Abstract
Abstract
Candida albicans is the main conditional pathogenic fungus among the human microbiome. Extracellular vesicles (EVs) secreted by C. albicans are important for its pathogenesis. However, the effects and mechanisms of EVs on C. albicans own growth are not clear. Here, we isolated EVs from C. albicans cells grown in four culture media, including RPMI 1640, DMEM, YPD, and YNB, and measured their effects on the own growth of C. albicans in these media. All the C. albicans EVs from the four media could promote the growth of C. albicans in RPMI 1640 and DMEM media, but had no effects in YPD and YNB media, indicating that the effects of EVs on C. albicans growth were dependent on some media contents. By comparing the media contents and transcriptome analysis, arginine was identified as the key factor for the growth promotion of C. albicans EVs. EVs activated the l-arginine/nitric oxide pathway to promote the growth of C. albicans through that EVs increased the NO levels and upregulated the expression of NO dioxygenase gene YHB1 to reduce the intracellular reactive oxygen species (ROS) and cell apoptosis. During the host cell infections, C. albicans EVs synergistically enhanced the destructive effects of C. albicans to host cells, including RAW264.7, HOK, TR146, and HGEC, suggesting that the growth promotion by EVs enhanced the pathogenesis of C. albicans. Our results demonstrated the important roles of EVs on C. albicans own growth for the first time and highlight its synergism with C. albicans to increase the pathogenesis.
Key points
• C. albicans extracellular vesicles (EVs) promoted its own growth.
• EVs activated the l-arginine/NO pathway to reduce ROS and apoptosis of C. albicans.
• EVs enhanced the damage to the host cell caused by C. albicans.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00253-022-12300-7.
Keywords: Fungal infection, Extracellular vesicles, Pathogenesis, Intracellular ROS, Cell apoptosis
Introduction
Candida albicans is one of the most common opportunistic fungal pathogens (Lee et al. 2021). It can colonize in the oral cavity, vagina, and digestive tract of healthy people, and become pathogenic when the host is under low immune conditions (Pappas et al. 2016), particularly in immunocompromised individuals, such as patients with AIDS, patients undergoing chemotherapy, and individuals receiving immunosuppressant therapies (Lohse et al. 2018). C. albicans infections include superficial mucosal infection, dermal infections, and disseminated bloodstream infections with mortality rates above 40% (Calderone and Fonzi 2001; Pappas et al. 2004; Wenzel 1995). It is also one of the most common coinfected fungal species with SARS-CoV-2 in COVID-19 (Chen et al. 2020b).
Extracellular vesicles (EVs) are naturally released nano-scale particles from different cells (Oliveira et al. 2010; van Niel et al. 2018). They were once considered carriers of cell “garbage” or debris shed by cells, but now, EVs have proved to be important for the cell communications in unicellular and multicellular organisms (Dawson et al. 2020; Rybak and Robatzek 2019). C. albicans EVs are composed of proteins, lipids, nucleic acids, and carbohydrates (de Toledo Martins et al. 2019), including proteins with 1, 3-β-glucosidase activity (Gow and Hube 2012), enolase, 3-phosphate dehydrogenase (Gpdh), phosphoglycerate kinase (Pgk), and phosphoglycerate mutase (Karkowska-Kuleta et al. 2011), which were found to be highly related to the fungal cell attachment and the interactions with the host (Sandini et al. 2011). C. albicans EVs were capable to regulate its pathogenic process and drug resistance (Rodrigues et al. 2013; Roszkowiak et al. 2019). An endosomal sorting complex required for transport (ESCRT)-deficient mutant of C. albicans reduced EVs production and greatly increased sensitivity to the antifungal drug fluconazole, indicating the effects of EVs on the antifungal drug responses of C. albicans (Zarnowski et al. 2018). C. albicans EVs also stimulated macrophages to produce NO and cytokine IL-10, and dendritic cells to produce cytokines such as TGF-β and TNF-α (Zamith-Miranda et al. 2018) indicating the crosstalk between C. albicans EVs and host immune cells. Recently, EVs produced by C. albicans were found to inhibit its biofilm formation and yeast to hyphae transition. The sesquiterpenes, diterpenes, and fatty acids from C. albicans EVs stopped its filamentation and promoted the formation of pseudo hyphae (Bitencourt et al. 2022; Honorato et al. 2022). However, the effects and mechanisms of C. albicans EVs on its own growth are still unclear.
Arginine is one of the most versatile amino acids in cells (Zou et al. 2019). It is a precursor not only for protein synthesis but also for the synthesis of nitric oxide, urea, polyamines, proline, glutamate, creatine, and agmatine (Wu and Morris 1998). Arginine has a variety of functions, including immunomodulatory, antioxidant, anti-inflammatory, regulation of cell proliferation, anti-apoptosis, and regulation of lipid metabolism. It has been also widely used in clinical nutritional therapy (Gogoi et al. 2016; Khalaf et al. 2019; Szefel et al. 2019). Currently, there are four main arginine metabolic pathways: (1) converting to creatine under the action of arginine-glycine transferase (Barcelos et al. 2016); (2) biosynthesizing agmatine through arginine decarboxylase (ADC) decarboxylation (Hyvönen et al. 2020); (3) producing bioactive nitric oxide (NO) and citrulline through nitric oxide synthase (eNOS) (Wu et al. 2021); (4) decomposing into ornithine and urea by arginase (Longo et al. 2020). C. albicans can metabolize arginine and grow on the media in which arginine was the sole nitrogen source (Schaefer et al. 2020). Arginine can promote the hyphal growth and biofilm formation of C. albicans and also enhance the cross-kingdom interactions with bacteria (Xiong et al. 2022). Arginine is a precursor of NO as well, while the endogenous NO produced by C. albicans protects itself against azoles (Li et al. 2016). However, the role of arginine in the actions of C. albicans EVs remains unknown.
In our study, aiming to reveal the effects of EVs on the growth of C. albicans, EVs from the C. albicans cells in four media were isolated. We found that EVs were proved to promote the growth of C. albicans through the l-arginine/nitric oxide pathway to reduce intracellular reactive oxygen species (ROS) and cell apoptosis for the first time. Our results highlighted the important roles of EVs on C. albicans own growth and pathogenesis.
Materials and methods
Strain and culture conditions
C. albicans SC5314 (ATCC MYA − 2876) was grown in YPD plates (4 g yeast extract, 8 g anhydrous glucose, 8 g peptone, 8 g agar dissolved in 400 mL deionized water) at 37 °C overnight (Chen et al. 2020a; Zhou et al. 2021; Zhu et al. 2021). For the treatment with EVs, the colonies of C. albicans were picked out and placed into phosphate buffered saline (PBS), adjust the final suspensions to 1 × 106 colony-forming unit (CFU)/mL concentration in different medium, including YPD, YNB (Solarbio, Beijing, China), RPMI 1640 (Thermo Fisher Scientific, Waltham, MA, USA), and DMEM (Gibco, Grand Island, NY, USA), and incubated at 37 °C.
Isolation of EVs by overspeed centrifugation
C. albicans EVs were isolated by overspeed centrifugation as described previously (Martínez-López et al. 2022). Briefly, C. albicans was incubated in YPD liquid medium overnight at 37 °C. The precipitates were collected by centrifugation at 4000 g 4 °C and resuspended in PBS. After being counted under a microscope using cell counting plates, C. albicans was inoculated in YPD/YNB/RPMI 1640/DMEM medium to a final concentration of 1 × 106 CFU/mL and cultivated at 30 °C and 150 rpm for 72 h. C. albicans supernatant was then collected by centrifugation at 15,000 g for 15 min at 4 °C. The supernatant was filtered by a filter with a pore size of 0.35 μm. Then, a 100 KD ultrafiltration tube was used to concentrate the supernatant to 1/20 of the original volume. The supernatant was removed by centrifugation at 4 °C and 100,000 g for 2 h, and the precipitate was rinsed with frozen PBS. The precipitate was centrifuged again at 4 °C and 100,000 g for 2 h. After the supernatant was removed, the precipitate was resuspended with 1 mL frozen PBS. The final EVs’ suspension was collected and filtered with a 0.22 μm filter, stored at 4 °C until used.
Identification of EVs
Scanning electron microscope (SEM) observation
EVs were observed by SEM as described previously (Chen et al. 2017). Ten microliters of EVs collected above was evenly spread on round cell climbing sheets, air-dried at room temperature, and fixed in 2.5% glutaraldehyde solution overnight at 4 °C. The fixed EVs’ samples were dehydrated successively with different concentrations of ethanol (50%, 60%, 70%, 80%, 90%, 95%, and absolute ethanol), and each concentration was dehydrated for 15 min. After drying and spraying gold, the samples were observed by scanning electron microscopy Tecnai G2 F20 S-TWIN (FEI Company, Hillsboro, OR, USA).
Transmission electron microscopy (TEM) observation
EVs were observed by TEM (Karkowska-Kuleta et al. 2020). Briefly, 100 μL of EVs was aspirated and dropped onto the copper grid for 10 min. Fifty microliters of 1% phosphotungstic acid was aspirated and stained on the copper grid for 2 min. Samples were rinsed with deionized water for 2 times, air dried, and observed on the transmission electron microscopy (FEI Company, Hillsboro, OR, USA).
Particle size detection
Particle size distribution of EVs was detected by nano-size analyzer (Honorato et al. 2022). Briefly, the suspension of EVs was diluted to 2 mL and added to the quartz dish of nano-size analyzer Zetasizer Nano ZS (Malvern Panalytical, Malvern, UK). The range of EVs’ particle size was detected at 25 °C.
EVs’ protein concentration detection
The concentrations of EVs’ proteins were measured according to the instructions of BCA protein quantification kit (Beyotime, Chengdu, China). Briefly, EVs’ suspensions were absorbed into a 96-well plate, and 200 μL of reaction solution was added to each well. The samples were then incubated at 37 °C for 30 min. The absorbance value of each well sample at A562 nm was detected by microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The absorbance values of different concentrations of EVs’ protein were calculated according to the BCA standard curves.
Colony forming unit count
C. albicans were treated with 15 μg/mL EVs from YNB or PBS control in different medium at 37 °C for 24 h. The influence of different amino acids on promoting proliferative effects was tested in 0.2% glucose YNB and YPD media in addition of 0.02%, 0.05%, 0.1%, 0.2%, 0.3% l-arginine, 0.2% l-cysteine, 0.2% l-proline, 0.2% l-leucine, 0.2% l-isoleucine, 0.2% l-valine, 0.2% l-methionine, 0.2% l-glutamic acid, 0.2% l-ornithine, 0.2% l-citrulline, and 0.2% l-histidine, respectively (all the amino acids were from Solarbio, Beijing, China). The influence of different glucose concentrations on promoting proliferative effects was tested using media including in RPMI 1640 medium with the addition of 0.2%, 2% glucose, and YNB medium in addition of 0.2%, 0.4%, 0.8%, and 2% glucose, respectively. The influence of oxidants and antioxidants on promoting proliferative effects was tested using media including 0.2% glucose YNB with 0.25 mM, 0.5 mM H2O2 (Boster, Wuhan, China) or 0.125 mM, and 0.25 mM glutathione (GSH) (Solarbio, Beijing, China). After being mixed in 96-well plates, C. albicans cells were serially diluted with PBS; then, 150 μL of C. albicans dilutions was spread on YPD plates. After 12 h of culture at 37 °C, the CFUs were counted. All the experiments were repeated in triple.
Growth curve of C. albicans
The growth curves of C. albicans were detected as described previously (Wang et al. 2021). C. albicans was treated with 15 μg/mL EVs and PBS, then inoculated in YNB medium to a concentration of 1 × 104 CFU/mL into 96-well plates, covered with 100 μL mineral oil (Sigma-Aldrich, Saint Louis, MO, USA) to prevent evaporation. The plates were incubated in a Multiskan Spectrum (Chro Mate1, Awareness Technology, Palm City, FL, USA) at 37 °C. The absorbance at OD600 nm was measured every 30 min. Absorbance at different time points was plotted to generate the growth curve. Three duplicate wells were set for each group.
Determination of cellular reactive oxygen species
Reactive Oxygen Species Assay Kit (Beyotime, Chengdu, China) was used for ROS (reactive oxygen species) detection. C. albicans was inoculated in 96-well plates with three replicate wells in each group. After the treatment with 15 μg/mL EVs, DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate) was added with a final concentration of 10 μM in 3 h, 6 h, 9 h, 12 h, and 24 h, and transferred to incubation in 37 °C for 1 h. PBS was served as control. A microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) was used to detect the fluorescence intensity with the wavelength of excitation light at 488 nm and emission light at 525 nm. After fluorescence intensity detection, CFU was counted for each well and the related ROS levels were normalized by CFU.
Fungal cell apoptosis analyzed by flow cytometry
Flow cytometry was performed using the Annexin V-FITC/PI Apoptosis Detection Kit (Yeason, Beijing, China). After the treatment of 15 μg/mL EVs and PBS control at 37 °C for 6 h, C. albicans was centrifuged at 300 g for 5 min at 4 °C. The precipitate was washed twice with pre-cooled PBS. After the centrifugation to collect the precipitate, PBS was discarded, and then resuspended with 100 μL binding buffer. Then, 5 μL Annexin V-FIFC and 10 μL PI were added and the samples were mixed well. After the reaction at room temperature for 10–15 min in the dark, 400 μL binding buffer was added. A flow cytometer (Beckman FC500, Carlsbad, CA, USA) was used for analysis, and the excitation and emission wavelengths were set to 488 and 525 nm, respectively. All experiments were performed in triplicate.
Transcriptomic analysis
Transcriptomic analysis was conducted as described previously (Yawen et al. 2022). Total RNA was extracted from the C. albicans cells treated with 15 μg/mL EVs or PBS control at 37 °C for 6 h by using TRIzol Reagent (Plant RNA Purification Reagent for plant tissue) according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Genomic DNA was removed using DNase I (Takara Inc., Chengdu, China). Then, RNA quality was determined by 2100 Bioanalyser (Agilent, Chengdu, China) and quantified using the ND-2000 (NanoDrop Technologies, Thermo Fisher Scientific, Waltham, MA, USA). Only high-quality RNA sample (OD 260/280 = 1.8 ~ 2.2, OD 260/230 ≥ 2.0, RIN ≥ 6.5, 28S:18S ≥ 1.0, > 1 μg) was used to construct a sequencing library (Hu et al. 2021). Then, sequencing is done using a sequencer Illumina Novaseq 6000 (Illumina, San Diego, CA, USA) in the Majorbio company (Shanghai, China).
Determination of nitric oxide content
Measurement of NO in C. albicans was conducted as described by Li et al. (2016). C. albicans was inoculated in 96-well plates with three replicate wells in each group. After the treatment with 15 μg/mL EVs and PBS control at 37 °C for 1 h, DAF-FM DA (3-amino,4-aminomethyl-2′,7′ -difluorescein, diacetate) (Beyotime, Chengdu, China) was added at a final concentration of 5 μM, and then incubated at 35 °C for 1 h in the absence of light. The excitation and emission light wavelengths were set as 495 and 515 nm, respectively, with microplate readers (Thermo Fisher Scientific, Waltham, MA, USA) to detect the fluorescence intensity. After fluorescence intensity detection, CFU was counted for each well and the related NO levels were normalized by CFU.
Lactate dehydrogenase (LDH) cytotoxicity assay
LDH cytotoxicity assay was conducted as described previously (Zhou et al. 2021). Macrophage RAW264.7, human oral keratinocytes (HOK), human squamous carcinoma cells (TR146), and human gingival epithelial cells (HGEC) were inoculated in 96-well plates at 1 × 105 cells/mL using DMEM medium without fatal bovine serum (FBS) and antibiotics. The cells were cultured overnight at 37 °C, 5% CO2. Then, PBS, C. albicans, 15 μg/mL EVs, and C. albicans + 15 μg/mL EVs were added and treated for 24 h. EVs were isolated from RPMI 1640 medium. Cytotoxicity LDH Assay Kit (Dojindo, Beijing, China) was used for LDH detection. After the treatment, 50 μL of Dye Mixture and Assay Buffer was added. After 30-min reaction, 25 μL stop solution was added. The absorbance of each well sample at A490 nm was detected by microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). All experiments were performed in triplicate.
RNA extraction and qPCR
qPCR was conducted as described previously (Zhu et al. 2021). C. albicans treated with 15 μg/mL EVs and PBS control at 37 °C for 6 h. The samples were collected by centrifugation at 4000 g for 10 min at 4 °C after treatment with EVs and PBS control in C. albicans and resuspended with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The total RNA was extracted after the wall was broken with liquid nitrogen. Excess DNA in RNA was removed and reverse transcribed using the PrimeScriptTM RT Reagent Kit Reverse Transcription Kit (Takara Inc., Chengdu, China) (Hu et al. 2021; Kong et al. 2022). Real-time PCR was performed using reverse transcription cDNA as template. Gene amplification was performed following the SYBR® PremixEx Taq™ kit (Takara Inc., Chengdu, China) two-step strategy: (1) 95 °C for 30 s; (2) 40 PCR cycles (95 °C for 5 s, a gene-specific annealing temperature for 30 s). The primer sequence was 18S-F: TGGAAGCTGCTGGTATTGAC, 18S-R: TCCTTTTGCATACGTTCAGC. YHB1-F: ATCGATTTAGAAGCCGCAGA, YHB1-R: GACCACGTTCAGGTTTTGGT. The qPCRs were run on LightCycler 480 II (Roche, Basel, Switzerland). The formula for calculating the relative value of gene expression was 2−△△Ct (Zhou et al. 2018). All experiments were performed in triplicate.
Statistical analysis
Data are represented as the mean ± standard deviations (SD) from at least three biological replicates and three technical replicates. The level of significance was analyzed by unpaired t-test and one-way ANOVA. p < 0.05 was considered significant. The Jin value method formula was used to calculate the collaboration index: EA+B = EA + EB-EA EB, Q > 1.15 indicates a synergistic effect (Jin 2004). All statistical analyses were performed using GraphPad Prism 8 v8.3.1 (GraphPad software, Beijing, China).
Results
EVs promoted the growth of C. albicans
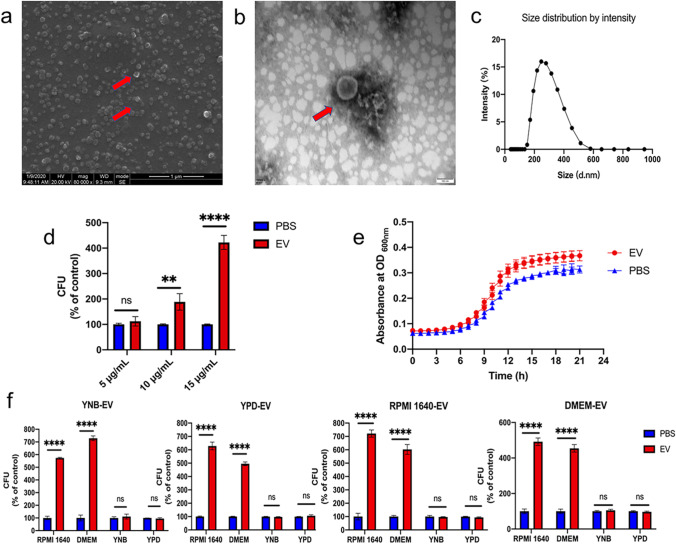
EVs were successfully isolated from C. albicans cells grown in different media by overspeed centrifugation. C. albicans EVs were nanoparticles with bilayer membranes (Fig. 1a, b) with the sizes arranging from 100 to 500 nm (Fig. 1c). The protein concentrations of EVs were 200–300 μg/mL. When C. albicans was treated with EVs (5, 10, and 15 μg/mL), EVs significantly promoted the growth of C. albicans at dose dependent manner (Fig. 1d, e) and 15 μg/mL was then selected for further evaluation. To determine whether different media would affect the C. albicans EVs’ promoting properties, EVs from C. albicans cells grown in four culture media including RPMI 1640, DMEM, YPD, and YNB media were isolated. Interestingly, all the isolated EVs significantly promoted the growth of C. albicans in RIMI 1640 and DMEM media but had no effects in YNB or YPD medium (Fig. 1f), indicating that the growth promotion of EVs was dependent on the contents of C. albicans growth media.
Fig. 1.
C. albicans EVs promoted its growth. a SEM of C. albicans EVs isolated from YNB medium. Scale bar, 1 μm. b TEM of C. albicans EVs isolated from YNB medium. Scale bar, 100 nm. c Range of size distribution of C. albicans EVs isolated from YNB medium measured by nanoparticle tracking analysis (NTA). d CFUs of C. albicans grown in RPMI 1640 medium treated with different concentrations of EVs and PBS were served as control. e Growth curves of C. albicans treated with 15 μg/mL EVs and PBS were served as control. f C. albicans treated with different EVs grew in different media. All of the experiments were performed in three distinct replicates, and the data are presented as the means ± SD, **p < 0.05, ****p < 0.0001, no significance (ns) p > 0.05
EVs regulated the arginine metabolism of C. albicans
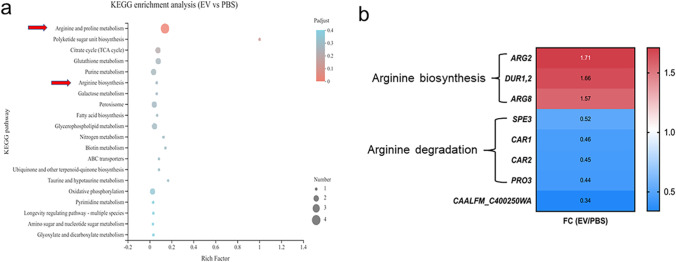
To identify how the EVs promoted the growth of C. albicans, transcriptomic analysis of C. albicans treated by EVs in RPMI 1640 medium was performed. EVs significantly upregulated 150 genes and downregulated 315 genes compared to the control group (Supplemental Fig. S1a). GO enrichment analysis indicated that the cellular and metabolic processes of EVs treated C. albicans were significantly changed (Supplemental Fig. S1b). KEGG pathway enrichment analysis indicated that the arginine and proline metabolism pathway and arginine biosynthesis pathway were significantly enriched after the EVs’ treatment (Fig. 2a). The genes related to arginine biosynthesis pathway were significantly upregulated, while the genes associated with arginine degradation were significantly downregulated (Fig. 2b, Supplemental Fig. S2a, b, Supplemental Table S1). The transcriptome data suggested that the growth promotion capability of EVs on C. albicans own growth was highly associated with C. albicans arginine metabolism related pathways.
Fig. 2.
Transcriptomic analysis of C. albicans treated by EVs. a The enriched KEGG pathways of C. albicans treated with 15 μg/mL EVs compared to that from C. albicans treated with PBS. b Heat map of shifted specific genes from the arginine biosynthesis and arginine degradation pathways
C. albicans growth promotion by EVs was dependent on arginine
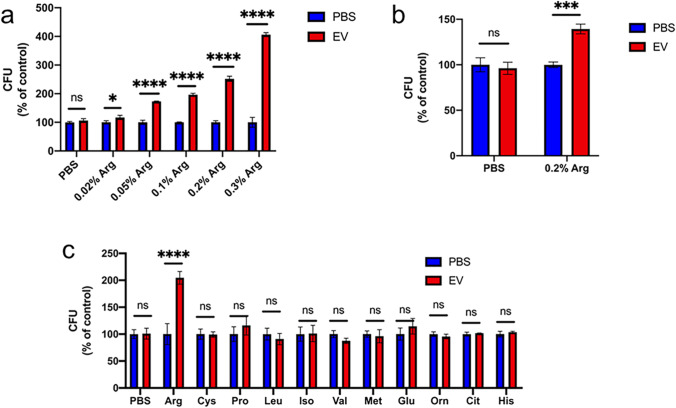
To confirm whether EVs’ growth promotion capability depended on arginine according to the transcriptomic analysis, the components of the four different media were firstly compared. The media differences between growth promotion media (RPMI 1640 and DMEM) and non-promotion media (YNB and YPD) were mainly glucose and amino acids (Supplemental Table S2). Then, glucose and amino acids were added to YNB and YPD media to verify whether they could affect the growth promotion capability of EVs. The addition of glucose did not affect the growth promotion characters of EVs on C. albicans in both promotion and non-promotion media, including YNB and RPMI 1640 media, respectively (Supplemental Fig. S3); however, when arginine was added to non-promotion media (YNB and YPD media), EVs significantly promoted the growth of C. albicans at dose dependent manner (Fig. 3a, b). Then the growth promotion was confirmed whether it was arginine specific by adding different amino acids into YNB media, and it turned out that EVs can only promote the growth of C. albicans under the addition of arginine (Fig. 3c) indicating that the promotion of C. albicans growth by EVs was dependent on arginine.
Fig. 3.
The growth promotion of EVs was dependent on arginine. a Effects of different arginine concentrations on the growth regulation induced by 15 μg/mL EVs. EVs were isolated from YNB medium and C. albicans grew in YNB medium. PBS was served as control. b Effects of the growth regulation induced by 15 μg/mL EVs in YPD medium with or without arginine. EVs were isolated from YNB medium. PBS was served as control. c Effect of media containing different amino acids on the growth regulation induced by 15 μg/mL EVs. EVs were isolated from YNB medium and C. albicans grew in YNB medium. All of the experiments were performed in three distinct replicates, and the data are presented as the means ± SD, *p < 0.05, ***p < 0.001, ****p < 0.0001, no significance (ns) p > 0.05
EVs activated l-arginine/nitric oxide pathway
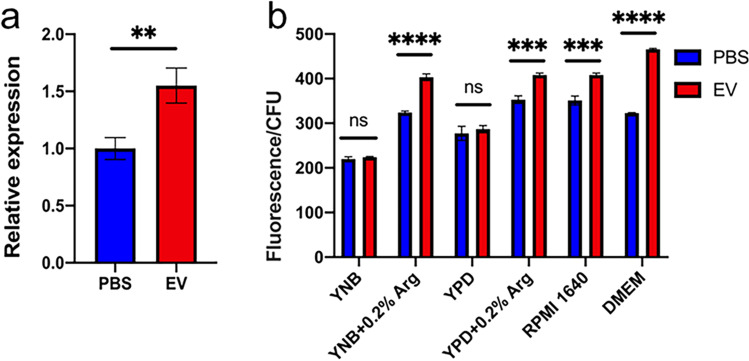
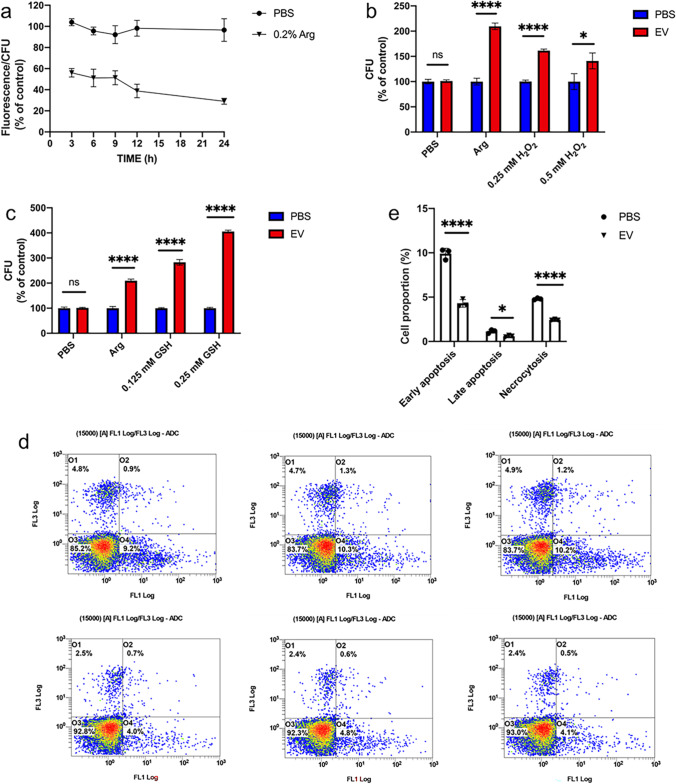
Since the arginine biosynthesis pathway was upregulated but the degradation pathway was downregulated, we then tested whether the upregulated arginine biosynthesis activated the l-arginine/nitric oxide pathway. YHB1, a nitric oxide dioxygenase gene, plays essential roles in nitric oxide scavenging/detoxification in C. albicans (Ullmann et al. 2004). Therefore, the expression of YHB1 was firstly measured. EVs significantly upregulated the expression of YHB1 (Fig. 4a). The intracellular nitric oxide (NO) levels of C. albicans treated by EVs in different media were then measured. EVs significantly increased the intracellular NO levels of C. albicans grown in RPMI 1640 and DMEM media, as well as the YNB and YPD media with the addition of arginine, but had no effects in YNB and YPD media (Fig. 4b), in line with the arginine dependent growth promotion of EVs, and also indicating that EVs promote the growth of C. albicans through the activation of the l-arginine/nitric oxide pathway.
Fig. 4.
EVs activated the l-arginine/NO pathway. a YHB1 mRNA expression in C. albicans after 15 μg/mL EVs’ treatment. EVs were isolated from YNB medium and C. albicans grew in YNB medium with 0.2% arginine. b Intracellular NO content in C. albicans after treatment with 15 μg/mL EVs in different medium. EVs were isolated from YNB medium. All of the experiments were performed in three distinct replicates, and the data are presented as the means ± SD, **p < 0.01, ***p < 0.001, ****p < 0.0001, no significance (ns) p > 0.05
EVs reduced the ROS level of C. albicans
The accumulation of intracellular NO levels plays important roles in the regulation of oxidative stress in cells (Araujo and Welch 2006; Förstermann et al. 2017). Then, the reactive oxygen species (ROS) were measured from the C. albicans cells since the EVs significantly increased the intracellular NO levels of C. albicans in the presence of arginine. EVs significantly reduced the intracellular ROS contents in C. albicans to 65% compared to that from the PBS control group at 3 h and 35% at 24 h (Fig. 5a). The addition of oxidant H2O2 and antioxidant GSH was employed to further validate the effects of EVs on ROS production. The addition of H2O2 significantly reduced the growth promotion abilities of EVs on C. albicans, while the addition of GSH significantly enhanced the growth promotion of EVs (Fig. 5b, c), further indicating that EVs promoted the growth of C. albicans by the reduction of the ROS level. ROS is an important inducer for cell apoptosis. Therefore, the fungal cell apoptosis of C. albicans treated by EVs was then measured. EVs significantly reduced the ratio of early apoptosis, late apoptosis, and cell necrosis in C. albicans (Fig. 5d, e), indicating that EVs inhibited the ROS production through the activation of l-arginine/nitric oxide pathway to reduce the cell apoptosis and to promote the growth of C. albicans.
Fig. 5.
EVs decreased ROS accumulation of C. albicans to reduce fungal cell apoptosis. a Intracellular ROS content in C. albicans. The triangle-labeled group was C. albicans grew in YNB medium with 0.2% arginine, and the circle-labeled group was C. albicans grew in YNB medium without arginine. The control group was C. albicans treated with PBS, and the experimental group was C. albicans treated with 15 μg/mL EVs. The percentages shown in the figure are C. albicans treated with EVs vs C. albicans treated with PBS. b Effect of oxidant (H2O2) treatment on the growth regulation of C. albicans by 15 μg/mL EVs. c Effect of antioxidant (GSH) treatment on the growth regulation of C. albicans by 15 μg/mL EVs. d Flow cytometry is used to detect the proportion of early apoptosis, late apoptosis, and cell necrosis in C. albicans. The first three figures are PBS-treated groups, and the last three figures are 15 μg/mL EV-treated groups. e Statistical analysis of flow cytometry. All of the experiments above were used EVs isolated from YNB medium and C. albicans grew in YNB medium with 0.2% arginine. All the experiments were performed in three distinct replicates, and the data are presented as the means ± SD, *p < 0.05, ****p < 0.0001, no significance (ns) p > 0.05
EVs enhanced the damage to the host cell caused by C. albicans
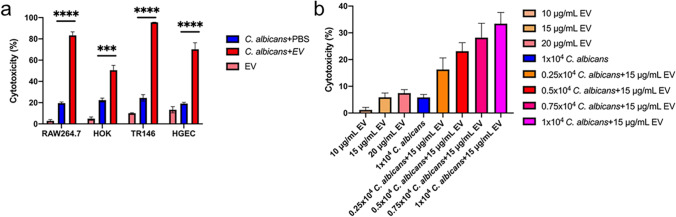
The pathogenesis of C. albicans affected the growth promotion of EVs was then evaluated. EVs alone showed weak capabilities on different host cells, including macrophage RAW264.7, human oral keratinocytes (HOK), human squamous carcinoma cells (TR146), and human gingival epithelial cells (HGEC). However, EVs significantly increased the cell damages caused by C. albicans (Fig. 6a). The synergistic effect between EVs and C. albicans was evaluated using the Jin value method (Jin 2004). The Q values of RAW264.7, HOK, TR146, and HGEC were respectively 3.82, 1.92, 2.98, and 2.35, indicating that EVs and C. albicans had a synergistic effect on cell destruction. Meanwhile, the host cell damage caused by EVs and C. albicans combinations was also positively related to the C. albicans cell numbers (Fig. 6b).
Fig. 6.
EVs’ synergies with C. albicans to destroy host cells. a Percentage of cytotoxicity of different cells after 15 μg/mL EVs and PBS control treatment. EVs were isolated from RPMI 1640 medium and the cells grew in DMEM medium. b Cytotoxicity of different concentrations of C. albicans and EVs in RAW264.7 cells. All of the experiments were performed in three distinct replicates, and the data are presented as the means ± SD, ***p < 0.001, ****p < 0.0001
Discussion
Fungal EVs were first reported in 1972 (Gibson and Peberdy 1972) and further studies have proved the important roles of fungal EVs in drug resistance, fungal pathogenicity, and host immune response (Liebana-Jordan et al. 2021; Reales-Calderón et al. 2017; Yang et al. 2020). Recently, Zarnowski et al. (2021) investigated the effects of C. albicans EVs on the biofilm development and found that EVs could promote the formation of extracellular matrix in C. albicans to increase the antifungal drug resistance and the adhesion and spread of C. albicans. Honorato et al. (2022) found that EVs inhibited C. albicans hyphal development and promoted pseudomycelial formation with multiple budding sites, indicating that fungal EVs were messengers affecting biofilm formation, morphogenesis, and virulence of C. albicans. Bitencourt et al. (2022) proposed that EVs released from filamentous C. albicans promoted the development of the C. albicans mycelial state, while EVs released from yeast-like C. albicans promoted the proliferation of the C. albicans yeast state. Different media greatly influenced the morphology of C. albicans and the contents of EVs (Brown et al. 2014). In our study, EVs isolated from different media, including YPD, YNB, DMEM, and RPMI 1640, could significantly promote the proliferation of C. albicans grown in RPMI 1640 and DMEM media, but not YNB and YPD media, suggesting that the EVs’ growth promotion capability dependents on the medium in which C. albicans growth, rather than the medium in which the EVs were isolated. According to previous studies, 1202 proteins were identified in C. albicans EVs (Dawson et al. 2020). The protein species of C. albicans EVs in mycelial and yeast states are different, while the protein content of C. albicans EVs from the yeast state was 10–100 times higher than that from mycelium state (Zarnowski et al. 2018). Lipids also play an important role due to the similarity of lipid composition within EVs (Rodrigues et al. 2007). The major lipids found in EVs are phospholipids, ergosterols, and ceramides, which are major components of cell membranes (Vargas et al. 2015). Ceramide, known as a “virulence regulator,” is an important immunogenic molecule, and antibodies against ceramide can inhibit fungal growth (Nimrichter and Rodrigues 2011). The protein contents including the proteins with 1, 3-β-glucosidase activity (Gow and Hube 2012), β-1, 6-glucan, mannanan, 3-phosphate dehydrogenase (Gpdh), phosphoglycerate kinase (Pgk), and phosphoglycerate mutase (Karkowska-Kuleta et al. 2011) from EVs can directly affect fungal growth, cell attachment, and host recognition (Sandini et al. 2011), and these protein components may be the key components to promote the growth of C. albicans. Further investigations are needed to identify the key factors from EVs that promote the own growth of C. albicans.
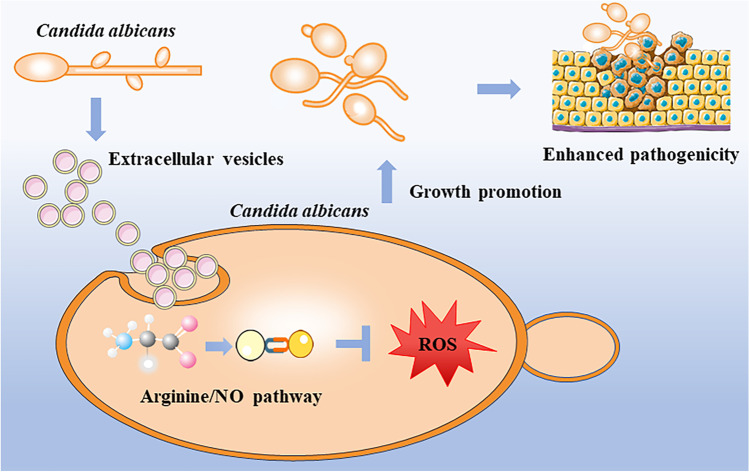
Arginine has a variety of functions, including antioxidant, anti-inflammatory, anti-apoptosis, proliferation promotion, and lipid metabolism regulation (Bronte and Zanovello 2005; Luiking et al. 2005; Popovic et al. 2007; Stechmiller et al. 2005). The l-arginine/nitric oxide pathway is widely recognized as an important regulator of cellular function and communication (Gogoi et al. 2016). It has been broadly applied in the development of septic shock, hypertension, and atherosclerosis, as well as the antihypertensive effect of invertase inhibitors (Palmer 1993; Wu et al. 2021). In this pathway, arginine acts as a substrate to generate NO by endothelial nitric oxide synthase (eNOS) (Moncada and Higgs 1993). NO is a free radical gas that can interact with biological free radicals. It is a potent free radical scavenger/terminator and antioxidant (Boudko 2007). The role of l-arginine/nitric oxide pathway in fungi has not been well studied. Li et al. (2016) identified the presence of endogenous NO in C. albicans and confirmed its participation in the oxidative stress response of C. albicans, but they failed to identify the classic eNOS sequences from the C. albicans genome indicating that C. albicans has a new type of enzyme with NOS-like activity. YHB1 encodes a nitric oxide dioxygenase with the function of nitric oxide scavenging/detoxification. YHB1 can be rapidly activated by NO, while the high level of intracellular NO can also upregulate its expression to enhance the reduction of intracellular oxidative stress (Ullmann et al. 2004). In our study, we proved that EVs upregulated the expression of YHB1 and increased the intracellular NO levels of C. albicans under arginine condition, while EVs also decreased the ROS accumulation and related cell apoptosis of C. albicans. Combining the transcriptome analysis, our results indicated that at the presence of arginine, EVs upregulated the arginine biosynthesis and activated the l-arginine/nitric oxide pathway to increase the intracellular NO levels, then inhibited the ROS accumulation to reduce the cell apoptosis (Fig. 7). In this study, we also found that although EVs had weak host cell damage abilities, but EVs significantly enhanced the cell damage abilities of C. albicans for the first time, indicating that EVs promoted the pathogenesis of C. albicans through the growth promotion. This might provide us with a new way to reduce the pathogenesis of pathogenic fungi.
Fig. 7.
Schematic diagram of growth promotion of EVs’ pathway. EVs activated the l-arginine/nitric oxide pathway to increase the intracellular NO levels, then inhibited the ROS accumulation to reduce the cell apoptosis and increased its pathogenicity
In conclusion, our study demonstrated that C. albicans could regulate its own growth through secreted EVs. EVs promoted C. albicans growth by reducing intracellular ROS accumulation and decreased cell apoptosis through l-arginine/nitric oxide pathway. EVs also enhanced the abilities of C. albicans to damage host cells. Our study highlighted the effects and mechanism of EVs on C. albicans itself and provided new information for fungal infections and treatment in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We greatly thank Professor Min Hu from Second Affiliated Hospital of West China School for the technical support of flow cytometry.
Author contribution
BR, LC, and XZ conceived and designed research. YW, ZW, YL, JW, and YS conducted experiments. YZ, ML, and BL contributed new reagents or analytical tools. YW and YL analyzed data. YW wrote the manuscript. LC and BR critically revised the manuscript. All authors read and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China grants (81870778, 82071106, 82271033, 81600858, 81991500, 81991501), Key Research and Development Projects of Science and Technology Department of Sichuan Province (2021YFQ0064), Applied Basic Research Programs of Sichuan Province (2020YJ0227), Technology Innovation R&D Project of Chengdu (2022-YF05-01401-SN), and the Research Funding from West China School/Hospital of Stomatology Sichuan University (RCDWJS2021-19).
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information included in this published article and its supplementary information files). The sequencing data from this study have been submitted to NCBI’s Sequence Read Archive under accession no. PRJNA877381.
Code availability
Not applicable.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors consent to the publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lei Cheng, Email: chenglei@scu.edu.cn.
Biao Ren, Email: renbiao@scu.edu.cn.
References
- Araujo M, Welch WJ. Oxidative stress and nitric oxide in kidney function. Curr Opin Nephrol Hypertens. 2006;15(1):72–77. doi: 10.1097/01.mnh.0000191912.65281.e9. [DOI] [PubMed] [Google Scholar]
- Barcelos RP, Stefanello ST, Mauriz JL, Gonzalez-Gallego J, Soares FA. Creatine and the liver: metabolism and possible interactions. Mini Rev Med Chem. 2016;16(1):12–18. doi: 10.2174/1389557515666150722102613. [DOI] [PubMed] [Google Scholar]
- Bitencourt TA, Hatanaka O, Pessoni AM, Freitas MS, Trentin G, Santos P, Rossi A, Martinez-Rossi NM, Alves LL, Casadevall A, Rodrigues ML, Almeida F. Fungal extracellular vesicles are involved in intraspecies intracellular communication. mBio. 2022;13(1):e0327221. doi: 10.1128/mbio.03272-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudko DY. Bioanalytical profile of the L-arginine/nitric oxide pathway and its evaluation by capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851(1–2):186–210. doi: 10.1016/j.jchromb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Brown GD, Netea MG, Gow NA. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22(11):614–622. doi: 10.1016/j.tim.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9(7):327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhou Y, Zhou X, Liao B, Xu HHK, Chu CH, Cheng L, Ren B. Dimethylaminododecyl methacrylate inhibits Candida albicans and oropharyngeal candidiasis in a pH-dependent manner. Appl Microbiol Biotechnol. 2020;104(8):3585–3595. doi: 10.1007/s00253-020-10496-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, Hu T, Li J, Zhou X, Ren B. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104(18):7777–7785. doi: 10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Han Q, Zhou X, Zhang K, Wang S, Xu HHK, Weir MD, Feng M, Li M, Peng X, Ren B, Cheng L (2017) Heat-polymerized resin containing dimethylaminododecyl methacrylate inhibits Candida albicans biofilm. Mater (Basel) 10(4). 10.3390/ma10040431 [DOI] [PMC free article] [PubMed]
- Dawson CS, Garcia-Ceron D, Rajapaksha H, Faou P, Bleackley MR, Anderson MA. Protein markers for Candida albicans EVs include claudin-like Sur7 family proteins. J Extracell Vesicles. 2020;9(1):1750810. doi: 10.1080/20013078.2020.1750810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Toledo Martins S, Szwarc P, Goldenberg S, Alves LR. Extracellular vesicles in fungi: composition and functions. Curr Top Microbiol Immunol. 2019;422:45–59. doi: 10.1007/82_2018_141. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713–735. doi: 10.1161/circresaha.116.309326. [DOI] [PubMed] [Google Scholar]
- Gibson RK, Peberdy JF. Fine structure of protoplasts of Aspergillus nidulans. J Gen Microbiol. 1972;72(3):529–538. doi: 10.1099/00221287-72-3-529. [DOI] [PubMed] [Google Scholar]
- Gogoi M, Datey A, Wilson KT, Chakravortty D. Dual role of arginine metabolism in establishing pathogenesis. Curr Opin Microbiol. 2016;29:43–48. doi: 10.1016/j.mib.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow NA, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol. 2012;15(4):406–412. doi: 10.1016/j.mib.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Honorato L, de Araujo JFD, Ellis CC, Piffer AC, Pereira Y, Frases S, de Sousa Araújo GR, Pontes B, Mendes MT, Pereira MD, Guimarães AJ, da Silva NM, Vargas G, Joffe L, Del Poeta M, Nosanchuk JD, Zamith-Miranda D, Dos Reis FCG, de Oliveira HC, Rodrigues ML, de Toledo Martins S, Alves LR, Almeida IC, Nimrichter L. Extracellular vesicles regulate biofilm formation and yeast-to-hypha differentiation in Candida albicans. mBio. 2022;13(3):e0030122. doi: 10.1128/mbio.00301-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Niu Y, Ye X, Zhu C, Tong T, Zhou Y, Zhou X, Cheng L, Ren B (2021) Staphylococcus aureus synergized with Candida albicans to increase the pathogenesis and drug resistance in cutaneous abscess and peritonitis murine models. Pathogens 10(8). 10.3390/pathogens10081036 [DOI] [PMC free article] [PubMed]
- Hyvönen MT, Keinänen TA, Nuraeva GK, Yanvarev DV, Khomutov M, Khurs EN, Kochetkov SN, Vepsäläinen J, Zhgun AA, Khomutov AR (2020) Hydroxylamine analogue of agmatine: magic bullet for arginine decarboxylase. Biomolecules 10(3). 10.3390/biom10030406 [DOI] [PMC free article] [PubMed]
- Jin Z. About the evaluation of drug combination. Acta Pharmacol Sin. 2004;25(2):146–147. [PubMed] [Google Scholar]
- Karkowska-Kuleta J, Kedracka-Krok S, Rapala-Kozik M, Kamysz W, Bielinska S, Karafova A, Kozik A. Molecular determinants of the interaction between human high molecular weight kininogen and Candida albicans cell wall: identification of kininogen-binding proteins on fungal cell wall and mapping the cell wall-binding regions on kininogen molecule. Peptides. 2011;32(12):2488–2496. doi: 10.1016/j.peptides.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Karkowska-Kuleta J, Kulig K, Karnas E, Zuba-Surma E, Woznicka O, Pyza E, Kuleta P, Osyczka A, Rapala-Kozik M, Kozik A (2020) Characteristics of extracellular vesicles released by the pathogenic yeast-like fungi Candida glabrata, Candida parapsilosis and Candida tropicalis. Cells 9(7). 10.3390/cells9071722 [DOI] [PMC free article] [PubMed]
- Khalaf D, Krüger M, Wehland M, Infanger M, Grimm D (2019) The effects of oral L-arginine and L-citrulline supplementation on blood pressure. Nutrients 11(7). 10.3390/nu11071679 [DOI] [PMC free article] [PubMed]
- Kong LX, Wang Z, Shou YK, Zhou XD, Zong YW, Tong T, Liao M, Han Q, Li Y, Cheng L, Ren B. The FnBPA from methicillin-resistant Staphylococcus aureus promoted development of oral squamous cell carcinoma. J Oral Microbiol. 2022;14(1):2098644. doi: 10.1080/20002297.2022.2098644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Puumala E, Robbins N, Cowen LE. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev. 2021;121(6):3390–3411. doi: 10.1021/acs.chemrev.0c00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DD, Yang CC, Liu P, Wang Y, Sun Y. Effect of nitric oxide on the antifungal activity of oxidative stress and azoles against Candida albicans. Indian J Microbiol. 2016;56(2):214–218. doi: 10.1007/s12088-016-0580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebana-Jordan M, Brotons B, Falcon-Perez JM, Gonzalez E (2021) Extracellular vesicles in the fungi kingdom. Int J Mol Sci 22(13). 10.3390/ijms22137221 [DOI] [PMC free article] [PubMed]
- Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16(1):19–31. doi: 10.1038/nrmicro.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo LM, Despotović D, Weil-Ktorza O, Walker MJ, Jabłońska J, Fridmann-Sirkis Y, Varani G, Metanis N, Tawfik DS. Primordial emergence of a nucleic acid-binding protein via phase separation and statistical ornithine-to-arginine conversion. Proc Natl Acad Sci U S A. 2020;117(27):15731–15739. doi: 10.1073/pnas.2001989117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiking YC, Poeze M, Ramsay G, Deutz NE. The role of arginine in infection and sepsis. JPEN J Parenter Enteral Nutr. 2005;29(1 Suppl):S70–74. doi: 10.1177/01486071050290s1s70. [DOI] [PubMed] [Google Scholar]
- Martínez-López R, Hernáez ML, Redondo E, Calvo G, Radau S, Pardo M, Gil C, Monteoliva L. Candida albicans hyphal extracellular vesicles are different from yeast ones, carrying an active proteasome complex and showing a different role in host immune response. Microbiol Spectr. 2022;10(3):e0069822. doi: 10.1128/spectrum.00698-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/nejm199312303292706. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Rodrigues ML. Fungal glucosylceramides: from structural components to biologically active targets of new antimicrobials. Front Microbiol. 2011;2:212. doi: 10.3389/fmicb.2011.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One. 2010;5(6):e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM. The L-arginine: nitric oxide pathway. Curr Opin Nephrol Hypertens. 1993;2(1):122–128. doi: 10.1097/00041552-199301000-00018. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38(2):161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic PJ, Zeh HJ, 3rd, Ochoa JB. Arginine and immunity. J Nutr. 2007;137(6 Suppl 2):1681s–1686s. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- Reales-Calderón JA, Vaz C, Monteoliva L, Molero G, Gil C. Candida albicans modifies the protein composition and size distribution of THP-1 macrophage-derived extracellular vesicles. J Proteome Res. 2017;16(1):87–105. doi: 10.1021/acs.jproteome.6b00605. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6(1):48–59. doi: 10.1128/ec.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Franzen AJ, Nimrichter L, Miranda K. Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr Opin Microbiol. 2013;16(4):414–420. doi: 10.1016/j.mib.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Roszkowiak J, Jajor P, Guła G, Gubernator J, Żak A, Drulis-Kawa Z, Augustyniak D (2019) Interspecies outer membrane vesicles (OMVs) modulate the sensitivity of pathogenic bacteria and pathogenic yeasts to cationic peptides and serum complement. Int J Mol Sci 20(22). 10.3390/ijms20225577 [DOI] [PMC free article] [PubMed]
- Rybak K, Robatzek S. Functions of extracellular vesicles in immunity and virulence. Plant Physiol. 2019;179(4):1236–1247. doi: 10.1104/pp.18.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandini S, Stringaro A, Arancia S, Colone M, Mondello F, Murtas S, Girolamo A, Mastrangelo N, De Bernardis F. The MP65 gene is required for cell wall integrity, adherence to epithelial cells and biofilm formation in Candida albicans. BMC Microbiol. 2011;11:106. doi: 10.1186/1471-2180-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer K, Wagener J, Ames RM, Christou S, MacCallum DM, Bates S, Gow NAR (2020) Three related enzymes in Candida albicans achieve arginine- and agmatine-dependent metabolism that is essential for growth and fungal virulence. mBio 11(4). 10.1128/mBio.01845-20 [DOI] [PMC free article] [PubMed]
- Stechmiller JK, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr Clin Pract. 2005;20(1):52–61. doi: 10.1177/011542650502000152. [DOI] [PubMed] [Google Scholar]
- Szefel J, Danielak A, Kruszewski WJ. Metabolic pathways of L-arginine and therapeutic consequences in tumors. Adv Med Sci. 2019;64(1):104–110. doi: 10.1016/j.advms.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Ullmann BD, Myers H, Chiranand W, Lazzell AL, Zhao Q, Vega LA, Lopez-Ribot JL, Gardner PR, Gustin MC. Inducible defense mechanism against nitric oxide in Candida albicans. Eukaryot Cell. 2004;3(3):715–723. doi: 10.1128/ec.3.3.715-723.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Vargas G, Rocha JD, Oliveira DL, Albuquerque PC, Frases S, Santos SS, Nosanchuk JD, Gomes AM, Medeiros LC, Miranda K, Sobreira TJ, Nakayasu ES, Arigi EA, Casadevall A, Guimaraes AJ, Rodrigues ML, Freire-de-Lima CG, Almeida IC, Nimrichter L. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2015;17(3):389–407. doi: 10.1111/cmi.12374. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou Y, Han Q, Ye X, Chen Y, Sun Y, Liu Y, Zou J, Qi G, Zhou X, Cheng L, Ren B. Synonymous point mutation of gtfB gene caused by therapeutic X-rays exposure reduced the biofilm formation and cariogenic abilities of Streptococcus mutans. Cell Biosci. 2021;11(1):91. doi: 10.1186/s13578-021-00608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel RP. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis. 1995;20(6):1531–1534. doi: 10.1093/clinids/20.6.1531. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(( Pt 1)(Pt 1)):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Meininger CJ, McNeal CJ, Bazer FW, Rhoads JM. Role of L-arginine in nitric oxide synthesis and health in humans. Adv Exp Med Biol. 2021;1332:167–187. doi: 10.1007/978-3-030-74180-8_10. [DOI] [PubMed] [Google Scholar]
- Xiong K, Zhu H, Li Y, Ji M, Yan Y, Chen X, Chi Y, Yang X, Deng L, Zhou X, Zou L, Ren B. The arginine biosynthesis pathway of Candida albicans regulates its cross-kingdom interaction with actinomyces viscosus to promote root caries. Microbiol Spectr. 2022;10(4):e0078222. doi: 10.1128/spectrum.00782-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Wang J, Jiang H, Lin H, Ou Z, Ullah A, Hua Y, Chen J, Lin X, Hu X, Zheng L, Wang Q. Extracellular vesicles derived from Talaromyces marneffei yeasts mediate inflammatory response in macrophage cells by bioactive protein components. Front Microbiol. 2020;11:603183. doi: 10.3389/fmicb.2020.603183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawen Z, Xiangyun C, Binyou L, Xingchen Y, Taiping L, Xuedong Z, Jiyao L, Lei C, Wenyue X, Biao R. The dynamic landscape of parasitemia dependent intestinal microbiota shifting and the correlated gut transcriptome during Plasmodium yoelii infection. Microbiol Res. 2022;258:126994. doi: 10.1016/j.micres.2022.126994. [DOI] [PubMed] [Google Scholar]
- Zamith-Miranda D, Nimrichter L, Rodrigues ML, Nosanchuk JD. Fungal extracellular vesicles: modulating host-pathogen interactions by both the fungus and the host. Microbes Infect. 2018;20(9–10):501–504. doi: 10.1016/j.micinf.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowski R, Sanchez H, Covelli AS, Dominguez E, Jaromin A, Bernhardt J, Mitchell KF, Heiss C, Azadi P, Mitchell A, Andes DR. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol. 2018;16(10):e2006872. doi: 10.1371/journal.pbio.2006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowski R, Noll A, Chevrette MG, Sanchez H, Jones R, Anhalt H, Fossen J, Jaromin A, Currie C, Nett JE, Mitchell A, Andes DR. Coordination of fungal biofilm development by extracellular vesicle cargo. Nat Commun. 2021;12(1):6235. doi: 10.1038/s41467-021-26525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang H, Zhou X, Luo H, Tang F, Yang J, Alterovitz G, Cheng L, Ren B. Lovastatin synergizes with itraconazole against planktonic cells and biofilms of Candida albicans through the regulation on ergosterol biosynthesis pathway. Appl Microbiol Biotechnol. 2018;102(12):5255–5264. doi: 10.1007/s00253-018-8959-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cheng L, Liao B, Shi Y, Niu Y, Zhu C, Ye X, Zhou X, Ren B. Candida albicans CHK1 gene from two-component system is essential for its pathogenicity in oral candidiasis. Appl Microbiol Biotechnol. 2021;105(6):2485–2496. doi: 10.1007/s00253-021-11187-0. [DOI] [PubMed] [Google Scholar]
- Zhu C, Liao B, Ye X, Zhou Y, Chen X, Liao M, Cheng L, Zhou X, Ren B. Artemisinin elevates ergosterol levels of Candida albicans to synergise with amphotericin B against oral candidiasis. Int J Antimicrob Agents. 2021;58(3):106394. doi: 10.1016/j.ijantimicag.2021.106394. [DOI] [PubMed] [Google Scholar]
- Zou S, Wang X, Liu P, Ke C, Xu S. Arginine metabolism and deprivation in cancer therapy. Biomed Pharmacother. 2019;118:109210. doi: 10.1016/j.biopha.2019.109210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information included in this published article and its supplementary information files). The sequencing data from this study have been submitted to NCBI’s Sequence Read Archive under accession no. PRJNA877381.
Not applicable.