Abstract
Nitro group containing xenobiotics include drugs, cancer chemotherapeutic agents, carcinogens (e.g., nitroarenes and aristolochic acid) and explosives. The nitro group undergoes a six-electron reduction to form sequentially the nitroso-, N-hydroxylamino- and amino-functional groups. These reactions are catalyzed by nitroreductases which, rather than being enzymes with this sole function, are enzymes hijacked for their propensity to donate electrons to the nitro group either one at a time via a radical mechanism or two at time via the equivalent of a hydride transfer. These enzymes include: NADPH-dependent flavoenzymes (NADPH: P450 oxidoreductase, NAD(P)H-quinone oxidoreductase), P450 enzymes, oxidases (aldehyde oxidase, xanthine oxidase) and aldo-keto reductases. The hydroxylamino group once formed can undergo conjugation reactions with acetate or sulfate catalyzed by N-acetyltransferases or sulfotransferases, respectively, leading to the formation of intermediates containing a good leaving group which in turn can generate a nitrenium or carbenium ion for covalent DNA adduct formation. The intermediates in the reduction sequence are also prone to oxidation and produce reactive oxygen species. As a consequence, many nitro-containing xenobiotics can be genotoxic either by forming stable covalent adducts or by oxidatively damaging DNA. This review will focus on the general chemistry of nitroreduction, the enzymes responsible, the reduction of xenobiotic substrates, the regulation of nitroreductases, the ability of nitrocompounds to form DNA adducts and act as mutagens as well as some future directions.
Graphical Abstract

1. INTRODUCTION
The NO2 group is found in a large variety of drugs, e.g., benzodiazepines, androgen receptor antagonists, nonsteroidal anti-inflammatory drugs, antibiotics, antiparasitic agents and chemotherapeutic pro-drugs. As nitrogen oxides are found in the atmosphere, the NO2 group is also introduced into polycyclic aromatic hydrocarbons (PAHs) to produce nitroarenes which have carcinogenic properties.1 The nitro group is also present in natural products, e.g., aristolochic acid2 (responsible for Balkan nephropathy) and other carcinogenic compounds, e.g., nitroquinolines. Due to the amount of military activity in the world, both past and present, explosives such as 2,4,6-trinitrotoluene (TNT) are found in contaminated water, and soil and remediation is necessary, to prevent their toxicity, see Figure 1.
Figure 1.

Common nitrated compounds.
The NO2 group is reduced to the corresponding NH2 group by a variety of enzymes collectively known as nitroreductases (NTRs). As NTRs have reducing capability this activity may be mediated by enzymes that mediate electron transfer that have other functions. NTRs are present in the gut microbiota, all other phyla and in mammalian cells.3–5 Interest exists in phenotyping the enzymes responsible since the introduction of the amine can eliminate the potency of a drug or lead to the activation of chemotherapeutic agents and chemical carcinogens. Air oxidation of the produced amines can generate reactive oxygen species (ROS). Because of these enzymatic and nonenzymatic transformations, the introduction of a NO2 group during drug development is considered a structural alert. However, this should not detract from the fact that many drugs used in the clinic contain this functional group, e.g., the benzodiazepines: clonazepam, flunitrazepam, nimetazepam; the antiandrogens: flutamide and nilutamide; and the NSAID: nimesulide, to name but a few.
This review will focus on the general chemistry of nitroreduction, the enzymes responsible, the reduction of xenobiotic substrates, the regulation of NTRs, the ability of nitrocompounds to form DNA adducts and act as mutagens as well as future directions.
2. GENERAL CHEMISTRY
When the NO2 group is encountered by the microbiota or mammalian cells, it can undergo a 6-electron reduction to the corresponding amine. This reduction can occur via radical chemistry, or by three successive 2-electron steps via the equivalent of a hydride transfer (2H+ + 2e). In the radical mechanism, there is successive production of the nitroanion radical, the nitroso compound, the hydronitroxide radical, the hydroxylamino derivative followed by formation of the amine, Figure 2A. When the reduction occurs by three successive two electron steps, the intermediates are the formation of the nitroso compound with elimination of water, the formation of the hydroxylamino compound followed by formation of the amine, Figure 2B. The reduction of the nitroso group to the hydroxylamino intermediate occurs at a rate 104 faster than the initial reduction of the NO2 group, and thus the nitroso intermediate can be difficult to detect.6 In both the radical mechanism and the 2-electron reduction mechanism, an intermediate of most interest is the hydroxylamine derivative. Here the hydroxyl group can undergo conjugation reactions either with acetate (catalyzed by an N,O-acetyl transferase, NAT)7,8 or sulfate (catalyzed by a sulfotransferase, SULT).9 These conjugation reactions introduce a good leaving group so that covalent DNA adducts can form via the tautomeric nitrenium or carbenium ion. Oxidation of the N-hydroxylamino group in the presence of Cu(II) leads to the formation of Cu(I) and its subsequent oxidation by molecular oxygen to yield superoxide anion. Oxidation of the other intermediate species formed during the nitroreduction by molecular oxygen can also lead to the production of superoxide anion. As superoxide anion can act as a reducing agent, a futile redox cycle can be established.10 The nitroso group can also be trapped with glutathione and the resultant adduct can undergo reduction to the hydroxylamine or amine. It may also rearrange to yield a sulfinic acid amide.11 The formation of N-hydroxylamines has been reported for numerous nitrosoarenes reacting with thiols.12 Similar outcomes are also seen when nitrosobenzene reacts with the Cys residues in hemoglobin, suggesting that the formation of thiol adducts in proteins may be a general phenomenon.11 The sulfinic acid amide can also undergo hydrolysis with acid or alkali to produce a sulfinic acid and amine, and this has been a method to detect 1-aminopyrene bound to hemoglobin.13 This was also developed as a general method to detect hemoglobin adducts of aromatic amines for biomonitoring.14
Figure 2.

Nitroreduction by radical chemistry (a) by successive 2 electron reduction (b).
An important feature of nitroreduction is the change in the electron-withdrawing and -donating properties that result. The Hammett equation predicts that the NO2 group σp = +0.78 (electron-withdrawing) when changed to the NH2 group σp = −0.66 (electron-donating) will have a dramatic effect on the chemistry of a nitroaromatic.15
3. BACTERIAL NITROREDUCTASES
Bacterial NTRs are NAD(P)H-dependent flavin mononucleotide (FMN) enzymes that are found in almost all bacterial genomes.5 The presence of the flavin prosthetic group allows NADPH to donate its two electrons to the flavin which can then be handed off as either two electrons or one electron at a time. There are two types of bacterial NTR. The type 1 NTR is oxygen insensitive, it transfers two electrons at a time, and the nitroso derivative is not detected since the second two electron reduction occurs very rapidly. The type 1 NTR can be further categorized into group A which is NADPH dependent (NrfsA) and group B which is NAD(P)H dependent (NrfsB). These enzymes are homodimers (monomers of 24–30 kDa), and the diaphorase activity is inhibited by dicoumarol. In these enzymes, the formation of the one electron reduced flavin semiquinone anion radical is not observed. These NTRs catalyze ping-pong reactions in which the enzyme cycles between a two electron reduced and oxidized FMN prosthetic group. The NrfsA gene is regulated by the soxRS and mar regulons. SoxRs sense low NAD(P)H:NAD(P)+ ratios and superoxide anion. By contrast the mar regulon is regulated in response to antibiotics, e.g., chloramphenicol and nitrated contaminants.
The type II NTRs are oxygen sensitive and catalyze nitroreduction via the radical mechanism, and futile redox cycling is favored (Figure 2). These enzymes are found in Escherichia coli (E. coli) and Clostridium. The bacterial enzymes are now being used in gene-directed enzyme pro-drug therapy (in which plasmids containing the bacterial NTRs are introduced into solid hypoxic tumors to activate nitro-pro-drug chemotherapeutic agents) and represent an exciting development in targeted drug delivery.16
4. MAMMALIAN NITROREDUCTASES
There are many reductive enzymes found in mammalian cells that are capable of nitroreduction and each is discussed below.
4.1. NADPH: P450 Oxidoreductase (POR).
POR is a membrane associated protein required for electron transport from NADPH to microsomal cytochrome P450 enzymes. The electron flow is from NADPH to FAD to FMN to the recipient P450. The P450 receives one electron at a time in the P450 cycle. The first electron reduces Fe3+ to Fe2+ so that the resting P450 substrate complex binds molecular oxygen, and the second electron reduces the oxygen bound in P450 to the superoxide intermediate. Because the two electrons of NADPH are distributed across the two flavin prosthetic groups in POR, flavin semiquinone anion radicals can pass on their electrons to other recipients so that POR can act as a NTR on its own. An analogy can be drawn between POR with the bacterial type II NTRs. The crystal structure of POR indicates that it has four structural domains including the NADPH binding domain and the FAD binding domain, a connecting domain and the FMN domain which aids separation of the electrons. POR has been implicated as an important NTR for the nitroreduction of flutamide, where the amine can undergo rearrangement to a diimine,17 and it has also been implicated in the nitroreduction of the nitrofurans,3 1-nitropyrene (1-NP),18 3-nitrobenzanthrone (3-NBA),19 and aristolochic acid.20
4.2. FAD-dependent NADPH: Adrenodoxin Reductase.
Adrenodoxin reductase is a mitochondrial enzyme that shuttles electrons from NADPH to mitochondrial P450s, unlike POR it contains only one flavin that donates its electrons to the 2Fe–S cluster of adrenodoxin which in turn passes its electrons to the recipient P450. The electrons in this transport chain can be a source of NTR activity for flutamide.21 Adrenodoxin reductase is also implicated in the reduction of 2-nitrofurans, 5-nitroimidazoles and para-substituted-derivatives of nitrobenzene to the corresponding amines in single electron steps under anaerobic conditions.22
4.3. Cytochrome P450s.
In the P450 reaction cycle the Fe2+ bound molecular oxygen is reduced to superoxide anion radical by POR. The superoxide anion can then act as a reductant for nitroreduction. P450s depending on their substrate binding pockets show distinct preferences for the reduction of different nitro-containing xenobiotics. For example, P450 3A4 has been implicated in the metabolic activation of 3-NBA,23 and P450 1A1, 1A2 and 1B1 have been implicated in the nitroreduction of other diesel exhaust constituents including 1-NP and 1,8-dinitropyrene (1,8-DNP) based on incubating E. coli membranes coexpressing POR and the respective P450 with Salmonella typhimurium TA1535/pK1002 to generate revertants.24 By contrast, mononitropyrenes preferentially underwent ring oxidation and not nitroreduction in MCF-10A cells, and similar products were observed with P4501A1 and P4501B1.25 It is possible that the difference in outcome may be attributed to the presence of POR acting as the NTR, and P4501A1 and 1B1 acting only as a monooxygenase. P4501A1, 1A2 and 1B1 have been implicated in the nitroreduction of aristolochic acid.26 The N-hydroxylamines of 4-aminobiphenyl, 2-naphthylamine, and 2-aminofluorene can be reduced by P450 2S1 under both anaerobic and aerobic conditions.27 Further, phenobarbital induced rat liver microsomes were able to activate nitroflouranthenes by nitroreduction, implicating P450 enzymes.28
4.4. Mitochondrial Nitroreductases.
Single electron transferring flavo-enzymes in the mitochondria have the potential to act as a source of electrons for NTR activity. NADH: ubiquinone reductase (complex 1 of the inner membrane of the mitochondria contains FMN, two Fe2S2 and at least three Fe4S4 clusters) transfers electrons from NADH to ubiquinone-10. The NTR activity of complex 1 is not inhibited by rotenone which binds the N2 Fe4S4 cluster, but is inhibited by NAD+ and ADP-ribose indicating that it is reduced FMN that is responsible for the reduction reaction.29
4.5. Aldehyde Oxidase (AOX).
AOX is predominately found in hepatic cytosol and catalyzes the conversion of an aldehyde in water and oxygen to give the corresponding acid and hydrogen peroxide. It has a similar amino acid sequence to xanthine oxidase. The enzyme requires FAD, molybdenum and has two 2Fe-2S clusters as cofactors. The formation of hydrogen peroxide occurs via the formation of two molecules of superoxide anion which can act as the reductant for NTR. AOX has been implicated in the nitroreduction of 1-NP and 3-nitroflouranthene in a FMN-dependent manner which is inhibited by oxygen.30 AOX is also implicated in the nitroreduction of clonazepam, flunitrazepam, flutamide, nilutamide, nimesulide and nimetazepam.31 AOX is also involved in the reduction of neonicotinoid insecticides which is inhibited by the AOX inhibitors potassium cyanide, menadione and promethazine and stimulated by the electron donor N-methylnicotinamide.32
4.6. Xanthine Oxidase (XO).
XO catalyzes the hydroxylation of hypoxanthine to xanthine and the hydroxylation of xanthine to uric acid. In doing so it generates superoxide anion which can be used to generate the reductant for NTR. It is potently inhibited by allopurinol. XO has been implicated in the nitroreduction of 1-NP, 2-nitrofluorene, 3-nitrofluoranthene and 2,7-dinitrofluorene.30,33
4.7. NADPH: Quinone Oxidoreductase (NQO1).
NQO1 is a FAD linked oxidoreductase which functions as a homodimer where each monomer is 274 amino acids in length. It acts primarily as an NADPH-dependent oxidoreductase for the reduction of quinones to hydroquinones without going through the reactive semiquinone anion radical intermediate. NQO1 cycles between a FAD/FADH2 form which donates electrons to the recipient quinone. It is potently inhibited by dicoumarol. The enzyme catalyzes a bisubstrate ping-pong reaction, and analogies can be drawn with the bacterial type 1 NTR. NQO1 has been implicated as a major NTR for the bioactivation of 3-nitrobenzanthrone34–36 and aristolochic acid.20,36 NQO1 has also been implicated in the reduction of nitroaromatic explosives but had very low kcat values versus its quinone substrates.6
4.8. Aldo-Keto Reductases (AKRs).
AKRs are mainly cytosolic NAD(P)(H)-dependent oxidoreductases which most often function as monomers of 34–37 kDa. They were originally found to catalyze the reduction of aldehydes and ketones to primary and secondary alcohols, respectively by hydride transfer.37 There are 11 proteins with this activity in humans. Of these, AKR1D1 was identified as steroid 5β-reductase expanding the repertoire of substrates to be reduced to those with double bonds in α,β-unsaturated ketones. Studies on the metabolic activation of the chemotherapeutic agent PR-104A using transfected cDNAs showed that surprisingly AKR1C3 and not NQO1 was the major NTR involved in its bioactivation.38 The NTR activity of AKR1C3 has since been expanded to include the nitroarenes, 3-nitrobenzanthrone39 and 1-NP.40 The AKRs catalyze an ordered bi–bi kinetic mechanism in which the NADPH is the first substrate to bind and NADP+ is last to leave.41,42 The final products of these reactions are amines, and the formation of the intermediate nitroso- and hydroxylamino compounds is difficult to observe and may be related to their rapid propensity to undergo further reduction.
5. SUBSTRATES FOR NITROREDUCTASES
Research has focused on identifying the major NTRs responsible for the metabolism and activation of the NO2-group containing substrates. Approaches have compared the catalytic efficiencies of recombinant enzymes, measured nitroreduction in cDNA transfection studies, and assessed the effect of genetic knockdown of target enzymes and the use of enzyme specific inhibitors. In considering the steady-state kinetic parameters for the NTRs, it is important to correlate Km values with the expected exposures to learn whether these activities are relevant. For example, following human exposure to nitro-containing pollutants, these will be at much lower concentrations than those seen following exposure to nitro-containing drugs. Thus, low Km enzymes would be preferred for nitroreduction of pollutants and high Km enzymes would be preferred for the nitroreduction of drugs. However, because many of the NTRs are pluripotent, it is important to compare kinetic parameters for a panel of different substrates for the same enzyme as well as across different enzymes and often such data are lacking. Values presented in Table 1 support the concept that drugs containing nitro groups have high Km values, by contrast lower Km values are observed for the nitroreduction of 3-NBA. Phenotyping the enzyme isoforms responsible can lead to the examination of allelic variants that through loss or gain of function may predispose individuals to susceptibility to nitroxenobiotic toxicity. Interest exists in the induction or repression of the genes for these enzymes since NTR activity may be regulated by the genome or epigenome.
Table 1.
Kinetic Parameters for Nitroreductases with Various Substratesa
| Pollutantb |
Drugc |
|||||||
|---|---|---|---|---|---|---|---|---|
| Kinetic constant | 3-NBA | Clonazepam | Flunitrazepam | Nimetazepam | Flutamide | Nilutamide | Nimesulide | |
| AKR1C1 | Km (μM) | 1.5 ± 0.27 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| kcat (min−1) | 0.012 ± 0.0004 | |||||||
| kcat/Km | 8.00 | |||||||
| AKR1C2 | Km (μM) | 1.1 ± 0.29 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| kcat (min−1) | 0.004 ± 0.0002 | |||||||
| kcat/Km | 3.63 | |||||||
| AKR1C3 | Km (μM) | 1.4 ± 0.43 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| kcat (min−1) | 0.012 ± 0.0007 | |||||||
| kcat/Km | 8.39 | |||||||
| NQO1 | Km (μM) | 8.8 ± 2.5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| kcat (min−1) | 0.058 ± 0.0066 | |||||||
| kcat/Km | 6.59 | |||||||
| AOX | Km (μM) | N.D. | 27.9 ± 1.6 | 62.3 ± 2.3 | 41.2 ± 7.7 | 60.8 ± 6.7 | 199.0 ± 10.9 | 201.4 ± 22.1 |
| Vmax (pmol/min/mg) | 42.5 ± 1.5 | 30.1 ± 0.8 | 55.2 ± 2.1 | 148.9 ± 10.9 | 41.2 ± 0.1 | 1.8 ± 0.1 | ||
| Vmax/Km | 1.5 ± 0.1 | 0.5 ± 0.0 | 1.3 ± 0.2 | 2.4 ± 0.1 | 0.2 ± 0.0 | 0.0089 ± 0.0 | ||
5.1. Nitroaromatics.
5.1.1. Nitroaromatic Explosives.
The toxic effects of TNT are well-known; it causes hemolytic anemia, methemoglobinemia, liver damage, splenomegaly, hyperchloestrolemia and testicular atrophy in rodents.6,43–46 TNT is one of more than 10 nitroaromatic explosives. Nitroreduction may contribute to their elimination and toxicity.6 TNT can undergo mononitroreduction on each of the three nitro groups via the standard, nitroso and hydroxylamino intermediates, giving rise to more than seven identified metabolites. Interest exists in the biodegradation of nitroaromatic explosives as a remediation strategy. There are two main pathways for the reduction of polynitroaromatic compounds in bacteria. First, the reduction of the aromatic ring through the addition of two hydride ions is possible due to the presence of three powerful electron-withdrawing groups to give rise to a dihydride-Mesenheimer complex which is catalyzed by hydride transferases or flavoreductases of the so-called old yellow enzyme family.6 Second, the reduction of the nitro groups can occur as before. In both mechanisms inorganic nitrogen species such as nitrite or ammonium ion are released (the latter by deamination of the reduced amine and subsequent protonation) and maintain bacteria cell growth by providing a source of nitrogen (Figure 3).
Figure 3.

Bacterial degradation of polynitroaromatics.
5.1.2. Nitro-PAH Pollutants.
1-NP, 1,3-dinitropyrene (1,3-DNP), 1,6-dinitropyrene (1,6-DNP), 1,8- DNP, 2,7-dinitroflourene, 6-nitrochrysene (6-NC) and 3-NBA are produced at high temperature from the nitration of PAH by nitrogen oxides in the atmosphere. They are present in the emissions of tail pipes from gasoline and diesel exhaust powered engines. They are ranked by the International Agency for Research on Cancer (IARC) as probable human carcinogens (group 2A) and possible human carcinogens (group 2B) since there is insufficient evidence to indicate that they are known human carcinogens.1
5.1.2.1. Nitropyrenes.
1-NP can be reduced to 1-aminopyrene by the intestinal flora in Fischer rats and was one of the early demonstrations that the gut microbiota could influence the metabolic activation of nitroarenes.47,48 1-NP is metabolically activated in mammals by nitroreduction mediated by xanthine oxidase/hypoxanthine.33 Comparison of the reduction of 1-NP and 1,3-DNP with 1,6- and 1,8-DNP showed that a dichotomy existed. 1,6- and 1,8-Dinitropyrene were reduced more readily by rat liver cytosolic and microsomal fractions and agreed with their relative higher mutagenicity and tumorigenicity.10 It was proposed that the reduction of 1-NP and 1,3-DNP may favor NTRs that transfer single electrons, while mononitroreduction of 1,6-DNP and 1,8-DNP may favor NTRs that transfer two electrons.49 However, the recent report that AKRs can efficiently reduce 1-NP counters this generalization.40 The formation of 1-amino-6-nitropyrene and 1-amino-8-nitropyrene was produced in greater quantities than 1-aminopyrene in human liver cytosolic and microsomal fractions from their respective parent compounds. The resulting DNA adducts were derived from N-acetylation of the N-hydroxylamino intermediate to form the N-acetoxy-intermediates and retained the nitro group at the 6- and 8-positions, implying that the reduction of the second nitro group was not important for DNA adduct formation. The reduction of the second nitro group may be hindered by two contributing mechanisms. First, the introduction of the electron donating 1-amino group may hinder further reduction of the remaining nitro group. Second, the 1-amino-nitropyrenes may undergo oxidation aerobically to the 1-nitrosonitropyrenes to generate superoxide anion, resulting in futile redox cycling of the intermediates.50
5.1.2.2. Nitroflourenes.
Nitroflourenes represent a significant fraction of nitro-PAH found in the environment. The carcinogenicity of 2,7-dinitrofluorene was greater than N-OH-2-acetylaminoflourene in Sprague–Dawley mammary gland.51 2,7-Dintrofluorene is metabolically activated by xanthine oxidase and NQO1.52 However, the level of DNA adducts using xanthine oxidase/hypoxanthine oxidation was 50 times higher when 1-NP was used as the substrate.53
5.1.2.3. 3-Nitrobenzanthrone.
There is considerable interest in the metabolic activation of 3-NBA since it is one of the most mutagenic compounds in the Ames test in the absence of S954 where activation is likely via a bacterial NTR and a N-hydroxylamino-O-acetyl transferase.55 The metabolic activation of 3-NBA was assigned to the NTR activity of POR, POR + P4501A1, and POR + P4501A2 as well as NQO136 (Figure 4). Comparison of the catalytic efficiencies for the reduction of 3-NBA by recombinant AKR1C1, AKR1C2 and AKR1C3 and NQO1 showed that surprisingly the AKR isoforms had comparable catalytic efficiency to NQO1 but that their Km values were 8 times lower, suggesting that they may be the more relevant NTRs for the low concentrations of nitroarenes found in air pollution.39 Using an in-cell fluorescence assay to measure 3-aminobenzanthrone (3-ABA), the pan-AKR1C inhibitor flufenamic acid inhibited 40% of the NTR activity and dicoumarol inhibited 20% of the NTR activity in human A549 lung adenocarcinoma cells.39 Similar results were seen in immortalized human bronchial epithelial cells (HBEC3-KT cells), suggesting that AKRs play a more important role than NQO1 in 3-NBA reduction in human lung cells.39 Similar results have been observed with 1-NP.40 Both 3-NBA and 3-ABA increased the production of ROS in A549 cells as measured by an increase in dichlorofluorescein-diacetate fluorescence that was attenuated by inhibition of P4501A1 with resveratrol and inhibition of complex-I with rotenone, suggesting the possibility of futile redox cycling being mediated by superoxide anion.56
Figure 4.

Metabolic activation of 3-NBA, dA-N6-3ABA = 2-(deoxyadensin-N6-yl)-3-aminobenzanthrone, dG-N2-3ABA = 2-(deoxyguanosin-N2-yl)-3-aminobenzanthrone, and dG-C8-N-3-ABA = N-(deoxyguanosine-8-yl)-3-aminobenzanthrone.
5.1.2.4. 6-Nitrochrysene (6-Nc).
6-NC is one of the most tumorigenic PAHs in the newborn mouse lung57 and is tumorigenic in the rat mammary gland.58 The metabolic activation of 6-NC is intriguing since it can be activated at the level of the parent hydrocarbon to 6-aminochrysene by the standard nitroreduction route.59 Alternatively, it can be activated via monooxygenation to the R,R- and S,S-1,2-trans-dihydrodiols by P450 enzymes60 which are then reduced to the corresponding 6-aminochrysene-1,2-dihydrodiols61 (Figure 5). The dihydrodiols also have the potential to form the corresponding diol-epoxides.
Figure 5.

Metabolic activation of 6-NC.
The tumorigenic activities of 6-NC and its metabolites were evaluated in the newborn mouse lung.57 It was found that 6-nitrosochrysene and 6-aminochyrsene were significantly less active in the lung than 6-NC. However, 1,2-dihydrodiol metabolites derived from 6-NC or 6-aminochrysene had tumorigenic activities comparable to that of 6-NC in the lung. In the liver, 1,2-dihydrodiol-6-NC was more active than 6-NC based on tumor multiplicity. These results favor the hypothesis that 6-NC is metabolically activated by both nitroreduction and ring oxidation in the newborn mouse lung. The ability of 6-NC metabolites to act as mammary gland tumorigens and their potency was ranked as 6-NC > 1,2-dihydrodiol-6-NC > 6-aminochrysene > 6-NC-diol-epoxide > 1,2-dihydrodiol-6-aminochrysene.58 Clearly the metabolic activation of 6-NC in the rat mammary gland differs from that observed in the newborn mouse lung.
When assessing the biological activities of nitroarenes, in addition to species differences, it is also important to consider other factors such as the number, position and the orientation of the nitro group with respect to the aromatic moiety.62–66 For example, when the tumorigenicity of nitrochrysene regioisomers were compared in the newborn mouse lung and liver,67 the carcinogenicity of 1-NC, 2-NC and 3-NC was not significantly different from that of DMSO that was used as the vehicle. The results indicate that nitro substitution at the 6-position of chrysene is critical for strong tumorigenicity. Structure–activity relationships show that nitroreduction is very facile when the NO2 group is parallel to the aromatic ring; however, the rate of reduction is decreased when the NO2 group is perpendicular to the aromatic moiety.64
As AKR1C enzymes possess dihydrodiol dehydrogenase activity in which the dihydrodiol is oxidized to a catechol and then an o-quinone,68 the prospect exists that these enzymes may form 6-NC-1,2-dione or 6-aminochrysene-1,2-dione, but this remains to be determined. 6-NC-1,2-Dione would be a very reactive electrophile due to the powerful electron-withdrawing properties of the nitro group, and thus we are currently focusing on the synthesis of these active electrophiles.
5.2. Nitrofurans.
The nitrofurans include antibiotics and antimicrobials. Antibiotics include difurazone, furazolidone, metronidazole, nifuroline, nifuroxazide and nitrofurantoin, and antimicrobials include furaltadone and furazidine. The general consensus is that their antibacterial action may be derived from the one electron reduction of the nitrogroup to produce the nitroanion radical most likely catalyzed by bacterial ferrodoxins.69,70 The representative compound nitrofurantoin can undergo one electron reduction by POR aerobically, but the rate is much faster when this occurs anaerobically, indicating that the 5-nitrofuran radical disproportionates to yield the nitroso compound and the parent drug.71 Nitrofurazone, nitrofurantoin, furazolidone and metronidazole can all undergo nitroreduction catalyzed by xanthine oxidase where the reductant is superoxide anion.3
5.3. Aristolochic Acid I and II.
Aristolochic acid, AAI (8-methoxy-6-nitro-phenthro-(3,4-d)-1,3-dioxole-5-carboxylic acid) and aristolochic acid, AAII (6-nitro-phenthro-(3,4-d)-1,3-dioxole-5-carboxylic acid) are major components of aristolochic acid derived from Aristolochia species. They are contaminants of wheat grown72 in the Balkans and are also constituents of herbal teas.73 AAI is the cause of a rapidly progressive renal fibrosis and predisposes exposed individuals to urothelial tract carcinoma and bladder cancer.74 AAI can be O-demethylated so that glucuronide and sulfate conjugates can form for elimination, and these conjugates are among the major metabolites.75 These transformations are unavailable for AAII. Upon nitroreduction, the cyclic N-hydroxyaristolactam I and II form which can generate a nitrenium ion for DNA adduct formation (Figure 6). It has been suggested that this nitroreduction occurs directly at the active site of NQO1 and that further involvement of SULT or NAT is not required for activation.76 However, others have found compelling evidence for the involvement of SULTs.77 Comparison of the metabolic activation of AAI and AAII in Wistar rats shows that oxidation of AAI is favored and reduction of AAII is more dominant, whereas coexposure increases the formation of AAI derived DNA adducts.78,79 This finding has been attributed to the inhibition of AAI oxidation by AAII.78 Deoxyadenosine (dA) and deoxyguanosine (dG)-aristolcholic DNA adducts were detected in the renal cortex of endemic nephropathy patients by 32P-postlabeling and were correlated with mutations in p53.74 When DNA adducts were measured in the presence of NQO1 + NADPH by 32P-postlabeling, adduct formation was suppressed by more than 99% by dicoumarol, indicting that NQO1 was responsible for the in vitro formation of DNA adducts.36 However, it is noteworthy that no incubations were reported using recombinant NQO1 and aristolochic acid to quantitate the formation of N-hydroxyaristolactam I and these experiments only identified DNA adducts by cochromatography of the 32P-labeled adducts with authentic standards.36 Subsequently, a UPLC-ESI/MS(n) method was developed to measure the stable DNA adducts 7-(deoxyadenosin-N6-yl) aristolactam I and 7-(deoxyguanosin-N2-yl) aristolactam I.80 The method was applied to tissues of rodents exposed to AAI and the renal cortex of patients with upper urinary tract carcinoma80 as well as to human bladder cells.80,81 In a separate study which measured aristolactam-DNA adducts in a variety of cells via stable isotope dilution LC-MS/MS, the maximum DNA adducts were formed in human kidney cells, yet this cell line had the lowest NQO1 expression of the various cell lines studied. This suggests that other NTRs may play a role in AAI bioactivation.82 Thus, the identification of the NTR responsible for aristolochic acid reduction in human renal cells requires more investigation.
Figure 6.
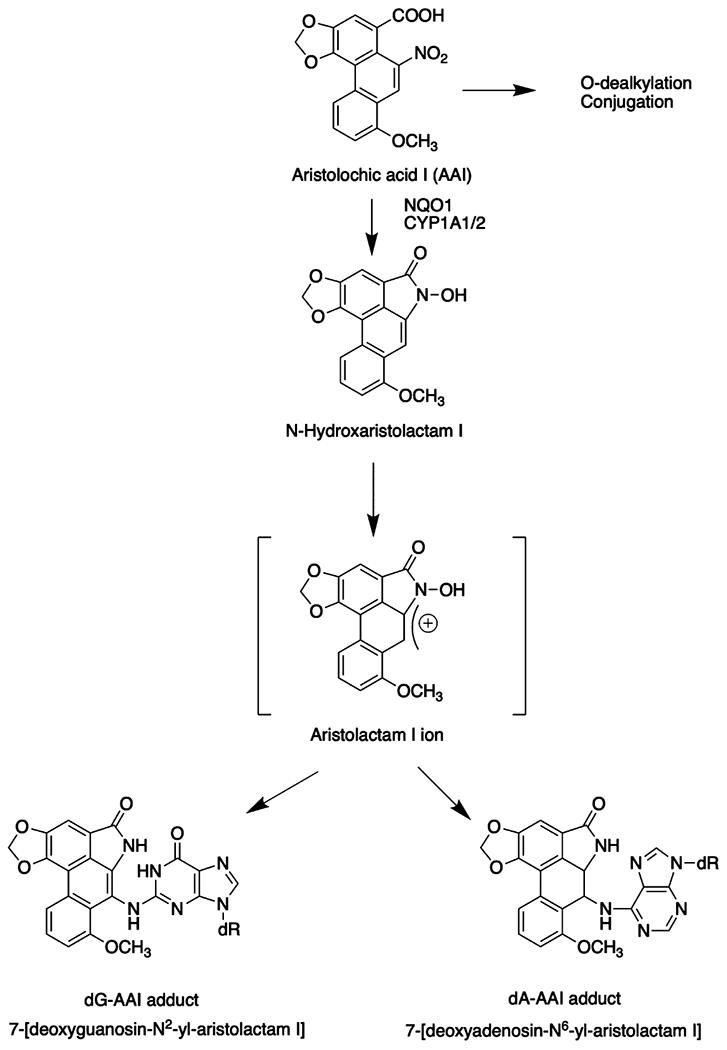
Metabolic activation of aristolochic acid.
5.4. Cancer Chemotherapeutics.
There is growing evidence that the gut microbiota can modulate the efficacy and toxicity of cancer chemotherapeutic agents with three main clinical outcomes: increased efficacy, compromised anticancer effects and mediation of toxicity (for a review, see ref 83). Nitroreduction can also be exploited in solid tumors that are hypoxic. PR-104 is a novel dinitrobenzamide phosphate ester prepro-drug designed to exploit tumor hypoxia. Following ester hydrolysis, bioactivation via one-electron reduction of the nitro group leads to DNA cross-links under anaerobic conditions (hypoxic conditions), where the reduction of the nitro group acts as a “trigger” so that the reduced intermediates activate the mustard for DNA crosslinking. However, PR-104A is also activated under aerobic conditions by nitroreduction. AKR1C3 was implicated in this process where its activity was much greater than NQO1.38 These findings were supported by measuring gain of activity with KEAP-1 RNAi or loss of activity by NRF2 RNAi,38 where AKR1C3 and NQO1 expressions are regulated by the KEAP1-NRF2 pathway. In transfection experiments, AKR1C3 produced 10 times more PR-104A reduced product than NQO1. Under these conditions, the selectivity sought for tumor hypoxia is lost, and the drug proved to be cytotoxic (Figure 7). This in turn has led to the development of analogs that are resistant to nitroreduction by AKR1C3 e.g., SN34507.16 The importance of the NRF2-KEAP1 system in regulating human NTR is discussed in the next section. These studies suggest that AKR1C3 expression could be used as a biomarker of chemotherapeutic sensitivity to PR-104, and this has been confirmed in patients with T cell acute lymphoblastic leukemia.84
Figure 7.

Metabolic activation of PR-104.
5.5. Other Drugs.
A number of drugs, e.g., benzodiazepines: clonazepam, flunitrazepam, nimetazepam; antiandrogens: flutamide and nilutamide; and the nonsteroidal antiinflammatory drug: nimesulide contain a nitro group. Nitroreduction of these drugs occurs via the standard route to the corresponding arylamines. A systematic study of the reduction of these compounds by AOX was performed by discontinuous HPLC assays in which formation of the arylamine was followed. Drug substrates showed a wide range of Km values and catalytic efficiencies varied by 24-fold (Table 1).31 These same panel of drugs have not been systematically examined as substrates for other NTRs.
6. GENE REGULATION
6.1. Bacetrial -SoxRS and MarRAB-Regulons.
The type 1 NTR NADPH-dependent nrfsA gene is part of the soxRS gene battery that responds to oxidative stress and protects against oxidative stress. The E. coli soxRS regulon is composed of 17 genes which respond to the redox state of the cell. When cells are subjected to oxidative stress by superoxide anion, which is produced during the oxidation of the nitroreduced products, the iron-sulfur clusters of SoxR become oxidized and SoxS gene expression is induced (Figure 8A). This induction leads to the transcription and translation of SoxS protein which interacts with MarA regulated by the mar locus.5
Figure 8.

Regulation of NTR expression in bacteria (a) and humans (b).
The mar locus in E. coli is associated with antibiotic resistance and activates genes related to oxidative stress. The mar locus consists of two divergent transcriptional units, marC and marRAB, regulated by the marO operator. In the marRAB operon, the marR gene represses mar gene transcription, whereas the marA gene activates gene expression. The MarR repressor protein binds aromatic and nitroaromatic compounds such as 2,4-dinitrophenol which prevent the binding of MarR to its promoter and allows expression of MarA. MarA is a transcriptional activator which shares 50% amino acid sequence identity with SoxS. SoxS binds to the E. coli nfsA promoter in a manner characteristic of a class I promoter that requires interaction between MarA and SoxS and the C-terminus of alpha-subunit of RNA polymerase to promote transcription. The MarRA/SoxRS regulatory systems likely control the expression of NTR genes in other bacteria as well (Figure 8A).5
6.2. Human Nitroreductases and NRF2-KEAP1.
Several enzymes that catalyze nitroreduction in humans are regulated by the nuclear factor-erythroid factor 2-related factor 2 (NRF2)-Kelch-like ECH-associated protein 1 (KEAP1) which is a major stress sensor that responds to electrophilic and oxidative stress (Figure 8B).85 NRF2 is sequestered from the cytosol by KEAP1 where it is targeted for ubiquitination and proteasomal degradation by a Culin3 containing ubiquitin ligase E3 complex.86,87 Upon activation by electrophiles or ROS, KEAP1 cysteines are modified leading to the release of NRF2 which can escape ubiquitination and translocate to the nucleus, where it binds with its heterodimeric partner small-maf on antioxidant response elements (AREs) in the promoter regions of responsive genes to cause new gene transcription.88,89 Human genes regulated by AREs that display NTR activity include NQO1, AKR1C1, AKR1C2, AKR1C3 and XO, which implies that this stress response system may enhance nitroreduction.90–93 This was demonstrated in the case of 3-NBA where heterozygous (NRF2 +/−) and homozygous (NRF2−/−) A549 cells were treated with the nitroarene. A549 cells constitutively express NRF2 and showed a high level of conversion of 3-NBA to 3-ABA that was reduced in the heterozygous cells and abolished in the homozygous knock down cells;94 similar results were obtained with 1-NP.40 These data suggest that the KEAP1-NRF2 pathway may induce bioactivation of the nitro group on a variety of xenobiotics.
7. ADDUCTOMICS
DNA lesions that arise from exposure to compounds containing the nitro group can include (i) stable covalent adducts formed with nucleobases, (ii) 8-oxo-guanine (8-oxo-G) derived from ROS generation and (iii) DNA strand breaks. The covalent DNA adducts are produced from the reactive nitrenium or carbenium ions which exist as tautomers that result from metabolic activation of the N-hydroxylamino derivative. O-Acetylation or O-sulfonation of the N-hydroxylamino derivative results in the cleavage of the N–O bond to produce the nitrenium and carbenium ion. This has also been observed following N-acetylation, so the reactive intermediate in this case would be an N-acetoxy-N-acetyl derivative, but this is considered a minor pathway with 3-NBA.95 The nitrenium ion preferentially gives rise to C8-dG adducts, whereas the carbenium ion preferentially gives rise to N2-dG and N6-dA adducts as described by Humphreys et al.96 (Figure 9).97 The nitrenium ions are attacked initially by the N7 atom of the purine ring of dG which then rearranges to yield the C8-dG adduct. By contrast, the carbenium ion is preferentially attacked by the N2-exocyclic amino group of dG or the N6-exocyclic amino group of dA;96 see Table 2 for adducts detected. These adducts have been detected in calf thymus DNA with bioactivation,53,98 in mammalian cells,59 and some have been detected in vivo.34,99 Both the acetylated and unacetylated DNA adducts have been observed with 3-NBA.95,100 Historically many of these adducts were detected by 32P-postlabeling with co-chromatography, but this approach has been superseded with stable isotope dilution liquid chromatography tandem mass spectrometry using internal standards for quantitation.
Figure 9.

Formation of nitrenium and carbenium ion DNA derived adducts from 3-NBA, where R1 = H or acetyl.
Table 2.
Nucleoside DNA Adducts Observed with Different Nitrocompounds
| Nitrocompound | Nitrenium Ion Derived | Carbenium Ion Derived |
|---|---|---|
| 1-Nitropyrene | N-(deoxyguanosin-8-yl)-1-aminopyrene98 | 6-(deoxyguanosin-N2-yl)-1-aminopyrene 8-(deoxyguanosin-N2-yl)-1-aminopyrene98 |
| 1,6-Dinitropyrene | N-(deoxyguanosin-8-yl)-1-amino-6-nitropyrene50 | |
| 2,7-Dinitroflourene | N-(deoxyguanosin-8-yl)-2-amino-7-nitrofuran53 | |
| 3-Nitrobenzanthrone | N-(deoxyguanosin-8-yl)-3-aminobenzanthrone149 N-acetyl (deoxyguanosin-8-yl)-3-aminobenzanthrone100 |
2-(deoxyguanosin-N2-yl)-3-aminobenzanthrone149 2-(deoxyadensin-N6-yl)-3-aminobenzanthrone150 |
| 6-Nitrochrysene | N-(deoxyguanosin-8-yl)-6-aminochsysene108 N-(deoxyguanosin-8-yl)-1,2-trans-dihydrodiol-6-aminochrsyene108,109 N-(deoxyadenosin-8-yl)-6-aminochrysene and N-(deoxyinosine-8-yl)-6-aminochrsyene109,151,152 N-(deoxyadenosin-8-yl)-1,2-trans-dihydrodiol-6-aminochrysene109 |
5-(deoxyguanosin-N2-yl)-6-AC153 5-(deoxyguanosin-N2-yl)-1,2-trans-dihydrodiol-6-aminochrysene153 |
| Aristolochic acid | 7-[deoxyadenosin-N6-yl]-aristolactam I, 7-[deoxyadenosin-N6-yl]-aristolactam II and 7-[deoxyguanosin-N2-yl]-aristolactam I)80,99 |
8-oxo-G is a nucleobase that is derived from ROS attack, and for nitroarenes, this ROS can be generated during the oxidation of the reduced products with molecular oxygen to produce superoxide anion radicals. These radicals can dismutate to produce H2O2 and under metal-catalyzed Fenton chemistry can produce hydroxyl radical attack.101 The oxidizing species to produce 8-oxo-G may be hydroxyl radical, a hydroperoxyl radical or singlet oxygen depending on the metal chemistry.101 The N-hydroxylamino-benzanthrone metabolite of 3-NBA increased the amount of 8-oxo-dG that was exacerbated by Cu(II) and NADH in vitro and increased 8-oxo-dG in HL-60 cells in comparison to H2O2 resistant cells.102 8-oxoG can also be formed when cells are exposed to 4-nitroquinoline oxide which may also form via the N7-dG intermediate, e.g., N4-(guanosin-7-yl)-4-aminoquinoline 1-oxide.103,104 8-oxo-G is usually measured as 8-oxo-dG following digestion of DNA to its deoxyribonucleosides. The challenge is that dG itself is prone to adventitious oxidation during sample work up, but this can be handled by using dual-labeled internal standards in liquid chromatography tandem mass spectrometry. In this approach, 8-oxo-[15N5]-dG is used as an internal standard for 8-oxo-dG, and [13C1015N5]-dG is used as an internal standard for the adventitious oxidation of dG.105
8. REPAIR OF DNA ADDUCTS
Two types of DNA adducts can be formed from nitroxenobiotics, covalent bulky DNA adducts and 8-oxo-G. Covalent bulky DNA adducts will be repaired by nucleotide excision repair or transcription coupled repair.106 If left unrepaired, the bulky DNA adducts may be bypassed by trans-lesion bypass DNA polymerases leading to mutation.107 The dG and dA adducts of 6-NC produced via nitroreduction are inefficiently repaired by nucleotide excision repair in human cells in vitro.108,109 These results suggest that these lesions may persist in mammalian tissues, and as a result, the persistence of various DNA adducts was examined in the mammary gland of female rats exposed to a single dose of 6-NC.110 It was found that four DNA adducts were detected in the mammary tissue and decreased over time, but the adduct derived from simple nitroreduction [N-(deoxyguanosin-8-yl)-6-aminochysene] persisted for 2 weeks and that obtained from nitroreduction and ring oxidation derived from trans-1,2-dihydrodiol-6-hydroxylaminochrysene [1,2-trans-dihydrodiol-6-NHOH-chrysene] persisted for 1 week as [N-(dG-8-yl)-1,2-trans-dihydrodiol-6-aminochrysene].
Error-prone and error-free translesion bypass has been observed with site-specifically synthesized DNA adducts of 3-NBA. 2-(Deoxyguanosin-N2-yl)-3-ABA allows for less error prone bypass than N-(deoxyguanosin-8-yl)-ABA which also blocks replication more strongly, but this may be sequence context dependent.111 It remains to be determined whether dG-C8yl adducts always block replication.
Translesion synthesis was examined for oligonucleotides containing the aristolochic derived DNA adducts (dA-AAI and dG-AAI, and dA-AAII and dG-AII adducts). These studies demonstrated that all AAI purine adducts blocked DNA replication and that guanine adducts were not efficient mutagenic lesions. By contrast, adenine adducts indicated a potential for AT to TA transversions due to translesion bypass consistent with activating c-ras mutations found in AA-induced tumors in rats.112 As a result, aristolactam-DNA adducts are being proposed as a biomarker of exposure to aristolochic acid.113
By contrast, 8-oxo-dG is repaired by base excision repair by monofunctional and bifunctional glycosylases. The monofunctional glycosylase is a mis-match repair enzyme (hMYH) which removes the incorrect base A opposite the lesion,114 while the bifunctional glycosylase (hOGG) removes 8-oxo-dG resulting in strand scission.115 8-oxo-dG has been detected in human lung A549 cells exposed to 1-NP.116 In rats exposed to 1,6-DNP, stable DNA adducts and 5-hydroxymethyl-2′-deoxyuridine resulting from oxidative DNA damage were detected in the liver, mammary glands and bladder.117 The detection of 5-hydroxymethyl-2′-deoxyuridine suggests nucleobase modification by ROS other than 8-oxo-dG. In this regard, Breen and Murphy have described over 22 oxidative modification of bases in DNA.118 The N-hydroxylamino-metabolite of 3-NBA also increased 8-oxo-dG formation in vitro in the presence of Cu(II) and NADH.102
9. MUTAGENESIS
9.1. Ames Test.
The nitroarenes are highly mutagenic in the Ames test. 1-NP and 1-nitrosopyrene are mutagenic in Salmonella typhimurium TA1538.119 3-NBA gave 208,000 revertants/nmol in Salmonella typhimurium TA 98 and 6,290,000 revertants in YG1024, a strain that over produces an N-hydroxylamine-O-acetyltransferase.120 By contrast 1,8-DNP gave 257,000 revertants per nmol in TA98 and 4,780,000 revertants in the YG1024 strain.54 In Salmonella, 3-NBA is a strong inducer of frame shift mutations. 3-NBA showed a 3.8-fold and 16.8-fold higher mutagenic activity in Salmonella strains expressing human N-acetyl transferase 1 and human N-acetyl transferase 2.121 These studies not only support the mutagenicity of the nitroarenes but also support the mechanism of activation involving acetylation of the N-hydroxylamino intermediate to form the reactive N-acetoxy-intermediate. A umu Salmonella tester strain has been engineered to harbor a bacteria NTR gene for nitroarene activation where the parental strain contained umuC’-lacz enabled genotoxicity to be quantified by measuring the induction of β-galactosidase.122,123 The assay showed that the strain was highly sensitive to 2-nitrofluorene, 1-nitronaphthalene, 2-nitronaphthalene, 1-NP, m-dinitrobenzene, 4,4′-dinitrobiphenyl, 3-nitrofluoranthene, 3,7-dinitrofluoranthene, 3,9-dinitrofluoranthene, 5-nitroacenaphthene and 2,4-dinitrotoluene, demonstrating the dependence of mutation on NTR.123 For a quantitative comparison of the mutagenicity of nitroarenes in various strains of Salmonella typhimurium, the reader is referred to a published review.124
9.2. Shuttle Vectors.
Shuttle vector plasmids have been used to examine the mutations caused by environmental mutagens and carcinogens. Mutations induced in the plasmids by mutagens reflect mutations in endogenous genes of mammalian cells in which the plasmids are propagated. Using the supF shuttle vector base substitutions were observed with N-acetoxy-N-acetyl-ABA adducts.125 These were observed at G:C sites resulting in G to T transversions (41–51%). In similar experiments, 61% G to T transversions were observed with 1-NP and 64% G to T transversions were observed with 1,6-DNP.126,127
9.3. Mammalian HPRT Assay.
1-NP and 3-nitroflouranthene were tested for mutagenicity on the hypoxanthineguanine phosphoribosyl transferase (HPRT) gene locus in Chinese hamster V79 cells. The mutagenicity was greatly enhanced by the addition of Aroclor induced rat liver S9 or S100.128 Similarly, the mutagenicity of 2-NBA was increased by the transfection of N-acetyl-transferase or SULT1A1 into the V79 cells.129 1,8-DNP was mutagenic in the HPRT locus in CHO cells but not V79 cells, and the difference was attributed to the lack of N-acetyltransferase activity in the V79 cells. Collectively, these data support the standard route of metabolic activation and that the resulting mutagenicity relies on the presence of N-acetyltransferase.130,130 A summary of investigations using V79 cells to study HPRT gene mutagenicity in response to exposure of nitro-containing compounds is included in Table 3. For a thorough account of HPRT mutagenesis studies performed in CHO cells following exposure to nitro-containing compounds, the reader is referred to the work of Li et al.131
Table 3.
Mutagenicity of Nitro-Containing Compounds at the HPRT Locus in V79 Chinese Hamster Lung Cells
| Nitro-Containing Compounda | V79 Cell Derivative Used | Mutant Frequency/106 Cells | Conclusion | Ref | IARC Classification |
|---|---|---|---|---|---|
| 1-Nitropyrene (1-NP) (3 μM) | V79-NH1A2 (V79 cells expressing NAT and CYP1A2) |
83 ± 34 (SD) 1-NP treated cells 7 ± 11 (SD) untreated cells |
1-NP was mutagenic in V79-NH1A2 cells containing NAT and CYP1A2 activity but not in V79-NH cells containing only NAT | 154 | 2A (probable human carcinogen) |
| 1-Nitropyrene (1-NP) (50 μM) | V79 cells in the presence or absence of rat S9 or rat S100 | 18.3 1-NP treated cells 72.8 1-NP treated cells + S9 15.3 1-NP treated cells + S100 10.4 untreated cells |
1-NP was only mutagenic in V79 cells in the presence of S9 but not S100 | 128 | 2A (probable human carcinogen) |
| 1,8-Dinitropyrene (1,8-DNP) (17.1 μm) | V79 (no additional enzymes expressed) | 14.2—20.2 1,8-DNP treated cells 1.4—27.6 in untreated cells |
1,8-DNP was not mutagenic in V79 cells at the conditions tested | 130 | 2B (possible human carcinogen) |
| 2-Nitrofluorene (2-NF) (10 μM, 30 μM) | V79-NH1A2, V79-NH2C9 (V79 cells expressing NAT activity and CYP1A2, or V79 cells expressing NAT activity and CYP2C9, respectively) | No statistically significant difference in V79-NH1A2 cells and V79-NH2C9 cells exposed to 2-NF when compared to untreated cells. The same was shown for V79-NH cells that contain NAT but do not express CYP1A2 or CYP2C9 | 2-NF was not mutagenic in V79-NH1A2, V79-NH2C9 or V79-NH cells at the conditions tested | 154 | 2B (possible human carcinogen) |
| 2-Nitrobenzanthrone (2-NBA) (1 μM) | V79-hSULT1A1 (V79 cells that express human SULT1A1) | 150 2-NBA treated cells 0 in V79 hSULT1A1 in untreated cells 0 in V79 cells lacking hSULT1A1 or hNAT2 |
SULT1A1 contributed to mutagenicity at the HPRT locus in cells exposed to 2-NBA. | 129 | N/A |
| 2-Nitrobenzanthrone (2-NBA) (1 μM) | V79-hNAT2 (V79 cells that express human NAT2) | 110 in 2-NBA treated cells 0 in V79-hNAT2 in untreated cells 0 in V79 cells lacking hSULT1A1 or hNAT2 |
NAT2 contributed to mutagenicity at the HPRT locus in cells exposed to 2-NBA. | 129 | N/A |
| 3-Nitrofluoranthene (3-NFA) (100 μM) | V79 cells in the presence or absence of rat S9 or rat S100 | 14.9 Cells treated with 3-NFA 25.0 Cells treated with 3-NFA + S9 175.7 Cells treated with 3-NFA + S100 10.4 Untreated cells |
3-NFA was mutagenic in V79 cells in the presence of S100 more so than in the presence of S9 | 128 | 3 (human carcinogenicity not classifiable) |
| 8-Nitrofluoranthene (8-NFA) (25 μM) | V79 cells in the presence or absence of rat S9 or rat S100 | 12.8 Cells treated with 8-NFA 21.5 Cells treated with 8-NFA + S9 1378 Cells treated with 8-NFA + S100 10.4 Untreated cells |
8-NFA was mutagenic in V79 cells in the presence of S100 more so than in the presence of S9 | 128 | N/A |
| Aristolochic acids (38% aristolochic acid I, 58% aristolochic acid II) (40 μM) | V79-hSULT1A1 (V79 cells that express human SULT1A1) | 250 in treated cells. 80 in V79 cells lacking hSULT1A1 0 in V79-hSULT1A1 in untreated cells |
SULT1A1 contributed to mutagenicity at the HPRT locus in cells exposed to a mixture of aristolochic acids | 155 | 1 (carcinogenic to humans) |
| Aristolochic acids (38% aristolochic acid I, 58% aristolochic acid II) (40 μM) | V79-hCYP2E1-hSULT1A1 (V79 cells that coexpress human CYP2E1 and SULT1A1) | 1300 in treated cells 350 in V79-hCYP2E1-hSULT1A1 cells treated additionally with 10 μM pentachlorophenol, a SULT1A1 inhibitor, 0 in V79-hCYP2E1-hSULT1A1 untreated cells |
Coexpression of SULT1A1 and CYP2E1 increased mutagenicity at the HPRT locus in cells exposed to a mixture of aristolochic | 155 | 1 (carcinogenic to humans) |
| Metronidazole, nimorazole, tinidazole, niridazole (each from 0 to 1 mM) | V79 cells and V79 cells cultured with freshly isolated and uninduced Sprague–Dawley male rat hepatocytes | No statistically significant difference for any of the compounds in V79 cells or V79 cells cultured with rat hepatocytes | Metronidazole, nimorazole, tinidazole, and niridazole were not mutagenic at the conditions tested. | 156 | Metronidazole (2B); nimorazole (N/A); tinidazole (N/A); niridazole (2B) |
The concentrations reported correspond to the highest concentrations used in the studies or the concentrations giving maximum or near maximum response observed. N/A in the IARC classification column indicates that the compound has not been classified by IARC for human carcinogenicity.
9.4. Trans-Gene Mutation.
The in vivo mutagenicity of 6-NC in female transgenic rats has been reported.110 The most common mutations in mammary tissues were GC → TA, GC → CG, AT → GC and AT → TA; the structures of the 6-NC-DNA adducts in the mammary tissues are consistent with these mutational patterns. Collectively this work defines the relationship between structures of DNA adducts, mutagenesis and carcinogenesis in the target organ (mammary gland) of rats treated with 6-NC.110 To identify the ultimate mutagenic metabolites, the mutational profile of 6-NC in the rat mammary gland with that of five of its known metabolites was compared in the cII gene of lacI mammary epithelial cells in vitro.132 The metabolite whose mutational profile was most similar to that of 6-NC in vivo was 1,2-trans-dihydrodiol-6-NHOH-chrysene derived from nitroreduction and ring oxidation, suggesting that this metabolite is the ultimate genotoxic metabolite of 6-NC.
Aristolochic acid was mutagenic in the BigBlue transgenic rat, and a strong linear relationship was observed between dose and mutation frequency in the transgenic cll gene. Sequence analysis showed that A:T to T:A transversions were the dominant mutations, consistent with the occurrence of the persistent 7-(deoxyadenosin-N6-yl)aristolactam adduct on the nontranscribed strand.133 These studies were extended to detect mutations in kidney and liver and correlate them with the occurrence of the major aristolochic acid adducts.99 Further, aristolochic acid was mutagenic in the lambda/lacz transgenic mouse. Mutation frequency was higher in target organs and sequence analysis of the cII gene also revealed a predominance of A:T to T:A transversions.134
9.5. Mutational Signatures in Humans.
The mutations observed in the mammary tissues of rats treated with 6-NC110 have also been reported in significant percentages among the mutations in the p53 gene in human breast tumors.135 Although the rats treated with 6-NC had comparable mutations in normal (adjacent) and tumor tissues of the mammary gland,110 it is possible that the normal tissue would have developed tumors if given time. Therefore, the possibility of 6-NC as a contributor to breast cancer development cannot be ruled out and deserves further investigation.
There have been significant inroads in developing mutational signatures in human tumors and relating them to environmental exposures.136 This has been achieved with aristolochic acid and provides a route to link exposures to DNA adducts to mutations. Aristolochic acid forms aristolactam-DNA adducts in the renal cortex and serves as a biomarker for internal exposure.113 DNA adducts are recognized not only as biomarkers for internal exposure but also as biomarkers for cancer risk assessment. The formation of the 7-(deoxyadenosin-N6-yl)aristolactam adduct accounts for A:T to T:A mutations that occur at hotspots on TP53 on codons 131 and 179 and the 5’-AG acceptor site of intron 6.137 UPLC-ESI/MS3 methods have been developed to detect the 7-(deoxyadenosin-N6-yl)aristolactam adduct in renal tissues and formalin-fixed paraffin embedded tissue.138 However, non-invasive and less expensive methodology will be required for biomonitoring. In this regard, a fluorescence-based assay has been developed for adduct detection.139
10. FUTURE DIRECTIONS
10.1. Challenges in the NTR Field.
One of the challenges in the NTR field is the assignment of detailed steady-state kinetic parameters for a panel of substrates for each NTR. Without this information, the enzyme which is preferred for each substrate is unknown. In addition, with a variety of one electron and two electron reducing activities available, it is difficult to determine which is the most dominant enzyme in a particular cell setting. This can be addressed with NTR-specific inhibitors and RNAi approaches. This is an important undertaking if differential susceptibility to nitro-xenobiotics is to be elucidated. For example, both POR140 and NQO1141 have allelic variants that have a high minor allelic frequency which cause a decrease in activity.
The question becomes why are there so many NTRs. It is unlikely that this activity arose to deal with nitrocompounds per se, but it more than likely reflects the redox half potential that exists between electron donor and recipient. Many nitroxenobiotics are drugs, and with the abundance of NTR in the gut microbiota, the efficacy of these drugs could be attenuated by microbial NTRs and affect individual response to drug therapy. A systematic examination of type 1 and type 2 NTRs and their ability to affect drug metabolism needs to be undertaken. The use of plasmids encoding these NTRs in transfection studies in hypoxic tumors is an exciting development in cancer chemotherapeutics and may be an important avenue for targeted therapeutics in difficult to treat tumors.16
Although there has been success relating aristolochic acid exposure with mutational signatures, it remains unknown if low levels of exposure to nitro-containing PAH and other environmental or dietary contaminants can be firmly linked to mutational signatures. Rats developing mutations when exposed to 6-NC were exposed to 100 μmol or 200 μmol of 6-NC once.110 Future studies should use a wider range of doses, inclusive of doses in the ng or μg range, to determine the extent to which relevant mutations still form. Together, evidence of mutational signatures at low levels of exposure coupled with the existence of low Km values for pollutants for NTRs would provide plausibility for the role of low exposure to nitro-containing pollutants in cancer causation.
10.2. Challenges in Biomonitoring of Environmental NO2-PAHs.
Human risk assessment to nitro-PAHs has not been clearly defined, despite their widespread occurrence in the environment and their possible involvement in the etiology of some human cancers.1 However, to assess their carcinogenic potential in humans requires more accurate exposure information on the internal dose than can be obtained from atmospheric measurements or surrogate exposure estimates such as employment records. Clearly, active biomonitoring programs aimed at assessing the internal dose in humans following exposure to sources (e.g., diesel exhaust) containing NO2-PAHs are urgently needed. Several methods are available for determining exposure uptake and metabolic activation of genotoxic carcinogens in humans. As described above, DNA adducts are regarded as the most direct biomarkers for genotoxicity and can be used for risk assessment. In contrast to DNA adducts, there is no repair mechanisms for protein adducts formed from active electrophiles such as intermediates (e.g., nitroso-PAHs) derived from nitroreduction of nitro-PAHs. Such protein adducts could accumulate during the lifespan of the protein.142–145 Nucleophilic sites in hemoglobin and serum albumin can react with electrophiles leading to the formation of carcinogen-protein adducts.14,143,146 In fact, previous preclinical studies conducted by us and by others have reported on the development of analytical methods to detect and quantify hemoglobin and serum albumin adducts derived from 1-NP, a major constituent of diesel exhaust.147,148 A number of hemoglobin adducts derived from 1-NP and 6-NC were found in blood drawn from nonsmoking male garage workers in Germany and interindividual variations were observed.13 Collectively, these results suggest that nitro-PAHs are widespread environmental contaminants and thus further development and fine-tuning of existing analytical methods to monitor human exposure to this class of carcinogens should be of high priority in future studies. However, surveillance programs that may be dependent on sophisticated and expensive mass spectrometry provide a challenge to this approach unless mass spectrometry assays can be limited to validate alternate less expensive assays.
ACKNOWLEDGMENTS
Supported by National Institutes Health grants P30-ES013508 (TMP) and R01-ES029294 (TMP and KEB) and by Training Grant T32-ES019851 (ALS) awarded by the National Institute of Environmental Health Sciences.
Biographies
Biographies
Trevor M. Penning, Ph.D., is the Thelma Brown and Henry Charles Molinoff Professor of Pharmacology, Professor of Biochemistry and Biophysics and OB/GYN, and Director of the Center of Excellence in Environmental Toxicology (CEET) at the University of Pennsylvania, Perelman School of Medicine. CEET is a P30 Environmental Health Sciences Core Center funded by the National Institute of Environmental Health Sciences. Dr. Penning is internationally recognized for his research on how hormones and chemicals cause cancer. He has published over 290 peer-reviewed articles. He is a member of The Johns Hopkins Society of Scholars, and he is a Fellow of the American Chemical Society.
Anthony L. Su received a Ph.D. in Toxicology from the University of Michigan, Ann Arbor. He is currently a postdoctoral researcher in the laboratory of Trevor M. Penning at the University of Pennsylvania. His research interests include the enzymology of nitroreduction of various nitro-containing compounds and regulation by cellular signaling mechanisms, including but not limited to NRF2/KEAP1. His interests include the use of high-performance liquid chromatography and liquid chromatography-mass spectrometry methods to characterize metabolites and products in nitroreduction and evaluation of carcinogenesis. He performed the work on the role of AKRs and NRF2 in 1-NP nitroreduction discussed in this review.
Karam El-Bayoumy, Ph.D. is a distinguished professor of Biochemistry and Molecular Biology and the Associate Director for Basic Research in the Cancer Institute (Penn State College of Medicine). His goals are to understand how chemicals found in tobacco, diet and the environment cause cancer and to develop means of cancer prevention. His laboratory is considered a leader in the field of NO2-PAH carcinogenesis; he co-chaired the WHO task group in Germany (2001) to evaluate the human risk associated with exposure to NO2-PAH and served as a member of the IARC Monograph 105: Diesel and Gasoline Exhausts and some Nitroarenes, 2013.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.chemrestox.2c00175
The authors declare the following competing financial interest(s): Member Expert Panel Research Institute of Fragrance Materials.
This paper is one of a series to appear in a special issue of Chemical Research in Toxicology to acknowledge 25 years of service to the journal by its inaugural Editor-in-Chief Dr. Larry J. Marnett.
Contributor Information
Trevor M. Penning, Center of Excellence in Environmental Toxicology and Department of Systems Pharmacology and Translational Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6160, United States
Anthony L. Su, Department of Systems Pharmacology and Translational Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6160, United States
Karam El-Bayoumy, Department of Biochemistry and Molecular Biology, Penn State College of Medicine, Pennsylvania State University, Hershey, Pennsylvania 17033-2360, United States.
REFERENCES
- (1).Benbrahim-Tallaa L; Baan RA; Grosse Y; Lauby-Secretan B; El Ghissassi F; Bouvard V; Guha N; Loomis D; Straif K Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012, 13 (7), 663–664. [DOI] [PubMed] [Google Scholar]
- (2).Sidorenko VS; Attaluri S; Zaitseva I; Iden CR; Dickman KG; Johnson F; Grollman AP Bioactivation of the human carcinogen aristolochic acid. Carcinogenesis 2014, 35, 1814–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Bartel LC; Montalto de Mecca M; Castro JA Nitroreductive metabolic activation of some carcinogenic nitro heterocyclic food contaminants in rat mammary tissue cellular fractions. Food Chem. Toxicol 2009, 47 (1), 140–144. [DOI] [PubMed] [Google Scholar]
- (4).McCoy EC; Rosenkranz HS; Mermelstein R Evidence for the existence of a family of bacterial nitroreductases capable of activating nitrated polycyclics to mutagens. Environ. Mutagen 1981, 3 (4), 421–427. [DOI] [PubMed] [Google Scholar]
- (5).Roldan M; Perez-Reinado E; Castillo F; Moreno-Vivian C Reduction of polynitroaromatic compounds: the bacterial nitroreductases. FEMS Microbiol Rev. 2008, 32 (3), 474–500. [DOI] [PubMed] [Google Scholar]
- (6).Nemeikaite-Ceniene A; Milukiene V; Sarlauskas J; Maldutis E; Cenas N Chemical spects of cytotoxicity of nitroaromaatic explosives: a review. CHEMIJA 2006, 17 (2–3), 34–41. [Google Scholar]
- (7).Hein DW Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mut. Res 2002, 506–507, 65–77. [DOI] [PubMed] [Google Scholar]
- (8).Savulescu MR; Mushtaq A; Josephy PD Screening and charactaerizing human NAT2 variants. Methods Enzymol 2005, 400, 192–215. [DOI] [PubMed] [Google Scholar]
- (9).Glatt H; Sabbioni G; Monien BH; Meinl W Use of genetically manipulated Salmonella typhimurium strains to evaluate the role of human sulfotransferases in the bioactivation of nitro- and aminotoluenes. Environ. Mol. Mutagen 2016, 57, 299–311. [DOI] [PubMed] [Google Scholar]
- (10).Djuric Z; Potter DW; Heflich RH; Beland FA Aerobic and anaerobic reduction of nitrated pyrenes in vitro. Chem. Biol. Interact 1986, 59 (3), 309–324. [DOI] [PubMed] [Google Scholar]
- (11).Neumann H-G Analysis of hemoglobin as a dose monitor for alkylating and arylating agents. Arch. Toxicol 1984, 56, 1–6. [DOI] [PubMed] [Google Scholar]
- (12).Eyer P; Gellemann D Reactions of Nitrosoarenes with SH Groups. In The Chemistry of Amino, Nitroso, Nitro and Related Groups; Patai S, Ed.; Wiley: Hoboken, NJ, 1996; Vol. 2, pp 999–1039. [Google Scholar]
- (13).Zwirner-Baier I; Neumann HG Polycyclic nitroarenes (nitro-PAHs) as biomarkers of exposure to diesel exhaust. Mutat. Res 1999, 441, 135–144. [DOI] [PubMed] [Google Scholar]
- (14).Bryant MS; Vineis P; Skipper PL; Tannenbaum SR Hemoglobin adducts of aromatic amines: associations with smoking status and type of tobacco. Proc. Natl. Acad. Sci. U. S. A 1988, 85 (24), 9788–9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Denny W Nitroaroamtic Hypoxia-Activated Prodrugs for Cancer Therapy. Pharmaceuticals (Basel) 2022, 15, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mowday AM; Ashoorzadeh A; Williams EM; Copp JN; Silva S; Bull MR; Abbattista MR; Anderson RF; Flanagan JU; Guise CP; et al. Rational design of an AKR1C3-resistant analog of PR-104 for enzyme-prodrug therapy. Biochem. Pharmacol 2016, 116, 176–187. [DOI] [PubMed] [Google Scholar]
- (17).Wen B; Coe KJ; Rademacher P; Fitch WL; Monshouwer M; Nelson SD Comparison of in vitro bioactivation of flutamide and its cyano analogue: evidence for reductive activation by human NADPH:cytochrome P450 reductase. Chem. Res. Toxicol 2008, 21 (12), 2393–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Saito K; Kamataki T; Kato R Participation of cytochrome P-450 in reductive metabolism of 1-nitropyrene by rat liver microsomes. Cancer Res. 1984, 44 (8), 3169–3173. [PubMed] [Google Scholar]
- (19).Arlt VM; Stiborova M; Hewer A; Schmeiser HH; Phillips DH Human enzymes involved in the metabolic activation of the environmental contaminant 3-nitrobenzanthrone: evidence for reductive activation by human NADPH:cytochrome p450 reductase. Cancer Res. 2003, 63 (11), 2752–2761. [PubMed] [Google Scholar]
- (20).Stiborová M; Frei E; Hodek P; Wiessler M; Schmeiser HH Human hepatic and renal microsomes, cytochromes P450 1A1/2, NADPH:cytochrome P450 reductase and prostaglandin H synthase mediate the formation of aristolochic acid-DNA adducts found in patients with urothelial cancer. Int. J. Cancer 2005, 113 (2), 189–197. [DOI] [PubMed] [Google Scholar]
- (21).Nemeikaitė-Čėnienė A; Marozienė A; Misevičienė L; Tamulienė J; Yantsevich AV; Čėnas N 5Flavoenzyme-catalyzed single-electron reduction of nitroaromatic antiandrogens: implications for their cytotoxicity. Free Radic Res. 2021, 55 (3), 246–254. [DOI] [PubMed] [Google Scholar]
- (22).Marcinkeviciene J; Cenas N; Kulys J; Usanov SA; Sukhova NM; Selezneva IS; Gryazev VF Nitroreductase reactions of the NADPH: adrenodoxin reductase and the adrenodoxin complex. Biomed Biochim Acta 1990, 49 (4), 167–172. [PubMed] [Google Scholar]
- (23).Bieler CA; Arlt VM; Wiessler M; Schmeiser HH DNA adduct formation by the environmental contaminant 3-nitrobenzanthrone in V79 cells expressing human cytochrome P450 enzymes. Cancer Lett. 2003, 200 (1), 9–18. [DOI] [PubMed] [Google Scholar]
- (24).Yamazaki H; Hatanaka N; Kizu R; Hayakawa K; Shimada N; Guengerich FP; Nakajima M; Yokoi T Bioactivation of diesel exhaust particle extracts and their major nitrated polycyclic aromatic hydrocarbon components, 1-nitropyrene and dinitropyrenes, by human cytochromes P450 1A1, 1A2, and 1B1. Mutat. Res 2000, 472 (1–2), 129–138. [DOI] [PubMed] [Google Scholar]
- (25).Sun YW; Guengerich FP; Sharma AK; Boyiri T; Amin S; el-Bayoumy K Human cytochromes P450 1A1 and 1B1 catalyze ring oxidation but not nitroreduction of environmental pollutant mononitropyrene isomers in primary cultures of human breast cells and cultured MCF-10A and MCF-7 cell lines. Chem. Res. Toxicol 2004, 17 (8), 1077–1085. [DOI] [PubMed] [Google Scholar]
- (26).Milichovský J; Bárta F; Schmeiser HH; Arlt VM; Frei E; Stiborová M; Martínek V Active Site Mutations as a Suitable Tool Contributing to Explain a Mechanism of Aristolochic Acid I Nitroreduction by Cytochromes P450 1A1, 1A2 and 1B1. Int. J. Mol. Sci 2016, 17 (2), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wang K; Guengerich FP Reduction of aromatic and heterocyclic aromatic N-hydroxylamines by human cytochrome P450 2S1. Chem. Res. Toxicol 2013, 26 (6), 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Belisario MA; Pecce R; Della Morte R; Arena AR; Cecinato A; Ciccioli P; Staiano N Characterization of oxidative and reductive metabolism in vitro of nitrofluoranthenes by rat liver enzymes. Carcinogenesis 1990, 11 (2), 213–218. [DOI] [PubMed] [Google Scholar]
- (29).Bironaite DA; Cenas NK; Kulys JJ The rotenone-insensitive reduction of quinones and nitrocompounds by mitochondrial NADH:ubiquinone reductase. Biochim. Biophys. Acta 1991, 1060 (2), 203–209. [DOI] [PubMed] [Google Scholar]
- (30).Bauer SL; Howard PC Kinetics and cofactor requirements for the nitroreductive metabolism of 1-nitropyrene and 3-nitrofluoranthene by rabbit liver aldehyde oxidase. Carcinogenesis 1991, 12 (9), 1545–1549. [DOI] [PubMed] [Google Scholar]
- (31).Ogiso T; Fukami T; Mishiro K; Konishi K; Jones JP; Nakajima M Substrate selectivity of human aldehyde oxidase 1 in reduction of nitroaromatic drugs. Arch. Biochem. Biophys 2018, 659, 85–92. [DOI] [PubMed] [Google Scholar]
- (32).Dick RA; Kanne DB; Casida JE Identification of aldehyde oxidase as the neonicotinoid nitroreductase. Chem. Res. Toxicol 2005, 18 (2), 317–323. [DOI] [PubMed] [Google Scholar]
- (33).Howard PC; Beland FA Xanthine oxidase catalyzed binding of 1-nitropyrene to DNA. Biochem. Biophys. Res. Commun 1982, 104 (2), 727–732. [DOI] [PubMed] [Google Scholar]
- (34).Stiborova M; Dracinska H; Mizerovska J; Frei E; Schmeiser HH; Hudecek J; Hodek P; Phillips DH; Arlt VM The environmental pollutant and carcinogen 3-nitrobenzanthrone induces cytochrome P450 1A1 and NAD(P)H:quinone oxidoreductase in rat lung and kidney, thereby enhancing its own genotoxicity. Toxicology 2008, 247 (1), 11–22. [DOI] [PubMed] [Google Scholar]
- (35).Stiborova M; Martinek V; Svobodova M; Sistkova J; Dvorak Z; Ulrichova J; Simanek V; Frei E; Schmeiser HH; Phillips DH; et al. Mechanisms of the different DNA adduct forming potentials of the urban air pollutants 2-nitrobenzanthrone and carcinogenic 3-nitrobenzanthrone. Chem. Res. Toxicol 2010, 23 (7), 1192–1201. [DOI] [PubMed] [Google Scholar]
- (36).Stiborova M; Frei E; Schmeiser HH; Arlt VM; Martinek V Mechanisms of enzyme-catalyzed reduction of two carcinogenic nitro-aromatics, 3-nitrobenzanthrone and aristolochic acid I: Experimental and theoretical approaches. Int. J. Mol. Sci 2014, 15 (6), 10271–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Penning TM; Drury JE Human aldo-keto reductases: Function, gene regulation, and single nucleotide polymorphisms. Arch. Biochem. Biophys 2007, 464 (2), 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Guise CP; Abbattista MR; Singleton RS; Holford SD; Connolly J; Dachs GU; Fox SB; Pollock R; Harvey J; Guilford P; et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010, 70, 1573–1584. [DOI] [PubMed] [Google Scholar]
- (39).Murray JR; Mesaros CA; Arlt VM; Seidel A; Blair IA; Penning TM Role of Human Aldo-Keto Reductases in the Metabolic Activation of the Carcinogenic Air Pollutant 3-Nitrobenzanthrone. Chem. Res. Toxicol 2018, 31 (11), 1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Su AL; Penning TM Role of nuclear factor erythroid 2-related factor 2 and aldo-keto reductases in the metabolic activation of 1-nitropyrene in human lung cells (A549 and HBEC3-KT). Proceedings from the 61st Society of Toxicology Annual Meeting and ToxExpo, March 27–31, 2022, San Diego, CA; Society of Toxicology: Reston, VA, 2022. [Google Scholar]
- (41).Jin Y; Penning TM Multiple steps determine the overall rate of the reduction of 5alpha-dihydrotestosterone catalyzed by human type 3 3alpha-hydroxysteroid dehydrogenase: implications for the elimination of androgens. Biochemistry 2006, 45 (43), 13054–13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Cooper WC; Jin Y; Penning TM Elucidation of a complete kinetic mechanism for a mammalian hydroxysteroid dehydrogenase (HSD) and identification of all enzyme forms on the reaction coordinate: the example of rat liver 3alpha-HSD (AKR1C9). J. Biol. Chem 2007, 282 (46), 33484–33493. [DOI] [PubMed] [Google Scholar]
- (43).Dilley JV; Tyson CA; Spanggord RJ; Sasmore DP; Newell GW; Dacre JC Short-term oral toxicity of 2,4,6-trinitrotoluene in mice, rats, and dogs. J. Toxicol Environ. Health 1982, 9 (4), 565–585. [DOI] [PubMed] [Google Scholar]
- (44).Levine BS; Furedi EM; Gordon DE; Barkley JJ; Lish PM Toxic interactions of the munitions compounds TNT and RDX in F344 rats. Fundam. Appl. Toxicol 1990, 15 (2), 373–380. [DOI] [PubMed] [Google Scholar]
- (45).Levine BS; Furedi EM; Gordon DE; Lish PM; Barkley JJ Subchronic toxicity of trinitrotoluene in Fischer 344 rats. Toxicology 1984, 32 (3), 253–265. [DOI] [PubMed] [Google Scholar]
- (46).Wyman JF; Serve MP; Hobson DW; Lee LH; Uddin DE Acute toxicity, distribution, and metabolism of 2,4,6-trinitrophenol (picric acid) in Fischer 344 rats. J. Toxicol Environ. Health 1992, 37 (2), 313–327. [DOI] [PubMed] [Google Scholar]
- (47).El-Bayoumy K; Reddy B; Hecht SS Identification of ring oxidized metabolites of 1-nitropyrene in the feces and urine of germfree F344 rats. Carcinogenesis 1984, 5 (10), 1371–1373. [DOI] [PubMed] [Google Scholar]
- (48).El-Bayoumy K; Sharma C; Louis YM; Reddy B; Hecht SS The role of intestinal microflora in the metabolic reduction of 1-nitropyrene to 1-aminopyrene in conventional and germfree rats and in humans. Cancer Lett. 1983, 19 (3), 311–316. [DOI] [PubMed] [Google Scholar]
- (49).Eddy EP; McCoy EC; Rosenkranz HS; Mermelstein R Dichotomy in the mutagenicity and genotoxicity of nitropyrenes: apparent effect of the number of electrons involved in nitroreduction. Mutat. Res 1986, 161 (2), 109–111. [DOI] [PubMed] [Google Scholar]
- (50).Beland FA The metabolic activation and DNA adducts of dinitropyrenes. Res. Rep. Health Eff Inst 1986, 4, 3–30. [PubMed] [Google Scholar]
- (51).Malejka-Giganti D; Niehans GA; Reichert MA; Bennett KK; Bliss RL Potent carcinogenicity of 2,7-dinitrofluorene, an environmental pollutant, for the mammary gland of female Sprague-Dawley rats. Carcinogenesis 1999, 20 (10), 2017–2023. [DOI] [PubMed] [Google Scholar]
- (52).Ritter CL; Malejka-Giganti D Nitroreduction of nitrated and C-9 oxidized fluorees in vitro. Chem. Res. Toxicol. 1998, 11, 1361–1367. [DOI] [PubMed] [Google Scholar]
- (53).Ritter CL; Culp SJ; Freeman JP; Marques MM; Beland FA; Malejka-Giganti D DNA adducts from nitroreduction of 2,7-dinitrofluorene, a mammary gland carcinogen, catalyzed by rat liver or mammary gland cytosol. Chem. Res. Toxicol 2002, 15 (4), 536–544. [DOI] [PubMed] [Google Scholar]
- (54).Enya T; Suzuki H; Watanabe T; Hirayama T; Hisamatsu Y 3-Nitrobenzanthrone, a powerful bacterial mutagen and suspected human carcinogen found in diesel exhaust and airborne particulates. Environ. Sci. Technol 1997, 31, 2772–2776. [Google Scholar]
- (55).Takamura-Enya T; Suzuki H; Hisamatsu Y Mutagenic activities and physiochemical properties of selected nitrobenzanthrones. Mutagenesis 2006, 21, 399–404. [DOI] [PubMed] [Google Scholar]
- (56).Hansen T; Seidel A; Borlak J The environmental carcinogen 3-nitrobenzanthrone and its main metabolite 3-aminobenzanthrone enhance formation of reactive oxygen intermediates in human A549 lung epithelial cells. Toxicol. Appl. Pharmacol 2007, 221 (2), 222–234. [DOI] [PubMed] [Google Scholar]
- (57).El-Bayoumy K; Shiue GH; Hecht SS Comparative tumorigenicity of 6-nitrochrysene and its metabolites in newborn mice. Carcinogenesis 1989, 10, 369–372. [DOI] [PubMed] [Google Scholar]
- (58).El-Bayoumy K; Desai D; Boyiri T; Rosa J; Krzeminski J; Sharma AK; Pittman B; Amin S Comparative tumorigenicity of the environmental pollutant 6-nitrochrysene and its metabolites in the rat mammary gland. Chem. Res. Toxicol 2002, 15 (7), 972–978. [DOI] [PubMed] [Google Scholar]
- (59).Boyiri T; Leszczynska J; Desai D; Amin S; Nixon DW; El-Bayoumy K Metabolism and DNA binding of the environmental pollutant 6-nitrochrysene in primary culture of human breast cells and in cultured MCF-10A, MCF-7 and MDA-MB-435s cell lines. Int. J. Cancer 2002, 100 (4), 395–400. [DOI] [PubMed] [Google Scholar]
- (60).El-Bayoumy K; Hecht SS Identification of trans-1,2-dihydro-1,2-dihydroxy-6-nitrochrysene as a major mutagenic metabolite of 6-nitrochrysene. Cancer Res. 1984, 44 (8), 3408–3413. [PubMed] [Google Scholar]
- (61).Chae YH; Yun CH; Guengerich FP; Kadlubar FF; el-Bayoumy K Roles of human hepatic and pulmonary cytochrome P450 enzymes in the metabolism of the environmental carcinogen 6-nitrochrysene. Cancer Res. 1993, 53 (9), 2028–2034. [PubMed] [Google Scholar]
- (62).Fu PP; Chou MW; Miller DW; White GL; Helflich RH; Beland FA The orientation of the nitro substituent predicts the direct-acting bacterial mutagenicity of nitrated polycyclic aromatic hydrocarbons. Mutat. Res 1985, 143 (3), 173–181. [DOI] [PubMed] [Google Scholar]
- (63).Fu PP; Heflich RH; Unruh LE; Shaikh AU; Wu YS; Lai CC; Lai JS Relationships among direct-acting mutagenicity, nitro group orientation and polarographic reduction potential of 6-nitrobenzo[a]pyrene, 7-nitrobenz[a]anthracene and their derivatives. Mutat. Res 1988, 209 (3–4), 115–122. [DOI] [PubMed] [Google Scholar]
- (64).Fu PP; Von Tungeln LS; Chiu LH; Zhan DJ; Deck J; Bucci T; Wang JC Structure, tumorigenicity, microsomal metabolism, and DNA binding of 7-Nitrodibenz[a,h]anthracene. Chem. Res. Toxicol 1998, 11 (8), 937–945. [DOI] [PubMed] [Google Scholar]
- (65).Hirayama T; Watanabe T; Akita M; Shimomura S; Fujioka Y; Ozasa S; Fukui S Relationships between structure of nitrated arenes and their mutagenicity in Salmonella typhimurium; 2- and 2,7-nitro substituted fluorene, phenanthrene and pyrene. Mutat. Res 1988, 209 (1–2), 67–74. [DOI] [PubMed] [Google Scholar]
- (66).Jung H; Heflich RH; Fu PP; Shaikh AU; Hartman PE Nitro group orientation, reduction potential, and direct-acting mutagenicity of nitro-polycyclic aromatic hydrocarbons. Environ. Mol. Mutagen 1991, 17 (3), 169–180. [DOI] [PubMed] [Google Scholar]
- (67).el-Bayoumy K; Desai D; Upadhyaya P; Amin S; Hecht SS Comparative tumorigenicity of nitrochrysene isomers in newborn mice. Carcinogenesis 1992, 13 (12), 2271–2275. [DOI] [PubMed] [Google Scholar]
- (68).Penning TM; Burczynski ME; Hung CF; McCoull KD; Palackal NT; Tsuruda LS Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active o-quinones. Chem. Res. Toxicol 1999, 12 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- (69).Scully BE Metronidazole. Med. Clin North Am 1988, 72, 613–621. [DOI] [PubMed] [Google Scholar]
- (70).Hof H. Antimicrobial therapy with nitroheterocyclic compounds, for example, metronidazole and nitrofurantoin. Immun Infekt 1988, 16, 220–225. [PubMed] [Google Scholar]
- (71).Holtzman JL; Crankshaw DL; Peterson FJ; Polnaszek CE The kinetics of the aerobic reduction of nitrofurantoin by NADPH-cytochrome P-450 !c reductase. Mol. Pharmacol 1981, 20 (3), 669–673. [PubMed] [Google Scholar]
- (72).Hranjec T; Kovac A; Kos J; Mao W; Chen JJ; Grollman AP; Jelaković B Endemic nephropathy: the case for chronic poisoning by aristolochia. Croat Med. J 2005, 46 (1), 116–125. [PubMed] [Google Scholar]
- (73).Ivković V; Karanović S; Fištrek Prlić M; Mišić M; Kos J; Jurić D; Vuković Lela I; Vitale K; Cvitković A; Laganović M; et al. Is herbal tea consumption a factor in endemic nephropathy. Eur. J. Epidemiol 2014, 29 (3), 221–224. [DOI] [PubMed] [Google Scholar]
- (74).Grollman AP; Shibutani S; Moriya M; Miller F; Wu L; Moll U; Suzuki N; Fernandes A; Rosenquist T; Medverec Z; et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (29), 12129–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Shibutani S; Bonala RR; Rosenquist T; Rieger R; Suzuki N; Johnson F; Miller F; Grollman AP Detoxification of aristolochic acid I by O-demethylation: less nephrotoxicity and genotoxicity of aristolochic acid Ia in rodents. Int. J. Cancer 2010, 127 (5) , 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Stiborová M; Mareš J; Frei E; Arlt VM; Martínek V; Schmeiser HH The human carcinogen aristolochic acid 1 is activated to form DNA adducts by human NAD(P)H:quinone oxidoreductase without the contribution of acetyltransferases or sulfotransferases. Environ. Mol. Mutagen 2011, 52 (6), 448–459. [DOI] [PubMed] [Google Scholar]
- (77).Hashimoto K; Zaitseva IN; Bonala R; Attaluri S; Ozga K; Iden CR; Johnson F; Moriya M; Grollman AP; Sidorenko VS Sulfotransferase-1A1-dependent bioactivation of aristolochic acid I and N-hydroxyaristolactam I in human cells. Carcinogenesis 2016, 37, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Bárta F; Dedíková A; Bebová M; Dušková Š; Mráz J; Schmeiser HH; Arlt VM; Hodek P; Stiborová M Co-Exposure to Aristolochic Acids I and II Increases DNA Adduct Formation Responsible for Aristolochic Acid I-Mediated Carcinogenicity in Rats. Int. J. Mol. Sci 2021, 22 (19), 10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Dedikova A; Barta F; Martinek V; Kotalik K; Duskova S; Mraz J; Arlt VM; Stiborova M; Hodek P In Vivo Metabolism of Aristolochic Acid I and II in Rats Is Influenced by Their Coexposure. Chem. Res. Toxicol 2020, 33, 2804–2818. [DOI] [PubMed] [Google Scholar]
- (80).Yun BH; Rosenquist TA; Sidorenko V; Iden CR; Chen CH; Pu YS; Bonala R; Johnson F; Dickman KG; Grollman AP; et al. Biomonitoring of aristolactam-DNA adducts in human tissues using ultra-performance liquid chromatography/ion-trap mass spectrometry. Chem. Res. Toxicol 2012, 25 (5), 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Bellamri M; Brandt K; Brown CV; Wu MT; Turesky RJ Cytotoxicity and genotoxicity of the carcinogen aristolochic acid I (AA-I) in human bladder RT4 cells. Arch. Toxicol 2021, 95 (6), 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Bastek H; Zubel T; Stemmer K; Mangerich A; Beneke S; Dietrich DR Comparison of Aristolochic acid I derived DNA adduct levels in human renal toxicity models. Toxicology 2019, 420, 29–38. [DOI] [PubMed] [Google Scholar]
- (83).Alexander JL; Wilson ID; Teare J; Marchesi JR; Nicholson JK; Kinross JM Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol Hepatol 2017, 14 (6), 356–365. [DOI] [PubMed] [Google Scholar]
- (84).Moradi Manesh D; El-Hoss J; Evans K; Richmond J; Toscan CE; Bracken LS; Hedrick A; Sutton R; Marshall GM; Wilson WR; et al. AKR1C3 is a biomarker of sensitivity to PR-104 in preclinical models of T-cell acute lymphoblastic leukemia. Blood 2015, 126 (10), 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Wakabayashi N; Dinkova-Kostova AT; Holtzclaw WD; Kang MI; Kobayashi A; Yamamoto M; Kensler TW; Talalay P Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. U. S. A 2004, 101 (7), 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Kang MI; Kobayashi A; Wakabayashi N; Kim SG; Yamamoto M Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. U. S. A 2004, 101 (7), 2046–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Kobayashi A; Kang MI; Okawa H; Ohtsuji M; Zenke Y; Chiba T; Igarashi K; Yamamoto M Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol 2004, 24 (16), 7130–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Dinkova-Kostova AT; Holtzclaw WD; Kensler TW The role of keap-1 in cellular protective responses. Chem. Res. Toxicol 2005, 18, 1779–1795. [DOI] [PubMed] [Google Scholar]
- (89).Tebay LE; Robertson H; Durant ST; Vitale SR; Penning TM; Dinkova-Kostova AT; Hayes JD Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol. Med 2015, 88, 108–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Burczynski ME; Lin H-K; Penning TM Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenases. Cancer Res. 1999, 59, 607–614. [PubMed] [Google Scholar]
- (91).Liu P; Kerins MJ; Tian W; Neupane D; Zhang DD; Ooi A Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. J. Biol. Chem 2019, 294 (48), 18131–18149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Sheikh T; Gupta P; Gowda P; Patrick S; Sen E Hexokinase 2 and nuclear factor erythroid 2-related factor 2 transcriptionally coactivate xanthine oxidoreductase expression in stressed glioma cells. J. Biol. Chem 2018, 293 (13), 4767–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Talalay P; Dinkova-Kostova AT Role of nicotinamide quinone oxidoreductase 1 (NQO1) in protection against toxicity of electrophiles and reactive oxygen intermediates. Methods Enzymol. 2004, 382, 355–364. [DOI] [PubMed] [Google Scholar]
- (94).Murray JR; de la Vega L; Hayes JD; Duan L; Penning TM Induction of the Antioxidant Response by the Transcription Factor NRF2 Increases Bioactivation of the Mutagenic Air Pollutant 3-Nitrobenzanthrone in Human Lung Cells. Chem. Res. Toxicol 2019, 32 (12), 2538–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Takamura-Enya T; Kawanishi M; Yagi T; Hisamatsu Y Structural identification of DNA adducts derived from 3-nitrobenzanthrone, a potent carcinogen present in the atmosphere. Chem. Asian J 2007, 2 (9), 1174–1185. [DOI] [PubMed] [Google Scholar]
- (96).Humphreys WG; Kadlubar FF; Guengerich FP Mechanism of C8 alkylation of guanine residues by activated arylamines: evidence for initial adduct formation at the N7 position. Proc. Natl. Acad. Sci. U. S. A 1992, 89 (17), 8278–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Rangiah K; Hwang WT; Mesaros C; Vachani A; Blair IA Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC-MS. Bioanalysis. 2011, 3, 745–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Herreno-Saenz D; Evans FE; Beland FA; Fu PP Identification of two N2-deoxyguanosinyl DNA adducts upon nitroreduction of the environmental mutagen 1-nitropyrene. Chem. Res. Toxicol 1995, 8 (2), 269–277. [DOI] [PubMed] [Google Scholar]
- (99).Mei N; Arlt VM; Phillips DH; Heflich RH; Chen T DNA adduct formation and mutation induction by aristolochic acid in rat kidney and liver. Mutat. Res 2006, 602 (1–2), 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Enya T; Kawanishi M; Suzuki H; Matsui S; Hisamatsu Y An unusual DNA adduct derived from the powerfully mutagenic environmental contaminant 3-nitrobenzanthrone. Chem. Res. Toxicol 1998, 11 (12), 1460–1467. [DOI] [PubMed] [Google Scholar]
- (101).Park JH; Troxel AB; Harvey RG; Penning TM Polycyclic aromatic hydrocarbon (PAH) o-quinones produced by the aldo-keto-reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8-oxo-dGuo via reactive oxygen species. Chem. Res. Toxicol 2006, 19 (5), 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Murata M; Tezuka T; Ohnishi S; Takamura-Enya T; Hisamatsu Y; Kawanishi S Carcinogenic 3-nitrobenzanthrone induces oxidative damage to isolated and cellular DNA. Free Radic Biol. Med 2006, 40 (7), 1242–1249. [DOI] [PubMed] [Google Scholar]
- (103).Kohda K; Tada M; Kasai H; Nishimura S; Kawazoe Y Formation of 8-hydroxyguanine residues in cellular DNA exposed to the carcinogen 4-nitroquinoline 1-oxide. Biochem. Biophys. Res. Commun 1986, 139 (2), 626–632. [DOI] [PubMed] [Google Scholar]
- (104).Tada M; Kohda K Identification of N4-(guanosin-7-yl)-4-aminoquinoline 1-oxide and its possible role in guanine C8 adduction. Nucleic Acids Symp. Ser 1993, 29, 25–26. [PubMed] [Google Scholar]
- (105).Mesaros C; Arora JS; Wholer A; Vachani A; Blair IA 8-Oxo-2’-deoxyguanosine as a biomarker of tobacco-smoking-induced oxidative stress. Free Radical Biol. Med 2012, 53, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Marteijn JA; Lans H; Vermeulen W; Hoeijmakers JH Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol 2014, 15, 465–481. [DOI] [PubMed] [Google Scholar]
- (107).Bridges BA DNA repair: Polymerases for passing lesions. Curr. Biol 1999, 9, R475–477. [DOI] [PubMed] [Google Scholar]
- (108).Krzeminski J; Kropachev K; Kolbanovskiy M; Reeves D; Kolbanovskiy A; Yun BH; Geacintov NE; Amin S; El-Bayoumy K Inefficient nucleotide excision repair in human cell extracts of the N-(deoxyguanosin-8-yl)-6-aminochrysene and 5-(deoxyguanosin-N(2)-yl)-6-aminochrysene adducts derived from 6-nitrochrysene. Chem. Res. Toxicol 2011, 24 (1), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Krzeminski J; Kropachev K; Reeves D; Kolbanovskiy A; Kolbanovskiy M; Chen KM; Sharma AK; Geacintov N; Amin S; El-Bayoumy K Adenine-DNA adduct derived from the nitroreduction of 6-nitrochrysene is more resistant to nucleotide excision repair than guanine-DNA adducts. Chem. Res. Toxicol 2013, 26 (11), 1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Boyiri T; Guttenplan J; Khmelnitsky M; Kosinska W; Lin JM; Desai D; Amin S; Pittman B; El-Bayoumy K Mammary carcinogenesis and molecular analysis of in vivo cII gene mutations in the mammary tissue of female transgenic rats treated with the environmental pollutant 6-nitrochrysene. Carcinogenesis 2003, 25 (4), 637–643. [DOI] [PubMed] [Google Scholar]
- (111).Yagi T; Fujikawa Y; Sawai T; Takamura-Enya T; Ito-Harashima S; Kawanishi M Error-Prone and Error-Free Translesion DNA Synthesis over Site-Specifically Created DNA Adducts of Aryl Hydrocarbons 3-Nitrobenzanthrone and 4-Aminobiphenyl. Toxicol Res. 2017, 33 (4), 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Broschard TH; Wiessler M; von der Lieth CW; Schmeiser HH Translesional synthesis on DNA templates containing site-specifically placed deoxyadenosine and deoxyguanosine adducts formed by the plant carcinogen aristolochic acid. Carcinogenesis 1994, 15 (10), 2331–2340. [DOI] [PubMed] [Google Scholar]
- (113).Jelakovic B; Karanovic S; Vukovic-Lela I; Miller F; Edwards KL; Nikolic J; Tomic K; Slade N; Brdar B; Turesky RJ; et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 2012, 81 (5), , 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Fromme JC; Banerjee A; Huang SJ; Verdine GL Structural basis for removal of adenine mispaired with 8-oxoguanine by MutY adenine DNA glycosylase. Nature 2004, 427, 652–656. [DOI] [PubMed] [Google Scholar]
- (115).Bruner SD; Norman DP; Verdine GL Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 2000, 403, 859–866. [DOI] [PubMed] [Google Scholar]
- (116).Kim Y-D; Ko Y-J; Kawamoto T; Kim H The effects of 1-nitropyrene on oxidative DNA damage and expression of DNA repair enzymes. J. Occup. Health 2005, 47, 261–266. [DOI] [PubMed] [Google Scholar]
- (117).Djurić Z; Potter DW; Culp SJ; Luongo DA; Beland FA Formation of DNA adducts and oxidative DNA damage in rats treated with 1,6-dinitropyrene. Cancer Lett. 1993, 71 (1–3), 51–56. [DOI] [PubMed] [Google Scholar]
- (118).Breen AP; Murphy JA Reactions of oxyl radicals with DNA. Free Radic Biol. Med 1995, 18, 1033–1077. [DOI] [PubMed] [Google Scholar]
- (119).Heflich RH; Howard PC; Beland FA 1-Nitrosopyrene: an intermediate in the metabolic activation of 1-nitropyrene to a mutagen in Salmonella typhimurium TA1538. Mutat. Res 1985, 149 (1), 25–32. [DOI] [PubMed] [Google Scholar]
- (120).Enya T; Suzuki H; Watanabe T; Hirayama T; Hisamatsu Y 3-Nitrobenzanthrone, a Powerful Bacterial Mutagen and Suspected Human Carcinogen Found in Diesel Exhaust and Airborne Particulates. Environ. Sci. Technol 1997, 31, 2772–2776. [Google Scholar]
- (121).Arlt VM; Glatt H; Muckel E; Pabel U; Sorg BL; Schmeiser HH; Phillips DH Metabolic activation of the environmental contaminant 3-nitrobenzanthrone by human acetyltransferases and sulfotransferase. Carcinogenesis 2002, 23 (11), 1937–1945. [DOI] [PubMed] [Google Scholar]
- (122).Oda Y; Yamazaki H; Watanabe M; Nohmi T; Shimada T Highly sensitive umu test system for the detection of mutagenic nitroarenes in Salmonella typhimurium NM3009 having high O-acetyltransferase and nitroreductase activities. Environ. Mol. Mutagen 1993, 21, 357–364. [DOI] [PubMed] [Google Scholar]
- (123).Oda Y; Shimada T; Watanabe M; Ishidate M Jr; Nohmi T A sensitive umu test system for the detection of mutagenic nitroarenes in Salmonella typhimurium NM1011 having a high nitroreductase activity. Mutat. Res 1992, 272 (2), 91–99. [DOI] [PubMed] [Google Scholar]
- (124).Purohit V; Basu AK Mutagenicity of nitroaromatic compounds. Chem. Res. Toxicol 2000, 13 (8), 673–692. [DOI] [PubMed] [Google Scholar]
- (125).Kawanishi M; Enya T; Suzuki H; Takebe H; Matsui S; Yagi T Mutagenic specificity of a derivative of 3-nitrobenzanthornr in the supF shuttle vector plasmids. Chem. Res. Toxicol 1998, 11, 1468–1473. [DOI] [PubMed] [Google Scholar]
- (126).Boldt J; Mah MC; Wang YC; Smith BA; Beland FA; Maher VM; McCormick JJ Kinds of mutations found when a shuttle vector containing adducts of 1,6-dinitropyrene replicates in human cells. Carcinogenesis 1991, 12 (1), 119–126. [DOI] [PubMed] [Google Scholar]
- (127).Yang JL; Maher VM; McCormick JJ Kinds and spectrum of mutations induced by 1-nitrosopyrene adducts during plasmid replication in human cells. Mol. Cell. Biol 1988, 8 (8), 3364–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Berry DL; Schoofs GM; Vance WA Mutagenicity of nitrofluoranthenes, 3-aminofluoranthene and 1-nitropyrene in Chinese hamster V79 cells. Carcinogenesis 1985, 6 (10), 1403–1407. [DOI] [PubMed] [Google Scholar]
- (129).Arlt VM; Glatt H; Gamboa da Costa G; Reynisson J; Takamura-Enya T; Phillips DH Mutagenicity and DNA adduct formation by the urban air pollutant 2-nitrobenzanthrone. Toxicol. Sci 2007, 98 (2), 445–457. [DOI] [PubMed] [Google Scholar]
- (130).O’Donovan MR 1,8-Dinitropyrene: comparative mutagenicity in Chinese hamster V79 and CHO cells. Mutagenesis 1990, 5 (3), 275–277. [DOI] [PubMed] [Google Scholar]
- (131).Li AP; Gupta RS; Heflich RH; Wassom JS A review and analysis of the Chinese hamster ovary/hypoxanthine guanine phosphoribosyl transferase assay to determine the mutagenicity of chemical agents. A report of phase III of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat. Res 1988, 196 (1), 17–36. [DOI] [PubMed] [Google Scholar]
- (132).Guttenplan JB; Zhao ZL; Kosinska W; Norman RG; Krzeminski J; Sun YW; Amin S; El-Bayoumy K Comparative mutational profiles of the environmental mammary carcinogen, 6-nitrochrysene and its metabolites in a lacI mammary epithelial cell line. Carcinogenesis 2007, 28 (11), 2391–2397. [DOI] [PubMed] [Google Scholar]
- (133).Chen L; Mei N; Yao L; Chen T Mutations induced by carcinogenic doses of aristolochic acid in kidney of Big Blue transgenic rats. Toxicol. Lett 2006, 165 (3), 250–256. [DOI] [PubMed] [Google Scholar]
- (134).Kohara A; Suzuki T; Honma M; Ohwada T; Hayashi M Mutagenicity of aristolochic acid in the lambda/lacZ transgenic mouse (MutaMouse). Mutat. Res 2002, 515 (1–2), 63–72. [DOI] [PubMed] [Google Scholar]
- (135).Greenblatt MS; Bennett WP; Holstein M; Harris CC Mutations in the p53 tumor supressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994, 54, 4855–4878. [PubMed] [Google Scholar]
- (136).Alexandrov LB; Kim J; Haradhvala NJ; Huang MN; Tian Ng AW; Wu Y; Boot A; Covington KR; Gordenin DA; Bergstrom EN; et al. The repertoire of mutational signatures in human cancer. Nature 2020, 578 (7793), 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Moriya M; Slade N; Brdar B; Medverec Z; Tomic K; Jelakovic B; Wu L; Truong S; Fernandes A; Grollman AP TP53 Mutational signature for aristolochic acid: an environmental carcinogen. Int. J. Cancer 2011, 129 (6), 1532–1536. [DOI] [PubMed] [Google Scholar]
- (138).Yun BH; Yao L; Jelaković B; Nikolić J; Dickman KG; Grollman AP; Rosenquist TA; Turesky RJ Formalin-fixed paraffin-embedded tissue as a source for quantitation of carcinogen DNA adducts: aristolochic acid as a prototype carcinogen. Carcinogenesis 2014, 35 (9), 2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Romanov V; Sidorenko V; Rosenquist TA; Whyard T; Grollman AP A fluorescence-based analysis of aristolochic acid-derived DNA adducts. Anal. Biochem 2012, 427 (1), 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Miller WL; Agrawal V; Sandee D; Tee MK; Huang N; Choi JH; Morrissey K; Giacomini KM Consequences of POR mutations and polymorphisms. Mol. Cell. Endocrinol 2011, 336 (1–2), 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Traver RD; Siegel D; Beall HD; Phillips RM; Gibson NW; Franklin WA; Ross D Characterization of a polymorphism in NAD(P)H: quinone oxidoreductase (DT-diaphorase). Br. J. Cancer 1997, 75 (1), 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Cook SF; King AD; Chang Y; Murray GJ; Norris HR; Dart RC; Green JL; Curry SC; Rollins DE; Wilkins DG Quantification of a biomarker of acetaminophen protein adducts in human serum by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: clinical and animal model applications. J. Chromatogr B Analyt Technol. Biomed Life Sci 2015, 985, 131–141. [DOI] [PubMed] [Google Scholar]
- (143).Gan J; Zhang H; Humphreys WG Drug-Protein Adducts: Chemistry, Mechanisms of Toxicity, and Methods of Characterization. Chem. Res. Toxicol 2016, 29 (12), 2040–2057. [DOI] [PubMed] [Google Scholar]
- (144).Hemminki K Significance of DNA and protein adducts. IARC Sci. Publ 1992, No. 116, 525–534. [PubMed] [Google Scholar]
- (145).Yang X; Bartlett MG Identification of protein adduction using mass spectrometry: Protein adducts as biomarkers and predictors of toxicity mechanisms. Rapid Commun. Mass Spectrom 2016, 30 (5), 652–664. [DOI] [PubMed] [Google Scholar]
- (146).Bryant MS; Skipper PL; Tannenbaum SR; Maclure M Hemoglobin adducts of 4-aminobiphenyl in smokers and nonsmokers. Cancer Res. 1987, 47 (2), 602–608. [PubMed] [Google Scholar]
- (147).el-Bayoumy K; Johnson B; Partian S; Upadhyaya P; Hecht SS In vivo binding of 1-nitropyrene to albumin in the rat. Carcinogenesis 1994, 15 (1), 119–123. [DOI] [PubMed] [Google Scholar]
- (148).van Bekkum YM; Scheepers PT; van den Broek PH; Velders DD; Noordhoek J; Bos RP Determination of hemoglobin adducts following oral administration of 1-nitropyrene to rats using gas chromatography-tandem mass spectrometry. J. Chromatogr B Biomed Sci. Appl 1997, 701, 19–28. [DOI] [PubMed] [Google Scholar]
- (149).Gamboa da Costa G; Singh R; Arlt VM; Mirza A; Richards M; Takamura-Enya T; Schmeiser HH; Farmer PB; Phillips DH Quantification of 3-nitrobenzanthrone-DNA adducts using online column-switching HPLC-electrospray tandem mass spectrometry. Chem. Res. Toxicol 2009, 22, 1860–1868. [DOI] [PubMed] [Google Scholar]
- (150).Nagy E; Zeisig M; Kawamura K; Hisamatsu Y; Sugeta A; Adachi S; Moller L DNA adduct and tumor formation in rats after intratracheal administration of the urban air pollutant 3-nitrobenzanthrone. Carcinogenesis 2005, 26 (10), 1821–1828. [DOI] [PubMed] [Google Scholar]
- (151).Delclos KB; Miller DW; Lay JO Jr.; Casciano DA; Walker RP; Fu PP; Kadlubar FF Identification of C8-modified deoxyinosine and N2- and C8-modified deoxyguanosine as major products of the in vitro reaction of N-hydroxy-6-aminochrysene with DNA and the formation of these adducts in isolated rat hepatocytes treated with 6-nitrochrysene and 6-aminochrysene. Carcinogenesis 1987, 8 (11), 1703–1709. [DOI] [PubMed] [Google Scholar]
- (152).Hosoya T; Maruyama A; Kang M-I; Kawatani Y; Shibata T; Uchida K; Itoh K; Yamamoto M Differential responses of the Nrf2-Keap1 systems to laminar and oscillatory shear stresses in endothelial cells. J. Biol. Chem 2005, 280, 27244–27250. [DOI] [PubMed] [Google Scholar]
- (153).El-Bayoumy K; Sharma AK; Lin JM; Krzeminski J; Boyiri T; King LC; Lambert G; Padgett W; Nesnow S; Amin S Identification of 5-(deoxyguanosin-N2-yl)- 1,2-dihydroxy-1,2-dihydro-6-aminochrysene as the major DNA lesion in the mammary gland of rats treated with the environmental pollutant 6-nitrochrysene. Chem. Res. Toxicol 2004, 17 (12), 1591–1599. [DOI] [PubMed] [Google Scholar]
- (154).Kappers WA; van Och FM; de Groene EM; Horbach GJ Comparison of three different in vitro mutation assays used for the investigation of cytochrome P450-mediated mutagenicity of nitro-polycyclic aromatic hydrocarbons. Mutat. Res 2000, 466 (2), 143–159. [DOI] [PubMed] [Google Scholar]
- (155).Meinl W; Pabel U; Osterloh-Quiroz M; Hengstler JG; Glatt H Human sulphotransferases are involved in the activation of aristolochic acids and are expressed in renal target tissue. Int. J. Cancer 2006, 118 (5), 1090–1097. [DOI] [PubMed] [Google Scholar]
- (156).Dayan J; Crajer MC; Deguingand S Mutagenic activity of 4 active-principle forms of pharmaceutical drugs. Comparative study in the Salmonella typhimurium microsome test, and the HGPRT and Na+/K+ ATPase systems in cultured mammalian cells. Mutat. Res 1982, 102 (1), 1–12. [DOI] [PubMed] [Google Scholar]


