Abstract
The lipid A portion of bacterial lipopolysaccharide (LPS) plays a central role in the production of endotoxic mediators. Different responses between human and murine macrophages to lipid A-like structures have been indicated. We investigated a series of structurally related monosaccharide lipid A analogues for their potency to activate human macrophage U937 cells and peripheral blood mononuclear cells for production of tumor necrosis factor-α and interleukin-6 compared with their potency to activate murine macrophage RAW264.7 cells. Two of the analogues were found to have sufficient potency to activate the human cells as well as the murine cells. These analogues comprise d-glucosamine, phosphoryl groups, and acyl groups of defined carbon chain lengths (C14 and C12) in a ratio of 1:1:3. This ratio of molecular constituents is proportional to that of the complete disaccharide structure of lipid A (2:2:6). Other analogues with two or four C14 acyl groups and with three acyl groups but including a C10 or a C16 acyl group, which are active to murine cells, showed no LPS-agonistic activity, but did show LPS-antagonistic activity, to human cells. An LPS-antagonistic analogue in the murine cells also showed antagonistic activity in human cells. These results reveal that lipid A analogues recognized as being LPS agonists by human macrophages have common structural features in monosaccharide and disaccharide structures which are more strict than those required for recognition by murine macrophages and that broad lipid A-like structures are recognized as being LPS antagonists by human cells but are recognized by murine cells as being either LPS agonists or antagonists.
During gram-negative infection, lipopolysaccharide (LPS), the major outer membrane constituent of the bacteria, is released by bacterial lysis. The LPS released is considered to be responsible for the induction of various pathophysiological reactions of an infected host such as fever, disseminated intravascular coagulation, and shock (29, 34). It has been shown that LPS activates host immune cells to release a variety of inflammatory mediators, such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-6, platelet-activating factor, and nitric oxide, and that cells of monocytic lineage are the major source of these mediators. These inflammatory mediators are thought to play a pivotal role in the mediation of LPS-triggered reactions and induce many of the physiological changes observed with endotoxemia and sepsis when they are present in excess.
Chemically, LPS has a hydrophilic polysaccharide region and a covalently linked hydrophobic glycolipid region, termed lipid A. The active region of LPS was concluded to be lipid A since free lipid A separated from polysaccharide by mild acid hydrolysis of LPS induced the same spectrum of activities as LPS and, furthermore, since chemically synthesized Escherichia coli-type lipid A (506) exhibited biological activity identical to that of the corresponding natural lipid A and LPS (7, 10, 19). By using chemically synthesized lipid A and its modified structures and partial structures, the relationship of structure to the biological activity of lipid A analogues has been intensively studied (11, 34). It was shown that endotoxic activity is optimally expressed by a molecule with the chemical structure of E. coli-type lipid A, 506 (Fig. 1), which is a bisphosphorylated glucosamine disaccharide carrying six acyl groups. Although this hexacylated lipid A acted as an LPS agonist in both murine and human cells, tetra-acylated disaccharide lipid A analogue 406 (Fig. 1) showed the unique ability to act as an LPS agonist in murine cells but as an LPS antagonist in human cells (9, 18, 20, 23, 37). These studies indicated differences between species in recognizing lipid A-like molecules and suggested structural differences between human and animal receptors for lipid A.
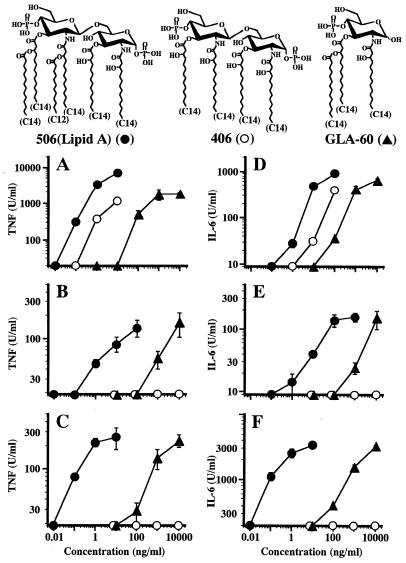
FIG. 1.
Induction of TNF-α and IL-6 release from murine RAW264.7 cells (A and D), human U937 cells (B and E), and human PBMC (C and F) by synthetic lipid A analogues. Murine RAW264.7 cells (5 × 105 cells/0.5 ml/well of a 48-well culture plate) were cultured in 5% FCS-RPMI medium for 2 h and washed three times with Hanks' balanced salt solution. The adherent cells were cultured in 5% FCS-RPMI medium with test samples. Human U937 cells (105 cells/0.5 ml/well of a 48-well culture plate) were cultured in 10% FCS-RPMI medium with 30 ng of PMA/ml for 3 days and washed once with 10% FCS-RPMI medium. The adherent cells were cultured in 10% FCS-RPMI medium with test samples. Human PBMC were isolated from heparinized peripheral blood of healthy volunteer donors by density gradient centrifugation with low-endotoxin Ficoll-Hypaque. The cells (5 × 105 cells/0.5 ml/well of a 48-well culture plate) were cultured in 10% FCS-RPMI medium with test samples. Culture supernatant from these three different types of cells obtained at 4 and 24 h after stimulation with test samples at the indicated concentrations was assayed for TNF-α (A, B, and C) and IL-6 (D, E, and F), respectively. Synthetic lipid A analogues tested were 506 with a hexacylated disaccharide structure (complete lipid A), 406 with a tetra-acylated disaccharide structure, and GLA-60 with a triacylated monosaccharide structure. Chemical structures of these analogues are shown at the top. Data are the means ± SEM of triplicate samples. A representative result of three independent experiments is shown.
We have been studying the biological activities of monosaccharide-type lipid A analogues and have shown that these analogues are nonpyrogenic but that some of them preserve LPS-mimetic activities such as induction of cytokines from murine macrophages (6, 21, 28) and induction of resistance to microbial infections and tumors in animal models (13, 14, 25, 31). In these monosaccharide lipid A analogues, acylation patterns as well as phosphorylation patterns were revealed to play an important role in the expression of biological activities, as was observed for disaccharide lipid A analogues (34). Concerning the action of monosaccharide lipid A analogues on human cells, a few studies have reported activity in some analogues (22, 24, 36). More detailed studies of the effects of monosaccharide analogues on human cells in relation to their structures are required for a further understanding of the characteristic features of human cells that allow them to recognize lipid A-like structures and for the development of beneficial applications of the analogues to human diseases.
In the present study, a series of structurally related monosaccharide lipid A analogues were examined for their ability to activate human monocytic cells to induce cytokines such as TNF-α and IL-6, in comparison with their ability to activate murine monocytic cells. Only the monosaccharide analogues with strong activity as LPS agonists in the murine cells were also active in the human cells, while the other analogues, with weaker agonistic activity and LPS-antagonistic activity in the murine cells, showed LPS-antagonistic activity in human cells.
MATERIALS AND METHODS
Reagents.
Monosaccharide lipid A analogues were synthesized chemically as described elsewhere (16, 17). The purity of the analogues was confirmed to be very high, without detectable contaminants such as disaccharide analogues, by reverse-phase high-performance liquid chromatography analysis. Synthetic complete lipid A 506 and disaccharide lipid A analogue 406 were obtained from Daiichi Kagaku Co. (Tokyo, Japan). The 3-hydroxytetradecanoic acids attached to glucosamine backbones in these monosaccharide and disaccharide compounds had an R configuration. These compounds were solubilized in triethylamine salt form and stabilized with bovine serum albumin in pyrogen-free distilled water as described previously (26) and stored at 4°C until use. The LPS used was a smooth-type LPS of Salmonella abortus equi which was purified and prepared in triethylamine salt form (8). This LPS was a kind gift from C. Galanos (Max-Planck-Institut für Immunbiology, Freiburg, Germany). The human U937 cell line and murine RAW264.7 cell line were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan) and from the American Type Culture Collection (Manassas, Va.), respectively. Phorbol myristate acetate (PMA) was purchased from Sigma Chemical Co. (St. Louis, Mo.).
Cell culture.
All cells were cultured in a humidified chamber at 37°C with 5% CO2. For culture of cells, RPMI 1640 medium (Flow Laboratories, Inc., Rockville, Md.) supplemented with 10 mM HEPES, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.2% NaHCO3 was used as the basic medium and heat-inactivated fetal calf serum (FCS; Flow Laboratories) was added at a concentration of 5 or 10% (5 or 10% FCS-RPMI medium). Murine RAW264.7 cells were suspended in 5% FCS-RPMI medium at 106 cells per ml. These cell suspensions were dispensed (0.5 ml) to each well of a 48-well culture plate (Sumitomo Bakelite Co. Ltd., Tokyo, Japan) and cultured for 2 h. The cells in each well were washed three times with 0.5 ml of Hanks' balanced salt solution (Flow Laboratories), and adherent cells were cultured with 5% FCS-RPMI medium in the presence of test samples (0.5 ml/well). Human U937 cells were prepared for experiments by adding PMA at a final concentration of 30 ng per ml in 10% FCS-RPMI medium (2 × 105 cells/ml) and by culturing cells for 3 days on a 48-well culture plate (0.5 ml/well) to induce differentiation into macrophage-like cells. The cells were washed once with 10% FCS-RPMI medium (0.5 ml/well), and adherent cells were cultured with 10% FCS-RPMI medium in the presence of test samples (0.5 ml/well). Human peripheral blood mononuclear cells (PBMC) were isolated from heparinized peripheral blood from healthy volunteer donors by density gradient centrifugation (2, 38) with low-endotoxin Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). The cells were cultured with 10% FCS-RPMI medium in the presence of test samples at 5 × 105 cells/0.5 ml/well of a 48-well culture plate.
Culture supernatant obtained at 4 and 24 h after stimulation with test samples was assayed for TNF-α and IL-6 activity, respectively. In inhibition experiments, test samples were added to the cultures 30 min before stimulation of the cells with LPS (10 ng/ml) and activity of TNF-α and IL-6 in the culture supernatant at 4 and 24 h after the addition of LPS, respectively, was assayed.
TNF-α assay.
The activity of TNF-α in culture supernatant was determined by a cytotoxic assay with L-929 cells (35). Briefly, L-929 cells were cultured with 5% FCS-RPMI medium on 96-well culture plates (Nunc, Roskilde, Denmark) for 2 to 3 h. Then, actinomycin D (Sigma Chemical Co.) at a final concentration of 1 μg/ml and serial dilutions of the test samples were added to the wells. After overnight culture, viable cells were stained with crystal violet, the blue color was extracted with 30% acetic acid solution, and absorbance at 540 nm was measured with the Biomek 1000 spectrophotometer. The activity of TNF-α (in units per milliliter) was calculated from the dilution factor of test samples necessary for 50% cell lysis, with correction by an internal standard of a recombinant human TNF-α in each assay.
IL-6 assay.
The activity of IL-6 in culture supernatant was determined by a proliferation assay of IL-6-dependent mouse hybridoma cell line B13.29 (1). Briefly, B13.29 cells were cultured with 2.5% FCS-RPMI medium on 96-well culture plates with serially diluted test samples for 3 days. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma Chemicals] was added to the culture for the last 4 h to form formazan blue crystals in viable cells (30). The supernatant was removed, and the precipitated formazan crystals were dissolved with isopropanol solution containing 5% formic acid for measurement of absorbance at 540 nm with the Biomek 1000 spectrophotometer. IL-6 activity (in units per milliliter) was calculated as the dilution factor required to induce 50% cell growth, with correction by an internal standard of recombinant human IL-6.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM) of triplicate samples. All experiments were performed two or three times.
RESULTS
Activity of monosaccharide lipid A analogue GLA-60 to induce release of TNF-α and IL-6 from murine RAW264.7 cells, human U937 cells, and human PBMC compared with those of disaccharide analogues 506 and 406.
Compounds 506 and 406 each have a disaccharide backbone with six and four acyl groups, respectively, and GLA-60 has a monosaccharide backbone with three acyl groups, as shown in Fig. 1. The activities of these compounds in murine RAW264.7 cells for the induction of TNF-α and IL-6 release were investigated. The strongest activity for induction of TNF-α release was seen in 506, and significant amounts of TNF-α were released by stimulation with 506 at concentrations over 0.1 ng/ml (Fig. 1A). A concentration about 1 order of magnitude higher than that of 506 was required for 406 to exhibit similar activity. For GLA-60, a concentration about 2 orders of magnitude higher than that of 406 was required, although significantly large amounts of TNF-α were released by stimulation with GLA-60 at concentrations over 100 ng/ml. For induction of IL-6 by murine cells, results similar to those for the induction of TNF-α were obtained (Fig. 1D). Significant amounts of IL-6 were released by stimulation with 506 at concentrations over 1 ng/ml. Concentrations about 1 and 2 orders of magnitude higher than that of 506 were required for 406 and GLA-60, respectively, to exhibit similar activity. These results show that in the induction of both cytokines from the murine cells, the strongest activity was exhibited by 506, followed by 406 and GLA-60 in that order.
Next, human U937 cells differentiated into macrophage-like cells by PMA and human PBMC were used as target cells, and the activities of the compounds to induce TNF-α and IL-6 release were investigated. Significant amounts of both TNF-α and IL-6 were released from U937 cells by stimulation with 506 at concentrations over 1 ng/ml (Fig. 1B and E) and from PBMC at concentrations over 0.1 ng/ml (Fig. 1C and F). GLA-60 stimulated U937 cells to release both cytokines at concentrations over 1,000 ng/ml and stimulated PBMC at concentrations over 100 ng/ml. The doses of 506 and GLA-60 required for activation of the human cells were comparable to those for activation of murine RAW264.7 cells. On the other hand, the activity of 406 in human cells for induction of the cytokines was not detectable up to the highest concentration tested (10,000 ng/ml), which was 1,000 to 10,000 times higher than that required to stimulate murine cells (1 to 10 ng/ml). These results indicate that 506 and GLA-60 are recognized by human cells as active stimulants just as they are recognized by murine cells but that 406 is not recognized by human cells as an active stimulant although it is recognized by murine cells. It is interesting to note that GLA-60 with a monosaccharide backbone could stimulate human cells as well as murine cells, as could 506, while 406 with a disaccharide backbone and stronger activity than GLA-60 to stimulate murine cells could not stimulate human cells.
Effect of acylation patterns in monosaccharide lipid A analogues on induction of TNF-α and IL-6 release from murine RAW264.7 cells, human U937 cells, and human PBMC.
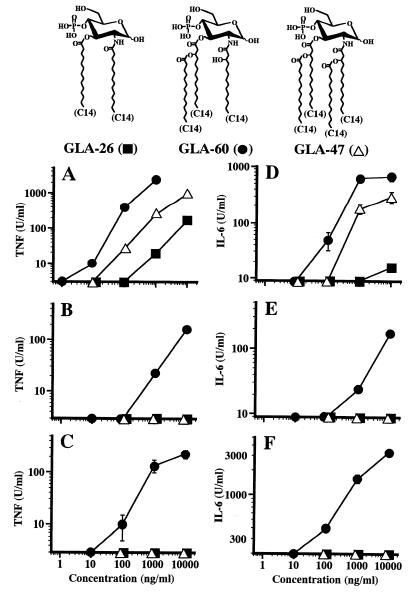
To clarify the structures of monosaccharide lipid A analogues required by human cells for induction of the cytokine release, the activities of monosaccharide lipid A analogues with two and four acyl groups of 14-carbon chain length (C14), GLA-26 and GLA-47, respectively, were compared with those of GLA-60 with three acyl groups of C14. As shown in Fig. 2A, both GLA-47 and GLA-26 exhibited significant activity to induce TNF-α release from murine RAW264.7 cells, although their activities were weaker than that of GLA-60. Concentrations of GLA-47 and GLA-26 about 1 and 2 orders of magnitude higher, respectively, than that of GLA-60 were required for activity similar to that of GLA-60. Neither GLA-47 nor GLA-26 exhibited significant activity in human U937 cells and PBMC to induce TNF-α release (Fig. 2B and C), while GLA-60 exhibited activity in both types of human cells, just as it did in murine cells. For induction of IL-6 from murine RAW264.7 cells, GLA-47 exhibited significant activity, although weaker than that of GLA-60 by about 1 order of magnitude, and GLA-26 exhibited very weak activity (Fig. 2D). As for TNF-α induction, neither GLA-47 nor GLA-26 exhibited significant activity in either type of human cells for induction of IL-6, while GLA-60 was as active in the human cells as in murine cells (Fig. 2E and F). These results indicate that the numbers of acyl groups attached to monosaccharide lipid A analogues are more strictly recognized by human cells for induction of those cytokines than by murine cells.
FIG. 2.
Effect of the number of attached acyl groups in monosaccharide lipid A analogues on induction of TNF-α and IL-6 release from murine RAW264.7 cells (A and D), human U937 cells (B and E), and human PBMC (C and F). Preparation and culture of cells were as described in the legend for Fig. 1. These cells were stimulated with monosaccharide lipid A analogues GLA-26 (two acyls), GLA-60 (three acyls), and GLA-47 (four acyls) at the indicated concentrations for 4 and 24 h, and the culture supernatant was assayed for TNF-α (A, B, and C) and IL-6 (D, E, and F), respectively. Chemical structures of these analogues are shown at the top. Data are the means ± SEM of triplicate samples. Similar results were obtained in another experiment.
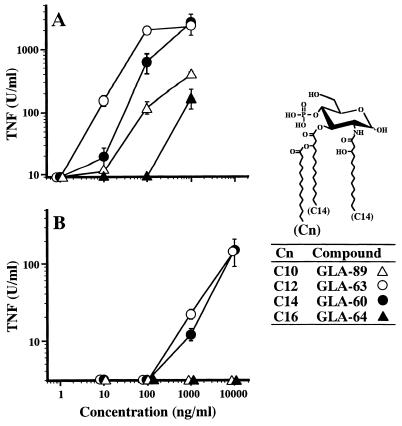
Effect of carbon chain length of a secondary acyl group in triacylated monosaccharide lipid A analogues on induction of TNF-α release from murine RAW264.7 cells and human U937 cells.
All three acyl groups including the secondary acyl group in GLA-60 are C14. Triacylated monosaccharide analogues with carbon chain lengths different from C14 in the secondary acyl group of GLA-60 were then examined for their capacity to induce TNF-α release from murine RAW264.7 cells and human U937 cells. The structures of the analogues are shown in Fig. 3. In RAW264.7 cells, GLA-63 with a C12 acyl group exhibited somewhat stronger activity than GLA-60 but GLA-89 with a C10 acyl group exhibited weaker activity than GLA-60 (Fig. 3A). The activity of GLA-64 with a C16 acyl group was less strong than that of GLA-89, although GLA-64 induced significant amounts of TNF-α at a concentration of 1,000 ng/ml. In the activation of human U937 cells, however, only GLA-63 and GLA-60 were found to be effective (Fig. 3B). They induced significant amounts of TNF-α release at concentrations over 1,000 ng/ml, but neither GLA-89 nor GLA-64 induced release even at the highest concentration tested (10,000 ng/ml). These results suggest that the carbon chain lengths of acyl groups in monosaccharide lipid A analogues are also recognized more strictly by human cells than by murine cells for the induction of cytokines.
FIG. 3.
Effect of the carbon chain length of a secondary acyl group in triacylated monosaccharide lipid A analogues on induction of TNF-α release from murine RAW264.7 cells (A) and human U937 cells (B). Preparation and culture of the cells were as described in the legend for Fig. 1. These cells were stimulated for 4 h with monosaccharide lipid A analogues of differing carbon chain lengths in their branched acyl side chains at the indicated concentrations, and the supernatants were assayed for TNF-α. As shown in the structures, GLA-89, GLA-63, GLA-60, and GLA-64 have secondary acyl groups with carbon chain lengths of C10, C12, C14, and C16, respectively. Data are the means ± SEM of triplicate samples. A representative result of three independent experiments is shown.
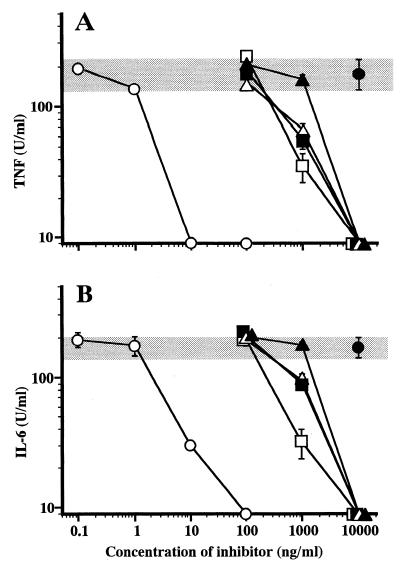
Antagonistic activity of lipid A analogues on LPS-induced cytokine release from human U937 cells.
As described above, several lipid A analogues were found to be inactive in human cells for induction of cytokine release but were found to be active in murine cells. The antagonistic activities of such analogues for LPS stimulation in human cells were then investigated. U937 cells were stimulated with LPS at 10 ng/ml in the presence of various concentrations of the analogues, and the activities of TNF-α and IL-6 released in the culture supernatant were measured. As shown in Fig. 4A, strong inhibitory activity of 406 against LPS-induced TNF-α release was observed. At concentrations over 10 ng of 406/ml, the activity of TNF-α in the culture supernatant decreased to an undetectable level. In monosaccharide analogues GLA-89, GLA-64, and GLA-47, a significant inhibitory effect was observed at 1,000 ng/ml. The inhibitory activity of GLA-26 was weaker and not significant at 1,000 ng/ml, but at higher concentrations, e.g., 10,000 ng/ml, it was strong enough to reduce the TNF-α activity to undetectable levels. The inhibitory effect of the analogues on LPS-induced IL-6 release is shown in Fig. 4B. In all the analogues tested, this inhibitory effect on IL-6 release showed close similarity to the observed effect on TNF-α release. Viability of U937 cells in these experiments was estimated by MTT assay, and no significant reduction in cell viability due to the presence of the analogues was found (data not shown). These results indicate that these analogues are recognized by human cells as LPS antagonists but not as LPS agonists.
FIG. 4.
Inhibitory effect of synthetic lipid A analogues on LPS-induced TNF-α and IL-6 release from human U937 cells. U937 cells were cultured in the presence of synthetic lipid A analogues at the indicated concentrations for 30 min and then stimulated with LPS at 10 ng/ml. Culture supernatant obtained at 4 and 24 h after LPS stimulation was assayed for TNF-α (A) and IL-6 (B), respectively. Lipid A analogues tested were 406 (○), GLA-26 (▴), GLA-47 (▵), GLA-89 (□), and GLA-64 (■). The levels of TNF-α and IL-6 production due to LPS stimulation alone in the absence of these analogues (gray bands) are (means ± SEM) 178 ± 46 and 160 ± 30 U/ml, respectively. Data are the means ± SEM of triplicate samples. A representative result of three independent experiments is shown.
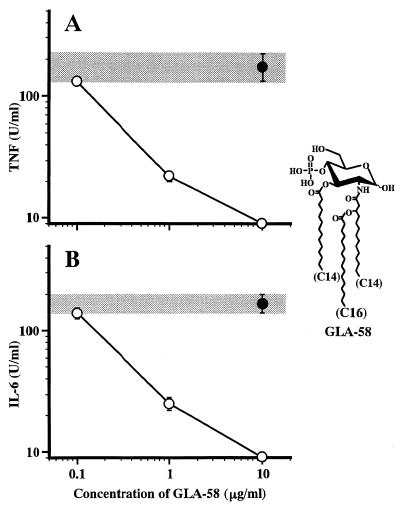
Antagonistic effect of monosaccharide lipid A analogue GLA-58 in human U937 cells on LPS-induced TNF-α and IL-6 release.
GLA-58 is a monosaccharide lipid A analogue which has been found to act as an LPS antagonist in murine cells (6, 27). The activity of this analogue as an LPS antagonist or agonist in human U937 cells was examined. As shown in Fig. 5, this analogue induced significant suppression, at concentrations above 1 μg/ml, of LPS-induced TNF-α and IL-6 release by human U937 cells. Neither of these cytokines was produced by the human cells upon stimulation with this analogue even at the highest concentration tested, 10 μg/ml (data not shown). These results indicate that GLA-58 is recognized as an LPS antagonist not only by murine cells but also by human cells.
FIG. 5.
Antagonistic action of monosaccharide lipid A analogue GLA-58 in human U937 cells. U937 cells were cultured in the presence of GLA-58 at the indicated concentrations for 30 min and then stimulated with LPS at 10 ng/ml. Culture supernatant obtained at 4 and 24 h after LPS stimulation was assayed for TNF-α (A) and IL-6 (B), respectively. The levels of TNF-α and IL-6 production by LPS stimulation alone in the absence of GLA-58 are shown as gray bands. The structure of GLA-58 is shown. Data are the means ± SEM of triplicate samples. A representative result of three independent experiments is shown.
DISCUSSION
In the present study, a series of monosaccharide lipid A analogues which differ in the acyl groups attached to the 4-O-phosphono-d-glucosamine backbone were examined for their potency to activate human monocytic cells for the production of cytokines such as TNF-α and IL-6. Among the analogues, GLA-60, carrying three acyl groups of C14, was revealed to have the potency to activate human monocytic cells. This analogue had previously been reported to have potency to activate human monocytes for IL-1 production (36) and for induction of tumoricidal properties (24). In addition, GLA-63 was found in this study to have potency comparable to that of GLA-60 for the activation of human cells (Fig. 3). This analogue also has three acyl groups, two of which are C14 and one of which is C12. The other monosaccharide analogues examined in this study carry two or four acyl groups of C14 (GLA-26 or GLA-47, respectively) or three acyl groups, two of which are C14 and one of which is C10 or C16 (GLA-89 or GLA-64, respectively). The potency of these analogues to activate human monocytic cells was undetectable, whereas the analogues showed significant potency to activate murine monocytic cells, although less than GLA-60 and GLA-63 (Fig. 2 and 3). A review (34) previously reported that monosaccharide structures are least effective for activation of human monocytic cells (reduction of activity by a factor of >107 compared to activity produced by 506), but the report was based on the activity of lipid X, which, like GLA-26 in this study, has two acyl groups. In that study, the relationship between the structures of monosaccharide lipid A analogues and activation of human monocytic cells was not studied in as much detail as was done in the present study. Based on a detailed study, we demonstrate here that, even in monosaccharide structures, analogues with three acyl groups of C14 and C12 such as GLA-60 and GLA-63 have sufficient potency to activate human monocytic cells, although they are less potent than 506 by a factor of about 103 (Fig. 1). These results indicate that the formation of a disaccharide structure is not a critical factor for lipid A-like structures to be recognized by human cells as LPS agonists.
It has been reported that monosaccharide lipid A analogue SDZ MRL 953 exhibited potency to activate human monocytes for TNF-α production (22) but that another one, lipid X, did not (39). SDZ MRL 953 and lipid X have a 1-O-phosphono-d-glucosamine structure with three and two acyl groups of C14, respectively. These results together with the results obtained in the present study indicate that monosaccharide structures constructed with d-glucosamine, phosphoryl groups, and acyl groups of defined carbon chain lengths (C14 and C12) in a ratio of 1:1:3 have potency to activate human monocytic cells. As for disaccharide lipid A analogues, it has been found that full endotoxic activity in human monocytic cells is expressed by the structure of E. coli-type lipid A (506), comprising d-glucosamine, phosphoryl groups, and acyl groups with defined carbon chain lengths (C14 and C12) in a ratio of 2:2:6 and that modification of this structure leads to less-active or inactive structures (34). The above-mentioned ratios of monosaccharide and disaccharide structures which are active for human cells are proportional (1:1:3 versus 2:2:6). These results indicate that the physicochemical properties of these molecules, produced by such a limited combination of constituents, play a more important role in the activation of human cells than the structure of the disaccharide backbone. It has been demonstrated for disaccharide lipid A analogues that an “endotoxic supramolecular conformation,” which is the particular organization of lipid A aggregates in physiological fluids, is required for optimal expression of biological effects (3). The above-mentioned physicochemical properties common to limited monosaccharide and disaccharide molecules may be the important factors to induce the endotoxic supramolecular conformation.
The above-mentioned ratio is also important for activation of murine cells, since 506 exhibits the strongest activity in murine cells of the disaccharide analogues (33) and GLA-60 exhibits the strongest activity in murine cells of the monosaccharide analogues (6, 11). However, a clear difference between the activation of murine cells and human cells by 406 was observed. The disaccharide analogue 406 exhibited greater potency than GLA-60 by a factor of 10 to 102 for the activation of murine cells but less potency than GLA-60 for the activation of human cells by a factor of >102, i.e., the potency of 406 was undetectable even at the highest concentration tested (10 μg/ml) (Fig. 1). 406 is a bisphosphorylated disaccharide with four acyl groups of C14, and the above-mentioned ratio for this molecule is 2:2:4. These results indicate that, for activation of murine monocytic cells, the structure of the disaccharide backbone is a more important factor than the factor that is important for the activation of human monocytic cells, namely, the proper acylation balance in lipid A-like structures.
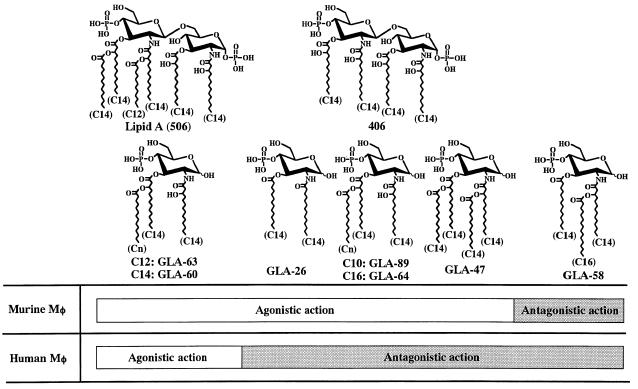
It was also demonstrated in the present study that the monosaccharide analogues which were active in murine cells but not in human cells, such as 406, have antagonistic activity to LPS-induced activation of human monocytic cells (Fig. 4) (9, 20, 23). The monosaccharide lipid A analogue GLA-58, which has been found to be an LPS antagonist in murine cells (6, 27), also exhibited antagonistic activity in human cells (Fig. 5). The results obtained in the present study are summarized in Fig. 6, showing the relationships between the chemical structures of lipid A analogues and their actions as LPS agonists or antagonists in human monocytic cells compared with their actions in murine cells. Paclitaxel (Taxol) is a compound which has been reported to act as an LPS agonist in murine macrophages although it is structurally unrelated to lipid A (5). This compound showed neither LPS-agonistic nor -antagonistic action in the human cells in this study (data not shown). It is clear that the structural requirements for human monocytic cells to recognize lipid A-like structures as LPS agonists are more strict than those for murine cells. But human cells recognize as LPS antagonists broad lipid A-like structures comparable to those recognized by the murine cells as LPS agonists or antagonists. This suggests that lipid A-like structures have greater potential for acting as LPS-antagonistic agents, with less toxicity for human use, than would be shown by mouse experiments and that the development of evaluation systems using human cells is necessary for discovering clinically useful LPS antagonistic agents.
FIG. 6.
Structures of synthetic lipid A analogues in relation to their LPS-agonistic and -antagonistic actions in murine and human macrophages (Mφ).
CD14, a glycosylphosphatidylinositol-anchored protein on the surface of monocytic cells, has been shown to bind LPS and subsequently initiate cellular activation (40). To determine whether the species specificity of 406 (agonist to murine cells and antagonist to human cells) occurred as a result of interactions with CD14, Delude et al. (4) examined the effects of 406 on cell activation with human, mouse, and hamster cell lines transfected with murine or human CD14 cDNA expression vectors. They also used another LPS antagonist in both human and murine cells, Rhodobacter sphaeroides lipid A (9), and showed that the cell lines respond to these lipid A analogues in a manner independent of the species from which the expressed CD14 was derived but dependent of the genetic background of the recipient cell line. It was proposed that a putative LPS recognition protein, which exists downstream of CD14, recognizes CD14-bound lipid A-like structures in a productive or nonproductive manner for signal transduction and that the differences in this protein among animal species causes species specificity in the response of the cells to lipid A-like structures. In the present study, such a protein can be assumed to play a role as the target molecule for lipid A analogues to transduce their signals as LPS agonists or antagonists. It is now an important subject in LPS studies to find and characterize such a protein and its homologues in different species. Recently, Toll-like receptor 4 (TLR-4) (12, 32) and TLR-2 (15, 41) were reported to be possible candidates for such proteins in murine and human cells, respectively. Based on the information obtained in the present study, further application of monosaccharide lipid A analogues as well as disaccharide analogues is promising in the search for valuable new information to elucidate such problems.
In summary, our results show that the structure of lipid A analogues recognized as LPS agonists by human monocytic cells comprises d-glucosamine, phosphoryl groups, and acyl groups with defined carbon chain lengths of C14 and C12 in a ratio proportional to 1:1:3; these constituents are common to monosaccharide and disaccharide molecules, and the elements necessary for recognition by human cells are more strict than those required by murine cells. In contrast, broad lipid A-like structures which are recognized by the murine cells as LPS agonists and antagonists are recognized as being LPS antagonists by human cells.
ACKNOWLEDGMENTS
This work was supported in part by grant 09670295 (M.M.) from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Aarden L A, de Groot E R, Shaap O L, Lansdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;7:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Boyum A. Isolation of mononuclear cells and granulocytes from human blood: isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 3.Brandenburg K, Mayer H, Koch M H J, Weckesser J, Rietschel E T, Seydel U. Influence of the supramolecular structure of free lipid A on its biological activity. Eur J Biochem. 1993;218:555–563. doi: 10.1111/j.1432-1033.1993.tb18409.x. [DOI] [PubMed] [Google Scholar]
- 4.Delude R L, Savedra R J, Zhao H, Thieringer R, Yamamoto S, Fenton M J, Golenbock D T. CD14 enhances cellular responses to endotoxin without imparting ligand-specific recognition. Proc Natl Acad Sci USA. 1995;92:9288–9292. doi: 10.1073/pnas.92.20.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding A, Porteu F, Sanchez E, Nathan C F. Shared actions of endotoxin and Taxol on TNF receptors and TNF release. Science. 1990;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- 6.Funatogawa K, Matsuura M, Nakano M, Kiso M, Hasegawa A. Relationship of structure and biological activity of monosaccharide lipid A analogues to induction of nitric oxide production by murine macrophage RAW264.7 cells. Infect Immun. 1998;66:5792–5798. doi: 10.1128/iai.66.12.5792-5798.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanos C, Lüderitz O, Rietschel E T, Westphal O, Brade H, Brade L, Freudenberg M, Shade U, Imoto M, Yoshimura H, Kusumoto S, Shiba T. Synthetic and natural Escherichia colilipid A express identical endotoxic activities. Eur J Biochem. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 8.Galanos C, Lüderitz O, Westphal O. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi. Zentbl Bakteriol Mikrobiol Hyg Abt 1 Orig A. 1979;243:226–244. [PubMed] [Google Scholar]
- 9.Golenbock D T, Hampton R Y, Qureshi N, Takayama K, Raetz C R H. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 10.Homma J Y, Matsuura M, Kanegasaki S, Kawakubo Y, Kojima Y, Shibukawa N, Kumazawa Y, Yamamoto A, Tanamoto K, Yasuda T, Imoto M, Yoshimura H, Kusumoto S, Shiba T. Structural requirements of lipid A responsible for the functions: a study with chemically synthesized lipid A and its analogues. J Biochem. 1985;98:395–406. doi: 10.1093/oxfordjournals.jbchem.a135294. [DOI] [PubMed] [Google Scholar]
- 11.Homma J Y, Matsuura M, Kumazawa Y. Studies on lipid A, the active center of endotoxin—structure-activity relationship. Drugs Future. 1989;14:645–665. [PubMed] [Google Scholar]
- 12.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lpsgene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 13.Ikeda K, Neyts J, Matsuura M, Kiso M, Hasegawa A, Nishimura C, De Clercq E. Protective activity of lipid A analogue GLA-60 against murine cytomegalovirus infection in immunodeficient mice. J Gen Virol. 1993;74:1399–1403. doi: 10.1099/0022-1317-74-7-1399. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda S, Neyts J, Matsuura M, Kiso M, Hasegawa A, Nishimura C, De Clercq E. Protective activity of lipid A analogue GLA-60 against murine cytomegalovirus infection in mice. J Med Virol. 1993;40:222–227. doi: 10.1002/jmv.1890400310. [DOI] [PubMed] [Google Scholar]
- 15.Kirschning C J, Wesche H, Ayres T M, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiso M, Tanaka S, Fujishima M, Ogawa Y, Hasegawa A. Synthesis of nonreducing sugar subunit analogues of bacterial lipid A carrying an amide-bound (3R)-3-acyloxytetradecanoyl group. Carbohydr Res. 1987;162:247–256. doi: 10.1016/0008-6215(87)80207-0. [DOI] [PubMed] [Google Scholar]
- 17.Kiso M, Tanaka S, Fujita M, Fujishima Y, Ogawa Y, Ishida H, Hasegawa A. Synthesis of the optically active 4-O-phosphono-D-glucosamine derivatives related to the non-reducing sugar subunit of bacterial lipid A. Carbohydr Res. 1987;162:127–140. doi: 10.1016/0008-6215(87)80207-0. [DOI] [PubMed] [Google Scholar]
- 18.Kitchens R L, Ulevitch R J, Munford R S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotani S, Takada H, Tsujimoto M, Ogawa T, Takahashi I, Ikeda T, Otsuka K, Shimauchi H, Kasai N, Mashimo J, Nagao S, Tanaka A, Tanaka S, Harada K, Nagaki K, Kitamura H, Shiba T, Kusumoto S, Imoto M, Yoshimura H. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coliRe mutant. Infect Immun. 1985;49:225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovach N, Yee E, Munford R S, Raetz C R H, Harland J M. Lipid IVa inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human blood ex vivo. J Exp Med. 1990;172:77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumazawa Y, Nakatsuka M, Takimoto H, Furuya T, Nagumo T, Yamamoto A, Homma J Y, Inada K, Yoshida M, Kiso M, Hasegawa A. Importance of fatty acid substituents of chemically synthesized lipid A-subunit analogues in the expression of immunopharmacological activity. Infect Immun. 1988;56:149–155. doi: 10.1128/iai.56.1.149-155.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liehl E, Lam C, Mayer P, Schütze E, Bahr G, Großmüller F, Stütz P, Hildebrandt J. SDZ MRL 953, a new cytokine inducing agent and stimulant for nonspecific immunity. In: Levin J, Alving C R, Munford R S, Stütz P I, editors. Bacterial endotoxin: regulation and effector mechanisms. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1993. pp. 399–412. [Google Scholar]
- 23.Loppnow H, Brade H, Durrbaum I, Dinarello C A, Kusumoto S, Rietschel E T, Flad H-D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989;142:3229–3238. [PubMed] [Google Scholar]
- 24.Maeda H, Saiki I, Yamamoto N, Takahashi T, Sekiguchi S, Kiso M, Hasegawa A, Azuma I. Activation by synthetic lipid A subunit analogues (GLA compounds) of tumoricidal properties in human blood monocytes. Vaccine. 1990;8:237–242. doi: 10.1016/0264-410x(90)90052-n. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura M, Homma J Y. Enhancement of nonspecific resistance against microbial infections with special reference to Pseudomonas aeruginosainfection by chemically synthesized lipid A-subunit analogs. Antibiot Chemother. 1991;44:203–208. doi: 10.1159/000420315. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura M, Kijima Y, Homma J Y, Kubota Y, Shibukawa N, Shibata M, Inage M, Kusumoto S, Shiba T. Interferon-inducing, pyrogenic and proclotting enzyme of horseshoe crab activation activities of chemically synthesized lipid A analogues. Eur J Biochem. 1983;137:639–642. doi: 10.1111/j.1432-1033.1983.tb07873.x. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura M, Kiso M, Hasegawa A, Nakano M. Multistep regulation mechanisms for tolerance induction to lipopolysaccharide lethality in the tumor-necrosis-factor-α-mediated pathway. Application of non-toxic monosaccharide lipid A analogues for elucidation of mechanisms. Eur J Biochem. 1994;221:335–341. doi: 10.1111/j.1432-1033.1994.tb18745.x. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura M, Shimada S, Kiso M, Hasegawa A, Nakano M. Expression of endotoxic activities by synthetic monosaccharide lipid A analogs with alkyl-branched acyl substituents. Infect Immun. 1995;63:1446–1451. doi: 10.1128/iai.63.4.1446-1451.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 30.Mosman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 31.Nakatsuka M, Kumazawa Y, Matsuura M, Homma J Y, Kiso M, Hasegawa A. Enhancement of nonspecific resistance to bacterial infections and tumor regressions by treatment with synthetic lipid A-subunit analogs. Critical role of N- and 3-O-linked acyl groups in 4-O-phosphono-D-glucosamine derivatives. Int J Immunopharmacol. 1989;11:349–358. doi: 10.1016/0192-0561(89)90080-5. [DOI] [PubMed] [Google Scholar]
- 32.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castanoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 33.Rietschel E T, Brade H, Brade L, Brandenburg K, Schade U, Seydel U, Zähringer U, Galanos C, Lüderitz O, Westphal O, Labishenski H, Kusumoto S, Shiba T. Lipid A, the endotoxic center of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Prog Clin Biol Res. 1987;231:25–53. [PubMed] [Google Scholar]
- 34.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, Padova F D, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 35.Ruff M R, Gifford G E. Purification and physiological characterization of rabbit tumor necrosis factor. J Immunol. 1980;125:1671–1677. [PubMed] [Google Scholar]
- 36.Saiki I, Maeda H, Murata J, Takahashi T, Sekiguchi S, Kiso M, Hasegawa A, Azuma A. Production of interleukin 1 from human monocytes stimulated by synthetic lipid A subunit analogues. Int J Immunopharmacol. 1990;12:297–305. doi: 10.1016/0192-0561(90)90085-2. [DOI] [PubMed] [Google Scholar]
- 37.Ulmer A J, Heine H, Feist W, Kusumoto S, Kusama T, Brade H, Schade U, Rietschel E T, Flad H-D. Biological activity of synthetic phosphonooxyethyl analogues of lipid A and lipid A partial structures. Infect Immun. 1992;60:3309–3314. doi: 10.1128/iai.60.8.3309-3314.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulmer A J, Scholz W, Ernst M, Brandt E, Flad H-D. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunology. 1984;166:238–250. doi: 10.1016/S0171-2985(84)80042-X. [DOI] [PubMed] [Google Scholar]
- 39.Wang M-H, Flad H-D, Feist W, Musehold J, Kusumoto S, Brade H, Gerdes J, Rietschel E T, Ulmer A. Inhibition of endotoxin or lipid A-induced tumor necrosis factor production by synthetic lipid A partial structures in human peripheral blood mononuclear cells. Lymphokine Cytokine Res. 1992;11:23–31. [PubMed] [Google Scholar]
- 40.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1432. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 41.Yang R-B, Mark M R, Gray A, Huang A, Xie M H, Zahng M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]