Abstract
BACKGROUND & AIMS:
Significant geographic variability in gastrointestinal (GI) cancer-related death has been reported in the United States. We aimed to evaluate both modifiable and nonmodifiable factors associated with intercounty differences in mortality due to GI cancer.
METHODS:
Data from the Centers for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research platform were used to calculate county-level mortality from esophageal, gastric, pancreatic, and colorectal cancers. Multivariable linear regression models were fit to adjust for county-level covariables, considering both patient (eg, sex, race, obesity, diabetes, alcohol, and smoking) and structural factors (eg, specialist density, poverty, insurance prevalence, and colon cancer screening prevalence). Intercounty variability in GI cancer-related mortality explained by these covariables was expressed as the multivariable model R2.
RESULTS:
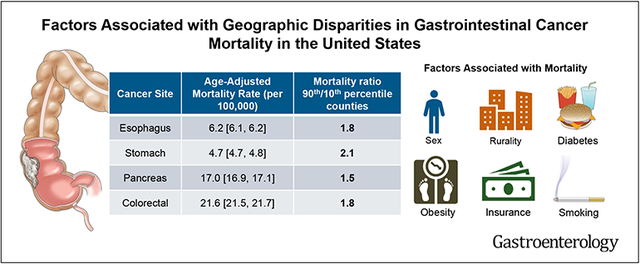
There were significant geographic disparities in GI cancer-related county-level mortality across the US from 2010–2019 with the ratio of mortality between 90th and 10th percentile counties ranging from 1.5 (pancreatic) to 2.1 (gastric cancer). Counties with the highest 5% mortality rates for gastric, pancreatic, and colorectal cancer were primarily in the Southeastern United States.Multivariable models explained 43%, 61%, 14%, and 39% of the intercounty variability in mortality rates for esophageal, gastric, pancreatic, and colorectal cancer, respectively. Cigarette smoking and rural residence (independent of specialist density) were most strongly associated with GI cancer–related mortality.
CONCLUSIONS:
Both patient and structural factors contribute to significant geographic differences in mortality from GI cancers. Our findings support continued public health efforts to reduce smoking use and improve care for rural patients, which may contribute to a reduction in disparities in GI cancer–related death.
Keywords: Cancer, Colon, Colorectal, County, Death
Graphical Abstract
Gastrointestinal (GI) cancers account for more than 25% of the global cancer incidence burden and in the United States ~1.5 million adults are diagnosed with GI cancers annually.1 These cancers are associated with a substantial burden of patient morbidity and mortality, with more than 35% of all cancer-related deaths attributable to GI malignancies.2 Although mortality has decreased during the past several decades,3 significant geographic variation in the risk of death still exists across the United States. Using data from the National Center for Health Statistics and the Human Mortality Database, Mokdad et al4 evaluated disparities in cancer mortality among different US counties. Using the difference in mortality rate between 90th and 10th percentile counties as a measure of geographic disparity, the authors showed that esophageal, gastric, pancreatic, and colorectal cancer all ranked among the top 10 malignancies with the highest county-level variation in risk of death. However, a detailed evaluation of potential factors associated with this variability in mortality rate by county was not performed. Understanding the patient- and system-level covariables that are associated with differences in mortality will help guide future public health interventions.
Several factors are potentially associated with geographic differences in cancer outcomes.5,6 For example, epidemiologic risk factors for cancer, such as smoking status and obesity, vary regionally across different counties in the United States.7,8 Similarly, access to high-quality health care is dependent on both patient location and socioeconomic status, with patients living in poverty, in rural areas, or without health insurance being particularly disadvantaged.6 Although the specific determinants of geographic variability in the risk of GI cancer–related death have not been well established, we hypothesize that both patient- and system-level factors are important in determining outcomes for GI malignancies. These include potentially modifiable patient characteristics (eg, obesity and risk of esophageal cancer9) as well as systemic factors, such as access to colorectal cancer screening, which vary by county.10,11
Understanding regional differences in mortality and reasons underlying these disparities is required to guide public health policy decisions at the local, state, and national levels. Furthermore, addressing potentially modifiable structural causes of mortality differences will require informed, contemporary, data-driven, system-level interventions. Therefore, we aimed to quantify county-level differences in mortality from GI cancer in the United States, identify hotspots of GI cancer mortality, and assess factors associated with intercounty variability in death rates using national data from the past decade.
Methods
Data Source
We estimated GI cancer–related mortality in all individual US counties using data from the Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) platform. The following GI malignancies were identified using diagnostic codes based on the International Classification of Diseases, 10th Revision: (1) esophageal cancer (C15); (2) gastric cancer (C16); (3) colorectal cancer (C18–C20); and (4) pancreatic cancer (C25). Detailed diagnostic coding is summarized in Supplementary Table 1. County-level mortality data were aggregated between 2010 and 2019 to maximize available data for multivariable modelling. In accordance with CDC guidelines, we censored counties reporting fewer than 20 deaths for each cancer subtype because these mortality rates are unreliable.12 Similarly, we excluded hepatobiliary cancer–related deaths because these occurrences were relatively uncommon. Data for liver cancer mortality have been previously evaluated and were not reanalyzed in this study.13
Study Outcomes and Covariables
The primary outcome was county-level, age-adjusted GI cancer-related mortality among adults aged ≥25 years (based on age categories defined in CDC WONDER). Covariables associated with GI cancer–related mortality for individual patients (eg, alcohol consumption, diabetes, obesity) and for all patients residing in a given county (eg, access to specialized care) were considered as potential confounders. Both patient- and system-level covariables were considered, although all covariables were modelled based on county-level aggregated data as individual patient data were not available. We considered the following structural covariables: (1) rural vs urban (large metropolitan or small/medium metropolitan) status as defined by the US Census Bureau14; (2) poverty, defined as the percentage of adults living below the federal poverty level based on the Small Area Income and Poverty Estimates Program15; (3) uninsured prevalence, defined as the mean annual percentage without health insurance based on the Small Area Health Insurance Estimates Program16; and (4) access to specialist care, modelled using the travel distance from the geographic centroid of a county to the closest National Cancer Center (NCI)-Designated Cancer Center17 and by the number of board-certified gastroenterologists, radiation oncologists, and colorectal surgeons (for colorectal cancer) per 100,000 adult population in 2015 using data from the Area Health Resources Files.18 We also considered the following patient-based covariables: (1) sex, race, and ethnicity using population estimates from the US Census Bureau in CDC WONDER19; (2) proportion of adults with heavy alcohol use (defined as an average consumption of >1 drink/d for women and >2 drinks/d for men in the past 30 days) from 2009–201220; (3) percentage of adults with diabetes from 2009–201221; (4) percentage of adults classified as obese in 20118; and (5) percentage of adults who currently or formerly used cigarettes in 2009–2012.7 In addition to these individual covariates, we assessed whether a composite risk score, the cumulative Community Health Score (CHS), was associated with the primary outcome (cancer mortality). The CHS is a composite indicator of proxy variables for community health, behavioral and environmental risks, social conditions, and access to care.22-24 This composite score includes: years of potential life lost, proportional low birth rate, poor or fair reported health, poor reported physical health days, poor reported mental health days, proportion of individuals reporting tobacco use, adult obesity and physical inactivity proportions, preventable hospital stays, and median annual household income.22-24 Finally, for colorectal cancer, we considered colon cancer screening prevalence per county based on the percentage of adults aged 50–75 who have ever had endoscopic or stool-based colon cancer screening as reported by the Behavioral Risk Factor Surveillance System and National Health Interview Survey.25
Statistical Analysis
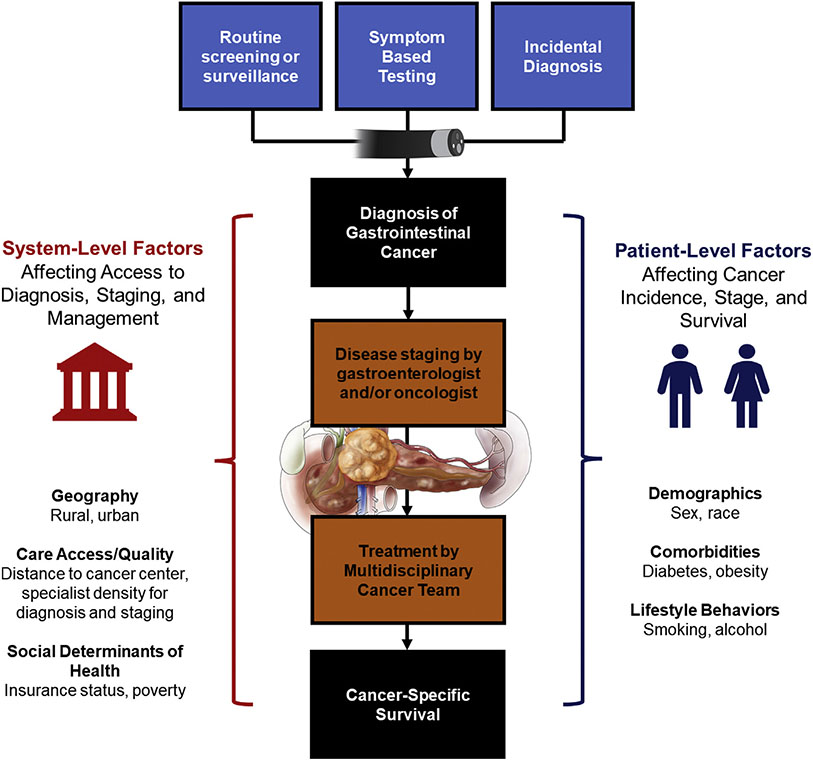
Age-adjusted mortality rates were obtained in CDC WONDER and were calculated using the direct method that applies age-specific death rates to the US standard population age distribution in 2000. This produced a weighted average of age-specific death rates, where the weight represented a fixed population by age allowing comparison of relative mortality risk across populations with different underlying age structures and over time. The year 2000 was considered the reference year for the standard population according to National Center for Health Statistics recommendations.26 Linear regression models were then fit to identify county-level factors associated with age-adjusted GI cancer–related mortality. Each cancer location (esophageal, gastric, pancreatic, and colorectal) was modelled separately. A backward selection process was used to fit covariables with P < .10 in univariable testing or covariables that increased the R2 of the final model for inclusion in the fully adjusted multivariable model. The β-coefficients within each model are presented as a measure of the strength of association between the covariable of interest and mortality. The adjusted R2 represents the proportion of variability in GI cancer mortality that is explained by the covariables in the model. The multivariable linear regression model was then used to predict adjusted, county-level GI cancer–related mortality rates that were mapped using the spmap function in STATA 16.0 (Stata-Corp, College Station, TX). Figure 1 presents a conceptual framework for interpreting these results; for an individual patient, the cancer journey includes initial diagnosis, staging, and management, ultimately resulting in either survival or death as the outcome. Cancer-related mortality rates are, therefore, a function of both incidence and death rates, and both are potentially influenced by patient- and system-level covariables included in our modelling.
Figure 1.
Conceptual framework of factors associated with mortality from GI cancers.
Hotspot analysis was conducted to assess for clusters of counties with high colorectal cancer–related mortality using ArcGIS 10.8 (Esri, West Redlands, CA). There were insufficient data to conduct hotspot analysis for esophageal, gastric, or pancreatic cancer. “High-high” clusters included counties with higher-than-expected mortality (although they may not have had the highest absolute mortality rates) that were surrounded by other counties with higher-than-expected mortalities. Age-adjusted and fully adjusted mortality rates from multivariable linear regression models were used as inputs for optimized hotspot analysis, using a distance band identified based on incremental spatial autocorrelation.13 Clusters were considered statistically significant at P < .05 and z score of 1.96 (corresponding to the 95% confidence level).
Finally, 2 sensitivity analyses were conducted to evaluate potential time trends in geographic disparity for GI cancer–related mortality. First, the cohort was divided into 2 equal 5-year periods (2010–2014 and 2015–2019), and similar methods were applied as above to assess for geographic disparities in age-adjusted mortality rate. Second, to understand if changes in the prevalence of covariables explained a different proportion of the variance in GI cancer–related mortality over time, we created multivariable models for each year and present annual β-coefficients and adjusted R2 for each cancer type. County-level data was not available for each covariable on an annual basis, so for this sensitivity analysis, we used state-level mortality and covariable prevalence data from 2011–2019.
This study was exempt from research ethics board review because it only included deidentified population-level data.
Results
Mortality From GI Cancers
A total of 3147 US counties were identified in CDC WONDER from 2010–2019. Among these, sufficient data to calculate reliable age-adjusted mortality rates were available for 1315, 826, 2174, and 2449 counties for esophageal, gastric, pancreatic, and colorectal cancer, respectively. Counties with unreliable counts were censored, although overall, the aggregated cohort captured 96.7% of all esophagus cancer–related deaths, 94.2% of stomach cancer–related deaths, 99.4% of pancreas cancer–related deaths, and 99.7% of colorectal cancer–related deaths (Supplementary Table 2).
Geographic Variability in GI Cancer–Related Mortality Rates
National and county-level age-adjusted mortality rates are summarized in Table 1. The median age-adjusted mortality rates for esophageal, gastric, pancreatic, and colorectal cancer from 2010–2019 were 7.1 deaths per 100,000 population (interquartile range [IQR], 6.0, 8.3), 4.4 (IQR 3.7, 5.4), 17.5 (IQR 15.7, 19.2), and 23.9 (IQR 20.7, 27.9), respectively. Geographic variability in GI cancer–related mortality is shown in Figure 2. The difference between the 90th and 10th percentile counties for age-adjusted GI cancer–related mortality ranged from 3.4–13.8 deaths per 100,000 population. Patients with gastric cancer living in counties greater than the 90th percentile of age-adjusted mortality had a 2.1-fold risk of death compared with those living in counties in the lowest 10%; this ratio was 1.8 for esophagus and colorectal cancer, and 1.5 for pancreas cancer. In a sensitivity analysis splitting the cohort into 2 time periods (2010–2014 and 2015–2019), there was no difference in these measures of geographic disparity for GI cancer–related mortality (Supplementary Table 3).
Table 1.
National Deaths due to GI Malignancies, Age-Adjusted Mortality Rate, and County-Level Differences in Age-Standardized Mortality
| Cancer type | National-level mortality |
County-level age-adjusted mortality rate (per 100,000) |
90th minus 10th percentile (deaths/100,000 population) |
90th/10th percentile ratio |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Total deaths (n) | Age-adjusted mortality rate (per 100,000) (95% CI) |
Minimum | 10th percentile | Median | 90th percentile | Maximum | |||
| Esophagus | 150,533 | 6.2 (6.1, 6.2) | 2.8 | 5.1 | 7.1 | 9.5 | 15.6 | 4.4 | 1.8 |
| Stomach | 112,036 | 4.7 (4.7, 4.8) | 2.0 | 3.2 | 4.4 | 6.6 | 14.6 | 3.4 | 2.1 |
| Pancreas | 411,531 | 17.0 (16.9, 17.1) | 6.2 | 14.2 | 17.5 | 21.5 | 45.5 | 7.2 | 1.5 |
| Colorectal | 516,517 | 21.6 (21.5, 21.7) | 10.9 | 18.3 | 23.9 | 32.1 | 73.1 | 13.8 | 1.8 |
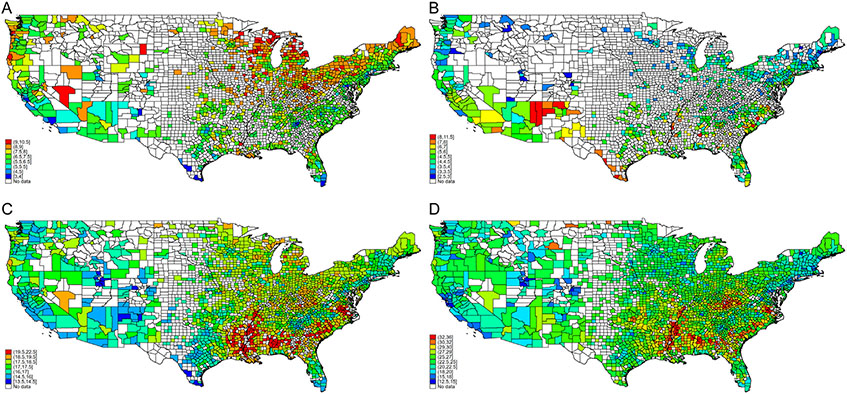
Figure 2.
County-level variability in esophagus (A), stomach (B), pancreas (C), and colorectal (D) cancer–related mortality rates, fully adjusted, 2010–2019. Mortality rates exclude counties with fewer than 20 observations because of unreliable estimates. Legend categories based on 1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th percentile of mortality. Mortality rates based on postestimation predictions of multivariable linear regression modeling.
Interstate and Intrastate Variability in County-Level Mortality
Counties in the top 5% of age-adjusted mortality rate for esophageal cancer were found in 25 states (n = 69 counties), although approximately half of these counties were in Ohio (n = 15; 21.7%), Indiana (n = 10; 14.5%), and Michigan (13.0%; n = 9). Similarly, for gastric cancer, 45.5% of the highest age-adjusted mortality rate counties (n = 44; 17 states) were in Texas (n = 8; 18.2%), Mississippi (n = 7; 15.9%), and South Carolina (n = 5; 11.4%). For pancreatic and colorectal cancer, counties with age-adjusted mortality rates in the highest 5% were more evenly distributed across 27 states, with no state contributing more than 15% of the total. However, the Southeastern states of Mississippi, Kentucky, Arkansas, Georgia, and Louisiana contributed to 41.2% (47/128) and 48.8% (62/127) of the highest 5% mortality counties for pancreas and colorectal cancer, respectively.
Within-state among-county variation in GI cancer–related mortality (among states with at least 10 counties with available data) is summarized in Figure 3. The ratio between the highest and lowest mortality rate counties within each state is summarized in Supplementary Table 4. Seven states (Georgia, Virginia, Texas, California, Maryland, New York, and Florida) had a greater than 2-fold difference in esophageal cancer mortality between the highest and lowest mortality counties. Two states (North Carolina and Virginia) had the greatest intrastate variability in mortality from gastric (ratio 2.3 and 2.0, respectively), pancreatic (ratio 1.4 and 1.4, respectively), and colorectal cancer (ratio 2.1 and 2.3, respectively).
Figure 3.
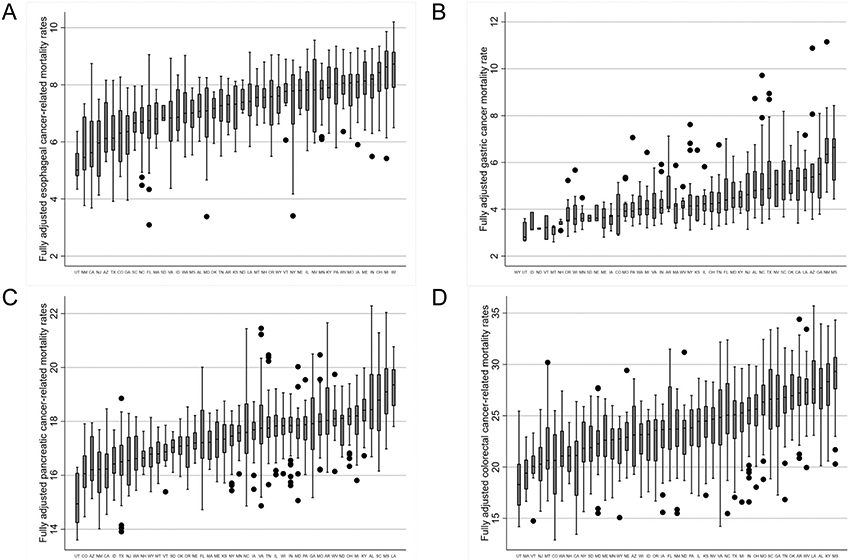
Within-state variability in fully adjusted, county-level mortality rates for esophagus (A), stomach (B), pancreas (C), and colorectal (D) cancer. Only states with ≥10 counties with available data were included, mortality rates based on postestimation predictions of multivariable linear regression modeling.
Factors Associated With Intercounty Mortality Disparity
Multivariable linear regression models were fitted to evaluate factors associated with county-level mortality from GI malignancies (Table 2). Both patient- and system-level factors were associated with county-level mortality from GI malignancies. The proportion of non-Hispanic black residents was associated with increased gastric, pancreatic, and colorectal cancer mortality, although this was inversely associated with esophageal cancer mortality. The proportion of cigarette smokers or former smokers was significantly associated with increased mortality from all considered GI malignancies, especially colorectal cancer. Other relevant metabolic comorbidities, including diabetes and obesity, were also associated with gastric/colorectal and esophagus/pancreas/colorectal cancer–related mortality, respectively. County rurality was the strongest system-level factor associated with death rates, although county density of gastroenterologists, radiation oncologists, colorectal surgeons, or travel time to the nearest NCI cancer center were not statistically significant. The proportion of uninsured patients was inversely associated with mortality from esophagus, stomach, and pancreas cancer. The colon cancer screening prevalence trended toward an association with intercounty variability in colorectal cancer mortality (β-coefficient −0.032; 95% confidence interval [CI], −0.066, 0.003; P = .07), although this was not statistically significant. The CHS was best modeled as a categorical variable, and, although it was significantly associated with mortality for esophageal (P < .0001) and gastric (P = .04) cancer, it was not included in the final models because it did not change the adjusted R2 by a measurable difference (ie, adding CHS to the models only increased our ability to explain county-level variability by <1% for all 4 of the studied cancers).
Table 2.
Multivariable Linear Regression Models Evaluating Factors Associated With County-Level Age-Adjusted GI Cancer–Related Mortality Rates
| Esophagus cancer |
Stomach cancer |
Pancreas cancer |
Colorectal cancer |
|||||
|---|---|---|---|---|---|---|---|---|
| County-level variable | Beta coefficient (95% CI) |
P value | Beta coefficient (95% CI) |
P value | Beta coefficient (95% CI) |
P value | Beta coefficient (95% CI) |
P value |
| Patient factors | ||||||||
| % male | 0.07 (0.01, 0.11) | <.01 | NS | - | NS | - | NS | - |
| Racial composition | ||||||||
| % White Hispanic | −0.02 (−0.04, −0.01) | <.001 | 0.05 (0.04, 0.06) | <.001 | NS | - | NS | - |
| % Black non-Hispanic | −0.03 (−0.03, −0.02) | <.001 | 0.05 (0.05, 0.06) | <.001 | 0.05 (0.04, 0.06) | <.001 | 0.03 (0.01, 0.05) | <.001 |
| % Black Hispanic | NS | - | NS | - | NS | - | NS | - |
| % Asian | −0.03 (−0.06, −0.003) | .003 | 0.06 (0.04, 0.08) | <.001 | NS | - | NS | - |
| % Native American | −0.04 (−0.06, −0.02) | <.001 | 0.09 (0.07, 0.10) | <.001 | −0.03 (−0.06, 0.004) | .09 | NS | - |
| % with diabetes | NS | - | 0.16 (0.09, 0.23) | <.001 | NS | - | 0.31 (0.12, 0.48) | <.001 |
| % with obesity | 0.12 (0.09, 0.15) | <.001 | NS | - | 0.10 (0.05, 0.15) | <.001 | 0.26 (0.17, 0.34) | <.001 |
| % cigarette use | 0.10 (0.06, 0.13) | <.01 | 0.06 (0.04, 0.09) | <.001 | 0.15 (0.11, 0.20) | <.001 | 0.40 (0.34, 0.46) | <.001 |
| % heavy alcohol use | 0.18 (0.14, 0.22) | <.001 | NS | - | 0.09 (0.02, 0.15) | .01 | NS | - |
| Systemic factors | ||||||||
| Rural/urban | ||||||||
| Large metropolitan | Reference | - | Reference | - | Reference | Reference | - | |
| Small/medium metropolitan | −0.07 (−0.29, 0.14) | .50 | −0.20 (−0.35, −0.05) | .008 | −0.36 (−0.75, 0.04) | .08 | −0.67 (−1.24, −0.10) | .02 |
| Rural | 0.56 (0.33, 0.79) | <.001 | 0.52 (0.32, 0.72) | <.001 | 0.13 (−0.26, 0.52) | .51 | 1.77 (1.22, 2.31) | <.001 |
| % uninsured | −0.05 (−0.07, −0.03) | <.001 | −0.03 (−0.05, −0.02) | <.001 | −0.08 (−0.11, −0.06) | <.001 | NS | - |
| % living below federal poverty level | NS | - | NS | - | NS | - | NS | - |
| Travel time to nearest NCI cancer centera | 0.03 (−0.005, 0.06) | .09 | NS | - | 0.04 (−0.01, 0.10) | .09 | NS | - |
| Specialist density per 100,000 population | ||||||||
| Gastroenterologists | NS | - | NS | - | NS | - | NS | - |
| Radiation oncologists | NS | - | NS | - | NS | - | NS | - |
| Colorectal surgeons | NA | - | NA | - | NA | - | NA | - |
| % colon cancer Screening | NA | - | NA | - | NA | - | −0.03 (−0.07, 0.003) | .07 |
| Overall model R2 | 0.43 | 0.61 | 0.14 | 0.39 | ||||
NOTE. Beta coefficients with a positive value associated with increased county-level cancer-related mortality. Covariables that were not included had P > .1 in univariable analysis or did not increase overall R2 in multivariable models fit by backward selection.
NA, not applicable; NS, not significant on univariable modelling and covariable not included in final model.
Travel time defined by per unit increase of 30 min from county centroid to nearest National Cancer Institute Comprehensive Cancer Center.
Together, the considered patient and systemic factors considered accounted for 43% of the observed variance in county-level mortality for esophagus cancer (as reflected in the adjusted R2), 61% for stomach cancer, 14% for pancreas cancer, and 39% for colorectal cancer.
Change in Factors Associated With State-Level Mortality Over Time
Variance in state-level mortality for GI cancers explained by patient- and system-level covariables over time is summarized in Supplementary Tables 5-8. There was minimal year-to-year variance in the model adjusted R2 and the magnitude of the effect size for individual covariables was also relatively stable. When modelled using state-level data as compared with county-level mortality, the covariables considered generally explained a greater proportion of the variance in cancer-related mortality rates with higher adjusted R2, particularly for colorectal cancer (adjusted R2, 0.51–0.72), pancreatic cancer (adjusted R2, 0.15–0.51), and gastric cancer (adjusted R2, 0.46–0.75). This suggests that there are unique factors associated with county-level mortality, consistent with our previous observations that there are significant variations in death rates even within the same state.
Geospatial Hotspot Analysis
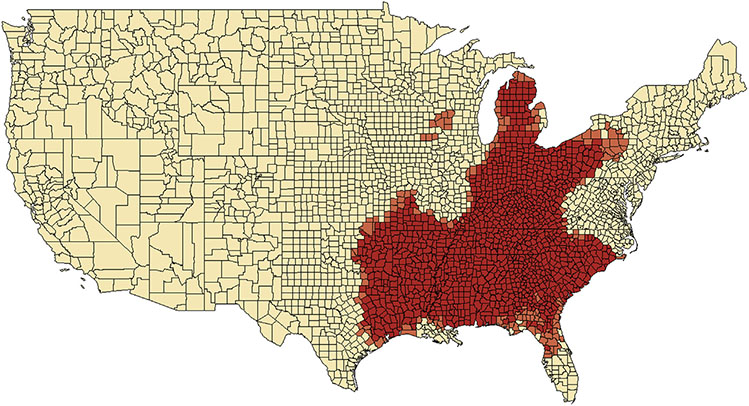
Sufficient data were only available to conduct spatial correlational analyses for colorectal cancer–related mortality rates. Mortality rates were highly spatially correlated (Figure 4), with a large hotspot encompassing most of the Southeastern states (including Arkansas, Louisiana, Kentucky, Tennessee, Mississippi, Alabama, Georgia, South Carolina, and northern Florida), and extending north through Appalachia and the eastern Midwest (including the eastern part of Texas, Oklahoma, Indiana, Ohio, and Michigan). A very small hotspot was also observed in western Wisconsin/northern Iowa, including 10 counties. These clusters of counties had higher than expected colorectal cancer mortality rates even after adjustment in multivariable models and were not explained by variation in county-level race, rurality, diabetes, obesity, or cigarette smoking.
Figure 4.
Geospatial hot spot analysis based on fully adjusted colorectal cancer–related mortality rates, estimated from multivariable linear regression models. Clusters with significantly increased mortality based on county-level z scores and P values (P < .05 and P < .01).
Discussion
In this analysis of 10 years of national county-level data, we highlight the significant variability in death rates from GI-related cancers, both within states and between counties, with an approximately 1.5- to 2-fold difference in age-adjusted mortality rates between the highest and lowest 10% of counties. Geographically, a greater proportion of counties with high levels of mortality from GI cancers are in the Southeastern US. Building on work by Mokdad et al,4 we then assessed for other potential covariables that are associated with variance in county-level GI cancer–related mortality. In multivariable modeling, potentially modifiable patient-related and structural factors were associated with county-level discrepancies in GI cancer–related death, with rurality and comorbidities (especially smoking and metabolic disorders) being important contributors, independent of specialist density. Our findings should be used to support local, targeted public health interventions, such as emphasizing smoking prevention and reducing the prevalence of metabolic syndrome, which may reduce future disparities in mortality from GI malignancies.
Rural residence was significantly associated with higher mortality from GI cancers. This has been similarly demonstrated for other medical conditions, including chronic pulmonary and cardiovascular diseases, inflammatory bowel disease, and peptic ulcer disease.27-30 The urban-rural disparity has progressively worsened during the past 20 years, with a more than 3-fold increase in the difference between urban and rural all-cause mortality in the United States.31 Rural counties face unique social, economic, and political challenges, particularly as the pace of urbanization increases. Historically, it was hypothesized that differences in mortality were related to poorer access to specialized medical care.32 However, we observed that differences in GI cancer–related mortality persisted even after adjusting for the density of gastroenterologists, colorectal surgeons, and radiation oncologists, and the distance to NCI cancer centers. Several hypotheses may explain these findings. First, specialist density is only a surrogate measure of care access. Beyond service availability, barriers to accessing health care are complex and include interactions between patient characteristics such as health literacy, financial consider-ations such as cost of treatment, and sociocultural and religious beliefs.33 Differences in health-care–seeking behaviors or treatment preferences also exist between rural vs urban populations.34 Second, accessing specialist care does not necessarily equate to better outcomes, and quantifying the quality of care provision is challenging. Indeed, patients with refractory or metastatic disease, who are inherently at higher risk for mortality, are more likely to be managed within specialist or subspecialist settings. Third, it is important to consider the different roles that specialists perform in the care pathway for patients with GI cancers. For example, although gastroenterologists are instrumental for diagnosing new cancers through endoscopy, they are typically less involved with surgical, radiation, or oncologic treatment decisions. Therefore, increased gastroenterologist density may be counterintuitively associated with higher cancer-related mortality counts due to the increased incidence detection rates.
Although we hypothesized that insurance status would be an important predictor of mortality, we showed that counties with higher prevalence of uninsured patients reported lower rates of GI cancer–related death. This is contradictory to findings that Medicaid expansion in the United States has been associated with increased rates of surgery and reductions in mortality for colorectal and lung cancer.35,36 There are several potential explanations for this seemingly paradoxical observation. First, in counties with low insurance prevalence, patients may be more likely to die due to other common and more preventable diseases such as myocardial infarctions or stroke.37,38 Second, given that insurance coverage is associated with access to cancer screening and diagnostic testing, counties with low insurance prevalence may be under-reporting cases that have not been detected.39 This is plausible because many GI malignancies have silent or minimally symptomatic presentations until late stages. An evaluation of patient-level data is required to definitively answer this question. Empirically, the potential role of changes in insurance structure under the Affordable Care Act has been assessed for esophageal and gastric cancer; Niroomand et al40 have demonstrated no significant change in gastric or esophageal cancer mortality pre- vs post-Medicaid expansion. Although our study captures the time period after enactment of the Affordable Care Act (2010–2019), it should also be recognized that there may be a time lag between changes in structural factors such as insurance coverage and cancer-related outcomes.
Several county-level patient factors were also significant predictors of GI cancer–related mortality. We focused on the effect of smoking, obesity, and diabetes because these are potentially modifiable and amenable to public health interventions. Smoking is highly correlated with mortality from all GI malignancies because the carcinogenic effects of cigarettes are an established risk factor for esophageal, gastric, pancreatic, and colorectal cancer.41 Although smoking prevalence in the United States has decreased, more than 34 million Americans remain active smokers, smoking cessation prevalence varies geographically with the highest prevalence of smokers in the US Midwest and South, and electronic cigarette use and vaping have increased significantly.42,43 Our findings support continued aggressive public health initiatives around smoking avoidance. Additionally, diabetes and obesity prevalence at the county-level were significantly associated with higher rates of GI cancer–related mortality, and both are associated with the development of GI malignancies.44-46 Similar to smoking, geographic variation in obesity has been consistently observed in the United States, with the highest prevalence in the Midwest (33.9%) and South (33.3%).47 In 2019, the prevalence of obesity in the adult population was >35% in Alabama, Arkansas, Indiana, Kansas, Kentucky, Louisiana, Michigan, Mississippi, Oklahoma, South Carolina, Tennessee, and West Virginia. There is substantial overlap with high mortality counties observed in our study. Furthermore, the rising prevalence of metabolic syndrome, particularly in adolescents and young adults, raises concerns for future epidemiologic trends in GI cancer–related mortality.
Differences in county-level mortality were only partially explained by the factors measured in our models, with the adjusted R2 ranging from 14% to >60%. Several hypotheses may explain both the absolute mortality variance captured by our models, as well as the differences in adjusted R2 observed between GI cancers. First, potentially important system-level determinants of cancer-related mortality may have been unmeasured. For instance, quality and timeliness of care is not easily quantifiable but likely influences mortality risk. Second, our analysis focuses on county-level predictive covariables and mortality, although cancer lethality is highly variable at an individual level based on factors such as tumor size, lymph node status, cancer grade and histology, immunologic phenotype, and patient age and functional status.48-51 The inherent variation in mortality based on tumor biology also likely explains the very low proportion of mortality variance for pancreatic cancer, where 5-year survival rate is only 10%,52 compared with the higher proportion of colorectal, gastric, and esophageal cancer mortality related to patient- and system-related covariables. For instance, colorectal cancer survival is highly stage dependent; although we did not observe a significant effect based on colon cancer screening prevalence in this analysis, this finding should be cautiously interpreted because the direction of effect is protective and high-quality randomized controlled trials have confirmed a protective benefit.53 Similarly, esophageal cancer survival is also stage dependent. Although we could not directly adjust for upper endoscopy screening prevalence for Barrett’s esophagus, some risk factors that trigger screening were included in our analysis (including age, sex, obesity, and smoking).54
The last key covariable associated with county-level mortality was racial composition. Higher rates of esophageal cancer–related mortality were observed among counties with a greater proportion of non-Hispanic whites (ie, lower mortality in counties with a higher proportion of under-represented minority populations), and, conversely, higher rates of colorectal, gastric, and pancreatic cancer-related mortality were observed in counties with a higher proportion of nonblack Hispanic or black residents. These findings are consistent with epidemiologic data demonstrating that the highest incidence of esophageal cancer is among white males,55 and that black patients are disproportionately at higher risk for colorectal cancer mortality.56-58 For counties with a higher proportion of Hispanic or Asians, there was a significantly higher mortality from gastric cancer, which also likely reflects epidemiologic studies demonstrating increased incidence and mortality from noncardia gastric cancer, likely associated with Helicobacter pylori infection.58
We conducted a hotspot analysis for colorectal cancer mortality. This is a spatial analysis and mapping technique used in public health research to identify geographic clusters where mortality rates are significantly higher than expected and compared with surrounding areas. Hotspot analysis provides important and actionable information for policymakers by identifying regions of greater-than-expected mortality, which is not explained by the covariables included in our models. For example, are there environmental exposures that lead to higher colorectal cancer–related mortality in the South and Ohio Valley, or are there geographic and/or financial barriers to cancer screening among certain populations living in these areas? The hotspots serve as a call to action for future studies in these areas to better understand the disparities in cancer-related mortality.
Our study has important strengths. We used a nationally representative data source with detailed county-level covariable and outcome data. Our analysis adjusts for important confounders associated with GI cancer–related mortality, allowing us to identify potentially modifiable patient, structural, and socioeconomic factors associated with mortality differences. However, we recognize some important limitations. First, we adjusted for county rather than patient-level data, and we are missing data granularity on cancer staging, histology, and treatment received. These factors are important predictors of an individual patient’s risk of death. Additionally, we could not adjust for the density of board-certified medical oncologists, although we used the distance to an NCI-designated cancer center as a surrogate measure for this type of specialist care. Second, data from administrative sources are subject to potential coding errors. Specifically, the accuracy of death certificates is limited by missingness, and, for patients with cancer, the reason for death may be miscoded if patients experience either iatrogenic or malignancy-related complications (eg, death from malignant thromboembolism). Historically, the accuracy of cause of death reporting has been unreliable as <10% of deaths in the US undergo autopsy for confirmation.59 However, this limitation may be mitigated in cancer-related deaths, where previous studies have suggested much higher sensitivity, and positive predictive values ranging from 85%–96%.60,61 Third, recognizing that counties with very low death counts would be censored in accordance with CDC recommendations and that these censored counties would more likely be rural, we aggregated data over 10 years to ensure reliable estimates of mortality rates across as many counties as possible. As a result, we captured >95% of all GI cancer–related deaths in the United States, including >99% of pancreatic and colorectal cancer deaths, even after censoring. Fourth, we recognize that cancer-related mortality rates are linked closely with incidence rates, and that many of the factors associated with mortality may be mediated through increased cancer incidence. Although state-level cancer incidence rates are available, we elected not to adjust for this because we demonstrated significant intrastate mortality variability. Adjustment for state-level incidence may, therefore, result in biased estimates of county-level mortality disparities. Similarly, we adjusted for other covariables using county-level data, although this was only collected cross-sectionally and precluded modeling year and changes in covariables over time. Lastly, our unit of analysis was the county, which is larger than smaller units such as census tract and zip code. Although using counties allowed us to account for covariates for which data are not available at the zip code level (eg, colorectal cancer screening), this did limit our analysis because aggregating zip codes (or census tracts) into counties may have resulted in a loss of nuance for certain variables (eg, zip code clustering of patients of a specific race that are overall low in a county).
In conclusion, this analysis of a decade of mortality data from counties across the United States demonstrated significant intercounty and within state differences in death from esophagus, stomach, pancreas, and colorectal cancer. Potentially modifiable patient and system-related factors are significantly associated with this geographic heterogeneity, and we propose that targeted public health interventions aimed at reducing smoking, curbing the obesity epidemic, and controlling the incidence of diabetes, especially among minorities living in rural counties may reduce the inequities observed in death from GI cancers in the United States.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
More than 35% of cancer-related deaths are from gastrointestinal (GI) malignancies. In the United States, there are significant differences in GI cancer–related mortality based on geographic location, yet the factors accounting for county-level differences in death rates are unclear.
NEW FINDINGS
Data from the Centers for Disease Control and Prevention (2010–2019) highlight that the highest rates of GI cancer–related death are in the Southeastern United States. Cigarette smoking, obesity, diabetes, and rural residence were most strongly associated with intercounty variability in death rates.
LIMITATIONS
Our models are adjusted for county- rather than individual patient-level covariables.
IMPACT
Both modifiable and nonmodifiable patient and structural factors are associated with geographic differences in GI cancer–related mortality. These findings should inform both national and local public health interventions, particularly those targeting smoking cessation and metabolic syndrome, to reduce disparities in GI cancer death.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CHS
Community Health Score
- CI
confidence interval
- GI
gastrointestinal
- IQR
interquartile range
- NCI
National Cancer Institute
Footnotes
Conflicts of interest
The authors disclose no conflicts.
CRediT Authorship Contributions
Christopher Ma, MD, MPH (Conceptualization: Lead; Data curation: Equal; Methodology: Equal; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead). Stephen E. Congly, MD, MSc (Conceptualization: Equal; Writing – review & editing: Supporting). Darius E. Chyou, BS (Formal analysis: Supporting; Writing – review & editing: Supporting). Katherine Ross-Driscoll, PhD, MPH (Formal analysis: Supporting; Writing – review & editing: Supporting). Nauzer Forbes, MD, MSc (Conceptualization: Supporting; Writing – review & editing: Supporting). Erica S. Tsang, MD, MPH (Methodology: Supporting; Writing – review & editing: Supporting). Daniel A. Sussman, MD, MSPH (Methodology: Supporting; Writing – review & editing: Supporting). David S. Goldberg, MD, MSCE (Conceptualization: Equal; Data curation: Lead; Formal analysis: Lead; Writing – review & editing: Equal).
Supplementary Material
To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://doi.org/10.1053/j.gastro.2022.04.019.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020;159:335–349.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidhu R, Powar P, Calla RP, et al. Gastrointestinal cancer mortality rate global trends over the last century. J Clin Oncol 2021;39. 457–457. [Google Scholar]
- 4.Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980-2014. JAMA 2017;317:388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lansdorp-Vogelaar I, Goede SL, Ma J, et al. State disparities in colorectal cancer rates: Contributions of risk factors, screening, and survival differences. Cancer 2015;121:3676–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radley DC, Schoen C. Geographic variation in access to care–the relationship with quality. N Engl J Med 2012;367:3–6. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer-Lindgren L, Mokdad AH, Srebotnjak T, et al. Cigarette smoking prevalence in US counties: 1996-2012. Popul Health Metr 2014;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwyer-Lindgren L, Freedman G, Engell RE, et al. Prevalence of physical activity and obesity in US counties, 2001-2011: a road map for action. Popul Health Metr 2013;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Zhuang H, Liu Y. The association between obesity factor and esophageal caner. J Gastrointest Oncol 2012;3:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106–1114. [DOI] [PubMed] [Google Scholar]
- 11.Joseph DA, King JB, Richards TB, et al. Use of colorectal cancer screening tests by state. Prev Chronic Dis 2018;15:E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. CDC WONDER Scientific Data Documentation. [Google Scholar]
- 13.Goldberg D, Ross-Driscoll K, Lynch R. County differences in liver mortality in the united states: impact of sociodemographics, disease risk factors, and access to care. Gastroenterology 2021;160:1140–1150.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Census Bureau. Urban and rural. [Google Scholar]
- 15.US Census Bureau. Small Area Income and Poverty Estimates (SAIPE) program. [Google Scholar]
- 16.US Census Bureau. Small Area Health Insurance Estimates (SAHIE). [Google Scholar]
- 17.National Cancer Institute. NCI-Designated Cancer Centers. [Google Scholar]
- 18.Health Resources & Services Administration. Area Health Resources Files. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Underlying cause of death. [Google Scholar]
- 20.Dwyer-Lindgren L, Flaxman AD, Ng M, et al. Drinking patterns in US counties from 2002 to 2012. Am J Public Health 2015;105:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwyer-Lindgren L, Mackenbach JP, van Lenthe FJ, et al. Diagnosed and undiagnosed diabetes prevalence by county in the U.S., 1999-2012. Diabetes Care 2016;39:1556–1562. [DOI] [PubMed] [Google Scholar]
- 22.Schold JD, Buccini LD, Kattan MW, et al. The association of community health indicators with outcomes for kidney transplant recipients in the United States. Arch Surg 2012;147:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross K, Patzer RE, Goldberg DS, et al. Sociodemographic determinants of waitlist and posttransplant survival among end-stage liver disease patients. Am J Transplant 2017;17:2879–2889. [DOI] [PubMed] [Google Scholar]
- 24.County Health Rankings 2011. 2017. [University of Wisconsin Population Health Institute; ]. [cited 2017 May 30]. Available at: www.countyhealthrankings.org. [Google Scholar]
- 25.Institute NC. State Cancer Profiles. [Google Scholar]
- 26.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes 2001;20:1–10. [PubMed] [Google Scholar]
- 27.Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease - United States, 2015. MMWR Morb Mortal Wkly Rep 2018;67:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross SH, Mehra MR, Bhatt DL, et al. Rural-urban differences in cardiovascular mortality in the US, 1999-2017. JAMA 2020;323:1852–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo H, Lam AY, Shaheen AA, et al. Urban-rural disparities and temporal trends in peptic ulcer disease epidemiology, treatment, and outcomes in the United States. Am J Gastroenterol 2021;116:296–305. [DOI] [PubMed] [Google Scholar]
- 30.Benchimol EI, Kuenzig ME, Bernstein CN, et al. Rural and urban disparities in the care of Canadian patients with inflammatory bowel disease: a population-based study. Clin Epidemiol 2018;10:1613–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross SH, Califf RM, Warraich HJ. Rural-urban disparity in mortality in the US from 1999 to 2019. JAMA 2021;325:2312–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue. JAMA Surg 2014;149:537–543. [DOI] [PubMed] [Google Scholar]
- 33.Dawkins B, Renwick C, Ensor T, et al. What factors affect patients’ ability to access healthcare? An overview of systematic reviews. Trop Med Int Health 2021;26:1177–1188. [DOI] [PubMed] [Google Scholar]
- 34.Haggerty JL, Roberge D, Levesque JF, et al. An exploration of rural-urban differences in healthcare-seeking trajectories: implications for measures of accessibility. Health Place 2014;28:92–98. [DOI] [PubMed] [Google Scholar]
- 35.Eguia E, Cobb AN, Kothari AN, et al. Impact of the Affordable Care Act (ACA) Medicaid expansion on cancer admissions and surgeries. Ann Surg 2018;268:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam MB, Phelan J, Orav EJ, et al. Medicaid expansion and mortality among patients with breast, lung, and colorectal cancer. JAMA Netw Open 2020;3:e2024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowler-Brown A, Corbie-Smith G, Garrett J, et al. Risk of cardiovascular events and death–does insurance matter? J Gen Intern Med 2007;22:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatana SAM, Bhatla A, Nathan AS, et al. Association of Medicaid expansion with cardiovascular mortality. JAMA Cardiol 2019;4:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews BA, Anderson RC, Nattinger AB. Colorectal cancer screening behavior and health insurance status (United States). Cancer Causes Control 2005;16:735–742. [DOI] [PubMed] [Google Scholar]
- 40.Niroomand E, Kumar SR, Goldberg DS, et al. S1416 Medicaid expansion is associated with decreased gastric cancer incidence. Am J Gastroenterol 2021;116:S650. [Google Scholar]
- 41.Jensen K, Afroze S, Munshi MK, et al. Mechanisms for nicotine in the development and progression of gastrointestinal cancers. Transl Gastrointest Cancer 2012;1:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornelius ME, Wang TW, Jamal A, et al. Tobacco product use among adults - United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villarroel MA, Cha AE, Vahratian A. Electronic cigarette use among U.S. adults, 2018. NCHS Data Brief 2020;365:1–8. [PubMed] [Google Scholar]
- 44.Yoon JM, Son KY, Eom CS, et al. Pre-existing diabetes mellitus increases the risk of gastric cancer: a metaanalysis. World J Gastroenterol 2013;19:936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut 2006;55:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog 2012;51:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. Adult Obesity Maps. [Google Scholar]
- 48.Yuequan J, Shifeng C, Bing Z. Prognostic factors and family history for survival of esophageal squamous cell carcinoma patients after surgery. Ann Thorac Surg 2010;90:908–913. [DOI] [PubMed] [Google Scholar]
- 49.Park JM, Ryu WS, Kim JH, et al. Prognostic factors for advanced gastric cancer: stage-stratified analysis of patients who underwent curative resection. Cancer Res Treat 2006;38:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dell’Aquila E, Fulgenzi CAM, Minelli A, et al. Prognostic and predictive factors in pancreatic cancer. Oncotarget 2020;11:924–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grande M, Milito G, Attina GM, et al. Evaluation of clinical, laboratory and morphologic prognostic factors in colon cancer. World J Surg Oncol 2008;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 53.Lin JS, Perdue LA, Henrikson NB, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021;325:1978–1998. [DOI] [PubMed] [Google Scholar]
- 54.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community-based study, 1994-2006. Gut 2009;58:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000-2010. Front Public Health 2015;3. 51–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashktorab H, Kupfer SS, Brim H, et al. Racial disparity in gastrointestinal cancer risk. Gastroenterology 2017;153:910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar S, Metz DC, Ellenberg S, et al. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology 2020;158:527–536.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med 2001;161:277–284. [DOI] [PubMed] [Google Scholar]
- 60.German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol 2011;35:126–131. [DOI] [PubMed] [Google Scholar]
- 61.Engel LW, Strauchen JA, Chiazze L Jr, et al. Accuracy of death certification in an autopsied population with specific attention to malignant neoplasms and vascular diseases. Am J Epidemiol 1980;111:99–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.