Abstract
In group-living species, cooperative tactics can offset asymmetries in resource-holding potential between individuals and alter the outcome of intragroup conflicts. Differences in the kinds of competitive pressures that males and females face might influence the benefits they gain from forming intragroup coalitions. We predicted that there would be a female bias in intragroup coalitions because females (1) are more like to live with kin than males are, and (2) compete over resources that are more readily shared than resources males compete over. We tested this main prediction using information about coalition formation across mammalian species and phylogenetic comparative analyses. We found that for nearly all species in which intragroup coalitions occur, members of both sexes participate, making this the typical mammalian pattern. The presence and frequency of female or male coalitions were not strongly associated with key socio-ecological factors like resource defensibility, sexual dimorphism or philopatry. This suggests that once the ability to form intragroup coalitions emerges in one sex, it is likely to emerge in the other sex as well and that there is no strong phylogenetic legacy of sex differences in this form of cooperation.
This article is part of the theme issue ‘Cooperation among women: evolutionary and cross-cultural perspectives’.
Keywords: coalition, collective action, comparative social evolution, hierarchy, intervention, sex differences
If cooperation can be analysed via natural selection operating on individuals, a new way to conceptualize the process emerges. Instead of viewing cooperation as distinct from competition, it becomes productive to regard them together. Students of animal behaviour have long recognized that an artificial dichotomy may exist insofar as animals frequently cooperate to compete with conspecifics. In taxa as diverse as insects, birds, and mammals, animals cooperate to obtain immediate or deferred fitness benefits.
Muller & Mitani 2005 [1]
1. Introduction
Competition over access to resources needed for individuals to survive and reproduce successfully is ubiquitous in nature. The outcome of contests between pairs of individuals (dyads) is expected to be influenced by asymmetries in the resource-holding potential of the participants [2,3] and the associated fitness consequences of fighting [4]. Resource-holding potential is based on a combination of morphological traits such as body size and weight, the size of weaponry, including antlers, horns, tusks and canines, and physical condition, which influences endurance capacity, strength and agility. For example, male red deer (Cervus elephas) compete over access to groups of females during the breeding season. Body size and condition influence males' success in contests and their ability to maintain access to groups of females [5]. Both body size and antler size are positively related to males’ lifetime breeding success [6]. In many group-living species, stable individual differences in resource-holding potential lead to predictable outcomes of contests between pairs of individuals, and individuals can be ordered in linear dominance hierarchies [7–9]. High-ranking animals generally have priority of access to monopolizable resources, and high rank is positively correlated with reproductive success in both sexes [10].
In group-living species, cooperative tactics can offset asymmetries in resource-holding potential between individuals and can alter the outcome of intragroup conflicts. One such tactic is intragroup coalition formation (figure 1), also called agonistic aiding and coalitionary aggression, which occurs when two or more group members join forces to collectively direct aggression toward one or more members of their own social group [11,12]. For example, high-ranking male yellow baboons (Papio cynocephalus) and olive baboons (Papio anubis) mate guard sexually receptive females and prevent lower-ranking rivals from mating with them [13–15]. Sometimes two or three lower-ranking males join up to challenge a mate-guarding male that outranks them both and often succeed in defeating him [13–15]. In some species, coalitionary aggression plays an important role in the acquisition and maintenance of dominance rank. For example, in some species, females form dominance hierarchies in which maternal kin occupy adjacent ranks (e.g. spotted hyenas, Crocuta crocuta [16,17]; white-faced capuchins, Cebus capucinus [18]; and several species of cercopithecine primates [19]). Maternal rank inheritance is the product of coalitionary support from kin. Mothers and other close kin consistently support related females in conflicts against members of other matrilines, and their support enables maturing females to defeat all of the females that their maternal relatives can defeat [19]. Coalitionary outcomes can also influence male dominance rank (African wild dogs, Lycaon pictus [20]; chimpanzees, Pan troglodytes [1,21]; Assamese macaques, Macaca assamensis [22]; Japanese macaques, Macaca mulatta [23]). In bonobos, Pan paniscus, coalitions of adult females often outrank males, and male dominance rank [24] and male access to female mates is influenced by the presence and support of their mothers [25–27]. In many human societies, coalitions are also crucial for both men and women for gaining social status, resource access and fitness [28–33].
Figure 1.
Numerous species of social mammals form intragroup coalitions. Intragroup coalitions involve two or more individuals joining forces to direct aggression toward another group member (a,c–e). Some coalitions initially also involve one individual (e.g. plains zebra in the lower right of panel b) joining an ongoing dyadic fight to intervene on behalf of others. For most of these species, members of both sexes form intragroup coalitions as is the case for (a) white-faced capuchins in Costa Rica, (b) plains zebra in South Africa, (c) olive baboons in Laikipia, Kenya and (d) spotted hyenas in Tanzania. Interestingly, only male (e) Indio-pacific bottlenose dolphins of Australia form coalitions. Male dolphins do so as part of their multifaceted set of mating strategies. Photographs reproduced with permission from Abid Karamali (Costa Rica), Kore Nordmann, Joan Silk (Uaso Ngiro Baboon Project), Oliver Höner (Ngorongoro Hyena Project) and Ewa Krzyszczyk (Shark Bay Dolphin Project).
Differences in the kinds of competitive pressures that males and females face might influence the benefits they gain from forming coalitions with adults of the same or opposite sex in within-group conflicts. In mammalian species, the primary focus of competition for males and females often differs. For mammalian females, which bear the energetic costs of internal gestation and lactation, fitness is usually expected to be more strongly influenced by the outcome of competition over access to material resources, such as food or dens, than access to mates [10,34,36–]. The obligate commitment of mammalian females to gestation and lactation makes them a limited resource for males, and male fitness is typically more strongly affected by the outcome of competition over access to females than other kinds of resources. Because males compete for access to fertile females [34–36], sexual selection tends to favour the evolution of traits that permit males to monopolize and gain mating opportunities with females [37–40]. The resources that females compete over, such as food and safety (e.g. dens or burrows), are more readily shared than paternities [41,42], and this can make the benefits of coalitions more evenly shared for females than males [43]. In addition, kinship is the primary foundation of cooperation in mammalian groups [44], and coalitionary activity is often nepotistic [11]. It is more common for adult female mammals to live in groups with close kin than for adult males to live with kin [10]. Thus, adult females might be more likely to intervene in ongoing fights or join forces to form coalitions with other adult group members because they are more likely to live with appropriate coalition partners, particularly when competing for access to spatially clumped food [45–47]. At the same time, males may benefit from intervening in support of females if this reduces the risk that their offspring will become victims of infanticide [48] or serves as a commodity that can be exchanged for other kinds of services, such as grooming [49–51], food-sharing [52], or future mating opportunities [53–55]. In species that form multi-male groups, males' participation in coalitionary aggression against other males within the group may help them to increase or maintain high-ranking positions in the dominance hierarchy (references above) or obtain mating concessions from more powerful males [56,57]. Males may also join forces to gain direct, immediate benefits during consortships [58]. In general, male conflict is also expected to be associated with sexual size dimorphism across species [59,60], as more intense male–male competition favours larger body size and weaponry.
In mammals, males are more likely to participate in intergroup conflicts than females [61–63], but the extent to which sex differences in intragroup coalitions exist is unknown, particularly beyond primates. Sex differences in patterns of dispersal may influence the propensity for intragroup coalitions to form. Among mammals, females are typically philopatric whereas males frequently disperse at sexual maturity, although in some species neither sex, both sexes or only males are philopatric [64]. These patterns may make kin-based coalitions more common among females than males [35,44,45,65,66]. However, coalitions are not limited to genetic relatives [11]; chimpanzee and bonobo females form intragroup coalitions even though females are the dispersing sex [67,68] (see also [69]). In some species, males do not limit coalitionary support to kin (e.g. chimpanzees [70], dolphins [71], stump-tailed macaques (Macaca artoides) [72]).
The goal of this paper is to evaluate sex differences in coalition formation during intragroup conflicts across social mammals. Whereas intergroup coalitions are well-recognized across taxa from ants and fiddler crabs to humans [61,73,74], historically, research on intragroup coalitions has focused primarily on primates, giving rise to the notion that intragroup coalitions may be more complex and frequent among primates than non-primates [75]. However, if the factors governing coalition formation are generalizable to social mammals overall, then we expected these patterns to be robust for primates and non-primate mammalian species. We hypothesized that females would cooperate in intragroup coalitions in more species than males because (1) females are more likely to live with kin than males are, and (2) the resources that females compete over are more readily shared than the resources that males compete over. We predicted that this would produce robust sex differences in intragroup coalitions even after controlling for shared phylogenetic history across the mammalian lineage. We also predicted that coalition formation would be more common in the philopatric sex than in the dispersing sex, and that female coalition formation should be present most often in species that rely on foods that can be monopolized and defended than in species that rely on foods that cannot be monopolized and defended. Finally, we predicted that males should form coalitions most often in species for which competition over access to females is most intense. Because the intensity of male–male competition is associated with sexual dimorphism in mammals, we predicted that the presence of male intragroup coalitions would be positively associated with the extent of sexual dimorphism in body size.
2. Methods
(a) . Literature search and data collection
To capture the breadth of empirical studies focusing on coalitions in non-human mammals, this study builds upon an initial review of intragroup coalition formation in group-living mammals [11], papers citing this review, including [43,76], and other papers identified via Google Scholar searches for species that engage in intragroup coalitions. We also communicated directly with researchers working on species for which there are reports of intragroup coalitions in one sex, but no information about the other sex. Captive studies were retained in our analysis to expand the number of species that we were able to include in the analyses. Although captivity is likely to influence the context and frequency of coalitions, it seems unlikely to generate false positives, i.e. produce evidence of coalitions in sexes/species where they are actually absent. Domesticated species were excluded from the analysis.
We scored each species as showing evidence of coalitions by females, males or both based upon whether or not adults of the focal sex intervened in ongoing fights on behalf of, and/or simultaneously formed coalitions to support, adult recipients of any sex. Specifically, same-sex and mixed-sex coalitions were both included as evidence for coalition formation for the focal sex. For example, male donors were scored as engaging in coalitions if males intervened on behalf of female recipients, male recipients, or both. This was used to assess the general pattern of sex differences in coalition formation, and also re-coded into presence/absence of female and male coalitions, respectively, as described below. In addition to presence/absence, a measure of the frequency of coalition formation by each sex was desirable. However, comparative data on the relative frequency of intragroup coalitions formed by each sex are rare, and this makes the direct assessment of coalition frequency by sex challenging. Ideally, each study would report on focal data collected on both sexes, making it possible to estimate the rate of coalition formation (events/time observed). Even then, it is not clear whether the relevant comparison would be based on per capita rates by males and females, or the absolute rates summed across individuals of each sex, or whether rates of coalition formation ought to be corrected for opportunities to intervene, which is a function of the frequency of aggression. Because coalitions are generally uncommon, almost all studies rely on ad libitum data or some combination of focal and ad libitum data, and these kinds of data are biased by differences in observability, conspicuousness and observer focus.
We attempted to overcome these methodological issues by implementing a bibliometric approach to assess the relative frequency of intragroup coalitions by sex. For each sex, we assessed whether intragroup coalitions are absent, present, or common for any species for which there is evidence that members of at least one sex are known to form intragroup coalitions. For example, females of a species were scored in one of the three following ways: (i) female coalitions are absent if there are papers mentioning male intragroup coalitions (i.e. somebody had studied coalitions in this species) but none mention female intragroup coalitions (or explicitly say that they are absent), (ii) female intragroup coalitions present if there is at least one study describing female intragroup coalitions, or (iii) female coalitions common if there are two or more published empirical studies describing female intragroup coalitions; the bibliometric method credits the number of original studies as evidence for the importance/frequency of a phenomenon.
We limited publication counts to original empirical studies, including dissertations and master's theses; review papers were omitted from these counts. Species for which intragroup coalitions by males or females were simply documented as an observation (but with no data analysis) in a published study or via personal communication with researchers were also deemed to be present (but uncommon) for a species if no additional published accounts were available. Information from multiple studies was typically combined to make this assignment. In most cases, a single study focused only on intrasexual coalition formation.
We used the two-step ratio [77] to assign sexual dimorphism using mean male and female body masses for each species (see electronic supplementary material, table S1 for references) [77]. Carnivores (i.e.. eat mostly meat), frugivores (i.e. eat mostly fruit) and gummivores (i.e. eat mostly gums and saps from trees) were scored as eating defensible food. Grazers, browsers, piscivores, omnivores, insectivores, herbivores and folivores (diet may also include fruits) were scored as eating non-defensible foods (see electronic supplementary material, table S1). We also described species based on patterns of philopatry (females only, males only, both sexes, or neither sex), adult integration of the sexes (mixed groups or sexually segregated) and presence of adults by sex (multiple males and/or females within groups; see electronic supplementary material, table S1 for references). A sample of 100 phylogenetic trees from VertLife.org [78] was downloaded to represent the evolutionary history of these species and its uncertainty.
(b) . Statistical analyses
To assess the general patterns of coalition formation by sex, i.e. to model the probability of female coalitions, male coalitions and coalitions by both sexes in a typical mammal, we used multinomial models, first only with an intercept (Model 1) and then with predictors to distinguish sex-segregated species from those living in mixed-sex groups (Model 2), and primates from non-primates (Model 3). To test socio-ecological predictors we used binomial models for the presence and absence of female coalitions (Model 4) and male coalitions (Model 5), and coded food defensibility as present (1) or absent (0) and centred sexual dimorphism on 1 (e.g. male and females of the same size). Philopatry was coded as ‘females philopatric’, ‘males philopatric’, ‘both sexes philopatric’ or ‘neither sex philopatric’. Finally, we repeated models 4 and 5 using our ordinal measure of coalition frequency with cumulative logit distributions.
These models were implemented as Bayesian phylogenetic generalized linear mixed models (GLMMs) [79] in R 4.2.0. [80] using the brms package v. 2.14.4 [81]. We also used functions from the phytools [82], rethinking [83], ape [84] and metbrewer [85] packages. To account for phylogenetic uncertainty, we looped all models over the sample of 100 phylogenetic trees and pooled the parameter estimates. Bayesian models yield a posterior probability distribution for each estimated parameter, which we here summarize by its median and 90% credible intervals; to directly quantify support for specific predictions, we report the proportion of the posterior that is consistent with the prediction. For instance, to test whether female coalitions are more likely in primates compared with non-primates, the model yields a posterior distribution of the difference between the probability of female coalitions in primates versus non-primates, which we expressed as an odds ratio (OR); the proportion of the posterior that lies above an OR of 1 quantifies the degree of support for the prediction. We calculated the phylogenetic signal as an intra-class correlation, i.e. the proportion of the total variance captured by the phylogenetic random effect [79,86], which is equivalent to Pagel's λ.
3. Results
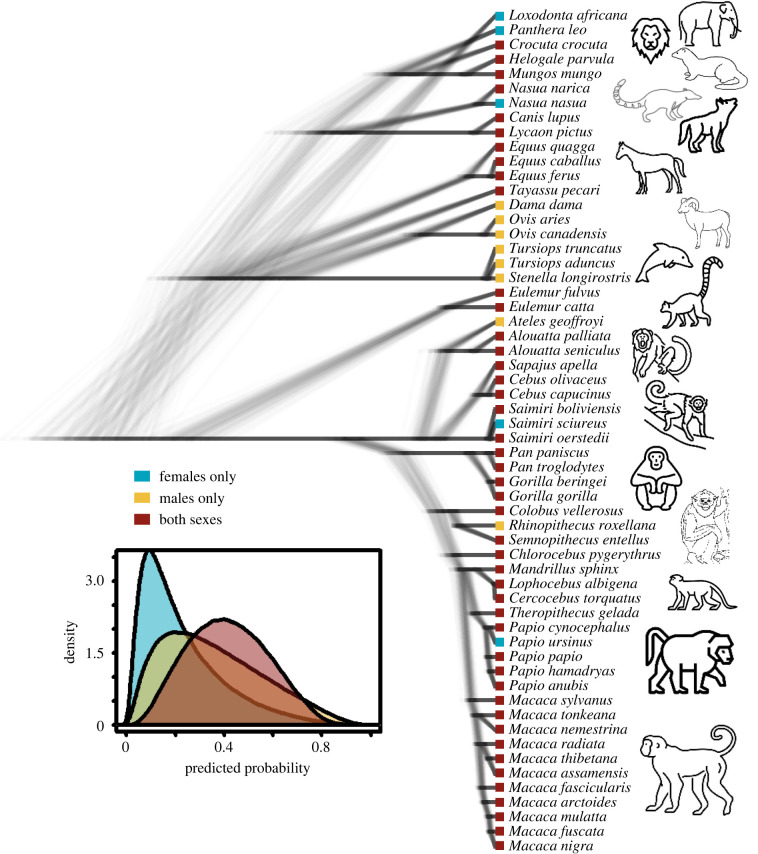
This study yielded evidence for intragroup coalitions in a total of 58 species, roughly two-thirds of which were primates (figure 2; electronic supplementary material, table S1). These species spanned five biological orders within the class Mammalia, including seven Artiodactyla (three species of deer and sheep, three dolphin species, and a peccary), three Perissodactyla (two species of horses and one zebra species), Proboscidea (one species of elephant), eight Carnivora (two species of dogs, one cat species, two species of mongooses, one hyena species and two species of coatis) and 39 Primata (39 species of primates). Roughly two-thirds of primate species reported to engage in intragroup coalitions belonged to the family Cercopithecidae (23 species of macaques and baboons). We also located primate data on intragroup coalitions formed by two species of lemurs, three species of spider and howler monkeys, seven species of capuchin and squirrel monkeys, and four species of apes.
Figure 2.
Phylogeny of 58 non-human social mammals that engage in intragroup coalition formation, showing some uncertainty in tree topology. The squares at the tips of the phylogeny indicate observed patterns of coalition formation: blue = females only, yellow = males only, red = both sexes. The inserted figure shows the posterior probability distributions for each type of coalition from Model 1, indicating the phylogenetic average, or typical mammalian species. Source for species icons: thenounproject.com.
We used multinomial models to estimate the overall predicted probabilities of coalitions by females only, males only or both sexes. Contrary to our first prediction, female-only coalitions were not more likely than male-only coalitions (figure 1, Model 1). In fact, the probability of female-only coalitions in a typical mammal (median = 0.18, 90% credible interval = 0.01–0.47) was lower than the probability of male-only coalitions (0.33, 0.04–0.67) and the most likely state was coalitions by both sexes (0.41, 0.14–0.68). Thus, only 34% of the posterior probability supported our prediction of female-only coalitions being more likely than male-only coalitions. The phylogenetic signal was moderate (median λ = 0.34, 90% CI = 0.14–0.54). In sum, conditional on having any coalitions at all, the typical extant mammal is just as likely to have female-only, male-only or both-sex coalitions.
This general pattern did not change appreciably when comparing species living in mixed-sex groups (n = 54 species) with sex-segregated ones (n = 4 species; Model 2), or primates (n = 39 species) with non-primates (n = 19 species; Model 3). Specifically, the odds of female-only coalitions and male-only coalitions were nearly the same in sex-segregated species compared with mixed-sex species (females only: median OR = 1.11, 90% CI = 0.35–2.15, probability OR > 1 = 58%; males only: median OR = 1.03, 90% CI = 0.32–2.02, probability OR > 1 = 53%) or primates compared with non-primates (females only: median OR = 0.93, 90% CI = 0.3–1.8, probability OR > 1 = 44%; males only: median OR = 1.01, 90% CI = 0.31–1.97, probability OR > 1 = 51%). We, therefore, did not stratify our subsequent analyses by these variables.
To test socio-ecological predictions about female coalitions, we combined the three categories ‘females only’, ‘males only’ and ‘both sexes’ into a binary variable for the presence (females only’ or ‘both sexes) or absence (males only) of female coalitions; philopatry was also re-coded as a binary variable indicating presence (females or both sexes philopatric) or absence (males or neither sex philopatric) of female philopatry. To test predictions about male coalitions, we analogously re-coded male coalitions as present (males only or both sexes) or absent (females only) and male philopatry as present (males or both sexes philopatric) or absent (females or neither sex philopatric). We then ran binomial models on the presence of female coalitions (Model 4), including food defensibility (yes/no) and female philopatry (yes/no) as predictors, and on the presence of male coalitions (Model 5), including sexual dimorphism and male philopatry (yes/no) as predictors. As a robustness check, we also modelled each of these competing causes on its own.
The probability of female coalitions was not higher in species with defensible food resources compared with species with non-defensible food resources (OR = 0.95, 90% CI = 0.3–1.85, probability OR > 1 = 46%), or in species with female philopatry compared with female dispersal (OR = 1.13, 90% CI = 0.36–2.18, probability OR > 1 = 59%). Likewise, the probability of male coalitions was not higher in sexually dimorphic species (OR for 1 unit change in dimorphism = 0.83, 90% CI = 0.28–1.61, probability OR > 1 = 36%), and was virtually the same whether males were philopatric or males dispersed (OR = 1.17, 90% CI = 0.39–2.21, probability OR > 1 = 63%). These inferences did not change when considering each predictor in a model on its own (see electronic supplementary material). Thus, the probability of female or male coalitions was not strongly associated with our predictors.
Finally, we tested socio-ecological predictors on coalition frequency—rather than just presence/absence—by analysing our ordinal scale data (absent, present, common) using cumulative logit distributions and the same predictors as Models 4 and 5. The frequency of female coalitions was not higher in species with defensible food resources compared with species with non-defensible food resources (OR = 0.76, 90% CI = 0.28–1.37, probability OR > 1 = 26%), though it was somewhat higher in species with female philopatry compared with female dispersal (OR = 1.47, 90% CI = 0.53–2.68, probability OR > 1 = 81%). Likewise, the frequency of male coalitions was not higher in sexually dimorphic species (OR for 1 unit change in dimorphism = 0.94, 90% CI = 0.34–1.7, probability OR > 1 = 44%), but somewhat higher in species with male philopatry compared with male dispersal (OR = 1.29, 90% CI = 0.51–2.27, probability OR > 1 = 73%).
4. Discussion
(a) . General patterns regarding sex bias in coalition formation
The comparative phylogenetic analysis indicates that among species that form intragroup coalitions, the typical pattern is for members of both sexes to form coalitions. These findings are quite consistent across taxa and were not influenced by food distribution, or the extent of sexual dimorphism. There was some rather weak support for dispersal patterns to be associated with the frequency of coalitions (with 81% confidence for female, and 73% for male coalitions), but not the presence/absence of coalitions. Thus, socio-ecology did not strongly affect coalition formation.
Sex bias in coalition formation was mainly clustered within the lineage that includes ungulates and dolphins. Although intragroup coalitions have been documented in relatively few ungulates and dolphin species, even-toed ungulates and dolphins accounted for 75% of the species in which only males form coalitions. Only males formed intragroup coalitions for fallow deer (Dama dama) [87–91], feral sheep (Ovis aries) [92], bighorn sheep (Ovis canadensis) [93], common bottlenose dolphins (Tursiops truncatus) [94], Indo-Pacific bottlenose dolphins (Tursiops aduncus) [[58,95,97] and Hawaiian spinner dolphins (Stenella l. longirostris) [98]. In all of these taxa, males formed coalitions primarily to protect groups of females from other males and to increase access to mating opportunities with females. Male Indo-Pacific bottlenose dolphins in Shark Bay, Australia are well known for forming complex, multilevel alliances to herd (female) mating partners [58]. Interestingly, although female Indo-Pacific dolphins do not engage in coalitionary aggression, non-cycling females do place their pelvic fins on the side of cycling females to initiate polyadic affiliative interactions with cycling females being harassed by males [99]. The odd-toed ungulates may represent an exception to this pattern of male-only coalition formation. Specifically, the dataset includes three odd-toed ungulate species in the family Equidae: the Przewalski horse (ferus przewalskii) [100,101], the wild horse (Equus caballus) [102–104] and the plains zebra (Equus burchellii quagga) [105,106]. In all three of these species, both sexes form coalitions. Males primarily intervene to interfere with other males' courtship while females most often intervene on behalf of their calves or other mares.
Outside of the ungulates and dolphins, there are relatively few species in which only one sex forms coalitions. An early account suggests that female African lions (Panthera leo leo) join forces to protect their offspring [107], but coalition formation has not been the subject of systematic study in lions [108]. Pairs of male lions that team up to compete against outside males for access to females are sometimes referred to as stable ‘coalitions’ (also called ‘alliances’), but to our knowledge male lions do not participate in intragroup coalitionary aggression [109,110]. Among another social carnivoran, ring-tailed coati (Nasua nasua) [111–113] adult females but not males form intragroup coalitions, generally to intervene on behalf of their juvenile offspring. Finally, female African bush elephants (Loxodonta africana) form intragroup coalitions to protect their offspring [114,115], but male elephants do not participate in intragroup coalitions. This may be related to the fact that bachelor males spend much of their time alone and, thus, have relatively few opportunities to form intragroup coalitions.
Among the 39 species of primates included in our dataset; intragroup coalitions were found within both sexes for most species (90%). Male-only and female-only coalitions are reported to occur in a maximum of two species for each sex. Only males form coalitions in spider monkeys (Ateles geoffroyi) [116–118] and golden snub-nosed monkeys (Rhinopithecus roxellana) [119]. In spider monkeys, the absence of female coalitions may be related to the fact that mixed-sex groups often split into temporary sexually segregated subgroups, and adult females typically travel alone or with their offspring [118]. Golden snub-nosed monkeys live in one-male, multi-female groups and female–female competition is intense [119]. One captive study reported that males frequently intervened in conflicts among females, but females did not form coalitions [119]. In snub-nosed monkeys, although females do not form coalitions with each other or simultaneously join forces with males to target other females, males frequently intervene in agonistic disputes to reduce conflict among females. In one study, males intervened in 93.6% of female fights [119]. Support from males reduces female infanticide prior to mothers transferring with their infants to an outside social unit [120]. Interestingly, female snub-nosed monkeys deviate from the typical mammalian pattern of male-biased participation in intergroup conflicts [61] as females join forces to attack outside males that pose an infanticidal risk to their infants [121]. By contrast, only females form intragroup coalitions in the chacma baboon (Papio ursinus), although their occurrence is apparently uncommon [122,123]. Nonetheless, these low rates of coalitionary interventions are likely sufficient to reinforce existing dominance rank relationships, as is the case for yellow baboons (Papio cynocephalus) [124]. Strikingly, multiple studies have explicitly documented the absence of male coalitions forming to take over consortships in chacma baboons [125,126]. Finally, in Guianan squirrel monkeys (Saimiri sciureus), only females form intragroup coalitions to support their kin in fights over food [127]; roughly 50% of aggressive interactions in fruiting trees involved coalition formation.
(b) . Limited evidence for intragroup coalitions at contested resources
We predicted that females would form coalitions more often than males because the resources that females compete over (e.g. food, dens) are more readily shared than the resources that males compete over (paternities). This prediction is supported by game-theoretical models that predict coalitions will evolve within groups when coalitionary strategies maximize individual fitness through competition for limited material resources [128]. Specifically, individuals are expected to join forces in coalitions when two or more group members may together increase each individual's chance of accessing a resource [129]. Coalitions are expected for species in which the strength of contestants is a highly reliable predictor of fight outcomes [130] and access to the rewards gained from winning [131]. Indeed, intergroup conflicts often occur directly over contested resources, including territories and resources contained within them (e.g. food, mates [61]).
Despite these theoretical predictions, empirical evidence for intragroup coalitionary aggression occurring directly over mates or food—for adults of either sex—is surprisingly limited. Instead, most intragroup coalitions form outside of circumstances involving an immediately contested resource [132]. On the whole, examples of coalitionary aggression that directly affect access to mates or food are limited. However, in male olive and yellow baboons and Barbary macaques [15,133,134], low- and mid-ranking males may join forces against higher-ranking males to take over a consortship, and male chimpanzees sometimes form coalitions to guard mates [135]. Male Camargue horses [103], Indo-Pacific bottlenose dolphins [97], and stump-tailed macaques [72] also form intragroup coalitions, often with non-kin, to gain access to sexually receptive females. However, in some cases, such as male fallow deer, coalition frequency fails to predict mating success [89]. Similarly, evidence for coalitions forming within the context of feeding competition is relatively sparse. Intragroup coalitions do increase the immediate access to food for female squirrel monkeys (Saimiri sciureus) [127], capuchin monkeys [136,137], savannah baboons [15], Barbary macaques [134] and chimpanzees [135]. Vervet monkeys of both sexes also form intragroup coalitions over food [137]. By contrast, spotted hyenas are significantly less likely to form coalitions when food is immediately available and coalitions that occur when food is available do not increase immediate feeding opportunities for coalitionary allies [11]. In many species, as in bonobos [25] and baboons [138], female coalitions, however, do protect females from male harassment or infanticide. Although intragroup coalitions often form in the absence of immediately contested resources, as we discuss in the next section, this form of cooperation can still have profound effects on the social structure (i.e. dominance status, social bonds) that in turn influence resource access in future situations.
(c) . Coalitions reinforce agonistic and affiliative social relationships
Detailed descriptions of coalition formation in the literature indicate that primates and non-primates gain direct as well as indirect benefits from forming coalitions. Mammalian coalitions are used widely to reinforce the status quo for species with dominance hierarchies [11], with examples ranging from carnivorans (e.g. spotted hyenas [16,139,140], African wild dogs (L. pictus) [20]) to ungulates (e.g. fallow deer [88]) and many species of primates (e.g. Assamese macaques [22], chimpanzees [21,141]). Across species, mammals also generally bias coalitionary support in favour of kin versus non-kin [11,44,142,143]. For instance, adult female baboons [124], and spotted hyenas [11] selectively support closely related maternal and paternal kin against less closely related kin and non-kin. Similarly, male white-lipped peccaries (Tayassu pecari) intervene more often on behalf of their closest genetic relatives during ongoing fights [144]. Male Barbary macaques are also more likely to respond to solicitations for support from (unrelated) males with whom they have close social bonds than from males with whom they have weak ties [145]. The finding that intragroup coalitions rarely form directly over access to contested resources (e.g. food, mates)—but rather generally serve to reinforce agonistic and affiliative social relationships within both sexes—runs counter to the assumptions of most theoretical models (see previous section) and likely contributes to the general lack of intraspecific sex differences in the tendency to form coalitions revealed in this study. That is, if the primary function of coalitions is to reinforce dominance status, and both sexes benefit from high rank, then this may explain why we found little evidence of sex biases in coalition formation.
(d) . Cognitive constraints and socio-ecological effects on coalition formation
The finding that members of both sexes usually form coalitions in species in which coalitions are observed suggests that the presence or absence of coalitionary behaviour may be more closely linked to species-level traits such as cognitive abilities, social organization and ecological factors than to sex differences in the benefits derived from coalitions. With regards to cognition, the constraints on intragroup coalitions may indeed differ from those of intergroup coalitions. Intergroup coalition formation likely requires an understanding of 'us versus them' and relative numbers and/or collective resource-holding power of the opposing group whereas intragroup coalitions may include ‘political’ decisions such as triadic awareness of rank, kinship or relationship quality.
Coalitions involve at least three parties, and individuals' decisions about whether to become involved in a coalition or who to support in an ongoing interaction may rely on simple heuristics (e.g. always support kin) or more complex calculus that integrates multiple costs and benefits (e.g. integrated knowledge of kinship, dominance rank and previous social history) [146–148]. Selective pressures requiring individuals to integrate third-party relationships based on two or more criteria (e.g. social rank and kinship) may favour the evolution of increased cognitive skills [149]. The social relationships among mammals are particularly multifaceted in groups of animals with dominance hierarchies and low average relatedness among adult females, as reflected by increases in conflicts of interest among group members, rates of coalition formation and brain sizes [76,150]. There is evidence that coalitionary behaviour is influenced by leverage and knowledge of the nature of relationships among other members of the group [125,131,132]. For example, male bonnet macaques (Macaca radiata) and stump-tailed macaques selectively recruit allies that outrank themselves and their opponents [125,131]. Similarly, spotted hyenas consistently intervene in fights to support the higher-ranking of two contestants, even when the dominant individual is losing [151]. All three of these species also preferentially support kin in intragroup coalitions [11]. Finally, revolutionary or levelling coalitions occur when both partners rank below their target, and can involve enormous immediate risks—but potentially high payoffs—and these forms of manipulation likely require sophisticated understanding of social dynamics [43,152,153].
The notion that cognitive constraints limit coalition formation is highly contested [12]. First, among male primates, measures of brain size fail to predict the intensity of coalition formation. Instead, the frequency of male coalitions in primates is best explained by their social organization (e.g. large group sizes, reduced contest competition) [154]. Second, in many species, patterns of coalition formation are explained by a simple set of rules and do not require complex social cognition. For example, simple rules of thumb could underlie the nepotistic patterns of support that are observed in many taxa. In many species, winner and loser effects occur, such that the winner of a fight is more likely to win again whereas the individual that lost a fight is more likely to lose in subsequent fights [8]. This phenomenon explains third-party interventions by fallow deer [87]; rates of coalition formation are predicted by the number of unique opponents encountered per day rather than more nuanced social measures requiring mental bookkeeping [89]. Moreover, male olive baboons form alliances with males close in rank to themselves to take over consortships from higher-ranking males [13], and partner choice may rely on males’ knowledge of their own rank relationships with other males [15], not third-party knowledge of rank relationships.
(e) . Limitations of the study
It is important to acknowledge that information about intragroup coalition formation is not available for many mammalian species that form social groups and could potentially form intragroup coalitions. Although coalitions are relatively conspicuous events, they are uncommon and difficult to study systematically. This means that they may occur in some species even though they have not been described in the literature.
Another limitation of our study is that the data are limited to those species for which individuals of at least one sex formed coalitions. Future analyses are needed to uncover if and how these mammalian species systematically differ from those for which intragroup coalitions are truly absent. It is possible that there are sex differences in the pattern, frequency and consequences of intragroup coalitions not uncovered in the current study. For example, male gorillas [155], bonobos [67,156] and spotted hyenas [11] form coalitions less often than females do. Moreover, additional sex differences may be detected from comparisons limited to patterns of intrasexual coalition formation. Our bibliometric measure of coalition frequency likely falls short of capturing some sex differences. Further empirical work is needed to address these issues.
(f) . Conclusions
Current evidence suggests that both sexes participate in coalitions in most mammalian species in which coalitions occur, and this is not clearly influenced by dispersal patterns, the extent of sexual dimorphism, or the distribution of food resources. Taken together, this suggests that there is not a strong phylogenetic legacy of sex differences in this form of cooperation. This contrasts with participation in intergroup conflict, which is strongly male-biased in mammals, including humans [61].
Animal ethics
No new data were collected from animals for this current study.
Acknowledgements
We are grateful to the guest editors for the invitation to contribute to this special issue, and to Maddie Buhbe and Chelsea Ortiz-Jimenez for contributions in the early phases of data extraction for this study. We also thank Janet Mann, John Robinson and John Hoogland for offering helpful insights.
Data accessibility
All data and R code to reproduce the results are publicly available at https://github.com/adrianjaeggi/Coalitions-by-sex-mammals.
The data are also provided in the electronic supplementary material [157].
Authors' contributions
J.E.S.: conceptualization, data curation, methodology, project administration, supervision, validation, writing—original draft, writing—review and editing; A.V.J.: formal analysis, visualization, writing—original draft, writing—review and editing; R.K.H.: data curation, writing—review and editing; J.B.S.: data curation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
The authors have no competing interests.
Funding
We received no funding for this study.
References
- 1.Muller MN, Mitani JC. 2005. Conflict and cooperation in wild chimpanzees. Adv. Study Behav. 35, 275-331. ( 10.1016/S0065-3454(05)35007-8) [DOI] [Google Scholar]
- 2.Parker GA. 1974. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223-243. ( 10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 3.Smith JM, Parker GA. 1976. The logic of asymmetric contests. Anim. Behav. 24, 159-175. ( 10.1016/S0003-3472(76)80110-8) [DOI] [Google Scholar]
- 4.Smith JM, Price GR. 1973. The logic of animal conflict. Nature 246, 15-18. ( 10.1038/246015a0) [DOI] [Google Scholar]
- 5.Clutton-Brock TH. 1982. The function of antlers. Behaviour 79, 108-124. ( 10.1163/156853982X00201) [DOI] [Google Scholar]
- 6.Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness F, Clutton-Brock T. 2002. Antler size in red deer: heritability and selection but no evolution. Evolution 56, 1683-1695. ( 10.1111/j.0014-3820.2002.tb01480.x) [DOI] [PubMed] [Google Scholar]
- 7.Schjelderup-Ebbe T. 1922. Beiträge zur Sozialpsychologie des Haushuhns [Contributions to the social psychology of domestic chickens]. Z. Psychol. 88, 225-252. [Google Scholar]
- 8.Dugatkin LA. 1997. Winner and loser effects and the structure of dominance hierarchies. Behav. Ecol. 8, 583-587. ( 10.1093/beheco/8.6.583) [DOI] [Google Scholar]
- 9.Hobson EA. 2022. Quantifying the dynamics of nearly 100 years of dominance hierarchy research. Phil. Trans. R. Soc. B 377, 20200433. ( 10.1098/rstb.2020.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clutton-Brock TH. 2016. Mammal societies. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 11.Smith JE, Van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE. 2010. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 284-303. ( 10.1093/beheco/arp181) [DOI] [Google Scholar]
- 12.Harcourt AH, de Waal FBM. 1992. Coalitions and alliances in humans and other animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Bercovitch FB. 1988. Coalitions, cooperation and reproductive tactics among adult male baboons. Anim. Behav. 36, 1198-1209. ( 10.1016/S0003-3472(88)80079-4) [DOI] [Google Scholar]
- 14.Packer C. 1977. Reciprocal altruism in Papio anubis. Nature 265, 441-443. ( 10.1038/265441a0) [DOI] [Google Scholar]
- 15.Noë R. 1992. Alliance formation among male baboons: shopping for profitable partners. In Coalitions and alliances in humans and other animals (eds de Waal FBM, Harcourt A), pp. 285-322. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Smale L, Laurence FG, Holekamp KE. 1993. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Anim. Behav. 46, 467-477. ( 10.1006/anbe.1993.1215) [DOI] [Google Scholar]
- 17.Holekamp K, Smith JE, Strelioff CC, Van Horn RC, Watts HE. 2012. Society, demography and genetic structure in the spotted hyena. Mol. Ecol. 21, 613-632. ( 10.1111/j.1365-294X.2011.05240.x) [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom ML, Fedigan LM. 2010. Dominance among female white-faced capuchin monkeys (Cebus capucinus): hierarchical linearity, nepotism, strength and stability. Behaviour 147, 889-931. ( 10.1163/000579510X497283) [DOI] [Google Scholar]
- 19.Chapais B. 1992. The role of alliances in social inheritance of rank among female primates. In Coalitions and alliances in humans and other animals (eds de Waal FBM, Harcourt A), pp. 29-60. Oxford, UK: Oxford University Press. [Google Scholar]
- 20.de Villiers MS, Richardson PRK, van Jaarsveld AS. 2003. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus). J. Zool. 260, 377-389. ( 10.1017/S0952836903003832) [DOI] [Google Scholar]
- 21.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2012. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373-381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207-2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 23.Kutsukake N, Hasegawa T. 2005. Dominance turnover between an alpha and a beta male and dynamics of social relationships in Japanese macaques. Int. J. Primatol. 26, 775-800. ( 10.1007/s10764-005-5308-4) [DOI] [Google Scholar]
- 24.Parish AR. 1994. Sex and food control in the ‘uncommon chimpanzee’: how Bonobo females overcome a phylogenetic legacy of male dominance. Ethol. Sociobiol. 15, 157-179. ( 10.1016/0162-3095(94)90038-8) [DOI] [Google Scholar]
- 25.Furuichi T. 2011. Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. 20, 131-142. ( 10.1002/evan.20308) [DOI] [PubMed] [Google Scholar]
- 26.Surbeck M, Mundry R, Hohmann G. 2011. Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus). Proc. R. Soc. B 278, 590-598. ( 10.1098/rspb.2010.1572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surbeck M, et al. 2019. Males with a mother living in their group have higher paternity success in bonobos but not chimpanzees. Curr. Biol. 29, R354-R355. ( 10.1016/J.CUB.2019.03.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess NH. 2017. Informational warfare: coalitional gossiping as a strategy for within-group aggression. In The Oxford handbook of women and competition (ed. Fisher ML), pp. 223-246. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Alami S, von Rueden C, Seabright E, Kraft TS, Blackwell AD, Stieglitz J, Kaplan H, Gurven M. 2020. Mother's social status is associated with child health in a horticulturalist population. Proc. R. Soc. B 287, 20192783. ( 10.1098/rspb.2019.2783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Rueden CR, Jaeggi AV. 2016. Men's status and reproductive success in 33 nonindustrial societies: effects of subsistence, marriage system, and reproductive strategy. Proc. Natl Acad. Sci. USA 113, 10 824-10 829. ( 10.1073/pnas.1606800113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patton JQ. 2005. Meat sharing for coalitional support. Evol. Hum. Behav. 26, 137-157. ( 10.1016/J.EVOLHUMBEHAV.2004.08.008) [DOI] [Google Scholar]
- 32.Macfarlan SJ, Walker RS, Flinn MV, Chagnon NA. 2014. Lethal coalitionary aggression and long-term alliance formation among Yanomamö men. Proc. Natl Acad. Sci. USA 111, 16 662-16 669. ( 10.1073/pnas.1418639111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seabright E, et al. 2022. Repercussions of patrilocal residence on mothers' social support networks among Tsimane forager–farmers. Phil. Trans. R. Soc. B 378, 20210442. ( 10.1098/rstb.2021.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 35.Kappeler PM, Van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707-740. ( 10.1023/A:1015520830318) [DOI] [Google Scholar]
- 36.Emlen S, Oring L. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215-223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 37.Payne HFP, Lawes MJ, Henzi SP. 2003. Fatal attack on an adult female Cercopithecus mitis erythrarchus: implications for female dispersal in female-bonded societies. Int. J. Primatol. 24, 1245-1250. ( 10.1023/B:IJOP.0000005990.39403.96) [DOI] [Google Scholar]
- 38.Cooper MA, Aureli F, Singh M. 2004. Between-group encounters among bonnet macaques (Macaca radiata). Behav. Ecol. Sociobiol. 56, 217-227. ( 10.1007/s00265-004-0779-4) [DOI] [Google Scholar]
- 39.Lewis RJ, Sandel AA, Hilty S, Barnett SE. 2020. The collective action problem but not numerical superiority explains success in intergroup encounters in Verreaux's sifaka (Propithecus verreauxi): implications for individual participation and free-riding. Int. J. Primatol. 41, 305-324. ( 10.1007/s10764-020-00155-6) [DOI] [Google Scholar]
- 40.Majolo B, Ventura R, Koyama NF. 2005. Sex, rank and age differences in the Japanese macaque (Macaca fuscata yakui) participation in inter-group encounters. Ethology 111, 455-468. ( 10.1111/j.1439-0310.2005.01087.x) [DOI] [Google Scholar]
- 41.van Hooff JARAM, van Schaik CP. 1992. Cooperation in competition: the ecology of primate bonds. In Coalitions and alliances in humans and other animals (eds de Waal FBM, Harcourt A), pp. 357-390. Oxford, UK: Oxford University Press. [Google Scholar]
- 42.Van Hooff JARAM, Van Schaik CP. 1994. Male bonds: affilliative relationships among nonhuman primate males. Behaviour 130, 309-337. ( 10.1163/156853994X00587) [DOI] [Google Scholar]
- 43.Bissonnette A, Perry S, Barrett L, Mitani JC, Flinn M, Gavrilets S, de Waal FBM. 2015. Coalitions in theory and reality: a review of pertinent variables and processes. Behaviour 152, 1-56. ( 10.1163/1568539X-00003241) [DOI] [Google Scholar]
- 44.Smith JE. 2014. Hamilton's legacy: kinship, cooperation and social tolerance in mammalian groups. Anim. Behav. 92, 291-304. ( 10.1016/j.anbehav.2014.02.029) [DOI] [Google Scholar]
- 45.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262-300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 46.Sterck EHM, Watts DP, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291-309. ( 10.1007/s002650050390) [DOI] [Google Scholar]
- 47.Van Schaik C. 1989. The ecology of social relationships among female primates. In Comparative socioecology: the behavioral ecology of humans and other mammals (eds Standen V, Foley R), pp. 195-218. Oxford, UK: Blackwell Scientific Publishing. [Google Scholar]
- 48.Palombit RA, Seyfarth RM, Cheney DL. 1997. The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Anim. Behav. 54, 599-614. ( 10.1006/anbe.1996.0457) [DOI] [PubMed] [Google Scholar]
- 49.Seyfarth RM, Cheney DL. 1984. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature 308, 541-543. ( 10.1038/308541a0) [DOI] [PubMed] [Google Scholar]
- 50.Cheney DL, Moscovice LR, Heesen M, Mundry R, Seyfarth RM. 2010. Contingent cooperation between wild female baboons. Proc. Natl Acad. Sci. USA 107, 9562-9566. ( 10.1073/pnas.1001862107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schino G. 2007. Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behav. Ecol. 18, 115-120. ( 10.1093/beheco/arl045) [DOI] [Google Scholar]
- 52.Jaeggi AV, Gurven M. 2013. Reciprocity explains food sharing in humans and other primates independent of kin selection and tolerated scrounging: a phylogenetic meta-analysis. Proc. R. Soc. B 280, 20131615. ( 10.1098/rspb.2013.1615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ostner J, Vigilant L, Bhagavatula J, Franz M, Schülke O. 2013. Stable heterosexual associations in a promiscuous primate. Anim. Behav. 86, 623-631. ( 10.1016/j.anbehav.2013.07.004) [DOI] [Google Scholar]
- 54.Seyfarth RM. 1978. Social relationships among adult male and female baboons. I. Behaviour during sexual consortship. Behaviour 64, 204-226. [Google Scholar]
- 55.Smuts BB. 1985. Sex and friendship in baboons. New York, NY: Aldine. [Google Scholar]
- 56.Duffy KG, Wrangham RW, Silk JB. 2007. Male chimpanzees exchange political support for mating opportunities. Curr. Biol. 17, R586-R587. ( 10.1016/j.cub.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 57.Feldblum JT, Krupenye C, Bray J, Pusey AE, Gilby IC. 2021. Social bonds provide multiple pathways to reproductive success in wild male chimpanzees. iScience 24, 102864. ( 10.1016/j.isci.2021.102864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krutzen M, Sherwin WB, Connor RC, Barre LM, Van de Casteele T, Mann J, Brooks R. 2003. Contrasting relatedness patterns in bottlenose dolphins (Tursiops sp.) with different alliance strategies. Proc. R. Soc. Lond. B 270, 497-502. ( 10.1098/rspb.2002.2229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plavcan JM, Van Schaik CP, Kappeler PM. 1995. Competition, coalitions and canine size in primates. J. Hum. Evol. 28, 245-276. ( 10.1006/jhev.1995.1019) [DOI] [Google Scholar]
- 60.Plavcan JM. 2012. Sexual size dimorphism, canine dimorphism, and male–male competition in primates: where do humans fit in? Hum. Nat. 23, 45-67. ( 10.1007/s12110-012-9130-3) [DOI] [PubMed] [Google Scholar]
- 61.Smith JE, Fichtel C, Holmes RK, Kappeler PM, van Vugt M, Jaeggi AV. 2022. Sex bias in intergroup conflict and collective movement among social mammals: male warriors and female guides. Phil. Trans. R. Soc. B 377, 20210142. ( 10.1098/rstb.2021.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitchen DM, Beehner JC. 2007. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144, 1551-1581. ( 10.1163/156853907782512074) [DOI] [Google Scholar]
- 63.Majolo B, deBortoli Vizioli A, Martínez-Íñigo L, Lehmann J. 2020. Effect of group size and individual characteristics on intergroup encounters in primates. Int. J. Primatol. 41, 325-341. ( 10.1007/s10764-019-00119-5) [DOI] [Google Scholar]
- 64.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140-1162. ( 10.1016/S0003-3472(80)80103-5) [DOI] [Google Scholar]
- 65.Smith JE, Lacey EA, Hayes LD. 2017. Sociality in non-primate mammals. In Comparative social evolution (eds DR Rubenstein, P Abbot), pp. 284-319. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 66.Silk JB, Kappeler PM. 2017. Sociality in primates. In Comparative social evolution (eds DR Rubenstein, P Abbot), pp. 253-283. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 67.Tokuyama N, Furuichi T. 2016. Do friends help each other? Patterns of female coalition formation in wild bonobos at Wamba. Anim. Behav. 119, 27-35. ( 10.1016/j.anbehav.2016.06.021) [DOI] [Google Scholar]
- 68.Surbeck M, et al. 2017. Sex-specific association patterns in bonobos and chimpanzees reflect species differences in cooperation. R. Soc. Open Sci. 4, 161081. ( 10.1098/rsos.161081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fox S, Muller MN, González NT, Enigk DK, Machanda ZP, Otali E, Wrangham R, Thompson ME. 2022. Weak, but not strong, ties support coalition formation among wild female chimpanzees. Phil. Trans. R. Soc. B 377, 20210427. ( 10.1098/rstb.2021.0427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Furuichi T, Ihobe H. 1994. Variation in male relationships in bonobos and chimpanzees. Behaviour 130, 211-228. ( 10.1163/156853994X00532) [DOI] [Google Scholar]
- 71.Möller LM, Beheregaray LB, Harcourt RG, Krützen M. 2001. Alliance membership and kinship in wild male bottlenose dolphins (Tursiops aduncus) of southeastern Australia. Proc. R. Soc. Lond. 268, 1941-1947. ( 10.1098/RSPB.2001.1756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toyoda A, Maruhashi T, Kawamoto Y, Matsudaira K, Matsuda I, Malaivijitnond S. 2022. Mating and reproductive success in free-ranging stump-tailed macaques: effectiveness of male–male coalition formation as a reproductive strategy. Front. Ecol. Evol. 10, 175. ( 10.3389/FEVO.2022.802012/BIBTEX) [DOI] [Google Scholar]
- 73.Harcourt AH, de Waal FBM. 1992. Cooperation in conflict: from ants to anthropoids. In Coalitions and alliances in humans and other animals (eds de Waal FBM, Harcourt A), pp. 493-510. Oxford, UK: Oxford University Press. [Google Scholar]
- 74.Backwell PRY, Jennions MD. 2004. Coalition among male fiddler crabs. Nature 430, 417. ( 10.1038/430417a) [DOI] [PubMed] [Google Scholar]
- 75.Harcourt A. 1992. Coalitions and alliances: are primates more complex than non-primates? In Coalitions and alliances in humans and other animals (eds FBM de Waal, A Harcourt), pp. 445-471. Oxford, UK: Oxford Science Publications. [Google Scholar]
- 76.Lukas D, Clutton-Brock T. 2018. Social complexity and kinship in animal societies. Ecol. Lett. 21, 1129-1134. ( 10.1111/ELE.13079) [DOI] [PubMed] [Google Scholar]
- 77.Smith RJ. 1999. Statistics of sexual size dimorphism. J. Hum. Evol. 36, 423-458. ( 10.1006/JHEV.1998.0281) [DOI] [PubMed] [Google Scholar]
- 78.Upham NS, Esselstyn JA, Jetz W. 2019. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494. ( 10.1371/journal.pbio.3000494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadfield JD, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494-508. ( 10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 80.R Development Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.r-project.org/. [Google Scholar]
- 81.Bürkner PC. 2017. Brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1-28. ( 10.18637/jss.v080.i01) [DOI] [Google Scholar]
- 82.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217-223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 83.McElreath R. 2020. Statistical rethinking: a Bayesian course with examples in R and Stan. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 84.Paradis E. 2012. Analysis of phylogenetics and evolution with R, 2nd edn. New York, NY: Springer New York. [Google Scholar]
- 85.Mills B. 2022. Color palettes inspired by works at the Metropolitan Museum of Art, version 0.1.0. See https://cran.r-project.org/.
- 86.Lynch M. 1991. Methods for the analysis of comparative data in evolutionary biology. Evolution 45, 1065-1080. ( 10.1111/j.1558-5646.1991.tb04375.x) [DOI] [PubMed] [Google Scholar]
- 87.Jennings DJ, Carlin CM, Gammell MP. 2009. A winner effect supports third-party intervention behaviour during fallow deer, Dama dama, fights. Anim. Behav. 77, 343-348. ( 10.1016/j.anbehav.2008.10.006) [DOI] [Google Scholar]
- 88.Jennings DJ, Carlin CM, Hayden TJ, Gammell MP. 2011. Third-party intervention behaviour during fallow deer fights: the role of dominance, age, fighting and body size. Anim. Behav. 81, 1217-1222. ( 10.1016/j.anbehav.2011.03.007) [DOI] [Google Scholar]
- 89.Jennings DJ, Boys RJ, Gammell MP. 2017. Investigating variation in third-party intervention behavior during a fallow deer (Dama dama) rut. Behav. Ecol. 28, 288-293. ( 10.1093/beheco/arw156) [DOI] [Google Scholar]
- 90.Jennings DJ, Boys RJ, Gammell MP. 2018. Suffering third-party intervention during fighting is associated with reduced mating success in the fallow deer. Anim. Behav. 139, 1-8. ( 10.1016/j.anbehav.2018.02.016) [DOI] [Google Scholar]
- 91.Jennings DJ, Amin B, Gammell MP. 2021. Third-party assessment of contestants during fallow deer fights increases with resource abundance and dominance rank. Anim. Behav. 177, 81-89. ( 10.1016/j.anbehav.2021.04.020) [DOI] [Google Scholar]
- 92.Rowell TE, Rowell CA. 1993. The social organization of feral Ovis aries ram groups in the pre-rut period. Ethology 95, 213-232. ( 10.1111/j.1439-0310.1993.tb00472.x) [DOI] [Google Scholar]
- 93.Pelchat GO. 2009. Coalitions, dominance and social structure of bighorn (Ovis canadensis) rams. Master's thesis, University of Calgary, Alberta, Canada. (doi:10.11575/PRISM/3045) [Google Scholar]
- 94.Parsons KM, Durban JW, Claridge DE, Balcomb KC, Noble LR, Thompson PM. 2003. Kinship as a basis for alliance formation between male bottlenose dolphins, Tursiops truncatus, in the Bahamas. Anim. Behav. 66, 185-194. ( 10.1006/anbe.2003.2186) [DOI] [Google Scholar]
- 95.Randic S, Connor RC, Sherwin WB, Krutzen M. 2012. A novel mammalian social structure in Indo-Pacific bottlenose dolphins (Tursiops sp.): complex male alliances in an open social network. Proc. R. Soc. B. 279, 3083-3090. ( 10.1098/rspb.2012.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Connor RC, Watson-Capps JJ, Sherwin WB, Krutzen M. 2011. A new level of complexity in the male alliance networks of Indian Ocean bottlenose dolphins (Tursiops sp.). Biol. Lett. 7, 623-626. ( 10.1098/rsbl.2010.0852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connor RC, Smolker RA, Richards AF. 1992. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl Acad. Sci. USA 89, 987-990. ( 10.1073/pnas.89.3.987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Norris KS, Johnson C. 1994. Schools and schooling. In The Hawaiian spinner dolphin (eds Norris KS, Würsig B, Well RS, Würsig M), pp. 232-242. Berkeley, CA: University of California Press. [Google Scholar]
- 99.Connor R, Mann J, Watson-Capps J. 2006. A sex-specific affiliative contact behavior in Indian Ocean bottlenose dolphins, Tursiops sp. Ethology 112, 631-638. ( 10.1111/j.1439-0310.2006.01203.x) [DOI] [Google Scholar]
- 100.Keiper RR. 1988. Social interactions of the Przewalski horse (Equus przewalskii Poliakov, 1881) herd at the Munich Zoo. Appl. Anim. Behav. Sci. 21, 89-97. ( 10.1016/0168-1591(88)90102-5) [DOI] [Google Scholar]
- 101.Krueger K, Schneider G, Flauger B, Heinze J. 2015. Context-dependent third-party intervention in agonistic encounters of male Przewalski horses. Behav. Process. 121, 54-62. ( 10.1016/j.beproc.2015.10.009) [DOI] [PubMed] [Google Scholar]
- 102.Feist D, Mccullough R, Dean J. 1976. Behavior patterns and communication in feral horses. Z. Tierpsychol. 41, 337-371. ( 10.1111/j.1439-0310.1976.tb00947.x) [DOI] [PubMed] [Google Scholar]
- 103.Feh C. 1999. Alliances and reproductive success in Camargue stallions. Anim. Behav. 1995, 705-713. ( 10.1006/anbe.1998.1009) [DOI] [PubMed] [Google Scholar]
- 104.Schneider G, Krueger K. 2012. Third-party interventions keep social partners from exchanging affiliative interactions with others. Anim. Behav. 83, 377-387. ( 10.1016/j.anbehav.2011.11.007) [DOI] [Google Scholar]
- 105.Schilder MBH. 1990. Intervention in a herd of semi-captive plains zebras. Behaviour 112, 53-83. ( 10.1163/156853990X00680) [DOI] [Google Scholar]
- 106.Klingel H. 1967. Social organization and behavior of wild plains zebras. J. Anim. Psychol. 24, 580-624. [PubMed] [Google Scholar]
- 107.Schaller GB. 1972. The Serengetti lion: a study of predator–prey relations. Chicago, IL: University of Chicago Press. [Google Scholar]
- 108.Packer C, Pusey AE, Elberly LE. 2001. Egalitarianism in female African lions. Science 293, 690-693. ( 10.1126/science.1062320) [DOI] [PubMed] [Google Scholar]
- 109.Dietrich G, Kalle K, Krauss W, Siedler G, Richardson PL. 1982. Cooperation and competition within coalitions of male lions: kin selection or game theory? Nature 296, 740-742. ( 10.1038/296740a0) [DOI] [Google Scholar]
- 110.Packer C, Scheel D, Pusey A. 1990. Why lions form groups: food is not enough. Am. Nat. 136, 1-19. ( 10.1086/285079) [DOI] [Google Scholar]
- 111.Hirsch BT. 2007. Spoiled brats: is extreme juvenile agonism in ring-tailed coatis (Nasua nasua) dominance or tolerated aggression? Ethology 113, 446-456. ( 10.1111/j.1439-0310.2007.01348.x) [DOI] [Google Scholar]
- 112.Romero T, Aureli F. 2008. Reciprocity of support in coatis (Nasua nasua). J. Comp. Psychol. 122, 19-25. ( 10.1037/0735-7036.122.1.19) [DOI] [PubMed] [Google Scholar]
- 113.Hirsch BT, Stanton MA, Maldonado JE. 2012. Kinship shapes affiliative social networks but not aggression in ring-tailed coatis. PLoS ONE 7, e37301. ( 10.1371/journal.pone.0037301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee PC. 1987. Allomothering among African elephants. Anim. Behav. 35, 278-291. ( 10.1016/S0003-3472(87)80234-8) [DOI] [Google Scholar]
- 115.Archie EA, Moss CJ, Alberts SC. 2006. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc. R. Soc. B 273, 513-522. ( 10.1098/rspb.2005.3361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valero A, Schaffner C, Vick L, Aureli F, Ramos-Fernandez G. 2006. Intergroup lethal aggression in wild spider monkeys. Am. J. Primatol. 68, 732-737. ( 10.1002/ajp.20263) [DOI] [PubMed] [Google Scholar]
- 117.Campbell CJ. 2006. Lethal intragroup aggression by adult male spider monkeys (Atles geoffroyi). Am. J. Primatol. 68, 1197-1201. ( 10.1002/ajp.20305) [DOI] [PubMed] [Google Scholar]
- 118.Fedigan LM, Baxter MJ. 1984. Sex differences and social organization in free-ranging spider monkeys (Ateles geoffroyi). Primates 25, 279-294. ( 10.1007/BF02382267) [DOI] [Google Scholar]
- 119.Ren R, Yan K, Su Y, Qi H, Liang B, Bao W, de Waal FBM. 1991. The reconciliation behavior of golden monkeys (Rhinopithecus roxellanae roxellanae) in small breeding groups. Primates 32, 321-327. ( 10.1007/BF02382673) [DOI] [Google Scholar]
- 120.Xiang Z, Yu Y, Yao H, Hu Q, Yang W, Li M. 2022. Female countertactics to male feticide and infanticide in a multilevel primate society. Behav. Ecol. 33, 679-687. ( 10.1093/BEHECO/ARAC022) [DOI] [Google Scholar]
- 121.Yao H, Yu H, Yang B, Yang W, Xu H, Grueter CC, Li M, Xiang Z. 2016. Male infanticide in the golden snub-nosed monkey (Rhinopithecus roxellana), a seasonally breeding primate. Int. J. Primatol. 37, 175-184. ( 10.1007/s10764-016-9892-2) [DOI] [Google Scholar]
- 122.Wittig RM, Crockford C, Seyfarth RM, Cheney DL. 2007. Vocal alliances in chacma baboons (Papio hamadryas ursinus). Behav. Ecol. Sociobiol. 61, 899-909. ( 10.1007/s00265-006-0319-5) [DOI] [Google Scholar]
- 123.Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. 2006. Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behav. Ecol. Sociobiol. 59, 469-479. ( 10.1007/s00265-005-0071-2) [DOI] [Google Scholar]
- 124.Silk JB, Alberts SC, Altmann J. 2004. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim. Behav. 67, 573-582. ( 10.1016/j.anbehav.2003.07.001) [DOI] [Google Scholar]
- 125.Weingrill T, Lycett JE, Henzi SP. 2000. Consortship and mating success in chacma baboons (Papio cynocephalus ursinus). Ethology 106, 1033-1044. ( 10.1046/j.1439-0310.2000.00616.x) [DOI] [Google Scholar]
- 126.Bulger JB. 1993. Dominance rank and access to estrous females in male savanna baboons. Behaviour 127, 67-103. ( 10.1163/156853993X00434) [DOI] [Google Scholar]
- 127.Mitchell CL, Boinski S, Van Schaik CP. 1991. Competitive regimes and female bonding in two species of squirrel monkeys (Saimiri oerstedi and S. sciureus). Behav. Ecol. Sociobiol. 28, 55-60. ( 10.1007/BF00172139) [DOI] [Google Scholar]
- 128.Kramer KL. 2022. Female cooperation: evolutionary, cross-cultural and ethnographic evidence. Phil. Trans. R. Soc. B 378, 20210425. ( 10.1098/rstb.2021.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Riker WH. 1962. The theory of political coalitions. New Haven, CT: Yale University Press. [Google Scholar]
- 130.Mesterton-Gibbons M, Sherratt TN. 2007. Coalition formation: a game-theoretic analysis. Behav. Ecol. 18, 277-286. ( 10.1093/beheco/arl084) [DOI] [Google Scholar]
- 131.Stamatopoulos G, Sengupta A, Vogel E, Janson C, Stamatopoulos G, Sengupta A, Vogel E, Janson C. 2009. A game-theoretic model of coalition formation among primates. J. Bioecon. 11, 165-183. ( 10.1007/s10818-009-9060-2) [DOI] [Google Scholar]
- 132.Silk JB. 2002. Practice random acts of aggression and senseless acts of intimidation: the logic of status contests in social groups. Evol. Anthropol. 11, 221-225. ( 10.1002/evan.10038) [DOI] [Google Scholar]
- 133.Bissonnette A, Bischofberger N, van Schaik CP. 2011. Mating skew in Barbary macaque males: the role of female mating synchrony, female behavior, and male–male coalitions. Behav. Ecol. Sociobiol. 65, 167-182. ( 10.1007/s00265-010-1023-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuester J, Paul A. 1992. Influence of male competition and female mate choice on male mating success in Barbary macaques (Macaca sylvanus). Behaviour 120, 192-216. ( 10.1163/156853992X00606) [DOI] [Google Scholar]
- 135.Watts DP. 1998. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 44, 43-55. ( 10.1007/s002650050513) [DOI] [Google Scholar]
- 136.Perry S. 1997. Male-female social relationships in wild white-faced capuchins (Cebus capucinus). Behaviour 134, 477-510. ( 10.1163/156853997X00494) [DOI] [Google Scholar]
- 137.Vogel ER, Munch SB, Janson CH. 2007. Understanding escalated aggression over food resources in white-faced capuchin monkeys. Anim. Behav. 74, 71-80. ( 10.1016/j.anbehav.2007.02.003) [DOI] [Google Scholar]
- 138.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231-1234. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 139.Zabel CJ, Glickman SE, Frank LG, Woodmansee KB, Keppel G. 1992. Coalition formation in a colony of prepubertal spotted hyaenas. In Coalitions and alliances in humans and other animals (eds de Waal FBM, Harcourt A), pp. 113-136. Oxford, UK: Oxford University Press. [Google Scholar]
- 140.Engh AL, Esch K, Smale L, Holekamp KE. 2000. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Anim. Behav. 60, 323-332. ( 10.1006/anbe.2000.1502) [DOI] [PubMed] [Google Scholar]
- 141.Watts DP. 2002. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour 139, 343-370. ( 10.1163/156853902760102708) [DOI] [Google Scholar]
- 142.Silk JB. 2002. Kin selection in primate groups. Int. J. Primatol. 23, 849-875. ( 10.1023/A:1015581016205) [DOI] [Google Scholar]
- 143.Widdig A. 2007. Paternal kin discrimination: the evidence and likely mechanisms. Biol. Rev. 82, 319-334. ( 10.1111/j.1469-185X.2007.00011.x) [DOI] [PubMed] [Google Scholar]
- 144.Leonardo DE, Nogueira-Filho SLG, de Góes Maciel F, Biondo C, Mendl M, Nogueira SS da C. 2021. Third-party conflict interventions are kin biased in captive white-lipped peccaries (Mammalia, Tayassuidae). Behav. Process. 193, 104524. ( 10.1016/j.beproc.2021.104524) [DOI] [PubMed] [Google Scholar]
- 145.Young C, Majolo B, Schülke O, Ostner J. 2014. Male social bonds and rank predict supporter selection in cooperative aggression in wild Barbary macaques. Anim. Behav. 95, 23-32. ( 10.1016/j.anbehav.2014.06.007) [DOI] [Google Scholar]
- 146.de Waal FBM, Luttrell LM. 1988. Mechanism of social reciprocity in three primate species: symmetrical relationship characteristics or cognition? Ethol. Sociobiol. 9, 101-118. ( 10.1016/0162-3095(88)90016-7) [DOI] [Google Scholar]
- 147.Aureli F, Schino G. 2019. Social complexity from within: how individuals experience the structure and organization of their groups. Behav. Ecol. Sociobiol. 73, 6. ( 10.1007/s00265-018-2604-5) [DOI] [Google Scholar]
- 148.Silk JB. 1999. Male bonnet macaques use information about third-party rank relationships to recruit allies. Anim. Behav. 58, 45-51. ( 10.1006/anbe.1999.1129) [DOI] [PubMed] [Google Scholar]
- 149.Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. 2003. Hierarchical classification by rank and kinship in baboons. Science 302, 1234-1236. ( 10.1126/science.1087513) [DOI] [PubMed] [Google Scholar]
- 150.Byrne RW, Whiten A. 1988. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford, UK: Oxford University Press. [Google Scholar]
- 151.Engh AL, Siebert ER, Greenberg DA, Holekamp KE. 2005. Patterns of alliance formation and postconflict aggression indicate spotted hyaenas recognize third-party relationships. Anim. Behav. 69, 209-217. ( 10.1016/j.anbehav.2004.04.013) [DOI] [Google Scholar]
- 152.Pandit SA, Van Schaik CP. 2003. A model for leveling coalitions among primate males: toward a theory of egalitarianism. Behav. Ecol. Sociobiol. 55, 161-168. ( 10.1007/s00265-003-0692-2) [DOI] [Google Scholar]
- 153.Gygax L, Harley N, Kummer H. 1997. A matrilineal overthrow with destructive aggression in Macaca fascicularis. Primates 38, 149-158. ( 10.1007/BF02382005) [DOI] [Google Scholar]
- 154.Bissonnette A, Franz M, Schülke O, Ostner J. 2014. Socioecology, but not cognition, predicts male coalitions across primates. Behav. Ecol. 25, 794-801. ( 10.1093/beheco/aru054) [DOI] [Google Scholar]
- 155.Stokes EJ. 2004. Within-group social relationships among females and adult males in wild western lowland gorillas (Gorilla gorilla gorilla). Am. J. Primatol. 64, 233-246. ( 10.1002/ajp.20074) [DOI] [PubMed] [Google Scholar]
- 156.Surbeck M, Hohmann G. 2013. Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 67, 1767-1780. ( 10.1007/s00265-013-1584-8) [DOI] [Google Scholar]
- 157.Smith JE, Jaeggi AV, Holmes RK, Silk JB. 2022. Sex differences in cooperative coalitions: a mammalian perspective. Figshare. ( 10.6084/m9.figshare.c.6251004) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Smith JE, Jaeggi AV, Holmes RK, Silk JB. 2022. Sex differences in cooperative coalitions: a mammalian perspective. Figshare. ( 10.6084/m9.figshare.c.6251004) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data and R code to reproduce the results are publicly available at https://github.com/adrianjaeggi/Coalitions-by-sex-mammals.
The data are also provided in the electronic supplementary material [157].