Abstract
Background:
The number of patients with breast cancer is increasing worldwide, resulting in a growing number of patients with chemotherapy-related cognitive impairment (CRCI), which seriously affects their quality of life. CRCI is associated with inflammatory factors and systemic inflammatory markers such as pan-immune-inflammation value (PIV) and monocyte-to-lymphocyte ratio (MLR), which can reflect the level of inflammation in the body. While the Managing Cancer and Living Meaningfully (CALM) intervention has been demonstrated to alleviate CRCI in patients with breast cancer, the specific mechanism remains unclear.
Objective:
This study evaluated the impact of the CALM intervention on systemic inflammation.
Methods:
Ninety patients with breast cancer with CRCI were enrolled and randomized into care as usual (CAU) and CALM intervention groups. All patients were assessed using the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog), Mini-Mental State Exam (MMSE), and Functional Assessment of Cancer Therapy-Breast (FACT-B) before and after the CAU/CALM intervention. The blood levels of inflammatory markers were also analyzed before and after the intervention.
Results:
Compared to the CAU group, the CALM group showed significantly improved cognitive function and significantly decreased PIV (P < .05). PIV was significantly negatively correlated with FACT-Cog (P < .05). The levels of other inflammatory markers, including MLR, neutrophil-to-lymphocyte ratio (NLR), granulocyte-to-lymphocyte ratio (GLR), and systemic immune-inflammation index (SII), were also reduced in the CALM group.
Conclusion:
PIV is an important marker of inflammation. The CALM intervention may improve the cognitive function of patients by regulating the systemic inflammation marker PIV through the neuroimmune axis.
Keywords: breast cancer, CRCI, CALM, PIV, FACT-Cog
Introduction
Breast cancer (BC) is the most common cancer in women worldwide.1 Therapy for BC has become increasingly complicated.2 However, chemotherapy remains the main therapeutic choice for patients with BC.3 Thus, chemotherapy-related cognitive impairment (CRCI) in patients with BC has become a growing area of clinical concern.4 The commonly used antimetabolite chemotherapeutic agent methotrexate (MTX) in BC is particularly related to CRCI, usually known as “chemofog.”5 The different degrees of impact on patient quality of life (QOL) range from objective to subjective cognitive complaints.6 Currently, the detailed mechanisms underlying CRCI remain unclear. Differential mechanisms, including changes in hormone levels, genetic susceptibility, and inflammation have been proposed and explored. One study reported that inflammation played a role in the pathogenesis of cognitive impairment in BCs.7 This finding suggests that the development of cognitive impairment is significantly associated with inflammation.
Several interventions have been developed to target CRCI. Tack et al8 demonstrated the great efficacy of the Emotional Freedom Techniques (EFT) in alleviating self-reported CRCI, distress, and depressive symptoms. Most recently, a kind of psychological intervention; namely, the Managing Cancer And Living Meaningfully (CALM) designed by Rodin et al has shown substantial effects on symptom management.9 This intervention is a manualized psychotherapeutic intervention used to treat and prevent depression and psychological distress in patients with cancer. Previous studies demonstrated the effects of the CALM intervention on alleviating cognitive impairment.10 However, the underlying mechanisms are poorly understood. Recently, inflammation has been suggested as a potential intervening mechanism in the pathogenesis of cognitive impairment in patients with cancer.11 Most studies have focused on cytokines and acute-phase proteins. However, these indicators have not been widely used in clinical practice. From a more general perspective, systemic inflammatory response markers measured in blood, including the pan-immune-inflammation value (PIV), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR), are increasingly used. Patients with cerebral small vessel disease with a high NLR may have a higher risk of cognitive impairment.12 A community-population study also explored the relationship between the SII and NLR in cerebral small vessel disease.13 Shang et al14 reported the diagnostic value of NLR for post-stroke cognitive impairment. Preoperative NLR, LMR, and PLR are also proven prognostic markers of survival in BC.15 The predictive function of NLR has also been explored in patients undergoing anti-programed cell death-1 (PD-1)/programed cell death ligand 1 (PD-L1) treatment.16 Systemic inflammatory response markers and cytokines are important body inflammation indices, both of which have been explored for their correlation with cognitive function in many diseases.17 A previous study noted that markers of systemic inflammation measures are correlated with circulating concentration of various cytokines, and the elevation of cytokines such as TNF- α, IL-1, and IL-6 may be earlier than the elevation of systemic inflammatory markers.18 Therefore, it is important to analyze the correlation between CALM interventions to improve cognitive function and changes in the levels of systemic inflammatory markers.
In this context, we recruited patients with BC to undergo the CALM intervention and analyzed the changes in their systemic inflammatory markers before and after the intervention. The correlation of these markers with cognitive abilities was also analyzed. The results suggested the potential value of systemic inflammatory markers in changes in cognitive function. Systemic inflammatory markers are readily available clinically; thus, our findings are applicable to general clinical practice.
Materials and Methods
Study and Randomization Procedure
The statistician in this study managed the randomization. The statisticians were not involved in the intervention process. Computer-generated random assignments were provided by the statistician after the patients’ baseline measurements were collected and age was used as a stratification variable. The doctors were blinded to the assignments written on cards and sealed in envelopes to be opened at the time of assignment. The doctors performing the assessments were blinded to the group assignments of the patients. All patients provided written informed consent for their participation in the study.
Participants
This study recruited patients with BC from the Cancer Center of the Second Affiliated Hospital of Anhui Medical University between Aug 2018and Sep 2019. Ninety patients with BC were enrolled, 45 of whom were randomized to the CALM intervention group and 45 to the care-as-usual (CAU) group. Ultimately, 31 and 38 participants in the 2 groups completed the study, respectively. All patients were assessed using neuropsychological tests, including the Mini-Mental State Exam (MMSE), Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog), and Functional Assessment of Cancer Therapy-Breast (FACT-B), before and after the intervention. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (Ethics number 2012088, date: Feb 26, 2018).
The inclusion criteria were:(1) Completion of at least 6 cycles of chemotherapy based on anthracyclines and taxanes and the last cycle of chemotherapy to first intervention less than 4 weeks, with no unbearable side effects (adverse effects grade of chemotherapy greater than 3, as assessed by the Common Terminology Criteria for Adverse Effects version 4.0). (2) Perceived cognitive impairment (PCI) score in FACT-Cog reflects CRCI.19,20 Complaints of decreased attention and memory function after chemotherapy. (3) Karnofsky performance status (KPS) score ≥80. (4) No impairment of hearing, vision, or language. The exclusion criteria were: (1) History of alcohol or drug addiction. (2) Distant metastases or advanced cachexia. (3) Other mental or physical disorders potentially leading to cognitive impairment.
CALM Intervention
The CALM intervention is an original, systematic, and tailored psychological intervention that was designed through collaborations at the Princess Margaret Cancer Center, Toronto, Canada.21 The patients in our study participated in the CALM intervention with 6 sessions occurring over 12 weeks. The intervention conducted by counselors with psychosocial counselor qualifications who received CALM intervention training. The whole process took place in a comfortable and quiet environment with soothing background music, and each session lasted approximately 30 to 60 minutes.
There are 4 main themes of the CALM intervention: (1) Managing symptoms of patients and talking with their healthcare providers. (2) Changes in themselves and relationships with close others. (3) Spiritual wellbeing and a sense of meaning and purpose. (4) Concerns about mortality and future (Figure 1). The sessions included communicating with the patient, popularizing BC-related knowledge, changing patients’ misunderstandings about BC, relieving nervousness, increasing the confidence of patients with BC in their treatment plans, helping patients with cancer to relax mentally and physically, encouraging patients with BC to communicate with friends and relatives, and reintegrating these patients into society. On the whole, CALM intervention is an individual intervention, which may involve multifarious techniques and is individualized to different patients in therapy. The detailed description for the implementation of the CALM intervention is shown in Supplemental Table 1.
Figure 1.
Four main themes of CALM intervention.
Neuropsychological Tests
MMSE: The MMSE was used to evaluate the objective cognitive ability of the patients. The MMSE includes tests of memory, language, attention, orientation, and visual-spatial skills.22 The MMSE is graded on a 30-point scale.23 Abebe et al24 assessed cognitive function in patients with BC. The MMSE has been widely applied in cognitive studies of Chinese patients25-27 and its reliability and validity have been verified previously.28,29 MMSE scores ≤26 were considered to indicate cognitive impairment, while scores of 27 to 30 were considered normal.
FACT-Cog: The FACT-Cog is a self-rated scale used to assess the subjective cognitive function of patients with cancer. The scale can be applied to evaluate the cognitive function of these patients based on 37 items, which include 4 main dimensions: perceived cognitive impairment (CogPCI), comments from others (CogOth), perceived cognitive ability (CogPCA), and cognitive changes on QOL (CogQOL). Every item is scored on a scale of 0 to 4, with a total of 5 grades. Cognitive function status can be reflected by the level of the scores. The English version of this scale has shown good validity and reliability. Binarelli et al30 used the FACT-Cog to assess CRCI in patients with BC. Cheung et al31 verified that the FACT-Cog is valid and reliable for clinical use in Chinese patients with BC. Therefore, it can be used to clinically assess the cognitive functional status of Chinese BC survivors.
FACT-B: QOL was evaluated using the FACT-B. The QOL scores were assessed at baseline and after 6 sessions of the CALM intervention. This study used vision 4.0 of the FACT-B, which includes 4 domains: family/social, physical, functional, and emotional well-being, as well as a subscale for BC, which has better validity and reliability. The FACT-B was used by Rosenberg et al32 to evaluate QOL in patients with BC receiving adjuvant chemotherapy33 and Wan et al33 demonstrated its good validity and reliability in Chinese patients with BC.
Acquisition and Analysis of Inflammatory Indexes
Laboratory data, including full blood count with white blood cells, hemoglobin, and platelets, were obtained before and after the intervention. PIV was defined as (neutrophil × platelet × monocyte)/lymphocyte count. The NLR was defined as neutrophil count/lymphocyte count. The PLR was defined as platelet count/lymphocyte count. The MLR was defined as monocyte count/lymphocyte count. GLR was defined as granulocyte count/lymphocyte count. The systemic immune-inflammation index (SII) was defined as (platelet count × neutrophil count)/lymphocyte count.
Statistical Analysis
All data were collected and analyzed using IBM SPSS Statistics for Windows, version 22.0, and expressed as means ± SD. Chi-squared tests were used to compare the classification data. Paired t and Wilcoxon signed-rank tests, respectively, were performed for normal and non-normal distributions in continuous variable data. Two independent-samples t-tests and Mann-Whitney U-tests were performed for normally and non-normally distributed continuous variable data, respectively. Differences were considered statistically significant for P < .05.
Results
Research Flow and Baseline Characteristics of the Patients with Breast Cancer
The research flowchart and baseline characteristics of patients are shown in Table 1 and Figure 2.
Table 1.
Baseline Data of Breast Cancer Patients.
| CALM group | CAU group | t/χ | P | |
|---|---|---|---|---|
| Age (y) | 51.645 ± 6.666 | 51.079 ± 6.403 | 0.359 | .721 |
| Education (y) | 8.355 ± 1.907 | 9.053 ± 2.217 | 1.092# | .275 |
| Tumor stage | 3.359 | .201 | ||
| I | 2 | 7 | ||
| II | 19 | 16 | ||
| III | 10 | 15 | ||
| ER status | 0.552 | .458 | ||
| Negative | 15 | 15 | ||
| Positive | 16 | 23 | ||
| PR status | 0.001 | .972 | ||
| Negative | 14 | 17 | ||
| Positive | 17 | 21 | ||
| Her-2 status | 0.062 | .803 | ||
| Negative | 5 | 7 | ||
| Positive | 26 | 31 | ||
| Pathological type | 1.822 | .401 | ||
| Invasive carcinoma no special type | 18 | 17 | ||
| Invasive carcinoma | 13 | 20 | ||
| Invasive carcinoma special type | 0 | 1 | ||
| KPS | 87.742 ± 4.250 | 87.895 ± 4.132 | 0.879# | .886 |
| Neuropsychological tests | ||||
| MMSE | 22.161 ± 2.66 | 22.395 ± 2.477 | 0.170# | .865 |
| FACT-Cog | 79.281 ± 7.239 | 82.892 ± 7.035 | 1.952# | .051 |
| FACT-B | 72.871 ± 6.130 | 73.132 ± 5.483 | 0.186 | .853 |
| Systemic inflammatory markers | ||||
| PIV | 307.910 ± 506.774 | 193.117 ± 158.699 | 0.820# | .412 |
| MLR | 0.340 ± 0.175 | 0.298 ± 0.119 | 0.784# | .433 |
| NLR | 2.655 ± 1.483 | 2.134 ± 0.948 | 1.062# | .288 |
| GLR | 2.750 ± 1.475 | 2.224 ± 0.973 | 1.140# | .254 |
| SII | 586.822 ± 563.074 | 425.448 ± 267.791 | 1.565 | .122 |
Abbreviations: CALM, managing cancer and living meaningfully; CAU, care as usual; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; KPS, Karnofsky performance status; MMSE, mini-mental state exam; FACT-Cog, functional assessment of cancer therapy-cognitive function; FACT-B, functional assessment of cancer therapy-breast; PIV, pan-immune-inflammation value; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; GLR, granulocyte-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Mann–Whitney U-test.
Figure 2.
Research flowchart.
Changes in PIV and its Correlations with Cognitive Function and QOL
As shown in Figure 3 and Table 2, after the CALM intervention, the PIV of patients with BC was significantly reduced (z = −2.932, P = .004), while the PIV of the CAU group patients did not change significantly (z = 1.066, P = .286). Our analysis of the correlations between PIV and MMSE, FACT-Cog, and FACT-B in the CALM group showed that PIV and FACT-Cog were closely related (r = −.297, P = .021).
Figure 3.
Changes in PIV and its correlations with cognitive function and quality of life. (A) Changes in PIV before and after CALM. (B) Changes of PIV before and after CAU. (C–E) Correlations between PIV and MMSE, FACT-Cog, and FACT-B, respectively, in the CALM group.
Abbreviations: PIV, pan-immune-inflammation value; CALM, Managing Cancer and Living Meaningfully; CAU, care as usual; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; MMSE, Mini-Mental State Exam; FACT-Cog, Functional Assessment of Cancer Therapy-Cognitive Function; FACT-B, Functional Assessment of Cancer Therapy-Breast.
Table 2.
Comparisons of the Levels of Markers of Systemic Inflammation Between the CALM and CAU Groups After the Intervention.
| CALM group | CAU group | z | P | |
|---|---|---|---|---|
| PIV | 111.926 ± 66.083 | 267.376 ± 290.563 | 3.331# | <.001 |
| MLR | 0.262 ± 0.128 | 0.351 ± 0.187 | 1.918# | .055 |
| NLR | 1.741 ± 0.903 | 2.666 ± 1.472 | 3.221# | <.001 |
| GLR | 1.837 ± 0.929 | 2.663 ± 1.355 | 3.011# | .003 |
| SII | 323.334 ± 161.184 | 477.133 ± 295.754 | 2.704# | .007 |
Abbreviations: CALM, managing cancer and living meaningfully; CAU, care as usual; PIV, pan-immune-inflammation value; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; GLR, granulocyte-to-lymphocyte ratio; SII, systemic immune-inflammation index.
Mann–Whitney U test.
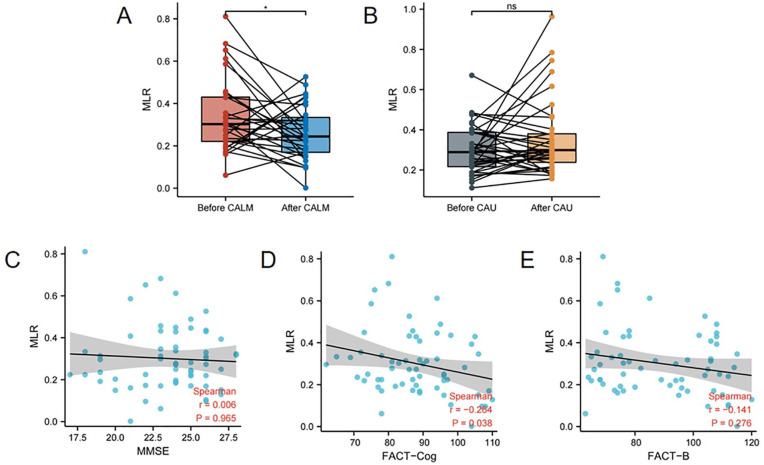
Changes in MLR and its Correlations with Cognitive Function and QOL
As shown in Figure 4 and Table 2, after the CALM intervention, the MLR of patients with BC was significantly reduced (t = −2.091, P = .045), whereas the MLR of the CAU group did not change significantly (z = 1.037, P = .300). Our analysis of the correlations between MLR and MMSE, FACT-Cog, and FACT-B in the CALM group showed that MLR and FACT-Cog were closely related (r = −.264, P = .038).
Figure 4.
Changes in MLR and its correlations with cognitive function and quality of life. (A) Changes in MLR before and after CALM. (B) Changes in MLR before and after CAU. (C–E) Correlations between MLR and MMSE, FACT-Cog, and FACT-B, respectively, in the CALM group.
Abbreviations: MLR, monocyte-to-lymphocyte ratio; CALM, Managing Cancer and Living Meaningfully; CAU, care as usual; MMSE, Mini-Mental State Exam; FACT-Cog, Functional Assessment of Cancer Therapy-Cognitive Function; FACT-B, Functional Assessment of Cancer Therapy-Breast.
Changes in NLR and its Correlations with Cognitive Function and Quality of Life
As shown in Figure 5 and Table 2, after the CALM intervention, the NLR of patients with BC was significantly reduced (z = 3.194, P < .001), whereas the NLR of the CAU group patients was upregulated (z = 2.618, P = .023). Our analysis of the correlations between NLR and MMSE, FACT-Cog, and FACT-B scores in the CALM group revealed no close relationships.
Figure 5.
Changes in NLR and its correlations with cognitive function and quality of life. (A) Changes in NLR before and after CALM. (B) Changes in NLR before and after CAU. (C–E) Correlation between NLR and, MMSE, FACT-Cog, and FACT-B, respectively, in the CALM group.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio CALM, managing cancer and living meaningfully; CAU, care as usual; MMSE, mini-mental state exam; FACT-Cog, functional assessment of cancer therapy-cognitive function; FACT-B, functional assessment of cancer therapy-breast.
Changes in GLR and its Correlations with Cognitive Function and QOL
As shown in Figure 6 and Table 2, after the CALM intervention, the GLR of patients with BC was significantly reduced (z = −3.175, P < .001), while the GLR of the CAU group was upregulated (z = 2.212, P = .027). Our analysis of the correlation between GLR and MMSE, FACT-Cog, and FACT-B scores in the CALM group revealed no close relationships.
Figure 6.
Changes in GLR and its correlation between cognitive function and quality of life. (A) Changes in GLR before and after CALM. (B) Changes in GLR before and after CAU. (C–E) Correlations between GLR and MMSE, FACT-Cog, and FACT-B, respectively, in the CALM group.
Abbreviations: GLR, granulocyte-to-lymphocyte ratio CALM, managing cancer and living meaningfully; CAU, care as usual; MMSE, mini-mental state exam; FACT-Cog, functional assessment of cancer therapy-cognitive function; FACT-B, functional assessment of cancer therapy-breast.
Changes in SII and its Correlation Between Cognitive Function and Quality of Life
As shown in Figure 7 and Table 2, after the CALM intervention, the SII of patients with BC was significantly reduced (t = −2.900, P = .007), while the SII of the CAU group did not change significantly (z = 0.935, P = .350). Our analysis of the correlation between the SII and MMSE, FACT-Cog, and FACT-B in the CALM group revealed no close relationships.
Figure 7.
Changes in SII and its correlations with cognitive function and QOL. (A) Changes in SII before and after CALM. (B) Changes in SII before and after CAU. (C–E) Correlations between SII and MMSE, FACT-Cog, and FACT-B, respectively, in the CALM group.
Abbreviations: SII, systemic immune-inflammation index; CALM, managing cancer and living meaningfully; CAU, care as usual; MMSE, mini-mental state exam; FACT-Cog, functional assessment of cancer therapy-cognitive function; FACT-B, functional assessment of cancer therapy-breast.
Discussion
In recent years, much attention has been paid to the negative correlation between systemic inflammatory markers and cognitive function. The results of the present study showed significantly reduced PIV and MLR and significantly improved cognitive function in patients after the CALM intervention compared to those in the control group. This is consistent with the observation that inflammation leads to cognitive decline in the brain.34,35
The CALM intervention is a psychological concept that helps improve the QOL of patients with cancer patients and has been proven to alleviate CRCI in patients with BC.10 The discovery of the mechanism by which it alleviates CRCI may benefit its promotion and improve its therapeutic effect. Usually, impairments in cognitive function are associated with aggravated inflammation in the body.36 Therefore, improvements in cognitive function may also be associated with reduced inflammation. Inflammatory markers such as PIV, MLR, and NLR are the most intuitive indicators that reflect the level of inflammation in the body. Our analysis of our findings revealed that PIV was the most important inflammatory marker for the alleviation of CRC by the CALM intervention.
In clinical research, CRCI usually includes subjective cognitive complaints and objective cognitive impairments.37 They have different effects on the QOL of patients with cancer. Lycke et al6 reported that subjective cognitive complaints impacted long-term QOL in patients with cancer. Therefore, we used the objective MMSE and subjective FACT-Cog to assess patient cognitive status in this study. A previous study found that high PIV was correlated with worse clinical outcomes in patients with cancer treated with immunocombination chemotherapy, suggesting the broad impact of systemic inflammatory markers on patients with cancer.38,39 Thus, PIV appears to be an important factor in CALM interventions that improves cognitive function.
Accumulating basic and clinical evidence suggests that inflammation underlies the mechanism of CRCI; thus, reducing systemic inflammatory marker levels may be an important mechanism and research direction for alleviating cognitive impairment in patients.40 The effect of CALM in improving cognitive function is obvious. This study is the first to analyze the changes in the levels of systemic inflammatory markers in patients and to provide a theoretical basis for the mechanism of action of the CALM intervention.
A recent study reported significantly increased NLR, MLR, and SII indicators in patients with postoperative cognitive decline (POCD) compared to those in patients without POCD. The authors also suggested that SII levels were prognostic biomarkers of POCD in elderly patients. Similarly, our findings indicated that PIV can be used as a marker for the effectiveness of CALM interventions.41 Increased systemic inflammatory marker levels also reflect a high risk of poststroke cognitive impairment (PSCI), whereas a decrease in systemic inflammatory markers reduces the risk of PSCI.42
Studies on the indicators of systemic inflammation have drawn increasing attention. PIV has been shown to be a prognostic marker in patients with BC after surgery.43 PIV is also an important indicator of poor prognosis in patients with BC.44 Patients with BC and high PIV showed significantly advanced AJCC tumor stages. High PIV also predicted worse survival and chemotherapeutic response in patients with BC treated with neoadjuvant chemotherapy.45 High PIV was also correlated with worse progression-free survival (PFS) in patients with human epidermal growth factor receptor 2-positive (HER2+) advanced BC treated with trastuzumab-pertuzumab-containing chemotherapy.46 These studies explored the effect of the systemic inflammatory index of PIV on survival and treatment efficacy in patients with BC. Through the correlation between PIV and CRCI, our study provides a theoretical basis for the role of PIV in psychological interventions, such as CALM (Figure 8).
Figure 8.
Overview of the research implications.
Limitations: The present study was subject to the boundedness inherent to a single-center study. The limitations of this research are the comparatively small number of patients with BC in each group. Age is also an important element that influences cognitive ability, and young female patients with breast cancer are reportedly to score better after receiving a psychosocial intervention. Therefore, future research should control the age range to reduce its impact on the results. After the initial exploration provided in this study, future studies are needed with increased numbers of cases and screening in suitable collaborating center units. The number of instructors also should be increased to reduce possible bias. After obtaining data from a large sample, functional gene enrichment pathway analysis of inflammatory factors and the identification of the molecular mechanisms of the CALM intervention will be performed.
Conclusion
This study analyzed changes in cognitive function and systemic inflammatory markers in patients with BC after the CALM intervention. The results suggested that the CALM intervention may alleviate CRCI in patients by reducing the level of inflammation in the body. This study also identified indicators that are easily detected and analyzed for the assessment of CRCI, which provides significant value for promoting the CALM intervention and improving cognitive function and QOL in patients with BC.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354221140498 for The Managing Cancer and Living Meaningfully (CALM) Intervention Alleviates Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer by Modulating Pan-Immune-Inflammation Values by Senbang Yao, Ke Ding, Shaochun Liu, Qianqian Zhang, Wen Li, Lingxue Tang, Sheng Yu, Lulian Pang, Xiangxiang Yin and Huaidong Cheng in Integrative Cancer Therapies
Acknowledgments
We thank the patients with cancer for their cooperation in this study. We thank the Pinclipart web site and relevant author for providing publicly available picture material. We also thank reviewers for valuable suggestions.
Footnotes
Author’s Note: Huaidong Cheng is also affiliated to Department of Oncology, Shenzhen Hospital of Southern Medical University, Shenzhen, Guangdong, China.
Author contributions: Conceptualization: Senbang Yao and Huaidong Cheng. Data collection: Senbang Yao, Ke Ding, and Shaochun Liu. Data analysis: Senbang Yao, Ke Ding, and Shaochun Liu. Original draft writing: Senbang Yao. Review and editing: Qianqian Zhang, Wen Li, Lingxue Tang, Sheng Yu, and Xiangxiang Yin. Senbang Yao, Ke Ding, and Shaochun Liu contributed equally to this study.
Data availability statement: All data are available from the corresponding author upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The National Natural Science Foundation of China (81872504).
ORCID iDs: Ke Ding  https://orcid.org/0000-0002-9682-4957
https://orcid.org/0000-0002-9682-4957
Xiangxiang Yin  https://orcid.org/0000-0002-5343-6348
https://orcid.org/0000-0002-5343-6348
Huaidong Cheng  https://orcid.org/0000-0001-6422-1257
https://orcid.org/0000-0001-6422-1257
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [DOI] [PubMed] [Google Scholar]
- 2. Greenup RA, Obeng-Gyasi S, Thomas S, et al. The effect of hospital volume on breast cancer mortality. Ann Surg. 2018;267:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller-Kleinhenz J, Guo X, Qian W, et al. Dual-targeting Wnt and uPA receptors using peptide conjugated ultra-small nanoparticle drug carriers inhibited cancer stem-cell phenotype in chemo-resistant breast cancer. Biomaterials. 2018;152:47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janelsins MC, Heckler CE, Peppone LJ, et al. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol. 2018;36:3231-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geraghty AC, Gibson EM, Ghanem RA, et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron. 2019;103:250-265.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lycke M, Lefebvre T, Pottel L, et al. The distress thermometer predicts subjective, but not objective, cognitive complaints six months after treatment initiation in cancer patients. J Psychosoc Oncol. 2017;35:741-757. [DOI] [PubMed] [Google Scholar]
- 7. van der Willik KD, Koppelmans V, Hauptmann M, Compter A, Ikram MA, Schagen SB. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 2018;20:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tack L, Lefebvre T, Lycke M, et al. A randomised wait-list controlled trial to evaluate emotional freedom techniques for self-reported cancer-related cognitive impairment in cancer survivors (EMOTICON). EClinicalMedicine. 2021;39:101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nissim R, Freeman E, Lo C, et al. Managing Cancer and living meaningfully (CALM): a qualitative study of a brief individual psychotherapy for individuals with advanced cancer. Palliat Med. 2012;26:713-721. [DOI] [PubMed] [Google Scholar]
- 10. Ding K, Zhang X, Zhao J, Zuo H, Bi Z, Cheng H. Managing cancer and living meaningfully (CALM) intervention on chemotherapy-related cognitive impairment in breast cancer survivors. Integr Cancer Ther. 2020;19:1534735420938450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel SK, Wong AL, Wong FL, et al. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. 2015;107:djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou L, Zhang S, Qi D, et al. Correlation between neutrophil/lymphocyte ratio and cognitive impairment in cerebral small vessel disease patients: a retrospective study. Front Neurol. 2022;13:925218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang L, Cai X, Yao D, et al. Association of inflammatory markers with cerebral small vessel disease in community-based population. J Neuroinflammation. 2022;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shang T, Ma B, Shen Y, et al. High neutrophil percentage and neutrophil-lymphocyte ratio in acute phase of ischemic stroke predict cognitive impairment: a single-center retrospective study in China. Front Neurol. 2022;13:907486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savioli F, Morrow ES, Dolan RD, et al. Prognostic role of preoperative circulating systemic inflammatory response markers in primary breast cancer: meta-analysis. Br J Surg. 2022; 1–10: znac319. [DOI] [PubMed] [Google Scholar]
- 16. Ma Y, Ma X, Wang J, Wu S, Wang J, Cao B. Absolute eosinophil count may be an optimal peripheral blood marker to identify the risk of immune-related adverse events in advanced malignant tumors treated with PD-1/PD-L1 inhibitors: a retrospective analysis. World J Surg Oncol. 2022;20:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tao R, Peng X, Liu X, et al. Outcome of lenalidomide treatment for cognitive impairment caused by immune reconstitution inflammatory syndrome in patients with HIV-related cryptococcal meningitis. J Inflamm Res. 2022;15:5327-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3:e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyu YR, Lee HY, Park HJ, et al. Electroacupuncture for cancer-related cognitive impairment: a clinical feasibility study. Integr Cancer Ther. 2022;21:15347354221098983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dyk KV, Crespi CM, Petersen L, Ganz PA. Identifying Cancer-related cognitive impairment using the FACT-Cog perceived cognitive impairment. JNCI Cancer Spectr. 2020;4:z099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodin G, Lo C, Rydall A, et al. Managing Cancer and living meaningfully (CALM): a randomized controlled trial of a psychological intervention for patients with advanced cancer. J Clin Oncol. 2018;36:2422-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petekkaya E, Kuş B, Doğan S, et al. Possible role of endocannabinoids in olfactory and taste dysfunctions in Alzheimer’s and Parkinson’s patients and volumetric changes in the brain. J Clin Neurosci. 2022;100:52-58. [DOI] [PubMed] [Google Scholar]
- 23. Schäfer N, Proescholdt M, Steinbach JP, et al. Quality of life in the GLARIUS trial randomizing bevacizumab/irinotecan versus temozolomide in newly diagnosed, MGMT-nonmethylated glioblastoma. Neuro Oncol. 2018;20:975-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abebe E, Tollesa T, Assefa M, et al. Cognitive functioning and its associated factors among breast cancer patients on chemotherapy at Tikur anbessa specialized hospital, Addis Ababa Ethiopia: an institution-based comparative cross-sectional study. BMC Cancer. 2021;21:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Ma W, Li M, et al. Relationship between physical performance and mild cognitive impairment in Chinese community-dwelling older adults. Clin Interv Aging. 2021;16:119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Yu X, Han P, et al. Gender-specific prevalence and risk factors of mild cognitive impairment among older adults in Chongming, Shanghai, China. Front Aging Neurosci. 2022;14:900523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ye S, Pan H, Li W, Wang B, Xing J, Xu L. High serum amyloid A predicts risk of cognitive impairment after lacunar infarction: development and validation of a nomogram. Front Neurol. 2022;13:972771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. 2017;389:1619-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lv X, Li W, Ma Y, et al. Cognitive decline and mortality among community-dwelling Chinese older people. BMC Med. 2019;17:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Binarelli G, Lange M, Dos Santos M, et al. Multimodal web-based intervention for cancer-related cognitive impairment in breast cancer patients: Cog-Stim feasibility study protocol. Cancers. 2021;13:4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheung YT, Lim SR, Shwe M, Tan YP, Chan A. Psychometric properties and measurement equivalence of the English and Chinese versions of the functional assessment of cancer therapy-cognitive in Asian patients with breast cancer. Value Health. 2013;16:1001-1013. [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg SM, O'Neill A, Sepucha K, et al. Quality of life following receipt of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer. JAMA Netw Open. 2022;5:e220254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan C, Zhang D, Yang Z, et al. Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Res Treat. 2007;106:413-418. [DOI] [PubMed] [Google Scholar]
- 34. McGrattan AM, McGuinness B, McKinley MC, et al. Diet and inflammation in cognitive ageing and Alzheimer’s Disease. Curr Nutr Rep. 2019;8:53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang RP, Ho YS, Leung WK, Goto T, Chang RC. Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav Immun. 2019;81:63-73. [DOI] [PubMed] [Google Scholar]
- 36. Luo J, le Cessie S, Blauw GJ, Franceschi C, Noordam R, van Heemst D. Systemic inflammatory markers in relation to cognitive function and measures of brain atrophy: a Mendelian randomization study. GeroScience. 2022;44:2259-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tulk J, Rash JA, Thoms J, Wassersug R, Gonzalez B, Garland SN. Androgen deprivation therapy and radiation for prostate cancer-cognitive impairment, sleep, symptom burden: a prospective study. BMJ Support Palliat Care. 2021; 1–10. [DOI] [PubMed] [Google Scholar]
- 38. Zeng R, Liu F, Fang C, et al. PIV and PILE score at baseline predict clinical outcome of Anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung cancer patients. Front Immunol. 2021;12:724443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ceran B, Alyamaç Dizdar E, Beşer E, Karaçağlar NB, Sarı FN. Diagnostic role of systemic inflammatory indices in infants with moderate-to-severe hypoxic ischemic encephalopathy. Am J Perinatol. 2021. doi: 10.1055/a-1673-1616. [DOI] [PubMed] [Google Scholar]
- 40. Oppegaard K, Harris CS, Shin J, et al. Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine. 2021;148:155653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu W, Zhang K, Chang X, Yu X, Bian J. The association between systemic immune-inflammation index and postoperative cognitive decline in elderly patients. Clin Interv Aging. 2022;17:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zha F, Zhao J, Chen C, et al. A high neutrophil-to-lymphocyte ratio predicts higher risk of poststroke cognitive impairment: development and validation of a clinical prediction model. Front Neurol. 2021;12:755011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin F, Zhang LP, Xie SY, et al. Pan-immune-inflammation value: a new prognostic index in operative breast cancer. Front Oncol. 2022;12:830138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demir H, Demirci A, Eren SK, Beypinar I, Davarcı SE, Baykara M. A new prognostic index in young breast cancer patients. J Coll Physicians Surg Pak. 2022;32:86-91. [DOI] [PubMed] [Google Scholar]
- 45. Şahin AB, Cubukcu E, Ocak B, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep. 2021;11:14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ligorio F, Fucà G, Zattarin E, et al. The Pan-Immune-Inflammation-Value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers (Basel). 2021;13:1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354221140498 for The Managing Cancer and Living Meaningfully (CALM) Intervention Alleviates Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer by Modulating Pan-Immune-Inflammation Values by Senbang Yao, Ke Ding, Shaochun Liu, Qianqian Zhang, Wen Li, Lingxue Tang, Sheng Yu, Lulian Pang, Xiangxiang Yin and Huaidong Cheng in Integrative Cancer Therapies