Abstract
Calcitroic acid, the excretory form of vitamin D, is the terminal product of a 5-step pathway catalyzed by CYP24A1, commencing with C24-hydroxylation of 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3). Catabolism of 25-hydroxyvitamin D3 (25-OH-D3) proceeds via analogous steps culminating in calcioic acid; however this C23-truncated acid has not been reported in the circulation. It has recently been shown that 24,25-dihydroxyvitamin D3 (24,25-(OH)2D3) is an important factor in optimal bone fracture healing acting via an effector molecule FAM57B2 to produce lactosylceramide. Administration of 24,25-(OH)2D3 was found to restore normal fracture repair in Cyp24a1−/− mice devoid of 24,25-(OH)2D3. We set out to study the multi-step catabolism of D3 metabolites in vivo using LC-MS/MS methods in vehicle or 24,25-(OH)2D3-treated mice. Vehicle-treated Cyp24a1+/− mice possessed normal levels of serum 24,25-(OH)2D3 (7 ng/mL) and 25-OH-D3-26,23-lactone (4 ng/mL). We also detected 24-oxo-25-OH-D3 (3 ng/mL) and 24-oxo-23,25-(OH)2D3 (0.4 ng/mL); which were not detectable in vehicle-treated Cyp24a1−/− mice. In 24,25-(OH)2D3-treated Cyp24a1+/− mice, serum 24,25-(OH)2D3 rose to 200 ng/mL while 25-OH-D3-26,23-lactone remained unchanged in comparison to vehicle-treated Cyp24a1+/− mice Concentration of serum 24-oxo-25-OH-D3 and 24-oxo-23,25-(OH)2D3 rose by 10-fold, when Cyp24a1+/− mice were treated with 24,25-(OH)2D3 Calcioic acid was increased to 0.030 ng/mL for 24,25-(OH)2D3-treated Cyp24a1+/− mice. In 24,25-(OH)2D3-treated Cyp24a1−/− mice, serum 24,25-(OH)2D3 rose further to a striking 830 ng/mL due to lack of catabolism of the 24,25-(OH)2D3 dose. Serum 1,25-(OH)2D3 levels were suppressed in 24,25-(OH)2D3-treated Cyp24a1+/− and Cyp24a1−/− mice. Circulating 1,24,25-(OH)3D3 rose from 73 pg/mL to 106 pg/mL when Cyp24a1+/− mice were treated with 24,25-(OH)2D3. While undetectable in vehicle-treated Cyp24a1−/− mice, 1,24,25-(OH)3D3 rose unexpectedly to 153 pg/mL in 24,25-(OH)2D3-treated nulls suggesting conversion of 24,25-(OH)2D3 to 1,24,25-(OH)3D3 via 1-hydroxylation. Taken together, amplification of 24,25-(OH)2D3 catabolism by exogenous doses of this metabolite have enabled detection of downstream C24-oxidation pathway products in vivo, including calcioic acid; and provides a platform for studying alternative routes of vitamin D metabolism that may occur in pathological states including hypervitaminosis D and idiopathic infantile hypercalcemia caused by mutations of CYP24A1.
Keywords: CYP24A1; LC-MS/MS; 24,25-dihydroxyvitamin D3; bone fracture healing; FAM57B2; knockout mouse
1. INTRODUCTION
Clearance of 25-hydroxyvitamin D (25-OH-D3) and 1,25-dihydroxyvitamin D (1,25-(OH)2D3) is mediated by the cytochrome P450, CYP24A1 via pathways commencing with C24- or C23-hydroxylation and culminating in either a C-23 carboxylic acid which leaves the body via bile, or a 26,23-lactone product which retains significant binding affinity to the serum vitamin D-binding protein (DBP)1. 25-OH-D3 and 1,25-(OH)2D3 are catabolized by analogous steps of either the C24- or C23-hydroxylation pathway, and the degree of pathway utilization is species-dependent. Humans, mice and rats catabolize vitamin D metabolites mainly via C24-hydroxylation, but opossums and guinea pigs catabolize vitamin D to lactone products arising from an initial C23-hydroxylation step2,3. While believed to have a primarily catabolic role, St-Arnaud and colleagues have identified an anabolic role for CYP24A1, revealing that 24,25-dihydroxyvitamin D3 (24,25-(OH)2D3) is essential for optimal bone fracture healing in mice. A role for 24,25-(OH)2D3 in bone fracture repair was first reported in the 1990s4,5,6, but the details of its vitamin D receptor (VDR)-independent mechanism of action remained elusive until recently. Using a combination of knockout mouse models and mRNA screening of the healing bone callus, St-Arnaud and colleagues revealed that 24,25-(OH)2D3 allosterically activates the membrane protein FAM57B2 which catalyzes the formation of lactosylceramide (LacCer) involved in downstream effects on bone healing7. Mice lacking either systemic CYP24A1 or FAM57B2 in chondrocytes exhibit impaired fracture healing. Administration of either 24,25-(OH)2D3 or LacCer restores optimal fracture healing in the Cyp24−/− mouse, but Col2-Cre Fam57bfl/fl mice respond only to LacCer and not exogenous 24,25-(OH)2D3 due to the absence of its downstream effector molecule7. Half of Cyp24a1−/− mice die post weaning of hypercalcemia due to accumulation of 1,25-(OH)2D; but the surviving animals retain a normal bone phenotype and are normocalcemic as they are able to circumvent lack of 1,25-(OH)2D catabolism by downregulating CYP27B1 involved in 1,25-(OH)2D formation8, thus permitting bone fracture healing studies on Cyp24a1-null mice devoid of 24,25-(OH)2D3. Using a sensitive liquid chromatography – tandem mass spectrometry (LC-MS/MS) assay, we set out to study serum vitamin D metabolites in Cyp24a1+/− and Cyp24a1−/− mice from the bone fracture healing study, given large doses of 24,25-(OH)2D37,8 to establish: What specific vitamin D metabolites can be detected in mice given 24,25-(OH)2D3? and How is vitamin D metabolism altered in the context of a large 24,25-(OH)2D3 dose?
2. MATERIALS AND METHODS
Development and treatment of Cyp24a1−/− and Cyp24A1+/− mice analyzed in this study have been described in previous work which identified the role of 24,25-(OH)2D3 in bone fracture repair.7,8. The animal use protocol (AUP #4138) and all standard operating procedures were approved by the Institutional Animal Care and Use Committee and followed the guidelines of the Canadian Council on Animal Care. Analytical methods used to assay mouse serum for a range of vitamin D metabolites have been described previously9,10,11. Briefly, 12-week old female Cyp24a1−/− and Cyp24A1+/− were subjected to osteotomy. Beginning 72-hours post surgery, mice were given rescue treatments of 6.7 μg/kg of 24,25-(OH)2D3 or 50 μL of propylene glycol daily for 15 days prior to sacrifice7. Vitamin D metabolites were measured in six mice from each of the four treatment groups. Cyp24A1+/− are identical to Cyp24A1+/+ in terms of body composition and metabolism. While kidney Cyp24a1 expression was not compared between Cyp24A1+/− and Cyp24A1+/+ mice, serum concentrations of 1,25-(OH)2D3, 1,24,25-(OH)3D3, 25-OH-D3, 24,25-(OH)2D3, 25-OH-D3-26,23-lactone were the same, suggesting similar CYP27B1 and CYP24A1 enzyme activities and that Cyp24A1+/− littermates served as good controls for Cyp24a1−/− mice7.
For analysis of 25-OH-D3 and its metabolites, serum was extracted by liquid-liquid extraction with methyl-tert butyl ether and hexane, after protein precipitation with zinc sulfate and methanol. For 1,25-(OH)2D3 and 1,24,25-(OH)3D3, serum was extracted with 100 μL of anti-1,25-(OH)2D antibody slurry (Immundiagnostik), and metabolites were eluted with ethanol. Both types of extract were evaporated to dryness and derivatized with 4-(2-(6,7-Dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalinyl)ethyl)1,2,4-triazoline-3,5-dione) (DMEQ-TAD), and analyzed by LC-MS/MS on ACQUITY/Xevo TQ-S instruments (Waters) using a 20-minute methanol/water-based gradient on a C18+ column as previously described10. Mobile phase A and B comprised either water or methanol supplemented with 0.1% (by vol.) formic acid and 2 mM methylamine respectvely. Composition of mobile phase B was raised from 50 to 77 % (by vol.) over 18 minutes using a linear (curve 6) gradient. Samples prepared by liquid-liquid extraction were also analyzed using a more rapid LC separation9. Metabolites 25-OH-D3, 24,25-(OH)2D3, 1,25-(OH)2D3 and 1,24,25-(OH)3D3 were quantified using specific deuterated internal standards and calibration lines, whereas concentration of the following metabolites was estimated on the basis of recovery of d6-24,25-(OH)2D3 and the calibration line for 24,25-(OH)2D3: 25-OH-D3-26,23-lactone, 24-oxo-25-OH-D3, 24-oxo-23,25-(OH)2D3, and calcioic acid. We previously expressed human CYP24A1 in V79–4 Chinese hamster lung fibroblast cells using a targeted integration method (Flp-In). Incubation of these cells with 25-OH-D3 using previously-described methods yielded a metabolite-rich extract which could be used as a LC-MS/MS standard12. Identity of vitamin D metabolites detected in mouse serum was based on a combination of criteria where appropriate, including: 1) Presence of both 6S and 6R isomers of DMEQ-TAD adducts using an MRM transition based on the theoretical molecular mass of the metabolite 2) Co-chromatography with extracts derived from human recombinant CYP24A1-expressing cells incubated with 25-OH-D312 3) Co-chromatography with synthetic standards for 24,25-(OH)2D3 and calcioic acid. 4) Absence of a peak when CYP24A1 was ablated. Student’s T test was used to determine significance of changes in vitamin D metabolite concentrations and ratios.
3. RESULTS
Vehicle-treated Cyp24a1+/− mice exhibited normal 25-OH-D3, and 24,25-(OH)2D3 levels typical of that observed over a range of mouse studies using the same LC-MS/MS platform13, giving a 25-OH-D3:24,25-(OH)2D3 ratio of 2.6 (Table 1, Figure 1A–C). With Cyp24a1 ablation, 24,25-(OH)2D3 levels decreased from 6.7 to 1.5 ng/mL, and 25-OH-D3 rose 6-fold from 17.2 to 101 ng/mL, indicating a marked lack of catabolism of endogenous 25-OH-D3 in the absence of CYP24A1, as such, the ratio of 25-OH-D3:24,25-(OH)2D3 rose from 2.6 to 70 in Cyp24a1−/− mice. The importance of CYP24A1 in the catabolism of 25-OH-D3 was also manifested by the reduction in serum 25-OH-D3-26,23-lactone in Cyp24a1−/− mice (Table 1, Figure 1G). When given a 24,25-(OH)2D3 dose, Cyp24a1+/− mice continued to exhibit normal 25-OH-D3 levels, but possessed very high serum 24,25-(OH)2D3 as was expected, at almost 200 ng/mL. 25-OH-D3 levels in 24,25-OH-D3-treated Cyp24a1−/− mice also appeared to accumulate in the absence of CYP24A1, and 24,25-(OH)2D3 was present at over 800 ng/mL, due to lack of catabolism of the exogenous 24,25-(OH)2D3 dose. Why the apparent accumulation of 25-OH-D3 is not as extreme when Cyp24a1−/− mice are treated with 24,25-(OH)2D3 as compared with vehicle remains unknown; but raises the possibility that extreme levels of 24,25-(OH)2D3 in Cyp24a1−/− mice affects the expression of CYP2R1/CYP27A1 or enzymes involved in CYP24A1-independent catabolism of 25-OH-D3. Because the metabolite 25-OH-D3-26,23-lactone arises from further catabolism of 23,25-(OH)2D3, concentrations of serum 25-OH-D3-26,23-lactone were identical between vehicle and 24,25-(OH)2D3-treated Cyp24a1+/− mice, at 3.8 ng/mL. Furthermore, absence of 25-OH-D3-26,23-lactone in the 24,25-(OH)2D3-treated Cyp24a1−/− mice confirmed from the metabolic standpoint, that accumulation of the exogenous 24,25-(OH)2D3 dose in Cyp24a1−/− mice was in fact due to a lack of CYP24A1.
Table 1:
Concentration of serum vitamin D metabolites in Cyp24a1+/− and Cyp24a1−/− mice.
| Genotype | Treatment | Na | [25-OH-D3] | [24,25-(OH)2D3] | R b | [25-D3-26,23-lactone] | R c | [24-oxo-25–OH-D3] | [24-oxo-23,25-(OH)2D3] | [Calcioic acid] | [1,25-(OH)2D3] | [1,24,25-(OH)3D3] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/mL | ng/mL | ng/mL | ng/mL | ng/mL | pg/mL | pg/mL | pg/mL | |||||
| Cyp24a1 +/− | vehicle | 6 | 17.2±2.4 | 6.7±1.1 | 2.6 | 3.8±0.7 | 4.5 | 2.8±0.4 | 0.4±0.1 | <5 | 27.8±4.2 | 72.8±12.1 |
| Cyp24a1 −/− | vehicle | 6 | 101.3±20.6d | 1.5±0.1d | 70.6d | <0.1d | 2000.2d | <0.1d | <0.1d | <5f | 34.0±6.2f | <4d |
| Cyp24a1 +/− | 24,25-D3 | 6 | 12.6±1.0e | 196.9±68.5d | 0.1d | 3.8±0.3f | 3.4d | 31.4±9.2d | 3.7±0.1d | 31±11d | <4d | 105.5±19.5d |
| Cyp24a1 −/− | 24,25-D3 | 6 | 56.8±12.3d | 827.9±327.8d | 0.1d | <0.1d | 2999.1d | 2.7±1.2f | <0.1d | <5f | <4d | 153.4±64.7g |
Number of mice in each treatment group.
Denotes the ratio 25-OH-D3:24,25-(OH)2D3 or
25-OH-D3:25-OH-D3–26,23-lactone.
p<0.001 based on comparison to vehicle-treated Cyp24a1+/− mice.
p<0.01 based on comparison to vehicle-treated Cyp24a1+/− mice.
p>0.05 based on comparison to vehicle-treated Cyp24a1+/− mice.
p<0.05 based on comparison to vehicle-treated Cyp24a1+/− mice.
Figure 1: LC-MS/MS-based metabolomics of serum vitamin D metabolites:
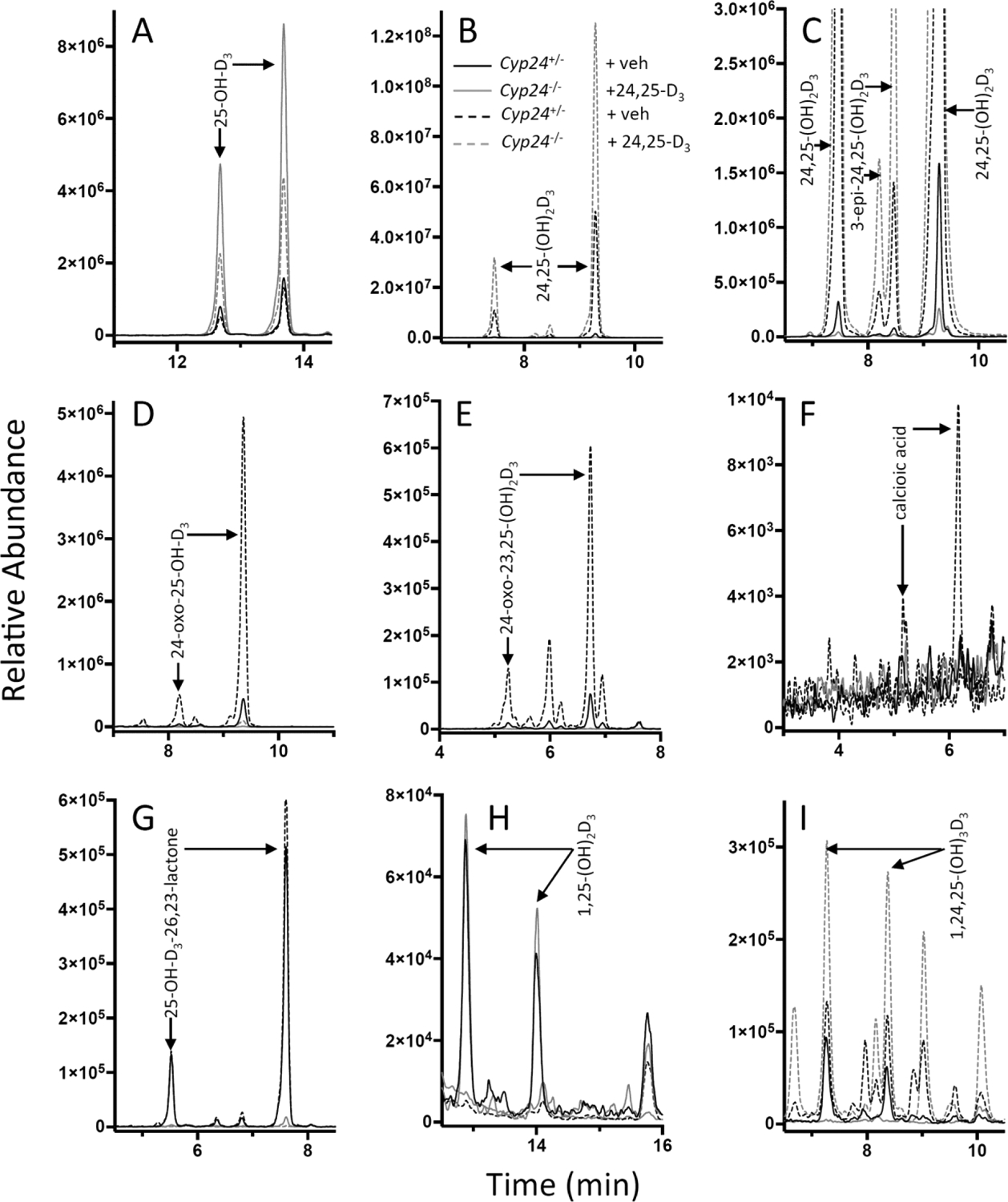
Panels A-I depict mass chromatograms based on MRM transitions used to detect DMEQ-TAD adducts of specific serum vitamin D metabolites in Cyp24a1−/− and Cyp24a1+/− mice given vehicle (veh) or 24,25-(OH)2D3 (24,25-D3), including: (A) 25-OH-D3 (MRM, m/z 746→468), (B & C) 24,25-(OH)2D3 (plotted on two different intensity scales; MRM, m/z 762→468), (D) 24-oxo-25-OH-D3 (MRM, m/z 760→468), (E) 24-oxo-23,25-(OH)2D3, (MRM, m/z 776→468), (F) calcioic acid (MRM, m/z 704→468), (G) 25-OH-D3-26,23-lactone (MRM, m/z 774→468), (H) 1,25-(OH)2D3, (MRM, m/z 762→468+484), and (I) 1,24,25-(OH)3D3, (MRM, m/z 778→468+484).
The high concentration of serum 24,25-(OH)2D3 in treated mice, prompted us to screen for downstream metabolites of the C24-oxidation pathway. Somewhat surprisingly, the metabolites 24-oxo-25-OH-D3 and 24-oxo-23,25-(OH)2D3 were easily detected at 2.8 and 0.4 ng/mL in vehicle-treated Cyp24a1+/− mice, and present at 10-fold greater concentrations in Cyp24a1+/− mice given 24,25-(OH)2D3 (Table, Figure 1D,E). While the absence of detectable peaks for these metabolites in most Cyp24a1−/− mice confirms the CYP24A1-dependent catabolism of 24,25-(OH)2D3 in vivo, the residual 24-oxo-25-OH-D3 in 24,25-(OH)2D3-treated Cyp24a1−/− animals imply the presence of alternative enzymes that might act to clear high doses of 24,25-(OH)2D3 in the absence of CYP24A1. The terminal catabolite in the C24-oxidation of 25-OH-D3, calcioic acid, could be detected at 30 pg/mL in 24,25-(OH)2D3-treated Cyp24a1+/− animals only (Figure 1F). In order to confirm the identity of calcioic acid observed in vivo, we generated more concentrated extracts of serum from 24,25-(OH)2D3-treated Cyp24a1+/− mice (Figure 2A) and a patient with hypervitaminosis D (Figure 2 B) and compared the LC-MS/MS profiles with extracts generated from human CYP24A1-expressing V79–4 cells incubated with 25-OH-D312, as well as a synthetic standard for calcioic acid (Figure 2C,D). While we are currently unable to detect calcioic acid in normal human subjects, we were able to identify calcioic acid in a patient with hypervitaminosis D, with serum 25-OH-D of 200 ng/mL and 24,25-(OH)2D3 of 10 ng/mL, and 19 pg/mL of 1,25-(OH)2D3 who presented with severe hypercalcemia and suppressed parathyroid hormone. As this patient possessed elevated 24,25-(OH)2D3 and a normal 25-OH-D3:24,25-(OH)2D3 ratio of 20, CYP24A1 mutation was ruled out, but patient history revealed prolonged and excessive cholecalciferol prophylaxis as the cause of hypercalcemia. These results reveal that amplified vitamin D catabolism through to calcioic acid can be observed both in mice given 24,25-(OH)2D3, as well as in a clinical case of hypercalcemia caused by over-supplementation with vitamin D3.
Figure 2: Detection of calcioic acid by LC-MS/MS.

MRM transition m/z 704→468 was used to screen for the presence of calcioic acid in (A) Cyp24a1+/− mice treated with 24,25-OH)2D3, (B) a patient with hypervitaminosis D, and (C) an extract generated from human CYP24A1-expressing V79–4 cells incubated with 25-OH-D3. Retention times were compared against a synthetic calcioic acid standard (D).
Since the mice used in our study comprised normocalcemic Cyp24a1−/− animals, 1,25-(OH)2D3 levels were normal, and did not differ from their heterozygotic littermates (28–34 pg/mL) However, super-physiological 24,25-(OH)2D3 concentrations appeared to suppress 1,25-(OH)2D3 levels to below the limit of detection of the assay, even in Cyp24a1−/− mice incapable of catabolizing 1,25-(OH)2D3 (Table 1, Figure 1H). While 1,24,25-trihydroxyvitamin D3 (1,24,25-(OH)3D3) was undetectable in vehicle-treated Cyp24a1−/− mice, it rose to 153 pg/mL when treated with 24,25-(OH)2D3. Seventy three pg/mL of 1,24,25-(OH)3D3 were found in Cyp24A1+/− mice when treated with vehicle, which increased to 106 pg/mL when treated with 24,25-(OH)2D3 (Table 1, Fig 1I). On the basis of apparent suppression of 1,25-(OH)2D3 formation in the 24,25-(OH)2D3-treated Cyp24a1+/− and Cyp24a1−/− mice, and absence of CYP24A1 in the 24,25-(OH)2D3-treated Cyp24a1−/− mice, we conclude that the 1,24,25-(OH)3D3 observed in these animals arose from inter-conversion via 1-hydroxylation of 24,25-(OH)2D3.
4. DISCUSSION
Growing sensitivity of LC-MS/MS methods as well as sample preparation techniques, has circumvented the need to pool mouse samples enabling studies of biological variability of vitamin D metabolism in small volumes of serum taken from individual mice. In vitro and ex vivo studies of CYP24A1 enzyme activity have been successful at delineating individual steps in the C24- and C23-hydroxylation of 25-OH-D3 and 1,25-(OH)2D3 using a combination of reconstituted enzyme systems and kidney perfusion models14,15,16, but most in vivo studies have been limited to metabolites of high-abundance, or have necessitated pooling of sera from multiple animals, or required use of a tritiated tracer. We have shown that detection of downstream metabolites of C24-oxidation of 25-OH-D3 is possible in individual mice, and represents the first report of detection of calcioic acid in both human and murine serum. Prior to calcioic acid formation, 24-oxo-23,25-(OH)2D3 undergoes cleavage at C23-C24 bond to form tetranor-23-OH-D3 alcohol as well as an aldehyde in a process called disproportionation, in a similar manner as previously described for side chain cleavage of 24-oxo-1,23,25-(OH)3D317. We were unable to detect these penultimate intermediates in mouse serum, presumably due to low stability and/or lack of sufficient binding affinity for the vitamin D-binding protein. While identification of the C24- and C23-oxidation products of 25-OH-D3, paralleled that of 1,25-(OH)2D3 in most cases, it was only in 2006 that the water-soluble products of 25-OH-D3 were specifically characterized using perfusates from vitamin D-intoxicated rats18. Reddy and co-workers concluded that calcioic acid was the major water-soluble product formed from 25-OH-D3, but also found that a C24-truncated carboxylic acid termed cholacalcioic acid was also produced as a minor product (approximately 20%)18, analogous to the C24-acid produced as the terminal catabolite of 20-epi-1,25-(OH)2D3 identified by Sakaki’s group19. Cholacalcioic acid has been proposed to be formed via a peroxy adduct of 24-oxo-25-OH-D3 leading to cleavage of the C24-C25 bond19. In vitro work conducted using the human CYP24A1 isoform where calcioic acid was identified as the major water-soluble product support our in vivo observations in the current study that detect calcioic acid in a patient with hypervitaminosis D (Figure 2B), as well as mice given 24,25-(OH)2D3 (Figures 1F, 2A). While chromatographic peaks potentially corresponding to cholacalcioic acid were identified in sera and cell extracts (MRM m/z 718→468), retention times and number of peaks were inconsistent between sample types, and we lacked a synthetic standard for this metabolite. On this basis we were unable to confirm the presence of cholacalcioic acid in serum, but we acknowledge that any cholacalcioic acid present in the relative amounts observed by Reddy and colleagues18 may be below the limit of detection of our serum assay3. The regioselectivity of CYP24A1 has been well documented based on in vitro studies. Different species of CYP24A1 have been noted for various degrees of C24- or C23-hydroxylation. It is the initial hydroxylation step on the side-chain that commits the substrate to a fate of either a C23-acid or a lactone product after multiple steps3,17 each catalyzed by CYP24A1. Our results confirm from an in vivo perspective that an exogenous dose of 24,25-(OH)2D3 continues to proceed through the C24-oxidation pathway to form calcioic acid and not 25-OH-D3-26,23-lactone which arises from 23,25-(OH)2D3, as lactone levels remained un-changed with administration of a 24,25-(OH)2D3 dose whereas metabolism via all other C24-pathway products was amplified (Figure 3).
Figure 3: Pathways of bioactivation, catabolism, and interconversion of detected vitamin D metabolites.

Vitamin D metabolites detected in mouse models using the current analytical platforms are indicated. Differential metabolism occurring as a result of Cyp24a1 ablation and/or 24,25-(OH)2D3 treatment are summarized in the legend by indicating the pathways that are either normal or unchanged (→), amplified (↑), or decreased (↓) in the specified test groups.
Treatment with 24,25-(OH)2D3 rescued Cyp24a1−/− animals from impaired bone fracture healing. These animals possessed serum 24,25-(OH)2D3 of over 800 ng/mL, raising the possibility that other metabolites formed from 24,25-(OH)2D3 may have contributed to biological function at this supraphysiological dose, especially as we observed marked suppression of 1,25-(OH)2D3 in 24,25-(OH)2D3-treated mice. CYP27B1 has been noted for its ability to catalyze 1-hydroxylation of metabolites of 25-OH-D3 in vitro20, and this appears consistent with our observation of increased 1,24,25-(OH)3D3 production with 24,25-(OH)2D3 treatment (Figure 1H,I, Table 1, Figure 3). While we cannot specifically distinguish 1,24,25-(OH)3D3 formed by CYP24A1 or CYP27B1, we observed continued 1,24,25-(OH)3D3 formation in the absence of CYP24A1. The metabolite 1,24,25-(OH)3D3 retains approximately one tenth of the VDR-binding affinity of 1,25-(OH)2D3, but it is unlikely that this metabolite contributed to the bone fracture healing properties attributed to 24,25-(OH)2D3 for several reasons, including (1) 1,24,25-(OH)3D3 and 1,25-(OH)2D3 were shown not to induce production of LacCer via activation of FAM57B2 in vitro7 (2) 24,25-(OH)2D3 treatment of Col2-Cre Fam57bfl/fl mice did not recover from bone fracture healing impairment, even with the presence of CYP24A17. We anticipate that measurement of an expanding range of vitamin D metabolites made possible by improvements to analytical methodology, used in combination with pre-clinical models of vitamin D metabolism will help to advance the understanding of the role of vitamin D metabolism in certain disease states.
5. CONCLUSIONS
Using a sensitive LC-MS/MS assay, we have demonstrated the importance of CYP24A1 in the catabolism of both 25-OH-D3, as well as exogenous doses of 24,25-(OH)2D3 using Cyp24a1+/− and Cyp24a1−/− mice used to establish a role for 24,25-(OH)2D3 in optimal bone fracture healing. The high sensitivity of our assay permitted assessment of CYP24A1 function on the basis of multi-step catabolism of 25-OH-D3 and 24,25-(OH)2D3 through to terminal pathway products in the C24- and C23-hydroxylation pathways including 24-oxo-25-OH-D3, 24-oxo-23,25-(OH)2D3, 25-OH-D3-26,23-lactone and calcioic acid. High doses of 24,25-(OH)2D3 resulted in suppression of 1,25-(OH)2D3 formation and interconversion to 1,24,25-(OH)2D3 via 1-hydroxylation (Figure 3). This combination of mouse models and LC-MS/MS tools can be used to identify alternative routes of vitamin D metabolism that might occur in clinical cases of hypervitaminosis D and in idiopathic infantile hypercalcemia (IIH) where CYP24A1 is mutated.
HIGHLIGHTS.
Serum calcioic acid can be detected in mice given 24,25-(OH)2D3.
CYP24A1 is integral for the clearance of 24,25-(OH)2D3 to water-soluble products.
•1,24,25-(OH)3D3 can be formed from 24,25-(OH)2D3 in vivo.
1,25-(OH)2D3 is suppressed when mice are given 24,25-(OH)2D3.
ACKNOWLEDGEMENTS
Through a Queen’s University-Waters Corporation research contract, Waters generously provided the LC-MS/MS instruments used in this study. This work was supported in part by grant 85600 from Shriners Hospitals for Children (to R.St-A.) and by NIH grant R01AR070544 (to R.St-A.). C.M. is a postdoctoral fellow of the Fonds de Recherche Québec – Santé. Synthetic calcioic acid was a generous gift from Prof. Leggy A. Arnold and Olivia B. Yu, University of Wisconsin – Milwaukee. Serum from a patient with hypervitaminosis D was provided by Dr. Joy Wu, Stanford University. We thank Linor Berezin (Queen’s University) for preparation of extracts from CYP24A1 cell lines.
Abbreviations:
- 25-OH-D3
25-hydroxyvitamin D3
- 1,25-dihydroxyvitamin D3
1,25-(OH)2D3
- 24,25-(OH)2D3
24,25-dihydroxyvitamin D3
- VDR
Vitamin D receptor
- LacCer
Lactosylceramide
- LC-MS/MS
liquid chromatography - tandem mass spectrometry
- DMEQ-TAD
4-(2-(6,7-Dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalinyl)ethyl)1,2,4-triazoline-3,5-dione)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
REFERENCES
- 1.Jones G, Strugnell SA & DeLuca HF Current understanding of the molecular actions of vitamin D. Physiol. Rev 78, 1193–1231 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Jones G, Prosser DE & Kaufmann M 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch. Biochem. Biophys 523, 9–18 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Prosser DE, Kaufmann M, O’Leary B, Byford V & Jones G Single A326G mutation converts human CYP24A1 from 25-OH-D3–24-hydroxylase into −23-hydroxylase, generating 1alpha,25-(OH)2D3–26,23-lactone. Proc. Natl. Acad. Sci. U. S. A 104, 12673–12678 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo EG & Norman AW Three-fold induction of renal 25-hydroxyvitamin D3–24-hydroxylase activity and increased serum 24,25-dihydroxyvitamin D3 levels are correlated with the healing process after chick tibial fracture. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 12, 598–606 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Seo EG, Einhorn TA & Norman AW 24R,25-dihydroxyvitamin D3: an essential vitamin D3 metabolite for both normal bone integrity and healing of tibial fracture in chicks. Endocrinology 138, 3864–3872 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Kato A, Seo EG, Einhorn TA, Bishop JE & Norman AW Studies on 24R,25-dihydroxyvitamin D3: evidence for a nonnuclear membrane receptor in the chick tibial fracture-healing callus. Bone 23, 141–146 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Martineau C et al. Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2. J. Clin. Invest 128, 3546–3557 (2018). doi: 10.1172/JCI98093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Arnaud R et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 141, 2658–2666 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann M et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J. Clin. Endocrinol. Metab 99, 2567–2574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann M et al. Improved Screening Test for Idiopathic Infantile Hypercalcemia Confirms Residual Levels of Serum 24,25-(OH)2 D3 in Affected Patients. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res (2017). doi: 10.1002/jbmr.3135 [DOI] [PubMed] [Google Scholar]
- 11.Meyer MB et al. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J. Biol. Chem 292, 17541–17558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann M, Prosser DE & Jones G Bioengineering anabolic vitamin D-25-hydroxylase activity into the human vitamin D catabolic enzyme, cytochrome P450 CYP24A1, by a V391L mutation. J. Biol. Chem 286, 28729–28737 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mass Spectrometry Assays of Vitamin D Metabolites. Vitam. D 909–923 (2018). doi: 10.1016/B978-0-12-809965-0.00050-1 [DOI] [Google Scholar]
- 14.Makin G, Lohnes D, Byford V, Ray R & Jones G Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem. J 262, 173–180 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy GS & Tserng KY Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry (Mosc.) 28, 1763–1769 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Beckman MJ et al. Human 25-hydroxyvitamin D3–24-hydroxylase, a multicatalytic enzyme. Biochemistry (Mosc.) 35, 8465–8472 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Tieu EW, Tang EKY & Tuckey RC Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 281, 3280–3296 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Reddy GS et al. 23-carboxy-24,25,26,27-tetranorvitamin D3 (calcioic acid) and 24-carboxy-25,26,27-trinorvitamin D3 (cholacalcioic acid): end products of 25-hydroxyvitamin D3 metabolism in rat kidney through C-24 oxidation pathway. Arch. Biochem. Biophys 455, 18–30 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Kusudo T et al. Metabolism of 20-epimer of 1alpha,25-dihydroxyvitamin D3 by CYP24: species-based difference between humans and rats. Biochem. Biophys. Res. Commun 309, 885–892 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Tang EKY, Tieu EW & Tuckey RC Expression of human CYP27B1 in Escherichia coli and characterization in phospholipid vesicles. FEBS J. 279, 3749–3761 (2012). [DOI] [PubMed] [Google Scholar]


