Abstract
Objectives
To analyse the available evidence and identify gaps in current knowledge regarding physical activity volume and intensity and their effects on pregnancy outcomes in female athletes.
Design
Scoping review.
Data sources
A structured literature search of three electronic databases (Embase, PubMed and Web of Science) was conducted on 25 February 2022, and a rerun search was conducted on 8 September 2022.
Eligibility criteria
Studies were eligible if they contained information on the relevant population (ie, elite or competitive amateur female athletes), intervention/exposure (ie, minimum of 10 hours of sport per week) and fetal and maternal outcomes. Eligible comparators included female recreational athletes and pregnant non-exercisers.
Risk of bias
The risk of bias was evaluated with the National Institutes of Health (National Heart, Lung and Blood Institute) quality assessment tool.
Results
The results revealed a discrepancy between the number of original research papers and the number of reviews and recommendations derived from them. The identified studies focused primarily on pregnant recreational athletes. Sixteen clinical studies met the inclusion criteria. No adverse effects on maternal or fetal outcomes were reported. Only during performance tests involving acute intensive exercise with the mother exercising at more than 90% of her maximal heart rate did some fetuses experience decelerations in heart rate.
Summary/conclusion
A lack of high-quality studies and direct evidence on pregnant elite and competitive amateur female athletes is evident. Further prospective observational cohort studies are needed using new monitoring methods (eg, non-invasive, wireless monitoring systems) aiming to gain a broader understanding of the stress tolerance of pregnant athletes and fetuses during exercise. Following that, interventional studies with stress tests in laboratory settings should be conducted. Therefore, technology plays a decisive role in gaining new knowledge and providing evidence-based recommendations on this topic.
PROSPERO registration number
CRD42022309541.
Keywords: elite performance, pelvic floor, physical activity, training, sports & exercise medicine
WHAT IS ALREADY KNOWN ON THIS TOPIC
The positive effects of moderately intense exercise during pregnancy are manifold (eg, prevention of excessive weight gain, reduction of the risk of pre-eclampsia, gestational diabetes and thrombosis, improvement of well-being and self-perception) and are supported by national and international guidelines.
There are solid data regarding recommendations on low to moderate physical activity for recreational female athletes.
Elite female athletes often state that they receive insufficient information during pregnancy regarding training recommendations that ensure the safety of both mother and child.
WHAT THIS STUDY ADDS
Training with higher intensities might be possible when carefully controlled and tolerated well by the mother.
There is knowledge transferred from the general population and consensus on best practices, but direct evidence for elite and competitive amateur female athletes, who have significantly higher and more intensive training loads, is sparse.
Currently, no generalisable recommendations can be made due to the limited direct evidence for elite and competitive amateur athletes.
Introduction
It is well known that both maternal and fetal morbidity can be reduced with appropriate physical activity.1–3 The positive effects of moderate-intensity exercise during pregnancy are manifold (eg, prevention of excessive weight gain, reduction of the risk of pre-eclampsia, gestational diabetes and thrombosis, improvement of well-being and self-perception) and broadly accepted in national and international guidelines for the general population.4–7
Of particular interest are the anatomical, hormonal, metabolic, cardiovascular and pulmonary adaptations that occur during pregnancy and their consequences for the load tolerance of pregnant elite and competitive amateur athletes and their unborn children.8–16 Some important ones are mentioned, such as an increased resting cardiac output and stroke volume related to anatomical changes as a progressive eccentric myocardial hypertrophy.13 14 17 18 In addition, increased ventilation, most likely triggered by escalating progesterone levels, results in up to 48% elevated minute ventilation during the first trimester.15 19 20 Although the current state of scientific evidence does not support the conclusion that the physiological changes described above increase peak performance during the first trimester of pregnancy, this topic is still controversial (especially in the lay press).21–24 However, some studies indicate that the aforementioned physiological changes in the first trimester of pregnancy at least allow top performance during this period, even in elite athletes.25
The evidence for pregnant athletes who also exercise at high intensity is sparse, and in current clinical practice, recommendations are usually based on best-practice knowledge and expert opinions.26 27 In current exercise guidelines, there are explicit recommendations for recreational athletes.28 29 Elite athletes or competitive amateur athletes are often only mentioned in side statements.28 29 Recently, however, interest in the relationship between exercise and pregnancy outcomes in elite athletes has increased, as the systematic reviews and meta-analysis by Wowdzia and Kimber indicate.30–32 The present reviews have investigated or focused on a subset of outcome parameters, which is also reflected in the selection of studies.32–34
An increasing number of elite and competitive amateur female athletes are reaching their career peak during the period of optimal fertility and do not want to postpone having children until they end their athletic careers. This is particularly the case in endurance disciplines where training age plays a relevant role.35 Moreover, if possible, these athletes want to time their pregnancy to not coincide with potential career highlights (eg, the Olympic cycle with a peak every 4 years).36 However, there is only sparse evidence and anecdotal reports of best-practice recommendations to help handle this issue in elite female athletes.25 Due to the lack of direct evidence for female athletes and the gaps in knowledge regarding the safe frequency, duration and intensity of training and competition, recommendations can only be made individually and under close observation of maternal and child well-being.37 The need for practical information regarding what type of sports and to what extent they can be continued safely and without risk for mother or child is of great importance, especially for female athletes who have great uncertainty in this regard,36 but also for trainers, coaches and healthcare providers. Moreover, such information is needed for both elite and competitive amateur female athletes.
In this scoping review, we aim to (1) identify and evaluate the current scientific evidence of literature regarding sport and exercise recommendations for elite and competitive amateur female athletes, (2) summarise the available evidence for the volume and intensity of physical activity for continuous exposure and acute exposure to physical exercise in terms of performance testing as well as for high-risk sports and their effects on pregnancy outcome parameters in female athletes and (3) point out existing knowledge gaps.
Methods
This scoping review was performed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines and PRISMA extension for searching (PRISMA-S).38 39 The protocol was registered in the PROSPERO database (CRD42022309541).40 The flow chart of the study selection process and the rerun are presented in figure 1 and figure 2.
Figure 1.

Flow chart of the study selection process (25 February 2022).
Figure 2.
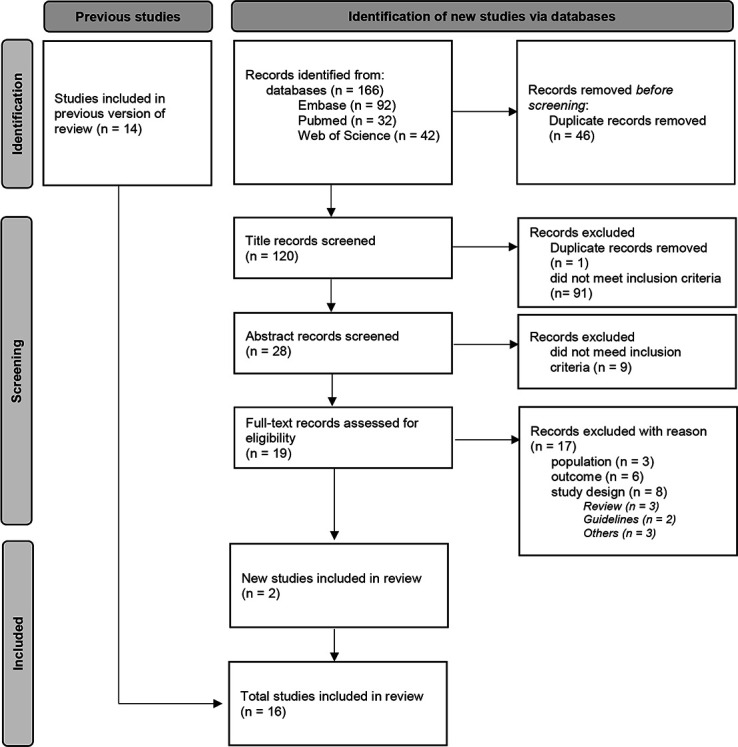
Flow chart of the update-search study selection process (8 September 2022).
Search strategy and study selection
Regarding the abovementioned aim of this review, a preliminary representative search was conducted in PubMed on 4 February 2022. The preliminary search covered the time span from 1950 to the present.
The main search of this review regarding the two other review questions was executed on 25 February 2022. For this, a structured search of three electronic databases (Embase, PubMed and Web of Science) was conducted. An identical rerun was performed on 8 September 2022. The search employed a sensitive search strategy, using a combination of database-specific thesaurus terms and free-text terms in the title and abstract related to sports and exercise during pregnancy and postpartum. The complete search strategy is presented in online supplemental appendix 1.
bmjsem-2022-001395supp001.pdf (32.9KB, pdf)
The search and study selection for the results of the main search was conducted by two raters independently (NW and AK). Endnote X9.2 (Clarivate Analytics, Philadelphia, Pennsylvania, USA) was used for data management. Citations and abstracts were listed, and duplicates were removed. The selected potentially eligible studies were screened for relevance in a two-step approach. First, two raters independently screened all studies for inclusion based on the title and abstract. In the case of uncertainty, full-text articles were retrieved. Additional information was sought from the authors where necessary. In the second step, the same authors independently evaluated the full-text articles for final inclusion. The reasons for the full-text exclusion can be found in online supplemental appendix 2. Any discrepancies were resolved via discussion and/or by discussion with a third reviewer (NK). Additional articles were retrieved through the bibliography lists of found literature, if applicable. Randomised clinical trials, longitudinal studies, case series and case reports in the English language were included.
bmjsem-2022-001395supp002.pdf (98.1KB, pdf)
Eligibility
For the literature of the main search, the PICO (population, intervention, comparison, outcome) framework was used to identify appropriate studies.41 The population of interest was pregnant athletes (ie, elite or competitive amateur female athletes), and the focused intervention/exposure was a minimum of 10 hours of sports activity per week in accordance with current guidelines (any type).42 Eligible comparators included female recreational athletes and pregnant non-exercisers. Relevant adverse pregnancy outcome parameters were (1) fertility, (2) miscarriage/pregnancy loss or intrauterine fetal death, (3) intrauterine fetal growth retardation/abnormal fetal birth weight (low birth weight <2500 g or macrosomia >4000 g), (4) impaired uteroplacental and fetal blood flow (assessed by Doppler ultrasound), (5) preterm birth (defined as birth <37+0 weeks of gestation), (6) low APGAR score (<7 at 5 min postpartum), (7) infant development, (8) non-planned caesarean/emergency c-section or instrumental vaginal delivery, (9) hypertensive pregnancy diseases (such as pre-eclampsia/haemolysis–elevated liver enzymes–low platelets (HELLP) syndrome), (10) maternal pelvic floor disorders and (11) maternal diastasis of the rectus abdominis muscles. We excluded all articles with a publication date before 1985 because of the limited availability of publications prior to 1985 and to not include outdated and irrelevant data.
Data extraction
Formal data extraction was performed for all included original articles from the main search and collected in a data spreadsheet. Data extraction was undertaken by two raters (NW and AK). The included articles were allocated into three groups of intervention/training patterns: (1) elite/competitive sports activity throughout the course of pregnancy, (2) performance testing during pregnancy reflecting an acute exposure to stress and (3) sports with specific risks for a pregnant athlete. In two case reports, continuous training and performance testing were reported at the same time.43 44 The following data were extracted: year of publication, study design, quality rating, sample size, type of sports, intervention/training patterns during pregnancy, comparators, type of assessed outcome parameter and major findings (table 1 and table 2).
Table 1.
Methodological characteristics, samples, quality and characteristics of the interventions/training patterns of the included studies
| Reference | Study design | SE | Sample | QA | Intervention/training patterns during/after pregnancy | Comparators |
| Almquist et al 52 | Case report | (a) | n=1 | g | ET and ST 4.2–6.9 hours/week prepregnancy, structured training during pregnancy, Week 1: 4 moderate-intensity interval training sessions (4×12 min); Week 2: 3–6 low-intensity training sessions (>60 min continuous cycling); :Week 3: 4 high-intensity interval training sessions (5×5 min); Week 4: recovery week, max. >20 hours/week at 18 weeks of pregnancy. | None |
| Beilock et al54 | Retrospective observational cohort (QNR) | (a) | n=26 age=30.65; competitive athletes (swimmers 41%, track and field/road racing 29%) |
f | 49% decreased participation in cardiovascular and resistance exercise during the first trimester, 72% during the second and 80% during the third. | None |
| Bo et al48 | Retrospective case–control study (QNR) | (a) | n=31 elite athletes (various types of sports) (response rate 77.5%) | f | Elite athletes’ sport volume: 14 hours/week before, 4 hours/week 6 weeks postpartum, 10.8 hours/week when filling out QNR; 38% started jogging within 6 weeks postpartum, 4.3% control; 77% of elite athletes continued to compete at the same level after childbirth and 45% were still at this level when answering the QNR. | n=46 age-matched controls (response rate 57.7%), age=34 years |
| Bubnjević et al53 | Case report | (a) | n=1 (marathon runner), age=33 years | f | During third trimester, reduction in the number of training sessions/week and the length of the runs; at 7 months, 6 sessions/week 7 km each; at 8 months, 5 km five times a week; at 9 months, 5 km four times a week; pace 4.30–6.15 km/hour. | None |
| Bung et al 43 |
Case report | (a), (b) | n=1 (competitive sports for 12 years, recently 400 m running), age=25 years | f | Training six times a week conditioning and performance (gymnastics/stretching, several sprints, running out, relaxation); during pregnancy focus on ET and ST; 24th pregnancy week, 28th week and then every 2 weeks, during puerperium, 6 weeks and 6 months postpartum: bicycle-ergometer testing up to 150 bpm mHR and running on a track (warming up, three 200 m and one 100 m sprint); <6 months after delivery personal records in various short-distance runs. | None |
| Darroch et al56 | Retrospective observational cohort (QNR) | (a) | n=42 (elite, world-class runners) age=31.7±3.8 years | g | 9±2 running sessions/week, running volume decreased from 63±34 km/week (first trimester) to 30±30 km/week (third trimester); significantly lower average training pace, return to activity/exercise at 6 weeks postpartum and to 80% of prepregnancy training volumes by 3 months. | None |
| Davies et al44 | Case report | (a), (b) | n=1 (marathon runner), age=33 years | g | Prior to conception: 155 km/week, 140–180 mHR; during pregnancy 107±19 km/week, 130–140 mHR; road and cross-country race first+second trimester; moderate cycling exercise was resumed 8 days postpartum; return to competitive racing 8.5 weeks postpartum; standardised submaximal field running test weekly during the first 32 weeks of pregnancy: three repetitions of 2.5 km road runs at a velocity HR of 140 mHR, 150 mHR, 160 mHR, separated by a 3 min recovery jog; standardised submaximal treadmill test 29 weeks antepartum and 10 weeks postpartum: four incremental stages (4 min), 30 s break (blood lactate), running velocities 3.33–4.17 m/s. | None |
| Drastig et al60 | Retrospective observational cohort (QNR) | (c) | n=32 (sport climbing), age=31 years; drop out=1; partly missing data n=17 | f | Sport climbing partly top ranking before pregnancy; climbing experience before pregnancy 2–24 years, skill level before pregnancy 4–7 (UIAA scale=International Climbing and Mountaineering Federation). 50% climbed until the 36th week; 90% adjusted climbing habits (reducing climbing difficulty, doing more top roping). | None |
| Kardel et al47 | Controlled intervention | (a) | n=42 (biathlon, bicycling, marathon; some at national and international levels); HEG n=20, age 28.8 years; MEG n=21, age 26.7 years drop out n=1, missing data n=9 |
p | HEG preconception: 8.2 hours/week; intervention: 8.6 hours/week; muscle ST 72 min two times a week, interval training 35 min two times a week mHR 170–180/min and ET 2.5 hours two times a week mHR 120–140/min), 1 woman stopped training 7 days before labour, 8 stopped 1–3 days before and 7 women exercised the day before labour. | MEG preconception: 4.8 hours/week; intervention: 6.2 hours/week (muscle ST 72 min two times a week; interval training 25 min two times a week mHR 170–180/min; ET 1.5 hours two times a week mHR 120–140/min), 1 woman stopped training 5 days before labour, 12 stopped 1 to 4 days before and 3 exercised the day before labour |
| Penttinen et al49 | Retrospective case–control study (QNR) | (a) | n=30 (endurance athletes, national top-level cross-country skiing, running, speed-skating or orienteering), age 28.1 years; missing data n=3 | p | Postpartum: 18 athletes continued to compete, the median interval being 8.2 months (range 2–24 months). Two (11%) achieved better condition than before the pregnancy, 11 (61%) reached the same level and 5 (28%) did not achieve the same performance level. | Controls (next primipara in the diaries formed a member of the control group) n=30; cave: no matched controls |
| Salvesen et al58 | Controlled intervention | (b) | n=6 (cross-country skiing, duathlon, long-distance running, race walking), age=28–37 years | p | Prior to conception 15–22 hours training/week; testing at 23–29 weeks of pregnancy; 10 min warm-up mHR 135 bpm; 3–5 submaximal workloads (5 min each) on a treadmill with 60%–90% of VO2max; 4-min pause (sampling capillary blood lactate concentration, ultrasound assessments of the maternal–fetal circulation). | None |
| Solli et al25 | Case report | (a) | n=1 (cross-country skier), age=34.5–36.8 years | g | Average training volume during pregnancy 14 hours/week, which included 79%, 86% and 49% of prepregnancy volumes during the first, second and third trimester; testing first, second trimester and postpartum. | None |
| Sigurdar-dottir et al50 | Retrospective case–control study | (a) | n=41 (low impact group: swimming, golf, riding/jockey, weightlifting, ballroom dancing, motocross, pole fitness); n=89 (high impact group: track and field, football, basketball, cross fit, team gymnastics, handball, racket sports, self-defence sport); missing data n=45 | f | Training during pregnancy: low impact 10.2 hours/week, high impact 8.5 hours/week. | n=118 non-athletic group (not compete in sports, physically active at recreational level); training during pregnancy 0.2 hours/week |
| Sundgot-Borgen et al51 | Case–control study | (a) | n=34 (elite athletes at international level; endurance, ball sports, aesthetic, weight or technical), age=33.1 years | f | ET prepregnancy 750 min/week, during pregnancy ~600–700 min/week, ST prepregnancy ~115 min/week, during pregnancy ~55–85 min/week. | n=34 regular active (>150 min/week) but not at an international level age=31.5 years; ET prepregnancy ~180 min/week, during pregnancy 150 min/week; ST prepregnancy 20 min/week, during pregnancy ~15 min/week |
| Szymanski et al59 | Controlled intervention | (b) | n=15 (highly active, predominantly runners), age=32.9 years | f | Vigorous activity >4 days/week; peak treadmill test to volitional fatigue; gestational age 30.3±1.0. | n=15 (regular active: >20 min per session±3 sessions/week), age=34.3 years, gestational age=30.2±0.9; n=15 (non-exercisers) age=32.9 years, gestational age 30.7±1.1; peak treadmill test to volitional fatigue |
| Szymanski et al57 | Controlled intervention | (b) | n=15 (highly active, predominantly runners), age=32.9 years | f | Vigorous activity >4 days/week; peak treadmill test to volitional fatigue; 30-min exercise session at moderate intensity on the treadmill (40%–59% of aerobic capacity reserve); target HR were calculated by the HRR method; 30-min vigorous-intensity session (60%–84% of HRR); gestational age 30.3±1.0. | n=15 (regular active: >20 min per session±3 sessions/week), age=34.3 years, gestational age 30.2±0.9; n=15 (non-exercisers) age=32.9 years, gestational age 30.7±1.1; peak treadmill test to volitional fatigue |
(a) Continuous exercise exposure, (b) acute exposure to intensive activity (performance testing) and (c) high-risk sports.
bpm, beats per minute; ET, endurance training; f, fair; g, good; HEG, high-exercise group; HR, heart rate; HRR, heart rate reserve; MEG, medium-exercise group; mHR, maternal heart rate; p, poor; QA, quality assessment; QNR, questionnaire; SE, type of sports exposure; ST, strength training; VO2max, maximal oxygen consumption.
Table 2.
Outcome measures and major findings of the included studies
| Reference | Outcomes | Major findings | |||||||||
| Fertility | Fetal birth weight including IUGR | Maternal/fetal circulation (mHR*, fHR**, umbilical/uterine) | APGAR/infant development | Preterm birth | Miscarriage/ intrauterine fetal death | Birth mode | Pelvic floor disorder | Hypertensive pregnancy disease | Diastasis muscle rectus abdominis | ||
| Almquist et al52 | x | x | Natural birth without complications, healthy child, 49 cm and 3238 g. | ||||||||
| Beilock et al54 | x | x | x | x | x | Maternal complications 8 (30.8%) (preterm labour 3, sinus problems 1, heavy bleeding 1, kidney stones 1, hypertension 1, extended labour 1); fetal complications during pregnancy 3 (11.55%; low birth weight 2, unhealthy 1); fetal complications during labour and delivery 6 (23.1%). | |||||
| Bo et al48 | x | x | x | x | No significant difference between prevalence of low back pain with or without sciatica; UI 39% athletes vs 37% control, BMI lower in athletes 6 weeks postpartum; no sign. Difference in preterm birth (39% athletes vs 44% controls) birth weight, instrumental births or caesarean sections; no sign. Difference in length of nursing period. | ||||||
| Bubnjević et al53 | x | x | x | x | x | x | x | Induction of labour because of low amniotic fluid; vaginal delivery 39+4; infant was born with jaundice, APGAR 10/10. | |||
| Bung et al43 | x | x | x | x | x | Weight gain 9 kg during pregnancy; delivery 11 days after expected day, healthy boy, 3200 g, APGAR 9/10, normal umbilical blood gases submaximal exercise: 150–165 W during pregnancy, 120 W puerperium, 165 W 6 wks postpartum; fHR changes after maternal submaximal exercise varied, generally increased by 1 to 15 bpm; uterine activity (contractions) also did not vary during and after strenuous exercise from the situation at rest. After one sprint (mHR 170 bpm) fetal bradycardia (70 bpm) recovered to 120 bpm within 3 min; athlete experienced dizziness and precollapse symptoms, uterine activity normal. | |||||
| Darroch et al56 | x | 21 injuries were reported postpartum (50% of the athletes), 6 bone stress injuries, 11 musculoskeletal injuries (muscle, tendon and/or ligament strain/sprain or rupture), 2 sciaticas, 2 injuries qualified as ‘other’. | |||||||||
| Davies et al44 | x | x | x | x | x | Normal fetal development (anthropometric, cardiovascular and metabolic measurements) obstetric cholestasis → elective caesarean section after a 36-week gestation period. Healthy twins: birth weights 2.2 kg and 2.3 kg; no complications during the clinical procedures; follow-up medical examinations: twins in good health, developing normally 12 months following birth. | |||||
| Drastig et al60 | x | x | x | One questionnaire was excluded from data analysis because of pre-eclampsia; mean duration of pregnancy 39.5±1.7 weeks (two preterm=13%, 36th week); mean weight of newborns was 3543±403 g; mean height 50.9±2.1 cm. All children were healthy after birth and 1 year later. No climbing accidents. | |||||||
| Kardel et al47 | x | fHR | x | x | x | x | No striae in any women (normally 30%); no differences between the MEG and HEG in duration of labour, birth weight, or 1-min and 5-min APGAR scores. HEG: greater maternal weight gain during pregnancy, earlier onset of labour for those women who gave birth to girls but not for those who gave birth to boys. | ||||
| Penttinen et al49 | x | x | x | x | 23 athletes (77%) regular menstrual cycles, 7 (23%) had irregularities, 4 hormonal treatments; 7 (23%) had experienced spontaneous abortion during first trimester in previous pregnancy; no significant differences in labour parameters between athletes and controls; ET had no harmful side effects on the pregnancies or deliveries of the athletes. | ||||||
| Salvesen et al58 | x | x | x | x | x | fHR was within the normal range (110–160 bpm) with mHR <90% of HRmax; fetal bradycardia (was observed in two pregnant women) and high umbilical artery pulsatile index (PI) occurred when mHR >90% of HRmax. When uterine blow flow below initial value+intensity >90% HRmax and mean uterine artery volume blood flow <50% of the initial value. fHR and umbilical artery pulsatility index normalised quickly after stopping the exercise. Four normal deliveries, one caesarean section, one vacuum delivery, two operative deliveries were due to obstructed labours. | |||||
| Solli et al25 | x | x | x | Increased muscle soreness around the hip after running sessions during third trimester; stopped running 6 weeks before giving birth; heavy strength and exercise training throughout whole pregnancy, volume reduction during third trimester; two sacrum fractures 13–18 weeks and 19–24 weeks postpartum. | |||||||
| Sigurdar-dottir et al50 | x | x | x | No significant difference in the incidence of emergency c-section, length of first and second stages of labour; the incidence of third to fourth degree perineal tears was significantly higher (23.7%) among low-impact athletes than among high-impact athletes (5.1%), no significant differences between athletes and controls. | |||||||
| Sundgot-Borgen et al51 | x | x | x | x | x | x | x | No group differences in fertility problems, miscarriage, preterm birth or low birth weight; both groups decreased training volume all trimesters and the first two postpartum periods compared with prepregnancy, more athletes returned to sport/exercise at weeks 0–6 postpartum, no difference in incontinence; no group differences in complications during pregnancy and delivery, athletes reported fewer common complaints. 4 athletes had a stress fracture postpartum; athletes had higher body dissatisfaction and drive for thinness (DT) postpartum, while controls had a reduced DT score. Number of athletes with clinical eating disorder was reduced postpartum, constant in controls. Athletes were not satisfied with advice related to ST and nutrition during pregnancy. | |||
| Szymanski et al59 | x | 5 highly active women with transient fHR decelerations (short duration (mean 2:37 min)) and alterations in umbilical and uterine artery Doppler indices immediately postexercise. | |||||||||
| Szymanski et al57 | x | x | x | x | x | Groups were similar in age, BMI, and gestational age. Maternal resting HR in the Highly Active group (61.6±7.2 bpm) was significantly lower than that in the non-exercise (79.0±11.6 and Regularly Active (71.9±7.4) groups, p<0.001. Treadmill time was longer in the Highly Active (22.3±2.9 min) group compared with the Regularly Active (16.6±3.4) and non-exercise (12.1±3.6) groups, p<0.001, reflecting higher fitness. With moderate exercise, all umbilical artery Doppler indices were similar pre-exercise and postexercise among groups. With vigorous exercise, Doppler indices were similar in Regularly and Highly Active women, with statistically significant decreases postexercise (p<0.05). Postexercise fetal heart tracings met criteria for reactivity within 20 min after all tests. | |||||
BMI, body mass index; bpm, beats per minute; ET, endurance training; fHR, fetal heart rate; HEG, high-exercise group; HRmax, maximal heart rate; IUGR, intrauterine growth restriction; MEG, medium-exercise group; mHR, maternal heart rate; ST, strength training; UI, urinary incontinence.
Quality assessment
Two raters (NW and AK) independently assessed the risk of bias in each study using the National Institutes of Health (NIH) study quality assessment tool.45 For any disagreements, a third rater (NK) was contacted for clarification. The tool includes items for evaluating potential flaws in study methods or implementation, including sources of bias confounding, study power, strength of causality in the association between interventions and outcomes, and other factors.46 The raters selected ‘yes,’ ‘no,’ or ‘cannot be determined/not reported/not applicable’ in response to each item. For each item where ‘no’ was selected, the raters were instructed to consider the potential risk of bias that could be introduced by that flaw in the study design or implementation. ‘Cannot determine’ and ‘not reported’ were also considered to represent potential flaws. The overall study quality was rated ‘good’, ‘fair’, or ‘poor’, according to the definition of the NIH study quality assessment tool.45
Patient and public involvement
Patients and the public were not directly involved in this review article. However, the questions addressed in this review were based on clinical questions that the authors are frequently confronted with when consulting with patients and for which no evidence-based answer could be given to date.
Results
Preliminary overview of the current literature
It was noted that in the last two decades, the amount of literature regarding sports during pregnancy has increased exponentially (online supplemental appendix 3 figure). Regarding the type of articles, a relatively large number of review articles compared with a small number of publications dealing with original data is evident (online supplemental appendix 4 figure).
bmjsem-2022-001395supp003.pdf (63.3KB, pdf)
bmjsem-2022-001395supp004.pdf (290.9KB, pdf)
Overview of the included articles along with their study characteristics
After the preliminary search, a similar distribution of articles applied to the results of the main search. Of the 1680 studies, only 16 original research manuscripts met the inclusion criteria (see ‘Eligibility’ section). Of the 16 papers considered for this scoping review, 4 were controlled interventional studies, 3 were observational cohort and cross-sectional studies, 4 were case–control studies and 5 were case reports. Evidence mapping gives an overview of the outcome parameters according to the number of female athletes and the study type (figure 3). The size of the bubbles varies depending on the number of female athletes who have been tested for this outcome parameter. The evidence for the parameters was based on the type of study. Here, in descending order the number of athletes for controlled interventions, cohorts, case‒control studies and case reports.
Figure 3.

Evidence mapping: outcome parameters are presented according to the level of evidence of the study type and the number of female athletes. (The number of studies is given in the parentheses except for the case studies, as here the number results from the study type. In the two studies by Szymanski et al, the data were given for the same study population.57 59 This results in the same number of female athletes (62) with the same number of studies.) IUGR, intrauterine growth restriction.
Quality assessment/risk of bias
Regarding the risk of bias in the intervention studies, three studies were rated as poor, nine studies were rated as fair and four papers were rated as good quality overall (table 1). The main reasons for downgrading were the small sample sizes, lack of control groups, lack of blinding and allocation concealment and randomisation problems.
Data synthesis
Continuous exercise exposure
In this category, 12 studies, 1 controlled intervention,47 4 case–control studies,48–51 5 case reports25 43 44 52 53 and 2 observational studies,54 55 including a total of 251 elite or competitive amateur female athletes of various types of sports, such as endurance, technical, weight class, aesthetic and ball sports, were identified. Typically, the training duration was more than 10 hours per week, with a maximum specified training duration of 14 hours per week on average during pregnancy.25 The study of Kardel et al reported a training duration of 8.4 hours/week in the high-exercise group (HEG). This is the only study to date that has investigated higher intensity training (heart rate between 170 and 180 beats/min during interval training) during pregnancy, so it was decided to include this study as well.47
Fertility
The average duration between becoming pregnant and ending contraception was 1.7 months (from 0 to 7 months) in the study of Penttinen et al.49 Contraceptive pills were used by 13 athletes for a mean of 4.5 years before their first pregnancy, and 1 athlete used an intrauterine device for 2 years.49 Sundgot-Borgen et al also observed no differences in fertility problems between 34 elite athletes and 34 active controls.51
Pregnancy disorders, complications and injuries
In three studies, pregnancy was described as uncomplicated.43 51 53 Beilock et al stated the following maternal complications, once each: sinus problems, heavy bleeding, hypertonia and kidney stones.54 Further physical complaints, such as upper respiratory tract infections, pelvic and back pain, nausea and fatigue, were reported, and one athlete presented with pneumonia, laryngitis and cholestasis.44 47 48 In the case–control study of Bo et al, low back pain, pelvic girdle pain and urinary or faecal incontinence during pregnancy and after childbirth were noticed, but no significant differences in the prevalence between the elite athletes and the control group could be observed. The rate of urinary stress incontinence symptoms at 6 weeks postpartum was 29% (n=9) in elite athletes and 30.4% (n=14) in the control group, and at the completion of the questionnaire, it was 35.5% (n=11) in elite athletes and 26.1% (n=26.1%) in the control group.48 It was stated that four athletes experienced training-induced injuries in the third trimester, and four athletes (two endurance-type sports; two team ball sports) suffered from a stress fracture postpartum.51 Two sacral fractures (13–18 weeks and 19–24 weeks postpartum) after rapid resumption of postpartum sports activities were described.25 Fifty per cent (n=21) of the athletes experienced an injury postpartum, of which 6 were bone stress injuries, 11 were musculoskeletal injuries (muscle, tendon and/or ligament strain/sprain or rupture), 2 were sciatic problems and 2 were not further specified.56
Miscarriage
Seven athletes (23%) experienced an abortion during their first trimester in a previous pregnancy.49 Sundgot-Borgen et al documented no differences in miscarriage between 34 elite athletes and 34 active controls (three miscarriages in 28 elite athletes (11%) and eight miscarriages in 29 inactive controls (28%), where three controls had two miscarriages).51
Preterm/post-term birth
No differences regarding preterm birth could be found in the case–control studies from Bo et al, Penttinen et al and Sundgot-Borgen et al.48 49 51 Based on the information about training provided, a birth at term can be assumed for another study.25 The athlete monitored in the case report of Bubnjević et al gave birth at 39 weeks+4 days.53 The twins were born after 36 weeks.44 Only one post-term birth (11 days) was reported.43 The average gestational age at delivery was 39.0±1.6 weeks in the study of Darroch et al, and no detailed information on preterm or post-term births was provided.56
Labour
Three preterm labours in the study of Beilock et al were observed.54 Kardel et al detected a significantly later onset of labour (mean difference of 1.2 weeks) in the medium-exercise group (MEG) than in the HEG, but only for mothers giving birth to girls.47
No significant differences could be found in the length of labour between the groups (6.7±5.5 hours in the MEG vs 9.4±5.9 hours in the HEG)47; first stage of labour in actives 626±332 min versus 576±322 min in controls and second stage of labour in actives 27.9±17.8 min versus 27.8±27.2 min versus in controls49; first stage of labour non-athletic 603 min (range 231–1069), high-impact 600 min (range 296–1386), low-impact group 613 min (range 331–1017), second stage of labour non-athletic 57 min (range 17–116), high-impact 56 min (range 32–106), low-impact group 65 min (range 23–153).50 Beilock et al confirmed one extended labour, providing no further details.54 Kardel et al reported that the duration of labour tended to be shorter in the MEG than in the HEG (normal delivery 6.7±5.5 vs 9.4±5.9 hours; instrumental delivery 8.0±2.6 vs 11.7±3.4 hours), although this was not statistically significant.47
No significant distinctions in the birth mode between the groups (vaginal, vacuum extraction, forceps or caesarean section) were stated.48 49 Other authors observed no significant differences with regard to the incidence of emergency caesarean section.50 The twins were delivered by elective caesarean section.44 Darroch et al reported 30 vaginal births and 11 caesarean sections.56 In the case report of Almquist et al, a natural birth without complications was stated.52
The rate of vaginal or perineal tears did not differ significantly (athletes 0 vs controls 0) in the study of Penttinen et al.49 Compared with the high-impact athletes, among the low-impact athletes, a significantly higher incidence of third-degree and fourth-degree perineal tears was detected, whereas no significant differences were seen in the comparison between the control group and the athletes (both low and high impact).50 One labour was induced due to a reduced amount of amniotic fluid, which was observed in the last sonography in the 39th week.53
Birth weight
Several authors revealed no significant differences in birth weight between the athletes and controls (elite athletes 3290.6±751 g vs controls 3466.4±529 g,48 MEG 3590.5±532 g vs HEG 3650.7±515.8 g,47 athletes 3460±484 g vs controls 3335±503 g,49 athletes 3607.1±544.7 g vs controls 3587.3±610.1 g).51 In one case report, the birth weight of twins was 2200 g and 2300 g (after a 36-week gestation period)44; in another, it was 3060 g (39 4/7 weeks).53 In the case report of Almquist et al, birth weight was 3238 g at 40 weeks of pregnancy.52 Beilock et al reported two children with low birth weight.54
APGAR score/infant development
The following information was provided on the health status of the children: during the twin pregnancy, fetal development was stated as normal, and after birth, the twins were in good health and showed normal development 12 months after birth.44 Likewise, Bung et al and Solli et al described athletes who gave birth to healthy infants.25 43 One study stated ‘unhealthy’ as a fetal complication during pregnancy, and further information was not provided.54 There were no reports of abnormal APGAR scores.43 47 51 53 57
Exercise behaviour during pregnancy and postpartum
The majority of the studies in this group confirmed a reduction in training volume and intensity during pregnancy.25 44 50 51 53 54 56 In the study of Beilock et al, women decreased cardiovascular and resistance exercise by 49% in the first trimester, by 72% in the second trimester and by 80% in the third trimester.54 Two case reports described a reduction in running volume from prior to pregnancy to the ninth month of pregnancy from 100 to 20 km/week,53 and from 155 to 72 km/week.44 Solli et al detected in a case report an increased training volume from a median of 10 hours/week in gestational weeks 1–12 to 18 hours/week in gestational weeks 13–28 but a decline from weeks 13–28 to weeks 29–40 (median 18 to 9.6 hours/week).25 Sundgot-Borgen et al revealed a decent strength and endurance training volume from prepregnancy to the first and third trimesters (p<0.001) and 0–3 months postpartum (p<0.001), but the exact values were not provided.51 Sigurdardottir et al differentiated only between before and during pregnancy and presented the following values: in the low-impact group 20.3±10.7 hours/week vs 10.2±12.7 hours/week and in the high-impact group 14.3±4.3 hours/week vs 8.5±7.4 hours/week.50 Darroch et al reported a significant reduction in running volume from the first (63±34 km/week) to the third trimester (30±30 km/week).56 Penttinen et al reported that 23 athletes (77%) continued their training until week 23 of gestation, and 18 (60%) still competed to week 13 of gestation.49 Postpartum, 18 athletes continued to compete at a median interval of 8.2 months (range 2–24 months).49 While 2 athletes achieved better condition than before pregnancy, 11 reached the same level of performance and 5 did not.49 Bo et al stated that 38% of athletes started running within 6 weeks postpartum. Sundgot-Borgen et al reported that most athletes (71%, n=24) returned to their training within 0–6 weeks, 24% (n=8) within 7–12 weeks and 6% (n=2) within 13–18 weeks postpartum.51 After delivery, in the case report of Bung et al, the athlete was able to resume her normal training within a few weeks, and after less than 6 months, she was able to beat her personal best time in various short distances.43 Another paper revealed that the mother returned to competitive racing 8.5 weeks after her delivery.44 In the study of Darroch et al, athletes returned to activity/exercise after approximately 6 weeks, and after 3 months, they reached 80% of their prepregnancy trainings volume.56
Acute exposure to intensive activity during performance testing
Three papers evaluated performance testing during pregnancy in 21 highly active women and elite athletes.57–59 Another two case reports of ‘continuous exercise exposure’ also mentioned performance testing during pregnancy.43 44
Salvesen et al evaluated performance testing at 23–29 weeks of pregnancy,58 and Szymanski et al evaluated performance testing at the 29th and 33rd weeks of gestation.59 In another paper, Szymanski et al described two treadmill sessions (moderate and vigorous intensity) in the same population, where women from the exercise group (regular and highly active) underwent an additional vigorous-intensity session (30-min treadmill) with a target heart rate of 60%–84% heart rate reserve.57 A case report described bicycle ergometer testing and track running from the 28th week of pregnancy every 2 weeks, during puerperium, 6 weeks and 6 months postpartum.43 Davies et al reported weekly standardised submaximal field running tests during the first 32 weeks of pregnancy as well as standardised submaximal treadmill tests 29 weeks antepartum and 10 weeks postpartum.44 The effect of the performance testing on different outcome parameters will be described in the following.
Umbilical and uterine blood flow/fetal heart rate
Uterine and umbilical arteries monitored by Doppler ultrasound showed a reduction in the mean uterine artery volume blood flow to 40%–75% of the initial value during testing.58 Fetal bradycardia (an unfavourable outcome indicator) and a high umbilical artery pulsatility index (PI) were detected, and the mean uterine artery volume blood flow was less than 50% of the initial value.58 In a highly active exercise group (n=15), five women (33.3%) experienced a deceleration of fetal heart rate (fHR) postexercise (lasting between minutes 2:08 and 3:12, pre-exercise 142.8±10.4 vs postexercise 81.8±10.4) and alterations in umbilical and uterine artery Doppler indices.59 In the same population, but after an additional vigorous-exercise-intensity session, Doppler indices (umbilical artery Doppler S/D ratio, resistance index, pulsatile index) decreased significantly (umbilical PI pre-exercise 0.85±0.07 vs postexercise 0.82±0.09).57 However, no significant differences in any umbilical artery Doppler indices were observed pre-exercise and postexercise with moderate exercise.57 Salvesen reported that both fHR and umbilical artery PI normalised quickly after exercise termination (figure 4).58 In the case report of Bung et al, the maternal heart rate reached values of over 170 beats per minute (bpm) during sprints.43 The fHR measured immediately after exercise indicated bradycardia (70 bpm), which recovered to 120 bpm within 3 min, and the athlete had dizziness and precollapse-like symptoms.43
Figure 4.
These data were extracted from a prior original study58 and are from six pregnant women before, during and after exercise. After a warm-up for 10 min (maternal heart rate (mHR) of ~135/min), the women ran three to five submaximal workloads (test 1 to test 5; 5 min each) on a treadmill with a VO2max (maximal oxygen consumption) of 60%–90%. Fetal heart rate (fHR) in beats per minute (data points in blue) and umbilical artery pulsatility index (PI) (data points in green) were measured before exercise, after warm-up, during (test 1 to test 5) and after exercise. Three women completed only three workloads, one woman completed four workloads and one woman completed even five workloads. An increase in PI and a drop in fHR could be observed in the two women (woman 5 and woman 6) who completed four or five workloads each. Woman 5 exercised at 95% of maximal mHR and woman 6 exercised at 92% maximal mHR. These results imply that exercise intensity over 90% of maximal mHR may have a negative impact on fetal well-being.
Pregnancy disorders, complications and injuries
One woman developed HELLP syndrome at 35 weeks.58 No other complications or injuries were reported.43 57–59
Preterm/post-term birth
The following durations of pregnancy were reported for six pregnant women: 35, 36, 39, 40, 41, 42.58 Szymanski et al recorded two preterm births (one in the highly active group and one in the non-exerciser group at 36 1/7 weeks each).57
Labour
Four normal deliveries (66.7%), one caesarean section (16.7%) and one vacuum delivery (16.7%) were reported,58 the last two due to obstructed labour.58 In the woman with HELLP syndrome, labour was induced, and the woman delivered vaginally.58
Birth weight
The baby of the woman with HELLP syndrome had a birth weight of 2285 g (35 weeks of pregnancy).58 The other babies weighed a mean of 3252±177.8 g.58 The birth weight relative to gestational age was in the lower normal range (86%–103%) for Norwegian children. Szymanski et al detected no significant differences in fetal birth weight between the groups (non-exercisers 3460±427 g, regularly active 3.408±426 g, highly active 3.167±299 g).57 One woman in the highly active group delivered a small-for-gestational age baby (2690 g at 39 3/7 weeks), whereas two women delivered large-for-gestational age babies (one woman in the regular active group (4700 g at 39 5/7 weeks) and one in the non-exerciser group (4451 g at 41 0/7 weeks)).57
AGPAR score/infant development
No significant differences in APGAR scores were reported.57 A healthy girl was born to a woman with HELLP syndrome.58
High-risk sports
Only one study with female sport climbers met the inclusion criteria for this category.60
Preterm birth
Preterm birth within the 36th week was reported in 2 out of 15 women (13.3%).60 The risk of preterm birth was stated not to be higher than in the general population in Germany.60
Pregnancy disorders, complications and injuries
Two women experienced preterm births (36th week of gestation) but reported no complications and suffered no injuries or accidents while climbing.60
APGAR score/infant development
Fifteen women provided information about the delivery and the newborn; they reported that the children were healthy at birth and throughout the first 12 months postpartum.60
Exercise behaviour during pregnancy
Fifty per cent of the women in this study climbed until the 36th week of gestation, and all of them reduced their level of difficulty in training voluntarily by shifting to safer training options (more top rope).60
Rectus diastasis
None of the included studies in all three categories stated any effects on maternal diastasis of the rectus abdominus muscle.
Discussion
Current recommendations for pregnant elite and competitive amateur female athletes regarding sports and exercise
Most of the included studies described a voluntary reduction in training volume and/or intensity during pregnancy,25 50 51 53 54 and only one case report stated that there was a reduction in training during pregnancy for medical reasons.44 This reduction in training may be caused by the lack of evidence-based recommendations regarding sports activity (frequency, intensity, type and duration) many pregnant competitive athletes receive.36 51 61 62 In general, the current literature recommends reducing training volume and/or intensity as a precautionary measure,63 64 which may also be due to medicolegal aspects, although in most cases, no significant differences were found between the groups.48–51 57 59 Athletic women are generally interested in being informed about evidence-based physical activity recommendations and sport-specific safety concerns during pregnancy and the postpartum period.36 62 65 66 However, in the study of Sundgot-Borgen et al, athletes reported receiving dissatisfying training recommendations, especially in the area of strength training,51 which could also be displayed in other studies.21 36 61 66 67 This might be amplified when confidence in medical staff is diminished following the provision of dissenting or contradictory recommendations. This can lead women to seek information on other platforms and to lose contact with healthcare providers, not noticing when there is a real risk.65 It was also shown that appropriate support is needed for female athletes to return to competition postpartum, and strategies for motivation and time management should be integrated into the training programmes as well as social support regarding childcare.54 66
The authors could not identify any significant disadvantages with respect to fetal and/or maternal conditions, supporting the possibility of training at higher intensities if it is subjectively tolerated and carefully monitored. The adverse effects of continuing to exercise during pregnancy compromised mild symptoms, such as urinary incontinence or back pain.48 A few cases indicated injuries, but no further information about cause and management was provided.25 51 60 Overall, there was a high rate of urinary stress incontinence symptoms (26.1%–35.5%) 6 weeks postpartum but also up to 17 years after giving birth, that rate did not differ significantly between the athletes and control groups.48 However, it is worth mentioning that almost one-third of the participants in the control group compared with only one-sixth of the female athletes performed regular pelvic floor exercises during pregnancy.48
The only exception may be performance tests during the third trimester, for which undesirable short-term risks at higher intensities, but no additional consequences, have been reported.57–59 Data regarding the long-term neurological consequences of children exposed to repeated high-intensity training are lacking. In addition, the data from a single study of high-risk sports during pregnancy (ie, climbing) did not detect any adverse events, and all children were healthy after birth and 12 months later.43 57–60
Where should we go from here?
As shown in this scoping review, there are a relatively large number of review articles and few guidelines or consensus statements regarding recreational sports and low to moderate exercise during pregnancy, which both result in a low level of evidence compared with a small number of publications dealing with original data (125 vs 16).68 Unfortunately, elite female athletes are often just mentioned in a side statement, and the lack of studies is briefly discussed, as is the need for more prospective studies.37 The gender data gap is amplified in the case of female competitive athletes, as women are under-represented in the scientific literature anyway, and there are only studies with small case numbers on the ‘fringe group’ of elite female athletes.69 With our review, we confirmed the lack of fundamental knowledge on this topic in elite and competitive amateur female athletes, and we support the idea of further prospective observations, particularly for higher intensity activities. Further high-quality studies with elite athletes are needed (using newly available technologies such as wearables) to provide evidence-based recommendations (table 3).70–73 Continuous monitoring of the fetal heart rate is conceivable here, for example, to anticipate problems under continuously high training loads as well as the pelvic floor stress of the mother. Observational studies should be carried out with large study populations to gain a broader understanding of the stress tolerance of pregnant competitive athletes. This could help to draw conclusions about the optimal volume and content of training and to define stress limits.
Table 3.
Future directions in research
| Study type | Variables to be captured |
| Observational cohort study (pregnant elite and competitive amateur athletes) | Exercise activity in daily live (wearables) |
| Training activities (wearables) | |
| Fetal heart rate (non-invasive, wireless monitoring systems, wearables) | |
| Interventional study | Fetal heart rate monitoring (non-invasive, wireless monitoring systems, wearables) with live feedback function (in case of fetal heart rate deceleration) |
| Maternal heart rate in daily live and during activity (wearables) | |
| Uterine contractions (non-invasive, wireless monitoring systems, wearables) |
Methodological considerations
This study has some limitations that should be considered when interpreting its findings. The data from this review were obtained from controlled interventions or case–control, case and observational studies. These study designs carry a high risk of bias, especially because questionnaires and self-reported data were used. Due to the retrospective study designs, recall bias must also be assumed. No studies of higher quality, such as randomised controlled trials, could be found, which lowers the validity of the current evidence base but at the same time emphasises the need for further, high-quality studies on this topic. The heterogeneity of the included papers impeded a direct comparison and the development of specific recommendations but allowed for various statements on several exercise interventions and gestational weeks. Due to ethical reasons, there is a lack of studies that evaluate maximal intensity exercise exposure during pregnancy, and the availability of such studies remains questionable for the future.
Conclusion
Although much is known about low to moderate physical activity during pregnancy, the study situation regarding elite and competitive amateur female athletes has enormous gaps. Randomised controlled trials and sufficiently powered observational studies with a large number of participants are missing. The effects of (high)-intensive strength and endurance training during pregnancy and the puerperium have only been examined to a limited extent thus far, so no scientifically reliable statement can be made.
Nonetheless, as in principle, there are no known significant negative consequences of physical activity for mothers or children; both individuals who adhere to exercise recommendations or engage in higher impact activities during pregnancy and pregnant elite and competitive athletes are encouraged to approach sporting activity with more confidence.
Acknowledgments
A special thanks goes to Sabine Klein and Jaqueline Huber from the University of Zurich Careum library, who helped define the search strategy and conducted the database searches. The authors also wish to convey that the information is presented clearly and truthfully.
Footnotes
Contributors: Conceptualisation: NW, AK, JSc and JSp; methodology: JSp; formal analysis: NW, AK and NK; investigation: NW, AK and NK; resources: JSp; data curation: NW and AK; writing—original draft preparation: NW and AK; writing—review and editing: NK, AK, JSc and JSp; visualisation: NW; supervision: JSc and JSp; project administration: NW. All authors revised the manuscript critically, approved the final version and agreed to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Blaize AN, Pearson KJ, Newcomer SC. Impact of maternal exercise during pregnancy on offspring chronic disease susceptibility. Exerc Sport Sci Rev 2015;43:198–203. 10.1249/JES.0000000000000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rich-Edwards JW, Fraser A, Lawlor DA, et al. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev 2014;36:57–70. 10.1093/epirev/mxt006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva SG, Ricardo LI, Evenson KR, et al. Leisure-time physical activity in pregnancy and maternal-child health: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Sports Med 2017;47:295–317. 10.1007/s40279-016-0565-2 [DOI] [PubMed] [Google Scholar]
- 4.Physical activity and exercise during pregnancy and the postpartum period: ACOG Committee opinion, number 804. Obstet Gynecol 2020;135:e178–88. 10.1097/AOG.0000000000003772 [DOI] [PubMed] [Google Scholar]
- 5.Bo K, Artal R, Barakat R, et al. Exercise and pregnancy in recreational and elite athletes: 2016 evidence summary from the IOC expert group meeting, Lausanne. Part 1-exercise in women planning pregnancy and those who are pregnant. Br J Sports Med 2016;50:571–89. 10.1136/bjsports-2016-096218 [DOI] [PubMed] [Google Scholar]
- 6.Bø K, Artal R, Barakat R, et al. Exercise and pregnancy in recreational and elite athletes: 2016 evidence summary from the IOC expert group meeting, Lausanne. Part 2-the effect of exercise on the fetus, labour and birth. Br J Sports Med 2016;50:1297–305. 10.1136/bjsports-2016-096810 [DOI] [PubMed] [Google Scholar]
- 7.Davies GAL, Wolfe LA, Mottola MF, et al. No. 129-exercise in pregnancy and the postpartum period. J Obstet Gynaecol Can 2018;40:e58–65. 10.1016/j.jogc.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Branco M, Santos-Rocha R, Aguiar L, et al. Kinematic analysis of gait in the second and third trimesters of pregnancy. J Pregnancy 2013;2013:718095 10.1155/2013/718095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilleard WL. Trunk motion and gait characteristics of pregnant women when walking: report of a longitudinal study with a control group. BMC Pregnancy Childbirth 2013;13:71. 10.1186/1471-2393-13-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cakmak B, Ribeiro AP, Inanir A. Postural balance and the risk of falling during pregnancy. J Matern Fetal Neonatal Med 2016;29:1623–5. 10.3109/14767058.2015.1057490 [DOI] [PubMed] [Google Scholar]
- 11.Duvekot JJ, Cheriex EC, Pieters FA, et al. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol 1993;169:1382–92. 10.1016/0002-9378(93)90405-8 [DOI] [PubMed] [Google Scholar]
- 12.Morris EA, Hale SA, Badger GJ, et al. Pregnancy induces persistent changes in vascular compliance in primiparous women. Am J Obstet Gynecol 2015;212:633.e1–633.e6. 10.1016/j.ajog.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cong J, Fan T, Yang X, et al. Structural and functional changes in maternal left ventricle during pregnancy: a three-dimensional speckle-tracking echocardiography study. Cardiovasc Ultrasound 2015;13:6. 10.1186/1476-7120-13-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe LA, Preston RJ, Burggraf GW, et al. Effects of pregnancy and chronic exercise on maternal cardiac structure and function. Can J Physiol Pharmacol 1999;77:909–17. 10.1139/y99-093 [DOI] [PubMed] [Google Scholar]
- 15.Weissgerber TL, Wolfe LA, Hopkins WG, et al. Serial respiratory adaptations and an alternate hypothesis of respiratory control in human pregnancy. Respir Physiol Neurobiol 2006;153:39–53. 10.1016/j.resp.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Heenan AP, Wolfe LA. Plasma acid-base regulation above and below ventilatory threshold in late gestation. J Appl Physiol 2000;88:149–57. 10.1152/jappl.2000.88.1.149 [DOI] [PubMed] [Google Scholar]
- 17.Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. Br Heart J 1992;68:540–3. 10.1136/hrt.68.12.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clapp JF, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol 1997;80:1469–73. 10.1016/S0002-9149(97)00738-8 [DOI] [PubMed] [Google Scholar]
- 19.Kolarzyk E, Szot WM, Lyszczarz J. Lung function and breathing regulation parameters during pregnancy. Arch Gynecol Obstet 2005;272:53–8. 10.1007/s00404-004-0691-1 [DOI] [PubMed] [Google Scholar]
- 20.Jensen D, Webb KA, Davies GAL, et al. Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: implications for respiratory sensation. J Physiol 2008;586:4735–50. 10.1113/jphysiol.2008.158154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melzer K, Schutz Y, Boulvain M, et al. Physical activity and pregnancy: cardiovascular adaptations, recommendations and pregnancy outcomes. Sports Med 2010;40:493–507. 10.2165/11532290-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 22.Kardel KR. Effects of intense training during and after pregnancy in top-level athletes. Scand J Med Sci Sports 2005;15:79–86. 10.1111/j.1600-0838.2004.00426.x [DOI] [PubMed] [Google Scholar]
- 23.Sorensen EA. Debunking the myth of pregnancy doping. J Intercolleg Sport 2009;2:269–85. 10.1123/jis.2.2.269 [DOI] [Google Scholar]
- 24.Aydin SB. A review on abortion doping discussion in athletes: is it a truth or a myth. Crimson Publishers 2021;4. 10.31031/PRM.2021.04.000599 [DOI] [Google Scholar]
- 25.Solli GS, Sandbakk Øyvind. Training characteristics during pregnancy and postpartum in the world's most successful cross country skier. Front Physiol 2018;9:595. 10.3389/fphys.2018.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson T, Bostock EL, Hassan A, et al. The legacy of pregnancy: elite athletes and women in arduous occupations. Exerc Sport Sci Rev 2022;50:14–24. 10.1249/JES.0000000000000274 [DOI] [PubMed] [Google Scholar]
- 27.Artal R. Exercise in pregnancy: guidelines. Clin Obstet Gynecol 2016;59:639–44. 10.1097/GRF.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 28.Mottola MF, Davenport MH, Ruchat S-M, et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br J Sports Med 2018;52:1339–46. 10.1136/bjsports-2018-100056 [DOI] [PubMed] [Google Scholar]
- 29.Meah VL, Davies GA, Davenport MH. Why can't I exercise during pregnancy? Time to revisit medical 'absolute' and 'relative' contraindications: systematic review of evidence of harm and a call to action. Br J Sports Med 2020;54:1395–404. 10.1136/bjsports-2020-102042 [DOI] [PubMed] [Google Scholar]
- 30.Wowdzia JB, McHugh T-L, Thornton J, et al. Elite athletes and pregnancy outcomes: a systematic review and meta-analysis. Med Sci Sports Exerc 2021;53:534–42. 10.1249/MSS.0000000000002510 [DOI] [PubMed] [Google Scholar]
- 31.Kimber ML, Meyer S, McHugh TL, et al. Health outcomes after pregnancy in elite athletes: a systematic review and meta-analysis. Med Sci Sports Exerc 2021;53:1739–47. 10.1249/MSS.0000000000002617 [DOI] [PubMed] [Google Scholar]
- 32.Wowdzia JB, Davenport MH. Cardiopulmonary exercise testing during pregnancy. Birth Defects Res 2021;113:248–64. 10.1002/bdr2.1796 [DOI] [PubMed] [Google Scholar]
- 33.Wowdzia JB, McHugh TL, Thornton J, et al. Elite athletes and pregnancy outcomes: a systematic review and meta-analysis. Med Sci Sports Exerc 2021;53:534–42. 10.1249/MSS.0000000000002510 [DOI] [PubMed] [Google Scholar]
- 34.Kimber ML, Meyer S, McHugh T-L, et al. Health outcomes after pregnancy in elite athletes: a systematic review and meta-analysis. Med Sci Sports Exerc 2021;53:1739–47. 10.1249/MSS.0000000000002617 [DOI] [PubMed] [Google Scholar]
- 35.Allen SV, Hopkins WG. Age of peak competitive performance of elite athletes: a systematic review. Sports Med 2015;45:1431–41. 10.1007/s40279-015-0354-3 [DOI] [PubMed] [Google Scholar]
- 36.Davenport MH, Nesdoly A, Ray L, et al. Pushing for change: a qualitative study of the experiences of elite athletes during pregnancy. Br J Sports Med 2022;56:452–7. 10.1136/bjsports-2021-104755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bo K, Artal R, Barakat R, et al. Exercise and pregnancy in recreational and elite athletes: 2016/2017 evidence summary from the IOC expert group meeting, Lausanne. Part 5. recommendations for health professionals and active women. Br J Sports Med 2018;52:1080–5. 10.1136/bjsports-2018-099351 [DOI] [PubMed] [Google Scholar]
- 38.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 39.Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev 2021;10:39. 10.1186/s13643-020-01542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NIHR . Available: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=309541
- 41.Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:16. 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelliccia A, Sharma S, Gati S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Rev Esp Cardiol 2021;74:545. 10.1016/j.rec.2021.05.003 [DOI] [PubMed] [Google Scholar]
- 43.Bung P, Huch R, Huch A. Maternal and fetal heart rate patterns: a pregnant athlete during training and laboratory exercise tests; a case report. Eur J Obstet Gynecol Reprod Biol 1991;39:59–62. 10.1016/0028-2243(91)90143-9 [DOI] [PubMed] [Google Scholar]
- 44.Davies B, Bailey DM, Budgett R, et al. Intensive training during a twin pregnancy. A case report. Int J Sports Med 1999;20:415–8. 10.1055/s-2007-971155 [DOI] [PubMed] [Google Scholar]
- 45.NHLBI . Study quality assessment tools. NIH. Available: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 46.Nose-Ogura S. Advancement in female sports medicine and preventive medicine. J Obstet Gynaecol Res 2021;47:476–85. 10.1111/jog.14523 [DOI] [PubMed] [Google Scholar]
- 47.Kardel KR, Kase T. Training in pregnant women: effects on fetal development and birth. Am J Obstet Gynecol 1998;178:280–6. 10.1016/S0002-9378(98)80013-6 [DOI] [PubMed] [Google Scholar]
- 48.Bø K, Backe-Hansen KL. Do elite athletes experience low back, pelvic girdle and pelvic floor complaints during and after pregnancy? Scand J Med Sci Sports 2007;17:480–7. 10.1111/j.1600-0838.2006.00599.x [DOI] [PubMed] [Google Scholar]
- 49.Penttinen J, Erkkola R. Pregnancy in endurance athletes. Scand J Med Sci Sports 1997;7:226–8. 10.1111/j.1600-0838.1997.tb00144.x [DOI] [PubMed] [Google Scholar]
- 50.Sigurdardottir T, Steingrimsdottir T, Geirsson RT, et al. Do female elite athletes experience more complicated childbirth than non-athletes? A case-control study. Br J Sports Med 2019;53:354–8. 10.1136/bjsports-2018-099447 [DOI] [PubMed] [Google Scholar]
- 51.Sundgot-Borgen J, Sundgot-Borgen C, Myklebust G, et al. Elite athletes get pregnant, have healthy babies and return to sport early postpartum. BMJ Open Sport Exerc Med 2019;5:e000652. 10.1136/bmjsem-2019-000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almquist NW, Sandbakk Øyvind, Solli GS. Performance-related physiological and haematological changes during pregnancy and postpartum in a well-trained cyclist performing endurance training. Front Physiol 2022;13:762950. 10.3389/fphys.2022.762950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bubnjević K, Ugarković D. Aerobic physical exercise in the third trimester in pregnant woman with Hashimoto’s thyroiditis: A case report. Vojnosanit Pregl 2017;74:687–92. 10.2298/VSP151013253B [DOI] [Google Scholar]
- 54.Beilock SL, Feltz DL, Pivarnik JM. Training patterns of athletes during pregnancy and postpartum. Res Q Exerc Sport 2001;72:39–46. 10.1080/02701367.2001.10608930 [DOI] [PubMed] [Google Scholar]
- 55.Darroch FE, Giles AR, Hillsburg H, et al. Running from responsibility: athletic governing bodies, corporate sponsors, and the failure to support pregnant and postpartum elite female distance runners. Sport in Society 2019;22:2141–60. 10.1080/17430437.2019.1567495 [DOI] [Google Scholar]
- 56.Darroch F, Schneeberg A, Brodie R, et al. Impact of pregnancy in 42 elite to World-class runners on training and performance outcomes. Med Sci Sports Exerc 2022. 10.1249/MSS.0000000000003025. [Epub ahead of print: 16 Aug 2022]. [DOI] [PubMed] [Google Scholar]
- 57.Szymanski LM, Satin AJ. Exercise during pregnancy: fetal responses to current public health guidelines. Obstet Gynecol 2012;119:603–10. 10.1097/AOG.0b013e31824760b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvesen Kjell Å, Hem E, Sundgot-Borgen J. Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. Br J Sports Med 2012;46:279–83. 10.1136/bjsm.2010.080259 [DOI] [PubMed] [Google Scholar]
- 59.Szymanski LM, Satin AJ. Strenuous exercise during pregnancy: is there a limit? Am J Obstet Gynecol 2012;207:179.e1–179.e6. 10.1016/j.ajog.2012.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drastig J, Hillebrandt D, Rath W, et al. Pregnant women in sport climbing - Is there a higher risk for preterm birth? Z Geburtshilfe Neonatol 2017;221:25–9. 10.1055/s-0042-119654 [DOI] [PubMed] [Google Scholar]
- 61.Martínez-Pascual B, Alvarez-Harris S, Fernández-de-Las-Peñas C, et al. Pregnancy in Spanish elite sportswomen: a qualitative study. Women Health 2017;57:741–55. 10.1080/03630242.2016.1202883 [DOI] [PubMed] [Google Scholar]
- 62.Franklin A, Mishtal J, Johnson T, et al. Rowers' self-reported behaviors, attitudes, and safety concerns related to exercise, training, and competition during pregnancy. Cureus 2017;9:e1534. 10.7759/cureus.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Downs DS, Chasan-Taber L, Evenson KR, et al. Physical activity and pregnancy: past and present evidence and future recommendations. Res Q Exerc Sport 2012;83:485–502. 10.1080/02701367.2012.10599138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leiferman J, Gutilla M, Paulson J, et al. Antenatal physical activity counseling among healthcare providers. OJOG 2012;02:346–55. 10.4236/ojog.2012.24073 [DOI] [Google Scholar]
- 65.Ohlendorf JM, Anklam AL, Gardner L. "I am a Runner": a qualitative analysis of women-runners' pregnancy experiences. Women Birth 2019;32:e307–14. 10.1016/j.wombi.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 66.Davenport MH, Ray L, Nesdoly A. We're not superhuman, we're human: a qualitative description of elite athletes' experiences of return to sport after childbirth. Sports Med 2022:1–11. 10.1007/s40279-022-01730-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connelly M, Brown H, van der Pligt P, et al. Modifiable barriers to leisure-time physical activity during pregnancy: a qualitative study investigating first time mother's views and experiences. BMC Pregnancy Childbirth 2015;15:100. 10.1186/s12884-015-0529-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305–10. 10.1097/PRS.0b013e318219c171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merone L, Tsey K, Russell D, et al. Sex inequalities in medical research: a systematic scoping review of the literature. Womens Health Rep 2022;3:49–59. 10.1089/whr.2021.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford A, Hayes D, Johnstone ED, et al. Women's experiences of continuous fetal monitoring - a mixed-methods systematic review. Acta Obstet Gynecol Scand 2017;96:1404–13. 10.1111/aogs.13231 [DOI] [PubMed] [Google Scholar]
- 71.Tamber KK, Hayes DJL, Carey SJ, et al. A systematic scoping review to identify the design and assess the performance of devices for antenatal continuous fetal monitoring. PLoS One 2020;15:e0242983. 10.1371/journal.pone.0242983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mhajna M, Sadeh B, Yagel S, et al. A novel, cardiac-derived algorithm for uterine activity monitoring in a wearable remote device. Front Bioeng Biotechnol 2022;10:933612. 10.3389/fbioe.2022.933612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarafan S, Le T, Ellington F, et al. Development of a home-based fetal electrocardiogram (ECG) monitoring system. Annu Int Conf IEEE Eng Med Biol Soc 2021;2021:7116–9. 10.1109/EMBC46164.2021.9630827 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2022-001395supp001.pdf (32.9KB, pdf)
bmjsem-2022-001395supp002.pdf (98.1KB, pdf)
bmjsem-2022-001395supp003.pdf (63.3KB, pdf)
bmjsem-2022-001395supp004.pdf (290.9KB, pdf)



