Graphical abstract
Keywords: Mitral valve prolapse, Papillary muscle rupture, Infective endocarditis
Highlights
-
•
Several conditions such as IE and papillary muscle rupture can cause acute MR.
-
•
A ruptured papillary muscle may in rare cases mimic an infectious vegetation.
-
•
Echocardiography can in most cases accurately diagnose the cause of MR.
-
•
Image quality should be considered in the diagnostic progression.
-
•
Papillary muscle rupture may be entangled by chordae preventing complete flail.
Introduction
Acute mitral regurgitation (MR) with subsequent acute pulmonary edema is a potentially life-threatening complication of both ST-elevation infarction (STEMI) and infective endocarditis (IE) that requires rapid diagnosis and management. In STEMI, rupture of a papillary muscle can cause acute MR, while in IE, destruction of the valve can cause a similar picture.1 The incidence of MR due to STEMI has been declining due to increased availability of timely percutaneous coronary intervention, whereas the incidence of IE is increasing.2, 3, 4 The optimal treatment differs based on the etiology of the MR, and thus it is important to identify the cause of the MR.1
Case Presentation
A 56-year-old man with a history of hypertension contacted emergency services due to acute dyspnea. When the ambulance arrived, the patient had a regular tachycardia of 133 bpm and SpO2 of 80% that did not respond to supplement of oxygen with a full-face mask with reservoir. In the hospital emergency room, the patient reported acute, severe chest pain 2 days prior and was found on exam to have a fever of 38.8 °C, blood pressure of 185/90 mm Hg, heart rate of 134 bpm, respiratory frequency of 34/min, and SpO2 of 87% with continuous positive airway pressure with an FiO2 of 0.5. Chest x-ray showed pulmonary edema. Electrocardiogram (ECG) showed sinus rhythm with ST elevation and Q waves in II, III, and aVF suggestive of a late-presentation inferior wall STEMI (Figures 1 and 2). Bedside transthoracic echocardiography (TTE) showed MR and a mass adjacent to the posterior mitral leaflet.
Figure 1.
ECG standard leads showing sinus rhythm with Q waves and ST elevations in II, III and aVF. Reciprocal ST depression in aVL.
Figure 2.
ECG precordial leads showing sinus rhythm and ST depression in V2-5.
Due to respiratory instability, the patient was sedated and intubated. By angiography, the left coronary artery was normal (Video 1, Figure 3). The right coronary artery was displayed only subselectively yet was interpreted as revealing no obstruction (Video 2, Figure 4).
Figure 3.

Left coronary arteriogram still frame without occlusions or stenoses.
Figure 4.

Subselective right coronary arteriogram still frame.
Blood test revealed elevated inflammatory markers with a C-reactive protein (CRP) of 260 mg/L and leukocytes of 25.0 × 109/L. Troponin I was 15,722 ng/L (normal range < 30 ng/L).
Transthoracic echocardiogram parasternal short-axis and apical 3-chamber views displayed akinesia of the inferior wall of the left ventricle and the posterior mitral leaflet flailing into the left atrium (Videos 3 and 4, Figure 5). Transesophageal echocardiography (TEE) showed a flailing mitral P2 segment with a pendulating mass (Videos 5 and 6, Figure 6), possibly thickened mitral leaflets (Video 7, Figure 7), and pulmonary vein flow reversal. The pendulating mass was also visualized with three-dimensional TEE (Video 8).
Figure 5.

Two-dimensional TTE apical 3-chamber view systolic frame, showing the posterior mitral leaflet flailing into the left atrium.
Figure 6.
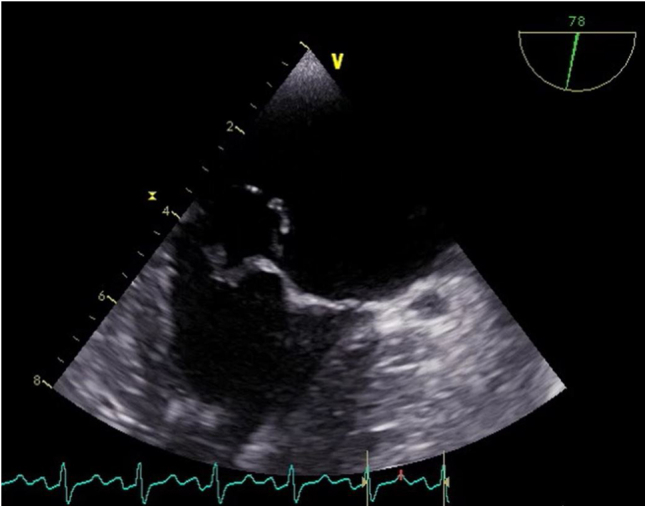
Two-dimensional TEE intercommissural view systolic frame showing mitral leaflet tissue billowing into the left atrium resembling a prolapse.
Figure 7.

Two-dimensional TEE 3-chamber view systolic frame of the mitral valve with an echogenic mass displaying the left atrium and a possibly thickened posterior mitral leaflet compared to the anterior mitral leaflet, suggestive of IE.
Clinical examination revealed poor dental status, and repeated blood test showed increasing infectious markers with CRP of 276 mg/L and leukocytes of 27.4 × 109/L.
Later the same evening the patient deteriorated, and possible treatment options included venoarterial extracorporeal membrane oxygenation or urgent high-risk surgery using standard extracorporeal circulation. In this setting, venoarterial extracorporeal membrane oxygenation would serve as bridge to surgery, postponing operative treatment until adequate control of infection and remission of pulmonary edema had been achieved. Emergency surgery with standard extracorporeal circulation was chosen as the most favorable option.
Upon inspection of the mitral leaflets intraoperatively, rupture of the posteromedial papillary muscle was detected. The torn muscular component was entangled in its own chordae tendineae. This intertwined mass was located close to the edge of the posterior leaflet in the P2 region, conveying an echocardiographic picture that could mimic a vegetation (Figure 8). This intraoperative finding diminished the suspicion of endocarditis. Consequently, the planned aortotomy to inspect the aortic valve for endocarditic lesions was no longer indicated.
Figure 8.
Picture of the removed mitral valve showing a papillary muscle entangled in its chordae tendineae. The ruptured surface is colored in pink.
The mitral leaflets were resected with exception of the P1 and its belonging chordae. A biological mitral valve prosthesis was implanted. Competency of the implanted prosthesis was verified by a saline test as well as intraoperative TEE. The patient was weaned successfully without mechanical support.
Transthoracic echocardiography on the seventh postoperative day showed satisfactory results: left ventricle with concentric hypertrophy, measured in the two-dimensional parasternal long-axis view by Devereux's formula; end-diastolic diameter, 5.2 cm; end-systolic diameter, 3.9 cm; mild, inferolateral hypokinesia; and ejection fraction, 55% calculated from biplane volumes. The biological mitral prosthesis had normal flow, mean gradient 6 mm Hg, and no pathological leakage.
The patient was clinically stable and discharged from hospital on the 19th postoperative day. Blood tests prior to discharge were as follows: Hgb, 11.5 g/dL; leukocytes, 6.9 10⁹/L; CRP, 48 mg/L (max 394 mg/L on second postoperative day); creatinine, 70 μmol/L; troponin T, 406 ng/L (max 3,774 ng/L on the first postoperative day). Blood cultures drawn upon admittance were incubated for 5 days and were negative. Chest x-ray showed a satisfactory postoperative status, with complete regress of the pulmonary edema.
Five months after surgery the patient reported full recovery and had returned to work. The patient provided written consent for this case report.
Discussion
Acute MR is a potentially life-threatening condition, and rapid recognition and management is of high importance. This case illustrates the potential difficulties in accurately diagnosing the cause of MR prior to surgical intervention. Initial anamnesis and ECG made late-presentation inferior wall STEMI with rupture of the posteromedial papillary muscle probable. Due to its single blood supply from the posterior descending artery, this is the most common site for papillary muscle rupture.5 However, the interpretation of the coronary angiogram as showing nonobstructive disease made STEMI less likely, although spontaneous reperfusion in STEMI is well described.6
Retrospectively, this turning point in the diagnostic progression underscores the importance of taking image quality into consideration and not relying more heavily on an image than the quality supports.
Fever and elevated blood inflammatory markers pointed in the direction of IE with the patient's poor dental status as a possible site of entry for bacteria. However, fever and elevated inflammatory markers are well-known in patients with STEMI.7 Another differential diagnosis is myocardial infarction with nonobstructive coronary arteries (MINOCA). This entity includes diagnoses such as coronary artery spasm and coronary embolism. In this case, the patient's age and sex were more indicative of atherosclerotic coronary artery disease, and yet, IE may cause an infectious coronary embolism. In general, MINOCA has fewer complications than coronary artery disease-acute myocardial infarction, although the incidence of mechanical complications is not well described in MINOCA.8
Several echocardiographic features may help distinguish between IE and a flailing mitral valve: regional left ventricular wall hypokinesia, surface of the potential vegetation, supernumerary mass, thickness of the native valve leaflets, and native cusp movement.
In this case, TTE showed akinesia of the inferior wall. This was in line with the ECG ST-elevation and the typical location that would threaten the posteromedial papillary muscle. The hypermobile structure was as sharp in the surface contour as the rest of the valve, opposite to the fluffy surface often seen in the acute phase of IE. An infective vegetation is an extra mass, supernumerary to the native structures in the heart. Even though our images showed no such structure unequivocally, some images (Video 4) were confusing. Moreover, the mass and particularly the tip of it appeared thicker than the adjacent valve, suggesting more than ruptured chordae alone and pointing toward IE. A large, preexisting mitral prolapse would provide a reasonable mechanism for MR, which eventually could become the site of IE, as IE normally originates from a structural defect. In our case, the hypermobile part of the posterior leaflet appeared to flail in some views (Video 4) and to prolapse in others (Video 5).
Conclusion
This case highlights challenges in the diagnostic workup in a patient presenting with acute MR and rapidly deteriorating clinical state. The inflammatory response in an acute STEMI may cause a presentation with both clinical and paraclinical overlap with IE. Even though ruptured chordae, papillary muscles, and IE vegetations usually present with typical appearances, different from one another, diseased and more or less degenerated mitral structures may take on any appearance.
Footnotes
Conflicts of Interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2022.08.002.
Supplementary Data
Left coronary arteriogram without occlusions or stenoses.
Subselective right coronary arteriogram. This was reported as revealing no obstruction.
Two-dimensional TTE parasternal basal short-axis view showing akinesia of the inferior and inferoseptal wall.
Two-dimensional TTE apical 3-chamber view showing akinesia of the inferolateral wall and the posterior mitral leaflet flailing into the left atrium.
Two-dimensional TEE intercommissural view showing mitral leaflet tissue billowing into the left atrium resembling valve prolapse.
Two-dimensional TEE intercommissural view slightly further to the right than Video 5 showing the appearance in the left atrium of a 5 mm large, pendulating mass.
Two-dimensional TEE 3-chamber view of an echogenic mass possibly attached to the valve tissue and raising the concern for partial papillary muscle rupture, tumor, or vegetation.
Three-dimensional TEE in surgical orientation of the mitral valve with an echogenic mass pendulating freely into the left atrium.
References
- 1.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 2.Figueras J., Alcalde O., Barrabés J.A., Serra V., Alguersuari J., Cortadellas J., et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation. 2008;118:2783–2789. doi: 10.1161/CIRCULATIONAHA.108.776690. [DOI] [PubMed] [Google Scholar]
- 3.Pant S., Patel N.J., Deshmukh A., Golwala H., Patel N., Badheka A., et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65:2070–2076. doi: 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- 4.Jordal S., Kittang B.R., Salminen P.R., Eide G.E., Kommedal Ø., Wendelbo Ø., et al. Infective endocarditis in Western Norway: a 20-year retrospective survey. Infect Dis (Lond) 2018;50:757–763. doi: 10.1080/23744235.2018.1482419. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2017;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 6.Fefer P., Beigel R., Atar S., Aronson D., Pollak A., Zahger D., et al. Outcomes of patients presenting with clinical indices of spontaneous reperfusion in ST-elevation acute coronary syndrome undergoing deferred angiography. J Am Heart Assoc. 2017;6:e004552. doi: 10.1161/JAHA.116.004552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reindl M., Reinstadler S.J., Feistritzer H.J., Klug G., Tiller C., Mair J., et al. Relation of inflammatory markers with myocardial and microvascular injury in patients with reperfused ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2017;6:640–649. doi: 10.1177/2048872616661691. [DOI] [PubMed] [Google Scholar]
- 8.Tamis-Holland J.E., Jneid H., Reynolds H.R., Agewall S., Brilakis E.S., Brown T.M., et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left coronary arteriogram without occlusions or stenoses.
Subselective right coronary arteriogram. This was reported as revealing no obstruction.
Two-dimensional TTE parasternal basal short-axis view showing akinesia of the inferior and inferoseptal wall.
Two-dimensional TTE apical 3-chamber view showing akinesia of the inferolateral wall and the posterior mitral leaflet flailing into the left atrium.
Two-dimensional TEE intercommissural view showing mitral leaflet tissue billowing into the left atrium resembling valve prolapse.
Two-dimensional TEE intercommissural view slightly further to the right than Video 5 showing the appearance in the left atrium of a 5 mm large, pendulating mass.
Two-dimensional TEE 3-chamber view of an echogenic mass possibly attached to the valve tissue and raising the concern for partial papillary muscle rupture, tumor, or vegetation.
Three-dimensional TEE in surgical orientation of the mitral valve with an echogenic mass pendulating freely into the left atrium.






