Abstract
Security and forensic applications employ test and reference materials to develop, calibrate, and validate analytical instrumentation such as mass spectrometry for the trace detection and chemical analysis of target analytes. An emerging class of target analytes includes homemade fuel oxidizer explosives based on pyrotechnics, propellants, and powder mixtures. Test materials for these compounds must appropriately and accurately embody the physical and chemical nature of the threat. Precision liquid deposition methods have long been employed for creation of trace level test materials. Mass spectral similarity and chemical signature differences between solid particulate and solution cast (i.e., liquid deposited) propellant samples were investigated by infrared thermal desorption direct analysis in real time mass spectrometry (IRTD-DART-MS). Differences in the mass spectra and ion distributions of solid and liquid deposited black powders and black powder substitutes were observed. These differences were attributed to chemical processes (e.g., degradation) and physical differences in the crystal formation, spatial distribution, morphology, and size. The production and deposition of test and reference materials remain critical to developing new technologies and detecting evolving threats.
Keywords: Propellants, Black powders, Black powder substitutes, Mass spectral similarity, Test materials, Homemade explosives, Security, Forensic science
Graphical Abstract
Mass spectral similarity and ion distribution differences of solid particulate versus solution cast propellant samples analyzed by IRTD-DART-MS.

Introduction
Fields such as checkpoint screening and forensic analysis commonly employ technologies for trace chemical detection and identification. These applications target small amounts (i.e., generally micrograms and below) of materials for presumptive identification purposes. Energetic materials encompass a major class of contraband compounds targeted, though many others exist such as illicit narcotics and toxic industrial chemicals.1, 2 Homemade fuel-oxidizer explosives based on readily available inorganic oxidizers, propellants, and pyrotechnics remain among the most common charges for improvised explosive devices (IEDs) in the United States.3 As the global threat from homemade explosives continues,4 research has focused on characterizing established instrumentation as well as developing novel technologies to detect these low explosives.5-14 A recent review provides details on the unique challenges presented by inorganic-based fuel-oxidizer mixtures and strategies for improved detection.15
Test and reference materials are critical for instrument development as well as characterizing system performance, verification, and calibration (research grade test materials are not as highly traceable to the International System of Units (SI) as standard reference materials). The measurement of trace amounts of explosive materials is often necessary for system calibration, library definitions, and alarm algorithms to achieve accurate identification. Traditionally, solution casting and drop-on-demand (i.e., inkjet printing) have been used for controlled, uniform, and precise test material production.16-22 In order to appropriately investigate the system response for a specific application, test materials must represent the actual threats to the maximum extent possible. However, potential differences in the chemical signatures of these samples presents an issue for trace detection technologies. The importance and impact of any such differences will depend on various aspects of the technology in question. For example, differences in polymorphic state of RDX (1,3,5-Trinitro-1,3,5-Triazine)23 or crystal structure of TNT (trinitrotoluene)24 can yield variability in Raman spectroscopy signatures. The need for dissolved explosives (commonly organic) to generate representative crystalline particles upon drying without degradation or decomposition is critical. The development of selective alarm algorithms also requires suitable test materials, representative of the nature of the actual threat. Though test materials for organic high explosives (e.g., RDX, TNT, PETN [pentaerythritol tetranitrate]) have long been studied, relatively little work has considered homemade fuel-oxidizer explosives based on inorganic oxidizers, propellants, and pyrotechnics.
We focus this work on the mass spectral similarity and chemical signature differences of solid particulate and solution cast (i.e., initially dissolved in liquid, deposited, and dried) propellant samples. We investigate these differences specifically using a recent technology that couples infrared thermal desorption (IRTD) for high temperature vaporization with direct analysis in real time mass spectrometry (DART-MS). This platform has demonstrated unique capabilities for thermally desorbing and detecting both more volatile organic and inorganic components as well as nonvolatile inorganic components (e.g., oxidizers) at each’s optimal temperature during a rapid ramp (i.e., 15 s).9, 25 The combination of high temperature desorption and soft ionization enabled the detection of intact salt adducts with DART-generated nitrate anions (e.g., [KClO4+NO3]−) for enhanced specificity. As new detection technologies are developed, specific considerations of appropriate test materials are critical. Here, we present an initial characterization of two sample classes (i.e., solid particulate vs solution cast) of propellants, including black powders and black powder substitutes.
Methods
Materials.
The Bureau of Alcohol, Tobacco, Firearms, and Explosives (ATF) forensic laboratory provided samples of commercial black powders and black powder substitutes. This included two black powders: Goex FFFg and Elephant Supreme FFFg, and four black powder substitutes: Pyrodex RS, Pyrodex P, Triple Seven FFFg, and Jim Shockey’s Gold. Propellant samples were prepared by two methods. The first consisted of weighing out solid grains (1-2 grains) of each propellant and crushing with a mortar and pestle. The resulting material was dry swipe sampled by a series of polytetrafluoroethylene (PTFE)-coated fiberglass weave wipes (DSA Detection, LLC, North Andover, MA, USA). Previous studies have measured collection efficiencies on the order of single percentages for dry swipe sampling with these wipes,26 yielding collection estimates on the order of single to tens of micrograms. The second method included the dissolution and suspension of each propellant in ultrapure deionized water to 1 mg/mL (gravimetrically prepared) and solution cast (pipetting 1 μL) directly onto PTFE-coated fiberglass wipes. Propellant components such as sulfur and carbon are not soluble in water and expected to be in suspension. Unfiltered liquid-deposited samples were allowed to dry prior to analysis.
Instrumentation.
Wipe-based propellant samples were analyzed by an IRTD-DART-MS platform. Details of the configuration and the infrared-based thermal desorber were described in the literature (Figure 1(a)).9, 27, 28 Briefly, near-infrared emission (15 s interval at 100% power) impinged a glass-mica ceramic, which absorbed the radiation and rapidly heated. The heated glass-mica ceramic component subsequently heated the inserted wipe. The IRTD unit was coupled to the DART ion source (Ionsense, Saugus, MA, USA) and mass spectrometer (AccuTOF, JEOL USA, Peabody, MA, USA) through a hybrid glass/ceramic/metal junction and hydrodynamic-assist interface (Vapur, Ionsense). Background subtracted (i.e., blank wipe) mass spectra were collected in negative ion mode and as done in previous work,25 extracted from multiple time-points in the desorption profile (Figure 1(b)). Blank spectra were dominated by a nitrate peak (NO3− m/z 62) generated by the nitrogen gas of the DART ion source.29 Specifically, spectra were collected across the entire 15 s infrared emission interval (Figure 1(b-i)), ‘early’ in the desorption period (i.e., from IRTD initiation to approximately 7 s) when more volatile species were observed (Figure 1(b-ii)), and ‘late’ in the desorption period (i.e., from approximately 8 s to 15 s of the emission interval) when the nonvolatile inorganic oxidizers were observed (Figure 1(b-iii)). Mass spectrometer settings, DART parameters, and additional instrumentation details can be found in the literature and Supporting Information.9, 25
Figure 1.
(a) Schematic representation of the IRTD-DART-MS configuration. Inset displays Section A-A of the infrared thermal desorber. (b) Demonstrative IRTD-DART-MS extracted ion chronograms of hydrogen sulfate (m/z 97, HSO4−) and potassium nitrate adduct with nitrate (m/z 163, [KNO3+NO3]−) from an Elephant black powder sample, overlaid with base plate temperature. Spectra were extracted from (i) the entire desorption period, (ii) ‘early’ (first 7 s of emission), and (iii) ‘late’ (final 7 s of emission) in the desorption period.
Spectral Similarity.
Mass spectral similarity was computed using the commonly employed cosine of the angle between them.30-32 Analogous similarity measurements and match factors are often used in library or database searching.33, 34 Each relevant mass spectrum was represented as a vector of intensity values (i.e., peak area). A nominal relative intensity lower limit of 10 % was used to simplify the high-resolution mass spectra and eliminate low intensity peaks or noise. In select cases, target ions of interest (e.g., known degradation products or ions previously identified in the literature) below this 10 % relative intensity threshold were included. The spectral similarity of each pair of spectra (i.e., vectors) was calculated as, , where xA and xB are the intensity vectors for spectrum A and B, and is the dot product of the vectors across all m/z values included. Spectral similarities were between 0 and 1, where 1 was a perfect match. Various weighting schemes and score scaling have been employed,30, 35 but were not considered here.
Results and Discussion
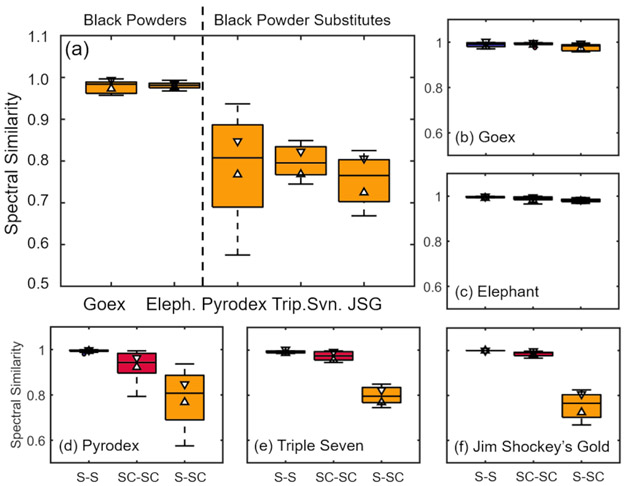
Two sets of wipe-based propellant samples were analyzed by IRTD-DART-MS. The first set consisted of solid particulate directly from crushed propellant grains (S). The second set consisted of crystallized propellant deposits from dried solution cast aqueous solutions/suspensions (SC). Though both sets of test materials resulted in solid deposits, they will be referred to by the deposition state for clear differentiation (i.e., ‘solid, (S)’ for solid deposited propellant particulate and ‘solution cast, (SC)’ for liquid deposited propellant). Five replicate spectra were collected for each sample characteristic ion distributions were identified in each spectrum based on the literature and past experience.25 A preliminary high-level comparison of the two sets of test samples was completed using a mass spectral similarity measure of the entire desorption profile. Here, each solid particulate sample mass spectrum was compared to each solution cast (i.e., liquid deposited) sample mass spectrum. Figure 2(a) displays the spectral similarities for solid deposited (S) relative to solution cast (SC) test samples of two black powders (Goex and Elephant) and three black powder substitutes (Pyrodex, Triple Seven, and Jim Shockey’s Gold). The two sets of black powder test samples exhibited very similar spectra with median similarity scores close to 1. In addition, the black powder mass spectral similarity scores between solid and solution cast samples were not significantly different (95 % confidence) than self-similarity scores – solid-to-solid and solution cast-to-solution cast (Figures 2(b) and 2(c)). Self-similarity scores compared spectra from the same test material preparation method, for example, solid deposited compared to other solid deposited samples (solid-to-solid, S-S) or solution cast deposited compared to other solution cast deposited samples (solution cast-to-solution cast, SC-SC).
Figure 2.
(a) Box-whisker plots of mass spectral similarity scores comparing solid or solution cast propellant samples for black powders and black powder substitutes. (b)-(f) Box-whisker plots of mass spectral self-similarity comparisons for each propellant – solid sample self-similarity (S-S: blue), solution cast self-similarity (SC-SC: red), and solid to solution cast spectral similarity (S-SC: yellow). Boxes, whiskers, and outliers (●) signify median ion ratio with lower and upper quartiles, 1.5× interquartile range, and ratios beyond that range. Triangles represent the 95% confidence intervals around each distribution’s median.
Black powder is a primitive propellant comprised of potassium nitrate, sulfur, and a carbon species (e.g., charcoal). The ion distribution for black powder was dominated by a range of sulfur species (Figure 3(a)). The IRTD platform enabled rapid and discrete heating profiles, temporally separating the more volatile components (e.g., sulfur) from the less volatile components (e.g., potassium nitrate). Figure 3(a) displays representative mass spectra for Goex black powder from both test materials early in the temperature ramp (identified in Figure 1(b)), exhibiting the sulfur ion distributions.36, 37 Though some differences in the ion distribution existed, hydrogen sulfate (m/z 97 HSO4−) was the overwhelmingly dominant peak for both solid and solution cast samples.
Figure 3.
(a) Representative mass spectra from (i) solid and (ii) liquid deposited (solution cast) Goex black powder, early in the desorption interval. (b) Differences in ion ratios for solid (blue) and liquid (red) test samples across all black powder samples. Boxes, whiskers, and outliers (●) signify median ion ratio with lower and upper quartiles, 1.5× interquartile range, and ratios beyond that range. Triangles represent the 95% confidence intervals around each distribution’s median.
Figure 3(b) exhibits box plots for a number of the main sulfur ions observed, normalized by HSO4−. Relative increases in a few of the sulfur ions were observed for black powder samples dissolved/suspended in water and recrystallized/solution cast. However, most still fell within 95 % confidence intervals of the solid particle medians. The minimal changes in sulfur distribution were not unexpected since elemental sulfur is not soluble in water and likely exhibited similar characteristics in both test sample sets. Two commercial black powders were investigated here, yet we expect these results to be similar for others given the relatively uniform composition of black powders (approximately 75% potassium nitrate, 15% carbon, and 10% sulfur). Applications such as checkpoint screening or on-site presumptive identification typically employ alarm algorithms that focus on the dominant ion (or ions). Given the more volatile nature of sulfur relative to potassium nitrate, traditional detection of black powders has focused on the detection of sulfur, in this case, HSO4−.36 Based on such a targeted detection scheme, these two test sample sets were effectively indistinguishable (Figure 3(a)). However, other applications such as forensic analysis may be interested in the full chemical signature of a sample. The elevated temperatures achievable by IRTD enabled the detection of the potassium nitrate oxidizer, commonly unachievable by traditional techniques.9, 36 Details characterizing the more nonvolatile oxidizers will be covered below.
Though black powder detection based on sulfur was independent of test material, numerous black powder substitutes have been formulated to reduce or eliminate sulfur and marketed as superior propellants. Black powder substitutes often include additional or alternative oxidizers (e.g., potassium perchlorate) and fuels (e.g., ascorbic acid). Solid particulate and solution cast black powder substitute samples exhibited median spectral similarities around 0.75 to 0.8 (Figure 2(a)), significantly lower (95% confidence) than the black powders. Unlike the black powders, the black powder substitutes demonstrated statistically different mass spectral similarity between the types of samples relative to self-similarity (Figure 2(d)-2(f)). These propellants were investigated further.
First, we considered the black powder substitutes containing dicyandiamide and sodium benzoate (e.g., Pyrodex and Triple Seven) in addition to potassium nitrate and potassium perchlorate oxidizers. Figure 4(a) displays representative mass spectra early in the IRTD heating profile (as identified in Figure 1(b)) for solid- and liquid-based Pyrodex P black powder substitute samples. The most notable differences came from the shift in the dicyandiamide ion distribution between sample sets. Specifically, the solid particulate test samples predominantly exhibited the nitrate adduct, m/z 146 [DCD+NO3]−, however, the liquid-deposited samples demonstrated similar abundances of the nitrate adduct and the deprotonated molecule, m/z 83 [DCD-H]−. Dicyandiamide also exhibited the loss of ammonia from the zwitterionic form to yield a dicyanamide anion, m/z 66 [N(CN)2]−, in liquid-based samples.
Figure 4.
Representative mass spectra from (i) solid and (ii) liquid deposited (a) Pyrodex P and (b) Jim Shockey’s Gold black powder substitutes, early in the desorption interval. Box-whisker plots of differences in ion ratios for solid (blue) and liquid (red) test samples of (c) Pyrodex and Triple Seven and (d) Jim Shockey’s Gold black powder substitutes. Triangles signify the 95% confidence intervals around each distribution’s median. Abbreviations: DCD: dicyandiamide, AA: ascorbic acid, TA: threonic acid, and OA: oxalic acid.
The shift in ion distribution and other relative differences in ions associated with dicyandiamide and sodium benzoate were captured across replicate analyses of Pyrodex P, Pyrodex RS, and Triple Seven black powder substitutes. Figure 4(c) demonstrates relative intensity differences from solid particulate and liquid deposited test samples for the dicyanamide anion (m/z 66), deprotonated dicyandiamide (m/z 83), benzoate anion (m/z 121), and dicyandiamide-benzoate adduct (m/z 205), all relative to the dicyandiamide nitrate adduct (m/z 146). The increase in deprotonated dicyandiamide observed in the spectra of liquid-based samples (Figure 4(a)) was quantified and significant (95 % confidence) across the relevant black powder substitutes. Similarly, significantly more (95 % confidence) benzoate was observed from the solution cast samples. Interestingly, there was little difference in the distribution of the dicyandiamide-benzoate adduct, m/z 205 [DCD + C6H5COO]−, between solid and liquid deposited samples. In addition to dicyandiamide and sodium benzoate, Triple Seven also contained nitrobenzoic acid. Solution cast Triple Seven samples exhibited an increase in the nitrobenzoate anion (m/z 166) and decrease in the dicyandiamide-nitrobenzoate adduct (m/z 250) relative to solid particulate samples (Figure S1).
The final black powder substitute investigated here, Jim Shockey’s Gold, was a mixture of ascorbic acid, potassium nitrate, and potassium perchlorate. Representative mass spectra from early (as defined in Figure 1(b)) in the heating profile demonstrated clear differences (Figure 4(b)) as eluded to by the spectral similarity measurements (Figure 2(a)). The ascorbic acid component (a common fuel in black powder substitutes38, 39) exhibited degradation products unique to the solution cast test materials. The degradation of ascorbic acid in solution has been widely investigated in the literature and many of the expected products, as well as other yet to be identified ions, were observed here.39, 40 Both sets of test materials, solid particulate and liquid deposited, demonstrated the ascorbate anion (m/z 175 [AA-H]−) and ascorbic acid nitrate adduct (m/z 238 [AA+NO3]−). However, similar to the prior examples with dicyandiamide, the ion distribution of solution cast Jim Shockey’s Gold samples shifted significantly toward the ascorbate anion (Figure 4(d)). In addition, degradation products, including threonic acid (threonate: m/z 135 [TA-H]−) and oxalic acid (oxalate: m/z 89 [OA-H]−), as well as known fragments C4H3O4− (m/z 115) and C3H3O3− (m/z 87), were observed for liquid-based samples (Figure 4(b)). A number of other unidentified peaks at m/z 145, m/z 210, and m/z 236 were also consistently observed with only the liquid deposited Jim Shockey’s Gold samples. As might be expected, the overall shift in the ascorbic acid ion distribution was significant between the sample types (95 % confidence).
Finally, we compared the chemical signatures of the nonvolatile oxidizers, specifically potassium nitrate and potassium perchlorate, desorbed at elevated temperatures later in the heating profile. Desorption of these nonvolatile oxidizers is typically unachievable by traditional thermal desorption temperatures for organic species (generally < 250 °C).9, 36 Above, the large sulfur ion distributions (i.e., numerous ions) dominated the mass spectra for black powders, generating overall mass spectral similarity between samples types near 1. However, when focusing solely on the potassium nitrate distribution (late in the heating profile – Figure 1(b)), we observed clear differences as seen in Figure 5(a). The solid particulate samples exhibited higher abundance of higher order intact salt clusters. Figure 5(c) displays the ratio of the potassium nitrate adduct with nitrate (m/z 163, [KNO3+NO3]−) relative to the dominant sulfur peak (hydrogen sulfate, m/z 97, HSO4−) for both sets of samples. Normalizing by the bare nitrate anion was not feasible due to the high concentrations of nitrate generated by the DART ion source. The solid deposited samples exhibited significantly (95% confidence) more of the intact salt in the mass spectra. Previous work on the IRTD-DART-MS platform demonstrated the ability to detect intact salts, providing both cation and anion information and improved specificity.9, 25
Figure 5.
Representative mass spectra from (i) solid and (ii) liquid deposited (a) Elephant black powder and (b) Triple Seven black powder substitute, late in the desorption interval. (c) Differences in oxidizer ion ratios for solid (blue) and liquid (red) test samples across all (i) black powder samples: potassium nitrate and (ii) black powder substitute samples: potassium perchlorate. Triangles signify the 95% confidence intervals around each distribution’s median.
The differences in the oxidizer distribution were also clearly evident for the black powder substitutes that incorporated potassium perchlorate. The main ions common to IRTD-DART-MS of potassium perchlorate were observed for both sets of samples, including perchlorate (m/z 99, ClO4−), potassium perchlorate-nitrate adduct (m/z 200, [KClO4+NO3]−), and potassium perchlorate-perchlorate adduct (m/z 237, [KClO4+ClO4]−). However, the distribution of these peaks varied (Figure 5(b)). The solution cast samples exhibited predominately the bare perchlorate anion. However, the solid deposited samples consistently resulted in a potassium perchlorate-nitrate adduct base peak. Figure 5(c) demonstrates the ratio of m/z 200 to m/z 99 (nitrate adduct/bare anion) across all tested black powder substitutes (Pyrodex RS, Pyrodex P, Triple Seven, and Jim Shockey’s Gold) for each sample type. The nature of the test materials and sample preparation (if present) played an important and significant role in the ion distribution produced. This may become more critical to certain applications when the dominant ions change, causing issues for predetermined or rigid alarm algorithms.
A number of the clear differences in mass spectra and ion distributions between sample sets (solid vs solution cast) were attributed to well documented compound degradation, e.g., ascorbic acid in solution.39, 40 However, other shifts in ion distribution were attributed to both the physical and chemical properties of the sample and thermal desorption process. For example, non-soluble elemental sulfur exhibited minimal differences between sample sets. However, more complex mixtures of organic (e.g., sodium benzoate) and inorganic salts (e.g., potassium perchlorate) dissociated in solution and their recombination and crystallization from a liquid deposit was a function of both the mixture composition and drying process. These factors, which were also a function of the solution and surface properties, yielded crystals with different morphology and size. The crystal formation and distribution across the deposit may play a role in both the desorption and ionization processes. Figure 6 displays SEM-EDS images of a crushed Pyrodex particle and solution cast Pyrodex. The solid propellant grains are often manufactured by pressing the components together. Here, larger potassium nitrate particles were coated in adhered smaller particles of potassium perchlorate, sulfur, and carbon with significant spatial overlap (Figure 6(a) inset). This intimate contact may have contributed to larger potassium perchlorate adducts with available nitrate from the surrounding potassium nitrate (and not solely nitrate generated by the DART source). However, the solution cast samples were distributed based on the drying process. Small sulfur and carbon particles pinned the drying solution drop and were found concentrated in this “coffee ring” region (Figure S2(b)). In addition, the potassium nitrate and potassium perchlorate crystals were more spatially separated (Figure 6(b)). This may have limited potassium perchlorate adducts with nitrate derived from the potassium nitrate component. The results demonstrated here reflect the crystal formation for drying from a water solution. The use of alternative solvents and ambient drying conditions or temperatures may result in vastly different crystal sizes and morphologies.
Figure 6.
Representative SEM-EDS images of (a) crushed solid and (b) solution cast Pyrodex RS black powder substitute samples. Mapped elements Cl, K, and S displayed as red, blue, and green. Inset displays further magnified solid Pyrodex.
In addition to the spatial distribution, the intimate interaction between the refractory inorganic oxidizer and certain fuels (e.g., ascorbic acid or nitrobenzoic acid) may have resulted in the acidification of potassium perchlorate. Similar to literature on acidic reagent chemical conversion of nonvolatile inorganic salts,6, 12 these acidic fuels may have converted a portion of the nonvolatile inorganic salt to the more volatile conjugate acid (i.e., perchloric acid). Unfortunately, the SEM-EDS images were generated from the full depth of each crystal and would not exhibit potential perchloric acid on the surface of these crystals. Potential contributions from perchloric acid would also yield an increase in the bare perchlorate anion.
Previous work visualized the thermal desorption process of pure potassium chlorate crystals and solution cast deposits by IRTD.9 The larger crystals melted and boiled, ejected large drops of molten salt. However, the liquid deposited crystal film ejected much smaller drops and direct vapor. These differences in the thermal desorption process may also directly affect the final transfer into the gas phase and ionization processes by DART. Considering a solution cast sample of pure potassium perchlorate, the ratio of the nitrate adduct to bare anion ([KClO4+NO3]−/ClO4−) was intermediate to either the solid particulate or liquid deposited black powder substitute samples (Figure S3). Relative to the solution cast Pyrodex, solution cast potassium perchlorate resulted in a focused agglomeration of large salt crystals (Figure S4). These differences in spatial distribution, morphology, and size may impact the desorption and subsequently resulting ion distributions. The intermediate salt/anion ratio from pure potassium perchlorate samples supported the role both chemical and physical processes have on the mass spectra of these sets of test samples.
Conclusions
Test and reference materials are critical for instrument development and performance evaluation. To accurately assess detection capabilities, test materials must accurately and precisely represent the physical (i.e., spatial distribution and crystal structure) and chemical (i.e., composition) characteristics of the target threat. Liquid deposition techniques have traditionally enabled more precise measurement of trace levels (i.e., micrograms and below), though advancements in positive displacement pipettes and systems for powder dispensing are approaching the microgram level.41, 42 However, when analyzed by thermal desorption mass spectrometry, dissolution of black powders and black powder substitutes resulted in shifts in the mass spectra and ion distributions relative to solid samples. Though this work focused on chemical signature differences resulting from the initial test material state, other aspects such as the ionization scheme also impact ion distributions. Alternative ionization methods may yield different ionization pathways or incorporate dopants driving specific adduct formation. Similarly, harsher ionization techniques (e.g., corona discharge or 63Ni) have demonstrated sufficient energy to fragment larger inorganic salt adducts (e.g., potassium perchlorate-nitrate adduct), shifting ion distributions toward the bare anion (e.g., perchlorate). Further, gas phase ionization will not play a role for other classes of analytical techniques, such as colorimetry or capillary electrophoresis. Nevertheless, the presence of degradation products, as demonstrated with the ascorbic acid fuel, may play a role in detection by these other techniques and must be investigated. As new technologies are developed and novel threats evolve, test materials and processes for their production and deposition will continue to play an important role.
Supplementary Material
Acknowledgments
The U.S. Department of Homeland Security Science and Technology Directorate sponsored a portion of the production of this material under Interagency Agreements IAA FTEN-18-00014 and FTPC-19-00040 with the National Institute of Standards and Technology.
Footnotes
Certain commercial equipment, instruments, or materials are identified in this article in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by NIST, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States.
The authors have declared the following potential conflict of interest: Thomas P. Forbes is an inventor on a patent application (describing infrared thermal desorption (IRTD)) to the United States of America as represented by the Secretary of Commerce, The National Institute of Standards and Technology. Greg Gillen declares no competing financial interests.
References
- 1.Evans-Nguyen K; Stelmack AR; Clowser PC; Holtz JM; Mulligan CC, Fieldable Mass Spectrometry for Forensic Science, Homeland Security, and Defense Applications Mass Spectrometry Reviews 2021, 10.1002/mas.21646. [DOI] [PubMed] [Google Scholar]
- 2.Klapec DJ; Czarnopys G; Pannuto J, Interpol review of detection and characterization of explosives and explosives residues 2016-2019. Forensic Science International: Synergy 2020, 2, 670–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Bomb Data Center (USBDC) Explosives Incident Report (EIR). https://www.atf.gov/resource-center/data-statistics (accessed March 23, 2020).
- 4.National Consortium for the Study of Terrorism and Responses to Terrorism (START), Global Terrorism Database https://www.start.umd.edu/gtd (accessed March 19, 2020).
- 5.Blanco GA; Nai YH; Hilder EF; Shellie RA; Dicinoski GW; Haddad PR; Breadmore MC, Identification of Inorganic Improvised Explosive Devices Using Sequential Injection Capillary Electrophoresis and Contactless Conductivity Detection. Analytical Chemistry 2011, 83 (23), 9068–9075. [DOI] [PubMed] [Google Scholar]
- 6.Peng L; Hua L; Wang W; Zhou Q; Li H, On-site Rapid Detection of Trace Non-volatile Inorganic Explosives by Stand-alone Ion Mobility Spectrometry via Acid-enhanced Evaporization. Scientific Reports 2014, 4, 6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters KL; Corbin I; Kaufman LM; Zreibe K; Blanes L; McCord BR, Simultaneous colorimetric detection of improvised explosive compounds using microfluidic paper-based analytical devices (μPADs). Analytical Methods 2015, 7 (1), 63–70. [Google Scholar]
- 8.Forbes TP; Sisco E, Recent advances in ambient mass spectrometry of trace explosives. Analyst 2018, 143 (9), 1948–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forbes TP; Sisco E; Staymates M, Detection of Nonvolatile Inorganic Oxidizer-Based Explosives from Wipe Collections by Infrared Thermal Desorption—Direct Analysis in Real Time Mass Spectrometry. Analytical Chemistry 2018, 90 (11), 6419–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezemer KDB; Forbes TP; Hulsbergen AWC; Verkouteren J; Krauss ST; Koeberg M; Schoenmakers PJ; Gillen G; van Asten AC, Emerging techniques for the detection of pyrotechnic residues from seized postal packages containing fireworks. Forensic Science International 2020, 308, 110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauss ST; Forbes TP; Lawrence JA; Gillen G; Verkouteren JR, Detection of fuel-oxidizer explosives utilizing portable capillary electrophoresis with wipe-based sampling. ELECTROPHORESIS 2020, 41 (16–17), 1482–1490. [DOI] [PubMed] [Google Scholar]
- 12.Kelley JA; Ostrinskaya A; Geurtsen G; Kunz RR, Reagent approaches for improved detection of chlorate and perchlorate salts via thermal desorption and ionization. Rapid Communications in Mass Spectrometry 2016, 30 (1), 191–198. [DOI] [PubMed] [Google Scholar]
- 13.Tsai C-W; Tipple CA; Yost RA, Application of paper spray ionization for explosives analysis. Rapid Communications in Mass Spectrometry 2017, 31 (19), 1565–1572. [DOI] [PubMed] [Google Scholar]
- 14.Frazier J; Benefield V; Zhang M, Practical investigation of direct analysis in real time mass spectrometry for fast screening of explosives. Forensic Chemistry 2020, 18, 100233. [Google Scholar]
- 15.Forbes TP; Krauss ST; Gillen G, Trace detection and chemical analysis of homemade fuel-oxidizer mixture explosives: Emerging challenges and perspectives. TrAC Trends in Analytical Chemistry 2020, 131, 116023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verkouteren RM; Verkouteren JR, Inkjet Metrology: High-Accuracy Mass Measurements of Microdroplets Produced by a Drop-on-Demand Dispenser. Analytical Chemistry 2009, 81 (20), 8577–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windsor E; Najarro M; Bloom A; Benner B; Fletcher R; Lareau R; Gillen G, Application of Inkjet Printing Technology to Produce Test Materials of 1,3,5-Trinitro-1,3,5 Triazcyclohexane for Trace Explosive Analysis. Analytical Chemistry 2010, 82 (20), 8519–8524. [DOI] [PubMed] [Google Scholar]
- 18.Holthoff E; Hankus M; Pellegrino P, Investigating a drop-on-demand microdispenser for standardized sample preparation. SPIE: 2011; Vol. 8018. [Google Scholar]
- 19.Verkouteren RM; Verkouteren JR, Inkjet Metrology II: Resolved Effects of Ejection Frequency, Fluidic Pressure, and Droplet Number on Reproducible Drop-on-Demand Dispensing. Langmuir 2011, 27 (15), 9644–9653. [DOI] [PubMed] [Google Scholar]
- 20.Holthoff EL; Farrell ME; Pellegrino PM, Standardized Sample Preparation Using a Drop-on-Demand Printing Platform. Sensors 2013, 13 (5), 5814–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillen G; Najarro M; Wight S; Walker M; Verkouteren J; Windsor E; Barr T; Staymates M; Urbas A, Particle Fabrication Using Inkjet Printing onto Hydrophobic Surfaces for Optimization and Calibration of Trace Contraband Detection Sensors. Sensors 2015, 15 (11), 29618–29634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verkouteren JR; Lawrence J; Brewer TM; Sisco E, New Particle-based Trace Explosive Test Material Produced by Drop-On-Demand Inkjet Printing for Quantitative Wipe-Sampling Studies. Analytical Methods 2017, 9 (23), 3441–3449. [Google Scholar]
- 23.Emmons ED; Farrell ME; Holthoff EL; Tripathi A; Green N; Moon RP; Guicheteau JA; Christesen SD; Pellegrino PM; Fountain AW, Characterization of Polymorphic States in Energetic Samples of 1,3,5-Trinitro-1,3,5-Triazine (RDX) Fabricated Using Drop-on-Demand Inkjet Technology. Appl. Spectrosc 2012, 66 (6), 628–635. [DOI] [PubMed] [Google Scholar]
- 24.Manrique-Bastidas C; Primera-Pedrozo O; Pacheco-Londono L; Hernandez-Rivera S, Raman microspectroscopy crystallization studies of 2,4,6-TNT in different solvents. SPIE: 2004; Vol. 5617. [Google Scholar]
- 25.Forbes TP; Verkouteren JR, Forensic Analysis and Differentiation of Black Powder and Black Powder Substitute Chemical Signatures by Infrared Thermal Desorption–DART-MS. Analytical Chemistry 2019, 91 (1), 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verkouteren JR; Coleman JL; Fletcher RA; Smith WJ; Klouda GA; Gillen G, A method to determine collection efficiency of particles by swipe sampling. Measurement Science and Technology 2008, 19 (11), 115101. [Google Scholar]
- 27.Forbes TP; Staymates M; Sisco E, Broad spectrum infrared thermal desorption of wipe-based explosive and narcotic samples for trace mass spectrometric detection. Analyst 2017, 142 (16), 3002–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes TP; Staymates M Infrared thermal desorber and performing infrared thermal desorption. U.S. Patent Application 17/144,232, 2021.
- 29.Sisco E; Staymates ME; Forbes TP, Optimization of confined direct analysis in real time mass spectrometry (DART-MS). Analyst 2020, 145 (7), 2743–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demuth W; Karlovits M; Varmuza K, Spectral similarity versus structural similarity: mass spectrometry. Analytica Chimica Acta 2004, 516 (1), 75–85. [Google Scholar]
- 31.Schollée JE; Schymanski EL; Stravs MA; Gulde R; Thomaidis NS; Hollender J, Similarity of High-Resolution Tandem Mass Spectrometry Spectra of Structurally Related Micropollutants and Transformation Products. Journal of the American Society for Mass Spectrometry 2017, 28 (12), 2692–2704. [DOI] [PubMed] [Google Scholar]
- 32.Wan KX; Vidavsky I; Gross ML, Comparing similar spectra: From similarity index to spectral contrast angle. Journal of the American Society for Mass Spectrometry 2002, 13 (1), 85–88. [DOI] [PubMed] [Google Scholar]
- 33.Moorthy AS; Kearsley AJ; Mallard WG; Wallace WE, Mass spectral similarity mapping applied to fentanyl analogs. Forensic Chemistry 2020, 19, 100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein SE, Chemical substructure identification by mass spectral library searching. Journal of the American Society for Mass Spectrometry 1995, 6 (8), 644–655. [DOI] [PubMed] [Google Scholar]
- 35.Stein SE; Scott DR, Optimization and testing of mass spectral library search algorithms for compound identification. Journal of the American Society for Mass Spectrometry 1994, 5 (9), 859–866. [DOI] [PubMed] [Google Scholar]
- 36.Crawford CL; Boudries H; Reda RJ; Roscioli KM; Kaplan KA; Siems WF; Hill HH, Analysis of Black Powder by Ion Mobility–Time-of-Flight Mass Spectrometry. Analytical Chemistry 2010, 82 (1), 387–393. [DOI] [PubMed] [Google Scholar]
- 37.Rahman MM; Jiang T; Tang Y; Xu W, A simple desorption atmospheric pressure chemical ionization method for enhanced non-volatile sample analysis. Analytica Chimica Acta 2018, 1002, 62–69. [DOI] [PubMed] [Google Scholar]
- 38.Bottegal M; Lang L; Miller M; McCord B, Analysis of ascorbic acid based black powder substitutes by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry 2010, 24 (9), 1377–1386. [DOI] [PubMed] [Google Scholar]
- 39.Lang Gui-hua L; Boyle KM, The Analysis of Black Powder Substitutes Containing Ascorbic Acid by Ion Chromatography/Mass Spectrometry*†. Journal of Forensic Sciences 2009, 54 (6), 1315–1322. [DOI] [PubMed] [Google Scholar]
- 40.Jansson PJ; Jung HR; Lindqvist C; Nordström T, Oxidative Decomposition of Vitamin C in Drinking Water. Free Radical Research 2004, 38 (8), 855–860. [DOI] [PubMed] [Google Scholar]
- 41.Alsenz J, PowderPicking: An inexpensive, manual, medium-throughput method for powder dispensing. Powder Technology 2011, 209 (1), 152–157. [Google Scholar]
- 42.Lemmo AV, Handheld powder handling devices and related methods. U.S. Patent Application 12/174,571: 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.