Abstract
In social species, individuals may be able to overcome competitive constraints on cooperation by leveraging relationships with familiar, tolerant partners. While strong social ties have been linked to cooperation in several social mammals, it is unclear the extent to which weak social ties can support cooperation, particularly among non-kin. We tested the hypothesis that weakly affiliative social relationships support cooperative coalition formation using 10 years of behavioural data on wild female chimpanzees. Female chimpanzees typically disperse and reside with non-kin as adults. Their social relationships are differentiated but often relatively weak, with few dyads sharing strong bonds. Females occasionally form aggressive coalitions together. Three measures of relationship quality—party association, five-metre proximity and whether a dyad groomed—positively predicted coalitions, indicating that relationship quality influenced coalition partnerships. However, dyads that groomed frequently did not form more coalitions than dyads that groomed occasionally, and kin did not cooperate more than expected given their relationship quality. Thus, strong bonds and kinship did not bolster cooperation. We conclude that cooperative coalitions among female chimpanzees depend on social tolerance but do not require strong bonds. Our findings highlight social tolerance as a distinct pathway through which females can cultivate cooperative relationships.
This article is part of the theme issue ‘Cooperation among women: evolutionary and cross-cultural perspectives’.
Keywords: cooperation, weak ties, coalitions, female chimpanzees, social bonds
1. Background
Cooperation requires that partners reconcile their competing interests to each gain a direct net benefit from the cooperative behaviour [1], making partner choice a critical challenge. Aggressive coalitions are a widespread example of cooperation in animal societies [2–5]. Because aggressive coalitions often entail the risk of injury or retaliation and potential asynchronies in payoff, individuals are expected to select reliable or effective coalition partners in this context [4]. In many social mammals, coalition formation mirrors other social dynamics, including kinship and affiliative social preferences [1,3–8]. Kin are expected to cooperate because the resulting direct benefits will be bolstered by inclusive fitness [9]. Individuals may also cooperate when partners mutually or reciprocally benefit, regardless of relatedness [10]. In any of these circumstances, we generally expect that impediments to cooperation, such as competition over resources or a need for coordination, are more easily overcome when partners have greater familiarity, tolerance and reliability, and therefore affiliative relationships are often correlated with cooperation [4,6,10–12]. That expectation has been regularly supported for social ties that are relatively strong [13–18]. It remains unclear, however, whether comparatively weak social ties can also support cooperation, particularly among non-kin [3,4,6]. We address this by examining patterns of coalition formation among adult female chimpanzees (Pan troglodytes schweinfurthii), who disperse to new groups at sexual maturity. These females engage in low rates of affiliation, such that their bond strength is weak in comparison to male chimpanzees and female cercopithecines. However, female chimpanzee social ties vary widely in quality [19–23], raising the possibility that partner preferences have functional significance [14,24].
Among social mammals, the propensity to establish networks of social ties is thought to have evolved to support cooperation in competitive contexts [6,8,14,25–28]. In females, cooperation is often leveraged to improve status and access to resources (e.g. ring-tailed coatis, Nasua nasua [29]; white-nosed coatis, Nasua narica [30]; spotted hyaenas, Crocuta crocuta [3]; African wild dogs, Lycaon pictus [31]; several primate species, reviewed in [3]), and in males, to improve status and defend mates and territories (e.g. Assamese macaques, Macaca assamensis [16]; bighorn sheep, Ovis canadensis [32]; bottlenose dolphins, Tursiops truncatus [13,33,34]; Camargue horses, Equus caballus [35]; chimpanzees, Pan troglodytes [36–39]; lions, Panthera leo [40]; geladas, Theropithecus gelada [41]; African wild dogs, Lycaon pictus [31]; Guinea baboons, Papio papio [42]; reviewed in [28]). Strong bonds may be necessary to support cooperation when risks associated with cooperating are high or the benefits are unevenly distributed, because strong bonds can help reduce the risk of partner defection and/or increase the likelihood that the support will be reciprocated in the future [1,4,43,44].
Patterns of philopatry reinforce the opportunity to form strong bonds among a given sex, by allowing individuals to build familiarity with same-sex partners and maximize inclusive fitness benefits. Within primates, female philopatry and female bonding are proposed to have evolved to support cooperation by female kin in the defense of food patches [2,45–49]. For example, among certain species of capuchins, squirrel monkeys and cercopithecine monkeys, females are philopatric and preferentially associate with and groom selected partners, particularly close kin, and selectively support these partners in agonistic conflicts (reviewed in [3,8]). By contrast to those ‘female-bonded’ species, females in other primate species that are not female philopatric, such as chimpanzees [50] and red colobus monkeys [51], tend to form comparatively weak bonds with other females and cooperate relatively rarely. However, some species do not follow the expected pattern, such as bonobos, where females groom and form coalitions frequently, despite living with unrelated individuals, and even interact affiliatively with females from other groups [52,53].
Strong bonds are also not always a prerequisite for cooperation. Weak social bonds, also described as weak ties or weak relationships, may refer to relationships that are weakly differentiated and/or unstable over time (e.g. red colobus, Piliocolobus tephrosceles [51]; some black and white colobus groups, Colobus vellerosus [54,55]), or relationships that are well differentiated and stable but exhibit a low frequency of overt indicators of social preference, such as grooming, in comparison to conspecifics (e.g. blue monkeys, Cercopithecus mitis stuhlmanni [56]; chacma baboons, Papio ursinus [57]; female chimpanzees, P. troglodytes [58]; some black and white colobus groups (C. vellerosus) [54]). In this paper, we refer to the latter, where weak ties are recognized by some degree of affiliation and tolerance but not by frequent interaction. These weak social bonds may be sufficient to support cooperation in specific contexts, such as when individuals have mutual interests. Establishing weak ties may help dyads overcome the costs of associating often, such as increased exposure to feeding competition [59,60], allowing a dyad to more frequently capitalize on chances for cooperation [61,62].
However, when the benefits of coalitions are mutual, coalitions could also arise opportunistically, where partner selection occurs entirely independently of differentiated ties. Such opportunistic coalitions are observed among female bonobos where coalition formation does not correlate with measures of dyadic relationship quality, including proximity and grooming, despite females' frequent affiliation and high rates of coalition formation [52,63]. Similarly, coalitions neither reflect measures of relationship strength nor kinship in crested macaques, who have a highly tolerant social style [64]. The direct effect of weak ties on partner preference is difficult to distinguish from the effect of opportunity. On the one hand, weak ties may simply influence the opportunity to form coalitions if higher rates of association, and the social tolerance accompanying this, make preferred social partners more likely to be nearby when the opportunity to form a coalition arises. On the other hand, weak ties could more directly support cooperation if individuals exhibit a preference among available partners based on their relationship quality. Teasing apart the extent to which weak ties influence opportunity and/or partner selection is central in understanding the mechanism through which weak ties may correlate with cooperation.
The behaviour of female chimpanzees presents a valuable opportunity to test whether weak ties can be leveraged for cooperation. In chimpanzees, females disperse and manage feeding competition largely by avoiding one another spatially, rather than engaging in aggressive inter- or intra-group competition over food patches [49,50,65,66] (but see [67]). As a result, both grooming and aggression are rare among females, relative to males or to females of many female philopatric primate species, and female chimpanzees are often described as having weak relationships [20,23,48,50,58,66]. While female dyads rarely meet the standard criteria of being ‘strong bonds’ based on relatively high levels of grooming, spatial measures of social preference reveal relationships that are stable over time and more differentiated than male relationships [20,21]. Additionally, cooperative coalitions between female chimpanzees have been documented at multiple sites, although they occur infrequently compared to male–male coalitions [50,68–70]. Previous research has reported that these coalitions are often in response to harassment from young, maturing males [71] or targeted against young females who are attempting to immigrate into the community [69]. Coalitions against the new females may function to suppress feeding competition from these females [69]. By contrast to bonobos, female chimpanzees rarely form coalitions in response to adult male aggression [69,70,72].
Given that social tolerance and predictability within a relationship are expected to reduce the challenges of cooperation among potential competitors and increase the reliability of coalition partners, we may expect female chimpanzees to form coalitions more often with individuals with whom they affiliate and associate more often. Some prior research supports this prediction. In a group of captive chimpanzees, pairs with more tolerant social relationships were more likely to cooperate in an experimental task, although this was observed in a context where less tolerant relationships meant less ability to directly benefit from the task [73]. Additionally, two studies of captive female chimpanzees reported that dyadic rates of coalition formation correlated with grooming and proximity behaviour [74,75]. And, among wild chimpanzees, Kahlenberg et al. [69] reported that female coalition partners frequently had similar range use, which may cultivate more tolerant relationships. In the Taï chimpanzee community, Boesch & Boesch-Achermann [68] anecdotally described frequent coalition formation among female dyads who associated frequently. These reports raise the possibility that relationships among female chimpanzees can influence the likelihood of cooperation.
Alternatively, given that relationships between female chimpanzees are subtle and cooperative coalitions occur rarely, we might expect coalition formation among female chimpanzees to be more opportunistic. That is, females could form a coalition with any available partner when the circumstances allow for mutual benefits, rather than forming coalitions that are supported by dyadic relationship quality. Supporting this perspective in chimpanzees, Newton-Fisher [70] reported that higher-ranking females typically assisted lower-ranking females and suggested that this may indicate that coalitions among female chimpanzees are not reciprocal. Thus, it is possible that female chimpanzees engage in coalitions opportunistically, like female bonobos, regardless of their relationship with the coalition partner.
In this study, we tested the hypothesis that female chimpanzees choose coalitionary partners based on relationship quality, as this may reflect differences in familiarity, tolerance or partner value. We conducted two analyses. First, we asked whether dyadic relationship strength predicted dyadic rates of coalition formation. We used four measures of relationship quality assessed during multiple-year study periods: the frequency that the dyad was seen in the same party given how often they were seen at all (party association index), the frequency that the dyad was within five metres of each other when in the same party (five-metre association index), whether the dyad was observed to groom (grooming presence) and the amount of time spent grooming when in the same party (grooming duration index). If female chimpanzees form coalitions opportunistically, like bonobo females, we predicted that only the party association index would predict coalition formation. However, if weak ties are sufficient to influence coalition partner choice, we expected that the five-metre association index and/or grooming presence would exert significant effects independently from the party-level association. Finally, if strong bonds are prerequisite to coalition formation, we expected all four measures to positively predict coalition formation, with grooming duration exerting a large effect.
If certain partners associate in parties at higher rates than others, it is possible that they naturally have more opportunity to form coalitions, even if they selected randomly from available partners at the time of coalition formation. To address this possibility, we conducted a second analysis to determine whether, at the time when a coalition was formed, females selected coalition partners with whom they associated and affiliated more often out of available females in the party. Our predictions for this analysis were the same as for the first analysis. In both analyses, we sought to determine the effect of relationship strength while controlling for the influence of dominance rank and kinship.
2. Methods
(a) . Study site and study subjects
We collected data on wild, habituated chimpanzees of the Kanyawara community in Kibale National Park, Uganda. During this study, the community ranged from 44–55 individuals, including 15–18 adult females in the community at any one time period. Of the 29 females in the community over the course of the study, N = 26 met our criteria (below) to be included in the study (see electronic supplementary material, table S1 for observation effort per female). Females were considered adults if they had ever been observed with a maximally tumescent sexual swelling.
(b) . Behavioural data
We used 10 years of behavioural data collected from 1 January 2010 to 31 December 2019. During this time, a team of two to five local field assistants collected daily observations of community-level party composition, aggression and social behaviour paired with full-day focal follows that rotated among all community members, where each individual was followed approximately once per month. The field assistants recorded party membership (all individuals within 50 m of any other individual) on scans every 15 minutes (party scans) and documented all observed occurrences of coalition formation ad libitum. During focal follows, field assistants recorded the presence of neighbours within 5 m on 15-minute scans, and recorded activity, including grooming, on 1-minute scans.
We assessed dyadic relationship quality and coalition formation during five 2-year (biennial) periods (following [20,58]), where ‘dyad-period’ refers to each occurrence of a unique dyad in a biennial period. We examined four measures of dyadic relationship quality indices: party association index, five-metre association index, presence of grooming and grooming duration index. We used the community-level data to determine the frequency of coalition formation and calculate the party association index and used scan data from the full-day focal follows to measure the five-metre association index and the presence and duration of grooming. The indices were calculated as:
where PAB is the number of party scans containing females A and B, PA is the number of party scans containing A and PB is the number of party scans containing B [20,58] (also called a simple ratio index [76]).
where is the number of 15-minute focal scans where A was the focal and B was within 5 m, is the number of scans where B was the focal and A was within 5 m, is the number of scans where A was the focal and B was in the party and is the number of scans where B was the focal and A was in the party [58].
where is the number of 1-min focal scans where A and B were seen grooming when A was the focal, is the number of scans where A and B were seen grooming when B was the focal and the denominator is the same as the five-metre association index. The presence of grooming was defined as the dyad grooming at least once during a focal follow during the biennial period.
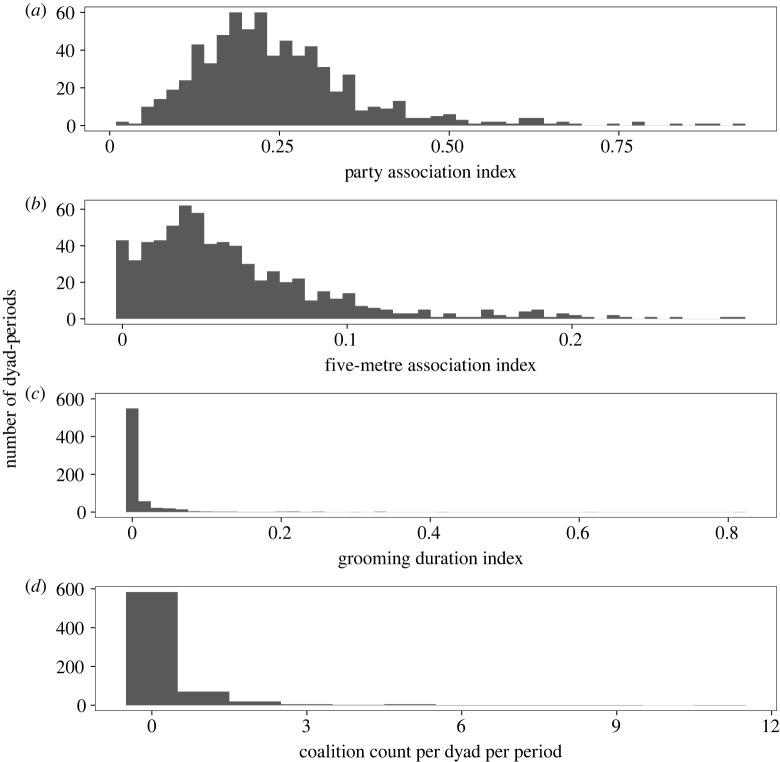
Grooming is a valuable indicator of social preference because it is a deliberate and targeted behaviour, and therefore a clear indicator of intent to associate. Including a binomial and continuous measure of grooming statistically accommodated the highly discontinuous and zero-inflated distribution of grooming among females [77,78] and allowed us to distinguish between the alternative predictions that social tolerance enables coalition formation or that strong social bonding is required. Because grooming is rare among female chimpanzees, even a low rate of grooming may be indicative of a higher-quality relationship compared with dyads who never groomed. However, a small number of dyads groomed frequently, indicating strong relationships (figure 1c). For example, in our dataset, 49% of dyads (N = 127) never groomed during the study, the median grooming duration index for all dyad-periods was 0, and the mean was 0.015 (±0.056 s.d., range 0–0.815). This translates to a mean of approximately 0.05 minutes of grooming per hour that a dyad was in the same party, while the dyad with the strongest grooming bond groomed for approximately 3.26 minutes per hour. Additionally, proximity is widely employed as a measure of social preference in primates and other social mammals [14,59,79], especially in animals that do not groom often, and spatial relationships are often correlated with grooming (e.g. female chimpanzees [20,21]; male and female Assamese macaques [16,80]; female red colobus [51]; female blue monkeys, Cercopithecus mitis [81]). In our data, histograms show that the distribution of five-metre association was more right-skewed compared to the party association, while grooming duration was even more skewed than five-metre association (figure 1). This suggests that these measures are increasingly meaningful indicators of relationship quality (party association, then five-metre association, then grooming), as females are more selective in the individuals with whom they engage in high frequencies of spatial proximity or grooming. Although measures were correlated (electronic supplementary material, table S2), variance inflation factors within our models were low (less than 3), indicating that it was acceptable to include these predictors together in a model.
Figure 1.
Histograms demonstrating the right-skewness of the three continuous relationship quality indices (a–c) and the frequency of coalitions (d).
We defined a coalition (N = 128 events) as two or more adult females cooperating in joint aggression to attack a common conspecific target [3,4,69,82] (N = 122), or as a joint non-vocal display with no clear target (N = 6). Non-vocal displays were included (following [72]) because they are a joint aggressive behaviour that likely functions to intimidate conspecifics [83]. Joint vocal displays were not included because they generally appear to represent long-distance communication rather than aggression. Twenty coalitions involved three to five females, and therefore more than one dyad, resulting in N = 197 dyadic coalition events. To avoid the issue that females might join a coalition together if they are simultaneously attracted to join an adult male in the coalition, we only analysed events that involved two or more adult females but no adult males as part of the coalition. Coalitions were considered independent events if they involved a different combination of female aggressors and/or targets or occurred at least 10 minutes apart. If two females were forming a coalition and were joined during the event by a third, this was counted as one coalition event.
High-ranking individuals may be generally more attractive affiliative and coalitionary partners, and high-ranking females have been found to form more coalitions overall ([70], our data). Therefore, we controlled for dominance rank in our analyses. We determined dominance ranks using an Elo rating method [84] to analyse long-term data on aggression and submission behaviour between females (see [71] for additional details). Elo scores were transformed into an ordinal rank per day and averaged for each female across each 2-year period. Immigrant females were incorporated into the dominance hierarchy after a six-month burn-in period starting from the date that they had their first agonistic encounter with another female. This delay, plus any time to the first agonistic encounter after the burn-in period, meant that in some cases we were unable to determine dominance rank for a new female during her first biennial period. Therefore, dyads including these females were excluded from the analysis during that period (see below), though this involved so few dyads that it is unlikely to have biased our results.
Additionally, it was necessary to assess the influence of kinship because some females in our study remained to reproduce in their natal community. Prior research suggests that when this occurs, related females tend to maintain strong relationships throughout adulthood [21,50]. We dichotomized kinship into two categories based on known pedigrees. Kin included mother–daughter dyads (N = 4) and maternally related sister dyads (N = 3), who contributed N = 14 dyad-periods to our dataset. All other dyads (N = 243 unique dyads) consisted of non-natal females and were categorized as non-kin. The assumption that immigrant females in this community are not related to each other has been supported for the females whose genetic data are available.
(c) . Long-term correlational analysis
To assess whether dyadic relationship quality correlated with coalition formation, we used multi-model inference to determine if any of the four relationship strength measures, or a combination of measures, best-predicted dyadic counts of coalitions at the level of the dyad-period. For each model, we used a generalized linear mixed-effects model with a Poisson distribution (glmer function, lme4 package [85]), where the dependent variable was the count of coalitions per dyad per biennial period and the biennial relationship measures were the predictors. In all candidate models, we included the number of scans where the dyad was seen in the same party (per biennial period) as a log-transformed offset to control for differences in observation, controlled for dominance rank by including the summed rank for the dyad, and included kinship as a control variable. We included a random effect for period in order to account for community-level differences in coalitions between years. To account for individual variation in sociality and tendency to form coalitions, we included two random effects for each chimpanzee ID in the dyad. Because the order of these random effects can affect results, we randomly assigned each ID to random effect 1 and 2 and then ran each model, permuting this process 1000 times (following [64]).
To identify which, if any, relationship strength measures best-predicted coalition formation, we compared a null model, which included only the control variables and random effects, with 15 candidate models that included all possible combinations of the relationship strength measures. We used AICC values to assess model fit [86–88] and identified which models occurred within the top 95% of cumulative model weights (dredge function, MuMin package [89]) [86,90]. However, to accommodate the permutations in our multi-model inference procedure, we also permuted the model comparison process 1000 times. We inspected the frequency with which each model occurred in the top 95% confidence set out of the 1000 model comparisons and rejected all models that did not occur within the 95% confidence set in more than 95% of permutations (similar to [91,92]). Having detected the ‘true’ top models, we retrieved these models and re-calculated the averaged model using only these models, thereby computing 1000 model averages (model.avg function, MuMIn package, [89]). Our results include (i) the top models, with average effect size estimates and standard errors from the 1000 permutations and (ii) the final averaged model, based only on the top models. For all predictors in the top models and the averaged model, we also report the frequency that 95% confidence intervals excluded zero across the 1000 iterations.
For this analysis, each dyad-period was included only if the dyad was seen in the same party for at least 200 party scans during the 2-year period (i.e. approx. 50 hours of observation time) to ensure enough observation hours where coalitions might be observed. This resulted in the removal of 98 dyad-periods (12% of all possible dyads) that did not meet this criterion.
We additionally removed 10 dyad-periods where the females were not observed in the same party during at least one scan when one was the focal animal. In most cases, these dyads were observed together infrequently due to demographic changes that took place during the time period, including immigration, maturation, death and disappearance of females. Three females were not seen frequently enough to be included in the analysis at all due to their short-term presence in the community (electronic supplementary material, table S1). Additionally, 14 dyad-periods were removed due to unavailable dominance data. Ultimately, N = 689 dyad-periods were included in this analysis (N = 257 unique dyads, N = 26 females).
(d) . Partner selection analysis
Our second analysis assessed whether, when females formed a coalition, they selected a partner with whom they had a higher-quality relationship than other females present at that time. We used a logistic linear mixed effect regression (glmer function, lme4 package [85]) to assess whether the selected partner(s) was predicted by higher dyadic relationship quality measures than other possible partners present at the time of a coalition. As above, we used multi-model inference to examine which combination of relationship strength measures, if any, best predicted partner selection. For each coalition, we identified all females in the party at the time of the event. The dyad(s) involved in the coalition was assigned an outcome of 1 for partner selection. Then we paired each of the females who were involved in the coalition with each other female available in the party at the time and assigned the outcome as 0. Thus, each dyad in the analysis included at least one female who was involved in the coalition paired with one potential partner. Predictors for each dyad were the dyadic relationship strength measures for the 2-year period during which the event occurred. We included a unique event ID, the ID of the potential partner and the time period as random effects. We additionally controlled for the number of females available in the party at the time of the event and the summed dominance rank of the dyad. We excluded six dyads due to unavailable dominance data. Additionally, we removed six coalitions (N = 18 dyad-events) where there were no females in the party besides those who were in the coalition. After these exclusions, our dataset included N = 122 coalitions, N = 215 unique dyads and N = 1815 dyad-events, which included N = 191 partner selection events. Across all coalitions, N = 25 different females were present as potential partners at the time of a coalition.
(e) . Model assessment
For both the long-term correlation analysis and the partner selection analysis, we evaluated our hypotheses by considering which predictors appeared among the top models. We assessed the relative importance of predictors by examining the effect size estimates and confidence intervals in the averaged model. All continuous variables were standardized as z-scores to allow for comparison between effect sizes.
We assessed model assumptions by testing for overdispersion and visually inspecting residual plots and qq-plots using the DHARMa package in R [93]. We calculated variance inflation factors using Zettersten & Lupyan's [94] vif.mer function for mixed effect models. We calculated conditional r-squared values for the top models using the r.squaredGLMM function from the MuMin package using the trigamma and delta method for the long-term correlation and partner selection analysis, respectively [89,95].
Although we controlled for kinship in the original analyses, we additionally ran both analyses after removing kin dyads to confirm that the patterns were not driven by these strong but rare relationships, which are effectively outliers. Finally, we conducted a post hoc analysis where we excluded dyads that included recent immigrant females to determine whether resident females distinguish among other resident females as potential coalition partners. Given that resident females target coalitions toward immigrating females [69], this tests whether the observed patterns in our original analysis could be explained by broad differences in social tolerance among residents versus between residents and new immigrants. Thus, we ran both analyses after removing new females (N = 8 immigrants from 2008 to 2019) as potential coalition partners during the 2-year period in which they immigrated and the subsequent 2-year period.
3. Results
Over the entirety of the study, 22 of 26 females were involved in at least one coalition. However, coalitions occurred rarely and were dispersed among dyads. The median number of coalitions per dyad per biennial period was 0, and for dyads that formed at least one coalition in a biennial period, the median was 1 (max = 11 coalitions per dyad per period). A mean of 16% of dyads formed at least one coalition during each biennial period, and 28% of all possible unique dyads formed a coalition during the study. No dyad was observed to form a coalition in all five of the biennial periods. Three dyads formed coalitions in four of the biennial periods.
In the targeted coalitions, females typically attacked one conspecific, but on five occasions two individuals were targeted and on one occasion three individuals were targeted. Of the 129 total targets, 56 (43.4%) were adult resident females, 18 (14.0%) were immigrant females (adult females who had immigrated but not yet conceived), 42 (32.6%) were males under age 15 years and 13 (10.1%) were adult males (over age 15 years). Of those targeted at adult males, five coalitions were directed toward males between the ages of 15–16 years, indicating that most female coalitions against males, even those against adults, are toward young males.
(a) . Long-term correlational analysis
In evaluating the association between relationship quality and the number of coalitions a dyad formed, top models retained all four measures of relationship quality. Grooming presence and the party association index were the most informative predictors, which both had strong, positive influences on the number of coalitions a dyad formed (table 1, full results in electronic supplementary material, table S3). In the averaged model, these predictors had relatively large effect sizes and confidence intervals that excluded zero in more than 95% of permutations. Grooming presence exerted the strongest statistical effect, with an effect size approximately 1.6 times the effect of the party association index. Though the five-metre association index was also included as a predictor in the two highest-ranked models, indicating it explained some variation in the data, confidence intervals for this predictor more frequently overlapped zero. Grooming duration did not explain much additional variation in coalition formation in these models.
Table 1.
Results of 1000 permutations of the multi-model inference procedure performed on four variables of dyadic relationship strength as predictors of the number of coalitions formed during biennial periods. The table contains the model diagnostics and estimates of predictors for the top three GLMM models, which fell within the top 95% cumulative weight confidence set on more than 95% of permutations, the null model and the weighted average estimates and error based on these top models. Bold values indicate where the confidence intervals around effect size estimates excluded zero in more than 95% of permutations. All continuous measures are z-scored.
| model | 1 | 2 | 3 | null | avg. |
|---|---|---|---|---|---|
| AICC mean | 683.055 | 684.877 | 685.67 | 727.442 | |
| d.f. | 9 | 10 | 8 | 6 | |
| ΔAICC | 0 | 1.822 | 2.615 | 44.388 | |
| weight | 0.474 | 0.192 | 0.139 | 0 | |
| cumulative weight | 0.474 | 0.666 | 0.805 | 1 | |
| freq. in 95% set | 1000 | 1000 | 979 | 0 | |
| conditional R2 | 0.093 | 0.093 | 0.094 | 0.101 | |
| predictors | |||||
| party association | |||||
| β | 0.315 | 0.324 | 0.453 | — | 0.340 |
| s.e.(β) | 0.119 | 0.121 | 0.102 | — | 0.127 |
| % CI exclude 0 | 96.4 | 97 | 100 | — | 98.4 |
| five-metre association | |||||
| β | 0.231 | 0.22 | — | — | 0.190 |
| s.e.(β) | 0.105 | 0.108 | — | — | 0.27 |
| % CI exclude 0 | 86.4 | 61.1 | — | — | 8.2 |
| grooming presence [Y] | |||||
| β | 0.532 | 0.515 | 0.667 | — | 0.550 |
| s.e.(β) | 0.193 | 0.196 | 0.184 | — | 0.199 |
| % CI exclude 0 | 99.6 | 98.4 | 100.0 | — | 99.5 |
| grooming duration | |||||
| β | — | 0.049 | — | — | 0.012 |
| s.e.(β) | — | 0.105 | — | — | 0.056 |
| % CI exclude 0 | — | 0 | — | — | 0 |
| control variables | |||||
| intercept | |||||
| β | −10.385 | −10.382 | −10.436 | −10.088 | −10.39 |
| s.e.(β) | 0.327 | 0.328 | 0.324 | 0.357 | 0.327 |
| % CI exclude 0 | 100.0 | 100.0 | 100.0 | 100.0 | 100 |
| summed dyad rank | |||||
| β | −0.403 | −0.415 | −0.366 | −0.467 | −0.400 |
| s.e.(β) | 0.132 | 0.134 | 0.133 | 0.146 | 0.133 |
| % CI exclude 0 | 99.8 | 100.0 | 98.8 | 100.0 | 99.8 |
| kinship | |||||
| β | −0.853 | −1.028 | −0.558 | 1.205 | −0.847 |
| s.e.(β) | 0.530 | 0.644 | 0.518 | 0.413 | 0.576 |
| % CI exclude 0 | 11.9 | 12.4 | 0.3 | 99.6 | 4.6 |
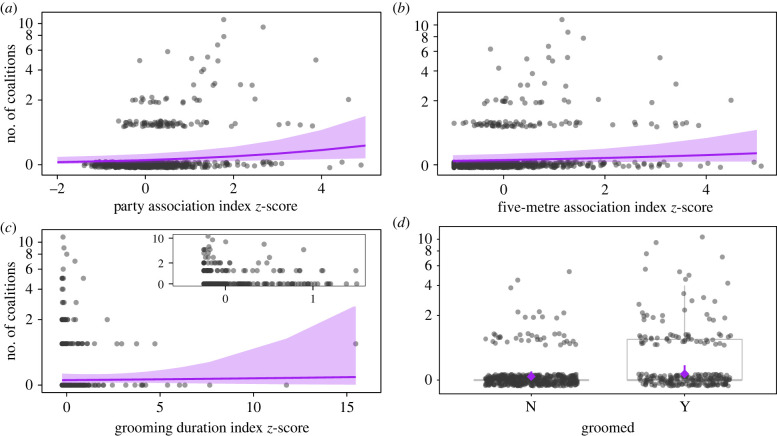
Because coalitions were rare overall, the model primarily predicted variation in coalition formation between 0 and 1, suggesting that as relationship quality increased, dyads were more likely to form a coalition but not necessarily many coalitions (figure 2, model predictions from the full model). For example, though dyads that groomed did not form a high number of coalitions (raw mean = 0.58 coalitions/2-yr period, range = 0–11), dyads that groomed still had a greatly increased likelihood of forming coalitions compared to dyads that did not groom (raw mean = 0.13 coalitions/2-yr period, range 0–5). Additionally, coalition formation did not increase with higher grooming duration. In other words, dyads that groomed each other were likely to form more coalitions than dyads that did not, but dyads that groomed frequently did not form more coalitions than those who groomed occasionally. Strong grooming relationships were rare. Although 32–65 dyads groomed per biennial period, only 13 out of 257 unique dyads ever groomed more than two standard deviations above the mean during a period. Of these, six were kin dyads.
Figure 2.
Results of the full model from one randomly selected permutation of the long-term correlation analysis showing the association between each dyadic relationship quality measure and number of coalitions. In all figures, grey data points represent one dyad-time period. In (a–c), purple lines show the model predictions with 95% confidence interval. In (d), purple diamonds show the predicted mean ± 95% confidence interval. All continuous x-axes are z-scored, and 0 represents the mean. In (c), note that most dyads never groomed nor formed coalitions, which is difficult to distinguish on the graph. Few dyads groomed frequently, making the raw mean of the grooming duration index close to 0 and, therefore, the grooming duration z-score data is heavily left skewed. The inset graph provides a close-up of the dyads who groomed slightly above or below average to help clarify this. (Online version in colour.)
Counter to expectations, kin dyads were no more or less likely to form coalitions when considering their relationship quality (table 1). Four of the seven kin dyads formed coalitions during the study. We confirmed that the patterns of this result remained consistent when we removed kin dyads from the analysis (electronic supplementary material, table S4).
(b) . Partner selection analysis
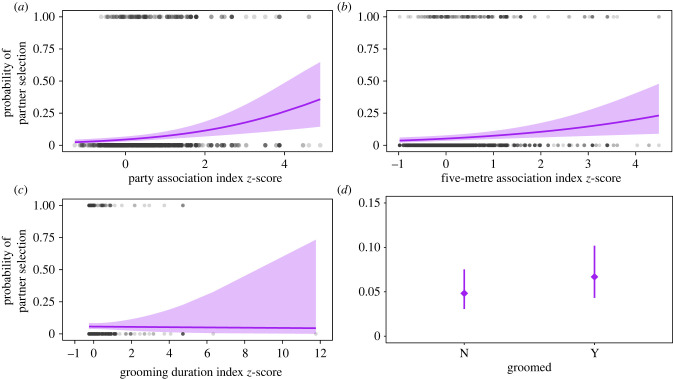
In evaluating females' choice of coalition partner among those present at the time of the coalition, again, all four measures were included among the top models, and the highest-ranked model included the same predictors. However, in this analysis, the party association index and five-metre association index were most informative, and both strongly and positively predicted partner selection in coalitions. These predictors had relatively large effect sizes and confidence intervals that excluded zero in the averaged model results (table 2, figure 3). Although grooming presence was included in two of the three top models, this predictor explained less variation compared to party association and five-metre association. And again, although grooming duration was included among the top models, this predictor had a negligible predictive effect. Kinship did not consistently influence partner choice and all patterns remained consistent when we removed kin dyads from the analysis (electronic supplementary material, table S5).
Table 2.
Results of multi-model inference procedure performed on four variables of dyadic relationship quality as predictors of partner selection at the time of a coalition event. The table contains the model diagnostics and estimates of predictors for the top three logistic LMM models, which fell within the top 95% cumulative weight confidence set, the null model and the weighted average estimates and error based on the top models. Bold values indicate that these predictors had confidence intervals that excluded zero. All continuous measures are z-scored.
| model | 1 | 2 | 3 | null | avg. |
|---|---|---|---|---|---|
| model diagnostics | |||||
| d.f. | 10 | 9 | 11 | 7 | |
| ΔAICc | 0 | 0.61 | 2.01 | 55.88 | |
| weight | 0.411 | 0.303 | 0.150 | 0.000 | |
| cumulative weight | 0.411 | 0.714 | 0.864 | ||
| conditional R2 | 0.110 | 0.108 | 0.110 | 0.091 | |
| predictors | |||||
| party association | |||||
| β | 0.508 | 0.525 | 0.505 | 0.514 | |
| s.e. | 0.129 | 0.132 | 0.131 | 0.131 | |
| 95% CI | 0.253 to 0.762 | 0.267 to 0.783 | 0.248 to 0.773 | 0.257 to 0.770 | |
| five-meter association | |||||
| β | 0.374 | 0.433 | 0.379 | 0.396 | |
| s.e. | 0.121 | 0.115 | 0.128 | 0.123 | |
| 95% CI | 0.138 to 0.610 | 0.208 to 0.658 | 0.129 to 0.630 | 0.154 to 0.637 | |
| grooming presence [Y] | |||||
| β | 0.340 | — | 0.344 | 0.222 | |
| s.e. | 0.207 | — | 0.210 | 0.234 | |
| 95% CI | −0.066 to 0.746 | — | −0.366 to 0.323 | −0.066 to 0.749 | |
| grooming duration | |||||
| β | — | — | −0.022 | −0.004 | |
| s.e. | — | — | 0.176 | 0.074 | |
| 95% CI | — | — | −0.366 to 0.323 | −0.367 to 0.323 | |
| control variables | |||||
| intercept | |||||
| β | −3.494 | −3.350 | −3.496 | −3.067 | −3.444 |
| s.e. | 0.255 | 0.239 | 0.256 | 0.256 | 0.259 |
| 95% CI | −3.995 to −2.993 | −3.818 to 2.882 | −3.999 to −2.994 | −3.650 to −2.585 | −3.952 to −2.936 |
| kinship [Y] | |||||
| β | −0.815 | −0.914 | −0.756 | 1.750 | −0.839 |
| s.e. | 0.617 | 0.621 | 0.780 | 0.496 | 0.652 |
| 95% CI | −3.995 to −2.993 | −2.103 to 0.303 | −2.84 to 0.773 | 0.739 to 2.735 | −2.118 to 0.440 |
| summed dyad rank | |||||
| β | −0.418 | −0.432 | −0.416 | −0.512 | −0.423 |
| s.e. | 0.129 | −0.432 | 0.129 | 0.125 | 0.129 |
| 95% CI | −0.670 to −0.166 | −0.684 to −0.180 | −0.669 to −0.163 | −0.770 to −0.266 | −0.675 to −0.170 |
| party size (females) | |||||
| β | −0.379 | −0.388 | −0.379 | −0.435 | −0.382 |
| s.e. | 0.103 | 0.102 | 0.103 | 0.099 | 0.103 |
| 95% CI | −0.580 to −0.178 | −0.588 to −0.187 | −0.580 to −0.178 | −0.639 to −0.240 | 0.583 to 0.323 |
Figure 3.
Results from the partner selection analysis full model showing the effect of each relationship quality measure on the probability of partner selection. In (a–c), each grey datapoint represents one dyad at the time of a coalition, and purple lines show model predictions with 95% confidence intervals. In (d), purple diamonds show the predicted mean ± 95% confidence interval (note different y-axis scaling in d). (Online version in colour.)
(c) . Post hoc analysis of resident dyads
Resident females may be expected to have higher tolerance and affiliation with other residents than new immigrants due to prolonged co-residency. Given the tendency of residents to form coalitions against immigrants, it is possible that our results were driven by a higher average relationship quality among residents, rather than a tendency to choose particular resident females over others. To address this possibility, we re-examined our models after removing newly immigrated females as coalition partners. This resulted in slightly different ranking of candidate models and less discrimination between top models. For the long-term analyses, the predictors in the top model and averaged model remained the same as they were reported in the original analysis (electronic supplementary material, table S6). However, the effect size for grooming presence decreased in the averaged model results, and the importance of grooming presence was less consistent across permutations (confidence intervals excluded zero in 90% of the averaged model permutations). In the partner selection analysis, the most notable difference was that the effect size for five-metre association was reduced in the averaged model, and its confidence intervals did not exclude zero (electronic supplementary material, table S7). This exercise suggests that selection between resident and immigrant partners can partly, but not completely, explain the effects of relationship quality on coalition formation.
4. Discussion
We evaluated the hypothesis that relationship quality influences coalition partner choice in female chimpanzees, as differences in proximity and affiliation may reflect differences in familiarity, tolerance or partner value. We compared this against the alternative hypothesis that cooperation is opportunistic, where alliances do not map onto social relationships. Consistent with the first hypothesis, party association, five-metre association and presence of grooming were important predictors of female coalitions. Party association and whether a dyad groomed independently predicted overall coalition rates, while party association and five-metre association independently predicted partner choice at the time of coalition formation. The few female dyads with strong bonds, characterized by high rates of grooming, did cooperate, but they did not form more coalitions nor were they more likely to choose each other as coalition partners compared to dyads with weaker affiliative bonds, characterized by occasional grooming. Our findings indicate that social tolerance facilitates cooperation in coalition formation for female chimpanzees, while kinship and strong social bonds do not bolster cooperation as it does in other female philopatric primates. This highlights a case where even relatively weak social ties, maintained largely through spatial association and occasional grooming, can support cooperation. This also adds to research emphasizing that relationships between female chimpanzees are differentiated and have downstream impacts on other aspects of female behaviour.
Five-metre association and grooming presence were important predictors of coalition formation in female chimpanzees, even when simultaneously considering the effect of party association, indicating that relationship quality is important for coalitionary partner choice independently of partner availability. However, it is conspicuous that high grooming duration did not result in more coalitions. This is consistent with anecdotal reports of female chimpanzees in the Taï community, where some female dyads associated intensely and formed coalitions more often but did not groom each other frequently, relative to other dyads [68]. On the one hand, the lack of influence of grooming duration on coalition formation demonstrates that cooperation among female chimpanzees can occur among dyads with even a low level of social affinity, in contrast to primate species where alliance partners groom often [15,96–98]. On the other hand, our results may point to functional differences for different types of social interactions among female chimpanzees. For example, some dyads in our dataset formed exceptionally strong grooming relationships, including kin, yet these dyads did not form proportionally more coalitions. Female dyads who groom frequently may have alternative motivations to invest in these social relationships, such as cultivating safe environments for their offspring to socialize with other families. Furthermore, most of the kin dyads in our dataset included one young female, a recently matured younger sister or daughter, and these females likely lack the rank, size and experience of an ideal coalition partner. Thus, while kinship promotes stronger relationships, demography may limit the occasions where kin make good coalition partners.
Effect sizes for grooming presence and five-metre association were reduced in models that excluded immigrant females, suggesting that a social divide between resident and immigrant females explained some of the variation in our data. This may explain why resident females, who are generally more familiar with one another, are able to form coalitions against immigrants relatively frequently [69]. However, in contrast to a report examining female coalitions from 1997 to 2006 in the same community [69], we documented more coalitions against resident females than immigrant females, indicating that our overall results cannot be explained solely by this phenomenon. Other factors shaping variation in social preference could include homophily in female age, offspring age and sex, timing of immigration or overlap in space use [19].
Female chimpanzees present a striking paradox where the threshold for cooperating in coalitions appears to be quite low, yet females rarely engage in them and do not appear to invest in stronger bonds to enhance the potential for cooperation. We identify two plausible explanations for this. One is that the opportunities for successful coalition formation in chimpanzees may be quite limited compared to the way that coalitions are used by females in other species. Unlike female-bonded cercopithecines, female chimpanzees rarely engage in direct competition over food patches, preferring to fission and avoid competition [48,66]. Unlike in bonobos, female chimpanzees rarely form coalitions to counteract aggression from adult males [52]. It is hard to say whether the definitive intersexual dominance of males over females in chimpanzees precludes female coalitions against males or results from the lack of coalitionary effort. However, female chimpanzees will form coalitions together in response to harassment from maturing males [71]. Additionally, females form more coalitions during periods when there is an influx of immigrant females [69]. These contexts occur sporadically over time due to demographic changes, raising the possibility that females might increase affiliative effort during particular periods in response to an influx of immigrant females or increased exposure to maturing males. Finally, like males, females may be using coalitions against immigrant and resident females to achieve higher dominance status. However, this has not been investigated and it is unclear how these opportunities might fluctuate over time.
Given these isolated coalitionary contexts, a second plausible explanation for the chimpanzee pattern is that the costs and benefits of these coalitions differ in fundamental ways from those documented in female-bonded species. In particular, where the risk and costs of losing a competition are high, we would expect that coalition partners would need to meet a relatively high threshold of relationship quality to ensure partner reliability. Additionally, if cooperation is driven at least partially by reciprocity, as opposed to mutualism, individuals should be more discriminating of partner selection, as they depend on partners for a long-term return on investment. Weaker, tolerant relationships may suffice if the coalition has lower risks and is mutually beneficial. For example, resident female chimpanzees may have a mutual interest in driving out immigrant females from high-quality foraging areas [69], as well as in quelling harassment from adolescent males. Both of these targets are still relatively small, less experienced or less able to attract coalition partners to defend themselves, making risks lower [69,72]. Among residents, it is possible that females choose to form coalitions when risks are low, such as high-ranking females engaging in coalitions against small, young or lower-ranking females.
Our results differ fundamentally from those reported for wild bonobos, where females frequently form aggressive coalitions, including against adult males. Coalition partner choice in bonobos appears to be opportunistic, unrelated to patterns of proximity or grooming [52,63]. However, it is possible that almost all bonobo female dyads achieve the level of social tolerance and affiliation needed to support coalition formation, which female chimpanzees achieve with only a select number of social partners, such that, for female bonobos, any female may be a viable coalition partner. Indeed, bonobo females both groom and form coalitions even with extra-group females [53,99]. As has been previously proposed, reduced feeding competition among female bonobos, extended sexual receptivity and increased gregariousness may have been critical in allowing bonobo females to form more widespread and available social connections and take advantage of them for cooperation [100–102]. Chimpanzee females clearly use coalitionary behaviour more conservatively, whereas the advantages of immediate and frequent coalitionary behaviour by female bonobos may outweigh any advantages of discriminating among available partners.
While most research has examined the benefits of strong social bonds, our study is not the first to consider the ways that weak ties can influence other aspects of behaviour. In humans, weak ties are thought to be beneficial for providing access to diverse knowledge and influencing group collective action because of the multitude of weak ties that a person can sustain and their effectiveness at bridging disparate groups [103,104]. Additionally, weak ties are thought to influence human health both directly, by influencing psychological well-being [105,106], and indirectly, by influencing overall social integration [105,107]. Among non-human primates, there is some evidence that weak ties can support cooperation. For example, grooming relationships among female black and white colobus monkeys, but not kinship, predicted co-participation in mutually beneficial intergroup encounters despite grooming occurring rarely in this species [54,108,109]. While our study has not attempted to connect social ties to fitness benefits in female chimpanzees, evidence demonstrating the fitness benefits of weak ties in other non-human primates has been mixed. Quantity, but not quality, of social ties predicted survival during an extreme cold season for barbary macaques (Macaca sylvanus), where individuals benefited from proximity to other warm bodies [110]. Female blue monkeys that were weakly tied to partners had higher survival than females with strong but inconsistent social ties [56]. And, studies on two separate populations of chacma baboons found conflicting results on whether the quantity of weak ties improved female fitness [57,111]. Whereas the benefits of weak ties may be difficult to detect against a backdrop of frequent social bonding, our findings suggest that weak ties may facilitate cooperation where strong ties are rare.
Our research highlights the diverse ways that social processes shape cooperation. The influence of weak ties on the formation of rare cooperative coalitions in female chimpanzees is distinct from the kinship-based interactions observed in many female philopatric primates (reviewed in [3]), from the strategic, strong bond-dependent coalitions seen in male chimpanzees [37,43,112], and from the opportunistic behaviour of female bonobos [52]. Increased attention to social tolerance and to the nuanced ways that individuals can cultivate cooperative relationships across ecological and phylogenetic contexts is needed to advance our understanding of the connection between social processes and the evolution of cooperation.
Acknowledgements
We are grateful to the field staff and project managers of the Kibale Chimpanzee Project for daily data collection and data entry. For database assistance, we thank Lindsey Hagberg, Jordan Lucore and Ashley Menante. We thank Joan Silk and two anonymous reviewers for their comments, which improved the manuscript.
Ethics
This study was approved by the Uganda National Council for Science and Technology, the Uganda Wildlife Authority and Makerere University Biological Field Station. The University of New Mexico, Tufts University and Harvard University Institutional Animal Care and Use Committees approved our research protocols.
Data accessibility
The data are provided in electronic supplementary material [113].
Authors' contributions
S.A.F.: conceptualization, formal analysis, funding acquisition, investigation, methodology, visualization, writing—original draft, writing—review and editing; M.N.M.: conceptualization, funding acquisition, methodology, project administration, supervision, writing—review and editing; N.T.G.: formal analysis, writing—original draft, writing—review and editing; D.K.E.: data curation, formal analysis, writing—review and editing; Z.P.M.: data curation, project administration, writing—review and editing; E.O.: data curation, project administration; R.W: data curation, project administration, writing—review and editing; M.E.T.: conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We have no competing interests.
Funding
This research was supported by the Leakey Foundation (grant no. 19-0066), the Wenner-Gren Foundation (grant no. Gr 9815), the Natural Sciences and Engineering Research Council of Canada (grant no. PGSD3-489996-2016), the U.S. National Science Foundation (grant nos NCS-FO-1926352, BCS-1355014, BCS-0849380), U.S. National Institute on Aging and NIH Office for Research on Women's Health (grant nos R01-AG049395 and R37AG049395) and the University of New Mexico.
References
- 1.Chapais B. 2006. Kinship, competence and cooperation in primates. In Cooperation in primates and humans: mechanisms and evolution (eds Kappeler PM, van Schaik CP), pp. 47-64. Berlin, Germany: Springer. [Google Scholar]
- 2.Harcourt AH. 1992. Coalitions and alliances: are primates more complex than non-primates? In Coalitions and alliances in humans and other mammals (eds Harcourt AH, deWall FB), pp. 445-471. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Smith JE, Van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE.. 2010. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 284-303. ( 10.1093/beheco/zzzzzarp181) [DOI] [Google Scholar]
- 4.Bissonnette A, Perry S, Barrett L, Mitani JC, Flinn M, Gavrilets S, de Waal FBM. 2015. Coalitions in theory and reality: a review of pertinent variables and processes. Behaviour 152, 1-56. ( 10.1163/1568539X-00003241) [DOI] [Google Scholar]
- 5.Chapais B. 1995. Alliances as a means of competition in primates: evolutionary, developmental, and cognitive aspects. Am. J. Phys. Anthropol. 38, 115-136. ( 10.1002/ajpa.1330380607) [DOI] [Google Scholar]
- 6.Hruschka DJ, Henrich J. 2006. Friendship, cliquishness, and the emergence of cooperation. J. Theor. Biol. 239, 1-15. ( 10.1016/j.jtbi.2005.07.006) [DOI] [PubMed] [Google Scholar]
- 7.Silk JB, Alberts SC, Altmann J. 2004. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim. Behav. 67, 573-582. ( 10.1016/j.anbehav.2003.07.001) [DOI] [Google Scholar]
- 8.Smith JE. 2014. Hamilton's legacy: kinship, cooperation and social tolerance in mammalian groups. Anim. Behav. 92, 291-304. ( 10.1016/j.anbehav.2014.02.029) [DOI] [Google Scholar]
- 9.Hamilton WD. 1964. The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17-52. ( 10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 10.Barrett L, Henzi P, Rendall D. 2007. Social brains, simple minds: does social complexity really require cognitive complexity? Phil. Trans. R. Soc. B 362, 561-575. ( 10.1098/rstb.2006.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berghänel A, Ostner J, Schröder U, Schülke O.. 2011. Social bonds predict future cooperation in male Barbary macaques, Macaca sylvanus. Anim. Behav. 81, 1109-1116. ( 10.1016/j.anbehav.2011.02.009) [DOI] [Google Scholar]
- 12.de Waal FBM. 1978. Exploitative and familiarity-dependent support strategies in a colony of semi-free living chimpanzees. Behaviour 66, 268-312. ( 10.1163/156853978X00143) [DOI] [Google Scholar]
- 13.Connor RC, Smolker RA, Richards AF. 1992. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl Acad. Sci. USA 89, 987-990. ( 10.1073/pnas.89.3.987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massen J, Sterck E, de Vos H. 2010. Close social associations in animals and humans: functions and mechanisms of friendship. Behaviour 147, 1379-1412. ( 10.1163/000579510X528224) [DOI] [Google Scholar]
- 15.Schino G, di Sorrentino EP, Tiddi B. 2007. Grooming and coalitions in Japanese macaques (Macaca fuscata): partner choice and the time frame reciprocation. J. Comp. Psychol. 121, 181-188. ( 10.1037/0735-7036.121.2.181) [DOI] [PubMed] [Google Scholar]
- 16.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207-2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 17.Gerber L, et al. 2021. Cooperative partner choice in multi-level male dolphin alliances. Sci. Rep. 11, 6901. ( 10.1038/s41598-021-85583-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St-Pierre A, Larose K, Dubois F. 2009. Long-term social bonds promote cooperation in the iterated Prisoner's Dilemma. Proc. R. Soc. B 276, 4223-4228. ( 10.1098/rspb.2009.1156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foerster S, McLellan K, Schroepfer-Walker K, Murray CM, Krupenye C, Gilby IC, Pusey AE. 2015. Social bonds in the dispersing sex: partner preferences among adult female chimpanzees. Anim. Behav. 105, 139-152. ( 10.1016/j.anbehav.2015.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilby IC, Wrangham RW. 2008. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behav. Ecol. Sociobiol. 62, 1831-1842. ( 10.1007/s00265-008-0612-6) [DOI] [Google Scholar]
- 21.Langergraber K, Mitani J, Vigilant L. 2009. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 71, 840-851. ( 10.1002/ajp.20711) [DOI] [PubMed] [Google Scholar]
- 22.Lehmann J, Boesch C. 2009. Sociality of the dispersing sex: the nature of social bonds in West African female chimpanzees, Pan troglodytes. Anim. Behav. 77, 377-387. ( 10.1016/j.anbehav.2008.09.038) [DOI] [Google Scholar]
- 23.Wakefield ML. 2013. Social dynamics among females and their influence on social structure in an East African chimpanzee community. Anim. Behav. 85, 1303-1313. ( 10.1016/j.anbehav.2013.03.019) [DOI] [Google Scholar]
- 24.Kummer H. 1978. On the value of social relationships to nonhuman primates: a heuristic scheme. Soc. Sci. Inf. 17, 687-705. ( 10.1177/053901847801700418) [DOI] [Google Scholar]
- 25.Apicella CL, Silk JB. 2019. The evolution of human cooperation. Curr. Biol. 29, R447-R450. ( 10.1016/j.cub.2019.03.036) [DOI] [PubMed] [Google Scholar]
- 26.Port M, Hildenbrandt H, Pen I, Schülke O, Ostner J, Weissing FJ. 2020. The evolution of social philopatry in female primates. Am. J. Phys. Anthropol. 173, 397-410. ( 10.1002/ajpa.24123) [DOI] [PubMed] [Google Scholar]
- 27.Walker RS, Bailey DH. 2014. Marrying kin in small-scale societies. Am. J. Hum. Biol. 26, 384-388. ( 10.1002/ajhb.22527) [DOI] [PubMed] [Google Scholar]
- 28.Cords M, Thompson NA. 2017. Friendships, coalitions, and alliances. In APA handbook of comparative psychology: basic concepts, methods, neural substrate, and behavior, vol. 1, pp. 899-913. Washington, DC: American Psychological Association. [Google Scholar]
- 29.Romero T, Aureli F. 2008. Reciprocity of support in coatis (Nasua nasua). J. Comp. Psychol. 122, 19-25. ( 10.1037/0735-7036.122.1.19) [DOI] [PubMed] [Google Scholar]
- 30.Gompper ME, Gittleman JL, Wayne RK. 1997. Genetic relatedness, coalitions and social behaviour of white-nosed coatis, Nasua narica. Anim. Behav. 53, 781-797. ( 10.1006/anbe.1996.0344) [DOI] [Google Scholar]
- 31.de Villiers MS, Richardson PRK, van Jaarsveld AS. 2003. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus). J. Zool. 260, 377-389. ( 10.1017/S0952836903003832) [DOI] [Google Scholar]
- 32.Pelchat GO. 2008. Buddies for life: male associations and coalitions in bighorn sheep. Master's of Science, University of Calgary. See https://scholar.google.com/scholar_lookup?title=Buddies%20for%20life%3A%20Male%20associations%20and%20coalitions%20in%20bighorn%20sheep%2C%20Ovis%20canadensis&publication_year=2008&author=G.O.%20Pelchat.
- 33.Gerber L, et al. 2020. Affiliation history and age similarity predict alliance formation in adult male bottlenose dolphins. Behav. Ecol. 31, 361-370. ( 10.1093/beheco/arz195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerber L, Connor RC, Allen SJ, Horlacher K, King SL, Sherwin WB, Willems EP, Wittwer S, Krützen M. 2022. Social integration influences fitness in allied male dolphins. Curr. Biol. 32, 1664-1669.e3. ( 10.1016/j.cub.2022.03.027) [DOI] [PubMed] [Google Scholar]
- 35.Feh C. 1999. Alliances and reproductive success in Camargue stallions. Anim. Behav. 57, 705-713. ( 10.1006/anbe.1998.1009) [DOI] [PubMed] [Google Scholar]
- 36.Duffy KG, Wrangham RW, Silk JB. 2007. Male chimpanzees exchange political support for mating opportunities. Curr. Biol. 17, R586-R587. ( 10.1016/j.cub.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 37.Feldblum JT, Krupenye C, Bray J, Pusey AE, Gilby IC. 2021. Social bonds provide multiple pathways to reproductive success in wild male chimpanzees. iScience 24, 102864. ( 10.1016/j.isci.2021.102864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373-381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langergraber KE, Mitani JC, Vigilant L. 2007. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786-7790. ( 10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bygott JD, Bertram BCR, Hanby JP. 1979. Male lions in large coalitions gain reproductive advantages. Nature 282, 839-841. ( 10.1038/282839a0) [DOI] [Google Scholar]
- 41.Snyder-Mackler N, Alberts SC, Bergman TJ. 2012. Concessions of an alpha male? Cooperative defence and shared reproduction in multi-male primate groups. Proc. R. Soc. B 279, 3788-3795. ( 10.1098/rspb.2012.0842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patzelt A, Kopp GH, Ndao I, Kalbitzer U, Zinner D, Fischer J. 2014. Male tolerance and male–male bonds in a multilevel primate society. Proc. Natl Acad. Sci. USA 111, 14 740-14 745. ( 10.1073/pnas.1405811111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitani JC. 2006. Reciprocal exchange in chimpanzees and other primates. In Cooperation in primates and humans: mechanisms and evolution (eds Kappeler PM, van Schaik CP), pp. 107-119. Berlin, Germany: Springer. [Google Scholar]
- 44.Ostner J, Schülke O. 2018. Chapter four - Linking sociality to fitness in primates: a call for mechanisms. Adv. Stud. Behav 50, 127-175. ( 10.1016/bs.asb.2017.12.001) [DOI] [Google Scholar]
- 45.Dunbar RIM. 1988. Primate social systems. London, UK: Croom Helm. [Google Scholar]
- 46.Sterck EHM, Watts DP, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291-309. ( 10.1007/s002650050390) [DOI] [Google Scholar]
- 47.Van Schaik CP. 1989. The ecology of social relationships amongst female primates. In Comparative socioecology: the behavioral ecology of humans and other mammals (eds Standen V, Foley RA), pp. 195-218. Oxford, UK: Blackwell Scientific. [Google Scholar]
- 48.Wrangham R. 1979. On the evolution of Ape Social Systems. Soc. Sci. Inf. 18, 336-368. ( 10.1177/053901847901800301) [DOI] [Google Scholar]
- 49.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262-300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 50.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 51.Kalbitzer U, Chapman CA. 2021. Patterns of female social relationships in a primate with female-biased dispersal. Anim. Behav. 177, 117-133. ( 10.1016/j.anbehav.2021.04.024) [DOI] [Google Scholar]
- 52.Tokuyama N, Furuichi T. 2016. Do friends help each other? Patterns of female coalition formation in wild bonobos at Wamba. Anim. Behav. 119, 27-35. ( 10.1016/j.anbehav.2016.06.021) [DOI] [Google Scholar]
- 53.Furuichi T. 2011. Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. 20, 131-142. ( 10.1002/evan.20308) [DOI] [PubMed] [Google Scholar]
- 54.Wikberg EC, Sicotte P, Campos FA, Ting N. 2012. Between-group variation in female dispersal, kin composition of groups, and proximity patterns in a black-and-white colobus monkey (Colobus vellerosus). PLoS ONE 7, e48740. ( 10.1371/journal.pone.0048740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wikberg EC, Ting N, Sicotte P. 2014. Kinship and similarity in residency status structure female social networks in black-and-white colobus monkeys (Colobus vellerosus). Am. J. Phys. Anthropol. 153, 365-376. ( 10.1002/ajpa.22435) [DOI] [PubMed] [Google Scholar]
- 56.Thompson NA, Cords M. 2018. Stronger social bonds do not always predict greater longevity in a gregarious primate. Ecol. Evol. 8, 1604-1614. ( 10.1002/ece3.3781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McFarland R, Murphy D, Lusseau D, Henzi SP, Parker JL, Pollet TV, Barrett L. 2017. The ‘strength of weak ties' among female baboons: fitness-related benefits of social bonds. Anim. Behav. 126, 101-106. ( 10.1016/j.anbehav.2017.02.002) [DOI] [Google Scholar]
- 58.Machanda ZP, Gilby IC, Wrangham RW. 2013. Male–female association patterns among free-ranging chimpanzees (Pan troglodytes schweinfurthii). Int. J. Primatol. 34, 917-938. ( 10.1007/s10764-013-9707-7) [DOI] [Google Scholar]
- 59.Carter AJ, Macdonald SL, Thomson VA, Goldizen AW. 2009. Structured association patterns and their energetic benefits in female eastern grey kangaroos, Macropus giganteus. Anim. Behav. 77, 839-846. ( 10.1016/j.anbehav.2008.12.007) [DOI] [Google Scholar]
- 60.Heesen M, Rogahn S, Macdonald S, Ostner J, Schülke O. 2014. Predictors of food-related aggression in wild Assamese macaques and the role of conflict avoidance. Behav. Ecol. Sociobiol. 68, 1829-1841. ( 10.1007/s00265-014-1792-x) [DOI] [Google Scholar]
- 61.DeTroy SE, Haun DBM, van Leeuwen EJC. 2022. What isn't social tolerance? The past, present, and possible future of an overused term in the field of primatology. Evol. Anthropol. 31.1, 30-44. ( 10.1002/evan.21923) [DOI] [PubMed] [Google Scholar]
- 62.Duboscq J, Micheletta J, Agil M, Hodges K, Thierry B, Engelhardt A. 2013. Social tolerance in wild female crested macaques (Macaca nigra) in Tangkoko-Batuangus Nature Reserve, Sulawesi, Indonesia. Am. J. Primatol. 75, 361-375. ( 10.1002/ajp.22114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surbeck M, Hohmann G. 2013. Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 67, 1767-1780. ( 10.1007/s00265-013-1584-8) [DOI] [Google Scholar]
- 64.Duboscq J, Neumann C, Agil M, Perwitasari-Farajallah D, Thierry B, Engelhardt A. 2017. Degrees of freedom in social bonds of crested macaque females. Anim. Behav. 123, 411-426. ( 10.1016/j.anbehav.2016.11.010) [DOI] [Google Scholar]
- 65.Pusey AE, Schroepfer-Walker K. 2013. Female competition in chimpanzees. Phil. Trans. R. Soc. B 368, 20130077. ( 10.1098/rstb.2013.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wakefield ML. 2008. Grouping patterns and competition among female Pan troglodytes schweinfurthii at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 29, 907. ( 10.1007/s10764-008-9280-7) [DOI] [Google Scholar]
- 67.Houle A, Wrangham RW. 2021. Contest competition for fruit and space among wild chimpanzees in relation to the vertical stratification of metabolizable energy. Anim. Behav. 175, 231-246. ( 10.1016/j.anbehav.2021.03.003) [DOI] [Google Scholar]
- 68.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï forest: behavioural ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 69.Kahlenberg SM, Emery Thompson M, Wrangham RW. 2008. Female competition over core areas in Pan troglodytes schweinfurthii, Kibale National Park, Uganda. Int. J. Primatol. 29, 931. ( 10.1007/s10764-008-9276-3) [DOI] [Google Scholar]
- 70.Newton-Fisher NE. 2006. Female coalitions against male aggression in wild chimpanzees of the budongo forest. Int. J. Primatol. 27, 1589-1599. ( 10.1007/s10764-006-9087-3) [DOI] [Google Scholar]
- 71.Enigk DK, Emery Thompson M, Machanda ZP, Wrangham RW, Muller MN. 2021. Female-directed aggression by adolescent male chimpanzees primarily constitutes dominance striving, not sexual coercion. Am. J. Phys. Anthropol. 176, 66-79. ( 10.1002/ajpa.24296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enigk DK, Thompson ME, Machanda ZP, Wrangham RW, Muller MN. 2020. Competitive ability determines coalition participation and partner selection during maturation in wild male chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 74, 89. ( 10.1007/s00265-020-02872-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melis AP, Hare B, Tomasello M. 2006. Engineering cooperation in chimpanzees: tolerance constraints on cooperation. Anim. Behav. 72, 275-286. ( 10.1016/j.anbehav.2005.09.018) [DOI] [Google Scholar]
- 74.de Waal FB. 1984. Sex differences in the formation of coalitions among chimpanzees. Ethol. Sociobiol. 5, 239-255. ( 10.1016/0162-3095(84)90004-9) [DOI] [Google Scholar]
- 75.Hemelrijk CK, Ek A. 1991. Reciprocity and interchange of grooming and ‘support’ in captive chimpanzees. Anim. Behav. 41, 923-935. ( 10.1016/S0003-3472(05)80630-X) [DOI] [Google Scholar]
- 76.Cairns SJ, Schwager SJ. 1987. A comparison of association indices. Anim. Behav. 35, 1454-1469. ( 10.1016/S0003-3472(87)80018-0) [DOI] [Google Scholar]
- 77.Hosmer DW Jr, Lemeshow S, Sturdivant RX. 2013. Applied logistic regression. New Jersey, USA: John Wiley & Sons. [Google Scholar]
- 78.Robertson C, Boyle P, Hsieh C, Macfarlane GJ, Maisonneuve P. 1994. Some statistical considerations in the analysis of case-control studies when the exposure variables are continuous measurements. Epidemiology 5, 164-170. ( 10.1097/00001648-199403000-00006) [DOI] [PubMed] [Google Scholar]
- 79.Cords M. 1997. Friendships, alliances, reciprocity and repair. In Machiavellian intelligence II: extensions and evaluations (eds Whiten A, Byrne RW), pp. 24-49. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 80.De Moor D, Roos C, Ostner J, Schülke O. 2020. Female Assamese macaques bias their affiliation to paternal and maternal kin. Behav. Ecol. 31, 493-507. ( 10.1093/beheco/arz213) [DOI] [Google Scholar]
- 81.Cords M. 2002. Friendship among adult female blue monkeys (Cercopithecus mitis). Behaviour 139, 291-314. ( 10.1163/156853902760102681) [DOI] [Google Scholar]
- 82.Harcourt AH, de Waal FBM, editors. 1992. Coalitions and alliances in humans and other animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 83.Muller MN, Mitani JC. 2005. Conflict and cooperation in wild chimpanzees. Adv. Stud. Behav. 35, 275-331. ( 10.1016/S0065-3454(05)35007-8) [DOI] [Google Scholar]
- 84.Albers PC, de Vries H. 2001. Elo-rating as a tool in the sequential estimation of dominance strengths. Anim. Behav. 61, 489-495. ( 10.1006/anbe.2000.1571) [DOI] [Google Scholar]
- 85.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Scheipl F, Grothendieck G. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 86.Burnham KP, Anderson DR (eds). 2002. Model selection and multimodel inference. A practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 87.Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101-108. ( 10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 88.Stephens PA, Buskirk SW, del Rio CM. 2007. Inference in ecology and evolution. Trends Ecol. Evol. 22, 192-197. ( 10.1016/j.tree.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 89.Bartoń K. 2020. MuMIn: multi-model inference. R package version 1.43.17. See https://CRAN.R-project.org/package=MuMIn
- 90.Symonds MR, Moussalli A. 2011. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13-21. ( 10.1007/s00265-010-1037-6) [DOI] [Google Scholar]
- 91.Farine DR. 2017. A guide to null models for animal social network analysis. Methods Ecol. Evol. 8, 1309-1320. ( 10.1111/2041-210X.12772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farine DR, Whitehead H. 2015. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144-1163. ( 10.1111/1365-2656.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hartig F. 2021. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package version 0.4.3. See https://CRAN.R-project.org/package=DHARMa
- 94.Zettersten M, Lupyan G. 2019. vif.mer R function. OSF. See https://osf.io/ezkpa/
- 95.Nakagawa S, Johnson PCD, Schielzeth H. 2017. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213. ( 10.1098/rsif.2017.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carne C, Wiper S, Semple S. 2011. Reciprocation and interchange of grooming, agonistic support, feeding tolerance, and aggression in semi-free-ranging Barbary macaques. Am. J. Primatol. 73, 1127-1133. ( 10.1002/ajp.20979) [DOI] [PubMed] [Google Scholar]
- 97.Matheson MD, Bernstein IS. 2000. Grooming, social bonding, and agonistic aiding in rhesus monkeys. Am. J. Primatol. 51, 177-186. () [DOI] [PubMed] [Google Scholar]
- 98.Ventura R, Majolo B, Koyama NF, Hardie S, Schino G. 2006. Reciprocation and interchange in wild Japanese macaques: grooming, cofeeding, and agonistic support. Am. J. Primatol. 68, 1138-1149. ( 10.1002/ajp.20314) [DOI] [PubMed] [Google Scholar]
- 99.Tokuyama N, Sakamaki T, Furuichi T. 2019. Inter-group aggressive interaction patterns indicate male mate defense and female cooperation across bonobo groups at Wamba, Democratic Republic of the Congo. Am. J. Phys. Anthropol. 170, 535-550. ( 10.1002/ajpa.23929) [DOI] [PubMed] [Google Scholar]
- 100.Malenky RK, Wrangham RW. 1994. A quantitative comparison of terrestrial herbaceous food consumption by Pan paniscus in the Lomako Forest, Zaire, and Pan troglodytes in the Kibale Forest, Uganda. Am. J. Primatol. 32, 1-12. ( 10.1002/ajp.1350320102) [DOI] [PubMed] [Google Scholar]
- 101.Surbeck M, Girard-Buttoz C, Samuni L, Boesch C, Fruth B, Crockford C, Wittig RM, Hohmann G. 2021. Attractiveness of female sexual signaling predicts differences in female grouping patterns between bonobos and chimpanzees. Commun. Biol. 4, 1-11. ( 10.1038/s42003-021-02641-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wrangham RW, White FJ. 1988. Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105, 148-164. ( 10.1163/156853988X00494) [DOI] [Google Scholar]
- 103.Aral S. 2016. The future of weak ties. Am. J. Sociol. 121, 1931-1939. ( 10.1086/686293) [DOI] [Google Scholar]
- 104.Granovetter MS. 1973. The strength of weak ties Am. J. Sociol. 78, 1360-1380. ( 10.1086/225469) [DOI] [Google Scholar]
- 105.Helliwell JF, Putnam RD. 2004. The social context of well-being. Phil. Trans. R. Soc. Lond. B 359, 1435-1446. ( 10.1098/rstb.2004.1522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sandstrom GM, Dunn EW. 2014. Social interactions and well-being: the surprising power of weak ties. Pers. Soc. Psychol. Bull. 40, 910-922. ( 10.1177/0146167214529799) [DOI] [PubMed] [Google Scholar]
- 107.Cohen S, Janicki-Deverts D. 2009. Can we improve our physical health by altering our social networks? Perspect. Psychol. Sci. 4, 375-378. ( 10.1111/j.1745-6924.2009.01141.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saj TL, Marteinson S, Chapman CA, Sicotte P. 2007. Controversy over the application of current socioecological models to folivorous primates: Colobus vellerosus fits the predictions. Am. J. Phys. Anthropol. 133, 994-1003. ( 10.1002/ajpa.20609) [DOI] [PubMed] [Google Scholar]
- 109.Wikberg EC, Gonzalez S, Rodriguez C, Sicotte P. 2022. Joint intergroup aggression in female colobus monkeys (Colobus vellerosus) is associated with grooming bonds, male participation, and group size. Am. J. Primatol. 84, e23355. ( 10.1002/ajp.23355) [DOI] [PubMed] [Google Scholar]
- 110.McFarland R, Majolo B. 2013. Coping with the cold: predictors of survival in wild Barbary macaques, Macaca sylvanus. Biol. Lett. 9, 20130428. ( 10.1098/rsbl.2013.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silk JB, Seyfarth RM, Cheney DL. 2018. Quality versus quantity: do weak bonds enhance the fitness of female baboons? Anim. Behav. 140, 207-211. ( 10.1016/j.anbehav.2018.04.013) [DOI] [Google Scholar]
- 112.Watts DP. 2002. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour 139, 343-370. ( 10.1163/156853902760102708) [DOI] [Google Scholar]
- 113.Fox S, Muller MN, González NT, Enigk DK, Machanda ZP, Otali E, Wrangham R, Thompson ME. 2022. Weak, but not strong, ties support coalition formation among wild female chimpanzees. Figshare. ( 10.6084/m9.figshare.c.6249961) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fox S, Muller MN, González NT, Enigk DK, Machanda ZP, Otali E, Wrangham R, Thompson ME. 2022. Weak, but not strong, ties support coalition formation among wild female chimpanzees. Figshare. ( 10.6084/m9.figshare.c.6249961) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in electronic supplementary material [113].