Abstract
During bacterial infection of the bovine mammary gland, large numbers of leukocytes migrate into the udder, resulting in the establishment of a host response against the pathogen. Currently, the specific leukocyte populations mediating this immune response are not well defined. In the studies described here, we analyzed blood and milk from healthy cows and cows with naturally occurring mastitis to determine if distinct αβ and γδ T-lymphocyte subsets were involved in the response of the udder to a mastitis pathogen and if the type of mastitis pathogen influenced the subset composition of these responding leukocytes. Although blood samples from cows with confirmed staphylococcal and streptococcal mastitis were characterized by increased numbers of γδ T cells, the most dramatic changes in leukocyte distributions occurred in milk samples from these cows, with a 75% increase in αβ T-cell levels and a 100% increase in γδ T-cell levels relative to the levels in milk samples from healthy animals. Interestingly, the increase in αβ T-cell numbers observed in milk from cows with staphylococcal mastitis was primarily due to increased numbers of CD4+ T cells, while the increase in αβ T-cell numbers observed in cows with streptococcal mastitis was due to a parallel increase in both CD4+ and CD8+ T-cell numbers. The increased numbers of γδ T cells in milk from cows with staphylococcal and streptococcal mastitis were due to a selective recruitment of a distinct γδ T-cell subset (GD3.1+), while no change in the numbers of GD197+ γδ T cells was observed. We also analyzed adhesion protein expression on blood and milk leukocytes and found that, in comparison to the situation for healthy cows, L-selectin was down-regulated and CD18 was up-regulated on leukocytes from cows with mastitis. Thus, shedding of L-selectin and up-regulation of CD18 by neutrophils may provide a sensitive indicator of early inflammatory responses during bovine mastitis. Overall, these studies suggest that distinct αβ and γδ T-cell subsets are involved in the host defense of the udder against mastitis infection and that selective recruitment of these T-cell subsets depends on the infectious agent involved.
Despite increased educational efforts and improved dairy herd management, mastitis still represents one of the most costly diseases of the dairy industry (53). In fact, the yearly loss due to mastitis has recently been estimated at about $2 billion for dairy producers in the United States alone (15, 25). In the common subclinical or chronic cases, mastitis can persist for months with little obvious inflammation. However, many of these infections eventually develop into clinical mastitis, which results in acute or slowly progressing inflammation and can later end in fibrosis of mammary tissue and loss of or decrease in milk production (53).
The most common bacterial pathogens associated with mastitis include staphylococcal, streptococcal, and coliform bacteria (15, 25). Staphylococcus aureus is currently one of the most difficult pathogens to control because it can spread rapidly among the herd and responds poorly to conventional antibiotic therapy (37). Members of another common group of mastitis-causing bacteria, Streptococcus spp., are frequently present on mucous membranes and are extremely infectious for the bovine mammary gland. Streptococcal mastitis causes a persistent type of infection that does not have a high self-cure rate, and undetected or untreated infected cattle can serve as reservoirs of infection (25, 60).
In efforts to prevent mastitis, a number of vaccines which can reduce the severity of mastitis have been generated; however, these vaccines still fail to effectively prevent the development of mastitis (67). Thus, the identification of alternative methods for combating mastitis is essential. In this regard, one of the most practical means for dealing with mastitis in the dairy industry may be to enhance the natural host defense mechanisms of the animal (29). Strategies aimed at enhancing the immune responses of the mammary gland during infection would significantly affect the ability of the animal to resist infection. Currently, the roles of various immune system components in the defense of the mammary gland against infection are not well understood. Both cytokine production and leukocyte adhesion play important roles during bacterial infection (29); however, the relative contributions of these factors to the pathogenesis of mastitis are not yet fully determined and will require more extensive studies. In addition, the contributions of various lymphoid and myeloid subsets to host defense in the mammary gland have not been extensively evaluated with naturally infected cows. Park et al. (41) reported that the presence of increased T-lymphocyte levels in bovine milk during lactation was due to an increase in the number of activated CD8+ T cells. In subsequent studies, Park et al. (42) showed that the number of activated CD8+ T cells was increased in milk obtained from cows experimentally infected with S. aureus and that these cells were responsible for suppressing the proliferative response of milk CD4+ T cells. Taylor et al. (56) also found that T cells in bovine milk were predominantly CD8+; however, as the number of days of lactation increased, the number of CD4+ T cells increased. In addition, Taylor et al. (57) also reported an increased percentage of CD4+ T cells in milk from cows with mastitis. Together, these previous reports demonstrate that changes in milk lymphocyte populations occur during mastitis; however, the nature of these changes seems to vary depending on the pathogen. In addition, because antibodies recognizing distinct γδ T-lymphocyte subsets have only recently been developed (66), the participation of these γδ T-lymphocyte subsets in mastitis has not been evaluated. Clearly, a more comprehensive understanding of these factors of the bovine immune response is essential to the development of effective treatments for the prevention of mastitis.
In the studies described here, we have investigated the hypothesis that distinct αβ and γδ T-lymphocyte subsets are involved in the response of the udder to a mastitis pathogen and that the type of pathogen may influence the subset composition of these responding leukocytes. To evaluate this hypothesis, we have used a panel of monoclonal antibodies to characterize the lymphocyte populations present in normal and mastitis milk with respect to leukocyte subset distribution and adhesion molecule expression. In addition, we have provided a comparison of these parameters for cows naturally infected with streptococcal or staphyloccocal bacteria. These studies have helped to delineate the role of the various leukocyte subsets in the host defense of the bovine mammary gland against infection with these different pathogens.
MATERIALS AND METHODS
Animals.
Thirty Holstein dairy cows from a local dairy were used throughout these studies. Control blood and milk samples were taken from apparently healthy animals. Acute mastitis was identified by detectable signs of inflammation of the infected udder and visual changes in infected milk. Clinical findings were confirmed by somatic cell counts in the foremilk and by bacteriologic culturing of milk from suspect quarters as described below. Animal care and handling were carried out in accordance with institutional guidelines.
Leukocyte isolation from blood.
Bovine lymphocytes and neutrophils were isolated from bovine blood as described by Sipes et al. (51). Briefly, blood was obtained by jugular venous puncture and collected in 20-ml Vacutainer tubes containing 0.2 ml of 0.5 M EDTA. After removal of erythrocytes by H2O lysis, leukocytes were resuspended in 20 ml of cold Dulbecco's phosphate-buffered saline (DPBS) and layered onto a two-step Histopaque gradient consisting of 15 ml of Histopaque 1077 over 15 ml of a 1:1 mixture of Histopaque 1077 and Histopaque 1119. After centrifugation at 2,500 × g for 25 min at room temperature, the two layers of cells were collected separately. The purified cells were washed two times with 20 ml of cold DBPS. Based on Wright staining and microscopic analysis, cells were routinely determined to be >95% pure; viability was determined to be >98% based on the exclusion of trypan blue. The final cell concentration used for flow cytometric analysis was 107 cells/ml.
Leukocyte isolation from milk.
Lymphocytes and neutrophils were isolated separately from the milk of 30 lactating cows. Briefly, milk was aseptically collected into sterile flasks and kept on ice until used. The milk was centrifuged at 1,500 × g for 20 min at 4°C, the cream layer was removed with a spatula, and the milk was gently decanted. The cell pellet was resuspended in 20 ml of ice-cold DPBS and filtered through 30-μm-pore-size Nitex to remove any remaining debris. The milk cell suspension was then applied to a Histopaque gradient as described above. Leukocytes or purified lymphocyte and neutrophil fractions were washed twice with DPBS and used for flow cytometric analysis.
Flow cytometry.
Single-color flow cytometric analysis was performed as described previously (21). Briefly, 106 cells were incubated with 50 μg of primary antibody per ml for 30 min on ice, washed with phosphate-buffered saline–2% goat serum, and incubated for 30 min with 1:250-diluted fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G secondary antibody (Jackson ImmunoResearch, West Grove, Pa.). The cells were washed again, resuspended in phosphate-buffered saline–2% goat serum, and analyzed by flow cytometry with a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). A total of 10,000 events were collected for each sample. All data files were further analyzed with CellQuest software (Becton Dickinson).
Two- and three-color flow cytometric analyses were performed by the methods of Wilson et al. (65). Briefly, 106 cells in 100 μl of DPBS–2% normal goat serum were incubated for 30 min on ice together with 100 μl of DPBS–2% goat serum containing one unconjugated primary antibody (diluted to 50 μg/ml). After being washed with DPBS–2% goat serum, the cells were incubated for 30 min on ice with 100 μl of secondary antibody (phycoerythrin-conjugated anti-mouse immunoglobulin G at 1:250) (Jackson ImmunoResearch). The cells were washed again with DPBS–2% goat serum, incubated with 100 μl of 10% normal mouse serum for 20 min on ice (to block available anti-mouse immunoglobulin binding sites on the second-stage reagent), and incubated for 30 min on ice with additional primary antibodies directly conjugated with a fluorochrome (e.g., FITC or phycoerythrin) or biotin. Avidin CyChrome (Becton Dickinson) was used to reveal biotin-labeled antibodies. The cells were then washed, resuspended in 500 μl of DPBS–2% goat serum, and analyzed on a FACSCalibur flow cytometer calibrated for three-color analysis with Calibright beads (Becton Dickinson). Negative controls included (i) cells alone, (ii) second-stage reagent alone, and (iii) single-color stains for the individual dyes. A minimum of 10,000 cells were analyzed for each sample. Marker placement for determination of the percentage of positive cells and for statistical comparisons was established by placing the marker outside the upper limit of background staining.
Monoclonal antibodies used in these studies included antibodies recognizing all γδ T-cell receptors (TCRs) (GD3.8) (66), γδ T-cell receptor subsets (GD3.1 and GD197) (18, 66), CD2 (CC42) (38), CD4 (CC30) (38), CD8 (CC58) (38), bovine neutrophils (BN15.6) (52), L-selectin (Dreg-56) (63), and CD18 (MHM23) (34). Although γδ T cells have been shown previously to express CD2 (31, 65, 66), this staining represents only a minor subset of γδ T cells (∼5%) (65). Additionally, we confirmed that the level of CD2+ γδ T cells was negligible in our samples (e.g., see Fig. 3). Therefore, we used CD2 staining to define and quantify the αβ T-cell population in these studies. Neutrophils were identified by their distinct forward and side light scatter profiles and by positive flow cytometric staining with antibody BN15.6, an antibody specific for bovine neutrophils (52).
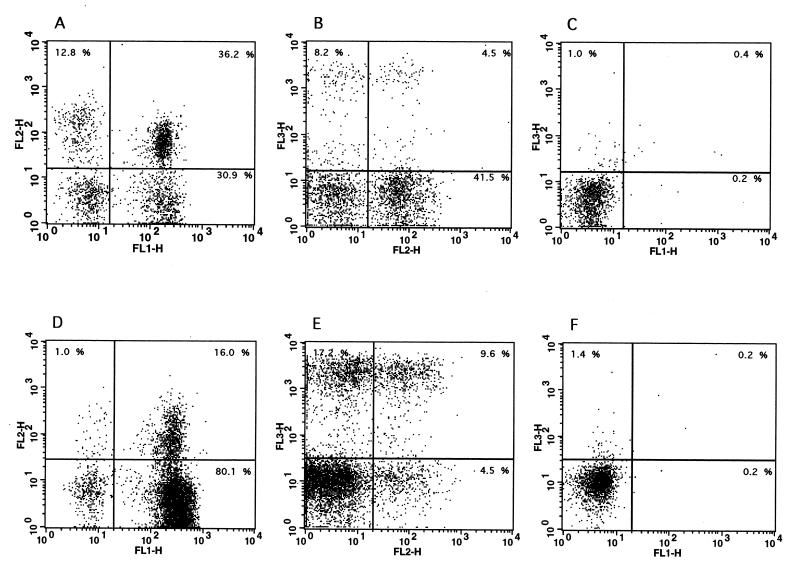
FIG. 3.
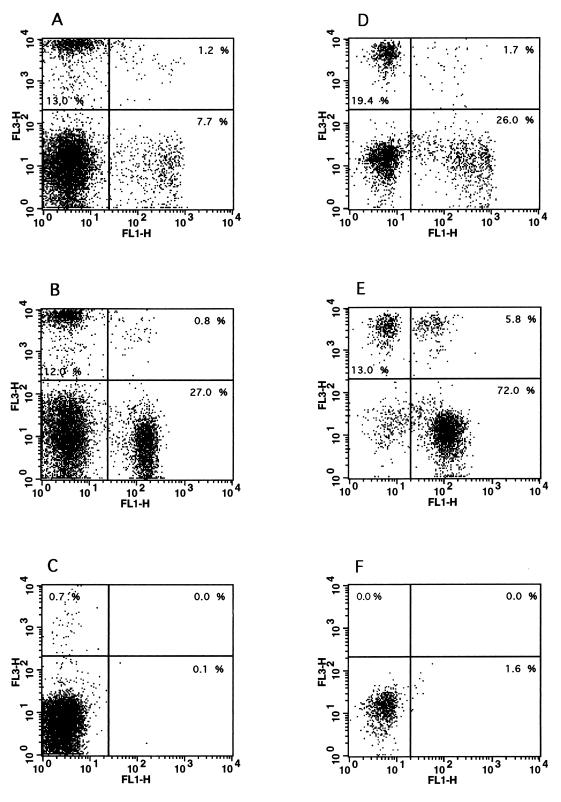
Two-color flow cytometric analysis of bovine blood and milk lymphocytes. Purified lymphocytes were isolated from the blood (A to C) and milk (D to F) of cows with mastitis, and two-color flow cytometric analysis was performed as described in Materials and Methods. (A and D) Staining of blood (A) and milk (D) lymphocytes with antibody GD3.8 (FL3, specific for γδ T cells) versus antibody CC58 (FL1, specific for bovine CD8). (B and E) Staining of blood (B) and milk (E) lymphocytes with antibody GD3.8 (FL3, specific for γδ T cells) versus antibody CC42 (FL1, specific for bovine CD2). (C and F) Control staining levels with secondary antibodies only in blood (C) and milk (F) samples. The data are representative of at least five independent experiments.
Somatic cell counting.
Somatic cell counts were determined by the State of Montana Veterinary Diagnostic Milk Laboratory (Bozeman). Cell counts were determined for all milk samples by use of a FOSSOMATIC electronic cell counter (Foss America Inc., Fishkill, N.Y.).
Bacteriology.
Bacteriologic analysis was performed on all milk samples by the State of Montana Veterinary Diagnostic Bacteriology Laboratory. Briefly, 0.01 ml of each milk sample was placed on tryptose agar containing 5% bovine blood. Plates were incubated for 48 h at 37°C, and the presence of pathogenic bacteria was considered a positive indicator of infection.
Statistical analysis.
Statistical analysis was performed by a paired Student t test, and a P value of <0.05 was considered significant.
RESULTS
Leukocyte distribution in blood and milk of healthy and mastitic cows.
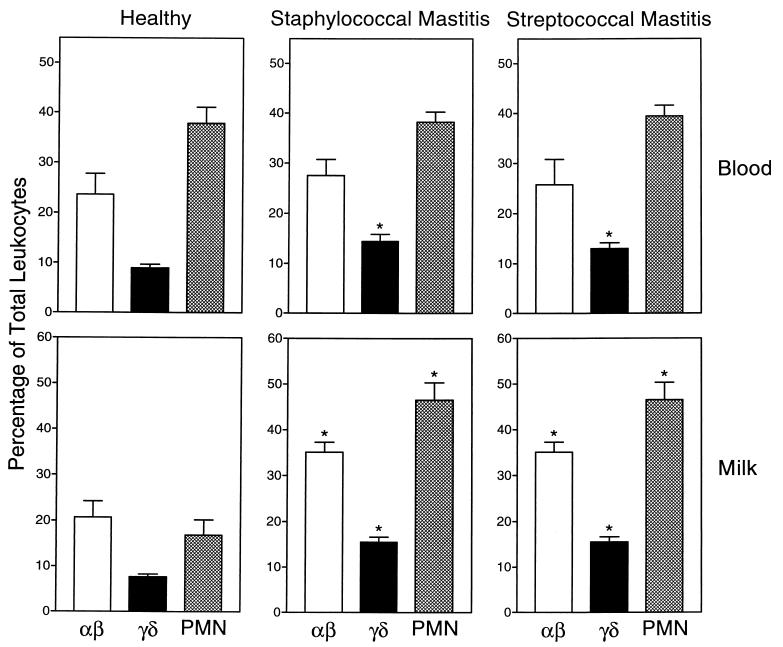
In blood from both healthy and infected animals, the overall total leukocyte counts were not significantly different, irrespective of the mastitis pathogen (Table 1). Furthermore, the leukocyte subset distribution in blood obtained from healthy cows was similar to that in blood obtained from cows with confirmed staphylococcal or streptococcal mastitis, with one important exception: blood from cows with mastitis had significantly increased numbers of γδ T cells (Fig. 1).
TABLE 1.
Total leukocyte counts in blood and milk from healthy cows and cows with mastitis
| Cows | Counts ofa:
|
|
|---|---|---|
| Leukocytes (105) | Somatic cells (104) | |
| Healthy | 142.6 ± 34.6 | 10.1 ± 1.9 |
| Mastitic | ||
| Staphylococcal | 147.5 ± 22.7 | 188.0 ± 86.2 |
| Streptococcal | 150.2 ± 23.4 | 82.4 ± 26.8 |
The data are presented as mean cells per milliliter ± standard error of the mean for five cows in each group.
FIG. 1.
Leukocyte subset distribution in blood and milk of healthy animals. Mixed bovine leukocytes were isolated from the blood and milk of 10 healthy lactating cows (left panels), 10 cows with staphylococcal mastitis (center panels), and 10 cows with streptococcal mastitis (right panels). The cells were labeled with monoclonal antibodies against αβ T-cell antigen CD2 (CC42), pan-γδ T-cell receptor (GD3.8), and bovine neutrophils (BN15.6) and analyzed by flow cytometry as described in Materials and Methods. Lymphocytes and neutrophils (PMN) were identified by their distinctive forward and side light scatter profiles, and the percentage of total leukocytes staining above the background (secondary antibody only) for the specific antigens listed above was determined. The data are expressed as mean ± standard error of the mean (n = 10). The asterisk indicates statistically significant differences (P, <0.05) between mastitis and healthy samples.
In contrast to the results obtained for blood samples very dramatic changes in the distribution of leukocytes were observed in milk samples from mastitic cows compared to milk samples from healthy cows. The number of somatic cells (leukocytes and epithelial cells) in milk from healthy animals did not exceed 2 × 105 cells/ml, and the samples cultured negative for the presence of bacteria. As expected, milk from mastitic cows was characterized by significant increases in total somatic cell counts (Table 1). Somatic cell counts in milk from cows with mastitis were increased 10- to 20-fold (Table 1). Analysis of these cells showed that the increase in somatic cell counts was due primarily to a dramatic increase in the number of neutrophils in the milk (Fig. 1), a result which is typical for mastitis (26). However, there were significant increases in both αβ and γδ T-cell numbers as well (Fig. 1). In addition, the relative γδ T-cell/αβ T-cell ratios increased from an average of 0.37 in healthy cows to 0.42 and 0.44 in cows with staphylococcal mastitis and streptococcal mastitis, respectively. These results suggested the interesting possibility that selective recruitment of T-cell subsets to the udder might occur during mastitis. Thus, we performed further studies to investigate the relative γδ and αβ T-cell subset distributions in milk from these animals.
Lymphocyte subset distribution in milk of healthy and mastitic cows.
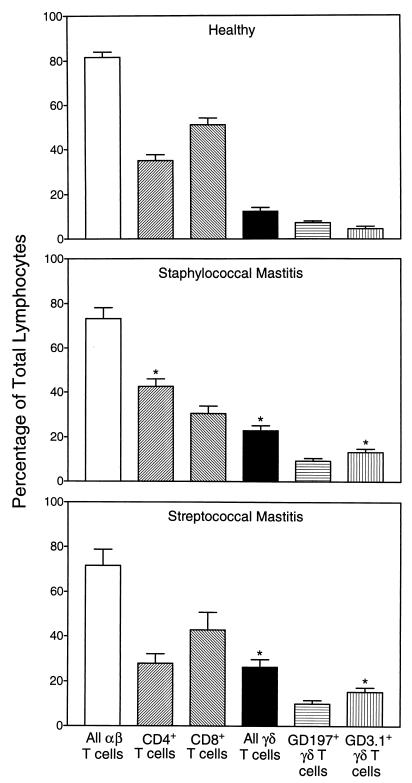
As shown in Fig. 1, milk samples from cows with staphylococcal and streptococcal mastitis contained approximately 75% more αβ T cells than and twice as many γδ T cells as samples from healthy cows. Further analysis of the T-cell subsets responsible for these changes showed that different αβ and γδ subsets were selectively recruited, depending on the mastitis pathogen (Fig. 2). In milk from cows with staphylococcal mastitis, the increase in αβ T-cell numbers was due primarily to a selective increase in CD4+ T-cell numbers, changing the CD4+/CD8+ ratio from 0.68 in healthy cows to 1.39 in mastitic cows (Fig. 2). In contrast, the increase in αβ T-cell numbers in milk from cows with streptococcal mastitis, which was similar in amplitude to that in cows with staphylococcal mastitis, was due to increases in both CD4+ and CD8+ T-cell numbers, without a major change in either subset relative to the other (CD4+/CD8+ ratio, 0.65).
FIG. 2.
Lymphocyte subset distribution in milk of cows with staphylococcal or streptococcal mastitis. Purified lymphocytes were isolated from the milk of 10 healthy lactating cows, 10 cows with staphylococcal mastitis, and 10 cows with streptococcal mastitis. The cells were labeled with monoclonal antibodies against CD2, CD4, CD8, pan-γδ TCR, GD3.1+ γδ TCR subset, and GD197+ γδ TCR subset and analyzed by flow cytometry as described in Materials and Methods. The percentage of total lymphocytes staining above the background (secondary antibody only) for the specific antigens listed above was determined. The data are expressed as mean ± standard error of the mean (N = 10). The asterisk indicates statistically significant differences (P, <0.05) between mastitis and healthy samples.
Analysis of γδ T-cell subsets in milk samples from cows with streptococcal and staphylococcal mastitis showed that the increase in γδ T-cell numbers shown in Fig. 1 was due, in part, to significantly increased levels of GD3.1+ γδ T cells, while the levels of GD197+ γδ T cells remained similar to those in milk samples from healthy cows (Fig. 2). The remaining increase in γδ T-cell numbers appeared to be due to a variable increase in CD8+ γδ T-cell numbers in the milk of mastitic cows (5 to 20%). This observation is consistent with the studies of Park et al. (43), who reported the presence of elevated numbers of CD8+ γδ T cells in the milk of cows with staphylococcal mastitis. However, not all animals consistently displayed increased levels of this subset of γδ T cells, which is negative for WC1, GD3.1, and GD197 (31, 65). For example, Fig. 3 shows that a relative increase in γδ T-cell numbers in the milk of a cow with mastitis is primarily due to an increase in CD8− cell numbers. Furthermore, the increase in CD8+ T-cell numbers in this animal is due primarily to an increase in αβ T-cell numbers, consistent with previous reports that the predominant T cells in bovine milk are CD8+ T cells (41, 56). In any case, our studies do show that different αβ and γδ T-cell subsets are recruited to the udder, depending on the mastitis pathogen and the host immune status.
Effect of mastitis on neutrophil and lymphocyte adhesion molecule expression.
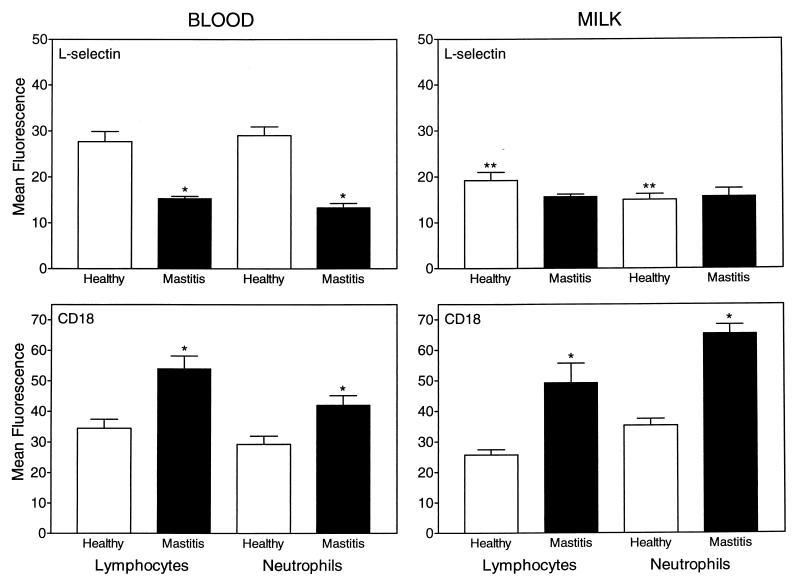
One of the earliest events observed after leukocyte priming or activation is a change in the level of cell surface adhesion molecule expression (4, 20, 27, 28, 30). Therefore, we analyzed how mastitis affected the level of expression of two adhesion molecules known to be sensitive indicators of cell activation: L-selectin and CD18. Using flow cytometric analysis, we found that mastitis infection caused a significant down-regulation of L-selectin expression on blood lymphocytes and neutrophils (Fig. 4). In milk samples from healthy cows, L-selectin expression was down-regulated on both lymphocytes and neutrophils compared to the levels of these cells in blood samples (Fig. 4), consistent with previous studies showing that L-selectin is shed during exudation (30). Interestingly, lymphocytes and neutrophils in the milk of cows with mastitis expressed levels of L-selectin similar to those isolated from healthy cows, indicating that maximal down-regulation of L-selectin may have occurred in the blood and/or during migration from the blood into the udder (Fig. 4). Analysis of CD18, the common β subunit of the β2 integrins (55), showed that CD18 was up-regulated on lymphocytes and neutrophils from the blood and milk of cows with mastitis compared to cells from the blood and milk of healthy cows (Fig. 4).
FIG. 4.
Adhesion molecule expression on blood and milk leukocytes obtained from healthy and infected cows. Purified lymphocytes and neutrophils isolated from the blood and milk of 10 healthy cows and 20 cows with acute mastitis were labeled with anti-L-selectin or anti-CD18 monoclonal antibodies followed by an FITC-labeled secondary antibody and then analyzed by flow cytometry as described in Materials and Methods. The results are expressed as mean fluorescence intensity ± standard error of the mean for cells staining positively for these antigens. ∗, statistically significant differences (P, <0.05) between mastitis and healthy samples; ∗∗, statistically significant differences (P, <0.05) between comparable milk and blood samples.
In contrast to our data showing L-selectin staining on milk leukocytes, Schmaltz et al. (49) reported that milk CD4+ and CD8+ T lymphocytes do not express Lam-1 (L-selectin), although closer inspection of their data does show staining of a small population of these cells. One possible explanation for this difference may be in the affinity of the antibody reagents used to stain L-selectin in these studies. In addition, staining of L-selectin on γδ T cells (except for possibly the small number of CD8+ γδ T cells) would have been missed. Therefore, we used three-color flow cytometric analysis to further investigate this issue. As shown in Fig. 5, a subpopulation of both αβ and γδ T cells stained positively for L-selectin (∼5 to 10%). In addition, the higher level of L-selectin staining on γδ T cells versus αβ T cells confirms the results of previous studies by Walcheck and Jutila (61), who reported that bovine γδ T cells express L-selectin at higher levels than αβ T cells. In any case, our results clearly show L-selectin staining on milk leukocytes.
FIG. 5.
Three-color flow cytometric analysis of L-selectin expression on bovine blood and milk lymphocytes. Purified lymphocytes were isolated from the blood (A to C) and milk (D to F) of cows with mastitis, and three-color flow cytometric analysis was performed as described in Materials and Methods. (A and D) Staining of blood (A) and milk (D) lymphocytes with antibody Dreg-56 (FL2, specific for L-selectin) versus antibody CC42 (FL1, specific for bovine CD2). (B and E) Staining of blood (B) and milk (E) lymphocytes with antibody GD3.8 (FL3, specific for γδ T cells) versus antibody Dreg-56 (FL2, specific for L-selectin). (C and F) Control staining levels with an isotype control antibody (same isotype as Dreg-56) in blood (C) and milk (F) samples. The data are representative of at least 10 independent experiments.
Since not all lymphocytes stain positive for L-selectin, we also analyzed whether mastitis infection caused changes in the relative numbers of L-selectin-positive lymphocytes. The relative percentages of L-selectin-positive lymphocytes were not significantly different (paired t test) in either blood (38.1% ± 7.0% versus 40.0% ± 10.2% [mean ± standard deviation; n = 5]) or milk (16.0% ± 2.4% versus 9.6% ± 1.8% [mean ± standard deviation; n = 5]) from healthy and mastitic cows, respectively. Thus, the changes observed in lymphocyte L-selectin expression appear to be due to the down-regulation of L-selectin expression and not to changes in the relative percentages of L-selectin-expressing cells. In addition, evaluation of milk leukocyte L-selectin expression showed that L-selectin down-regulation primarily occurred on αβ T cells (Fig. 5), while little L-selectin down-regulation was observed for γδ T cells. Consistent with this observation, the down-regulation of L-selectin on blood lymphocytes from mastitic cows was also due primarily to the down-regulation of L-selectin on αβ T cells rather than γδ T cells (data not shown).
DISCUSSION
The interaction between invading bacteria and the host immune system is a key factor in determining the outcome of an infection (29). Consequently, an effective immune response against any pathogen requires the migration of various sets of myeloid and lymphoid cells into the tissues during the inflammatory response (19). An essential component of the defense of the bovine udder against infection involves the recruitment of large numbers of neutrophils (40); however, it is clear that lymphoid cells are also involved in this process. Currently, little is known about the types of lymphoid cells recruited to the udder during mastitis. Therefore, to better understand the host defense process in mastitis, we have characterized the changes in lymphocyte subset populations present in the blood and milk of cows with naturally occurring staphylococcal and streptococcal mastitis.
The distributions of lymphocyte subsets were similar in blood samples obtained from cows with mastitis due to naturally occurring staphylococcal and streptococcal infections. However, in both cases, we observed a significant increase in the total number of γδ T cells in the blood of infected animals compared to the blood of healthy animals. In milk samples from infected animals, we observed even larger increases in the numbers of αβ and γδ T cells. In samples obtained from cows with staphylococcal mastitis, the increase in αβ T-cell numbers was due primarily to an increase in CD4+ T-cell numbers. In contrast, the increase in αβ T-cell numbers in milk from cows with streptococcal mastitis appeared to be due to parallel increases in both CD4+ and CD8+ T-cell numbers. This difference in responding αβ T-cell subsets is most likely due to the nature of the toxins released by staphylococcal and streptococcal bacteria during the acute stages of infection (44, 59). Indeed, recent studies by Ferens et al. (11) showed that treatment of bovine peripheral blood mononuclear cell cultures with staphylococcal enterotoxin C1 (SEC1) led to preferential activation and proliferation of CD4+ T cells. In addition, they found that activation of CD4+ and CD8+ T cells by SEC1 was significantly influenced by the proportion of γδ T cells present in the cultures and suggested that the γδ T-cell/αβ T-cell ratio might play a role in modulating the immune response to SEC1 (11). In support of this hypothesis, our present studies demonstrate a significant increase in the γδ T-cell/αβ T-cell ratio during naturally occurring mastitis infections.
The mechanisms of antigen recognition and stimulatory signals involved in the activation and proliferation of γδ T cells are not yet clearly defined (24). In humans and rodents, γδ T cells represent a minor T-cell population. In contrast, γδ T cells represent the predominant T-cell population in the circulation of newborn ruminants (16, 62). Despite the variable numbers of circulating γδ T cells in different species, recent data suggest that these cells play an important role in the initial host response to infectious agents (8). It has been proposed that γδ T cells complement αβ T cells during the host defense process by providing a rapid response before the αβ T-cell response has fully developed, i.e., “the first line of defense” (8, 64). γδ T cells have been shown to respond to antigen in the context of major histocompatibility complex molecules; however, most of these cells do not always require antigen processing to recognize bacterial antigens (3, 5, 32, 48). Interestingly, some activated γδ T cells also express high levels of major histocompatibility complex class II molecules on their surface and are able to present antigen to CD4+ T cells (6) and prime bacterial antigen-specific CD8+ T cells (39). These cells also appear to produce costimulatory molecules as well as cytokines, demonstrating that γδ T cells do indeed have the capability of influencing αβ T-cell function (6). Thus, the presence of increased levels of γδ T cells in milk obtained from cows with mastitis is consistent with their putative role in modulating the inflammatory response.
Although γδ T cells have been shown to play a role in contributing to the inflammatory response (54, 68), recent studies by several groups have suggested that γδ T cells may have a protective or anti-inflammatory function (10, 35, 36, 47). For example, γδ TCR gene knockout mice infected with Mycobacterium tuberculosis were able to control early infection in a manner similar to wild-type mice; however, a substantial pyogranulomatous response was observed in the knockout mice but not in the wild-type controls (10). These authors concluded that γδ T cells do not directly protect against infection but instead play a role in modulating local cellular traffic by promoting the influx of lymphocytes and monocytes and limiting the access of inflammatory cells that do not contribute to protection but can cause tissue damage (10). In support of this idea, Mukasa et al. (35) found that depletion of γδ T cells accelerated testicular inflammation in mice injected with Listeria monocytogenes. In addition, studies by Park et al. (42, 43) showed that a subset of CD8+ γδ T cells in bovine milk was responsible for the down-regulation of the response of CD4+ T cells to staphylococcal antigens. Finally, γδ T cells have also been found to produce growth factors that may play a role in the healing of epithelia damaged by infection or by inflammation (2, 22). Thus, it is clear that γδ T cells do play a role in modulating the inflammatory response; however, it is currently not known whether the primary role of increased levels of γδ T cells is to regulate the magnitude of the immune response or to directly contribute to host tissue protection. Studies are in progress to investigate this issue.
Several different γδ T-cell subsets have been identified, as defined by their respective TCR expression (18, 66), and recent studies have demonstrated that the distinct γδ T-cell subsets localize to specific tissues. For example, murine γδ T cells homing to the intestinal epithelium, skin, vagina, uterus, and tongue utilize a distinct γδ TCR (17). Recently, Wilson et al. (65) showed that the CD8+ CD2+ γδ T-cell subset exhibited a defined tissue tropism for the spleen but did not accumulate efficiently at sites of inflammation. In addition, Wilson et al. (66) described three novel anti-bovine γδ TCR antibodies (GD3.8, GD197, and GD3.1) and showed that these antibodies could be used to define a distinct γδ T-cell subset that preferentially localized in inflamed lymph node tissue. Specifically, GD3.1+ γδ T cells were found to be preferentially enriched at the site of inflammation (66). The data presented here support this observation, as we found that the increase in γδ T-cell numbers in milk from cows with acute mastitis was also primarily due to a preferential increase in GD3.1+ γδ T-cell numbers.
The recruitment of leukocytes is one of the first steps in the host response to infection, and leukocyte emigration from the blood to sites of inflammation involves a sequential interaction of adhesion molecules expressed by leukocytes and endothelial cells (1, 13). Two groups of adhesion molecules known to play important roles in this process are the selectins, which mediate leukocyte rolling on the endothelium (reviewed in reference 23), and the β2 integrins, which mediate tight adhesion and diapedesis (reviewed in reference 12). Both of these groups of adhesion molecules have been found to be sensitive indicators of leukocyte activation (20, 27). Depending on the treatment, neutrophil priming or activation can cause the shedding of L-selectin from the cell surface or the up-regulation of CD11b/CD18 or both (4, 7, 20, 27). Recently, it has also been shown that the exudation of human neutrophils into skin chambers in vivo causes the shedding of L-selectin and the modest up-regulation of CD11b/CD18 (30, 50). In the present studies, we found that lymphocytes and neutrophils from the milk of healthy cows expressed significantly lower levels of L-selectin than cells obtained from the blood of these cows; however, we observed very little up-regulation of CD18 in milk leukocytes compared to blood leukocytes. In contrast, lymphocytes and neutrophils obtained from the milk of cows with mastitis exhibited significant up-regulation of CD18, consistent with an activated state due to the presence of bacterial pathogens. Since these cells already down-regulated L-selectin during migration into the udder, little or no further down-regulation or shedding of L-selectin was observed in milk leukocytes isolated from cows with mastitis. In support of these findings, a similar decrease in L-selectin expression has been observed for caprine milk leukocytes (14). Interestingly, we observed the down-regulation or shedding of L-selectin and the up-regulation of CD18 in blood lymphocytes and neutrophils obtained from cows with mastitis compared to blood leukocytes obtained from healthy cows. Thus, changes in the expression of these adhesion molecules on blood leukocytes might represent a potential diagnostic indicator of mastitis that could be easily and rapidly evaluated.
The changes in adhesion molecule expression observed on cells from cows with mastitis reflect the overall host response to infection, where increased adhesion mediated through the β2 integrins would facilitate adherence to the pathogen, phagocytosis, and killing (12). Enhanced expression of β2 integrins would also facilitate leukocyte migratory potential through increased adhesive interactions with the endothelium, i.e., more effective transition from rolling to adherent cells. Shedding of L-selectin occurs during the process of diapedesis and may be required to dissociate the cells from ligands associated with the transmigration process and induce unresponsiveness to extracellular ligands (58). The role of L-selectin shedding by cells in the blood is not yet clear. One possibility is that soluble L-selectin plays a cytokine-like role in priming or even stimulating the host defense response. Soluble forms of L-selectin have been detected in the plasma of patients at risk for adult respiratory distress syndrome, and a correlation between reduced levels of soluble L-selectin and progression to this syndrome has been observed (9). In addition, recent studies by Ruchaud-Sparagano and coworkers (46) showed that soluble E-selectin exerted a proinflammatory effect on neutrophil function. Another possible function of L-selectin shedding by blood leukocytes is to limit the number of leukocytes accumulating at sites of inflammation and, thereby, to limit excessive tissue injury caused by these inflammatory cells. This idea is supported by the studies of McGill et al. (33), who reported that intravascular shedding of L-selectin might be a mechanism for controlling neutrophil exudation in patients with systemic inflammatory response syndrome. It has also been proposed that intravascular L-selectin shedding may be a general mechanism used by the human body to control inflammation, thus explaining the normal neutrophilia observed during inflammatory disease (45). In a similar manner, the intravascular shedding of L-selectin by leukocytes in mastitic cows may be a mechanism for controlling the level of leukocyte accumulation in the mammary gland and preventing further tissue damage due to inflammation.
In summary, we have characterized lymphocyte subset distributions and adhesion molecule expression on leukocytes from the blood and milk of healthy cows and cows with naturally acquired mastitis of staphylococcal and streptococcal origin. Our studies show that, depending on the type of mastitis pathogen, differential T-cell subset recruitment to the udder occurs. Both αβ and γδ T cells were recruited to the mammary gland; however, depending on the infectious agent, various ratios of CD4+ and CD8+ αβ T cells and distinct γδ T-cell subsets were found in the milk. In addition, the shedding of L-selectin and the up-regulation of CD18 by leukocytes in the blood may provide a sensitive indicator of early inflammatory responses during bovine mastitis. Further studies are necessary to define the specific roles of the various leukocyte subsets in the host immune response as well as the contributions of the pathogens involved. Understanding the role of specific T-cell subsets in mastitis may have a significant impact on the development of effective treatments or vaccines.
ACKNOWLEDGMENTS
We thank Mark Jutila and Eric Wilson (Montana State University, Bozeman) for generously providing antibodies for this research and for reviewing the manuscript. We also thank David Bos and Marilyn Bos (Faith Dairy, Bozeman, Mont.) for allowing us to study cows from their dairy herd.
This work was supported in part by USDA/NRICGP grant 9502274 (to M.T.Q.), USDA/NRICGP grant 9903508 (to J.S.), NSF equipment grant DBI-9604797 (to M.T.Q.), NIH equipment grant S10 RR11877, an equipment grant from the M. J. Murdock Charitable Trust, USDA Animal Health Formula Funds, and the Montana State University Agricultural Experimental Station. Mark T. Quinn is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Ali H, Haribabu B, Richardson R M, Snyderman R. Mechanisms of inflammation and leukocyte activation. Med Clin N Am. 1997;81:1–28. doi: 10.1016/s0025-7125(05)70503-4. [DOI] [PubMed] [Google Scholar]
- 2.Boismenu R, Havran W L. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 3.Boismenu R, Havran W L. An innate view of gamma delta T cells. Curr Opin Immunol. 1997;9:57–63. doi: 10.1016/s0952-7915(97)80159-8. [DOI] [PubMed] [Google Scholar]
- 4.Borregaard N, Kjeldsen L, Sengelov H, Diamond M S, Springer T A, Anderson H C, Kishimoto T K, Bainton D F. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leukoc Biol. 1994;56:80–87. doi: 10.1002/jlb.56.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Chien Y H, Jores R, Crowley M P. Recognition by gamma/delta T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 6.Collins R A, Werling D, Duggan S E, Bland A P, Parsons K R, Howard C J. γδ T cells present antigen to CD4+ αβ T cells. J Leukoc Biol. 1998;63:707–714. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- 7.Condliffe A M, Chilvers E R, Haslett C, Dransfield I. Priming differentially regulates neutrophil adhesion molecule expression/function. Immunology. 1996;89:105–111. doi: 10.1046/j.1365-2567.1996.d01-711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Libero G. Sentinel function of broadly reactive human γδ T cells. Immunol Today. 1997;18:22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly S C, Haslett C, Dransfield I, Robertson C E, Carter D C, Ross J A, Grant I S, Tedder T F. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994;344:215–219. doi: 10.1016/s0140-6736(94)92995-5. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza C D, Cooper A M, Frank A A, Mazzaccaro R J, Bloom B R, Orme I M. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 11.Ferens W A, Davis W C, Hamilton M J, Park Y H, Deobald C F, Fox L, Bohach G. Activation of bovine lymphocyte subpopulations by staphylococcal enterotoxin C. Infect Immun. 1998;66:573–580. doi: 10.1128/iai.66.2.573-580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gahmberg C G, Tolvanen M, Kotovuori P. Leukocyte adhesion. Structure and function of human leukocyte β2-integrins and their cellular ligands. Eur J Biochem. 1997;245:215–232. doi: 10.1111/j.1432-1033.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 13.Gahmberg C G, Valmu L, Fagerholm S, Kotovuori P, Ihanus E, Tian L, Pessa-Morikawa T. Leukocyte integrins and inflammation. Cell Mol Life Sci. 1998;54:549–555. doi: 10.1007/s000180050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiguen F, Greenland T, Pardo E, Panaye G, Mornex J F. Flow cytometric analysis of goat milk lymphocytes: subpopulations and adhesion molecule expression. Vet Immunol Immunopathol. 1996;53:173–178. doi: 10.1016/0165-2427(96)05553-5. [DOI] [PubMed] [Google Scholar]
- 15.Harmon R J. Physiology of mastitis and factors affecting somatic cell counts. J Dairy Sci. 1994;77:2103–2112. doi: 10.3168/jds.S0022-0302(94)77153-8. [DOI] [PubMed] [Google Scholar]
- 16.Hein W R, Mackay C R. Prominence of γδ T cells in the ruminant immune system. Immunol Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- 17.Itohara S, Farr A G, Lafaille J J, Bonneville M, Takagaki Y, Haas W, Tonegawa S. Homing of a γδ thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 18.Jones W M, Walcheck B, Jutila M A. Generation of a new γδ T cell-specific monoclonal antibody (GD3.5). Biochemical comparison of GD3.5 antigen with the previously described workshop cluster 1 (WC1) family. J Immunol. 1996;156:3772–3779. [PubMed] [Google Scholar]
- 19.Jutila M A. Recruitment of γ/δ T-cells and other T-cell subsets to sites of inflammation. In: Serhan C N, Ward P A, editors. Molecular and cellular basis of inflammation. Totowa, N.J: Humana Press, Inc.; 1999. pp. 193–214. [Google Scholar]
- 20.Jutila M A, Rott L, Berg E L, Butcher E C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989;143:3318–3324. [PubMed] [Google Scholar]
- 21.Jutila M A, Watts G, Walcheck B, Kansas G S. Characterization of a functionally important and evolutionarily well-conserved epitope mapped to the short consensus repeats of E-selectin and L-selectin. J Exp Med. 1992;175:1565–1573. doi: 10.1084/jem.175.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagnoff M F. Current concepts in mucosal immunity. III. Ontogeny and function of γδ T cells in the intestine. Am J Physiol. 1998;274:G455–G458. doi: 10.1152/ajpgi.1998.274.3.G455. [DOI] [PubMed] [Google Scholar]
- 23.Kansas G S. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 24.Kaufmann S H. γδ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keefe G P. Streptococcus agalactiaemastitis: a review. Can Vet J. 1997;38:429–437. [PMC free article] [PubMed] [Google Scholar]
- 26.Kehrli M E, Jr, Shuster D E. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J Dairy Res. 1994;77:619–627. doi: 10.3168/jds.S0022-0302(94)76992-7. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto T K, Jutila M A, Berg E L, Butcher E C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto T K, Jutila M A, Butcher E C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotwal G J. Microorganisms and their interaction with the immune system. J Leukoc Biol. 1997;62:415–429. doi: 10.1002/jlb.62.4.415. [DOI] [PubMed] [Google Scholar]
- 30.Kuhns D B, Priel D A L, Gallin J I. Loss of L-selectin (CD62L) on human neutrophils following exudation in vivo. Cell Immunol. 1995;164:306–310. doi: 10.1006/cimm.1995.1174. [DOI] [PubMed] [Google Scholar]
- 31.MacHugh N D, Mburu J K, Carol M J, Wyatt C R, Orden J A, Davis W C. Identification of two distinct subsets of bovine γδ T cells with unique cell surface phenotype and tissue distribution. Immunology. 1997;92:340–345. doi: 10.1046/j.1365-2567.1997.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matis L. Specificity and selection of gamma-delta receptor-expressing T cells. Immunol Res. 1991;10:5–14. doi: 10.1007/BF02918163. [DOI] [PubMed] [Google Scholar]
- 33.McGill S N, Ahmed N A, Hu F, Michel R P, Christou N V. Shedding of L-selectin as a mechanism for reduced polymorphonuclear neutrophil exudation in patients with the systemic inflammatory response syndrome. Arch Surg. 1996;131:1141–1146. doi: 10.1001/archsurg.1996.01430230023005. [DOI] [PubMed] [Google Scholar]
- 34.McMichael A J, Gotch F M, Hildreth J E. Lysis of allogeneic human lymphocytes by nonspecifically activated T-like cells. Eur J Immunol. 1982;12:1002–1005. doi: 10.1002/eji.1830121204. [DOI] [PubMed] [Google Scholar]
- 35.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of αβ and γδ T cells. J Immunol. 1995;155:2047–2056. [PubMed] [Google Scholar]
- 36.Mukasa A, Lahn M, Pflum E K, Born W, O'Brien R L. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794. [PubMed] [Google Scholar]
- 37.Myllys V, Ridell J, Björkroth J, Biese I, Pyörälä S. Persistence in bovine mastitis of Staphylococcus aureusclones as assessed by random amplified polymorphic DNA analysis, ribotyping and biotyping. Vet Microbiol. 1997;57:245–251. doi: 10.1016/s0378-1135(97)00137-5. [DOI] [PubMed] [Google Scholar]
- 38.Naessens J, Howard C J, Hopkins J. Nomenclature and characterization of leukocyte differentiation antigens in ruminants. Immunol Today. 1997;18:365–368. doi: 10.1016/s0167-5699(97)81055-9. [DOI] [PubMed] [Google Scholar]
- 39.Nomura A, Matsuzaki G, Takada H, Hiromatsu K, Nabeshima S, Nakamura T, Kishihara K, Nomoto K. The role of γδ T cells in induction of bacterial antigen-specific protective CD8+ cytotoxic T cells in immune response against the intracellular bacteria Listeria monocytogenes. Immunology. 1998;95:226–233. doi: 10.1046/j.1365-2567.1998.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paape M J, Wergin W P, Guidry A J, Pearson R E. Leukocytes—second line of defense against invading mastitis pathogens. J Dairy Sci. 1979;62:135–153. doi: 10.3168/jds.S0022-0302(79)83215-4. [DOI] [PubMed] [Google Scholar]
- 41.Park Y H, Fox L K, Hamilton M J, Davis W C. Bovine mononuclear leukocyte subpopulations in peripheral blood and mammary gland secretions during lactation. J Dairy Sci. 1992;75:998–1006. doi: 10.3168/jds.S0022-0302(92)77842-4. [DOI] [PubMed] [Google Scholar]
- 42.Park Y H, Fox L K, Hamilton M J, Davis W C. Suppression of proliferative response of BoCD4+ T lymphocytes by activated BoCD8+ T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis. Vet Immunol Immunopathol. 1993;36:137–151. doi: 10.1016/0165-2427(93)90103-b. [DOI] [PubMed] [Google Scholar]
- 43.Park Y H, Jung S C, Moon J S, Ku B G, Wyatt C R, Hamilton M J, Fox L K, Davis W C. A subset of mammary gland γδ T lymphocytes downregulates BoCD4 T lymphocyte response to Staphylococcus aureusin cattle with intramammary infection. Korean J Immunol. 1994;16:19–27. [Google Scholar]
- 44.Rago J V, Schlievert P M. Mechanisms of pathogenesis of staphylococcal and streptococcal superantigens. Curr Top Microbiol Immunol. 1998;225:81–97. doi: 10.1007/978-3-642-80451-9_5. [DOI] [PubMed] [Google Scholar]
- 45.Rogowski O, Sasson Y, Kassirer M, Zeltser D, Maharshak N, Arber N, Halperin P, Serrov J, Sorkin P, Eldor A, Berliner S. Down-regulation of the CD62L antigen as a possible mechanism for neutrophilia during inflammation. Br J Haematol. 1998;101:666–669. doi: 10.1046/j.1365-2141.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- 46.Ruchaud-Sparagano M H, Drost E M, Donnelly S C, Bird M I, Haslett C, Dransfield I. Potential pro-inflammatory effects of soluble E-selectin upon neutrophil function. Eur J Immunol. 1998;28:80–89. doi: 10.1002/(SICI)1521-4141(199801)28:01<80::AID-IMMU80>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 47.Saunders B M, Frank A A, Cooper A M, Orme I M. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium aviuminfection in mice. Infect Immun. 1998;66:5508–5514. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schild H, Mavaddat N, Litzenberger C, Ehrich E W, Davis M M, Bluestone J A, Matis L, Draper R K, Chien Y H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 49.Schmaltz R, Bhogal B, Wang J, Wang Y Y, Mackay C R, Chen S S, Larson L. Characterisation of leucocytic somatic cells in bovine milk. Res Vet Sci. 1996;61:179–181. doi: 10.1016/s0034-5288(96)90099-5. [DOI] [PubMed] [Google Scholar]
- 50.Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- 51.Sipes K M, Edens H, Kehrli M E, Jr, Cutler J E, Miettinen H M, Jutila M A, Quinn M T. Analysis of surface antigen expression and host defense function in leukocytes from calves with bovine leukocyte adhesion deficiency: comparison of heterozygous and homozygous animals. Am J Vet Res. 1999;60:1255–1261. [PubMed] [Google Scholar]
- 52.Soltys J, Swain S D, Sipes K M, Nelson L K, Jutila M A, Quinn M T. Isolation of bovine neutrophils with biomagnetic beads: comparison with standard Percoll density gradient isolation methods. J Immunol Methods. 1999;226:71–84. doi: 10.1016/s0022-1759(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 53.Sordillo L M, Shafer-Weaver K, DeRosa D. Immunobiology of the mammary gland. J Dairy Sci. 1997;80:1851–1865. doi: 10.3168/jds.S0022-0302(97)76121-6. [DOI] [PubMed] [Google Scholar]
- 54.Spinozzi F, Agea E, Bistoni O, Forenza N, Bertotto A. γδ T cells, allergen recognition and airway inflammation. Immunol Today. 1998;19:22–26. doi: 10.1016/s0167-5699(97)01182-1. [DOI] [PubMed] [Google Scholar]
- 55.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 56.Taylor B C, Dellinger J D, Cullor J S, Stott J L. Bovine milk lymphocytes display the phenotype of memory T cells and are predominantly CD8+ Cell Immunol. 1994;156:245–253. doi: 10.1006/cimm.1994.1169. [DOI] [PubMed] [Google Scholar]
- 57.Taylor B C, Keefe R G, Dellinger J D, Nakamura Y, Cullor J S, Stott J L. T cell populations and cytokine expression in milk derived from normal and bacteria-infected bovine mammary glands. Cell Immunol. 1997;182:68–76. doi: 10.1006/cimm.1997.1215. [DOI] [PubMed] [Google Scholar]
- 58.Tedder T F, Steeber D A, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 59.Torres B A, Johnson H M. Modulation of disease by superantigens. Curr Opin Immunol. 1998;10:465–470. doi: 10.1016/s0952-7915(98)80122-2. [DOI] [PubMed] [Google Scholar]
- 60.Villanueva M R, Tyler J W, Thurmond M C. Recovery of Streptococcus agalactiae and Staphylococcus aureus from fresh and frozen bovine milk. J Am Vet Med Assoc. 1991;198:1398–1400. [PubMed] [Google Scholar]
- 61.Walcheck B, Jutila M A. Bovine γδ T cells express high levels of functional peripheral lymph node homing receptor (L-selectin) Int Immunol. 1994;6:81–91. doi: 10.1093/intimm/6.1.81. [DOI] [PubMed] [Google Scholar]
- 62.Walcheck B, Watts G, Jutila M A. Bovine γ/δ T cells bind E-selectin via a novel glycoprotein receptor: first characterization of a lymphocyte/E-selectin interaction in an animal model. J Exp Med. 1993;178:853–863. doi: 10.1084/jem.178.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walcheck B, White M, Kurk S, Kishimoto T K, Jutila M A. Characterization of the bovine peripheral lymph node homing receptor: a lectin cell adhesion molecule (LECAM) Eur J Immunol. 1992;22:469–476. doi: 10.1002/eji.1830220227. [DOI] [PubMed] [Google Scholar]
- 64.Williams N. T cells on the mucosal frontline. Science. 1998;280:198–200. doi: 10.1126/science.280.5361.198. [DOI] [PubMed] [Google Scholar]
- 65.Wilson E, Aydintug M K, Jutila M A. A circulating bovine γδ T cell subset, which is found in large numbers in the spleen, accumulates inefficiently in an artificial site of inflammation: correlation with lack of expression of E-selectin ligands and L-selectin. J Immunol. 1999;162:4914–4919. [PubMed] [Google Scholar]
- 66.Wilson E, Walcheck B, Davis W C, Jutila M A. Preferential tissue localization of bovine γδ T cell subsets defined by anti-T cell receptor for antigen antibodies. Immunol Lett. 1998;64:39–44. doi: 10.1016/s0165-2478(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 67.Yancey R J J. Vaccines and diagnostic methods for bovine mastitis: fact and fiction. Adv Vet Med. 1999;41:257–273. doi: 10.1016/s0065-3519(99)80020-2. [DOI] [PubMed] [Google Scholar]
- 68.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig B B, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]