Abstract
Helicobacter pylori has been shown to agglutinate erythrocytes in a sialic acid-dependent manner. However, very few studies have examined relevant target cells in the human stomach. Neutrophils are required for the onset of gastritis, and the inflammatory reaction may be induced on contact between bacteria and neutrophils. In the present work, glycolipids and glycoproteins were isolated from neutrophils and were studied for binding by overlay with radiolabeled bacteria on thin-layer chromatograms and on membrane blots. There was a complex pattern of binding bands. The only practical binding activity found was sialic acid dependent, since treatment of glycoconjugates with neuraminidase or mild periodate eliminated binding. As shown before for binding to erythrocytes and other glycoconjugates, bacterial cells grown on agar bound to many glycoconjugates, while growth in broth resulted in bacteria that would bind only to polyglycosylceramides, which are highly heterogeneous and branched poly-N-acetyllactosamine-containing glycolipids. Approximately seven positive bands were found for glycoproteins, and the traditional ganglioside fraction showed a complex, slow-moving interval with very strong sialic-acid-dependent binding, probably explained by Fuc substitutions on GlcNAc.
Helicobacter pylori, the recently discovered human-specific gastric pathogen (5), has been shown to have complex carbohydrate-binding specificities (20). The first binding found was a sialic-acid-dependent hemagglutination which was inhibitable by the addition of neuraminidase or soluble sialylated glycoconjugates (6). Recently, evidence was provided for two separate recognitions of sialic acid, based on solid-phase binding to various glycoconjugates and bacterial growth in different media (26, 29). However, questions remain about the expression of such binding epitopes in the two major target cells for the bacterium, gastric epithelial cells and neutrophils. We have found extremely low levels of sialylated glycoconjugates in human gastric epithelium (50a). In contrast, human neutrophils appear rich in receptor-positive sialylated glycoconjugates, which is the subject of the present paper.
The close association of H. pylori with massive polymorphonuclear infiltration in the human antrum was first observed by Warren and Marshall (53), and this finding has been repeatedly documented and discussed (11, 14, 32, 42). The inflammation caused by the bacteria predisposes patients to ulceration (4) and may lead to gastric adenocarcinoma (39) or gastric lymphoma (40, 54). The pathogenesis and mechanisms of these diseases are, however, not clear. There are reports on exacerbation of gastritis by H. pylori (1a, 9, 13, 35), and the gastric inflammation may be advantageous for the pathogen (1a). H. pylori synthesizes a neutrophil-activating protein, Hp-NAP (9), which upregulates adhesion molecules of the CD11b/CD18 series on human neutrophils, increasing binding of these cells to the endothelium. Upon contact of H. pylori with neutrophils, there is an oxidative burst (32, 42) followed by phagocytosis, but the bacterial cells are not necessarily killed (3, 24, 42), and the infection persists. Some strains of H. pylori have the ability to agglutinate human leukocytes (3).
H. pylori binds to a variety of membrane components, including phospholipids, glycolipids, and glycoproteins (20, 52). Binding to sialylated glycoconjugates has been suggested to be of importance for H. pylori resistance to phagocytosis (3), and sialic acid binding has been shown to be a constant feature of fresh clinical isolates of the bacterium (44). We report that sialylated glycoconjugates are abundant in human neutrophils, providing numerous binding sites for the bacterial cells.
MATERIALS AND METHODS
Preparation of granulocyte cells.
Human neutrophils were prepared from buffy coats of venous blood of healthy donors as described (10). The procedure was a modification of the method of Bøyum (2) and included centrifugation of cells in Ficoll medium, washing cells in phosphate-buffered saline (PBS)-glucose-gelatin solution, and sedimentation of the mixture in dextran solution. Erythrocytes remaining in the granulocyte fraction were removed by lysis in a 0.8% solution of NH4Cl in H2O. After incubation in NH4Cl for at least 10 min, the cells were centrifuged at 400 × g, and the supernatant was discarded. The lysis and centrifugation were repeated until the preparation was free from erythrocytes. This procedure usually results in granulocyte fractions with neutrophil contents of greater than 95%. Cell fractions referred to in the text as total leukocytes and with a granulocyte content of 70 to 85% were prepared from unseparated buffy coats, which were lysed in NH4Cl solution (for the removal of erythrocytes) as described above. For comparison, we also obtained a smaller, highly pure, neutrophil (polymorphonuclear leukocytes, 100%) preparation from Claes Dahlgren, Institute of Medical Microbiology and Immunology, Göteborg University (18).
H. pylori strains.
Bacterial strains used in these studies were 17874 and 17875 (CCUG) and 032 (a gift from Dan Danielsson, Örebro Medical Center, Sweden). H. pylori cultivation and labeling on agar plates or in broth were performed as described (26).
Preparation of glycosphingolipids.
Gangliosides were prepared according to standard procedures (21). Ganglioside and carbohydrate nomenclature is according to recommendations by the IUPAC-IUB Commission on Biochemical Nomenclature. The method included extraction of glycolipids from lyophilized cells with mixtures of chloroform and methanol as well as alkaline degradation, dialysis, DEAE-cellulose column chromatography, and silicic acid column chromatography. The polyglycosylceramide fraction was isolated by the peracetylation method (28) as follows. The dry tissue residue (1.2 g) left after extraction of lipids and common glycolipids was peracetylated in formamide-pyridine-acetic anhydride (10:5:4, by volume; 22.8 ml) followed by extraction with an excess of chloroform (50 ml). After filtration, the extract was washed three times with water (17 ml each time), and the chloroform phase was evaporated to near dryness. The oily residue was applied to a Sephadex LH-20 column followed by Sephadex LH-60 chromatography. The crude polyglycosylceramide (PGC) preparation was de-O-acetylated in 0.1 M NaOH in water overnight at room temperature and was dialyzed against distilled water for 2 days. The sample was next freeze-dried, extracted with 2-propanol–hexane–water (55:25:20, by volume; 2 ml), and centrifuged. Complex glycosphingolipids were recovered from the supernatant. The total sphingosine content in this fraction was 136 nmol.
Extraction of proteins.
Membranes from fresh neutrophils were prepared by the method of Moore et al. (33). The outer membrane fragment fraction was collected and dissolved in 25 mM Tris-HCl containing 2.5% sodium dodecyl sulfate (SDS) and 1 mM EDTA, pH 8.0, heated to 95°C for 10 min and centrifuged at 10,000 × g for 10 min.
The protein concentration was determined by bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.). The sample was diluted to 2 to 4 mg of protein per ml, and 2-mercaptoethanol (5%) was added prior to SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Overlay on TLC plates with 35S-labeled bacteria.
Overlay of glycosphingolipids on thin-layer chromatography (TLC) silica gel plates with [35S]methionine-labeled bacteria was done essentially as described previously (22). Briefly, the silica gel plates with separated glycolipids were treated with 0.5% polyisobutylmethacrylate (high molecular weight) (Aldrich Chemical Company, Inc., Milwaukee, Wis.) in diethyl ether–n-hexane (3:1, by volume) for 1 min and were dried. The plates were then soaked in 2% bovine serum albumin (BSA) and 0.1% Tween in PBS for 2 h and were overlaid with radiolabeled cells. The plates were incubated under normal atmospheric conditions for an additional 2 h, were washed five times with PBS, were dried, and were exposed to Kodak X-OMAT AR films (Kodak Eastman Co., Rochester, N.Y.) for 1 to 4 days.
Electrophoresis and bacterial overlay.
SDS-PAGE and Coomassie staining were carried out with Pharmacia PhastSystem according to the protocols of the manufacturer (Amersham Pharmacia Biotech, Uppsala, Sweden). Briefly, samples were heated to 95°C for 5 min and were centrifuged at 10,000 × g for 2 min before electrophoresis. A homogeneous gel of 12.5% polyacrylamide was used, and 2 to 4 μg of protein was applied for each lane. After electrophoresis, the gel was either stained with Coomassie R 350 (PhastGel Blue R; Pharmacia) or was electroblotted to a polyvinylidene difluoride (PVDF) (0.2-μm) membrane according to manufacturers instructions. The transfer buffer consisted of 20% methanol, 192 mM glycine, and 25 mM Tris-HCl at pH 8.3.
The PVDF membrane was preincubated in blocking solution (3% BSA, 50 mM Tris-HCl, 200 mM NaCl, 0.1% NaN3, pH 8.0) for 1.5 h. The membrane was then incubated with 35S-labeled H. pylori in PBS. After 1.5 to 2 h, the membrane was washed in a solution of 50 mM Tris-HCl, 200 mM NaCl, and 0.05% Tween 20, pH 8.0, was dried at room temperature, and was exposed to Kodak film overnight.
Ceramide glycanase digestion of glycolipids.
Ceramide glycanase (from the leech Macrobdella decora [55]; Boehringer Mannheim GmbH, Mannheim, Germany) digestion was performed at 37°C overnight. The reaction mixture contained 100 μg of PGCs, 75 μg of sodium cholate, and 0.5 mU of enzyme in 60 μl of 50 mM acetate buffer, pH 5.0. After digestion, the sample was mixed with 240 μl of water and 1,500 μl of chloroform-methanol (2:1, by volume), was shaken, and was centrifuged. The lower and upper phases contained free ceramides and free oligo- and polysaccharides, respectively. The hydrolysis was complete, and the recovery of the material after digestion was practically quantitative, as judged by TLC. Both phases were evaporated under nitrogen. The saccharides were desalted using a Sephadex G-15 column (Pharmacia, Uppsala, Sweden) which was packed and run in distilled water. The sugar-positive material (detected on TLC plates by anisaldehyde) was collected, evaporated, redissolved in a small volume of water, and used for TLC analysis, as described for polyglycosylceramides of human erythrocytes (28). The ceramides were redissolved in a small volume of 2:1 (by volume) chloroform-methanol and were used for TLC analysis (28). The released ceramides were further analyzed by fast atom bombardment mass spectrometry (28).
Neuraminidase hydrolysis and periodate oxidation.
For neuraminidase (from Clostridium perfringens; Sigma Chemical Co., St. Louis, Mo.) treatment of glycoproteins on blots, the PVDF membranes were washed twice after blocking in 50 mM sodium acetate buffer (pH 5.5) containing 0.1% BSA and 5 mM CaCl2 and were incubated in the same buffer (0.1 ml/cm2) with or without neuraminidase (50 mU/ml) at 37°C for 30 h (38).
Glycolipid desialylation and mild periodate oxidation were performed as described (26).
Colorimetric tests.
Quantitative determination of hexose, sialic acid, and sphingosine was performed as described (30).
RESULTS
Three groups of glycoconjugates of human neutrophils, gangliosides, PGCs, and glycoproteins, were tested for binding by radiolabeled H. pylori on TLC plates and membrane blots. The surfaces were overlaid with 35S-radiolabeled bacteria, and the bound radioactivity was detected by autoradiography.
We use the term gangliosides to define a ganglioside fraction prepared by extraction with organic solvents and other standard procedures. More complex glycosphingolipids recovered from the extracted residue are referred to as PGCs.
Binding to glycolipids.
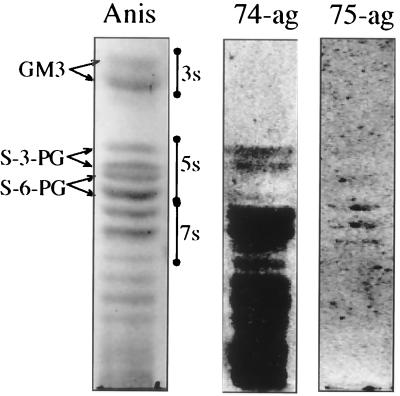
Binding to gangliosides on TLC plates is shown in Fig. 1. This particular ganglioside fraction was prepared from total leukocytes (see Materials and Methods), but the same binding pattern was obtained for highly purified neutrophils. Polymorphonuclear leukocytes have been shown to contain a series of sialylated glycosphingolipids based on neolacto carbohydrate core chains, with predominant species located in three-, five-, and seven-sugar regions (16, 23, 34, 50). H. pylori recognized components in five- and seven-sugar regions, as well as in more complex fractions. Experiments with desialylated and periodate-oxidized gangliosides revealed a strict dependence of the binding on NeuAc, and the sialic-acid-independent H. pylori strain CCUG 17875 practically did not bind (Fig. 1, 75-ag). The weak reaction seen for CCUG 17875 in the seven-sugar region was not dependent on sialic acid and did not disappear after desialylation or mild oxidation and reduction. There was a preference of binding to NeuAcα3Galβ4GlcNAc compared to NeuAcα6Galβ4GlcNAc (16, 27), which is in agreement with results of other groups (15, 44). There was also a stronger binding to some complex fractions, confirmed by binding to a dilution series of gangliosides (not shown). The binding to S-3-PG and other gangliosides was strong after bacterial growth on solid media (agar plates). After growth in liquid media, this specificity was lost (26, 29) and only sialic-acid-dependent binding to PGCs was observed.
FIG. 1.
Gangliosides of human leukocytes separated on silica gel TLC plates and visualized by anisaldehyde (Anis), 35S-labeled H. pylori CCUG 17874 grown on agar (74-ag), or 35S-labeled H. pylori CCUG 17875 grown on agar (75-ag). 3s, 5s, and 7s indicate migration regions for three-, five-, and seven-sugar-containing monosialogangliosides, respectively. The TLC plates were developed in chloroform–methanol–0.25% KCl in water (50:40:10, by volume).
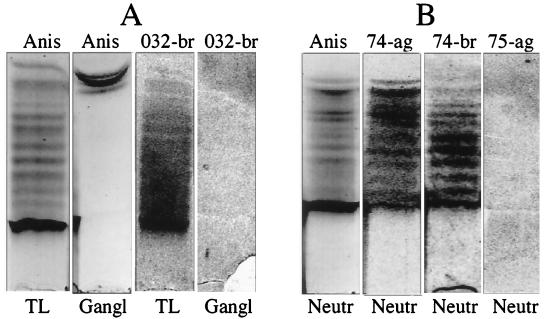
Figure 2 illustrates the binding to PGCs isolated from two cell fractions, total leukocyte fraction, panel A, and neutrophil fraction, panel B. Most of the components migrated below reference brain gangliosides. H. pylori from agar bound strongly to the whole chromatographic interval, including less polar components, while H. pylori from broth bound less strongly to less polar components. Three H. pylori strains were used in these studies, two classified as sialic-acid-dependent binders and one classified as a sialic-acid-independent binder. The sialic-acid-independent strain CCUG 17875 did not bind to PGCs, and the binding by the other two strains disappeared after desialylation of PGCs or after mild periodate oxidation and reduction, which shortens the sialic acid glycerol tail by one or two carbon atoms (31).
FIG. 2.
PGCs, prepared from total human human leukocytes (TL, panel A) and human neutrophils (Neutr, panel B), were separated on silica gel TLC plates and visualized by anisaldehyde (Anis), 35S-labeled H. pylori 032 from broth (032-br), 35S-labeled H. pylori CCUG 17874 from agar (74-ag), 35S-labeled H. pylori CCUG 17874 from broth (74-br), or 35S-labeled H. pylori CCUG 17875 from agar (75-ag). Bovine brain gangliosides (a mixture of GM1, GD1a, GD1b, and GT1b) (Gangl, panel A) were used as a control of nonspecific binding. TLC plates were developed in chloroform-methanol-water (50:55:19, by volume).
The glycosphingolipid nature of the PGC material was proved by ceramide glycanase digestion. Ceramide glycanase specifically cleaves the glycosidic bond between ceramides and carbohydrate chains in glycolipids, and the cleavage of leukocyte PGCs was monitored by TLC using polar solvent systems (28, 30).
The released oligo- and polysaccharides changed their speeds of migration compared to undigested PGCs. The released ceramides were analyzed separately by TLC with less-polar solvent (28). The digestion eliminated binding of H. pylori on TLC plates due to loss of ceramides, which are necessary for a hydrophobic anchoring of the saccharide chains in the plastic-treated silica gel during washing procedures. The structure of the saccharide part of the leukocyte PGC has not yet been analyzed. These compounds are not identical to myeloglycans, oligofucosylated gangliosides with up to 12 monosaccharides per core chain, described in human leukocytes by others (34, 49, 50). Glycolipids defined as myeloglycans are less polar than PGCs and are extracted together with gangliosides (16), in contrast to PGCs which remain in the residue after extraction (28). Our preliminary data from colorimetric tests and mass spectrometry showed the presence in leukocyte PGCs of ceramides (mainly d18:1-C16:0 and C24:1), hexose, hexosamine, sialic acid, and minor amounts of fucose. Electron impact ionization mass spectrometry analysis of the permethylated material revealed the presence of an abundant fragment ion corresponding to NeuAc1Hex1HexNAc1 (m/z 825) (31). The same sequence is present in H. pylori-binding PGCs from human erythrocytes (31). We have shown using matrix-assisted laser desorption ionization–time of flight mass spectrometry (19) that PGCs of human erythrocytes contain sialylated molecules with more than 40 monosaccharides per ceramide. Mass spectrometry after degradation with endo-β-galactosidase indicated that the sialylated sequence is present entirely in the form of a three-sugar nonextended side branch (31a). A hydrogen bond formation between the sialic acid glycerol tail and GlcNAcs of the neighboring branches may be necessary for the proper presentation of the binding epitope, as interpreted from the effect on binding of mild periodate and molecular modeling studies (1). Leukocyte PGCs are presently being analyzed in our laboratory. So far, the pattern of binding by the bacteria when grown on agar or in broth indicates that the binding epitope should be the same in leukocyte and erythrocyte PGCs.
Binding to glycoproteins.
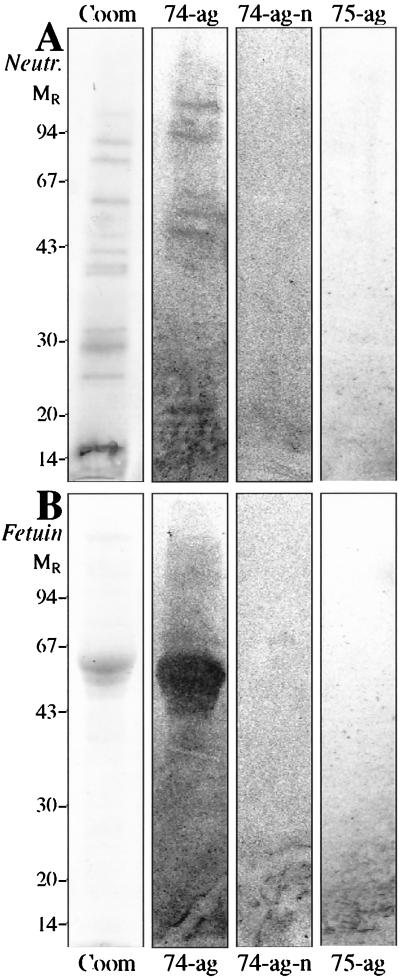
Binding of H. pylori to protein extracts obtained from fresh neutrophil outer membranes is shown in Fig. 3. Recognition of at least seven protein bands could be observed and these were sensitive to neuraminidase treatment (compare 74-ag and 74-ag-n in panel A). This binding was only observed for strains grown on agar and known to bind sialic acid, as illustrated by the control experiment performed on calf fetuin (Fig. 3, panel B). The sialic-acid-binding strain CCUG 17874 and the nonbinding strain CCUG 17875 were used for comparison.
FIG. 3.
SDS-PAGE of human neutrophil protein extract from fresh membranes (Neutr, panel A) and of calf fetuin (Fetuin, panel B) on a 12.5% polyacrylamide homogeneous gel stained with Coomassie brilliant blue (Coom) and the corresponding autoradiograms after binding of 35S-labeled H. pylori CCUG 17874 on PVDF membrane blot (74-ag), strain CCUG 17874 on neuraminidase-treated PVDF membrane blot (74-ag-n), and strain CCUG 17875 on PVDF membrane blot (75-ag). The numbers on the left denote apparent relative molecular masses in kilodaltons.
DISCUSSION
Human leukocytes apparently contain a variety of sialylated glycoconjugates with high potency to bind H. pylori. Binding on artificial surfaces was demonstrated for glycolipids and glycoproteins isolated from mixtures of human leukocytes as well as for neutrophils. We will report elsewhere (in collaboration with C. Dahlgren and A. Karlsson) the binding to glycoconjugates isolated from subcellular fractions of neutrophils.
The simplest binding-active molecule was shown elsewhere (16, 27) to be a five-sugar monosialoganglioside, S-3-PG, NeuAcα3Galβ4GlcNAcβ3Galβ4GlcCer (Fig. 1), having sialyl-N-acetyllactosamine as the terminal trisaccharide. Sialyl-N-acetyllactosamine is structurally related to the sialyllactose (NeuAcα3Galβ4Glc) present in the negative three-sugar glycolipid (3s in Fig. 1). Other groups using various methods have, however, reported this saccharide or glycolipid to be active (6, 7, 15, 25, 43, 44, 46). A stronger binding was observed by us for some complex gangliosides, and this could depend on the presence of extended carbohydrate chains with repeated lactosamine units and/or fucose branches. Neolacto carbohydrate chains with Fucα3GlcNAc substitutions have been shown to be present in human leukocytes, in both glycolipids (34, 49, 50) and glycoproteins (48).
Binding to PGCs apparently represents a separate sialic acid-dependent specificity, as indicated by the results obtained with different growth media as shown before for erythrocytes (26, 29). The agar-dependent binding may be to NeuAcα3Galβ4GlcNAc, as indicated in the original paper (6), and the binding remaining after growth in broth may be a novel epitope unique for PGCs. Binding to PGCs was observed for both broth- and agar-grown bacteria, with the difference that the binding to some rapid-moving fractions was weaker by broth-grown bacteria (compare 74-ag with 74-br in Fig. 2). This is not surprising, as PGCs and gangliosides may overlap, and, as mentioned, the broth-grown bacteria lose their binding to gangliosides. The binding to the slow-moving bands was apparently strong, with binding detected in the presence of low levels of PGCs.
Sialylated molecules with affinity for H. pylori were also found among glycoproteins. This binding was only expressed by bacteria grown on agar and was compared with the binding to fetuin (Fig. 3), apparently representing the same specificity as the binding to gangliosides. Fetuin is known to contain both NeuAcα3Galβ4GlcNAc and NeuAcα6Galβ4GlcNAc (36). As discussed earlier (26, 29), the binding to gangliosides and fetuin seems to be separate from the binding to PGCs, as shown by the effects of different growth media on binding.
Due to the apparent abundance of the H. pylori-binding molecules, human neutrophils may make contact with this bacterium. The sialylated epitopes are present in molecules of different complexities, and this may be of importance for steric presentation of the binding sites on the membrane surface and for in vivo events. The calculated ganglioside content of granulocytes was about 17 nmol per 108 cells (calculation based on molecular content of sialic acid [23]), and the PGC content was about 0.8 nmol per 108 cells (calculation based on sphingosine content). The PGCs are, therefore, less abundant than gangliosides in terms of concentration but may provide efficient multivalent epitope structures. The effectiveness of binding to glycolipids may be improved by the formation of plasma membrane microdomains with locally concentrated receptor structures, as reported for lymphocytes (47) and other cells (12, 41, 45, 51).
The biological significance of the sialic acid-dependent binding of neutrophil glycoconjugates by H. pylori is still unclear. Although established reference bacterial strains may be positive or negative binders of sialic acid, it is of interest that fresh clinical isolates expressed a consistent binding (44). Future experiments may test if a sialic acid-mediated interaction between bacterial cells and neutrophils is essential for a strong inflammation. Noteworthy in this respect is our finding of a very low content of sialylated glycoconjugates of gastric epithelium (data not shown). Therefore, the expression of a sialic-acid-binding adhesin(s) may support bacterial homing to neutrophils rather than to epithelial cells. An adhesin related to sialyllactose-inhibitable hemagglutinin was reported in 1993 (8). However, later reports claimed that this cloned protein was not an adhesin but rather a lipoprotein (17, 37). Therefore, the adhesin(s) responsible for the bindings reported in this work remains to be identified.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Medical Research Council (No. 3967, 10435, and 12628), grants from the Swedish Research Council for Engineering Sciences, and grants from the Swedish Cancer Foundation.
REFERENCES
- 1.Ångström, J., et al. Unpublished data.
- 1a.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 2.Bøyum A. Separation of blood leukocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4:269–274. [PubMed] [Google Scholar]
- 3.Chmiela M, Lelwala-Guruge J, Wadström T. Interaction of cells of Helicobacter pyloriwith human polymorphonuclear leukocytes: possible role of hemagglutinins. FEMS Immunol Med Microbiol. 1994;9:41–48. doi: 10.1111/j.1574-695X.1994.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 4.Dooley C P. Helicobacter pyloriinfection and peptic ulcer disease. Curr Opin Gastroenterol. 1993;9:112–117. [Google Scholar]
- 5.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans D G, Evans D J, Jr, Moulds J J, Graham D Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988;56:2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans D G, Evans D J., Jr Adhesion properties of Helicobacter pylori. Methods Enzymol. 1995;253:336–360. doi: 10.1016/s0076-6879(95)53029-0. [DOI] [PubMed] [Google Scholar]
- 8.Evans D G, Karjalainen T K, Evans D J, Jr, Graham D Y, Lee C-H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans D J, Jr, Evans D G, Takemura T, Nakano H, Lampert H C, Graham D Y, Granger D N, Kvietys P R. Characterization of a Helicobacter pylorineutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredlund H, Olcén P, Danielsson D. A reference procedure to study chemiluminescence induced in polymorphonuclear leukocytes by Neisseria meningitidis. APMIS. 1988;96:941–949. doi: 10.1111/j.1699-0463.1988.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 11.Graham D Y. Campylobacter pyloriand peptic ulcer disease. Gastroenterology. 1989;96:615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- 12.Hakomori S-i, Handa K, Iwabuchi K, Yamamura S, Prinetti A. New insights in glycosphingolipid function: “glycosignaling domain”, a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology. 1998;8:xi–xix. doi: 10.1093/oxfordjournals.glycob.a018822. [DOI] [PubMed] [Google Scholar]
- 13.Harris P R, Mobley H L T, Perez-Perez G I, Blaser M J, Smith P D. Helicobacter pyloriurease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–425. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 14.Hatz R A, Brooks W P, Krämling H-J, Enders G. Stomach immunology and Helicobacter pyloriinfection. Curr Opin Gastroenterol. 1992;8:993–1001. [Google Scholar]
- 15.Hirmo S, Kelm S, Schauer R, Nilsson B, Wadström T. Adhesion of Helicobacter pyloristrains to α-2,3-linked sialic acids. Glycoconj J. 1996;13:1005–1011. doi: 10.1007/BF01053196. [DOI] [PubMed] [Google Scholar]
- 16.Johansson L, Miller-Podraza H. Analysis of 3- and 6-linked sialic acids in mixtures of gangliosides using blotting to polyvinylidene difluoride membranes, binding assays, and various mass spectrometry techniques with application of recognition by Helicobacter pylori. Anal Biochem. 1998;265:260–268. doi: 10.1006/abio.1998.2920. [DOI] [PubMed] [Google Scholar]
- 17.Jones A C, Logan R P H, Foynes S, Cockayne A, Wren B W, Penn C W. A flagellar sheath protein of Helicobacter pylori is identical to HpaA, a putative N-acetylneuraminyllactose-binding hemagglutinin, but is not an adhesin for AGS cells. J Bacteriol. 1997;179:5643–5647. doi: 10.1128/jb.179.17.5643-5647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson A, Dahlgren C. Secretion of type-1-fimbriae binding proteins from human neutrophil granulocytes. Inflammation. 1996;20:389–400. doi: 10.1007/BF01486741. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson H, Johansson L, Miller-Podraza H, Karlsson K-A. Fingerprinting of large oligosaccharides linked to ceramide by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: highly heterogeneous polyglycosylceramides of human erythrocytes with receptor activity for Helicobacter pylori. Glycobiology. 1999;9:765–778. doi: 10.1093/glycob/9.8.765. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson K-A. Meaning and therapeutic potential of microbial recognition of host glycoconjugates. Mol Microbiol. 1998;29:1–11. doi: 10.1046/j.1365-2958.1998.00854.x. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson K-A. Preparation of total non-acid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 1987;138:212–220. doi: 10.1016/0076-6879(87)38018-8. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson K-A, Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- 23.Kiguchi K, Henning-Chubb C B, Huberman E. Glycosphingolipid patterns of peripheral blood lymphocytes, monocytes, and granulocytes are cell specific. J Biochem. 1990;107:8–14. doi: 10.1093/oxfordjournals.jbchem.a123016. [DOI] [PubMed] [Google Scholar]
- 24.Kist M, Spiegelhalder C, Moriki T, Schaefer H E. Interaction of Helicobacter pylori (strain 151) and Campylobacter coliwith human peripheral polymorphonuclear granulocytes. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;280:58–72. doi: 10.1016/s0934-8840(11)80941-2. [DOI] [PubMed] [Google Scholar]
- 25.Lelwala-Guruge J, Ljungh Å, Wadström T. Hemagglutination patterns of Helicobacter pylori. APMIS. 1992;100:908–913. [PubMed] [Google Scholar]
- 26.Miller-Podraza H, Abul Milh M, Bergström J, Karlsson K-A. Recognition of glycoconjugates by Helicobacter pylori: an apparently high affinity binding of human polyglycosylceramides, a second sialic acid-based specificity. Glycoconj J. 1996;13:453–460. doi: 10.1007/BF00731478. [DOI] [PubMed] [Google Scholar]
- 27.Miller-Podraza H, Abul Milh M, Teneberg S, Karlsson K-A. Binding of Helicobacter pylorito sialic acid-containing glycolipids of various origins separated on thin-layer chromatograms. Infect Immun. 1997;65:2480–2482. doi: 10.1128/iai.65.6.2480-2482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller-Podraza H, Andersson C, Karlsson K-A. New method for the isolation of polyglycosylceramides from human erythrocyte membranes. Biochim Biophys Acta. 1993;1168:330–339. doi: 10.1016/0005-2760(93)90190-k. [DOI] [PubMed] [Google Scholar]
- 29.Miller-Podraza H, Bergström J, Abul Milh M, Karlsson K-A. Recognition of glycoconjugates by Helicobacter pylori. Comparison of two sialic acid-dependent specificities based on haemagglutination and binding to human erythrocyte glycoconjugates. Glycoconj J. 1997;14:467–471. doi: 10.1023/a:1018599401772. [DOI] [PubMed] [Google Scholar]
- 30.Miller-Podraza H, Stenhagen G, Larsson T, Andersson C, Karlsson K-A. Screening for the presence of polyglycosylceramides in various tissues: partial characterization of blood group-active complex glycosphingolipids of rabbit and dog small intestines. Glycoconj J. 1997;14:231–239. doi: 10.1023/a:1018545922728. [DOI] [PubMed] [Google Scholar]
- 31.Miller-Podraza H, Larsson T, Nilsson J, Teneberg S, Matrosovich M, Johansson L. Epitope dissection of receptor-active gangliosides with affinity for Helicobacter pyloriand influenza virus. Acta Biochim Pol. 1998;45:439–449. [PubMed] [Google Scholar]
- 31a.Miller-Podraza, H., et al. Unpublished data.
- 32.Mooney C, Keenan J, Munster D, Wilson I, Allardyce R, Bagshaw P, Chapman B, Chadwick V. Neutrophil activation by Helicobacter pylori. Gut. 1991;32:853–857. doi: 10.1136/gut.32.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore K L, Stults N L, Diaz S, Smith D F, Cummings R D, Varki A, McEver R P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müthing J. Influenza A and Sendai viruses preferentially bind to fucosylated gangliosides with linear poly-N-acetyllactosaminyl chains from human granulocytes. Carbohydrate Res. 1996;290:217–224. doi: 10.1016/0008-6215(96)00149-8. [DOI] [PubMed] [Google Scholar]
- 35.Nedrud J G, Czinn S J. Helicobacter pylori. Curr Opin Gastroenterol. 1997;13:71–78. [Google Scholar]
- 36.Nilsson B, Nordén N E, Svensson S. Structural studies on the carbohydrate portion of fetuin. J Biol Chem. 1979;254:4545–4553. [PubMed] [Google Scholar]
- 37.O'Toole P W, Janzon L, Doig P, Huang J, Kostrzynska M, Trust T J. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pyloriCCUG 17874 is a lipoprotein. J Bacteriol. 1995;177:6049–6057. doi: 10.1128/jb.177.21.6049-6057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkkinen J, Rogers G N, Korhonen T, Dahr W, Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986;54:37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pyloriinfection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 40.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman H J, Friedman G D. Helicobacter pyloriinfection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 41.Parton R G. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 42.Rautelin H, Blomberg B, Fredlund H, Järnerot G, Danielsson D. Incidence of Helicobacter pyloristrains activating neutrophils in patients with peptic ulcer disease. Gut. 1993;34:599–603. doi: 10.1136/gut.34.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitoh T, Natomi H, Zhao W, Okuzumi K, Sugano K, Iwamori M, Nagai Y. Identification of glycolipid receptors for Helicobacter pyloriby TLC-immunostaining. FEBS Lett. 1991;282:385–387. doi: 10.1016/0014-5793(91)80519-9. [DOI] [PubMed] [Google Scholar]
- 44.Simon P M, Goode P L, Mobasseri A, Zopf D. Inhibition of Helicobacter pyloribinding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun. 1997;65:750–757. doi: 10.1128/iai.65.2.750-757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 46.Slomiany B L, Piotrowski J, Samanta A, VanHorn K, Murty V L N, Slomiany A. Campylobacter pyloricolonization factor shows specificity for lactosylceramide sulfate and GM3 ganglioside. Biochem Int. 1989;19:929–936. [PubMed] [Google Scholar]
- 47.Sorice M, Parolini I, Sansolini T, Garofalo T, Dolo V, Sargiacomo M, Tai T, Peschle C, Torrisi M R, Pavan A. Evidence for the existence of ganglioside-enriched plasma membrane domains in human peripheral lymphocytes. J Lipid Res. 1997;38:969–980. [PubMed] [Google Scholar]
- 48.Spooncer E, Fukuda M, Klock J C, Oates J E, Dell A. Isolation and characterization of polyfucosylated lactosaminoglycan from human granulocytes. J Biol Chem. 1984;259:4792–4801. [PubMed] [Google Scholar]
- 49.Stroud M R, Handa K, Ito K, Salyan M E K, Fang H, Levery S B, Hakomori S-i, Reinhold B B, Reinhold V N. Myeloglycan, a series of E-selectin-binding polylactosaminolipids found in normal human leukocytes and myelocytic leukemia HL60 cells. Biochem Biophys Res Commun. 1995;209:777–787. doi: 10.1006/bbrc.1995.1568. [DOI] [PubMed] [Google Scholar]
- 50.Stroud M R, Handa K, Salyan M E K, Ito K, Levery S B, Hakomori S-i. Monosialogangliosides of human myelogenous leukemia HL60 cells and normal human leukocytes. 2. Characterization of E-selectin binding fractions, and structural requirements for physiological binding to E-selectin. Biochemistry. 1996;35:770–778. doi: 10.1021/bi952461g. [DOI] [PubMed] [Google Scholar]
- 50a.Teneberg, S., et al. Unpublished data.
- 51.Tillack T W, Alietta M, Moran R E, Young W W., Jr Localization of globoside and Forssman glycolipids on erythrocyte membranes. Biochim Bophys Acta. 1983;733:15–24. doi: 10.1016/0005-2736(83)90086-x. [DOI] [PubMed] [Google Scholar]
- 52.Wadström T, Hirmo S, Nilsson B. Biochemical aspects of H. pyloriadhesion. J Physiol Pharmacol. 1997;48:325–331. [PubMed] [Google Scholar]
- 53.Warren J R, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 54.Wotherspoon A C, Doglioni C, Diss T C, Pan L, Moschini A, de Boni M, Isaacson P G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 55.Zhou B, Li S-C, Laine R A, Huang R T C, Li Y-T. Isolation and characterization of ceramide glycanase from leech Macrobdella decora. J Biol Chem. 1989;264:12272–12277. [PubMed] [Google Scholar]