Abstract
Background
It is 1 of the standard treatment options for metastasis pancreatic cancer to receive nab-paclitaxel (125 mg/m2) plus gemcitabine (1000 mg/m2) on days 1, 8 and 15 every 28 days. Some patients showed intolerance and inconvenience to this therapeutic regimen. Thus, we conducted this retrospective real-world study to determine the efficacy and tolerability of a modified 21-day nab-paclitaxel plus gemcitabine (nab-P/Gem) regimen for the first-line treatment of locally advanced or metastatic pancreatic cancer.
Methods
Patients with locally advanced and metastatic pancreatic cancer treated with nab-paclitaxel (125 mg/m2) plus gemcitabine (1000 mg/m2) on days 1 and 8 every 21-day at West China Hospital and Shang Jin Hospital of Sichuan University from Mar 2018 to Dec 2021 were reviewed retrospectively. Clinical characteristics of patients were collected. The progression-free survival, overall survival, objective response rate, disease control rate, and toxicity were evaluated.
Results
A total of 113 patients who received the modified regimen of 21-day nab-P/Gem chemotherapy were included. The median overall survival was 9.3 months and the median progression-free survival was 4.4 months. The objective response rate and disease control rate were 18.6% and 56.7%, respectively. The median relative dose intensity for this modified regimen was 65%. The adverse events were mild to moderate, and the most common grade 3 or 4 treatment-related adverse events were neutropenia (21%) and leukopenia (16%).
Conclusions
Our study showed that this modified regimen of 21-day nab-P/Gem for locally advanced and metastatic pancreatic cancer had comparable efficacy and tolerable toxicity. This treatment may provide a considerable option for pancreatic cancer patients who desire a modified schedule. The modified regimen of 21-day nab-P/Gem is also an option worth considering during the coronavirus disease 2019 pandemic for minimizing the number of visits and limiting the risk of exposure.
Keywords: nab-paclitaxel, gemcitabine, pancreatic cancer, overall survival, progression-free survival
Introduction
Pancreatic cancer patients are often faced with the dilemma of an unresectable tumor or metastasis, and the 5-year survival of pancreatic cancer is less than 10%.1,2 After tumor resection, less than 5% of patients had more than 10 years of survival.3 Chemotherapy is the primary option for patients with locally advanced or metastatic pancreatic cancer, which, however, is 1 of the most chemotherapy-resistant tumors.4,5 Therefore, it is urgent to develop different regimens to improve the prognosis.
Some clinical trials have verified the function of chemotherapy drugs, such as gemcitabine or fluorouracil, which have shown a modest survival benefit for pancreatic cancer patients.6,7 The MPACT and LAPACT clinical trials tested the effect of nab-paclitaxel plus gemcitabine (nab-P/Gem) as a valuable choice for patients with locally advanced and metastatic pancreatic cancer.8–10 However, the traditional 28-day regimen, 1, 8 and 15-day administration of nab-P/Gem treatment, presented many adverse events, such as hematological toxicity and neurotoxicity, and some patients experienced dose reduction or discontinuation due to adverse events (AEs), which remained a concern.9,10 According to the CSCO guideline, most Chinese medical institutions chose 21-day nab-P/Gem regimen to decrease the potential AEs and increase tolerability.11 Thus, we conducted this retrospective real-world study to determine the efficacy and tolerability of a modified 21-day nab-P/Gem regimen for the first-line treatment of locally advanced or metastatic pancreatic cancer.
Materials and Methods
Patients and Study Design
This study retrospectively collected patients with locally advanced or metastatic pancreatic cancer from West China Hospital and Shang Jin Hospital of Sichuan University between Mar 2018 and Dec 2021. All patients were reviewed according to the inclusion criteria and patients would be included if they met the key inclusion criteria: (1) patients with locally advanced or metastatic pancreatic cancer confirmed by pathology; (2) not receiving any prior systematic anti-tumor treatment; (3) age ≥18 years old and ≤80 years old; (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. Eligible patients’ clinical characteristics were abstracted from clinical records, including age, sex, body mass index (BMI), ECOG PS score, tumor location, distant metastasis site, tumor stage, time of first diagnosis, treatment regimen, number of chemotherapy cycles, best tumor response, progression-free survival (PFS), overall survival (OS), relative dose intensity (RDI), and AEs. The study was approved by Chinese Ethics Committee of Registering Clinical Trials (ChiECRCT20190240) on November 14, 2019 and registered at Chinese Clinical Trial Registry (ChiCTR1900027729) on November 24, 2019. The Chinese Ethics Committee of Registering Clinical Trials is located at Chinese Clinical Trial Registry Hong Kong Center, Hong Kong Baptist University Road, Hong Kong SAR, China. Written informed consents were obtained from all participants. All patient details had been de-identified. The reporting of this study conforms to STROBE guidelines.12
Treatment and Evaluation
Patients received nab-paclitaxel at 125 mg/m2 and gemcitabine at 1000 mg/m2 on days 1 and 8 of a 21-day cycle. Preventive use of antiemetic drugs was allowed. Treatment was continued until documented disease progression or unacceptable toxicity. AEs were evaluated according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) 5.0. Radiographic evaluation was performed by computerized tomography or magnetic resonance imaging every 2 cycles of the treatment following Response Evaluation Criteria In Solid Tumors (RECIST) 1.1. The best tumor response during the first-line 21-day nab-P/Gem chemotherapy would be recorded for evaluating the efficacy. The PFS, OS, objective response rate (ORR), disease control rate (DCR), and toxicity were evaluated. PFS was defined as the time from the start of treatment to the date of documented disease progression, treatment discontinuation due to intolerable AEs, or death. OS was defined as the time from the start of treatment to the death from any cause or the date of last known follow up. ORR was defined as the proportion of the total number of complete response (CR) + partial response (PR) patients according to the RECIST 1.1. DCR was defined as the sum of ORR and stable disease (SD). During the study, the inclusion and exclusion criteria of patients were strictly followed to address potential selection bias.
Statistical Analysis
Categorical data were described using frequencies and percentages. Normally distributed continuous data were reported as mean ± standard deviation. The PFS and OS survival curves were estimated by Kaplan-Meier method. Estimated median with 95% confidence intervals (CIs) of PFS and OS were also reported. All statistical analyses were performed using SPSS 22.0 and GraphPad Prism 8.0.
Results
Patient Characteristics
In total, 113 patients with locally advanced or metastatic pancreatic cancer were included. Of the 113 patients who received 21-day nab-P/Gem chemotherapy, 66 were male (58.4%) and 47 were female (41.6%). Age and BMI are conformed to the normal distribution. The mean age was 59 ± 10.0 years, with a range of 22-79. The mean BMI was 20.8 ± 2.8, ranging from 15.6-29. All patients had a ECOG PS of 0 or 1. Among all of patients, 51 patients (45.1%) had the tumor in the head of the pancreas while for 62 patients (54.9%) it was in the body and tail of the pancreas. Twenty-seven patients (23.9%) were at a locally advanced stage and 86 patients (76.1%) had tumor metastasis. Among these, 54 (62.8%) and 14 (16.3%) patients had liver and peritoneal metastasis, respectively. The details of patients’ characteristics were summarized in Table 1.
Table 1.
Patient characteristics (n = 113).
| Variables | n (%) |
|---|---|
| Age | |
| ≤60 | 54 (47.8) |
| >60 | 59 (52.2) |
| Sex | |
| Male | 66 (58.4) |
| Female | 47 (41.6) |
| ECOG PS | |
| 0 | 44 (38.9) |
| 1 | 69 (61.1) |
| Tumor location | |
| Head | 51 (45.1) |
| Body and tail | 62 (54.9) |
| BMI | |
| ≤20 | 47 (41.6) |
| >20 | 66 (58.4) |
| Disease stage | |
| Metastasis | 86 (76.1) |
| Locally advanced | 27 (23.9) |
| Liver metastasis | |
| Yes | 54 (47.8) |
| No | 59 (52.2) |
| Peritoneal metastasis | |
| Yes | 14 (12.4) |
| No | 99 (87.6) |
Abbreviations: BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status.
Efficacy Analysis
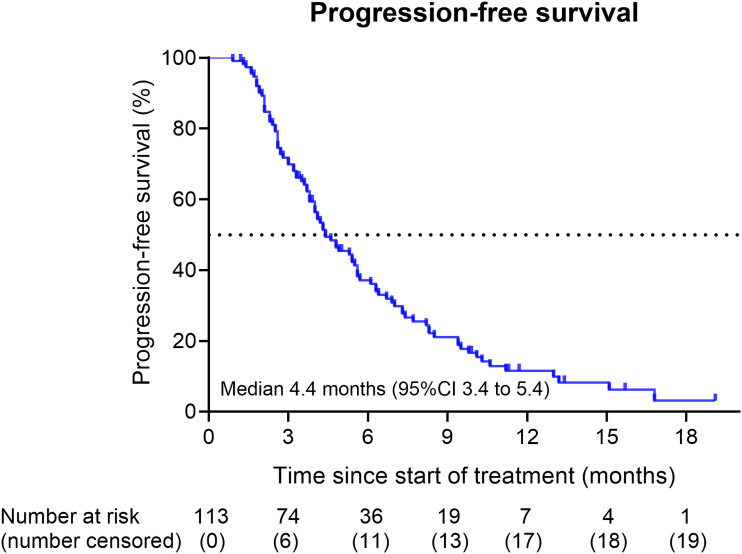
The final follow-up time was 31 December 2021. The median number of chemotherapy cycles administered was 3 (range of 1-13 cycles). The median duration of treatment was 4.3 months and 38 patients (33.6%) received the first-line treatment for at least 6 months. The median RDI for this modified regimen was 65%. Until the final follow-up time, 93 patients (82.3%) showed disease progression after first-line 21-day nab-P/Gem chemotherapy and 100 patients (88.5%) had died. Among all of 113 patients, the median OS was 9.3 months (95% CI 8.2-10.4) (Figure 1), and the median PFS was 4.4 months (95% CI 3.4-5.4) (Figure 2). Best tumor response evaluation data were available for all patients at the time of the analysis. One patient achieved CR, while 20 patients achieved PR, 43 patients achieved SD, and 49 patients showed PD. The ORR was 18.6%, and the DCR was 56.7%.
Figure 1.
Kaplan–Meier curves for overall survival (OS) in all treated patients (n = 113). The median OS was 9.3 months (95% confidence interval 8.2-10.4).
Figure 2.
Kaplan–Meier curves for progression-free survival (PFS) in all treated patients (n = 113). The median PFS was 4.4 months (95% confidence interval 3.4-5.4).
Toxicity Analysis
The toxicity of the modified 21-day nab-P/Gem regimen was analyzed and no treatment-associated deaths were recorded. AEs were recorded in 105 patients during the fisrt-line 21-day nab-P/Gem chemotherapy outlined in Table 2. The most common occurring hematologic AE of any grade was anemia (89%). The most common grade ≥3 hematologic AEs were neutropenia (17%). The most frequent nonhematologic AEs of any grade was increased AST (41%). The most common grade ≥3 nonhematologic AEs was increased ALT (6%). No death or grade 5 AEs occurred during the treatment.
Table 2.
Treatment-Related Adverse Events.
| Adverse events | All Grades, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % |
|---|---|---|---|---|---|
| Leukopenia | 44 | 8 | 20 | 15 | 1 |
| Neutropenia | 47 | 12 | 15 | 17 | 4 |
| Anemia | 89 | 52 | 29 | 8 | 0 |
| Thrombocytopenia | 35 | 15 | 16 | 3 | 1 |
| Bilirubin | 18 | 7 | 6 | 4 | 0 |
| ALT | 36 | 25 | 5 | 6 | 0 |
| AST | 41 | 28 | 9 | 4 | 0 |
| Diarrhea | 8 | 6 | 2 | 0 | 0 |
| Fatigue | 26 | 15 | 9 | 2 | 0 |
| Vomiting | 27 | 19 | 8 | 0 | 0 |
| Peripheral neuropathy | 20 | 15 | 5 | 0 | 0 |
| Skin allergy | 7 | 7 | 0 | 0 | 0 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Discussion
It has been confirmed that the 28-day regimen of nab-P/Gem treatment is primary option for patients with locally advanced or metastatic pancreatic cancer.8–10 Here, to our knowledge, for the first time the safety and efficacy of a 21-day regimen of nab-P/Gem was assessed. In this study, the median OS and PFS were 9.3 months and 4.4 months, respectively. The ORR was 18.6% and the DCR was 56.7%. Most of the treatment-related AEs were grade 1 or 2 and the most common grade 3 or 4 treatment-related AEs were neutropenia (21%) and leukopenia (16%).
In a phase Ⅰ/II clinical trial,13 a total of 34 Japanese patients with metastatic pancreatic cancer were administered nab-paclitaxel and gemcitabine on days 1, 8, and 15 every 28 days. The ORR was 58.8%, and the PFS and OS were 6.5 months and 13.5 months, respectively. In another phase I/II study,14 44 patients with advanced pancreatic cancer received the 28-day regimen treatment. The OS and PFS were 12.2 and 7.9 months respectively, and the DCR was 68%. In the phase III MPACT trial, 431 patients with metastatic pancreatic cancer were treated with 28-day regimen. The median OS was 8.5 months and the median PFS was 5.5 months.10 In the phase Ⅱ LAPACT trial, 107 patients with locally advanced pancreatic cancer were treated with a 28-day regimen of nab-P/Gem. The median OS was 18.8 months and the median PFS was 10.9 months.9 In this study, DCR, median PFS and median OS were 56.7%, 4.4 and 9.3 months, respectively. The results may relate to the difference in disease stage of included patients. The MPACT trial included patients with metastatic pancreatic cancer and the LAPACT trial included patients with locally advanced disease. In our study, patients with either metastatic disease (76.1%) or locally advanced disease (23.9%) were both included, which may explain that OS in this study was longer than the MPACT trial but shorter than the LAPACT trial. However, the median RDI for this modified regimen was lower than that reported in the MPACT trial. At this stage, we can only speculate that this controversial result may relate with the inherent risk of confounding and selection bias. The efficacy of 21-day nab-P/Gem chemotherapy was consistent with published retrospective studies,15,16 and the OS of this modified regimen seemed to be comparable with the OS of the traditional 28-day regimen reported by recent real-world studies listed in Table 3.17,18
Table 3.
Comparison of Efficacy Among Retrospective studies.
| Variable | The Modified Regimen | The Classic Regimen | |||
|---|---|---|---|---|---|
| Our Study (n = 113) | Cui H 2020 (n = 75) | Quan Q 2020 (n = 62) | Riedl JM 2021 (n = 297) | Fernández a 2018 (n = 210) | |
| Objective response rate (%) | 18.6 | 18.7 | 24 | 31 | 24.6 |
| Disease control rate (%) | 56.7 | 69.3 | 82 | 58 | 46.9 |
| Median PFS (months) | 4.4 | 4 | 4.9 | 4.6 | 5.0 |
| Median OS (months) | 9.3 | 9 | 11.1 | 10.1 | 7.2 |
Abbreviations: PFS, progression-free survival; OS, overall survival.
In addition to efficacy, the safety is also important for patients. In the MPACT trial,10 the most common adverse events of grade 3 or 4 were neutropenia (38%), fatigue (17%), and neuropathy (17%) in the nab-P/Gem group. In the LAPACT trial,9 the most common adverse events of grade 3 or 4 were also neutropenia (33%), anemia (11%), and fatigue (10%). However, our data showed that the most common grade 3 or 4 treatment-related AEs were neutropenia (21%) and leukopenia (16%), and the incidence of AEs listed in Table 4 was lower than that reported in the MPACT or LAPACT trial.9,10 In addition, no patients experienced a toxicity that required a reduction in the 21-day regimen of nab-P/Gem, whereas the MPACT trial reported 41% required a nab-paclitaxel dose reduction and 47% required a gemcitabine dose reduction.10 However, the incidence of grade 3 or 4 treatment-related AEs reported in the modified 21-day regimen was similar to the incidence reported in the classic regimen at real-world setting, and both were lower than in prospective trials. The reason for this discrepancy could be related to the differences in study design. Therefore, the 21-day regimen of nab-P/Gem may be a considerable option for advanced pancreatic cancer patients who are less tolerant of the 28-day regimen, especially for elderly patients or those in poor physical condition.
Table 4.
Comparison of Grade 3 or Higher Treatment-Related Adverse Events.
| Adverse events | The Modified Regimen | The Classic Regimen | Clinical Trials | ||||
|---|---|---|---|---|---|---|---|
| Our Study (n = 113) | Cui H 2020 (n = 75) | Quan Q 2020 (n = 62) | Riedl JM 2021 (n = 297) | Fernández a 2018 (n = 210) | MPACT Trial (n = 421) | LAPACT Trial (n = 106) | |
| Leukopenia | 16% | 19.7%* | 25% | 26%** | NR | 31% | 5% |
| Neutropenia | 21% | NR | 32% | NR | 18.1% | 38% | 33% |
| Anemia | 8% | NR | 14% | NR | 3.3% | 13% | 11% |
| Thrombocytopenia | 4% | NR | 4% | NR | 6.2% | 13% | 4% |
| Bilirubin | 4% | NR | NR | NR | NR | NR | 2% |
| ALT | 6% | NR | NR | NR | NR | NR | 6% |
| AST | 4% | NR | NR | NR | NR | NR | 2% |
| Diarrhea | 0 | 0 | NR | 22%** | NR | 6% | 4% |
| Fatigue | 2% | NR | 9% | 40%** | 6.2% | 17% | 10% |
| Vomiting | 0 | 1.3% | NR | NR | 1.4% | NR | 3% |
| Peripheral neuropathy | 0 | 2.6% | 13% | 31%** | 2.4% | 17% | 1% |
| Skin allergy | 0 | 0 | NR | NR | NR | NR | 1% |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; NR, not report.
*Myelosuppression; **any grade.
Findings of this study provide new insights into the management of cancer patients during the coronavirus disease 2019 (COVID-19) pandemic. Previous studies confirmed that patients with cancer had a higher infection rate than the general population, and patients with both cancer and COVID-19 had a higher mortality and the severe illness rate than that in the overall COVID-19-positive population.19–24 In addition, anti-epidemic measures during the COVID-19 pandemic may result in a delay in cancer diagnosis or treatment, causing negative impact on cancer care.25 Therefore, it is of great significance to explore the modifications of the management of patients with cancer under the background of COVID-19 pandemic. The 21-day regimen of nab-P/Gem proposed in this study could minimize the number of visits and limit the risk of exposure for patients without compromising the efficacy. At the same time, the modified regimen did not increase the treatment-related toxicities, and the susceptibility of patients with cancer was also controlled. Thus, the modified regimen of 21-day nab-P/Gem is an option worth considering during the COVID-19 pandemic.
The main limitation of this study is its retrospective design and its small sample size. Considering the limited information obtained from electronic records, the information bias could not be excluded. We should underline the fact that this study was performed at a single center, the extrapolation needs to be further explored in future studies.
Conclusion
The modified regimen of 21-day nab-P/Gem was a safe, tolerated and effective treatment for patients with locally advanced or metastatic pancreatic cancer. The AEs were much more moderate and tolerable. Our analysis represents the possibility of a modified 21-day regimen of nab-P/Gem treatment. This modified regimen may be an option worth considering during the COVID-19 pandemic for minimizing the number of visits and limiting the risk of exposure. We also agree that patient tolerability and schedule convenience should weigh into treatment decision making for metastatic incurable malignancies, in which therapy is for palliative intent and such modified regimens should be used after a discussion between doctors and patients.
Acknowledgments
The authors would like to thank CSPC OUYI PHARMACEUTICAL CO., Ltd for study assistance.
Footnotes
Author contributions: (I) Conception and design: Ji Ma and Dan Cao; (II) Administrative support: Ji Ma and Dan Cao; (III) Provision of study materials or patients: Chen Chang, Lingwei Meng, Xiaofen Li, Ke Cheng, Cheng Yi, and Bing Peng; (IV) Collection and assembly of data: Chen Chang, Lingwei Meng, Xiaofen Li, Ke Cheng, Cheng Yi, and Bing Peng; (V) Data analysis and interpretation: Chen Chang and Ji Ma; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the Sichuan Province Science and Technology Support Program (2021YFS0047 to Dan Cao), Key Research and Development Project of Sichuan Province (2020YFS0273 to Ji Ma), and Scientific Research Project of Sichuan Health Commission (20PJ008 to Ji Ma).
Data availability: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Statement: The study was approved by Chinese Ethics Committee of Registering Clinical Trials (ChiECRCT20190240) on November 14, 2019 and registered at Chinese Clinical Trial Registry (ChiCTR1900027729) on November 24, 2019. The Chinese Ethics Committee of Registering Clinical Trials is located at Chinese Clinical Trial Registry Hong Kong Center, Hong Kong Baptist University Road, Hong Kong SAR, China. Written informed consents were obtained from all participants.
ORCID iDs
Chen Chang https://orcid.org/0000-0001-8408-0906
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [DOI] [PubMed] [Google Scholar]
- 2.Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paniccia A, Hosokawa P, Henderson W, Schulick RD, Edil BH, McCarter MD, et al. Characteristics of 10-Year Survivors of Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2015;150:701-710. [DOI] [PubMed] [Google Scholar]
- 4.Adamska A, Elaskalani O, Emmanouilidi A, Kim M, Abdol Razak NB, Metharom P, et al. Molecular and cellular mechanisms of chemoresistance in pancreatic cancer. Adv Biol Regul. 2018;68:77-87. [DOI] [PubMed] [Google Scholar]
- 5.Lemstrova R, Melichar B, Mohelnikova-Duchonova B. Therapeutic potential of taxanes in the treatment of metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;78:1101-1111. [DOI] [PubMed] [Google Scholar]
- 6.Bachet JB, Chibaudel B, Bonnetain F, Validire P, Hammel P, Andre T, et al. A randomized phase II study of weekly nab-paclitaxel plus gemcitabine or simplified LV5FU2 as first-line therapy in patients with metastatic pancreatic cancer: the AFUGEM GERCOR trial. BMC Cancer. 2015;15:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413. [DOI] [PubMed] [Google Scholar]
- 9.Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285-294. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui J, Zhang X, Qu S, et al. Cost-effectiveness analysis of nab-paclitaxel plus gemcitabine versus in the treatment of metastatic pancreatic cancer in china. Expert Rev Pharmacoecon Outcomes Res. 2021;21:691-697. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595-603. [DOI] [PubMed] [Google Scholar]
- 14.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui H, Guan J, Deng G, Yuan J, Lou C, Zhang W, et al. A Chinese Retrospective Multicenter Study of First-Line Chemotherapy for Advanced Pancreatic Cancer. Med Sci Mon Int Med J Exp Clin Res. 2020;26:e927654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan Q, Wang Y, Wang F, Zhang D, Chen X, He W, et al. Real World First-Line Treatments and Outcomes of Nab-Paclitaxel Plus Gemcitabine, mFOLFIRINOX and GEMOX in Unresectable Pancreatic Cancer from a Chinese Single Institution. Curr Oncol. 2020;28:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riedl JM, Posch F, Horvath L, Gantschnigg A, Renneberg F, Schwarzenbacher E, et al. Gemcitabine/nab-Paclitaxel versus FOLFIRINOX for palliative first-line treatment of advanced pancreatic cancer: A propensity score analysis. Eur J Cancer. 2021;151:3-13. [DOI] [PubMed] [Google Scholar]
- 18.Fernández A, Salgado M, García A, Buxo E, Vera R, Adeva J, et al. Prognostic factors for survival with nab-paclitaxel plus gemcitabine in metastatic pancreatic cancer in real-life practice: the ANICE-PaC study. BMC Cancer. 2018;18(1):1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10:783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020;6:1108-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong X, Qi Y, Huang J, Zhao Y, Zhan Y, Qin X, et al. Epidemiological and clinical characteristics of cancer patients with COVID-19: A systematic review and meta-analysis of global data. Cancer Lett. 2021;508:30-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]