Abstract
Neutrophils are abundant, short-lived myeloid cells that are readily recruitable to sites of inflammation, where they serve as first-line defense against infection and other types of insult to the host. In recent years, there has been increased understanding on the involvement of neutrophils in chronic inflammatory diseases, where they may act as direct effectors of destructive inflammation. However, destructive tissue inflammation is also instigated in settings of neutrophil paucity, suggesting that neutrophils also mediate critical homeostatic functions. The activity of neutrophils is regulated by a variety of local tissue factors. In addition, systemic metabolic conditions, such as hypercholesterolemia and hyperglycemia, affect the production and mobilization of neutrophils from the bone marrow. Moreover, according to the recently emerged concept of innate immune memory, the functions of neutrophils can be enhanced through the process of trained granulopoiesis. This process may have both beneficial and potentially destructive effects, depending on context, that is, protective against infections and tumors, while destructive in the context of chronic inflammatory conditions. Although we are far from a complete understanding of the mechanisms underlying the regulation and function of neutrophils, current insights enable the development of targeted therapeutic interventions that can restrain neutrophil-mediated inflammation in chronic inflammatory diseases, such as periodontitis.
Keywords: myeloid cells, metabolic dysregulation, trained immunity, host modulation, periodontitis, complement
Introduction
Neutrophils are the most abundant white blood cells in the circulation, albeit relatively short lived, and have been historically regarded as the first line of the host’s defense against pathogens. They have thus been extensively studied in the context of acute inflammation, infection, or injury. In the past 2 decades, neutrophils have been increasingly appreciated for their role in chronic inflammatory pathologies, including periodontitis (Hajishengallis et al. 2015; Ley et al. 2018; Burn et al. 2021).
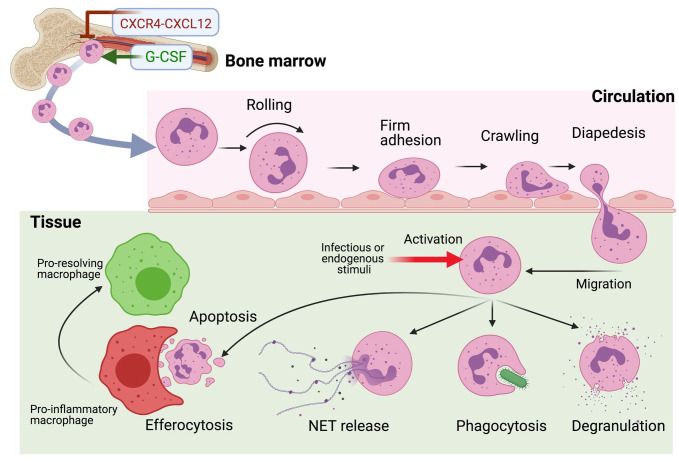
Neutrophils are generated in high numbers (~109 cells per kg bodyweight) daily in the bone marrow (BM) under steady-state conditions, although their production may increase 10-fold under hematopoietic stress, for instance, due to systemic infection or inflammation (“emergency granulopoiesis”) (Manz and Boettcher 2014; Ley et al. 2018). Upon their release from the BM, neutrophils circulate in the vasculature and can migrate to sites of inflammation through a well-established cascade of low- and high-affinity rolling and adhesive interactions with receptors on the vascular endothelium (Fig. 1). β2-Integrins play critical roles in the final steps of the adhesion cascade, leading to neutrophil extravasation, by mediating firm adhesion (integrin αLβ2; leukocyte function-associated antigen-1[LFA-1]) and crawling (integrin αΜβ2; Mac-1) of the neutrophils on the endothelial surface (Hajishengallis and Chavakis 2013). Intraluminal crawling allows the neutrophil to “select” an appropriate site for transmigration, which occurs mainly between endothelial cells (paracellularly). Transmigrating neutrophils follow gradients of chemokines, for example, CXC-chemokine ligand 1 (CXCL1) or CXCL2, as well as gradients generated deeper into the infected/inflamed tissue as a result of activation of the complement system (C5a chemoattractant gradient) or bacterial infection (N-formyl-methionyl-leucyl-phenylalanine gradient) (Ley et al. 2018).
Figure 1.
Mobilization, recruitment, and functions of neutrophils in infected/inflamed tissue. The granulocyte colony-stimulating factor (G-CSF) plays a key role in promoting neutrophil mobilization and release from the bone marrow, while antagonizing the neutrophil-retentive interaction between the CXC-chemokine receptor 4 (CXCR4) and CXC-chemokine ligand 12 (CXCL12) (Manz and Boettcher 2014). After their release into the blood circulation, the neutrophils can be recruited to peripheral tissues in response to infection and/or inflammation. In this process, the circulating neutrophils undergo a series of tightly regulated rolling, adhesive, and crawling interactions with the endothelium followed by their extravasation (or diapedesis) into the tissue (Ley et al. 2018). Once in the tissue, neutrophils migrate in response to chemotactic gradients of host-derived chemokines and inflammatory mediators and/or pathogen-derived molecules, and they perform a variety of defensive functions, as indicated (Burn et al. 2021). The clearance via efferocytosis of dying (apoptotic) neutrophils by tissue macrophages is crucial for the resolution of inflammation (Hajishengallis and Chavakis 2019). NET, neutrophil extracellular trap.
Neutrophils respond to a variety of stimuli, including microbial products (e.g., bacterial LPS or lipoproteins via distinct Toll-like receptors), complement activation products (e.g., C3a and C5a via specific G-protein coupled receptors), arachidonic acid–derived proinflammatory lipid mediators (e.g., the eicosanoid leukotriene B4 via the BLT1 receptor), cytokines, chemokines and growth factors (via cognate receptors), and endogenous danger-associated molecules, such as extracellular ATP (via purinergic receptors), extracellular cold-inducible RNA-binding protein (via the triggering receptor expressed on myeloid cell [TREM]–1), and N-formylated mitochondrial peptides (via the formyl-peptide receptor 1 [FPR1]) (Dorward et al. 2015; Hajishengallis et al. 2015; Ley et al. 2018; Wang and Chen 2018; Fine et al. 2020). To perform their defensive functions (Fig. 1), neutrophils are equipped with diverse and powerful cytotoxic and proinflammatory mechanisms, which, however, may cause more harm than benefit to the host if not properly controlled. Specifically, activated neutrophils can release reactive oxygen species, different granular proteolytic enzymes, proinflammatory lipid mediators and cytokines, and “neutrophil extracellular traps” (NETs), that is, web-like chromatin scaffolds that concentrate released antimicrobial molecules and proteases resulting in the trapping and killing of bacteria but also potentially involved in inflammatory/autoimmune disorders (Ley et al. 2018). It is therefore important that the numbers and activity of neutrophils be properly regulated. The focus of this review is to discuss mechanisms of neutrophil regulation as well as potential host modulation approaches to control or mitigate unwarranted neutrophil-mediated inflammation.
Neutrophil Production and Clearance: Consequences of Dysregulation
To maintain a balance between the beneficial and potentially detrimental effects of neutrophils, several homeostatic mechanisms modulate their generation, trafficking, activation, and function as well as clearance in peripheral tissues or in the BM upon homing back. In both mice and humans, the granulocyte colony-stimulating factor (G-CSF) is a key player in both granulopoiesis and neutrophil mobilization from the BM to the periphery (Manz and Boettcher 2014). Specifically, G-CSF acts on myeloid progenitors and drives the expression of myeloid lineage-specific transcription factors (PU.1 and CCAAT/enhancer-binding protein-β). Moreover, G-CSF disrupts the interaction between the CXC-chemokine receptor 4 (CXCR4) and CXC-chemokine ligand 12 (CXCL12), a major mechanism of neutrophil retention within the BM. Besides the aforementioned action, G-CSF can contribute to neutrophil mobilization via triggering the release from BM megakaryocytes and endothelial cells of ligands for the CXC-chemokine receptor 2 (CXCR2), such as CXCL1, 2, and 3, which in turn stimulate neutrophil migration from the BM (Köhler et al. 2011). Thus, CXCR2-binding chemokines not only chemoattract neutrophils to sites of peripheral inflammation, but when produced in the BM, the same chemokines promote neutrophil mobilization to the circulation.
G-CSF can be produced in response to interleukin (IL)–17 derived from adaptive or innate-like T cells (e.g., T helper 17 and γδ T cells, respectively). Thus, IL-17 can orchestrate granulopoiesis and neutrophil mobilization and recruitment by upregulating the expression of G-CSF as well as of CXCR2-binding chemokines (e.g., CXCL1, CXCL2) from stromal cells (Hajishengallis et al. 2015; Ley et al. 2018; Williams et al. 2021). At least in the setting of human periodontitis, fibroblasts are particularly proactive in the expression of neutrophil-recruiting chemokines (CXCL1, 2, 5, 8) (Williams et al. 2021). The production of IL-17 is in turn regulated by IL-23 released from tissue-resident macrophages. This IL-23→IL-17→G-CSF axis links neutrophil clearance in peripheral tissues to regulation of granulopoiesis in the BM (Ley et al. 2018). In particular, “efferocytosis,” the phagocytosis of apoptotic neutrophils by tissue macrophages, triggers liver X receptor (LXR) proresolution signals in the latter that diminish their production of IL-23, in turn leading to reduction of T cell–derived IL-17 and eventually of stromal cell–derived G-CSF, thus limiting a key granulopoietic factor (Ley et al. 2018) (Fig. 2).
Figure 2.

The IL-23→IL-17→G-CSF axis and its regulation. The IL-23–IL-17–G-CSF cytokine cascade and cellular sources of the cytokines involved (left panel). Apoptotic neutrophils are cleared by efferocytosis by tissue macrophages, leading to suppression of IL-23 production in the latter, in turn, downregulating the production of IL-17 and hence of G-CSF (right panel). The proper function of the IL-23→IL-17→G-CSF axis helps maintain steady-state neutrophil counts (Ley et al. 2018). Dashed arrows indicate reduced activation and production. IL, interleukin; G-CSF, granulocyte colony-stimulating factor.
This regulatory feedback mechanism is disrupted in β2 integrin deficiency (leukocyte adhesion deficiency type 1 [LAD1]) since circulating neutrophils fail to transmigrate; the resulting paucity of apoptotic neutrophils in the tissues limits the efferocytosis-associated regulatory, proresolution signals in macrophages, thereby leading to upregulation of IL-23 and the downstream cytokines IL-17 and G-CSF (Moutsopoulos et al. 2014; Kajikawa et al. 2020). Whereas G-CSF is responsible for the elevated granulopoiesis and neutrophilia in LAD1 patients, the local excessive production of IL-17 in the periodontium causes pronounced inflammation and alveolar bone loss (Moutsopoulos et al. 2014). Thus, neutrophils do not cause inflammation simply by their increased numbers and activities but also by their absence from the tissue. The same IL-23→IL-17→G-CSF circuit is also dysregulated in case of impaired efferocytosis, as in the absence of the critical proefferocytic protein developmental endothelial locus 1 (DEL-1). Indeed, DEL-1–deficient mice display defective efferocytosis and overexpression of IL-23, IL-17, and G-CSF and develop IL-17–driven inflammatory bone loss (Eskan et al. 2012; Kourtzelis et al. 2019).
Neutrophils in the Setting of Metabolic Dysregulation
Neutrophil homeostasis may be adversely affected under conditions of metabolic dysregulation. Hypercholesterolemia and hyperglycemia are not only risk factors for cardiometabolic disorders but also linked to other chronic inflammatory disorders, including periodontitis (Silvestre-Roig et al. 2020; Hajishengallis and Chavakis 2021). Indeed, hypercholesterolemia and hyperglycemia may instigate chronic inflammation, at least in part through alterations in neutrophil production, activation, and function (Tall and Yvan-Charvet 2015; Silvestre-Roig et al. 2020).
Under hypercholesterolemic conditions, defective cholesterol efflux and increased cholesterol content in the plasma membrane alter the differentiation potential of hematopoietic stem and progenitor cells (HSPCs) in the BM (Tall and Yvan-Charvet 2015). Specifically, increased cholesterol levels in the HSPC plasma membrane cause an increase in the cell surface abundance of and signaling via CD131, the common β-subunit of the receptor for IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF) (Tall and Yvan-Charvet 2015). The enhanced activation of the GM-CSF/CD131 axis, in turn, leads to elevated proliferation and myeloid-biased differentiation of HSPCs and hence increased generation of monocytes and neutrophils (Tall and Yvan-Charvet 2015). Importantly, in the setting of hypercholesterolemia, neutrophils exhibit increased mobilization from the bone marrow and circulate in a “primed” state, typified by increased expression of CD11b and enhanced release of reactive oxygen species (ROS) and myeloperoxidase upon stimulation (Soehnlein et al. 2017).
Impaired cholesterol efflux and cholesterol accumulation in the plasma membrane also affect the immunoinflammatory status of mature myeloid cells (Westerterp et al. 2012). Consistently, the production of IL-23 is enhanced in macrophages lacking ATP-binding cassette transporters (ABCA1 and ABCG1) that promote the efflux of cholesterol. This is followed by upregulation of IL-17 and G-CSF, which is released to the circulation (Westerterp et al. 2012), and can thus act in the BM to stimulate the production and mobilization of neutrophils (Manz and Boettcher 2014). Interestingly, efferocytosis-induced LXR signaling in macrophages not only inhibits IL-23 but also stimulates increased expression of ATP-binding cassette transporters (Kourtzelis et al. 2019). Therefore, the inhibitory effect of LXR signaling on the induction of IL-23 (and hence downstream suppression of IL-17 and G-CSF) is probably mediated, at least in part, by its ability to upregulate ABCA1 and ABCG1 and cholesterol efflux. How cholesterol efflux downregulates proinflammatory signaling in macrophages is uncertain; however, it is thought that cellular cholesterol levels crucially regulate cholesterol-enriched membrane microdomains that function as signaling platforms for TLRs and other inflammatory receptors (Tall and Yvan-Charvet 2015).
Under hyperglycemic conditions, neutrophils release increased levels of the danger-associated molecules S100A8 and S100A9, which, via interaction with the receptor for advanced glycation end products (RAGE) on common myeloid progenitors in the BM, promote nuclear factor–κB–dependent production of macrophage colony-stimulating factor (M-CSF) and GM-CSF. These factors in turn stimulate proliferation and expansion of granulocyte-monocyte progenitors, thereby augmenting granulopoiesis (Nagareddy et al. 2013). Moreover, hyperglycemia primes human and mouse neutrophils for increased ROS production and NET release (Omori et al. 2008; Wong et al. 2015). Conversely, disruption of glucose transporter type 1 (and hence glucose uptake) in hematopoietic progenitors causes a reduction in myelopoiesis and thus the counts of circulating neutrophils, at least in hyperlipidemic (APOE-deficient) mice (Sarrazy et al. 2016). Interestingly, analogous metabolic alterations (involving glycolysis and cholesterol biosynthesis) underlie the induction of trained innate immunity.
Trained Immunity and Neutrophils
Trained immunity, a form of innate immune memory (Netea et al. 2020), can be initiated in the BM through long-lived metabolic and epigenetic alterations in HSPCs upon their activation by microbial stimuli (e.g., the fungal cell wall constituent β-glucan) or vaccines/adjuvants (e.g., the bacillus Calmette–Guérin [BCG] vaccine) (Kaufmann et al. 2018; Mitroulis et al. 2018; Hajishengallis, Li and Chavakis 2021). Epigenetic adaptations (e.g., histone acetylation or methylation) that unfold chromatin and promote transcription factor access to promoter or enhancer regions are critical for enhanced innate immune cell preparedness for future challenges and thus the expression of a trained immune phenotype (Netea et al. 2020). β-Glucan–induced trained immunity triggers IL-1β signaling in HSPCs, accompanied by increased glycolysis and cholesterol biosynthesis, leading to enhanced GM-CSF/CD131 signaling and myeloid-biased differentiation, giving rise to increased numbers of neutrophils (“trained granulopoiesis”) (Mitroulis et al. 2018; Chavakis et al. 2019). In β-glucan–induced trained granulopoiesis, epigenetic adaptations in granulocyte-monocyte progenitors are transmitted to neutrophils and confer a potent antitumor phenotype, which enables the neutrophils to kill tumors in an ROS-dependent manner (Kalafati et al. 2020) (Fig. 3). Trained myeloid cells, including neutrophils, can mediate protection against future infections (Netea et al. 2020), although in the context of chronic inflammation, they may exacerbate tissue damage (Fig. 3). In this regard, experimental periodontitis-associated systemic inflammation results in maladaptive trained myelopoiesis (i.e., the generation of elevated numbers of hyperinflammatory monocytes and neutrophils); these trained cells populate oral and nonoral tissues, thereby not only exacerbating periodontitis but also increasing the severity of inflammatory comorbidities, such as arthritis (Li et al. 2022). Trained myeloid cells are also suspected to contribute to the pathogenesis of atherosclerosis and other inflammatory disorders (Hajishengallis and Chavakis 2021). In the setting of atherosclerosis, the focus has been on the role of trained monocytes/macrophages. However, given that neutrophils are implicated in the initiation of atherosclerosis, in part by secreting chemokines for monocyte recruitment as well as ROS and proteases that activate vascular endothelial cells (Silvestre-Roig et al. 2020), further enhancement of these functions in trained neutrophils could aggravate atherosclerosis.
Figure 3.

Trained granulopoiesis. Trained innate immunity, a form of epigenetic immune/inflammatory memory, can be induced in the bone marrow by certain inflammatory and microbial stimuli, such as the fungally derived β-glucan (Mitroulis et al. 2018; Netea et al. 2020). Trained hematopoietic stem and progenitor cells display myeloid-biased differentiation and give rise to increased numbers of neutrophils with enhanced immune and inflammatory responsiveness (“trained granulopoiesis”) (Mitroulis et al. 2018; Chavakis et al. 2019). Epigenetic adaptations in granulocyte-monocyte progenitors (GMPs) are transmitted to neutrophils, which exhibit enhanced capacity to kill tumors (Kalafati et al. 2020). Trained myeloid cells, including neutrophils, also display enhanced antimicrobial activities and contribute to protection from future infections (Netea et al. 2020). However, the enhanced immune responsiveness of trained neutrophils is also thought to contribute to increased inflammatory tissue damage in the setting of inflammatory diseases, such as atherosclerosis and periodontitis (Hajishengallis and Chavakis 2021). HSC, hematopoietic stem cell; MPP, multipotent progenitor.
Therapeutic Modulation of Neutrophil-Mediated Inflammation
The above-discussed mechanistic insights into the activation and function of neutrophils enable targeted therapeutic interventions that can inhibit the initiation and persistence of tissue inflammation that leads to chronic inflammatory diseases. We focus on different and representative approaches that target the recruitment, activation, and clearance of neutrophils (Fig. 4). The discussion is not exhaustive and the interested reader is referred to a recent dedicated review on this topic (Németh et al. 2020).
Figure 4.
Therapeutic modulation of neutrophil-mediated inflammation. Targeted therapeutic interventions that can potentially restrain the initiation and persistence of neutrophil-mediated tissue inflammation by suppressing the recruitment (A) or the activation (B) of neutrophils as well as their ability to release neutrophil extracellular traps (NETs) (C). In addition, or alternatively, approaches to enhance the apoptosis and/or the efferocytosis of neutrophils by macrophages can promote inflammation resolution (D). See text for details. BLT1, leukotriene B4 receptor 1; CDK9, cyclin-dependent kinase 9; DEL-1, developmental endothelial locus 1; FPR1, formyl-peptide receptor 1; GDF-15, growth differentiation factor 15; ICAM-1, intercellular adhesion molecule 1; LXR, liver X receptor; MPO, myeloperoxidase; PAD-4, protein-arginine deiminase type 4.
Inhibition of Neutrophil Recruitment
As β2 integrins, and particularly LFA-1, are critical for neutrophil firm adhesion to the endothelium and hence recruitment to tissues (Hajishengallis and Chavakis 2013), molecules that can block either the binding interactions of β2 integrins or their inside-out activation (which induces their high-affinity binding state) are potential therapeutics to control neutrophil-mediated inflammation. Certain endogenous regulatory molecules fulfill these criteria. The 52-kDa protein DEL-1 antagonizes LFA-1 binding to intercellular adhesion molecule 1 (ICAM-1) (Fig. 4A) and restrains neutrophil recruitment in vivo (e.g., to the periodontium or the lungs of mice) (Choi et al. 2008; Eskan et al. 2012). Whereas DEL-1 is secreted by distinct resident cell types, its antirecruitment function is associated with secretion from endothelial cells (Kourtzelis et al. 2019). Treatment with recombinant DEL-1 in mice (Eskan et al. 2012; Choi et al. 2015) and nonhuman primates (Shin et al. 2015) protects from inflammatory disorders wherein neutrophil infiltration contributes to their pathogenesis (e.g., experimental periodontitis and multiple sclerosis). The transforming growth factor β (TGFβ)–related growth differentiation factor 15 (GDF-15) and the neutrophil-derived protein annexin A1 can both suppress the activation of LFA-1 (as assessed by flow cytometry using the activation-specific epitope reporter MAb24) induced by different chemokines, including CXCL1 (Fig. 4A). Mechanistically, both GDF-15 and annexin A1 inhibit the activity of the Rap1-GTPase, which mediates chemokine-triggered β2 integrin activation (Kempf et al. 2011; Drechsler et al. 2015). Upon myocardial infarction, GDF-15 is upregulated in cardiomyocytes and attenuates neutrophilic inflammation and myocardial damage in mice (Kempf et al. 2011), whereas annexin A1 (and specifically the fragment Ac2-26) inhibits arterial recruitment of neutrophils and atherogenesis in APOE-deficient mice (Drechsler et al. 2015). Besides chemokines, deposition of complement C5a on the endothelium also activates β2 integrin-dependent neutrophil adhesion and extravasation, at least in the context of joint inflammation (Miyabe et al. 2017). Thus, at least in principle, complement inhibition or antagonistic blockade of the C5a receptor 1 could block neutrophil infiltration of the joints in inflammatory arthritis. Specialized proresolving lipid mediators (SPMs), such as resolvins and lipoxins, also suppress neutrophil infiltration by acting via multiple mechanisms, including downregulation of adhesion molecule expression on both neutrophils and endothelial cells (Serhan 2014).
Blocking Neutrophil Activation or Its Effects
Besides the aforementioned receptors for sensing complement activation and chemokines, multiple other receptors mediate neutrophil activation, including Toll-like receptors (Sabroe et al. 2005), the leukotriene B4 receptor 1 (BLT1) (Saeki and Yokomizo 2017), TREM-1 (Radsak et al. 2004), purinergic receptors (e.g., P2Y2 and P2X), and FPR1 that sense, respectively, extracellular nucleotides (e.g., ATP) and N-formylated mitochondrial peptides, which act as endogenous danger signals (Dorward et al. 2015; Wang and Chen 2018). Pharmacological inhibition of these receptors with specific antagonists was protective in different mouse models of inflammation (e.g., inflammatory arthritis, periodontitis, cardiovascular inflammation, lethal shock-like syndrome), although the effect of these inhibitors on additional leukocyte types (other than neutrophils) cannot be ruled out (Némethet al. 2020). A recent study in mice showed that microbe-induced fibrin deposits in the periodontal tissue are indispensable substrates for Mac-1 integrin-dependent full activation of neutrophils that release tissue-destructive ROS and NETs in periodontitis. Plasmin-induced fibrinolysis prevents excessive neutrophil activation, whereas in humans, genetic polymorphisms in plasminogen, the precursor of plasmin, are associated with severe periodontitis (Silva et al. 2021). This work suggests that targeting the interaction of neutrophil Mac-1 integrin with fibrin may control the destructive inflammatory activity of neutrophils.
In addition to blocking activation receptors (many of which act in synergy), another strategy is to block exocytosis using neutrophil-specific inhibitors (Uriarte et al. 2011; Ley et al. 2018) or to inhibit the activity of key neutrophil-released mediators of inflammatory tissue damage, such as elastase, proteinase 3, myeloperoxidase, and matrix metalloproteinases (Németh et al. 2020) (Fig. 4B). In this regard, neutrophil elastase is particularly involved in neutrophil-driven inflammation in the context of pulmonary disorders. A phase 2 study showed that the elastase inhibitor AZD9668 led to reduction of inflammatory markers in the sputum and improvement of lung function in patients with bronchiectasis (Stockley et al. 2013), although no efficacy was shown in another phase 2 study using the elastase inhibitor BAY 85-8501 (Watz et al. 2019). Treatment of patients with periodontitis using systemically delivered doxycycline (a metalloproteinase inhibitor at sub-antimicrobial doses), as an adjunctive host-modulation therapy, provided modest protection (improved clinical attachment gain and reduction in probing depths) over what could be accomplished by mechanical removal of the dental-plaque biofilm alone (Caton and Ryan 2011). The C3-targeting drug AMY-101, which showed efficacy in reducing gingival inflammation in a recent phase 2a clinical trial, caused a significant decrease in the gingival crevicular concentrations of matrix metalloproteinase 8 and 9, which are indicators of periodontal tissue destruction (Hasturk et al. 2021).
Inhibition of NET formation, in response to activation by pathogens or even endogenous danger signals, such as immune complexes and monosodium urate crystals (Papayannopoulos 2018), may also be relevant for treating neutrophil-mediated tissue injury (Fig. 4C). NET formation requires decondensation of chromatin, a process driven by the nuclear enzyme protein-arginine deiminase type 4 (PAD4) through citrullination of histones. Neutrophil elastase and myeloperoxidase (MPO) are not only components of NETs but are also involved in their formation and are thus potential targets along with PAD4 for the treatment of inflammatory/autoimmune diseases, such as psoriasis, systemic lupus erythematosus, and rheumatoid arthritis (Papayannopoulos 2018). Another potential detrimental effect of NET release is the thrombotic occlusion of the vasculature. In this regard, acute thrombosis is associated with reduced DNAse I activity in the plasma of patients, whereas treatment of mice with PAD4 inhibitors or DNAase I blocks deep vein thrombosis in mice (Papayannopoulos 2018). Pharmacological blockade of complement by a C3-targeted inhibitor (AMY-101) inhibits NET release in the plasma of patients with COVID-19 (Mastellos et al. 2020), and this function could also contribute to the documented ability of the same drug to block periodontal inflammation in nonhuman primates and human patients (Hajishengallis, Hasturk, et al. 2021; Hasturk et al. 2021).
Promotion of Inflammation Resolution
Efferocytosis of apoptotic neutrophils by tissue phagocytes is critical for efficient inflammation termination and tissue repair (Hajishengallis and Chavakis 2019). Indeed, efficient efferocytosis is not simply “waste disposal” preventing secondary neutrophil necrosis and inflammation but also rewires the efferocytic macrophage to adopt a proresolving phenotype, that is, to decrease expression of proinflammatory cytokines (e.g., IL-23 and IL-6) and upregulate the expression of regulatory cytokines (e.g., TGFβ and IL-10) and of SPM (Serhan 2014; Hajishengallis and Chavakis 2019). SPMs—which include lipoxins, derived from arachidonic acid, as well as resolvins and protectins, derived from ω-3 polyunsaturated fatty acids—block further neutrophil recruitment but also promote neutrophil apoptosis and their efferocytosis by macrophages (Serhan 2014). During inflammation resolution, resolvins restore the expression of DEL-1 (which was diminished during inflammation); in turn, DEL-1 further promotes resolution by acting as an efferocytic opsonin (Maekawa et al. 2015; Kourtzelis et al. 2019). Specifically, by means of its C-terminal discoidin-like domains, DEL-1 binds the “eat-me” signal phosphatidylserine on apoptotic neutrophils, which are bridged to an efferocytic receptor (αvβ3 integrin) on macrophages by DEL-1’s Arg-Gly-Asp site at the second EGF-like repeat of the molecule (Kourtzelis et al. 2019). This interaction facilitates apoptotic cell clearance both in vitro and in vivo and induces macrophage reprogramming to a proresolving phenotype via a LXR signaling-dependent manner (Kourtzelis et al. 2019) (Fig. 4D). Synthetic LXR agonists may not only promote resolution of neutrophil-mediated inflammation but can also compensate for the diminished efferocytosis-induced signals in LAD1 deficiency, thereby resolving destructive periodontal inflammation (Kajikawa et al. 2020). Moreover, given that LXR signaling upregulates ABCA1 and ABCG1 and cholesterol efflux, LXR agonists can also be used to counteract the detrimental effects of excessive cholesterol accumulation in HSPCs and macrophages, thus restraining the overproduction of neutrophils and their mobilization from the BM in patients with cardiometabolic disorders (obesity, insulin resistance, and atherosclerotic heart disease). Both resolvins and DEL-1 have also been shown to promote Treg cell responses (Chiurchiu et al. 2016; Li et al. 2020) and regeneration of soft tissue and bone during the resolution of periodontitis (Van Dyke et al. 2015; Yuh et al. 2020). In the presence of adequate efferocytosis potential, the promotion of neutrophil apoptosis can contribute to inflammation resolution. In this regard, inhibitors of cyclin-dependent kinase 9 promote neutrophil apoptosis (Fig. 4D) and enhance neutrophil-mediated inflammation in different mouse models, including inflammatory arthritis and bleomycin-induced lung injury (Rossi et al. 2006). Similarly, annexin A1, which is released from neutrophils undergoing cell death, promotes neutrophil apoptosis (Solito et al. 2003) and, along with peptide derivatives thereof, also enhances the efferocytosis of dead neutrophils (Scannell et al. 2007).
Conclusion and Perspective
Once seen as merely antimicrobial effector cells armed with cytotoxic substances, neutrophils are now appreciated as a key component in tissue inflammation associated with a variety of chronic disorders. Recent research presented in this review has attempted to attain in-depth understanding of the local and systemic factors that regulate the production, trafficking, memory status, and function of neutrophils. Such knowledge can facilitate, at least in principle, the pharmacologic manipulation of neutrophils in a targeted manner that would promote host defense and mitigate their destructive effects. Although not specifically discussed in this review, neutrophils exhibit significant phenotypic and functional heterogeneity. Such diverse phenotypes are thought unlikely to represent bona fide subsets generated from progenitors exposed to differential stimuli (Fine et al. 2020; Rubio-Ponce et al. 2020). Rather, mature neutrophils may undergo substantial phenotypic changes either as a consequence of aging (Rubio-Ponce et al. 2020) or due to their exposure to different local microenvironments (e.g., tumor-associated neutrophils with antitumor or protumorigenic phenotype), depending on their exposure to type 1 interferons or TGFβ, respectively (Kalafati et al. 2020; Rubio-Ponce et al. 2020). Besides local factors, the activity of neutrophils is also profoundly affected by systemic factors, such as the metabolic status of the host. As discussed above, both hypercholesterolemia and hyperglycemia are associated with increased production of neutrophils that may also bear a hyperinflammatory phenotype, thereby linking metabolic dysregulation to chronic inflammation (Nagareddy et al. 2013). The numbers and function of neutrophils can also be manipulated by either promoting or inhibiting trained granulopoiesis, the latter approach being a promising one for the treatment of inflammatory comorbidities, such as periodontitis and cardiovascular disease (Hajishengallis and Chavakis 2021). Targeting neutrophil infiltration and/or overactivation may not be pertinent to all forms of periodontal disease (e.g., not relevant for LAD1 periodontitis where the disease results from neutrophil paucity). However, it should be a promising approach for the majority of patients (adult-type chronic periodontitis), as long as safety can be established. In principle, the restraining per se of neutrophil infiltration and function should not entail a significant risk in an inflammatory disease; however, each drug should be evaluated carefully for potential off-target or other unanticipated adverse effects. Future basic research is expected to lead to new insights into the basic biology of neutrophils and their role in preclinical models of disease. Of higher importance would be the translation of these findings into clinical interventions and ultimately practice.
Author Contributions
G. Hajishengallis, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript; T. Chavakis, contributed to conception, design, and data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank all colleagues whose work made this review possible and regret that several important studies could only be cited indirectly through comprehensive reviews, owing to reference number limitations. The figures were prepared using Biorender.com.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G. Hajishengallis is the inventor of a patent that describes the use of complement inhibitors for therapeutic purposes in periodontitis (“Methods of Treating or Preventing Periodontitis and Diseases Associated with Periodontitis,” patent no. 10,668,135). Other than that, the authors declared no other potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors’ research is supported by grants from the US National Institutes of Health (DE029436, DE031206 to G. Hajishengallis; DE026152 and DE028561 to G. Hajishengallis and T. Chavakis) and the German Research Foundation (CRC1181 to T. Chavakis).
ORCID iD: G. Hajishengallis  https://orcid.org/0000-0001-7392-8852
https://orcid.org/0000-0001-7392-8852
References
- Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. 2021. The neutrophil. Immunity. 54(7):1377–1391. [DOI] [PubMed] [Google Scholar]
- Caton J, Ryan ME. 2011. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol Res. 63(2):114–120. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Mitroulis I, Hajishengallis G. 2019. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol. 20(7):802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. 2016. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 8(353):353ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, et al. 2008. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 322(5904):1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Lim JH, Neuwirth A, Economopoulou M, Chatzigeorgiou A, Chung KJ, Bittner S, Lee SH, Langer H, Samus M, et al. 2015. Developmental endothelial locus-1 is a homeostatic factor in the central nervous system limiting neuroinflammation and demyelination. Mol Psychiatry. 20(7):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, Rossi AG. 2015. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol. 185(5):1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, et al. 2015. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 116(5):827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, et al. 2012. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 13(5):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine N, Tasevski N, McCulloch CA, Tenenbaum HC, Glogauer M. 2020. The neutrophil: constant defender and first responder. Front Immunol. 11:571085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Chavakis T. 2013. Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 34(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Chavakis T. 2019. DEL-1-regulated immune plasticity and inflammatory disorders. Trends Mol Med. 25(5):444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Chavakis T. 2021. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 21(7):426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. 2015. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol. 98(4):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Hasturk H, Lambris JD, Apatzidou DA, Belibasakis GN, Bostanci N, Corby PM, Cutler CW, D’Aiuto F, Hajishengallis E, et al. 2021. C3-targeted therapy in periodontal disease: moving closer to the clinic. Trends Immunol. 42(10):856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Li X, Chavakis T. 2021. Immunometabolic control of hematopoiesis. Mol Aspects Med. 77:100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Hajishengallis G; Forsyth Institute Center for Clinical and Translational Research staff; Lambris JD, Mastellos DC, Yancopoulou D. 2021. Phase IIa clinical trial of complement C3 inhibitor AMY-101 in adults with periodontal inflammation. J Clin Invest. 131(23):e152973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa T, Wang B, Li X, Wang H, Chavakis T, Moutsopoulos NM, Hajishengallis G. 2020. Frontline science: activation of metabolic nuclear receptors restores periodontal tissue homeostasis in mice with leukocyte adhesion deficiency-1. J Leukoc Biol. 108(5):1501–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, Hagag E, Sinha A, Has C, Dietz S, et al. 2020. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. 183(3):771–785.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonca LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, et al. 2018. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 172(1–2):176–190.e19. [DOI] [PubMed] [Google Scholar]
- Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, et al. 2011. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 17(5):581–588. [DOI] [PubMed] [Google Scholar]
- Köhler A, De Filippo K, Hasenberg M, van den Brandt C, Nye E, Hosking MP, Lane TE, Männ L, Ransohoff RM, Hauser AE, et al. 2011. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 117(16):4349–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzelis I, Li X, Mitroulis I, Grosser D, Kajikawa T, Wang B, Grzybek M, von Renesse J, Czogalla A, Troullinaki M, et al. 2019. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat Immunol. 20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, Catz SD. 2018. Neutrophils: new insights and open questions. Sci Immunol. 3(30):eaat4579. [DOI] [PubMed] [Google Scholar]
- Li X, Colamatteo A, Kalafati L, Kajikawa T, Wang H, Lim J-H, Bdeir K, Chung K-J, Yu X, Fusco C, et al. 2020. The DEL-1/β3 integrin axis promotes regulatory T cell responses during inflammation resolution. J Clin Invest. 130 (12):6261–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang H, Yu X, Saha G, Kalafati L, Ioannidis C, Mitroulis I, Netea MG, Chavakis T, Hajishengallis G. 2022. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell. 185(10):1709–1727.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, Hajishengallis G. 2015. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun. 6:8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz MG, Boettcher S. 2014. Emergency granulopoiesis. Nat Rev Immunol. 14(5):302–314. [DOI] [PubMed] [Google Scholar]
- Mastellos DC, Pires da, Silva BGP, Fonseca BAL, Fonseca NP, Auxiliadora-Martins M, Mastaglio S, Ruggeri A, Sironi M, Radermacher P, Chrysanthopoulou A, et al. 2020. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin Immunol. 220:108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, et al. 2018. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 172(1–2):147–161.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe Y, Miyabe C, Murooka TT, Kim EY, Newton GA, Kim ND, Haribabu B, Luscinskas FW, Mempel TR, Luster AD. 2017. Complement C5a receptor is the key initiator of neutrophil adhesion igniting immune complex–induced arthritis. Sci Immunol. 2(7):eaaj2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, et al. 2014. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17–driven inflammatory bone loss. Sci Transl Med. 6(229):229ra240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. 2013. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 17(5):695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh T, Sperandio M, Mócsai A. 2020. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 19(4):253–275. [DOI] [PubMed] [Google Scholar]
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, et al. 2020. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 20(6):375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K, Ohira T, Uchida Y, Ayilavarapu S, Batista EL, Jr, Yagi M, Iwata T, Liu H, Hasturk H, Kantarci A, et al. 2008. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol. 84(1):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. 2018. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 18(2):134–147. [DOI] [PubMed] [Google Scholar]
- Radsak MP, Salih HR, Rammensee HG, Schild H. 2004. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol. 172(8):4956–4963. [DOI] [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, et al. 2006. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 12(9):1056–1064. [DOI] [PubMed] [Google Scholar]
- Rubio-Ponce A, Hidalgo A, Ballesteros I. 2020. How to bridle a neutrophil. Curr Opin Immunol. 68:41–47. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Dower SK, Whyte MKB. 2005. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis. 41(Suppl 7):S421–S426. [DOI] [PubMed] [Google Scholar]
- Saeki K, Yokomizo T. 2017. Identification, signaling, and functions of LTB4 receptors. Semin Immunol. 33:30–36. [DOI] [PubMed] [Google Scholar]
- Sarrazy V, Viaud M, Westerterp M, Ivanov S, Giorgetti-Peraldi S, Guinamard R, Gautier EL, Thorp EB, De Vivo DC, Yvan-Charvet L. 2016. Disruption of Glut1 in hematopoietic stem cells prevents myelopoiesis and enhanced glucose flux in atheromatous plaques of ApoE–/– mice. Circ Res. 118(7):1062–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C, Maderna P. 2007. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 178(7):4595–4605. [DOI] [PubMed] [Google Scholar]
- Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Maekawa T, Abe T, Hajishengallis E, Hosur K, Pyaram K, Mitroulis I, Chavakis T, Hajishengallis G. 2015. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci Transl Med. 7(307):307ra155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LM, Doyle AD, Greenwell-Wild T, Dutzan N, Tran CL, Abusleme L, Juang LJ, Leung J, Chun EM, Lum AG, et al. 2021. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science. 374(6575):eabl5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. 2020. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 17(6):327–340. [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Steffens S, Hidalgo A, Weber C. 2017. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 17(4):248–261. [DOI] [PubMed] [Google Scholar]
- Solito E, Kamal A, Russo-Marie F, Buckingham JC, Marullo S, Perretti M. 2003. A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J. 17(11):1544–1546. [DOI] [PubMed] [Google Scholar]
- Stockley R, De Soyza A, Gunawardena K, Perrett J, Forsman-Semb K, Entwistle N, Snell N. 2013. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir Med. 107(4):524–533. [DOI] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L. 2015. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 15(2):104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriarte SM, Rane MJ, Luerman GC, Barati MT, Ward RA, Nauseef WM, McLeish KR. 2011. Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J Immunol. 187(1):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, Serhan CN. 2015. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. 94(1):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen D. 2018. Purinergic regulation of neutrophil function. Front Immunol. 9:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watz H, Nagelschmitz J, Kirsten A, Pedersen F, van der Mey D, Schwers S, Bandel T-J, Rabe KF. 2019. Safety and efficacy of the human neutrophil elastase inhibitor BAY 85-8501 for the treatment of non-cystic fibrosis bronchiectasis: a randomized controlled trial. Pulm Pharm Ther. 56:86–93. [DOI] [PubMed] [Google Scholar]
- Westerterp M, Gourion-Arsiquaud S, Murphy Andrew J, Shih A, Cremers S, Levine Ross L, Tall Alan R, Yvan-Charvet L. 2012. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 11(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, Webb S, Martin D, Hajishengallis G, Divaris K, et al. 2021. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 184(15):4090–4104.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. 2015. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 21(7):815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh DY, Maekawa T, Li X, Kajikawa T, Bdeir K, Chavakis T, Hajishengallis G. 2020. The secreted protein DEL-1 activates a β3 integrin-FAK-ERK1/2-RUNX2 pathway and promotes osteogenic differentiation and bone regeneration. J Biol Chem. 295(21):7261–7273. [DOI] [PMC free article] [PubMed] [Google Scholar]