Background
Cutaneous reactions after Coronavirus 2019 (COVID-19) vaccination with mRNA-1273 (Moderna, Cambridge, MA, USA), and BNT162b2 (Pfizer-BioNTech, New York, NY, USA) have been commonly reported [1–4]. In contrast, Ad26.COV2.S (Johnson & Johnson, New Brunswick, NJ, USA), which uses a nonreplicating viral vector, seems to have relatively fewer dermatological side effects [5].
Case presentation
We report the case of a 30-year-old woman who showed localized itching coin-like erythematous macules with scaling beginning at her left breast and left shoulder region 24 h after Ad26.COV2.S vaccination (Fig. 1) on her left upper arm. After another 24 h, her right breast and right shoulder showed similar cutaneous alterations (Fig. 2). Any involvement at the injection site was lacking. Unfortunately, a lesional biopsy was not approved by the patient. Systemic prednisolone and bilastine, and lesional topical application of mometason fuorate cream resulted in a full restitutio ad integrum. Nevertheless, 4 weeks after Ad26.COV2.S vaccination, the patient was infected by corona virus (as demonstrated by positive SARS-CoV‑2 PCR test). She encountered a moderate COVID-19 symptom severity score with fever and cough resolving at home without new cutaneous alterations.
Fig. 1.
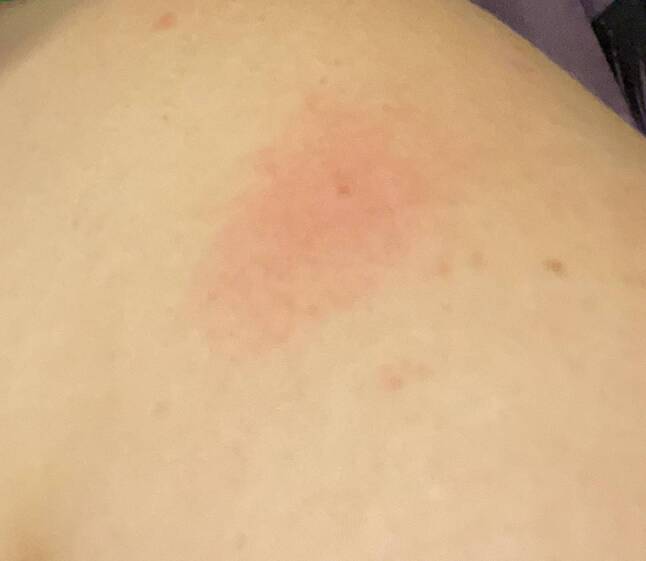
Coin-like eczema-like erythematous lesion affecting left shoulder starting 24 h after first Ad26.COV2.S shot
Fig. 2.

Coin-like eczema-like erythematous lesion affecting right shoulder starting 48 h after first Ad26.COV2.S shot
Patient’s medical history revealed a body mass index (BMI) > 25 kg/m2, allergic asthma, allergic rhinoconjunctivitis, drug allergies (paracetamol, ibuprofen), blood hypertension, endometriosis, and sleep apnoea syndrome. In regard to type IV allergic reactions, a positive sensitization to diethylenetriamine, a solvent for plastics and dyes and in chemical synthesis was confirmed by patch test. In regard to drugs, telmisartan 80 mg/amlodipine 5 mg tablet, dienogest 2 mg tablet, and formoterol 400 mcg/budesonide 12 mcg inhaler had been in continuous use for over 1 year. For sleep apnoea syndrome, the patient used an auto-adjusting positive airway pressure device during night hours. In regard to skin alterations in the past, the patient denied any cutaneous lesions except acne pustules during puberty.
In a large sample of cutaneous COVID-19 vaccine reactions to mRNA-1273, BNT162b2, and Ad26.COV2.S, McMahon et al. found robust papules with overlying crusts, pityriasis rosea-like eruptions, pink papules with fine scale (V-REPP), bullous pemphigoid-like lesions, dermal hypersensitivity, herpes zoster, lichen-planus-like lesions, urticarial, neutrophilic dermatosis, leukocytoclastic vasculitis, morbilliform, delayed large local reactions, erythromelalgia, and others [1].
Although macroscopic lesional inspection in our patient was consistent with the diagnosis of nummular eczema, dermal hypersensitivity, and id reaction, (defined as dermatitis distant to an initial site of inflammation or infection) must also be considered.
Conclusion
The cause of the encountered localized eczematous reactions remain unclear. It is possible that Ad26.COV2.S vaccine may act as an environmental trigger in a genetically susceptible individual. In our case, the patient suffered from allergic asthma and rhinoconjunctivitis, had a positive type IV sensitization to diethylenetriamine, and had a medical history of drug intolerances, perhaps making her more susceptible to an eczematous reaction. It is a matter of discussion whether this cutaneous reaction would have occurred in a similar way after administration of Moderna’s mRNA-1273 and Pfizer’s BNT162b2 vaccine. Further recording of cutaneous reactions following vaccination with Ad26.COV2.S seem mandatory to provide a complete picture of possible side effects.
Conflict of interest
N. Anasiewicz, C. Seeli, M.-C. Brüggen and M. Möhrenschlager declare that they have no competing interests.
References
- 1.McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113–121. doi: 10.1016/j.jaad.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oulee A, Salem S, Yahia R, Yang K, Garcia D, Holmes A, et al. Cutaneous reactions due to Pfizer’s BNT162b2 mRNA and Moderna’s mRNA-1273 vaccine. J Eur Acad Dermatol Venereol. 2022;36:e332–4. doi: 10.1111/jdv.17925. [DOI] [PubMed] [Google Scholar]
- 3.Leasure AC, Cowper SE, McNiff J, Cohen JM. Generalized eczematous reactions to the Pfizer-Biontech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35:e716–7. doi: 10.1111/jdv.17494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt V, Blum R, Möhrenschlager M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA-1273 vaccine in a 84-year-old female with polymorbidity and polypharmacy. J Eur Acad Dermatol Venereol. 2022;36:e88–90. doi: 10.1111/jdv.17722. [DOI] [PubMed] [Google Scholar]
- 5.Sadoff J, Gray A, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]


