fluorescence intensity for FITC. (B) Representative immunofluorescence images (above), and their human IgG immunohistochemistry images (below) converted by Inform software. Scale bar, 100 μm. (C) Integrated density of FTIC in mouse livers, and A-431 tumors analyzed with ImageJ. (D) Integrated density ratio of FITC in A-431 tumors and related mouse livers. (C and D) Data are means ± SE; statistical significance was determined using two-tailed unpaired Student’s t tests.
When assessing human IgG signals in livers of mice treated with G532 or G532Ctrl, we found that the average intensity of the human IgG signal in the G532 group appeared to be much lower (∼9-fold) than the G532Ctrl group (Figures 4B, 4C, and S9). In addition, calculating the human IgG signal ratio between A-431 tumors and mice livers showed that all of the A-431 tumors in the G532 group had higher human IgG signals than mice livers (intensity ratio, tumor/liver >1); in contrast, all of the A-431 tumors in the G532Ctrl group had lower human IgG signals than mice livers (intensity ratio, tumor/liver <1); (Figure 4D). These results indicate that the pH-dependency of the G532 antibody increases its tumor selectivity relative to G532Ctrl.
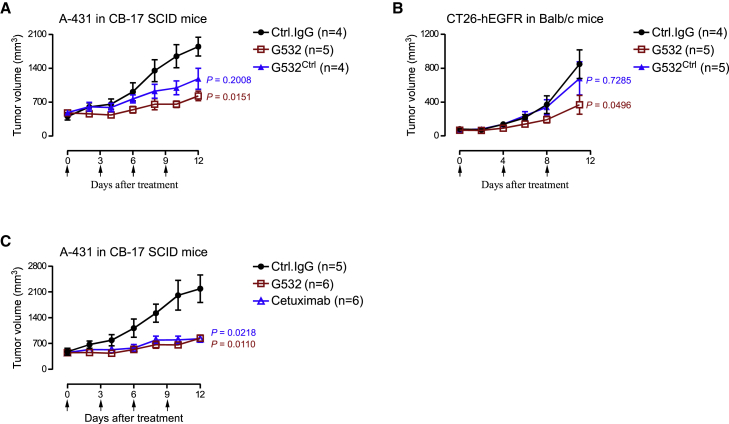
We next evaluated the antitumor activity of G532 and G532Ctrl in A-431 xenograft tumor models established with CB-17 SCID mice, as well as CT26-EGFR syngeneic tumor models established with Balb/c mice. In A-431 xenograft tumor models, both G532 and G532Ctrl inhibited tumor growth (Figure 5A). However, a significant difference was only observed between the Control IgG treatment group and the G532 treatment group (Figure 5A). In CT26-EGFR syngeneic tumor models, G532Ctrl failed to induce significant tumor growth inhibition; in contrast, G532 treatment significantly inhibited tumor growth (Figure 5B). These results indicate that G532’s superior tumor penetration increases its antitumor performance. Finally, we found that G532 elicited comparable antitumor activity with the FDA-approved therapeutic anti-EGFR mAb Cetuximab (an antibody that cannot recognize mouse EGFR) in A-431 xenograft models (Figure 5C), supporting that G532 is an anti-EGFR antibody with clinically relevant antitumor activity.
Figure 5.
Characterization of antitumor activity of G532
(A) Antitumor activity of G532 and G532Ctrl in A-431 tumor models. CB-17 SCID mice bearing A-431 tumors were randomized into three groups with similar tumor volumes: Control IgG, G532, or G532Ctrl (10 mg/kg, in human IgG1 form). (B) Antitumor activity of G532 and G532Ctrl in CT-26-hEGFR tumor models. Balb/c mice bearing CT-26-hEGFR tumors were randomized into three groups with similar tumor volumes: Control IgG, G532, or G532Ctrl (10 mg/kg, in human IgG1 form). (C) Antitumor activity of G532, and Cetuximab in A-431 tumor models. CB-17 SCID mice bearing A-431 tumors were randomized into three groups with similar tumor volumes: Control IgG, G532, or Cetuximab (10 mg/kg, in human IgG1 form). (A–C) Antibodies were tested at 10 mg/kg. The time points for antibody treatment are marked by arrows. Data are means ± SE; statistical significance was determined using two-way ANOVA.
Discussion
On-target/off-tumor binding-induced toxicity is a major obstacle limiting the utility of anti-EGFR antibody-based therapies in clinical cancer treatment. Developing pH-dependent anti-EGFR antibodies could enable selective antibody binding in the acidic tumor microenvironment. In the present study, using a combination of phage display library selection and structure-guided antibody engineering, we generated pH-dependent anti-EGFR antibodies with cross-reactivity between human and mouse EGFR. Subsequently, with characterization in immune-compromised and immune-competent mice models, we found that compared with the control antibody lacking binding selectivity at acidic pH, a pH-dependent anti-EGFR antibody elicited improved tumor selectivity, tumor penetration, and antitumor activity. Our study thus demonstrates that pH-dependent anti-EGFR antibodies can overcome on-target/off-tumor binding issues of anti-EGFR antibodies by eliciting pH-dependent tumor selectivity. Beyond that, our study indicates that developing pH-dependent antibodies apparently represents a general strategy for improving antibody penetration into tumors.
Previous studies have shown that protonation of ionizable residues like histidine can contribute to pH-dependent binding, and several strategies have been reported to introduce pH-dependency into antibody-antigen binding. Note that in former studies, the development of pH-dependent antibodies was either based on large-scale histidine scanning, or was highly dependent on histidine-rich epitopes on the antigen.30,42,51,53,54,55 Sulea et al. (2020) reported a structure-guided computational approach implemented with dual-pH histidine-scanning mutagenesis to engineer an anti-HER2 antibody into variants capable of selective binding with HER2 at acidic pH in in vitro experiments.42 Johnston et al. (2019) reported engineering an anti-VISTA antibody with selective binding in acidic environments by taking advantage of a histidine-rich epitope (over 10 histidines within amino acid residues 94–165 of VISTA ECR) of VISTA.30
In our study, we hypothesized that increasing the extent of interactions between H and acidic amino acids (D or E) may promote pH-dependent antibody-antigen binding. Integration of an antibody-antigen structure helped us to engineer our antibodies in two ways: (1) We identified a candidate hotspot (H433 in hEGFR domain III) determining binding pH-dependency, and introduced an H-D/E interaction at the hotspot, which did increase the binding pH-dependency of the antibody. (2) We engineered an antibody CDR that does not participate in antibody-antigen pH-dependent binding to improve the binding affinity and avoid disturbing binding pH-dependency. Ultimately, an antibody named G532 with adequate binding pH-dependency and binding affinity was generated with this two-pronged structure-guided engineering strategy. It bears mentioning that manual positioning of an antibody structure model near the antigen structure to construct an antibody-antigen structure cannot be assumed to yield reliably accurate epitope information for developing pH-dependent antibodies, and such efforts require personal experience. Where possible, solving the structure of an antibody-antigen complex can be reliably expected to give better guidance for pH-dependent antibody engineering.
Similar to previous reports about the development of pH-dependent antibodies, our study indicates that (1) even a single histidine at the antibody-antigen binding interface can contribute to pH-dependent binding; (2) specifically introducing H-D/E interactions between the antibody and antigen can efficiently adjust binding pH-dependency; and (3) beyond histidine, aspartate and glutamate residues on the antibody-antigen binding interface can be used to develop pH-dependent antibodies.
Binding pH-dependency, affinity, and cross-species reactivity are three assessed properties of our final anti-EGFR antibodies: Binding pH-dependency and affinity are two properties that were engineered for; the cross-species reactivity was obtained serendipitously. Our strategy is to engineer binding pH-dependency at first to select antibodies with greatly improved binding pH-dependency, then engineer binding affinity to improve the binding affinity of the pH-dependent antibodies at pH 6.5.
Investigators have reported attempts to develop tumor-selective anti-EGFR antibodies and related therapies by developing antibodies targeting tumor-specific EGFR. Antibodies, and antibody-drug conjugates (ADCs) with selective binding for tumor-specific EGFR mutations or overexpressed EGFR, have shown potent antitumor activity in tumor models constructed with immunodeficient mice.14,15,56,57,58,59 In the present study, we developed a pH-dependent anti-EGFR antibody (G532) with selective binding for EGFR in acidic tumor microenvironments. Distinct from previously reported tumor-selective anti-EGFR antibodies, binding of G532 with EGFR is not limited to certain EGFR mutations or EGFR overexpression. Thus, G532 is apparently a universally applicable tumor-selective anti-EGFR antibody for cancer patients.
Beyond tumor selectivity, we observed that G532 penetrated more readily into tumors than the non-pH-dependent variant. Poor tissue penetration is a major obstacle for successful usage of antibodies and antibody-based therapies60,61,62,63; one study showed that the antitumor activity of Panitumumab (an FDA-approved anti-EGFR antibody) is limited by its poor penetration into tumor tissue.64 Improving penetration has been demonstrated as useful for enhancing the antitumor activity of antibodies and antibody-based therapies,64,65,66 but this strategy can raise safety concerns related to increasing on-target/off-tumor binding in normal tissues. Notably, our study illustrates that developing pH-dependent antibodies can enable a strategy for improve tumor antibody penetration that takes advantage of the decreased pH gradient with increasing distance away from capillaries in tumors.
Considering the advantages of tumor selectivity and improved tumor penetration of G532 over traditional non-pH-dependent antibodies, developing antibody-based therapies (ADC or Chimeric antigen receptor [CAR] T cell) based on G532 seems like an attractive prospect. Such therapies could be reasonably expected to reduce safety concerns while also conferring excellent therapeutic effects. Finally, we know that many tumor-associated proteins are also expressed in normal tissues, and on-target/off-tumor binding and poor tumor penetration are major limitations for antibodies targeting these proteins.66,67 Thus, beyond developing an anti-EGFR antibody with pH-dependent tumor selectivity, our study provides insights and illustrates practical strategies for developing tumor-selective antibodies targeting other tumor-associated antigens expressed in normal tissues.
Materials and methods
Animals
All mouse experiments were conducted following the National Guidelines for Housing and Care of Laboratory Animals in China and were performed under the approved IACUC protocols at the National Institute of Biological Sciences, Beijing. Balb/c and CB-17 SCID mice were purchased from Charles River.
Cell lines
A-431 cells were provided by Dr. Zhiyuan Zhang (National Institute of Biological Sciences, Beijing); CT26 cells were from the Cell Bank of Type Culture Collection, Chinese Academy of Sciences. The CT26-hEGFR cell line (expressing human EGFR) was generated via transfection followed by G418 selection. A-431 and CT26 cells were cultured with RPMI 1640 medium supplemented with 10% fetal bovine serum. These cells were cultured at 37°C in a humidified incubator with a 5% CO2 atmosphere. The FreeStyle 293F cells were from Life Technologies and were cultured following the manufacturer’s instructions.
Expression and purification of proteins
The ECR of human and mouse EGFR were produced as His6-Avi-tagged fusion proteins by transient transfection of FreeStyle 293F cells and were purified by affinity chromatography. For full-length IgG antibodies, the coding sequences of the VH and VL were subcloned into a human IgG1 H chain (HC) expression vector and an L chain (LC) expression vector, respectively. The 293F cells were co-transfected with the two IgG expression plasmids (HC + LC plasmids) at a 1:1 ratio. After 3 to 6 days of transfection, the cell culture supernatants were collected for purification of IgG1 via protein A bead affinity chromatography.
Antibody library panning and screening of pH-dependent antibodies
hEGFR ECR was fused with an His6-Avi tag and biotinylated using BirA ligase. Then an hEGFR ECR fusion protein was used an antigen in the panning experiments with a non-immune phage display human scFv antibody library (Library size, 1.1 × 1010).47 For screening pH-dependent antibodies from the human non-immune antibody library, we incubated the library with antigens in a pH 6.0 solution, and washed with pH 6.0 washing buffer, followed by elution with pH 7.4 buffer (Figure S1). Then single clones were randomly picked and screened for binding to the corresponding antigen at pH 6.0 and pH 7.4 with ELISA. Similar strategies were used for screening of sub-libraries derived from 14C07 or G5V2, except that pH of acidic buffer was changed from pH 6.0 to pH 6.5 (Figure S6). Amino acid numbering and CDR definition were conducted based on Kabat, using the abYsis tool (http://www.abysis.org/abysis/).
ELISA-based binding assays
For the ELISA-based analysis of IgG antibody binding with the antigens, biotinylated protein antigens (1 μg/mL) were captured with streptavidin (3 μg/mL) (Sigma-Aldrich, Cat # 189730) coated 96-well plates (Thermo Fisher Scientific, Cat # 449824). Then, serially diluted antibodies were added, and detected by adding an horseradish peroxidase (HRP)-labeled goat polyclonal anti-Human IgG Fc (Thermo Fisher Scientific, Cat # 31413). For the ELISA-based analysis of IgG antibody competition with EGF, biotinylated EGFR protein antigens (1 μg/mL) were captured with streptavidin (3 μg/mL) (Sigma-Aldrich, Cat # 189730) coated 96-well plates (Thermo Fisher Scientific, Cat # 449824). Then serially diluted antibodies that had been mixed with EGF-mFc (50 nM at pH 6.0–6.5, 15 nM at pH 7.4) were added, followed by detection based on adding an HRP-conjugated anti-mouse Fc secondary antibody (Thermo Fisher Scientific, Cat # 31437).
For the ELISA-based analysis of phage-scFv or phage-Fab antibody binding with the antigens, biotinylated protein antigens (1 μg/mL) were captured with streptavidin (3 μg/mL) (Sigma-Aldrich, Cat # 189730) coated 96-well plates (Thermo Fisher Scientific, Cat # 449824). Then serially diluted antibodies were added, and detected by adding an HRP-conjugated anti-M13 antibody (GE Healthcare, Cat # 27-9421-01). For the ELISA-based analysis of antibody binding with EGFR and its variants, antibodies (5 μg/mL) were coated on 96-well plates (Thermo Fisher Scientific, Cat # 449824). Then serially diluted EGFR and its variants were added, followed by detection based on adding an HRP-labeled His-Tag antibody (Mei5Bio, Cat # MF082-HRP-01). A nonlinear regression model was used to fit ELISA curves; each curve was fitted independently.
Construction of a G5V2-hEGFR complex structure model
The Cetuximab/EGFR complex structure (PDB: 1YY9) and the G5V2 (in scFv form) structure model that we generated using ABodyBuider were imported in PyMOL. After hiding Cetuximab in the Cetuximab/EGFR complex, we moved and rotated G5V2 and hEGFR to form a model of G5V2-hEGFR complex using the 3-Button Editing mode, based on the following steps: (1) G5V2 exhibited comparable binding affinity with the hEGFR ECR and hEGFR domain III (Figure S6), indicating that the binding epitope of G5V2 is likely located on hEGFR domain III. Thus, we moved G5V2 to face hEGFR domain III. (2) The EGFR H433A mutation abolished 14C07-hEGFR binding, while the EGFR H370A mutation reduced 14C07-hEGFR binding (Figure 1C), indicating that the H433 and H370 residues are likely located on the binding interface between hEGFR and 14C07 (the parent antibody of G5V2). Thus, we presented EGFR H370 and H433 at the binding interface, ensuring that EGFR H433 is positioned closer to G5V2 than EGFR H370 (since EGFR H433 contributes more to the binding than EGFR H370 according to EGFR H433A mutation exhibiting stronger impact on decreasing binding affinity than EGFR H370A mutation [Figure 1C]). (3) G5V2 exhibits comparable binding affinity to human and mouse EGFR (Figure 1D), indicating that the binding epitope of G5V2 conserves the same or highly similar residues between human and mouse EGFR. Thus, we adjusted the position of G5V2 and hEGFR to avoid the presentation of non-conserved residues between human EGFR and murine EGFR to the greatest extent (Figure 2B).
Binding kinetic analysis by surface plasmon resonance
Kinetics analyses of the binding of anti-EGFR antibodies to the ECR of mouse and human EGFR were performed on a Biacore T200 instrument (Biacore, GE Healthcare). Anti-hFc Ab or protein A/G (Thermo Fisher Scientific) was covalently attached to the surface of a CM5 sensor chip using an amine coupling kit (GE Healthcare). Antibodies (1 μg/mL) were captured on the chip and the analytes were then injected at serially diluted concentrations (3200 nM, 1600 nM, 800 nM, 400 nM, 200 nM, 100 nM, 50 nM). Binding kinetics were evaluated using a 1:1 Langmuir binding model. The dissociation constant (KD) values were calculated with a steady-state affinity model using the Biacore T200 evaluation software.
Flow cytometry analysis of cell lines
For examining binding of antibodies with cells, cells were stained with G532, G532Ctrl, or Cetuximab followed by staining with a goat polyclonal anti-Human IgG Fc fluorescein isothiocyanate (FITC) (Thermo Fisher Scientific, Cat # A18830).
In vivo tumor assays
Six- to 8-week-old mice were inoculated subcutaneously with A-431 or CT26-hEGFR tumor cells (in 100 μL DPBS or medium). CB-17 SCID mice were inoculated with 5 × 106 A-431 cells, Balb/c mice were inoculated with 1 × 105 CT26-hEGFR cells. Tumor volume was measured with a digital caliper and calculated using the modified ellipsoid formula 1/2 × (length × width2). Based on similar mean tumor volumes, mice were randomized into groups (n = 4–6/group) and received intraperitoneal injection of the examined therapeutic antibodies. When the tumor reached 2 cm in length or when weakness was observed, the mice were killed.
Immunofluorescence assays
For detecting enrichment of antibodies in mice livers and A-431 tumors, CB-17 SCID mice bearing A-431 tumors were killed 48 h after injection with Control IgG, G532, or G532Ctrl. Then mice livers and tumors were harvested to prepare frozen sections. To detect antibody accumulation, sections were fixed with pre-cold pH 6.5 4% PFA buffer, then sections were stained with FITC-conjugated anti-human IgG secondary antibody or Alexa Fluor 633-conjugated anti-human IgG secondary antibody (Thermo Fisher Scientific, Cat # A-21091). To evaluate tumor vasculature, sections were fixed with pre-cold pH 6.5 4% PFA buffer, then sections were incubated with Rat anti-mouse CD31 (BD Pharmingen, Cat # 557355) prior to staining with Alexa Fluor 647-conjugated anti-Rat IgG secondary antibody (Thermo Fisher Scientific, Cat # A-21247). Antibody enrichment was assessed based on the fluorescence intensity for FITC (Thermo Fisher Scientific, Cat # A18830). Immunofluorescence intensities were analyzed with ImageJ.
Statistics
GraphPad Prism 6 was used for comparisons throughout the manuscript, with p values indicated in the relevant figures. Two-way ANOVA or two-tailed unpaired Student’s t tests were applied. Two-way ANOVA was applied for analyzing antitumor activity. p values <0.05 were regarded as statistically significant.
Data availability
The data that support the findings of this study are included within the article and its supplemental information. The raw and analyzed datasets generated during the study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank W. Chen, Z. Wang, F. Yang, and L. Zhao in the Sui lab for their technical assistance. We would also like to thank the NIBS Animal Facility for their help in the handling and care of mice, and thank the NIBS Biological Resource Center for DNA sequencing, and the NIBS imaging facility for assistance with the microscope experiment. This work was supported by grants from Ministry of Science and Technology of the People's Republic of China (973 Program #2012CB837600 to J.S.), Beijing Municipal Science and Technology Commission, and Beijing Key Laboratory of Pathogen Invasion and Immune Defense (Z171100002217064 to J.S.).
Author contributions
X.L., X.T., and J.S. conceptualized this study, interpreted the results, and drafted the manuscript. X.L., X.T., X.H., H.Z., K.W., Z.W., X.W., and Y.L. performed experiments. X.T. and X.L. prepared the figures. J.S. supervised the study. All authors commented on the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omto.2022.11.001.
Supplemental information
References
- 1.Ullrich A., Coussens L., Hayflick J.S., Dull T.J., Gray A., Tam A.W., Lee J., Yarden Y., Libermann T.A., Schlessinger J., et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 2.Ogiso H., Ishitani R., Nureki O., Fukai S., Yamanaka M., Kim J.H., Saito K., Sakamoto A., Inoue M., Shirouzu M., Yokoyama S. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 3.Harris R.C., Chung E., Coffey R.J. EGF receptor ligands. Exp. Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 4.Freed D.M., Bessman N.J., Kiyatkin A., Salazar-Cavazos E., Byrne P.O., Moore J.O., Valley C.C., Ferguson K.M., Leahy D.J., Lidke D.S., Lemmon M.A. EGFR ligands differentially stabilize receptor dimers to specify signaling kinetics. Cell. 2017;171:683–695.e18. doi: 10.1016/j.cell.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess A.W. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 6.Roskoski R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C., et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E., Peeters M., Siena S., Humblet Y., Hendlisz A., Neyns B., Canon J.L., Van Laethem J.L., Maurel J., Richardson G., et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 9.Jonker D.J., O'Callaghan C.J., Karapetis C.S., Zalcberg J.R., Tu D., Au H.J., Berry S.R., Krahn M., Price T., Simes R.J., et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 10.Thatcher N., Hirsch F.R., Luft A.V., Szczesna A., Ciuleanu T.E., Dediu M., Ramlau R., Galiulin R.K., Bálint B., Losonczy G., et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 11.Cai W.Q., Zeng L.S., Wang L.F., Wang Y.Y., Cheng J.T., Zhang Y., Han Z.W., Zhou Y., Huang S.L., Wang X.W., et al. The latest battles between EGFR monoclonal antibodies and resistant tumor cells. Front. Oncol. 2020;10:1249. doi: 10.3389/fonc.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplon H., Reichert J.M. Antibodies to watch in 2021. MAbs. 2021;13:1860476. doi: 10.1080/19420862.2020.1860476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dienstmann R., Patnaik A., Garcia-Carbonero R., Cervantes A., Benavent M., Roselló S., Tops B.B.J., van der Post R.S., Argilés G., Skartved N.J., et al. Safety and activity of the first-in-class Sym004 anti-EGFR antibody mixture in patients with refractory colorectal cancer. Cancer Discov. 2015;5:598–609. doi: 10.1158/2159-8290.CD-14-1432. [DOI] [PubMed] [Google Scholar]
- 14.Anderson M.G., Falls H.D., Mitten M.J., Oleksijew A., Vaidya K.S., Boghaert E.R., Gao W., Palma J.P., Cao D., Chia P.L., et al. Targeting multiple EGFR-expressing tumors with a highly potent tumor-selective antibody-drug conjugate. Mol. Cancer Ther. 2020;19:2117–2125. doi: 10.1158/1535-7163.MCT-20-0149. [DOI] [PubMed] [Google Scholar]
- 15.Reilly E.B., Phillips A.C., Buchanan F.G., Kingsbury G., Zhang Y., Meulbroek J.A., Cole T.B., DeVries P.J., Falls H.D., Beam C., et al. Characterization of ABT-806, a humanized tumor-specific anti-EGFR monoclonal antibody. Mol. Cancer Ther. 2015;14:1141–1151. doi: 10.1158/1535-7163.MCT-14-0820. [DOI] [PubMed] [Google Scholar]
- 16.Spangler J.B., Neil J.R., Abramovitch S., Yarden Y., White F.M., Lauffenburger D.A., Wittrup K.D. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc. Natl. Acad. Sci. USA. 2010;107:13252–13257. doi: 10.1073/pnas.0913476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Zhang X., Mortenson E.D., Radkevich-Brown O., Wang Y., Fu Y.X. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol. Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen M.W., Jacobsen H.J., Koefoed K., Hey A., Pyke C., Haurum J.S., Kragh M. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70:588–597. doi: 10.1158/0008-5472.CAN-09-1417. [DOI] [PubMed] [Google Scholar]
- 19.Lammerts van Bueren J.J., Bleeker W.K., Bøgh H.O., Houtkamp M., Schuurman J., van de Winkel J.G.J., Parren P.W.H.I. Effect of target dynamics on pharmacokinetics of a novel therapeutic antibody against the epidermal growth factor receptor: implications for the mechanisms of action. Cancer Res. 2006;66:7630–7638. doi: 10.1158/0008-5472.CAN-05-4010. [DOI] [PubMed] [Google Scholar]
- 20.Kimura H., Sakai K., Arao T., Shimoyama T., Tamura T., Nishio K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98:1275–1280. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincenzi B., Schiavon G., Silletta M., Santini D., Tonini G. The biological properties of cetuximab. Crit. Rev. Oncol. Hematol. 2008;68:93–106. doi: 10.1016/j.critrevonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava R.M., Lee S.C., Andrade Filho P.A., Lord C.A., Jie H.B., Davidson H.C., López-Albaitero A., Gibson S.P., Gooding W.E., Ferrone S., Ferris R.L. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin. Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano S., Kondo K., Yamaguchi M., Richmond G., Hutchison M., Wakeling A., Averbuch S., Wadsworth P. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res. 2003;23:3639–3650. [PubMed] [Google Scholar]
- 24.Olayioye M.A., Neve R.M., Lane H.A., Hynes N.E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baselga J., Swain S.M. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 26.Hansel T.T., Kropshofer H., Singer T., Mitchell J.A., George A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 27.Shitara K., Yokota T., Utsunomiya S. Cetuximab for patients with colon cancer and hepatic metastasis complicated by liver dysfunction and icterus. Gastrointest. Cancer Res. 2009;3:171–172. [PMC free article] [PubMed] [Google Scholar]
- 28.Achermann Y., Frauenfelder T., Obrist S., Zaugg K., Corti N., Günthard H.F. A rare but severe pulmonary side effect of cetuximab in two patients. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-03-2012-5973. bcr0320125973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jungbluth A.A., Stockert E., Huang H.J.S., Collins V.P., Coplan K., Iversen K., Kolb D., Johns T.J., Scott A.M., Gullick W.J., et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA. 2003;100:639–644. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston R.J., Su L.J., Pinckney J., Critton D., Boyer E., Krishnakumar A., Corbett M., Rankin A.L., Dibella R., Campbell L., et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature. 2019;574:565–570. doi: 10.1038/s41586-019-1674-5. [DOI] [PubMed] [Google Scholar]
- 31.Scott A.M., Wolchok J.D., Old L.J. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 32.Drent E., Themeli M., Poels R., de Jong-Korlaar R., Yuan H., de Bruijn J., Martens A.C.M., Zweegman S., van de Donk N.W.C.J., Groen R.W.J., et al. A rational strategy for reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Mol. Ther. 2017;25:1946–1958. doi: 10.1016/j.ymthe.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazor Y., Sachsenmeier K.F., Yang C., Hansen A., Filderman J., Mulgrew K., Wu H., Dall'Acqua W.F. Enhanced tumor-targeting selectivity by modulating bispecific antibody binding affinity and format valence. Sci. Rep. 2017;7:40098. doi: 10.1038/srep40098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crombet Ramos T., Mestre Fernández B., Mazorra Herrera Z., Iznaga Escobar N.E. Nimotuzumab for patients with inoperable cancer of the head and neck. Front. Oncol. 2020;10:817. doi: 10.3389/fonc.2020.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrido G., Tikhomirov I.A., Rabasa A., Yang E., Gracia E., Iznaga N., Fernández L.E., Crombet T., Kerbel R.S., Pérez R. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol. Ther. 2011;11:373–382. doi: 10.4161/cbt.11.4.14097. [DOI] [PubMed] [Google Scholar]
- 36.Gan H.K., Burgess A.W., Clayton A.H.A., Scott A.M. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–2930. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 37.Hills D., Rowlinson-Busza G., Gullick W.J. Specific targeting of a mutant, activated FGF receptor found in glioblastoma using a monoclonal antibody. Int. J. Cancer. 1995;63:537–543. doi: 10.1002/ijc.2910630414. [DOI] [PubMed] [Google Scholar]
- 38.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 40.Corbet C., Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat. Rev. Cancer. 2017;17:577–593. doi: 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 41.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 42.Sulea T., Rohani N., Baardsnes J., Corbeil C.R., Deprez C., Cepero-Donates Y., Robert A., Schrag J.D., Parat M., Duchesne M., et al. Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment. MAbs. 2020;12:1682866. doi: 10.1080/19420862.2019.1682866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerin M.V., Finisguerra V., Van den Eynde B.J., Bercovici N., Trautmann A. Preclinical murine tumor models: a structural and functional perspective. Elife. 2020;9:e50740. doi: 10.7554/eLife.50740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shultz L.D., Ishikawa F., Greiner D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 45.Du X., Liu M., Su J., Zhang P., Tang F., Ye P., Devenport M., Wang X., Zhang Y., Liu Y., Zheng P. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res. 2018;28:433–447. doi: 10.1038/s41422-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Threadgill D.W., Dlugosz A.A., Hansen L.A., Tennenbaum T., Lichti U., Yee D., LaMantia C., Mourton T., Herrup K., Harris R.C., et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 47.Li D., He W., Liu X., Zheng S., Qi Y., Li H., Mao F., Liu J., Sun Y., Pan L., et al. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. Elife. 2017;6:26738. doi: 10.7554/eLife.26738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang J.C., Sun W., Khare P., Karimi M., Wang X., Shen Y., Ober R.J., Ward E.S. Engineering a HER2-specific antibody-drug conjugate to increase lysosomal delivery and therapeutic efficacy. Nat. Biotechnol. 2019;37:523–526. doi: 10.1038/s41587-019-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leem J., Dunbar J., Georges G., Shi J., Deane C.M. ABodyBuilder: automated antibody structure prediction with data-driven accuracy estimation. MAbs. 2016;8:1259–1268. doi: 10.1080/19420862.2016.1205773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S., Schmitz K.R., Jeffrey P.D., Wiltzius J.J.W., Kussie P., Ferguson K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Chaparro-Riggers J., Liang H., DeVay R.M., Bai L., Sutton J.E., Chen W., Geng T., Lindquist K., Casas M.G., Boustany L.M., et al. Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9. J. Biol. Chem. 2012;287:11090–11097. doi: 10.1074/jbc.M111.319764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helmlinger G., Yuan F., Dellian M., Jain R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 53.Sarkar C.A., Lowenhaupt K., Horan T., Boone T.C., Tidor B., Lauffenburger D.A. Rational cytokine design for increased lifetime and enhanced potency using pH-activated "histidine switching". Nat. Biotechnol. 2002;20:908–913. doi: 10.1038/nbt725. [DOI] [PubMed] [Google Scholar]
- 54.Igawa T., Ishii S., Tachibana T., Maeda A., Higuchi Y., Shimaoka S., Moriyama C., Watanabe T., Takubo R., Doi Y., et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 2010;28:1203–1207. doi: 10.1038/nbt.1691. [DOI] [PubMed] [Google Scholar]
- 55.Wei W., Corbeil C.R., Gaudreault F., Deprez C., Purisima E.O., Sulea T. Antibody mutations favouring pH-dependent binding in solid tumour microenvironments: insights from large-scale structure-based calculations. Proteins. 2022;90:1538–1546. doi: 10.1002/prot.26340. [DOI] [PubMed] [Google Scholar]
- 56.Garrett T.P.J., Burgess A.W., Gan H.K., Luwor R.B., Cartwright G., Walker F., Orchard S.G., Clayton A.H.A., Nice E.C., Rothacker J., et al. Antibodies specifically targeting a locally misfolded region of tumor associated EGFR. Proc. Natl. Acad. Sci. USA. 2009;106:5082–5087. doi: 10.1073/pnas.0811559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips A.C., Boghaert E.R., Vaidya K.S., Mitten M.J., Norvell S., Falls H.D., DeVries P.J., Cheng D., Meulbroek J.A., Buchanan F.G., et al. ABT-414, an antibody-drug conjugate targeting a tumor-selective EGFR epitope. Mol. Cancer Ther. 2016;15:661–669. doi: 10.1158/1535-7163.MCT-15-0901. [DOI] [PubMed] [Google Scholar]
- 58.Phillips A.C., Boghaert E.R., Vaidya K.S., Falls H.D., Mitten M.J., DeVries P.J., Benatuil L., Hsieh C.M., Meulbroek J.A., Panchal S.C., et al. Characterization of ABBV-221, a tumor-selective EGFR-targeting antibody drug conjugate. Mol. Cancer Ther. 2018;17:795–805. doi: 10.1158/1535-7163.MCT-17-0710. [DOI] [PubMed] [Google Scholar]
- 59.Hamblett K.J., Kozlosky C.J., Siu S., Chang W.S., Liu H., Foltz I.N., Trueblood E.S., Meininger D., Arora T., Twomey B., et al. AMG 595, an anti-EGFRvIII antibody-drug conjugate, induces potent antitumor activity against EGFRvIII-expressing glioblastoma. Mol. Cancer Ther. 2015;14:1614–1624. doi: 10.1158/1535-7163.MCT-14-1078. [DOI] [PubMed] [Google Scholar]
- 60.Ackerman M.E., Pawlowski D., Wittrup K.D. Effect of antigen turnover rate and expression level on antibody penetration into tumor spheroids. Mol. Cancer Ther. 2008;7:2233–2240. doi: 10.1158/1535-7163.MCT-08-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurber G.M., Schmidt M.M., Wittrup K.D. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu G., Fakurnejad S., Martin B.A., van den Berg N.S., van Keulen S., Nishio N., Zhu A.J., Chirita S.U., Zhou Q., Gao R.W., et al. Predicting therapeutic antibody delivery into human head and neck cancers. Clin. Cancer Res. 2020;26:2582–2594. doi: 10.1158/1078-0432.CCR-19-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y., Li Q., Kong Y., Wang Z., Lei C., Li J., Ding L., Wang C., Cheng Y., Wei Y., et al. A highly stable human single-domain antibody-drug conjugate exhibits superior penetration and treatment of solid tumors. Mol. Ther. 2022;30:2785–2799. doi: 10.1016/j.ymthe.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freeman D.J., McDorman K., Ogbagabriel S., Kozlosky C., Yang B.B., Doshi S., Perez-Ruxio J.J., Fanslow W., Starnes C., Radinsky R. Tumor penetration and epidermal growth factor receptor saturation by panitumumab correlate with antitumor activity in a preclinical model of human cancer. Mol. Cancer. 2012;11:47. doi: 10.1186/1476-4598-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ponte J.F., Lanieri L., Khera E., Laleau R., Ab O., Espelin C., Kohli N., Matin B., Setiady Y., Miller M.L., et al. Antibody Co-administration can improve systemic and local distribution of antibody-drug conjugates to increase in vivo efficacy. Mol. Cancer Ther. 2021;20:203–212. doi: 10.1158/1535-7163.MCT-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cilliers C., Menezes B., Nessler I., Linderman J., Thurber G.M. Improved tumor penetration and single-cell targeting of antibody-drug conjugates increases anticancer efficacy and host survival. Cancer Res. 2018;78:758–768. doi: 10.1158/0008-5472.CAN-17-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu G., Nishio N., van den Berg N.S., Martin B.A., Fakurnejad S., van Keulen S., Colevas A.D., Thurber G.M., Rosenthal E.L. Co-administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat. Commun. 2020;11:5667. doi: 10.1038/s41467-020-19498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are included within the article and its supplemental information. The raw and analyzed datasets generated during the study are available from the corresponding author upon reasonable request.