CONSPECTUS:
Membranes are multifunctional supramolecular assemblies that encapsulate our cells and the organelles within them. Glycerophospholipids are the most abundant component of membranes. They make up the majority of the lipid bilayer and play both structural and functional roles. Each organelle has a different phospholipid composition critical for its function that results from dynamic interplay and regulation of numerous lipid-metabolizing enzymes and lipid transporters. Because lipid structures and localizations are not directly genetically encoded, chemistry has much to offer to the world of lipid biology in the form of precision tools for visualizing lipid localization and abundance, manipulating lipid composition, and in general decoding the functions of lipids in cells.
In this Account, we provide an overview of our recent efforts in this space focused on two overarching and complementary goals: imaging and editing the phospholipidome. On the imaging front, we have harnessed the power of bioorthogonal chemistry to develop fluorescent reporters of specific lipid pathways. Substantial efforts have centered on phospholipase D (PLD) signaling, which generates the humble lipid phosphatidic acid (PA) that acts variably as a biosynthetic intermediate and signaling agent. Though PLD is a hydrolase that generates PA from abundant phosphatidylcholine (PC) lipids, we have exploited its transphosphatidylation activity with exogenous clickable alcohols followed by bioorthogonal tagging to generate fluorescent lipid reporters of PLD signaling in a set of methods termed IMPACT.
IMPACT and its variants have facilitated many biological discoveries. Using the rapid and fluorogenic tetrazine ligation, it has revealed the spatiotemporal dynamics of disease-relevant G protein-coupled receptor signaling and interorganelle lipid transport. IMPACT using diazirine photo-cross-linkers has enabled identification of lipid–protein interactions relevant to alcohol-related diseases. Varying the alcohol reporter can allow for organelle-selective labeling, and varying the bioorthogonal detection reagent can afford super-resolution lipid imaging via expansion microscopy. Combination of IMPACT with genome-wide CRISPR screening has revealed genes that regulate physiological PLD signaling.
PLD enzymes themselves can also act as tools for precision editing of the phospholipid content of membranes. An optogenetic PLD for conditional blue-light-stimulated synthesis of PA on defined organelle compartments led to the discovery of the role of organelle-specific pools of PA in regulating oncogenic Hippo signaling. Directed enzyme evolution of PLD, enabled by IMPACT, has yielded highly active superPLDs with broad substrate tolerance and an ability to edit membrane phospholipid content and synthesize designer phospholipids in vitro. Finally, azobenzene-containing PA analogues represent an alternative, all-chemical strategy for light-mediated control of PA signaling.
Collectively, the strategies described here summarize our progress to date in tackling the challenge of assigning precise functions to defined pools of phospholipids in cells. They also point to new challenges and directions for future study, including extension of imaging and membrane editing tools to other classes of lipids. We envision that continued application of bioorthogonal chemistry, optogenetics, and directed evolution will yield new tools and discoveries to interrogate the phospholipidome and reveal new mechanisms regulating phospholipid homeostasis and roles for phospholipids in cell signaling.
Graphical Abstract

INTRODUCTION
Cellular membranes are selectively permeable barriers and platforms for signaling, and their composition and behavior are highly dynamic.5–7 Membranes comprise hydrophobic proteins embedded in or adhered to a lipid bilayer containing glycerophospholipids, sphingolipids, and sterols. Membrane lipid compositions are unique in different tissues, cell types, and even organelles, with the lipid content matched to specialized physiological demands.8,9 Among lipid classes, the most abundant are glycerophospholipids (phospholipids), which contain glycerol, acyl chains at the sn-1 and sn-2 positions, and a phosphodiester-linked headgroup at the sn-3 position (Figure 1A).8,10
Figure 1.
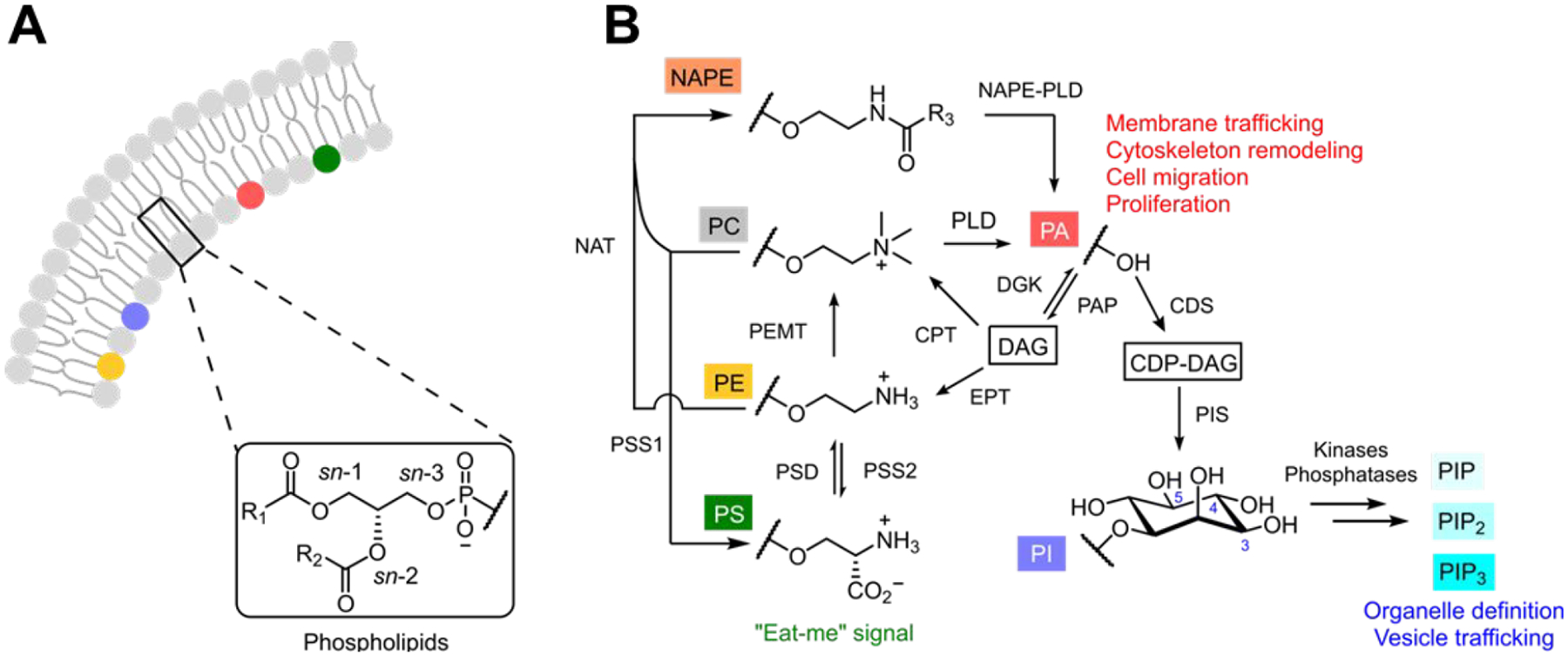
Overview of mammalian phospholipid metabolism. (A) Cartoon structure of a typical lipid bilayer, highlighting the chemical structure of glycerophospholipids. R1 and R2 denote fatty acyl tails at the sn-1 and sn-2 positions that can be saturated, monounsaturated, or polyunsaturated, and a phosphate-linked headgroup is located at the sn-3 position. (B) Simplified metabolic pathway showing biosynthesis and interconversion of the major glycerophospholipids and selected roles in signaling.
Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are the most abundant glycerophospholipids, and they play mainly structural roles. Lipids present at lower concentrations can function in specific physiological events, e.g., cargo transport, ion flux, and signaling. For example, phosphoinositides (PIPs), the phosphorylated derivatives of phosphatidylinositol (PI), decorate certain organelle membranes and act as cofactors for physiological processes, including endocytosis, vesicle trafficking, ion channel activity, actin dynamics, and interorganelle and transbilayer transport of lipids, including cholesterol and phosphatidylserine (PS).11 The latter of these is restricted to the cytosolic face of membranes, including the inner leaflet of the plasma membrane (PM), but during apoptosis and other conditions such as neurite development, it becomes externalized to the extracellular leaflet, serving as an “eat-me” signal to promote phagocytosis.12
Central among phospholipid metabolism and signaling but often overlooked is phosphatidic acid (PA), perhaps the simplest glycerophospholipid, with only a phosphomonoester headgroup. Formed directly from glycerol 3-phosphate and fatty acyl-CoAs derived from glycolysis and fat metabolism, PA is the precursor of essentially all other glycerophospholipids. Beyond this de novo biosynthetic pathway, PA is formed from other lipids by diacylglycerol kinases (DGKs), phospholipase Ds (PLDs), and N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD). Some of these enzymes are stimulated by cell-surface receptors, causing signaling by enabling activation of PA-binding proteins. Through these various mechanisms, many downstream events can be stimulated by PA (Figure 1B).13,14
A major challenge in lipid cell biology is to deconvolute the intricate and overlapping mechanisms by which cells tune machineries that maintain the fine balance of membrane composition and lipid signaling. These include lipid-metabolizing enzymes, lipid transporters, and lipid-binding proteins, and they are relevant for both abundant bulk lipids like PC and PE and low-abundance signaling lipids like PA and PIPs. It will be crucial to image precise locations, quantify transport rates, and understand how local lipid pools affect distinct signaling pathways, as exemplified by our work on PA.13 Motivated by the limitations of traditional toolsets for interrogating lipid biology, which lack spatiotemporal precision in introducing perturbations to cell membranes, we have harnessed molecular engineering approaches, including optochemical probes and optogenetic enzymes, metabolic probes using bioorthogonal chemistry, and photoaffinity labeling.
Using these strategies, we have developed tools for precisely manipulating the phospholipid content of membranes, a process that we term membrane editing in analogy to other biomolecular editors, e.g., base editing of nucleic acids or protein editors that add or remove post-translational modifications. We have also developed complementary methods for visualizing the spatiotemporal dynamics of phospholipid signaling and transport at organelle-level resolution and in some cases using super-resolution methods. Furthermore, we have developed photoaffinity labeling probes for identifying pathophysiologically relevant interactions of phospholipids with the proteome. In this Account, we describe the motivation and development of these tools for interrogating the phospholipidome and highlight applications to reveal new mechanisms regulating phospholipid homeostasis and roles for phospholipids in signaling.
MANIPULATING SIGNALING WITH PHOTOSWITCHABLE PA ANALOGUES
The most straightforward way to perturb membrane composition is to incubate cells with a natural or unnatural lipid, leading to its uptake and incorporation into cellular membranes, a procedure known as bulk dosing. This approach has the downside that if the delivered lipid is bioactive, it will always be in the “on” state, even during uptake, when it may be in an undesirable organelle location. Photoswitchable lipids that have “on” and “off” states controllable by light can partially solve this problem. These unnatural lipids bear azobenzene groups that replace one or two acyl tails and are capable of photoinduced isomerization between the thermally stable (or 450 nm light-induced) trans isomer and the 365 nm light-induced cis isomer.15–20 As the former mimics a saturated tail and the latter an unsaturated tail, photoswitchable lipid analogues can undergo light- and shape-dependent interactions with protein effectors and lipid-metabolizing enzymes, hence allowing their bioactivity to be controlled by UV light.
To afford light-mediated control of PA signaling, we designed and synthesized photoswitchable PA analogues featuring either one or two azobenzene-containing acyl tails, termed AzoPA and dAzoPA, respectively, in a collaboration with the Trauner group (Figure 2A).21 Interestingly, unsaturated forms of PA can activate mitogenic signaling through the mammalian target of rapamycin (mTOR), a central regulator of cell growth and proliferation.22 Indeed, we found that cis-dAzoPA but not trans-dAzoPA stimulated mTOR signaling in cells.23 Beyond mTOR signaling, we also found that the cis forms of AzoPA and dAzoPA could selectively repress the Hippo pathway, a growth-restrictive pathway that prevents nuclear translocation of the progrowth transcription factor YAP. By turning off the Hippo pathway, the cis forms of AzoPA and dAzoPA mimic the role of natural PA in promoting growth but in a manner that depends upon the conformation of the hydrophobic azobenzene groups.3,24 Overall, these photoswitchable PA analogues add to the growing compendium of photoswitchable lipids25 for light-dependent control of lipid signaling pathways and provide specific tools for manipulating two therapeutically relevant oncogenic signaling pathways.
Figure 2.
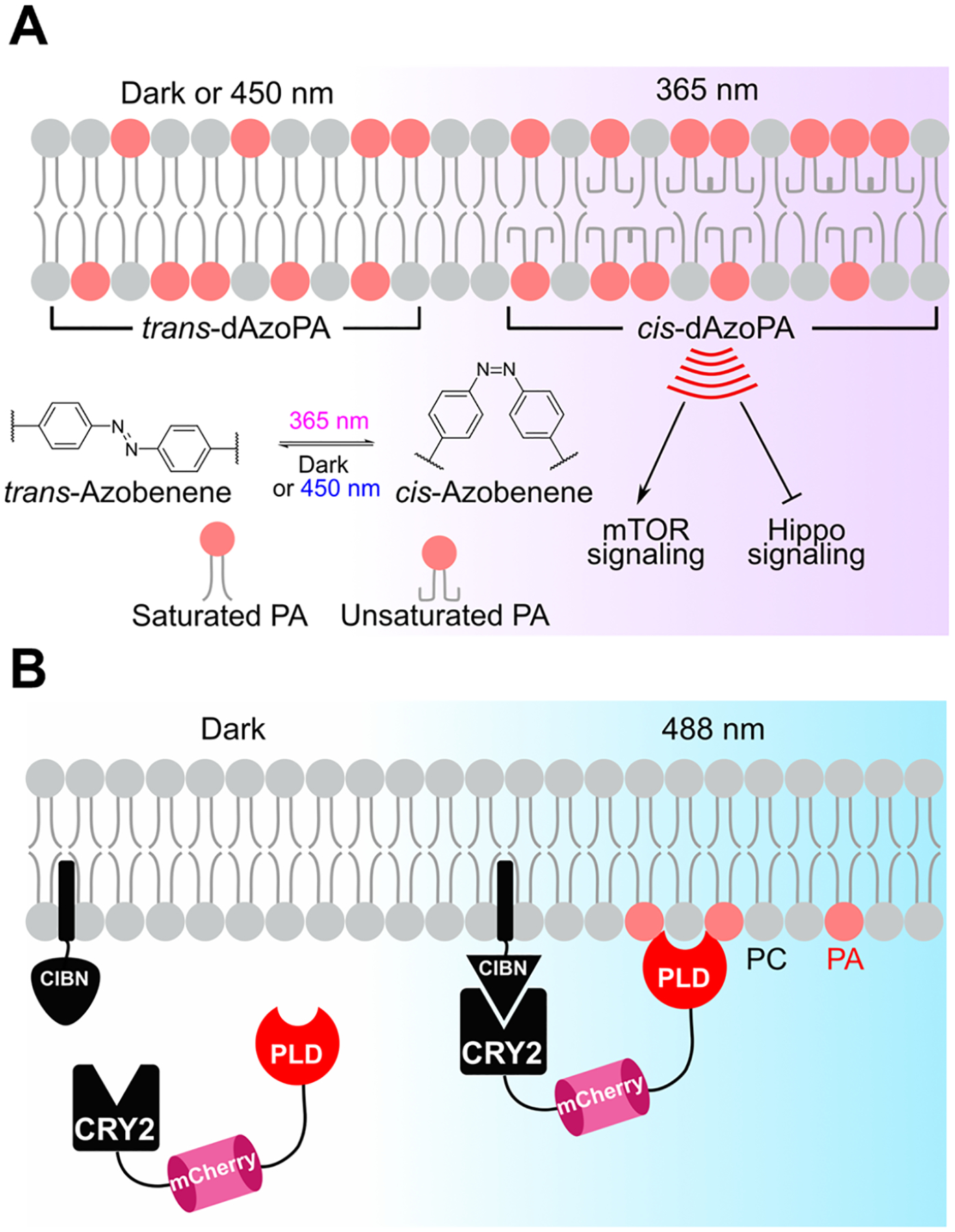
Small-molecule and engineered enzyme-based tools for optical control of PA signaling. (A) Isomerization of the photoswitchable PA analogues AzoPA and dAzoPA (dAzoPA is shown) from the trans configuration to the cis configuration by 365 nm light leads to stimulation of mTOR signaling and suppression of Hippo signaling. The trans configuration can be regenerated by exposure to 450 nm light. (B) Optogenetic phospholipase Ds (optoPLDs) enable light-dependent generation of PA by recruitment of a PLD enzyme from Streptomyces sp. PMF to a desired organelle membrane mediated by CRY2–CIBN heterodimerization, followed by PLD-catalyzed hydrolysis of PC to generate PA.
AN OPTOGENETIC PHOSPHOLIPASE D ENABLES SPATIOTEMPORALLY CONTROLLED PA PRODUCTION
Directly delivered lipids are generally imported by endocytosis, and thereby the incorporated lipid labels membranes of many intracellular organelles.26 As an alternative to delivery of intact lipids, enzyme-based systems afford high precision and tunability for engineering and editing membrane composition. Notably, chemical- and light-mediated heterodimerization tools, also known as induced proximity tools, have recently gained popularity.27 Classically, the rapamycin-dependent heterodimerization of FKBP and FRB has enabled recruitment of PIP-modifying enzymes to specific organelle membranes for local editing of PIP composition.28–30 On the other hand, optogenetic systems such as CRY2–CIBN have the added advantages of being reversible and allowing spatially addressable dimerization. Typically, CIBN is linked to a constitutive membrane-targeting sequence to control its location, and CRY2 is fused to a lipid-modifying enzyme. Upon blue-light stimulation, the CRY2 fusion protein rapidly translocates to the CIBN-containing target membrane to mediate local lipid metabolism.31
Because of the diverse metabolic sources of PA and its pleiotropic effects on signaling, we exploited the CRY2–CIBN optogenetic dimerization system to generate tools for light-controlled local synthesis of PA.3 Critically, since PLDs hydrolyze PC into PA, for the PA-generating enzyme we used a bacterial PLD from Streptomyces sp. PMF. This PLD is a soluble, constitutively active enzyme that is not subject to regulation by endogenous mammalian factors. Our optogenetic PLD (optoPLD) consists of a membrane-anchored CIBN and a fusion of CRY2 to both mCherry and PLD. When both constructs are expressed in mammalian cells, CRY2–CIBN heterodimerization upon blue-light stimulation recruits PLD to the desired membrane to produce PA (Figure 2B). OptoPLD enabled PA generation on several organelle membranes, including the PM, endosomes, the endoplasmic reticulum (ER), and the trans-Golgi network in our initial study3 and in subsequent work additional organelles such as mitochondria32 and lysosomes.33 To demonstrate the biological relevance of the optoPLD-derived PA, we established that PM-targeted optoPLD caused translocation of YAP into the nucleus under starvation, providing evidence of a suppressive effect of PM pools of PA on Hippo signaling. Interestingly, this finding not only corroborated a previous study indicating that PA can antagonize the Hippo pathway24 but also concluded that PA generated at the PM and not on other organelle membranes is the main driver of this phenotype, driving home the importance of spatial regulation of PA-dependent signaling.
DIRECTED EVOLUTION OF SUPERPLDS AS HIGHLY EFFICIENT MEMBRANE EDITORS
Despite the power, precision, and mild nature of optogenetic recruitment, the first-generation optoPLD had modest activity due to its use of an unoptimized secreted bacterial enzyme in the mammalian cytosolic compartment. To address this shortcoming, we developed an activity-based directed enzyme evolution strategy to increase the activity of optoPLD in mammalian cells (Figure 3A).33 Key to this strategy was the use of a method for fluorescent labeling of PLD activity within intact cells that we developed, termed IMPACT (for Imaging PLD Activity with Clickable Alcohols via Transphosphatidylation; Figure 3B), which we will describe further in the following section. The directed evolution strategy began with generating a plasmid library of mutant PLDs using error-prone PCR and expression of this library in HEK 293 cells following lentiviral delivery. IMPACT labeling was performed in the transfected cells, yielding cellular fluorescence proportional to the PLD activity, followed by fluorescence-activated cell sorting (FACS) to enrich high-fluorescence cells, expansion, iteration over several cycles of evolution, and ultimately isolation of PLD clones bearing high activity for characterization (Figure 3A). These evolution campaigns yielded numerous PLD mutants exhibiting activities up to 100-fold higher than that of wild-type PLD. We named these evolved PLDs as superPLDs, where a “×n” superscript denotes an n-fold increase in PLD activity.
Figure 3.
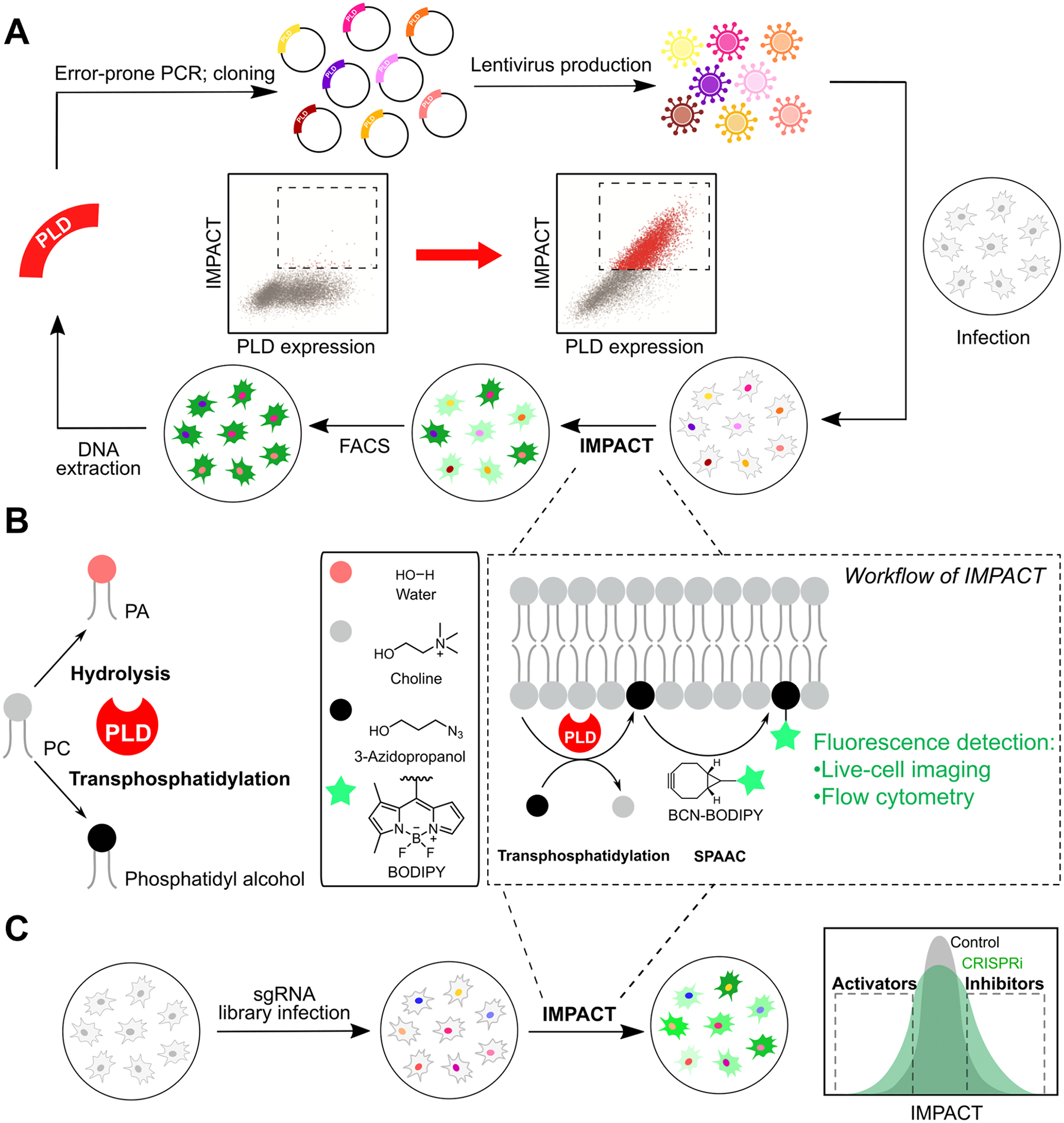
Imaging PLD Activity with Clickable Alcohols via Transphosphatidylation (IMPACT). (A) Activity-based directed evolution of superPLDs with higher catalytic activities in mammalian cells using error-prone PCR and an IMPACT-based enrichment strategy. (B) The concept underlying IMPACT. PLDs can catalyze two reactions of PC: hydrolysis to form PA and transphosphatidylation with primary alcohols to form phosphatidyl alcohols. By using transphosphatidylation with bioorthogonal alcohols such as 3-azido-1-propanol followed by tagging with a bicyclononyne (BCN)-BODIPY fluorophore via the strain-promoted azide–alkyne cycloaddition (SPAAC) bioorthogonal reaction, IMPACT enables generation of fluorescent phosphatidyl alcohol lipids as reporters of cellular PLD activity. (C) Platform for discovery of new activators and inhibitors of mammalian PLD signaling by coupling pooled, genome-wide CRISPR interference (CRISPRi) screening with IMPACT labeling, fluorescence-activated cell sorting (FACS) enrichment, and short guide RNA (sgRNA) sequencing.
Encouragingly, various superPLD clones, when incorporated into the optoPLD system, exhibited an enhanced ability to produce PA on desired organelle membranes relative to PLDWT. We demonstrated that superPLD-generated PA is bioactive, as it can regulate three different PA-dependent signaling pathways: AMPK signaling (assessed by membrane recruitment of the LKB1 kinase and phosphorylation of AMPK), mTOR signaling (assessed by phosphorylation of S6 kinase), and Hippo signaling (assessed by nuclear translocation of YAP). This enhanced activity is necessary to overcome continuous PA metabolism and highlights the use of superPLDs as optimized membrane editors for local PA production. To understand the source of the vastly improved activity, we performed X-ray crystallography on two of the most highly active superPLDs and found that they had an expanded catalytic pocket due to rearrangements of loops containing bulky residues that gated the active site. The superPLDs were also more tolerant to mutation of their disulfide bonds, highlighting the importance of using mammalian cells as the host cells for the directed evolution. In fact, to the best of our knowledge, the evolution of superPLDs represents the first example of using a direct fluorescent reporter as a readout of enzymatic activity for FACS-based directed enzyme evolution in mammalian cells. Furthermore, using the biochemically purified superPLDs, we found that they were excellent in vitro biocatalysts for the chemoenzymatic synthesis of a variety of natural and unnatural phospholipids, underlining a second key application of the superPLDs—beyond membrane editing for cell biology—in the production of designer lipids for various applications not only for basic research but also in the food, cosmetic, and pharmaceutical industries.
BIOORTHOGONAL CHEMISTRY ENABLES IMPACT-FUL LIPID IMAGING TOOLS
Up to this point, we have been discussing tools for manipulating PA signaling, but equally important are tools to monitor endogenous PA signaling pathways, ideally in a minimally perturbative manner. Because of the importance of PLD-dependent PA signaling to many fundamental signaling pathways and disease processes, we have focused our efforts on tools for visualizing these pathways. Chief among these are the tools that we have termed IMPACT (Figure 3B). The basis for IMPACT is that PLDs, which naturally catalyze hydrolysis of PC to generate PA, can also catalyze efficient transphosphatidylation of PC with exogenous primary alcohols such as ethanol or n-butanol to form phosphatidyl alcohols.34
We envisioned that using clickable, or bioorthogonally tagged, primary alcohols would enable subsequent click chemistry tagging of the resultant phosphatidyl alcohols with fluorophores or other useful probes for in situ visualization to enable single-cell- and subcellular-level measurements of PLD activity rather than bulk biochemical measurements by TLC or LC–MS as with classic transphosphatidylation assays, where all of the spatial information is lost during sample preparation.35 Indeed, we found that a variety of alkynyl and azido primary alcohols are PLD transphosphatidylation substrates, enabling visualization of their localization in fixed or live cells following Cu-catalyzed azide–alkyne cycloaddition (CuAAC) or strain-promoted azide–alkyne cycloaddition (SPAAC) labeling to introduce a fluorescent tag onto the lipid headgroup (Figure 3B).1,36,37 Visualization of the fluorescent lipids can be accomplished by fluorescence microscopy imaging to examine subcellular localizations, and the intensity can be quantified by either fluorescence microscopy or flow cytometry. Further, IMPACT may be used for bulk biochemical measurements similar to butanol transphosphatidylation, with readout by either LC–MS or fluorescence-coupled HPLC. Initially, we used strong and pleiotropic stimulation with phorbol esters to activate endogenous PLDs, but in subsequent studies we determined that IMPACT has sufficient sensitivity to detect PLD signaling downstream of native receptor signaling pathways.1,2,38
APPLICATIONS OF IMPACT FOR BIOLOGICAL DISCOVERY
A key unique feature of IMPACT relative to traditional PLD assays is its ability to enable single-cell measurements of PLD activity. One application that capitalizes upon this property was our use of IMPACT labeling and FACS enrichment of IMPACThigh cells for the directed evolution of superactive forms of Streptomyces PLDs33 (Figure 3A). In another line of study, we combined IMPACT with pooled, genome-wide CRISPR screens to identify genes that regulate endogenous PLD signaling (Figure 3C). The motivation for this work was that despite decades of studies on PLD and PA signaling, our understanding of how cells regulate the activation of PLD enzymes in response to different physiological stimuli is still incomplete. We reasoned that by performing knockdown of every gene in a large, pooled population of cells, followed by IMPACT labeling and FACS sorting of IMPACThigh and IMPACTlow subsets of cells, we would be able to identify genes whose knockdown either enhanced or suppressed PLD activity. In practice, we chose to use CRISPR interference (CRISPRi) to perform knockdown due to the accessibility of genome-wide libraries and suitability for such screening, even for essential genes. Following FACS enrichment of desired cell populations, short guide RNAs (sgRNAs) enriched in each population were identified by next-generation sequencing, and this information led to the identification of genes whose knockdown modulated PLD activity (Figure 3C).4
Our first IMPACT–CRISPRi screen explored regulation of protein kinase C (PKC)-stimulated PLD signaling using phorbol ester stimulation, a pathway that naturally occurs downstream of several types of cell-surface receptors such as G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs). Among the ~160 statistically significant hits, a secondary validation on a subset established a false positive rate of ~20%, suggesting that ~80% of the predicted hits would be bona fide PLD regulators. We performed mechanistic follow-up on glycogen synthase kinase 3A (GSK3A), a therapeutically relevant kinase involved in metabolism, cell proliferation, and cell death, which was predicted to be a novel PLD activator from the screen.39 We found that prolonged, but not acute, pharmacological inhibition of GSK3A (and its paralogue GSK3B) reduced not only PLD activity but also the protein and mRNA levels of both PLD isoforms (PLD1 and PLD2) and a major PKC isoform. These results support a model wherein GSK3 activation synergistically primes cells for PKC–PLD signaling by stimulating de novo PKC and PLD expression. The model also allows for negative feedback because PKCs can carry out inhibitory phosphorylation of GSK3 to downregulate its activity.39,40 We are currently exploring both the mechanistic basis for how other gene hits from this screen might regulate PLD signaling and how screens with additional stimuli might reveal general or context-specific PLD regulation. Beyond PLD signaling, our combination of IMPACT with CRISPR screening joins a small but growing set of studies that highlight the power of combining bioorthogonal labeling and genome-wide CRISPR screening for discovering new regulators of specific enzyme-driven metabolic and signaling pathways.
Another application of IMPACT is for the discovery of physiologically relevant lipid–protein interactions. By using bifunctional primary alcohols bearing both an alkyne and a photo-cross-linkable diazirine group for so-called cross-linking IMPACT (XL-IMPACT) followed by azido-biotin tagging by CuAAC, streptavidin-based enrichment, and LC–MS/MS-based proteomics, we identified many phosphatidyl alcohol-interacting proteins (Figure 4).41 Critically, the bifunctional phosphatidyl alcohols generated by XL-IMPACT closely resemble phosphatidylethanol (PEth), a lipid produced by endogenous PLDs following alcohol consumption. PEth is a widely used biomarker for alcohol intake, but mechanisms underlying potential pathophysiological effects of this lipid, suggested by studies in model organisms, remain unknown. Fortunately, we found that global proteome cross-linking by XL-IMPACT-derived lipids was competed by PEth, suggesting that the former could mimic the interactions of the latter. We validated one such interaction in detail with basigin/CD147, a single-pass transmembrane protein, using mutagenesis and site mapping to identify a PEth-binding site on this protein. Studies are currently underway to understand the pathophysiological significance of this and other PEth interactions revealed by our XL-IMPACT-based chemoproteomics studies.
Figure 4.
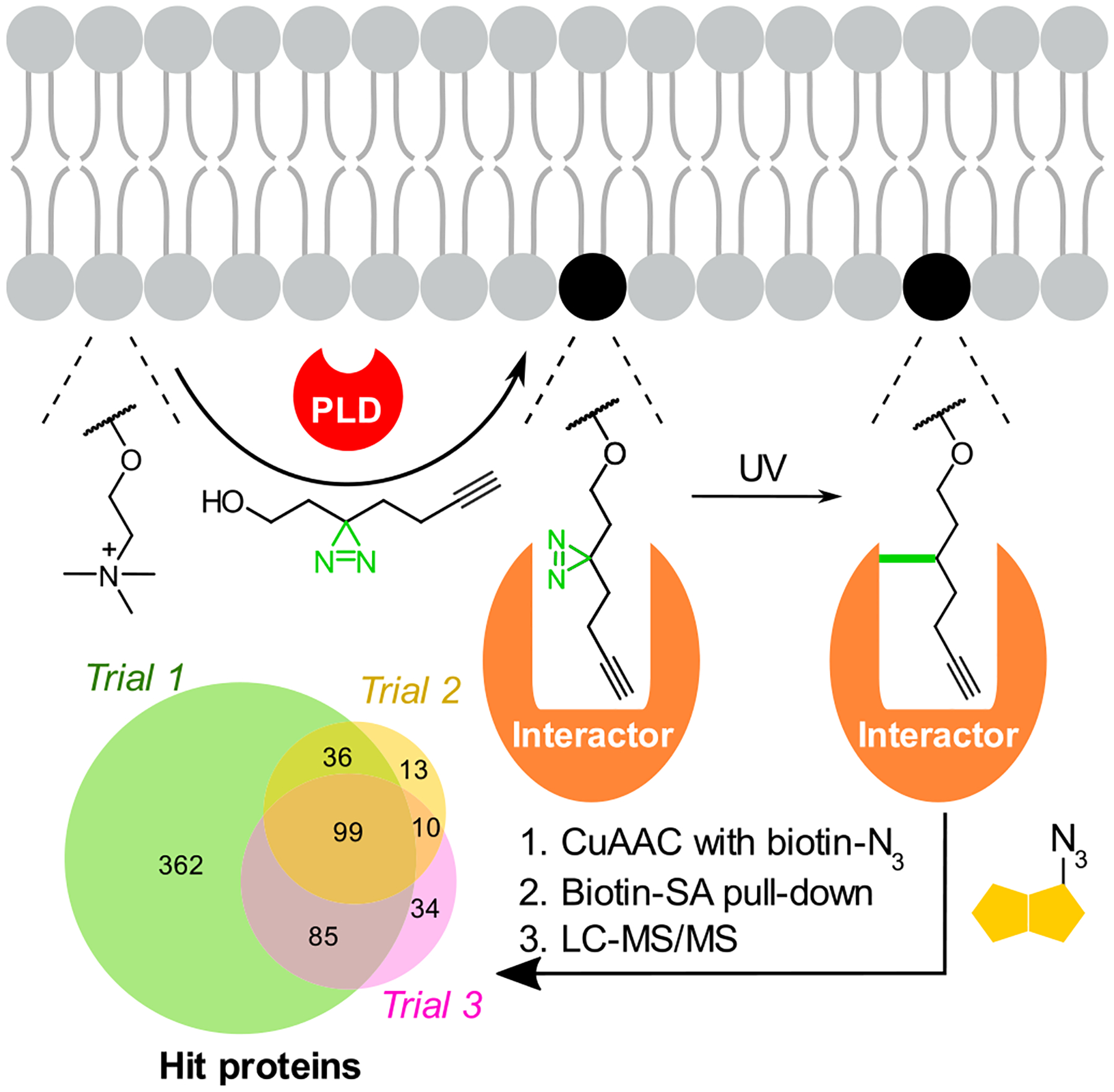
XL-IMPACT, a photoaffinity labeling variant for discovery of phospholipid–protein interactions. PLD-mediated transphosphatidylation of PC with a minimalist diazirine alkyne alcohol yields a photo-cross-linkable mimic of phosphatidylethanol (PEth), a phospholipid formed following alcohol consumption. Following CuAAC tagging with azido-biotin, streptavidin (SA)-based enrichment, and LC–MS/MS-based chemoproteomics, XL-IMPACT enabled the identification of the protein interactome of PEth, a biomarker of alcohol consumption.
REAL-TIME IMPACT USING THE TETRAZINE LIGATION FOR IMAGING PLD AND GPCR SIGNALING
The above-described IMPACT methods are most useful as single-cell measurements of PLD activity. However, because the SPAAC tagging and rinse-out steps require 20–30 minutes, IMPACT-derived fluorescent lipids can redistribute within the cell, making them suboptimal reporters of PLD activity at the subcellular, organelle level. Notably, fluorescent lipids were not observed at the PM following phorbol ester stimulation, which activates PLDs at this membrane.1,42–44 To overcome this limitation and improve the temporal resolution of IMPACT, we took advantage of the rapid kinetics of inverse-electron-demand Diels–Alder (IEDDA) cycloadditions between tetrazines and strained alkene dienophiles. After screening several dienophiles, we identified an oxo-trans-cyclooctene primary alcohol ((S)-oxoTCO)45 as a suitable PLD transphosphatidylation substrate. The resultant oxoTCO-containing lipid can be tagged with a fluorogenic tetrazine-BODIPY reagent in a rapid click reaction that can be monitored in real time (Figure 5A). Using this real-time IMPACT (RT-IMPACT) approach, we observed PM fluorescence mere seconds after the initiation of the IEDDA reaction as well as rapid trafficking of the fluorescent lipids to the ER and other organelles on the minute time scale. By performing colocalization with an ER-tracker dye, we quantified the rates of interorganelle lipid transfer from the PM to the ER, which occurs by nonvesicular pathways. We are currently using RT-IMPACT as a tracer of not only the subcellular sites of PLD signaling but also of intracellular lipid transport pathways mediated by lipid transfer proteins at membrane contact sites.46
Figure 5.
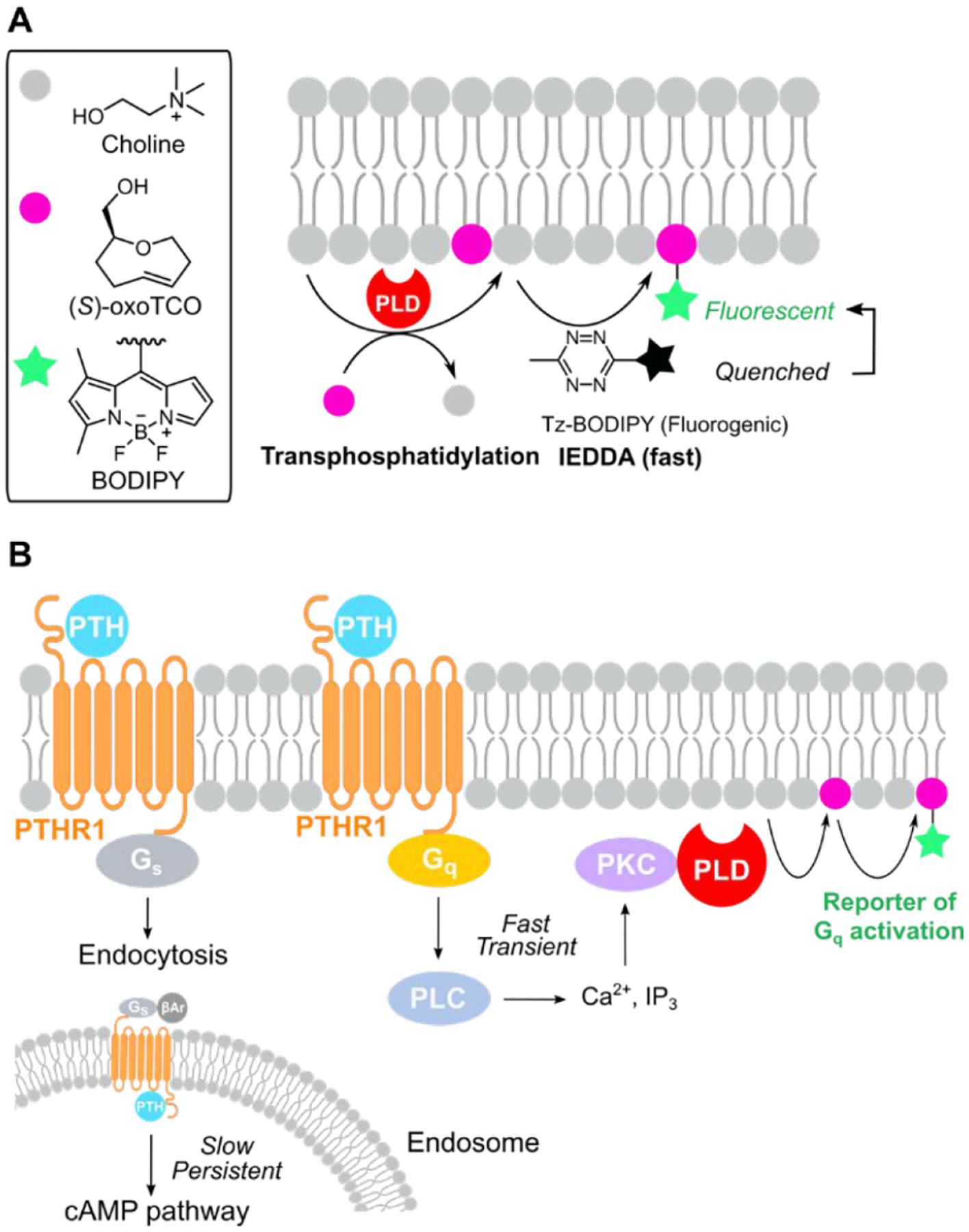
Real-time IMPACT (RT-IMPACT) using the tetrazine ligation for rapid visualization of PLD and GPCR–Gq signaling. (A) RT-IMPACT involves a rapid inverse-electron-demand Diels–Alder (IEDDA) reaction between a strained alkene, (S)-oxoTCO, and a fluorogenic tetrazine-BODIPY (Tz-BODIPY). (B) RT-IMPACT is a useful tool for reporting on the spatiotemporal dynamics of GPCR–Gq signaling, and we applied it to elucidate the relationship between Gs and Gq signaling triggered by the parathyroid hormone (PTH) receptor PTHR1. βAr, β-arrestin.
We recently used the high spatiotemporal precision of RT-IMPACT to investigate the dynamics of GPCR–Gq signaling, as PLD is activated selectively downstream of Gq (via the PLC–PKC–PLD pathway) and not other major Gα forms such as Gs and Gi. Using the disease-relevant parathyroid hormone receptor PTHR1, which can signal via two Gα proteins, Gs and Gq, we established that PTHR1–Gq signaling occurs transiently and exclusively at the PM, in contrast to the Gs pathway, which unconventionally occurs primarily on endosomes and for prolonged periods.38 This work not only determined important parameters surrounding physiologically relevant PTHR–Gq signaling but also established RT-IMPACT as an important complement to Ca2+ imaging as a useful method for imaging and quantifying the dynamics of GPCR–Gq signaling more generally.
BEYOND PA: PHOSPHATIDYLCHOLINE AS A TARGET FOR SUPER-RESOLUTION AND ORGANELLE-SELECTIVE IMAGING OF ABUNDANT PHOSPHOLIPIDS
PC is an appealing target for imaging because it is the most abundant component of cellular membranes. Alkynyl47,48 and azido49,50 choline analogues can be taken up via ubiquitously expressed choline transporters and metabolically incorporated into PC analogues through the Kennedy pathway for de novo PC biosynthesis. To better visualize narrow membranous structures and membrane contact sites, we developed a lipid-compatible version of expansion microscopy to enable super-resolution imaging of PC-containing membranes. Key to this approach, termed lipid expansion microscopy (LExM), are trifunctional detection probes equipped with an azide for tagging alkynyl PCs, a fluorophore for visualization, and a methacrylamide group for covalent incorporation into hydrogels that are ultimately expanded after sample clearing (Figure 6A).51 A defining feature of LExM is the preservation of signal due to cross-linking of tagged lipids to the hydrogel prior to detergent-assisted permeabilization, which is a required step in expansion microscopy and otherwise rinses away the lipids. Using either metabolic labeling of PC with an alkynyl choline analogue47 or IMPACT with an alkynyl alcohol, we demonstrated that LExM can enable visualization of very narrow membrane-containing structures, such as nuclear-membrane-derived channels whose dimensions are below the diffraction limit, using widely available confocal microscopes. LExM adds to the growing compendium of expansion-microscopy-based techniques for visualizing different types of biomolecules.52–55
Figure 6.
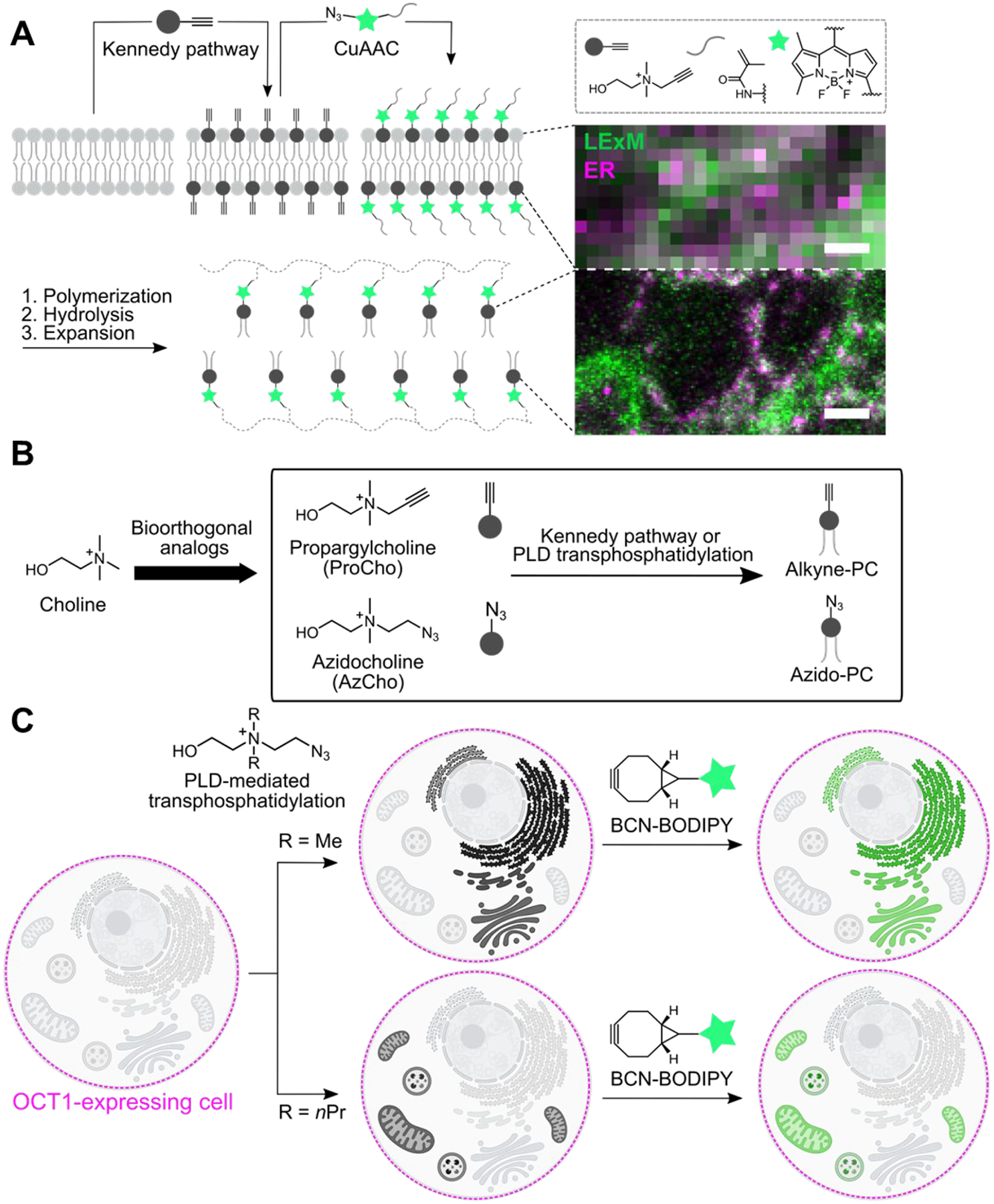
Imaging phosphatidylcholine analogues at super-resolution with organelle-level precision. (A) Lipid expansion microscopy (LExM) uses clickable, polymerizable fluorophores that can tag alkyne-PC analogues metabolically labeled via the Kennedy pathway. Following hydrogel generation and expansion, super-resolution imaging of lipids can be achieved using standard confocal microscopes. Shown are (top) conventional confocal microscopy and (bottom) LExM images of adjacent regions of a HeLa cell, where lipids metabolically labeled with propargylcholine (ProCho) and then tagged with an azido-BODIPY-methacrylamide reagent via CuAAC are shown in green and an ER marker is shown in magenta. Scale bar: 400 nm. Adapted from ref 51. Copyright 2022 American Chemical Society. (B) Clickable choline analogues can metabolically label PC via either the Kennedy pathway or PLD-mediated transphosphatidylation. (C) Organelle-selective labeling with PC analogues generated via PLD transphosphatidylation. IMPACT labeling using N,N-dimethylazidocholine and BCN-BODIPY tagging results in ER- and Golgi-selective labeling, whereas the extension to N,N-dipropylazidocholine leads to selective mitochondrial and lysosomal labeling despite using the same BCN-BODIPY tagging reagent.
The alkyne- or azide-bearing PC analogues labeled via the Kennedy pathway are distributed in essentially all membranes without appreciable selectivity,47,49 but organelle-targeting clickable dyes can be used to visualize subsets of azido PC analogues in specific organelle membranes, including the PM, ER, and mitochondria.56,57 Beyond the Kennedy pathway, we showed that an alkynyl choline derivative can also be converted to the corresponding PC analogue through a single PLD transphosphatidylation step (Figure 6B).37 Because PLD activity can be controlled by external stimuli to activate endogenous PLDs or via exogenous optoPLDs, we envisioned that diverse bioorthogonal choline analogues might permit differential organelle labeling if installed selectively by PLD enzymes.
Accordingly, we synthesized a panel of azidocholine derivatives by replacing the two N-methyl groups at the ammonium center with ethyl, n-propyl, or 2-hydroxyethyl.58 Though all were PLD substrates in vitro, these new analogues were not taken up into wild-type cells. To circumvent the permeability challenge, we expressed organic cation transporter 1 (OCT1/SLC22A1), a promiscuous transporter of cationic metabolites, to facilitate the entry of our bioorthogonal choline analogues into cells.59–61 Gratifyingly, OCT1 expression enabled robust phospholipid labeling via PLD activity and SPAAC tagging with BCN-BODIPY. Even more excitingly, the fluorescent lipids made from different choline probes displayed distinct and remarkably stable labeling patterns. The N,N-dimethyl lipid localized to the ER and Golgi complex, whereas the N,N-dipropyl lipid decorated mitochondria and lysosomes, with the N,N-diethyl lipid exhibiting an intermediate labeling pattern, despite the use of the same SPAAC detection reagent, BCN-BODIPY, in all cases (Figure 6C). This trend reveals substantial differences in affinities of PC analogues for different membranes and potentially for machinery that transports these lipids from their site of synthesis, most likely at the PM, to their final destinations.
Through the development of this organelle-selective labeling, we revealed that expression of OCT1 or other promiscuous metabolite transporters might offer a generalizable solution to a recurring problem of cell permeability in chemical probe development. This work also describes a potentially useful tool for membrane engineering, namely that the different lipid analogues could be used to anchor functional molecules on selected organelle membranes to locally modulate cellular events.62,63
CONCLUSIONS AND OUTLOOK
Lipid metabolism is complex and intertwined, and lipid structures and localizations are not directly genetically encoded. Thus, we believe that chemical tools will continue to play a major role in elucidating biological functions of lipids and mechanisms that underlie lipid homeostasis and signaling. One major avenue is bioorthogonal metabolic labeling, which can be achieved for several classes of lipids, with some still outstanding.47 By combining bioorthogonal labeling with substrates that are more selective for certain elements of lipid metabolism, i.e., enzymes and transporters, we may achieve greater selectivity of labeling in numerous contexts beyond what we have already shown for abundant PC lipids. Furthermore, lipid probes with additional light-dependent functionalities, including photo-cross-linking and photoswitchable lipids,25,64,65 will continue to be fertile strategies for elucidating lipid–protein interactions and affording spatiotemporal control over lipid signaling and metabolism.
Many of our advances have focused on PLD enzymes. IMPACT was originally developed as a bioorthogonal metabolic strategy to visualize endogenous PLD signaling, but it has evolved in unexpected ways to encompass a bevy of tools and to enable many applications. Rapid and fluorogenic RT-IMPACT enables spatiotemporal dissection of PLD-related pathways, including GPCR–Gq signaling, and interorganelle lipid transport pathways. A chemoproteomics variant, XL-IMPACT, has revealed lipid–protein interactions relevant to diseases of excessive alcohol consumption that derive from potentially pathological interactions of ethanol with PLD enzymes. Moreover, with an assist from a promiscuous cation transporter, diverse choline analogues can label discrete organelle compartments via a combination of PLD activity and selective intracellular transport. Bespoke trifunctional bioorthogonal detection reagents enable super-resolution imaging of lipids using expansion microscopy and may be combined with organelle-selective tagging approaches in the future. Finally, single-cell labeling using our original IMPACT probes allows for unbiased biological discovery via CRISPR screening and directed enzyme evolution of PLD-based membrane editors. Though bioorthogonal/fluorescent lipids offer tremendous opportunities, one drawback is the rather large tag size, which can lead to behavior different from that of native lipids. To gain information on native lipid trafficking and dynamics, alternative approaches may be desirable, including minimalist tags or label-free methodologies.66,67
A bacterial PLD has emerged as a malleable and versatile chemoenzymatic catalyst for precise editing of phospholipid content in cellular membranes. By using optogenetics to control the enzyme activity, we can acutely produce PA on defined compartments. Directed evolution campaigns have increased PLD activity toward generation of not only PA but also other phospholipids, with superPLDs poised to act as general editors of the phospholipidome. Orthogonal inducible dimerization strategies with different editors could afford spatiotemporally controlled multiplex membrane editing. Collectively, we envision many applications of these tools, including for the biological discovery of regulators of lipid signaling, new lipid–protein interactions, and ultimately new biological functions for localized pools of bioactive lipids.
ACKNOWLEDGMENTS
Work in the Baskin lab is supported by the NIH (R01GM131101 and R01GM143367), the NSF (CAREER CHE-1749919), a Beckman Young Investigator Award, and a Sloan Research Fellowship.
Biographies
Din-Chi Chiu is a Ph.D. candidate in Prof. Jeremy Baskin’s group in the Department of Chemistry and Chemical Biology and the Weill Institute for Cell and Molecular Biology at Cornell University. He received a B.S. in Materials Science and Engineering from National Chiao Tung University and an M.S. in Chemistry from National Taiwan University. His research is focused on development of bioorthogonal probes for imaging intracellular lipid distribution and elucidation of physiologically relevant lipid–protein interactions.
Jeremy M. Baskin is Associate Professor and Nancy and Peter Meinig Family Investigator in the Life Sciences at Cornell University, with appointments in the Department of Chemistry and Chemical Biology and the Weill Institute for Cell and Molecular Biology. Born and raised in Montreal, Canada, he holds S.B and Ph.D. degrees in Chemistry from the Massachusetts Institute of Technology and the University of California, Berkeley, respectively, the latter with Carolyn Bertozzi, where he developed new bioorthogonal chemistries. He received postdoctoral training in lipid cell biology with Pietro De Camilli at Yale University. His research interests encompass the chemical biology and cell biology of lipid signaling, including development of tools for imaging lipid signaling events and editing membrane composition as well as elucidation of (patho)physiological mechanisms underlying lipid metabolism, transport, and signaling.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.accounts.2c00510
The authors declare no competing financial interest.
Contributor Information
Din-Chi Chiu, Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, United States;; Weill Institute for Cell and Molecular Biology, Cornell University, Ithaca, New York 14853, United States
Jeremy M. Baskin, Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, United States; Weill Institute for Cell and Molecular Biology, Cornell University, Ithaca, New York 14853, United States;
REFERENCES
- (1).Bumpus TW; Baskin JM Clickable Substrate Mimics Enable Imaging of Phospholipase D Activity. ACS Cent. Sci 2017, 3 (10), 1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that azido primary alcohols could replace water as a substrate of phospholipase D (PLD) enzymes, enabling the generation of fluorescent lipid reporters of PLD signaling in live cells in a method termed IMPACT (Imaging PLD Activity with Clickable Alcohols via Transphosphatidylation).
- (2).Liang D; Wu K; Tei R; Bumpus TW; Ye J; Baskin JM A Real-Time, Click Chemistry Imaging Approach Reveals Stimulus-Specific Subcellular Locations of Phospholipase D Activity. Proc. Natl. Acad. Sci. U.S.A 2019, 116 (31), 15453–15462. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study developed a real-time variant of IMPACT harnessing a rapid and fluorogenic tetrazine ligation for imaging the subcellular localization of endogenous PLD signaling, revealing that such signaling via the protein kinase C–PLD pathway occurs at the plasma membrane and also visualizing rapid interorganelle bulk phospholipid transport from the plasma membrane to the endoplasmic reticulum on the second-to-minute time scale.
- (3).Tei R; Baskin JM Spatiotemporal Control of Phosphatidic Acid Signaling with Optogenetic, Engineered Phospholipase Ds. J. Cell Biol 2020, 219 (3), No. e201907013. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study developed a light-controlled, optogenetic PLD that upon blue-light illumination could be recruited to target organelles to enable local synthesis of phosphatidic acid (PA) lipids and applied this tool to discover that pools of PA at the plasma membrane can selectively suppress oncogenic Hippo signaling.
- (4).Bumpus TW; Huang S; Tei R; Baskin JM Click Chemistry-Enabled CRISPR Screening Reveals GSK3 as a Regulator of PLD Signaling. Proc. Natl. Acad. Sci. U.S.A 2021, 118 (48), No. e2025265118. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study combined IMPACT with pooled, genome-wide CRISPR interference screening to elucidate GSK3 as an activator of protein kinase C-stimulated PLD signaling, expanding the scope of unbiased CRISPR screening to the discovery of new regulators of enzyme-driven signaling pathways using bioorthogonal fluorescent labeling as a readout.
- (5).Holthuis JCM; Menon AK Lipid Landscapes and Pipelines in Membrane Homeostasis. Nature 2014, 510 (7503), 48–57. [DOI] [PubMed] [Google Scholar]
- (6).Bernardino de la Serna J; Schütz GJ; Eggeling C; Cebecauer M There Is No Simple Model of the Plasma Membrane Organization. Front. Cell Dev. Biol 2016, 4, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ingólfsson HI; Melo MN; van Eerden FJ; Arnarez C; Lopez CA; Wassenaar TA; Periole X; de Vries AH; Tieleman DP; Marrink SJ Lipid Organization of the Plasma Membrane. J. Am. Chem. Soc 2014, 136 (41), 14554–14559. [DOI] [PubMed] [Google Scholar]
- (8).Harayama T; Riezman H Understanding the Diversity of Membrane Lipid Composition. Nat. Rev. Mol. Cell Biol 2018, 19 (5), 281–296. [DOI] [PubMed] [Google Scholar]
- (9).Vance JE Phospholipid Synthesis and Transport in Mammalian Cells. Traffic 2015, 16 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- (10).Hishikawa D; Hashidate T; Shimizu T; Shindou H Diversity and Function of Membrane Glycerophospholipids Generated by the Remodeling Pathway in Mammalian Cells. J. Lipid Res 2014, 55 (5), 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Posor Y; Jang W; Haucke V Phosphoinositides as Membrane Organizers. Nat. Rev. Mol. Cell Biol 2022, DOI: 10.1038/s41580-022-00490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sapar ML; Ji H; Wang B; Poe AR; Dubey K; Ren X; Ni J-Q; Han C Phosphatidylserine Externalization Results from and Causes Neurite Degeneration in Drosophila. Cell Rep. 2018, 24 (9), 2273–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wang X; Devaiah S; Zhang W; Welti R Signaling Functions of Phosphatidic Acid. Prog. Lipid Res 2006, 45 (3), 250–278. [DOI] [PubMed] [Google Scholar]
- (14).Tanguy E; Wang Q; Moine H; Vitale N Phosphatidic Acid: From Pleiotropic Functions to Neuronal Pathology. Front. Cell. Neurosci 2019, 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Frank JA; Moroni M; Moshourab R; Sumser M; Lewin GR; Trauner D Photoswitchable Fatty Acids Enable Optical Control of TRPV1. Nat. Commun 2015, 6 (1), 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hüll K; Morstein J; Trauner D In Vivo Photopharmacology. Chem. Rev 2018, 118 (21), 10710–10747. [DOI] [PubMed] [Google Scholar]
- (17).Lichtenegger M; Tiapko O; Svobodova B; Stockner T; Glasnov TN; Schreibmayer W; Platzer D; de la Cruz GG; Krenn S; Schober R; Shrestha N; Schindl R; Romanin C; Groschner K An Optically Controlled Probe Identifies Lipid-Gating Fenestrations within the TRPC3 Channel. Nat. Chem. Biol 2018, 14 (4), 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Frank JA; Yushchenko DA; Hodson DJ; Lipstein N; Nagpal J; Rutter GA; Rhee J-S; Gottschalk A; Brose N; Schultz C; Trauner D Photoswitchable Diacylglycerols Enable Optical Control of Protein Kinase C. Nat. Chem. Biol 2016, 12 (9), 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Morstein J; Dacheux MA; Norman DD; Shemet A; Donthamsetti PC; Citir M; Frank JA; Schultz C; Isacoff EY; Parrill AL; Tigyi GJ; Trauner D Optical Control of Lysophosphatidic Acid Signaling. J. Am. Chem. Soc 2020, 142 (24), 10612–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Pernpeintner C; Frank JA; Urban P; Roeske CR; Pritzl SD; Trauner D; Lohmüller T Light-Controlled Membrane Mechanics and Shape Transitions of Photoswitchable Lipid Vesicles. Langmuir 2017, 33 (16), 4083–4089. [DOI] [PubMed] [Google Scholar]
- (21).Tei R; Morstein J; Shemet A; Trauner D; Baskin JM Optical Control of Phosphatidic Acid Signaling. ACS Cent. Sci 2021, 7 (7), 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yoon M-S; Rosenberger CL; Wu C; Truong N; Sweedler JV; Chen J Rapid Mitogenic Regulation of the MTORC1 Inhibitor, DEPTOR, by Phosphatidic Acid. Mol. Cell 2015, 58 (3), 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Magnuson B; Ekim B; Fingar DC Regulation and Function of Ribosomal Protein S6 Kinase (S6K) within MTOR Signalling Networks. Biochem. J 2012, 441 (1), 1–21. [DOI] [PubMed] [Google Scholar]
- (24).Han H; Qi R; Zhou JJ; Ta AP; Yang B; Nakaoka HJ; Seo G; Guan K-L; Luo R; Wang W Regulation of the Hippo Pathway by Phosphatidic Acid-Mediated Lipid-Protein Interaction. Mol. Cell 2018, 72 (2), 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Morstein J; Impastato AC; Trauner D Photoswitchable Lipids. ChemBioChem 2021, 22 (1), 73–83. [DOI] [PubMed] [Google Scholar]
- (26).Hussey SL; Peterson BR Efficient Delivery of Streptavidin to Mammalian Cells: Clathrin-Mediated Endocytosis Regulated by a Synthetic Ligand. J. Am. Chem. Soc 2002, 124 (22), 6265–6273. [DOI] [PubMed] [Google Scholar]
- (27).Tei R; Baskin JM Induced Proximity Tools for Precise Manipulation of Lipid Signaling. Curr. Opin. Chem. Biol 2021, 65, 93–100. [DOI] [PubMed] [Google Scholar]
- (28).Suh B-C; Inoue T; Meyer T; Hille B Rapid Chemically Induced Changes of PtdIns(4,5)P2 Gate KCNQ Ion Channels. Science 2006, 314 (5804), 1454–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Varnai P; Thyagarajan B; Rohacs T; Balla T Rapidly Inducible Changes in Phosphatidylinositol 4,5-Bisphosphate Levels Influence Multiple Regulatory Functions of the Lipid in Intact Living Cells. J. Cell Biol 2006, 175 (3), 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zoncu R; Perera RM; Sebastian R; Nakatsu F; Chen H; Balla T; Ayala G; Toomre D; De Camilli PV Loss of Endocytic Clathrin-Coated Pits upon Acute Depletion of Phosphatidylinositol 4,5-Bisphosphate. Proc. Natl. Acad. Sci. U.S.A 2007, 104 (10), 3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Idevall-Hagren O; Dickson EJ; Hille B; Toomre DK; De Camilli P Optogenetic Control of Phosphoinositide Metabolism. Proc. Natl. Acad. Sci. U.S.A 2012, 109 (35), E2316–E2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Chang Y-C; Su Y-A; Chiu H-Y; Chen C-W; Huang XR; Tei R; Wang H-C; Chuang M-C; Lin Y-C; Hsu J-C; Baskin JM; Chang Z-F; Liu Y-W NME3 Binds to Phosphatidic Acid and Tethers Mitochondria for Fusion. SSRN Electron. J 2021, DOI: 10.2139/ssrn.3808299. [DOI] [Google Scholar]
- (33).Tei R; Bagde SR; Fromme JC; Baskin JM Activity-Based Directed Evolution of a Membrane Editor in Mammalian Cells. bioRxiv 2022, DOI: 10.1101/2022.09.26.509516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Selvy PE; Lavieri RR; Lindsley CW; Brown HA Phospholipase D: Enzymology, Functionality, and Chemical Modulation. Chem. Rev 2011, 111 (10), 6064–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Brown HA; Henage LG; Preininger AM; Xiang Y; Exton JH Biochemical Analysis of Phospholipase D. Methods Enzymol. 2007, 434, 49–87. [DOI] [PubMed] [Google Scholar]
- (36).Bumpus TW; Baskin JM A Chemoenzymatic Strategy for Imaging Cellular Phosphatidic Acid Synthesis. Angew. Chem 2016, 128 (42), 13349–13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Bumpus TW; Liang FJ; Baskin JM Ex Uno Plura: Differential Labeling of Phospholipid Biosynthetic Pathways with a Single Bioorthogonal Alcohol. Biochemistry 2018, 57 (2), 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Liang D; Cheloha RW; Watanabe T; Gardella TJ; Baskin JM Activity-Based, Bioorthogonal Imaging of Phospholipase D Reveals Spatiotemporal Dynamics of GPCR-Gq Signaling. Cell Chem. Biol 2022, 29 (1), 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Beurel E; Grieco SF; Jope RS Glycogen Synthase Kinase-3 (GSK3): Regulation, Actions, and Diseases. Pharmacol. Ther 2015, 148, 114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Patel P; Woodgett JR Glycogen Synthase Kinase 3. Curr. Top. Dev. Biol 2017, 123, 277–302. [DOI] [PubMed] [Google Scholar]
- (41).Yu W; Lin Z; Woo CM; Baskin JM A Chemoproteomics Approach to Profile Phospholipase D-Derived Phosphatidyl Alcohol Interactions. ACS Chem. Biol 2021, DOI: 10.1021/acschem-bio.1c00584. [DOI] [PubMed] [Google Scholar]
- (42).Conricode KM; Brewer KA; Exton JH Activation of Phospholipase D by Protein Kinase C. Evidence for a Phosphorylation-Independent Mechanism. J. Biol. Chem 1992, 267 (11), 7199–7202. [PubMed] [Google Scholar]
- (43).Du G; Altshuller YM; Vitale N; Huang P; Chasserot-Golaz S; Morris AJ; Bader M-F; Frohman MA Regulation of Phospholipase D1 Subcellular Cycling through Coordination of Multiple Membrane Association Motifs. J. Cell Biol 2003, 162 (2), 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kim Y; Han JM; Han BR; Lee K-A; Kim JH; Lee BD; Jang I-H; Suh P-G; Ryu SH Phospholipase D1 Is Phosphorylated and Activated by Protein Kinase C in Caveolin-Enriched Microdomains within the Plasma Membrane. J. Biol. Chem 2000, 275 (18), 13621–13627. [DOI] [PubMed] [Google Scholar]
- (45).Lambert WD; Scinto SL; Dmitrenko O; Boyd SJ; Magboo R; Mehl RA; Chin JW; Fox JM; Wallace S Computationally Guided Discovery of a Reactive, Hydrophilic Trans-5-Oxocene Dienophile for Bioorthogonal Labeling. Org. Biomol. Chem 2017, 15 (31), 6640–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Prinz WA; Toulmay A; Balla T The Functional Universe of Membrane Contact Sites. Nat. Rev. Mol. Cell Biol 2020, 21 (1), 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Jao CY; Roth M; Welti R; Salic A Metabolic Labeling and Direct Imaging of Choline Phospholipids in Vivo. Proc. Natl. Acad. Sci. U.S.A 2009, 106 (36), 15332–15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Wang D; Du S; Cazenave-Gassiot A; Ge J; Lee J-S; Wenk MR; Yao SQ Global Mapping of Protein-Lipid Interactions by Using Modified Choline-Containing Phospholipids Metabolically Synthesized in Live Cells. Angew. Chem., Int. Ed 2017, 56 (21), 5829–5833. [DOI] [PubMed] [Google Scholar]
- (49).Jao CY; Roth M; Welti R; Salic A Biosynthetic Labeling and Two-Color Imaging of Phospholipids in Cells. ChemBioChem 2015, 16 (3), 472–476. [DOI] [PubMed] [Google Scholar]
- (50).Chen Q; Chu T A Two-Step Strategy to Radiolabel Choline Phospholipids with 99m Tc in S180 Cell Membranes via Strain-Promoted Cyclooctyne-Azide Cycloaddition Reaction. Bioorg. Med. Chem. Lett 2016, 26 (22), 5472–5475. [DOI] [PubMed] [Google Scholar]
- (51).White BM; Kumar P; Conwell AN; Wu K; Baskin JM Lipid Expansion Microscopy. J. Am. Chem. Soc 2022, 144 (40), 18212–18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sun D; Fan X; Shi Y; Zhang H; Huang Z; Cheng B; Tang Q; Li W; Zhu Y; Bai J; Liu W; Li Y; Wang X; Lei X; Chen X Click-ExM Enables Expansion Microscopy for All Biomolecules. Nat. Methods 2021, 18 (1), 107–113. [DOI] [PubMed] [Google Scholar]
- (53).Götz R; Kunz TC; Fink J; Solger F; Schlegel J; Seibel J; Kozjak-Pavlovic V; Rudel T; Sauer M Nanoscale Imaging of Bacterial Infections by Sphingolipid Expansion Microscopy. Nat. Commun 2020, 11 (1), 6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Wen G; Vanheusden M; Acke A; Valli D; Neely RK; Leen V; Hofkens J Evaluation of Direct Grafting Strategies via Trivalent Anchoring for Enabling Lipid Membrane and Cytoskeleton Staining in Expansion Microscopy. ACS Nano 2020, 14 (7), 7860–7867. [DOI] [PubMed] [Google Scholar]
- (55).Damstra HGJ; Mohar B; Eddison M; Akhmanova A; Kapitein LC; Tillberg PW Visualizing Cellular and Tissue Ultrastructure Using Ten-Fold Robust Expansion Microscopy (TREx). eLife 2022, 11, No. e73775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Tamura T; Fujisawa A; Tsuchiya M; Shen Y; Nagao K; Kawano S; Tamura Y; Endo T; Umeda M; Hamachi I Organelle Membrane-Specific Chemical Labeling and Dynamic Imaging in Living Cells. Nat. Chem. Biol 2020, 16 (12), 1361–1367. [DOI] [PubMed] [Google Scholar]
- (57).Tsuchiya M; Tachibana N; Nagao K; Tamura T; Hamachi I Organelle-Selective Click Labeling Coupled with Flow Cytometry Allows High-Throughput CRISPR Screening of Genes Involved in Phosphatidylcholine Metabolism. bioRxiv 2022, DOI: 10.1101/2022.04.18.488621. [DOI] [PubMed] [Google Scholar]
- (58).Chiu D-C; Baskin JM Organelle-Selective Membrane Labeling through PLD-Mediated Transphosphatidylation. ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-4wj4j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Andreev E; Brosseau N; Carmona E; Mes-Masson A-M; Ramotar D The Human Organic Cation Transporter OCT1Mediates High Affinity Uptake of the Anticancer Drug Daunorubicin. Sci. Rep 2016, 6 (1), 20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Koepsell H Organic Cation Transporters in Health and Disease. Pharmacol. Rev 2020, 72 (1), 253–319. [DOI] [PubMed] [Google Scholar]
- (61).Koepsell H Polyspecific Organic Cation Transporters: Their Functions and Interactions with Drugs. Trends Pharmacol. Sci 2004, 25 (7), 375–381. [DOI] [PubMed] [Google Scholar]
- (62).Dzijak R; Galeta J; Vázquez A; Kozák J; Matoušová M; Fulka H; Dračínskyý M; Vrabel M Structurally Redesigned Bioorthogonal Reagents for Mitochondria-Specific Prodrug Activation. JACS Au 2021, 1 (1), 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Miyamoto T; Rho E; Sample V; Akano H; Magari M; Ueno T; Gorshkov K; Chen M; Tokumitsu H; Zhang J; Inoue T Compartmentalized AMPK Signaling Illuminated by Genetically Encoded Molecular Sensors and Actuators. Cell Rep. 2015, 11 (4), 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Farley S; Laguerre A; Schultz C Caged Lipids for Subcellular Manipulation. Curr. Opin. Chem. Biol 2021, 65, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Yu W; Baskin JM Photoaffinity Labeling Approaches to Elucidate Lipid-Protein Interactions. Curr. Opin. Chem. Biol 2022, 69, 102173. [DOI] [PubMed] [Google Scholar]
- (66).John Peter AT; Petrungaro C; Peter M; Kornmann B METALIC Reveals Interorganelle Lipid Flux in Live Cells by Enzymatic Mass Tagging. Nat. Cell Biol 2022, 24 (6), 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Folick A; Min W; Wang MC Label-Free Imaging of Lipid Dynamics Using Coherent Anti-Stokes Raman Scattering (CARS) and Stimulated Raman Scattering (SRS) Microscopy. Curr. Opin. Genet. Dev 2011, 21 (5), 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]


