Abstract
Purpose of Review
In this review, we define health equity, disparities, and social determinants of health; the different components of digital health; the barriers to digital health equity; and cardiovascular digital health trials and possible solutions to improve health equity through digital health.
Recent Findings
Digital health interventions show incredible potential to improve cardiovascular diseases by obtaining longitudinal, continuous, and actionable patient data; increasing access to care; and by decreasing delivery barriers and cost. However, certain populations have experienced decreased access to digital health innovations and decreased representation in cardiovascular digital health trials.
Summary
Special efforts will need to be made to expand access to the different elements of digital health, ensuring that the digital divide does not exacerbate health disparities. As the expansion of digital health technologies continues, it is vital to increase representation of minoritized groups in all stages of the process: product development (needs findings and screening, concept generation, product creation, and testing), clinical research (pilot studies, feasibility studies, and randomized control trials), and finally health services deployment.
Introduction
Intentional and targeted use of digital health innovations can advance and promote health equity. The COVID-19 pandemic drove an increased uptake of telemedicine services of up to 1.5x that of the pre-pandemic period [1]. However, certain populations experienced barriers to telemedicine access [2, 3]. These disparities are rooted in structural inequities that can in term impact how individuals can benefit from the promise of digital health. However, during the COVID-19, we also witnessed an increased collaboration between technology and health industry to expand access to transportation services, educational programs in digital health, and cloud programs to aid with vaccine distribution to historically marginalized groups [4]; federal funded programs increased access to broadband internet, digital health education, and devices [5]. Special efforts will need to be made to expand access to the different elements of digital health, ensuring that the digital divide (the economic, educational, and social inequalities between those that have or do not have access to information and communication technology) does not exacerbate health disparities [6]. In this review, we define health equity, disparities, and social determinants of health; the different components of digital health; the barriers to digital health equity; and cardiovascular digital health trials and possible solutions to improve health equity through digital health.
Health Equity
In 1985, the Department of Health and Human Services published the Heckler Report on Black and Minority Health, exposing for one of the first times that race and ethnicity may be an independent contributor to health outcomes [7]. Specifically for cardiovascular care, it was clear that Black Americans had fewer office visits, diagnosis and interventions for coronary artery disease than White individuals. Subsequently, ample research has elucidated the inequities in cardiovascular care for racial and ethnic minorities [8]. The National Institute on Minority Health and Health Disparities defines a health disparity as a health difference that adversely affects disadvantaged populations, based on one or more health outcomes: higher incidence, prevalence or earlier onset of disease, higher prevalence of risk factors, higher rates of condition-specific symptoms, premature or excessive mortality, and greater global burden of disease [9]. In order to reduce health disparities, we need to understand the different social determinants of an individual’s health or the conditions in which individuals live work and play that impact their health. Many of these factors are outside of the health care systems and include housing, income, and education. Not everyone has the same opportunity to be healthy [10]. To properly address these disparities, we should strive for health equity, not equality. Equality entails the distribution of the same resources and opportunities to every individual across a population—regardless of achieving the same outcome. Equity, on the other hand, is delivering these resources and opportunities tailored to the specific needs of a group to achieve equal outcome in the population [11]. Black individuals have higher rates of uncontrolled cardiovascular risk factors and higher age-adjusted death from cardiovascular disease compared with the general US population [12]. Improving health equity involves directing resources to communities that are underrepresented in research. For instance, Brewer et al. partnered with Black communities in all phases of product development to create and test a digital health cardiovascular disease prevention program that led to an improvement in the intervention Heart Association (AHA) Life-Simple 7 score (components: smoking, healthy diet, physical activity, BMI, blood pressure, cholesterol, and glucose) [13, 14].
Digital Health Elements
To better understand how digital health could address health inequities, we first need to define the different components involved in the use of information and communication technology for the delivery of health care:
Digital health: commonly referred as eHealth, is the use of information and communication technology to manage patients and their health [15]. Digital health includes consumer products such as smart devices or connected equipment that have not undergone rigorous clinical studies [16].
Digital medicine: the subset of digital health that pertains to high-quality hardware and software products developed through evidence-based clinical studies for medical care and treatment [17].
Telemedicine: the specific use of information and communication technologies to deliver health care, clinical and administrative services, and medical education, remotely, from one site to another.
mHealth: the use of mobile communication devices to exchange data or information between doctors and patients [18].
Remote patient monitoring: the use of digital health devices to capture and serially monitor vital signs and biometrics with upload of such patient generated data to a digital platform for review by patients and clinical teams [18].
Wearable and consumer technologies: such as commercially available activity tracking, sleep monitoring devices, smartwatches [18].
Barriers to Digital Health Inclusion
The COVID-19 pandemic propelled the use of digital health throughout the world, predominantly through telemedicine and remote patient monitoring [19]. But due to lack of preparedness, many historically marginalized groups were left with less access to care from the lack of infrastructure to support digital health care delivery in these communities [3]. Telemedicine has the ability to decrease health inequities by surpassing the limitations of access to care such as the cost of transportation, inability to leave the house due to disability, and loss of time at work. But in order for patients to benefit from telemedicine services, patients require internet or broadband access, a device (smartphone, computer, or tablet), applications, and a minimum digital health literacy.
In general terms, broadband refers to a set of networked data transmission technologies which permit internet communication and access to digital information. In the USA, 15% of households do not have internet service [20]. The states with majority Black residents or lowest median incomes had the lowest broadband adoption rates. For example, areas with majority White residents had an average adoption rate of 84% compared with 67% of majority-Black residential areas [20]. Decreased access to broadband internet use has been linked to decreased patient portal access. An observational study from a large tertiary-care center hospital showed that Black and Hispanic individuals had lowers odds of initiating a patient portal account or messaging their providers related to their decreased access to the internet [21]. Federal, state, and local programs specifically targeted at underserved communities have the ability to increase access to broadband. The Rural Digital Opportunity Fund program established in 2019 will award a total of $9.2 billion dollars over 10 years. In its 2021 report, the number of Americans without broadband access decreased from 30% in 2016 to 16% in 2019. Additionally, three-quarters of those with new access to broadband lived in rural areas [22].
Gaps remain in computer ownership, with just 69% of Black adults and 67% of Hispanic adults owning a computer as compared with 80% of White individuals [23]. On the other hand, smartphone ownership has steadily increased over the years and data from a 2021 Pew Research survey demonstrates that gaps in smartphone ownership by race are decreasing. Still, nearly one-half of older adults and 30% of those earning less than $30,000 own a smartphone and many low-income households share devices [24]. As an example, home-based cardiac rehabilitation programs can decrease health disparities by expanding care to patients with difficulties attending specialized rehabilitation centers. But if patients from low socioeconomic status, older adults or minoritized groups continue to lack access to computers, broadband, or digital health literacy, then such interventions will continue to exacerbate the digital divide. We need programs that increase access to device ownership and education. If passed, The Device Access for Every American Act introduced at the end of 2021 has the potential to reduce disparities by increasing access to a connected device (desktop, laptop, tablet) by providing a $400 voucher to low-income individuals [25]. In addition, we should extend federal programs enacted during the COVID-19 pandemic to help schools and libraries increase access to devices and education [26].
With regard to wearable ownership, survey data from the Pew Research Center (65% White participants and annual income above 30,000) revealed that in 2020, 21% of US adults report regular use of a smartwatch [24]. Ownership was similar between participants of different ethnic backgrounds, but Black and Hispanic individuals were less likely to approve sharing of their data for heart disease research. On the other hand, surveys performed in Federally Qualified Health Centers with predominantly underrepresented population (70% nonwhite, 70% learning less than 30,000) report lower wearable ownership of 21% [27]. Cost was the main barriers for those who did not own a fitness tracker but would like to own one. With multiple imbedded sensors (accelerometers, photoplethysmography, electrocardiography, etc.), wearable devices can collect a plethora of data for the prevention, diagnosis, and treatment of cardiovascular diseases. Researchers and device companies should continue to collaborate to improve device accuracy and define meaningful use criteria. To increase access, device companies should lower costs; insurance companies must expand reimbursement for biometric data collection and develop programs that increase access to wearable devices [28].
Universal design principles have been present at least since the 1990s to aid designers in developing products for the widest possible range of individuals [29]. The equitable use principle states that products should “Provide the same means of use for all users: identical whenever possible; equivalent when not” and to “avoid segregating or stigmatizing any users” [30]. Unfortunately, the COVID-19 pandemic provided important insight in the gaps in the design of products for people from different cultural, social, educational backgrounds. Most of the apps were available only in English (65%) and 69% had a readability above 9th grade [31]. Several design standards and guides are now available aimed specifically at digital health tools [32]. Two commonly used design processes include the Universal Design Principles and User-Centered Design. While these approaches possess important differences, both aim at maximizing the usability of products to a diverse group of users [33]. Community-based participatory research is another approach that aims to create partnerships between intended end users from the community, academic, and research institutions across all the design stages [34].
Digital Health Interventions for Cardiovascular Care
Hypertension
Hypertension affects close to a third of the adult population worldwide [35]. Black individuals have a higher prevalence of hypertension and lower levels of hypertension awareness and control [36]. Additionally, low-middle-income countries and racial and ethnic minorities in high-income countries tend to have decreased awareness, control, and worse outcomes [35]. Some of the barriers in hypertension control are related to frequent follow up visits, cost of multiple follow-up appointments, the cost transportation, and in certain places the distance required to reach a health care institution. With telemedicine the health care system has the ability to obtain more blood pressure readings that are transferable to the health care team (nurse, pharmacist, physician, etc.) to allow more frequent medication titration without the need for time or cost spent in transportation or additional follow up appointments.
Randomized trials have tested different ways to improve hypertension treatment and control before and after the internet era, with a few trials specifically targeting low-resource setting and diverse patient populations (Table 1). In the Effects of Nurse-Managed Telemonitoring on Blood Pressure at 12-Month Follow-Up Among Urban African Americans study, a nurse-managed telemonitoring intervention (telephonically transmitted BP measurements, nurse intervention, and counseling) showed greater reduction in systolic blood pressure at 12 months compared with usual care alone (13 vs 7.5 mm Hg) [37]. Blood pressure reduction was also achieved with a similar intervention in a subgroup analysis of uncontrolled patients in the Durham VA internal medicine clinics (49% black) [38]. The “Nurse-led Disease Management for Hypertension Control in a Diverse Urban Community” Randomized Controlled Trial (RCT) looked at the response to different levels of intervention (HBPM alone, HBPM + nurse telephone counseling and usual care). At 9 months, systolic blood pressure was − 7.0 mm Hg lower (confidence interval [CI] − 13.4 to − 0.6) in the nurse management plus home blood pressure monitor arm relative to usual care. There was no statistical difference in the home blood pressure only arm versus usual care [39]. Bove et al. studied a telemedicine plus physician intervention versus usual care in a predominantly African American population with 53% at or below the poverty line. Blood pressure control was similar in both groups at 6-month follow-up (52.3% intervention versus 54.5% usual care, P = 0.43) [40].
Table 1.
Digital health randomized control trials in hypertension
| First author | Country | Age mean (SD) | N | Race ethnicity* | Duration (months) | Inclusion criteria | Primary outcome& | Results | Device | Intervention | Medication titration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Artinian et al. (2007) [37] | US | 59.1 (13) vs 60.2 (12.3) | 394 | 100% Black | 12 months | AA with HTN at community centers | Reduction in BP at 12 months | − 13 vs − 7.5 mm Hg, P = 0.04 | HBPM + LifeLink Monitor |
•TM** + nurse tele counseling •Enhanced UC$ |
No |
| Green et al. (2008) [41] | US | 59.1 (8.5) | 778 | 83% Black | 12 months | 25–75 years of age, uncontrolled BP, no diabetes, no cardiovascular or renal disease | Change in SBP, DBP and control at 12 months | TM + pharmacist versus UC: 56% vs 31%, P = < 0.001 |
HBPM (Omron Hem-705-CP) |
3 arms: •TM + Web •TM + pharmacist •Usual care |
Yes (Pharmacist) |
|
Parati et al (2009) [42] |
Italy |
57.2 (10.7) in IT 58.1 (10.8) in UC |
288 | Not reported | 6 months | 18–75 years with office SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg and ABPM SBP ≥ 130 or DBP ≥ 80 mm Hg | Proportion of patients reaching control | 62% vs 50% (P < 0.05) IT vs UC | HBPM (Tensiomed) + Tensiophone | TM + BP data telephone transmitter + Nurse intervention if BP above safety thresholds | No (physician contacted if BP above safety threshold, i.e., ≥ 180/110 mm Hg) |
| McManus (2010) [43] | UK | 66 (8.8) | 480 | 96% White, 1.5% Black, 2.1% Asian | 12 months | Age 38–85 years with BP ≥ 140/90 mm Hg | SBP difference at 6 and 12 months | 6-month SBP difference of 3.7 (0.8–6.6, P = 0.013); 12-month SBP difference 5.5 (2.2 to 8.8) in the IT vs control | HBPM (Omron 705IT) | TM via telephone device (i-modem, Netmedical) | Yes (physician) |
| Wakefield et al. (2011) [44] | US | 66 (10) | 302 | 96% White, 3% African American | 18 months | T2DM and HTN treated by VA PCP | SBP at 6 months |
High IT vs UC: − 6.05 vs + 4.48, P = 0.001 High IT vs low intensity: − 6.05 vs − 0.29, P = 0.9 |
HBPM + telephone line for data transfer (Viterion-Bayer Panasonic) |
3 arms: •TM + high intensity education •TM + low intensity education Usual care |
Yes, for high intensity (Physician based treatment algorithm) |
| Bosworth et al. (2011) [38] | US | 64 (10) | 591 |
49% White, 48% Black |
18 months | Diagnosis of HTN, using BP-lowering meds, BP > 140/90 mmHg | BP control at 18 months |
12.8%, P = 0.03 (behavioral vs usual care) 12.5%, P = 0.03 (combined intervention vs usual care) |
HBPM (UA-767PC, A&D Medical Digital BP) |
4 arms: •TM •TM + behavioral management •TM + behavioral management + nurse and software intervention •Usual |
Yes (Physician aided by nurse from study team with web-based algorithm) |
| Piette et al. (2012) [45] | Mexico and Honduras | 57.6 (0.8) | 200 | Not reported | 6 weeks | Age 18–80 and SBP ≥ 140 mm Hg or ≥ 130 mm Hg with diagnosis of T2DM | SBP difference at 6 weeks | SBP difference of − 4.2 mm Hg (− 9.1 to 0.7, P = 0.09) IT vs UC | HBPM | TM via telephone calls + adherence monitoring and behavioral change | No |
| Hebert et al. (2012) [39] | US | 60.8 (11.6) | 416 | 59% Black, 37% Hispanic | 18 months | Self-described black or Hispanic, community dwelling at enrollment, BP ≥ 140/90 mm Hg | Change in SBP and DBP at 9 | Difference of − 7 mm Hg (− 13.4 to − 0.6 IT vs UC at 9 months | HBPM (Omron HEM-712C) | TM + nurse counseling and contacting physician to suggest treatment changes | Yes (Physician aided by nurse from study team) |
| Margolis et al. (2013) [46] | US | 61.1 (12) | 450 | 82% White, 12% Black, 2% Asian | 18 months | HTN with BP above 140/90 mmHg | Proportion of patients controlled at 6- and 12-month visit |
6 months: 71.8 vs 45.2% P ≤ 0.001 12 months: 71.2 vs 52.8% P = 0.005 |
HBPM (A&D Medical 767PC) | TM + web services + pharmacist calls | Yes (pharmacist with web-based algorithm) |
| Bove et al. (2013) [40] | US | 59.6 (13.5) | 241 | 81% African American, 15% White, 2.5% Hispanic | 6 months | SBP ≥ 140 mmHg | Proportion of BP control | 54.5% versus 52.3%, P = 0.430 |
HBPM (Microlife USA Scale (Taylor Digital) Pedometer (Digi-Walker SW-200) |
TM + patients uploaded data via web or telephone + monthly report to physicians | No (usual clinical decision) |
| Magid et al. (2013) [47] | US | 60 (11) | 348 | 84% White, 8% Black, 6% Hispanic | 6 months | 18–79 years of age, HTN with SBP ≥ 140 or DBP ≥ 90 mmHg | Proportion of BP control | 54.1 versus 35.4%, P ≤ 0.05 | HBPM (Omron HEM-790IT) | TM + automatic upload data to Heart360 Web Account + clinical pharmacist | Yes (pharmacist) |
| Kerry et al. (2013) [48] | UK | 72 (12) | 381 | 77% White, 14.9% Black, 7.2% Asian | 12 months | History of stroke or TIA 9 months prior and BP > 140/85 mm Hg or on antihypertension medication | Change in SBP at 12 months | Difference of 0.3 mm Hg (CI − 3.6 to 4.2) at 12 months | HBPM (Omron 705CP) | Self-monitoring + nurse counseling | No |
| McKinstry et al. (2013) [49] | UK | 61 (11) | 401 | Not reported | 6 months | ≥ 18 years + SBP > 145 mm Hg or DBP > 85 mm Hg | Mean change in SBP at 6 months | Difference of − 4.3 (− 2 to − 6.5, P = 0.0002) IT vs UC | HBPM (Sabil-O-Graph) | TM + automatic transmission of BP readings via smart phone + feedback to patients and HCT*** | No |
| Stewart et al. (2014) [50] | Australia | 67 (12) | 395 | 71% born in Australia, race/ethnicity not reported | 6 months | ≥ 18 years + HTN + on HTN meds | Change in SBP and DBP at 6 months (2ry outcome) | SBP difference of − 5.3 mm Hg (0.0 to 10.6, P = 0.05) IT vs UC | HBPM (Omron HEM-790IT) | TM + pharmacist medication review and adherence check | No (pharmacist could referred to GP) |
| Leiva et al. (2014) [51] | Spain | 65 (10.7) | 114 | Not reported | 12 months | 18–80 years with SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg | SBP at 12 months | SBP 151.3 vs 153.7, P = 0.294 IT vs UC | HBPM | TM + education and motivational interview + pillbox organized + pharmacist intervention | Yes (pharmacist) |
| McManus et al. (2014) [52] | UK |
69.3 (9.3) in IT 69.6 (9.7) in UC |
552 | 96.6% White, 1.5% Black, 1.5% Asian, 0.5% others | 12 months | Age ≥ 35 years, SBP ≥ 130 or DBP ≥ 80 mm Hg plus at least one high risk condition# | SBP difference at 12 months | SBP difference 9.2 (5.7–12.7) IT vs UC | HBPM (MicroLife Watch BP Home) | TM plus self-titration (3 step plan prespecified by their physician) | Yes (participant) |
| Ogedegbe et al. (2014) [53] | US | 56 (12.1) | 1039 | 100% African American | 12 months | Self-identified black or African American, uncontrolled HTN | Rate of BP control at 12 months | 50.2 vs 45.3%, P = 0.18 | HBPM |
TM + lifestyle counseling Physicians received monthly feedback |
No (usual clinical decision) |
| Yi et al. (2015) [54] | US | 61.3 (12) | 900 | 11% White, 26% Black, 63% Hispanic | 6 months | ≥ 18 years, HTN ≥ 6 months, last clinic visit SBP ≥ 140 or DBP ≥ 90 mmHg | Change in SBP and DBP at 7–10 months | Mean change in SBP of 14.7 vs 14.1 mmHg P = 0.70 | HBPM | TM + education | No |
| Bobrow et al. (2016) [55] | South Africa | 54.3 (11.5) | 1256 | 57.6% Black, 42.4% other | 12 months | Age ≥ 21 years and HTN diagnosis | Change in SBP at 12 months | Difference SBP: interactive vs UC − 1.6 (− 3.7 to 0.6, P = 0.16); information only vs UC − 2.2 (− 4.4 to − 0.04, P = 0.046) | SMS text messages |
3 arms: •Interactive two-way SMS messages •One-way information only SMS messages •Usual care |
No |
| Frias et al. (2017) [56] | US |
IT: 57.8 (1.1) UC: 61.6 (1.7) |
109 | 66% White, 47% Hispanic, 16% African American, 14% Asian | 1–3 months | Adults with HTN and BP ≥ 140/90 mmHg | Change in SBP at 4 weeks | − 21.8 vs − 12.7 mm Hg* | DMO*** | Digital medicine + wearable sensor + app | No |
| McManus et al. (2018) [57] | UK | 66.9 (9.4) | 1182 | 95% White, 1.7% Black, 1.4% Asian, 0.6% mixed | 12 months | Age ≥ 35 years, SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg | SBP difference at 12 months | Adjusted difference: self-monitoring − 3.5 mm Hg, P = 0.0029; TM − 4.7 mm Hg, P = 0.0001 vs usual care. No difference between self- and TM | HBPM (Omron M10-IT) |
3 arms: •TM via text or web + physician titration and web interface •Self-monitoring + mail readings + physician titration •Usual care |
Yes (physician) |
| McManus et al. (2021) [58] | UK | 66 (10) | 622 | 94% White, 1.4% Black, 1.1% Asian, 3.4% Other | 12 months | Age ≥ 18 years, SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg | SBP difference at 12 months | Mean SBP difference of − 3.4 (− 6.1 to − 0.8) IT vs UC | HBPM (Omron M3) | TM + patient and physician integrated online intervention + behavioral and lifestyle education | Yes (physician) |
$Enhance UC: provided with list of PCPs (if did not have one), enrolled in pharmacy assistance program and AHA pamphlet “Silent Stalker”
*As collected or reported in the original manuscript
**TM = BP Telemonitoring
**DMO = includes digital medicines, the wearable sensor patch, and the mobile device app
***HCT = health care team
&All office BP unless otherwise stated
#Stroke or transient ischemic attack; diabetes; stage 3 chronic kidney disease (estimated glomerular filtration rate, 30–59 ml/min/m2); coronary artery bypass graft surgery; myocardial infarction or angina
The “Counseling African Americans to Control Hypertension” trial enrolled 1039 patients from 30 community health centers in the New York City area. In the intervention arm, patients received computerized education, behavioral counseling sessions, and HBMP-validated devices; clinicians received monthly on-site education, hypertension case rounds, and quarterly chart audits of their patient office blood pressure readings. The BP control rate was similar in intervention (49.3%) versus control (44.5%) groups. In prespecified subgroup analyses, the intervention was associated with greater BP control in patients without diabetes mellitus (intervention 54.0% versus usual care 44.7%; odds ratio, 1.45 [CI, 1.02–2.06]); and small-sized community health centers (intervention 51.1% versus usual care 39.6%; odds ratio, 1.45 [CI, 1.04–2.45]) [53].
Researchers and companies developing patient-facing platforms should collaborate with communities throughout the entire design process, test and validate these technologies, and integrate them into the electronic health record for seamless transfer of data. Insurers should expand coverage of home blood pressure monitors and reimburse clinicians for using digital health technology to deliver care. Developing provider-facing platforms for low-resource settings, imbedding tools into regular clinician workflow, and providing concise and actionable data aiming at improving care with no added cost or effort should be national priorities.
Cardiovascular Health
The use of digital health to improve cardiovascular risk factors such as dyslipidemia by improving adherence or promoting lifestyle changes is an important public health intervention, both for primary and secondary prevention of cardiovascular diseases. Unfortunately, there is paucity of data regarding the benefits of digital health interventions targeted specifically at lower socioeconomic status, elderly, Black, or Hispanic populations for risk factor modification in cardiovascular health (Table 2). Furthermore, studies have shown that patients with a self-reported history of atherosclerotic cardiovascular disease are less likely to use health information technology to manage their health [59].
Table 2.
Digital health trials in ASCVD and cardiac rehabilitation
| First author | Country | Age mean (SD) | N | Race ethnicity | Duration | Inclusion criteria | Primary outcome | Results | Device | Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Brath et al. (2013) [60] | Austria | 69 (4.8) | 53 | Not reported | 40 weeks | At least 2 diagnoses: HTN, DM2, HLD | Intake rate at 20 weeks | Significant difference in Metformin adherence. No difference in the other 3 medications | Electronic blister + NFC capable smartphone | Adherence text reminders to participants and adherence information to physicians |
| Petrella et al. (2014) [61] | Canada | 56.7 (9.4) | 149 | 100% Caucasian | 12 weeks | At least 2 risk factors* | SBP at 12 weeks. Secondary outcome: waist circumference, HBA1c, HDL, LDL | SBP mean change greater in IT vs control. No difference in secondary outcomes | Smartphone, app, glucometer, HBPM, weight scale, pedometer | Individualized exercise program + home monitoring kit |
| Chow et al. (2016) [62] | Australia | 58 (9.2) | 710 | 66.6% European, 10.7% South Asian, 10.1% other Asian, 9.9% Arab | 6 months | ≥ 18 years of age and documented CHD** | LDL-C level at 6 months | Significant difference in LDL-C of − 5 mg/dL (− 9 to 0, P = 0.4) | Text messages | Semi-personalized text messages with motivation to improve diet, exercise, and smoking cessation |
| Anand et al. (2016) [63] | Canada | 50.6 (11.4) | 343 |
100% South Asian (90% India, 2.3% Pakistan, 5.2% Sri Lanka) |
1 year | South Asian ≥ 30 years of age | MI scores at 12 months | Relative change between IT and control was not significant (− 0.27, − 1.12 to 0.58, P = 0.53) | Email messages | Change-oriented motivational, diet, and physical activity messages |
| Salisbury et al. (2016) [64] | UK | 67.4 (4.8) | 641 | 99% White | 1 year | 40–74 years of age + QRISK2 10-year risk score of ≥ 20% and modifiable diseases*** | Maintaining or decreasing QRISK2 score at 12 months |
Proportion that maintained or improved was not significantly different in IT vs control 50 vs 42% (OR 1.3, 1.0–1.9, P = 0.08) |
Telephone calls + web portal | Health advisor plus computerized behavioral management program |
| Skobel et al. (2017) [65] | UK, Germany, Spain | 59 (14) | 132 | Not reported | 6 months | Hx of acute MI or CAD s/p PCI, LVEF ≥ 30% | Peak VO2 max at 6 months in HBCR$ vs CBCR# national standards | Peak VO2 max change 1.76 ± 4.1 ml/min/kg in HBCR vs − 0.4 ± 2.7 ml/min/kg in CBCR, P = 0.005 |
•Smartphone •ECG •Vest •Vital sign senor •Physician-facing platform |
Asynchronous home-based cardiac rehabilitation |
| Hwang et al. (2017) [66] | Australia | 67 | 53 | 92% Caucasian | 12 weeks | ≥ 18 years of age and recent heart failure admission, diagnosis confirmed by echocardiogram | Non-inferiority: change in 6-min walk distance HBCR vs CBCR | At 12 weeks, there was no between-group difference 15 m (95% CI − 28 to 59); F = 1.39, P = 0.24 |
•Laptop •Mobile broadband •HBPM •Pulse oximeter •Weight and resistance bands |
Synchronous videoconference home-based cardiac rehabilitation |
| Harzand et al. (2018) [67] | US | 65 (5) | 18 | 50% African American | 12 weeks | ≥ 18 years with coronary heart disease plus on indication for cardiac rehabilitation | BP and functional capacity (single arm feasibility study) | Improvement in metabolic equivalent from 5.3 to 6.3, P = 0.008; mean BP at rest decreased from 1401 to 130.5, P = 0.039 |
•Smartphone platform •Hospital-facing dashboard |
Asynchronous home-based cardiac rehabilitation |
| Peng et al. (2018) [68] | China | 66.3 (10.5) | 98 | Not reported | 4 months | ≥ 18 years, heart failure for at least 3 month and NYHA class I–III | Primary: QoL, secondary: 6-min walking distance, LVEF and heart rate | Statistically significant changes in QoL scores, 6-min walk distance and heart rate | Web-based platform | Synchronous videoconference home-based cardiac rehabilitation |
| Maddison et al. (2019) [69] | New Zealand | 61 (13) | 162 | 75.3% NZ European, 4.3% NZ Maori, 2.5% Pacific, 8% Asian | 12 weeks | ≥ 18 years with coronary heart disease within 6 months | Non-inferiority outcomes: VO2 max at 12 weeks | Adjusted mean VO2 max difference = 0.46, 95% CI − 0.92 to 1.84 ml/kg/min, P = 0.51 |
•Smartphone •Chest-word wearable sensor •Apps and Web Platform |
Synchronous home-based cardiac rehabilitation |
| Tekkesin et al. (2021) [70] | Turkey | Mean:59 (53–64) | 283 | Not reported | 1 year | 20–79 years of age with 10 years ASCVD score ≥ 7.5% | ASCVD scores at one year | IT vs UC reduced ASCVD score by difference of − 2.7% (− 2.2 to − 3.3, P ≤ 0.0001) | Smartphone, weight scale, smart wrists band and HBPM | Daily upload of data with motivational messages and feedback |
| Bae et al. (2021) [71] | Korea | 60.4 (10.5) | 879 | Not reported | 6 months | CHD and underwent PCI | LDL-C, SBP and BMI change at 6 months | No significant difference in any outcome: LDL-C, SBP, and BMI | Text messages | Semi-personalized text messages with motivation to improve diet, exercise, and smoking cessation |
*Waist circumference ≥ 88 cm (women) or 102 cm (men); SBP ≥ 135 mmHg and/or DBP ≥ 85 mmHg; fasting plasma glucose ≥ 6.1 mmol/l; fasting triglycerides ≥ 1.7 mmol/l; fasting HDL ≤ 1.29 mmol/l (women) or 1.02 mmol/l (men)
**Defined as documented prior myocardial infarction, coronary artery bypass graft surgery, percutaneous coronary intervention, or 50% or greater stenosis in at least 1 major epicardial vessel on coronary angiography
***Systolic blood pressure ≥ 140 mm Hg, body mass index ≥ 30, being a current smoker, or any combination of these
$Home-based cardiac rehabilitation
#Center-based cardiac rehabilitation
Bae et al. randomized 879 patients with a history of coronary heart disease who underwent percutaneous coronary intervention to a semi-personalized support website and a short message service (SMS) with lifestyle modifications versus usual care (regular clinic follow-up without text messages). At 6 months, there was no significant difference in the cardiometabolic risk profiles between the groups [71]. A higher intensity intervention in a Turkish population that remotely monitored patients’ diet, weight, steps, and blood pressure with additional motivational messages to improve healthy lifestyle showed a significant reduction in ASCVD score of − 2.7% (adjusted treatment effect − 2.7, 95% CI − 2.2 to − 3.3, P < 0.0001) [70]. Brewer et al. randomized churches with predominantly African American adults to test an app-based cardiovascular health promotion intervention. The FAITH! App provided educational models, diet, and physical activity self-monitoring and social networking. Educational material focused on all American Heart Association (AHA) Life-Simple 7 components: smoking, healthy diet, physical activity, BMI, blood pressure, cholesterol, and glucose. The primary outcome was the average change in mean AHA Life-Simple 7 score between the immediate and delayed intervention groups. At 6 months, the mean AHA Life-Simple 7 score of the intervention group increased by 1.9 (SD 1.9) points compared with 0.7 (SD 1.7) point in the control group (P < 0.0001) [14].
Cardiac rehabilitation programs are important components in cardiovascular health and secondary prevention. It is a Class Ia recommendation for secondary prevention after myocardial infarction (MI), percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), stable angina, or symptomatic peripheral arterial disease [72–75]. Uptake continues to be low, especially in minoritized groups. An observational study from the Veterans Affairs Health Care System and Medicare administrative data showed that cardiac rehabilitation after MI, PCI, or CABG in Medicare patients was 16.3% and in VA patients was 10.3%. In Medicare, participation rates were 17.6% Whites, 7.3% Blacks, and 3.8% Hispanics, whereas in VA, participation rates were 10.4% Whites, 8.9% Blacks, and 12.0% Hispanics [76]. Some of the barriers to access cardiac rehabilitation include lack of insurance coverage or high co-payments [77], language barriers [78], and transportation [79]. However, home-based cardiac rehabilitation has the potential to improve participation across all population by addressing these barriers.
Virtual world technology can support home-based cardiac rehabilitation with programs not only tailored to the patient’s comorbidity but also to the patient’s social, cultural, and language background [80]. In China, Peng et al. randomized 98 participants to receive home-based cardiac rehabilitation versus usual care that included education and regular clinic follow-up. At 4 months, there was a statistically significant change in QoL scores, 6-min walk distance, and heart rate [68]. In a single-arm feasibility study in a US Veterans Affairs Center, Harzand et al. evaluated the change from baseline in blood pressure metabolic equivalent of an asynchronous home-based cardiac rehabilitation program. At 12 weeks, participants (50% Black) showed improvement in metabolic equivalent (5.3 to 6.3, P = 0.008) and mean systolic blood pressure (140.1 to 130.5, P = 0.039). Studies have demonstrated non-inferiority when compared to center-based cardiac rehabilitation, but with limited representation of minorities groups [65, 66, 69, 81]. Home-based cardiac rehabilitation shows potential for the future of cardiac rehabilitation; nevertheless, data in minoritized patient populations are limited and we should strive for increased representation in future trials.
Heart Failure
Heart failure is one of the leading causes of death worldwide [82]. There is a higher prevalence and increased mortality in Black and Hispanic individuals compared with White individuals [83, 84]. Racial and ethnic minoritized groups are less likely to receive appropriate medical therapy and to be included in cardiovascular trials [85, 86]. Digital health tools have the ability to improve heart failure management by obtaining important health-related information such as blood pressure, heart rate, EKGs, weight, and symptoms. It can also address health disparities by remotely monitoring data, thereby decreasing time lost from work or travel, and expenses in travel or follow up visits. Unfortunately, only a few clinical trials report or include diverse populations and even fewer have been done specifically in minoritized groups (Table 3).
Table 3.
Digital health randomized control trials in heart failure
| First author | Country | Age mean (SD) | N | Race ethnicity | Duration | Inclusion criteria | Primary outcome | Results | Device | Intervention | Health care team intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Koehler et al. (2011) [87] | Germany | 66.9 (10.8) | 710 | Not reported | 18 months | Stable HF with LVEF ≤ 35% and admission in previous 2 yrs OR LVEF ≤ 25% | Death from any cause | 8.4 in RTM group vs 8.7 in UC (HR 0.97, P = 0.87) | 3-lead EKG, HBPM, weight scale, smartphone | Daily transmission of biometric data | Cardiologist or family doctor |
| Dendale et al. (2012) [88] | Belgium | 76 (10) | 160 | Not reported | 6 months | Decompensated HF | All-cause mortality | Reduced all-cause mortality in the IT vs usual care | Bluetooth scale and HBPM | Automatic transfer of data to website + emails with alerts above threshold to clinicians | GPs, Cardiologist and Nurse follow-up |
| Villani et al. (2014) [89] | Italy | 58 (12) | 94 | Not reported | 6 months | HF with LVEF < 35%, NYHA ≥ 2 | Number of HF-related hospital days | No difference in HF-related hospital days | Weight scale, HBPM, mobile phone | Upload of measurements and survey to software app that provides machine-based feedback + weekly nurse evaluation | Nurse |
| Vuorinen et al. (2014) [90] | Finland | 55 (13.7) | 100 |
62% Caucasian 9% African Canadian 7% Asian 12% other |
6 months | HF with LVEF < 40% | BNP, self-care, and quality of life measured by MLHFQ* | Significantly improved self-care score | Weight scale, HBPM, single-lead ECG recorder, MLHFQ* | Automatic upload of readings and questionnaire by email to cardiologist | Cardiologist |
| Dang et al. (2017) [91] | US | 53 (9.4) | 61 | 76% White Hispanics, 21% AA | 3 months | HF, smartphone ownership, survival expected > 6 months | Self-care efficacy | Improved self-care | Smartphone (provided by study team) | Daily surveys including weight + feedback to physicians | Study coordinator providing data to Heart Failure Clinic |
| Koehler et al. (2018) [92] | Germany | 70 (10) | 1571 | Not reported | 393 days | HF with LVEF ≤ 45% plus hospital admission in last 12 months | Percentage of days lost due to a cardiovascular admission or death | 4.88 versus 6.64% lost days (P = 0.046), all cause death 7.9 vs 11.3 100 person years (P = 0.028) | EKG device, HBPM, weight scales and oximeter, smartphone | Daily transmission of biometric data and surveys plus nurse or physician intervention | Physician or nurse |
| Melin et al. (2018) [93] | Sweden | 75 (8) | 72 | Not reported | 6 months | Admitted HF patients | Self-care behavior based on 9-item European HF Self-care Behavior Scale | Better self-care behaviors in the intervention (16.5 versus 23.5 P ≤ 0.5) | Weight scale and tablet computer | Patient education, transmission of surveys and weight | NA |
|
Chen et al (2019) [94] |
China | 61 (15) | 767 | Not reported | 180 days | Decompensated CHF, mobile phone ownership, life expectancy > 1 year | All-cause mortality | SMS and STS significantly reduced the composite endpoint and readmission in 180 days | Smartphone | Structured telephone support (STS) vs short message service (SMS) vs usual care | No |
*Minnesota Living with Heart Failure Questionnaire
Koehler et al. randomized 1571 participants in Germany to a telemedicine intervention versus usual care. Participants in the intervention arm received an EKG device, HBPM, weight scales, oximeter, and smartphone that provided daily transmission of data to a telemedicine center where algorithms aided clinicians in patient care. The primary outcome, the percentage of days lost due to unplanned cardiovascular hospital admissions, and all-cause death was 4.88% (95% CI 4.55–5.23) in the remote patient management group and 6.64% (6.19–7.13) in the usual care group (ratio 0.80, 95% CI 0·65–1.00; P = 0.0460) [92]. Chen et al. evaluated mortality 180 days after discharge in participants randomized to a two-telemedicine telephone support system versus usual care. The first-level intervention group received education and reminders via a SMS; the second group received SMS plus structured telephone support system managed by research nurses who called patients every 30 days and allowed patients to call nurses on an as needed basis. The 180-day composite event rate was significantly lower in the SMS and STS groups (50.4 vs 41.3% and 36.5%, both P < 0.05) than in the usual care group, but no difference was observed between the two phone-based intervention groups (P = 0.268) [94].
Unfortunately, there are a lack of on the distribution of racial and ethnic populations in heart failure RCTs. There are limited data on Hispanic, Black, and other minoritized populations and the few studies available did not evaluate hard clinical outcomes. The “Mobile Phone Intervention for Heart Failure in a Minority Urban County Hospital Population” was a feasibility study in Hispanic (76%) and Black (21%) individuals that evaluated a mobile phone intervention to test the system’s usability (ease of use, navigation, readability, confidence, and motivation). The study team provided patients with a telemonitoring program (mobile phone, data usage and free 30-min calls per month) for participants to provide daily heart failure symptoms and weight. At 3 months, participant satisfaction scores was excellent, with a mean score of 6.84 ± 0.46 (rating scale of 1–7); 94% of participants thought that the program was easy to use and 84% thought that navigating the system was not complicated [91].
Arrhythmia Detection
Over the last few years, there has been a growing interest in the use of wearables or home ECG devices as an adjunct to usual care for the detection of arrythmias, most commonly atrial fibrillation. Wearables have the ability to detect irregular rhythm such as atrial fibrillation through continuous monitoring of irregular pulse variation with the use of photoplethysmography or on-demand ECG recording. There is a growing interest in the medical field to test commercially available remote patient monitors in multiple clinical or real-world settings for the detection of cardiac arrhythmias (Table 4).
Table 4.
Digital health randomized control trials in arrhythmia detection or management
| First author | Country | Age mean (SD) | Design | N | Race ethnicity | Duration | Inclusion criteria | Primary outcome | Results | Device | Intervention | Health care team interpretation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
William et al (2018) [95] |
US | 68 years | Single-Center Non-Randomized | 52 | Not reported | NA | AF admitted for anti-arrhythmic drug initiation, 38–85 yrs, hx of paroxysmal AF | Sensitivity and specificity | 96.6% sensitivity and 94.1% specificity of 225 recordings | Kardia Mobile Cardiac Monitor coupled to Wi-Fi smart device (iPod) | 30 s recordings of lead I ECG + automatic analysis by KMCM algorithm | I-lead ECG reviewed by blinded electrophysiologist |
| Steinhubl et al. (2018) [96] | US | 72.4 (7.3) | RCT + prospective matched cohort | 2659 | Not reported | 4 months | ≥ 75 yrs, male > 55 yrs or female > 65 yrs* | Incidence of new AF diagnosis at 4 months immediate vs delayed group | 3.9% in the immediate versus 0.9% delayed group | iRhythm ZioXT | Stored data analyzed by an FDA approved algorithm | I-lead ECG adjudication by blinded committee |
| Guo et al. (2019) [97] | China | 54 | Prospective cohort | 187,912 | Not reported | At least 14 days of monitoring | ≥ 18 yrs and smartphone ownership | AF detection efficacy | PPV of 91.6% (91.5–91.8) | Smartphone plus smart wrist band | AF detection using PPG in the smartphone or wrist band | Confirmed by patient’s provider with use of ECG or 24-h Holter monitoring |
| Perez et al. (2019) [98] | US | 41 (13) | Prospective single group pragmatic study | 219,297** |
68% White 12% Hispanic 7.7% Black 6.2% Asian |
Median 117 days of monitoring | ≥ 22 years without prior AF diagnosis or AC use | Proportion of notified participants with AF on ECG patch and PPV of irregular pulse intervals | PPV 84% (0.76–0.92) | Apple Watch + iPhone | AF detection by app with irregular pulse notification algorithm | Confirmed by ECG patch worn for 7 days |
| Goldenthal et al. (2019) [99] | US | 61 (12) | RCT | 238 |
77% White, 3% Black 1% Asian 20%, 9% Hispanic |
6 months | AF patients who underwent RFA or DCCV | AF recurrence detection | Likelihood of recurrent significantly greater IT*** vs control (HR = 1.56, 1.06–2.3) | KardiaMobile + iPhone + cellular servce plan | Record ECG daily or with symptoms plus motivational texts | Confirmation was determined by patient’s provider |
| Koh et al. (2021) [100] | Malaysia | 65.3 (7.4) | RCT | 203 | Not reported | 30 days | ≥ 55 years without AF and ischemic stroke or TIA within the preceding 12 months | AF detection 30-day monitor KardiaMobile vs 24-h Holter | 9.5 vs 2% IT vs control (P = 0.024) | KardiaMobile | Use KardiaMobile monitor 3 times a day for 30 days | I-lead ECG adjudicated by blinded electrophysiologist |
| Lubitz et al. (2022) [101] | US | 74 (7) | Cluster RCT | 30,715 | 82.4% White, 5.3% Black, 2.2% Hispanic | 1 year | ≥ 65 years | New AF diagnosis at 1 year | 1.72% vs 1.59% IT vs control P = 0.38 | KardiaMobile + iPad | Screening AF at primary care clinic with tracings reviewed by cardiologist |
I-lead ECG reviewed by independent cardiologist Confirmation with 12-lead ECG determined by patient’s PCP |
*And any of the following diagnosis: prior stroke or TIA, heart failure, diabetes and hypertension, mitral valve disease, left ventricular hypertrophy, COPD on home O2, sleep apnea, history of pulmonary embolism, history of myocardial infarction or obesity
**450 returned patches
***IT = intervention
The Huawei Heart Study screened for atrial fibrillation using a PPG monitoring app on a smartphone and or smartwatch in adults across China (mean age 35 years, 86.7% male). In total, 424 participants (mean age 54 years, 87.0% male) received a “suspected AF” notification, which was confirmed in 227 individuals (positive predictive value of PPG signals being 91.6%, CI 91.5 to 91.8%) [97]. The Apple Heart Study enrolled 219,297 participants with average age of 41 years (± 13 years) (68% White, 12% Hispanic, 7.7% Black, and 6.2% Asian) to evaluate the efficacy of the Apple Watch detect atrial fibrillation in a real-world setting. Over a median monitoring time of 117 days, irregular pulse notifications were received by 2161 participants (0.52%), ranging from 3.1% of those 65 years of age or older to 0.16% of those 22 to 40 years of age. Of the 2089 irregular tachograms sampled from participants who had received a notification for analysis, 1489 showed simultaneous atrial fibrillation on ECG patch monitoring, resulting in a positive predictive value of the individual tachogram of 0.71 (97.5% CI, 0.69 to 0.74) [98].
Currently, there are less data in the Hispanic or Black populations. Lubitz et al. looked at the ability of the KardiaMobile device to screen for atrial fibrillation in patients older than 65 years of age without prevalent atrial fibrillation attending a primary care visit [101]. The KardiaMobile was not superior to usual care in the detection of new onset atrial fibrillation. Data are also lacking for other racial or ethnic populations. The “Mobile Health Intervention for Rural Atrial Fibrillation” study aims to test the efficacy of a mobile health application virtual coach coupled to a heart rhythm monitor (Kardia) in patients with atrial fibrillation to improve adherence to oral anticoagulation in a rural population of Western Pennsylvania. This will be one of the first studies using commercially available heart rate and rhythm monitors to be tested in rural underserved populations. The Fitbit Heart Study will be the largest study to date, enrolling 450,000 participants across the USA [102]. Part of the inclusion criteria include ownership of a Fitbit and smartphone device. Large-scale studies that include a greater number of diverse participants could provide important information regarding the accuracy of wearable devices for the detection of atrial fibrillation in across all racial and ethnic populations. If a difference exists, further studies should be aimed at determining how we can improve the accuracy in hardware or software.
There has been an increase in the use of wearable devices such as fitness trackers and smart watches (e.g., Fitbit, Apple Watch) to track activity, sleep, oxygenation, and heart rate. As we expand the use of wearables into clinical practice, it is crucial that we provide access to wearable devices to all communities who may benefit from early arrythmia detection and guideline directed treatments.
Technology companies and the medical community developing and testing these devices with its algorithms should ensure they provide reliable information in patients of all skin tones and age groups. The accuracy of wearables to detect certain metrics, such as oxygenation or heart rate, continues to be an issue. Oxygen saturation and heart rate in fitness trackers are measured by photoplethysmography green light signaling. Research in the last decade revealed that dark brown skin type showed significantly lower modulation, perhaps due to absorption of the light by melanin [103]. Wearables devices have been shown to be less accurate in darker skin tones [104]. Unfortunately, recent data show that this issue is not restricted to fitness trackers. A retrospective multi-center study in the Veterans Health Administration showed that Black patients had higher probability of having occult hypoxemia in the inpatient setting when oxygen saturation is measured by pulse oximeter [105]. It is important for fitness tracking companies to be forthcoming with the limitations present to measure certain metrics in population with darker skin tones [106].
Digital Inclusion
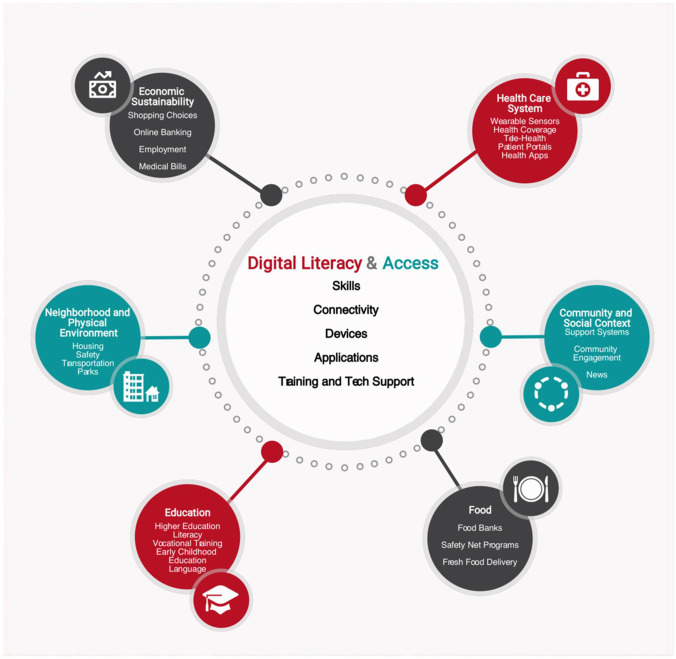
The National Digital Inclusion Alliance (NDIA) defines digital inclusion as the “activities necessary to ensure that all individuals and communities, including the most disadvantaged, have access to and use of Information and Communication Technologies.” It is clear that the elderly, certain racial and ethnic, and lower socioeconomic populations continue to be underrepresented in clinical trials of digital health interventions in cardiovascular disease. There is a continued need to test digital health solutions in underrepresented communities to better understand which interventions provide the most benefit. Beyond clinical trials, there also continues to be a gap in the access to key digital infrastructure in these populations. Attaining digital health equity in cardiovascular care not only requires increased representation of vulnerable populations in clinical trials but also ensured access to the different component of information and communication technologies. In order to improve our current state, proposed solutions should involve multilevel interventions at the individual, family, community, services, and policy level (Fig. 1) [107].
Fig. 1.
Digital literacies and social determinants of health. Digital literacy and access, including skills, connectivity, devices, and training and technical support, relate to all other domains of social determinants of health. With permission from Sieck et al., with no changes made [91]. https://creativecommons.org/licenses/by/4.0/
At the individual level, patients require access to affordable devices (smartphone, computer, tablets, etc.), continued access to digital health literacy education, and applications to be created to their level of education, cultural, racial, and ethnic background. The Affordable Connectivity Program, which replaces the Emergency Broadband Benefit, provides the Federal Communications Commission with funds to provide broadband monthly discounts to eligible households in the hopes to improve internet access to communities in need [108]. It is crucial to provide continued, affordable, and easy to access digital education in settings where an individual feels comfortable to address the skills and knowledge gaps in digital health. The American Library Association Digital Literacy Task Force has provided recommendations and online resources to aid schools, academic, and public libraries increase the access to digital health education in their respective communities [109].
Technology companies should partner with communities starting with the inception of the design process. Involvement of these key stakeholders from underrepresented communities in product design and software development will ensure that products are made for and used by a broader audience [110]. Additionally, there needs to be increased representation of diverse employees in the tech sector; in many if the big technology companies, less than 5% identify as Hispanic or Black [111, 112]. Increased representation can not only increase trust when engaging underrepresented communities but enrich the design team’s knowledge of the environment where these products are expected to be deployed. In order to advance digital health equity, we should design and develop products aimed at including underrepresented communities with consideration to social determinant of health—based on where people are born, grow, live, work and age.
Perhaps one of the most important domains in order to effect change across all levels is policy changes. Existing programs have expanded the access to devices and broadband internet coverage in low-income communities. The Emergency Connectivity Fund (ECF) is a $7 billion program targeted at helping schools and libraries acquire devices and broadband equipment. Since its first cycle in 2021, the ECF has funded close to 12 million devices and over 7 million broadband connections [26]. Additionally, the Digital Inclusion Act programs not only aim to increase internet access, but support programs to provide underrepresented communities with skills and training necessary to successfully use the internet [113]. To accelerate health equity, we should continue to collaborate with policy makers to renew and expand available programs [5].
Conclusion
Digital health interventions show incredible potential to improve cardiovascular disease detection, prevention and management by obtaining longitudinal, continuous, and actionable patient data; increasing access to care; and decreasing delivery barriers and cost. As the expansion of digital health technologies continues, it is vital to increase representation of minoritized groups in all stages of the process: product development (needs findings and screening, concept generation, product creation and testing), clinical research (pilot studies, feasibility studies, and randomized control trials), and finally health services deployment.
Author Contribution
MFH drafted the manuscript. FR contributed to draft, edit, and supervisory role of the manuscript.
Funding
Dr. Rodriguez was funded by grants from the NIH National Heart, Lung, and Blood Institute (1K01HL144607), the American Heart Association/Harold Amos Faculty Development program, and the Doris Duke Charitable Foundation (Grant #2022051).
Declarations
Conflict of Interest
Dr. Fatima Rodriguez reports equity from HealthPals and Carta and consulting fees from Novartis, Novo Nordisk, and Amgen outside the submitted work. Dr. Mario Funes Hernandez has no conflict of interest to disclose.
Footnotes
This article is part of the Topical Collection on Technology and Cardiovascular Health
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koonin LM, Hoots B, Tsang CA, Leroy Z, Farris K, Jolly T, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595–1599. doi: 10.15585/mmwr.mm6943a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eberly LA, Kallan MJ, Julien HM, Haynes N, Khatana SAM, Nathan AS, et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID-19 pandemic. JAMA Netw Open. 2020;3(12):e2031640. doi: 10.1001/jamanetworkopen.2020.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe DH, Lee L, Huynh S, Haskell TP. Health inequalities in the use of telehealth in the United States in the lens of COVID-19. Popul Health Manag. 2020;23(5):368–377. doi: 10.1089/pop.2020.0186. [DOI] [PubMed] [Google Scholar]
- 4.Durocher K, Boparai N, Jankowicz D, Strudwick G. Identifying technology industry-led initiatives to address digital health equity. Digit Health. 2021;7:20552076211056156. doi: 10.1177/20552076211056156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Policy - National Digital Inclusion Alliance 2022 [Available from: https://www.digitalinclusion.org/policy/.
- 6.Merriam-Webster.com Dictionary sv. “Digital Divide”: @MerriamWebster; 2022 [Available from: https://www.merriam-webster.com/dictionary/digital+divide.
- 7.United States. Department of Health and Human Services. Task Force on Black and Minority Health. Report of the Secretary’s Task Force on Black & Minority Health. Washington, D.C.: U.S. Dept. of Health and Human Services; 1985. <v. 1, 3–4, pts. 1–2 5–8 in > p.
- 8.Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;336(7):480–6. [DOI] [PubMed]
- 9.Borrell LN, Vaughan R. An AJPH supplement toward a unified research approach for minority health and health disparities. Am J Public Health. 2019;109(S1):S6–S7. doi: 10.2105/AJPH.2019.304963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics (U.S.). Healthy People 2010: final review. Hyattsville, MD: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2012.
- 11.Equity vs. equality: what’s the difference? 2021 [Available from: https://onlinepublichealth.gwu.edu/resources/equity-vs-equality/.
- 12.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular Health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 13.Brewer LC, Hayes SN, Caron AR, Derby DA, Breutzman NS, Wicks A, et al. Promoting cardiovascular health and wellness among African-Americans: community participatory approach to design an innovative mobile-health intervention. PLoS ONE. 2019;14(8):e0218724. doi: 10.1371/journal.pone.0218724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewer LC, Jenkins S, Hayes SN, Kumbamu A, Jones C, Burke LE, et al. Community-based, cluster-randomized pilot trial of a cardiovascular mobile health intervention: preliminary findings of the FAITH! trial. Circulation. 2022;146(3):175–190. doi: 10.1161/CIRCULATIONAHA.122.059046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omboni S, Benczur B, McManus RJ. Editorial: Digital health in cardiovascular medicine. Front Cardiovasc Med. 2021;8:810992. doi: 10.3389/fcvm.2021.810992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kario K, Harada N, Okura A. Digital therapeutics in hypertension: evidence and perspectives. Hypertension. 2022:HYPERTENSIONAHA12219414. [DOI] [PMC free article] [PubMed]
- 17.Coravos A, Goldsack JC, Karlin DR, Nebeker C, Perakslis E, Zimmerman N, et al. Digital medicine: a primer on measurement. Digit Biomark. 2019;3(2):31–71. doi: 10.1159/000500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnaswami A, Beavers C, Dorsch MP, Dodson JA, Masterson Creber R, Kitsiou S, et al. Gerotechnology for older adults with cardiovascular diseases: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76(22):2650–2670. doi: 10.1016/j.jacc.2020.09.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uscher-Pines L, Sousa J, Jones M, Whaley C, Perrone C, McCullough C, et al. Telehealth use among safety-net organizations in california during the COVID-19 pandemic. JAMA. 2021;325(11):1106–1107. doi: 10.1001/jama.2021.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomer A, Fishbane, L., Siefer, A., & Callahan, B. How broadband can deliver health and equity to all communities. Brookings Metro; 2020.
- 21.Perzynski AT, Roach MJ, Shick S, Callahan B, Gunzler D, Cebul R, et al. Patient portals and broadband internet inequality. J Am Med Inform Assoc. 2017;24(5):927–932. doi: 10.1093/jamia/ocx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sophia Campbell JRC, and David Wessel. The benefits and costs of broadband expansion: @BrookingsInst; 2021 [updated 2021–08–18. Available from: https://www.brookings.edu/blog/up-front/2021/08/18/the-benefits-and-costs-of-broadband-expansion/.
- 23.Atske S PA. Home broadband adoption, computer ownership vary by race, ethnicity in the U.S.: @pewresearch; 2022 [Available from: https://www.pewresearch.org/fact-tank/2021/07/16/home-broadband-adoption-computer-ownership-vary-by-race-ethnicity-in-the-u-s/.
- 24.E V. Digital divide persists even as Americans with lower incomes make gains in tech adoption. 2022.
- 25.Warnock RG. S.2729 - 117th Congress (2021–2022): device access for every American Act [webpage]. 2021 [updated 09/14/2021. Available from: https://www.congress.gov/bill/117th-congress/senate-bill/2729.
- 26.Emergency Connectivity Fund Federal Communications Commission. 2021 [updated 11-16-2022]. Retrieved October 24, 2022 from: https://www.fcc.gov/emergency-connectivity-fund.
- 27.Holko M, Litwin TR, Munoz F, Theisz KI, Salgin L, Jenks NP, et al. Wearable fitness tracker use in federally qualified health center patients: strategies to improve the health of all of us using digital health devices. NPJ Digit Med. 2022;5(1):53. doi: 10.1038/s41746-022-00593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021;18(8):581–599. doi: 10.1038/s41569-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Story MF. Maximizing usability: the principles of universal design. Assist Technol. 1998;10(1):4–12. doi: 10.1080/10400435.1998.10131955. [DOI] [PubMed] [Google Scholar]
- 30.The 7 principles. Centre for excellence in universal design. (n.d.). Retrieved October 24, 2022, from https://universaldesign.ie/What-is-Universal-Design/The-7-Principles/.
- 31.Blacklow SO, Lisker S, Ng MY, Sarkar U, Lyles C. Usability, inclusivity, and content evaluation of COVID-19 contact tracing apps in the United States. J Am Med Inform Assoc. 2021;28(9):1982–1989. doi: 10.1093/jamia/ocab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyles CR, Adrian Aguilera, Oanh Nguyen, Urmimala Sarkar. Bridging the digital health divide: how designers can create more inclusive digital. 2022.
- 33.Astbrink G, Beekhuyzen J. The synergies between universal design and user-centred design. Brisbane, Australia: Griffith University School of Computing and Information Technology; 2003. [Google Scholar]
- 34.Unertl KM, Schaefbauer CL, Campbell TR, Senteio C, Siek KA, Bakken S, et al. Integrating community-based participatory research and informatics approaches to improve the engagement and health of underserved populations. J Am Med Inform Assoc. 2016;23(1):60–73. doi: 10.1093/jamia/ocv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.(NCD-RisC) NRFC. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–80. [DOI] [PMC free article] [PubMed]
- 36.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artinian NT, Flack JM, Nordstrom CK, Hockman EM, Washington OG, Jen KL, et al. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res. 2007;56(5):312–322. doi: 10.1097/01.NNR.0000289501.45284.6e. [DOI] [PubMed] [Google Scholar]
- 38.Bosworth HB, Powers BJ, Olsen MK, McCant F, Grubber J, Smith V, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171(13):1173–1180. doi: 10.1001/archinternmed.2011.276. [DOI] [PubMed] [Google Scholar]
- 39.Hebert PL, Sisk JE, Tuzzio L, Casabianca JM, Pogue VA, Wang JJ, et al. Nurse-led disease management for hypertension control in a diverse urban community: a randomized trial. J Gen Intern Med. 2012;27(6):630–639. doi: 10.1007/s11606-011-1924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bove AA, Homko CJ, Santamore WP, Kashem M, Kerper M, Elliott DJ. Managing hypertension in urban underserved subjects using telemedicine–a clinical trial. Am Heart J. 2013;165(4):615–621. doi: 10.1016/j.ahj.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299(24):2857–2867. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parati G, Omboni S, Albini F, Piantoni L, Giuliano A, Revera M, et al. Home blood pressure telemonitoring improves hypertension control in general practice. The TeleBPCare study. J Hypertens. 2009;27(1):198–203. doi: 10.1097/HJH.0b013e3283163caf. [DOI] [PubMed] [Google Scholar]
- 43.McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376(9736):163–172. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 44.Wakefield BJ, Holman JE, Ray A, Scherubel M, Adams MR, Hillis SL, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E Health. 2011;17(4):254–261. doi: 10.1089/tmj.2010.0176. [DOI] [PubMed] [Google Scholar]
- 45.Piette JD, Datwani H, Gaudioso S, Foster SM, Westphal J, Perry W, et al. Hypertension management using mobile technology and home blood pressure monitoring: results of a randomized trial in two low/middle-income countries. Telemed J E Health. 2012;18(8):613–620. doi: 10.1089/tmj.2011.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46–56. doi: 10.1001/jama.2013.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magid DJ, Olson KL, Billups SJ, Wagner NM, Lyons EE, Kroner BA. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes. 2013;6(2):157–163. doi: 10.1161/CIRCOUTCOMES.112.968172. [DOI] [PubMed] [Google Scholar]
- 48.Kerry SM, Markus HS, Khong TK, Cloud GC, Tulloch J, Coster D, et al. Home blood pressure monitoring with nurse-led telephone support among patients with hypertension and a history of stroke: a community-based randomized controlled trial. CMAJ. 2013;185(1):23–31. doi: 10.1503/cmaj.120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKinstry B, Hanley J, Wild S, Pagliari C, Paterson M, Lewis S, et al. Telemonitoring based service redesign for the management of uncontrolled hypertension: multicentre randomised controlled trial. BMJ. 2013;346:f3030. doi: 10.1136/bmj.f3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart K, George J, Mc Namara KP, Jackson SL, Peterson GM, Bereznicki LR, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial) J Clin Pharm Ther. 2014;39(5):527–534. doi: 10.1111/jcpt.12185. [DOI] [PubMed] [Google Scholar]
- 51.Leiva A, Aguiló A, Fajó-Pascual M, Moreno L, Martín MC, Garcia EM, et al. Efficacy of a brief multifactorial adherence-based intervention in reducing blood pressure: a randomized clinical trial. Patient Prefer Adherence. 2014;8:1683–1690. doi: 10.2147/PPA.S66927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312(8):799–808. doi: 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- 53.Ogedegbe G, Tobin JN, Fernandez S, Cassells A, Diaz-Gloster M, Khalida C, et al. Counseling African Americans to control hypertension: cluster-randomized clinical trial main effects. Circulation. 2014;129(20):2044–2051. doi: 10.1161/CIRCULATIONAHA.113.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi SS, Tabaei BP, Angell SY, Rapin A, Buck MD, Pagano WG, et al. Self-blood pressure monitoring in an urban, ethnically diverse population: a randomized clinical trial utilizing the electronic health record. Circ Cardiovasc Qual Outcomes. 2015;8(2):138–145. doi: 10.1161/CIRCOUTCOMES.114.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bobrow K, Farmer AJ, Springer D, Shanyinde M, Yu LM, Brennan T, et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-text adherence support [StAR]): a single-blind, randomized trial. Circulation. 2016;133(6):592–600. doi: 10.1161/CIRCULATIONAHA.115.017530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res. 2017;19(7):e246. doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391(10124):949–959. doi: 10.1016/S0140-6736(18)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McManus RJ, Little P, Stuart B, Morton K, Raftery J, Kelly J, et al. Home and Online Management and Evaluation of Blood Pressure (HOME BP) using a digital intervention in poorly controlled hypertension: randomised controlled trial. BMJ. 2021;372:m4858. doi: 10.1136/bmj.m4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nwokeji U, Spaulding EM, Shan R, Turkson-Ocran RA, Baptiste D, Koirala B, et al. Health information technology use among persons with self-reported atherosclerotic cardiovascular disease: analysis of the 2011–2018 National Health Interview Survey. J Med Internet Res. 2021;23(8):e23765. doi: 10.2196/23765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brath H, Morak J, Kästenbauer T, Modre-Osprian R, Strohner-Kästenbauer H, Schwarz M, et al. Mobile health (mHealth) based medication adherence measurement - a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(Suppl 1):47–55. doi: 10.1111/bcp.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrella RJ, Stuckey MI, Shapiro S, Gill DP. Mobile health, exercise and metabolic risk: a randomized controlled trial. BMC Public Health. 2014;14:1082. doi: 10.1186/1471-2458-14-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- 63.Anand SS, Samaan Z, Middleton C, Irvine J, Desai D, Schulze KM, et al. A digital health intervention to lower cardiovascular risk: a randomized clinical trial. JAMA Cardiol. 2016;1(5):601–606. doi: 10.1001/jamacardio.2016.1035. [DOI] [PubMed] [Google Scholar]
- 64.Salisbury C, O’Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. BMJ. 2016;353:i2647. doi: 10.1136/bmj.i2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skobel E, Knackstedt C, Martinez-Romero A, Salvi D, Vera-Munoz C, Napp A, et al. Internet-based training of coronary artery patients: the Heart Cycle Trial. Heart Vessels. 2017;32(4):408–418. doi: 10.1007/s00380-016-0897-8. [DOI] [PubMed] [Google Scholar]
- 66.Hwang R, Bruning J, Morris NR, Mandrusiak A, Russell T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother. 2017;63(2):101–107. doi: 10.1016/j.jphys.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Harzand A, Witbrodt B, Davis-Watts ML, Alrohaibani A, Goese D, Wenger NK, et al. Feasibility of a smartphone-enabled cardiac rehabilitation program in male veterans with previous clinical evidence of coronary heart disease. Am J Cardiol. 2018;122(9):1471–1476. doi: 10.1016/j.amjcard.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng X, Su Y, Hu Z, Sun X, Li X, Dolansky MA, et al. Home-based telehealth exercise training program in Chinese patients with heart failure: a randomized controlled trial. Medicine (Baltimore) 2018;97(35):e12069. doi: 10.1097/MD.0000000000012069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart. 2019;105(2):122–129. doi: 10.1136/heartjnl-2018-313189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tekkeşin A, Hayıroğlu M, Çinier G, Özdemir YS, İnan D, Yüksel G, et al. Lifestyle intervention using mobile technology and smart devices in patients with high cardiovascular risk: a pragmatic randomised clinical trial. Atherosclerosis. 2021;319:21–27. doi: 10.1016/j.atherosclerosis.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 71.Bae JW, Woo SI, Lee J, Park SD, Kwon SW, Choi SH, et al. mHealth interventions for lifestyle and risk factor modification in coronary heart disease: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9(9):e29928. doi: 10.2196/29928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;143(1):4–34. doi: 10.1016/j.jtcvs.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 73.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82(1):E1–27. doi: 10.1002/ccd.24776. [DOI] [PubMed] [Google Scholar]
- 74.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 76.Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in medicare and veterans affairs populations: opportunity for improvement. Circulation. 2018;137(18):1899–1908. doi: 10.1161/CIRCULATIONAHA.117.029471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mead H, Ramos C, Grantham SC. Drivers of racial and ethnic disparities in cardiac rehabilitation use: patient and provider perspectives. Med Care Res Rev. 2016;73(3):251–282. doi: 10.1177/1077558715606261. [DOI] [PubMed] [Google Scholar]
- 78.Brady S, Purdham D, Oh P, Grace S. Clinical and sociodemographic correlates of referral for cardiac rehabilitation following cardiac revascularization in Ontario. Heart Lung. 2013;42(5):320–325. doi: 10.1016/j.hrtlng.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. Cardiac rehabilitation barriers by rurality and socioeconomic status: a cross-sectional study. Int J Equity Health. 2013;12:72. doi: 10.1186/1475-9276-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Content VG, Abraham HM, Kaihoi BH, Olson TP, Brewer LC. Novel virtual world-based cardiac rehabilitation program to broaden access to underserved populations: a patient perspective. JACC Case Rep. 2022;4(14):911–914. doi: 10.1016/j.jaccas.2022.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol. 2019;74(1):133–153. doi: 10.1016/j.jacc.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682–1690. doi: 10.1093/eurjpc/zwaa147. [DOI] [PubMed] [Google Scholar]
- 83.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 84.Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol. 2019;73(18):2354–2355. doi: 10.1016/j.jacc.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 85.Prasanna A, Miller HN, Wu Y, Peeler A, Ogungbe O, Plante TB, et al. Recruitment of Black adults into cardiovascular disease trials. J Am Heart Assoc. 2021;10(17):e021108. doi: 10.1161/JAHA.121.021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vilcant V, Ceron C, Verma G, Zeltser R, Makaryus AN. Inclusion of under-represented racial and ethnic groups in cardiovascular clinical trials. Heart Lung Circ. 2022;31(9):1263–1268. doi: 10.1016/j.hlc.2022.06.668. [DOI] [PubMed] [Google Scholar]
- 87.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 88.Dendale P, De Keulenaer G, Troisfontaines P, Weytjens C, Mullens W, Elegeert I, et al. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur J Heart Fail. 2012;14(3):333–340. doi: 10.1093/eurjhf/hfr144. [DOI] [PubMed] [Google Scholar]
- 89.Villani A, Malfatto G, Compare A, Della Rosa F, Bellardita L, Branzi G, et al. Clinical and psychological telemonitoring and telecare of high risk heart failure patients. J Telemed Telecare. 2014;20(8):468–475. doi: 10.1177/1357633X14555644. [DOI] [PubMed] [Google Scholar]
- 90.Vuorinen AL, Leppänen J, Kaijanranta H, Kulju M, Heliö T, van Gils M, et al. Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: randomized controlled trial. J Med Internet Res. 2014;16(12):e282. doi: 10.2196/jmir.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dang S, Karanam C, Gómez-Orozco C, Gómez-Marín O. Mobile phone intervention for heart failure in a minority urban county hospital population: usability and patient perspectives. Telemed J E Health. 2017;23(7):544–554. doi: 10.1089/tmj.2016.0224. [DOI] [PubMed] [Google Scholar]
- 92.Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392(10152):1047–1057. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 93.Melin M, Hägglund E, Ullman B, Persson H, Hagerman I. Effects of a tablet computer on self-care, quality of life, and knowledge: a randomized clinical trial. J Cardiovasc Nurs. 2018;33(4):336–343. doi: 10.1097/JCN.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 94.Chen C, Li X, Sun L, Cao S, Kang Y, Hong L, et al. Post-discharge short message service improves short-term clinical outcome and self-care behaviour in chronic heart failure. ESC Heart Fail. 2019;6(1):164–173. doi: 10.1002/ehf2.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.William AD, Kanbour M, Callahan T, Bhargava M, Varma N, Rickard J, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: The iREAD Study. Heart Rhythm. 2018;15(10):1561–1565. doi: 10.1016/j.hrthm.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 96.Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320(2):146–155. doi: 10.1001/jama.2018.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74(19):2365–2375. doi: 10.1016/j.jacc.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 98.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goldenthal IL, Sciacca RR, Riga T, Bakken S, Baumeister M, Biviano AB, et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J Cardiovasc Electrophysiol. 2019;30(11):2220–2228. doi: 10.1111/jce.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koh KT, Law WC, Zaw WM, Foo DHP, Tan CT, Steven A, et al. Smartphone electrocardiogram for detecting atrial fibrillation after a cerebral ischaemic event: a multicentre randomized controlled trial. Europace. 2021;23(7):1016–1023. doi: 10.1093/europace/euab036. [DOI] [PubMed] [Google Scholar]
- 101.Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan W, et al. Screening for atrial fibrillation in older adults at primary care visits: VITAL-AF randomized controlled trial. Circulation. 2022;145(13):946–954. doi: 10.1161/CIRCULATIONAHA.121.057014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lubitz SA FA, Atlas SJ, McManus DD, Singer DE, Pagoto S, Pantelopoulos A, Foulkes AS. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: The Fitbit heart study. Am Heart J2021. p. 16–26. [DOI] [PubMed]
- 103.Fallow BA, Tarumi T, Tanaka H. Influence of skin type and wavelength on light wave reflectance. J Clin Monit Comput. 2013;27(3):313–317. doi: 10.1007/s10877-013-9436-7. [DOI] [PubMed] [Google Scholar]
- 104.Shcherbina A, Mattsson CM, Waggott D, Salisbury H, Christle JW, Hastie T, et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med. 2017;7(2). [DOI] [PMC free article] [PubMed]
- 105.Valbuena VSM, Seelye S, Sjoding MW, Valley TS, Dickson RP, Gay SE, et al. Racial bias and reproducibility in pulse oximetry among medical and surgical inpatients in general care in the Veterans Health Administration 2013–19: multicenter, retrospective cohort study. BMJ. 2022;378:e069775. doi: 10.1136/bmj-2021-069775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Colvonen PJ, DeYoung PN, Bosompra NA, Owens RL. Limiting racial disparities and bias for wearable devices in health science research. Sleep. 2020;43(10). [DOI] [PMC free article] [PubMed]
- 107.Sieck CJ, Sheon A, Ancker JS, Castek J, Callahan B, Siefer A. Digital inclusion as a social determinant of health. NPJ Digit Med. 2021;4(1):52. doi: 10.1038/s41746-021-00413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Affordable Connectivity Program 2021 [updated 2021–12–15. Available from: https://www.fcc.gov/acp.
- 109.Force DLT. Digital literacy, libraries, and public policy 2013 [Available from: http://hdl.handle.net/11213/16261.
- 110.Equity-centered design framework — Stanford d.school 2022 [Available from: https://dschool.stanford.edu/resources/equity-centered-design-framework.
- 111.D M. Diversity in tech — information is beautiful: @infobeautiful; 2022 [Available from: https://informationisbeautiful.net/visualizations/diversity-in-tech/.
- 112.Fry R, Kennedy, B., Funk, C. STEM jobs see uneven progress in increasing gender, racial and ethnic diversity. 2021.
- 113.Digital Equity Act Programs | Internet for All 2022 [Available from: https://www.internetforall.gov/program/digital-equity-act-programs.