Abstract
Background:
Enhanced recovery after surgery (ERAS) protocols in orthopaedic surgery have garnered significant focus due to their ability to control pain adequately in the immediate postoperative window, allowing for earlier mobilization, shorter hospital stays, and fewer complications. Virginia Commonwealth University created a multimodal pain management approach in which patients receive a preoperative femoral nerve block followed by periarticular intraoperative local injection anesthesia consisting of bupivacaine, ketamine, and ketorolac.
Hypothesis:
We hypothesized that implementation of the ERAS protocol will decrease postoperative pain scores, decrease recovery time in the postanesthesia care unit (PACU), and decrease opioid use in anterior cruciate ligament (ACL) reconstruction.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Two patient cohorts were involved: before ERAS implementation (pre-ERAS) and after (post-ERAS). Patients with ACL reconstruction only and patients with ACL reconstruction with meniscal repair were analyzed separately. Post-ERAS patients received an intraoperative periarticular injection of bupivacaine, ketamine, and ketorolac and a postoperative multimodal pain regimen. Outcomes included time spent in the PACU, short-term and long-term opioid consumption, and pain score at discharge from the PACU.
Results:
Compared with pre-ERAS patients, post-ERAS patients had decreased pain (2.1 vs 0.84 out of 10, respectively), spent less time in the PACU (79.4 vs 62.8 minutes, respectively), and had less opioid consumption in the immediate postoperative period (4.55 vs 2.26 total morphine milligram equivalents [MMEs], respectively) (P < .001 for all). After ERAS implementation, long-term MME use decreased from 410 to 321 between 0 and 2 weeks postoperatively, 92.6 to 1.69 between 2 and 6 weeks, and 494.5 to 323 between 0 and 6 weeks (P < .001 for all). All domains showed significant improvements for both the ACL and the ACL plus meniscal repair cohorts, with the exception of pain at discharge in the ACL plus meniscal repair group.
Conclusion:
The study findings suggest that an enhanced recovery pathways protocol that includes a standardized intraoperative periarticular bupivacaine, ketamine, and ketorolac injection improves pain scores in the immediate postoperative window, decreases opioid consumption, and reduces recovery time in the PACU for patients undergoing ACL reconstruction.
Keywords: ACL, anesthesia, knee, pain management
Historically, opioids have been a mainstay of perioperative pain management regimens. 2 In orthopaedic surgery specifically, they are considered the most widely used pain control mechanism postoperatively. 11 Overprescription of opioids in the orthopaedic surgery setting has been linked to the opioid epidemic, leading to the search for other effective forms of pain management postoperatively, 11 especially in the ambulatory setting.
For anterior cruciate ligament (ACL) reconstruction, many localized pain management options have been employed to decrease the need for oral opioids. The most popular preoperative anesthetic strategies include a femoral nerve block (FNB), adductor canal block (ACB), and local injection anesthesia (LIA). 1 Different studies have shown mixed results. A 2017 study by Kirkham et al 8 showed that periarticular anesthetic (lidocaine, ketamine, bupivacaine) use was effective but to a lesser extent than regional nerve blocks. However, more recent research has shown strong evidence for pain relief with LIA. 1 Similarly, a 3-part systematic review also showed improved outcomes with periarticular anesthetics, suggesting that nerve blocks used in conjunction with periarticular LIA may have limited utility. 12,13,14 Studies have shown conflicting evidence regarding the effectiveness of ACB. One prospective randomized control showed ACB to be equally effective as FNB with improved quadriceps strength long term. 10 In another study, ACB was shown to have almost no effect on postoperative pain or opioid consumption compared with placebo. 12 Further research has shown encouraging results for use of LIA for ACL reconstruction pain management. 14 In the absence of a consensus in the literature, further investigation into the optimal perioperative anesthetic regimen for patients undergoing ACL reconstruction is warranted.
Through enhanced recovery after surgery (ERAS) protocol implementation, beginning in May 2021, the Sports Medicine and Regional Anesthesia Departments at Virginia Commonwealth University created a standardized, multimodal pain management approach in which patients received a preoperative nerve block and a novel intraoperative periarticular injection of bupivacaine, ketamine, and ketorolac (BKK). The goal of this study was to determine the efficacy of this new ERAS protocol. It was hypothesized that this novel intraoperative periarticular BKK injection, when used in conjunction with a preoperative nerve block, would improve patient-reported pain scores in the immediate postoperative window, decrease opioid consumption in the postanesthesia care unit (PACU), reduce recovery time in the PACU, and reduce the amount of opioid prescribed following surgery in patients undergoing elective primary ACL reconstruction.
Methods
Study Design
This was a retrospective cohort study completed by reviewing patient charts through the electronic medical record system. Institutional review board approval was waived because the study only collected and analyzed identifiable health information when the research use was regulated by the Health Insurance Portability and Accountability Act. The surgeries were performed by 1 of 4 sports medicine fellowship–trained orthopaedic surgeons at Virginia Commonwealth University. Surgeries performed before ERAS protocol initiation (2020-2021; pre-ERAS) were compared with those after implementation (post-ERAS).
ERAS Protocol
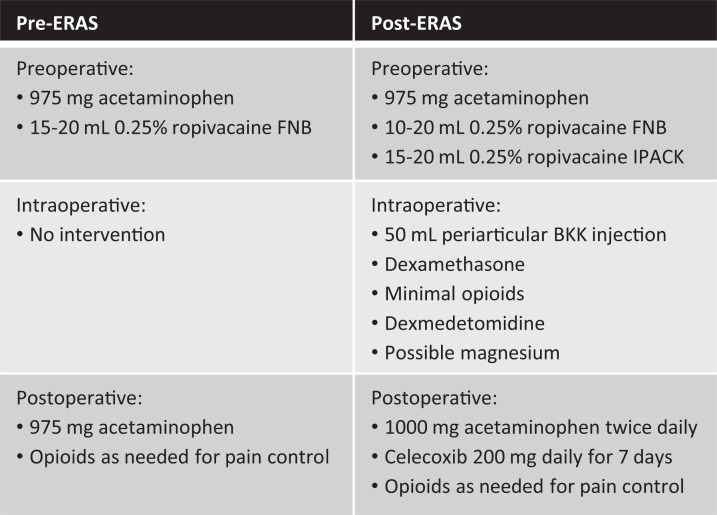
Before the ERAS protocol, patients preoperatively received 975 mg acetaminophen in addition to a 15- to 20-mL 0.25% ropivacaine FNB. Postoperatively, acetaminophen and opioids were used for pain management. The ERAS protocol included the addition of preoperative, intraoperative, and postoperative measures.
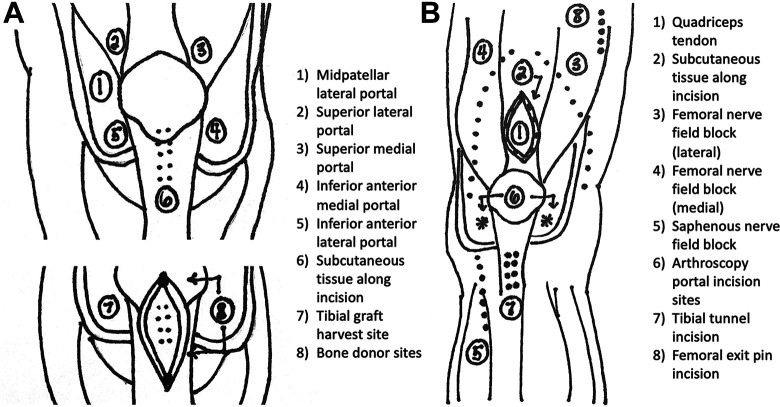
Preoperatively, patients were given a multimodal pain relief regimen including acetaminophen (975 mg), celecoxib (200 mg), and a scopolamine patch in addition to a 10- to 20-mL 0.25% ropivacaine FNB and 15- to 20-mL 0.25% ropivacaine infiltration between popliteal artery and capsule of the knee (IPACK). Intraoperatively, patients were given varying doses of dexamethasone, minimal opioids and dexmedetomidine, and possible magnesium. Dexmedetomidine activates alpha-2 adrenoceptors, which helps decrease sympathetic pain response to surgery. Magnesium has been shown in other clinical studies to aid in treatment of neuropathic pain. A 50-mL periarticular BKK injection was also introduced intraoperatively during the time of closing (Figure 2). This injection contained 150 mg bupivacaine (3 mg/mL), 60 mg ketorolac (1.2 mg/mL), and 60 mg ketamine (1.2 mg/mL). Possible complications of this BKK injection were a very low risk of local site reaction or possible infection. Postoperatively, multimodal pain management included acetaminophen (1000 mg) twice a day and celecoxib (200 mg) daily for 7 days, with opioids prescribed only for breakthrough pain. Before and after ERAS implementation, patients were initially prescribed 42 pills of 5 mg oxycodone with additional doses if requested. Patients were instructed to take the oxycodone every 6 hours as needed for pain. Differences in pain intervention between pre- and post-ERAS protocol can be seen in Figure 1.
Figure 2.
Bupivacaine, ketamine, and ketorolac injection sites for (A) bone-tendon-bone graft and (B) quadriceps and hamstring autografts.
Figure 1.
Chart comparing pre-ERAS and post-ERAS interventions. BKK, bupivacaine, ketamine, and ketorolac; ERAS, enhanced recovery after surgery; FNB, femoral nerve block; IPACK, infiltration between popliteal artery and capsule of the knee.
Study Participants
Individuals aged between 18 and 80 years who underwent a primary ACL reconstruction with or without meniscal surgery (including repair or meniscectomy) from January 2020 to December 2021 were eligible for inclusion in the study. ACL reconstructions with and without meniscal repair were analyzed separately to reduce risk of confounding factors related to the procedure conducted. Patients with other concomitant injuries such as other ligamentous injury or fractures were excluded to ensure pre- and post-ERAS patients were adequately paired. Patients with a history of chronic pain, fibromyalgia, or history of narcotic use were excluded; polytrauma patients were also excluded because other injuries sustained could affect pain scores. Our aim was to exclude those patients who were not naïve to opioid pain medication or who had higher levels of pain at baseline, as these may add bias to the outcomes of interest.
Data Extraction
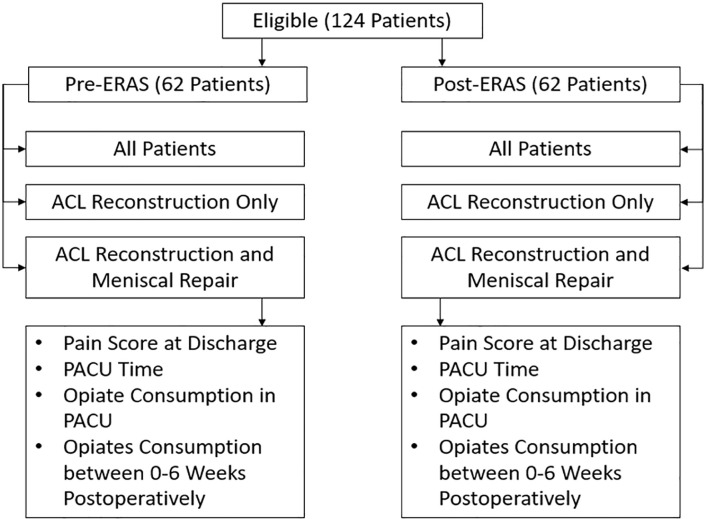
There were 124 patients analyzed for this study. One author (P.P.) randomly selected 62 patients whose procedures were completed before ERAS implementation, and another author (M.T.) randomly selected 62 patients who underwent ACL reconstruction after ERAS protocol initiation (Figure 3). ACL reconstruction without meniscal repair was performed in 30 pre-ERAS and 29 post-ERAS patients, and ACL reconstruction with meniscal repair was performed in 32 pre-ERAS and 33 post-ERAS patients. This breakdown can be seen in Figure 3.
Figure 3.
Patient breakdown. ACL, anterior cruciate ligament; ERAS, enhanced recovery after surgery; PACU, postanesthesia care unit.
The pre-ERAS and post-ERAS patients were then matched for type of graft used and surgeon who performed the procedure to reduce the risk of significant findings to be secondary to surgical technique. In the pre-ERAS cohort, 27 patients received a bone-tendon-bone (BTB) autograft, 7 received a hamstring autograft, and 28 received an allograft. Surgeon 1 completed 28 procedures, surgeon 2 completed 24 (A.V.), and surgeons 3 and 4 each completed 5 procedures (R.O.). Comparably, in the post-ERAS cohort, 30 patients received a BTB autograft, 6 received a hamstring autograft, and 26 received an allograft. Surgeon 1 completed 24 procedures, surgeon 2 completed 26 (A.V.), surgeon 3 completed 8 procedures (R.O.), and surgeon 4 completed 4 procedures.
Patient data were extracted manually from the electronic medical record by members of the study team and recorded in an Excel (Microsoft Corp) spreadsheet. Information collected included date of surgery, surgery performed, surgeon, nerve blocks used, postoperative pain at discharge (on a scale from 0 [best] to 10 [worst]), PACU time, dosage of opioids consumed in PACU, and opioids prescribed between 0 and 6 weeks after discharge. Postoperative pain scores were recorded as pain reported at discharge. PACU time was calculated from surgery end time to PACU discharge.
Statistical Analysis
The 4 outcome variables of interest were PACU time, pain at discharge, opioids consumed in the PACU, and opioids prescribed outpatient after surgery between pre- and post-ERAS cohorts (Figure 3). Patients were analyzed based on 3 cohorts: all patients undergoing ACL reconstruction, primary ACL reconstruction only (ACL only), and ACL reconstruction with concomitant meniscal repair (ACL plus meniscal repair). Independent 2-sided t tests were conducted for each variable within PACU time, pain at discharge, and immediate opioid consumption. A separate set of independent 2-sided t tests were completed comparing total morphine milligram equivalents (MMEs) between pre-ERAS and post-ERAS patients between 0 and 2 weeks, 2 and 6 weeks, and 0 and 6 weeks postoperatively. Differences between BTB and hamstring grafts were also evaluated for possible subgroup interactions. Missing data were not used in the statistical calculations. Alpha was set to 0.05. Microsoft Excel was used for statistical analysis.
Results
Compared with the pre-ERAS group, the mean pain rating at discharge was significantly lower in the post-ERAS group (2.1 ± 2.45 [range, 0-8] vs 0.84 ± 2.19 [range, 0-8], respectively; P < .001). Similar results were seen in the ACL-only cohort (pre- vs post-ERAS: 1.97 ± 2.65 [range 0-8] vs 0.14 ± 0.74 [range 0-4]; P < .001). In the ACL plus meniscal repair group, the post-ERAS pain rating was also lower (pre- vs post-ERAS: 2.31 ± 2.27 [range 0-8] vs 1.45 ± 2.8 [range 0-8]); however, the difference was not statistically significant (P = .08). All these findings are illustrated in Figure 4.
Figure 4.
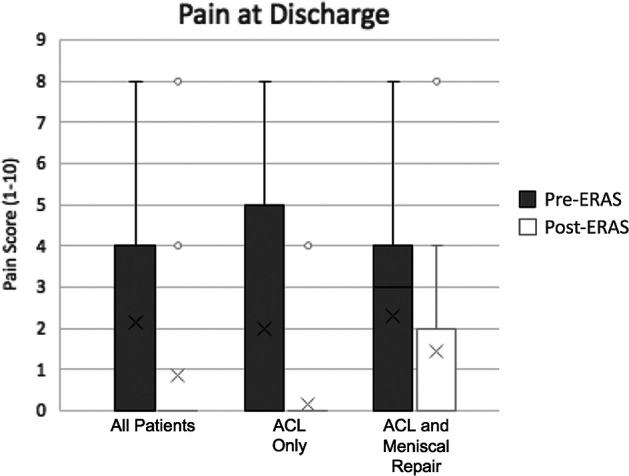
Pain score at discharge. The X represents the mean. The horizontal lines of the bars represent the 1st quartile, median, and 3rd quartile. The error bars represent the 4th quartile. The circles denote outliers. ACL, anterior cruciate ligament; ERAS, enhanced recovery after surgery.
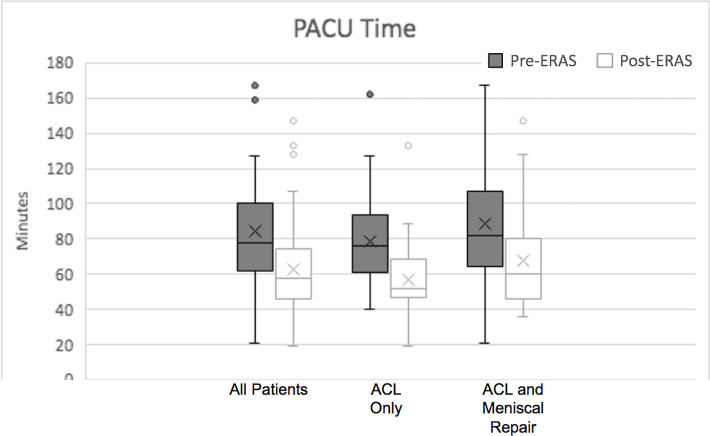
As seen in Figure 5, the mean time spent recovering in the PACU before discharge was significantly less for the post-ERAS group (pre- vs post-ERAS: 79.4 ± 32.7 vs 62.8 ± 24.9 minutes; P < .001). Similar results were seen in the ACL-only cohort (74.1 ± 29.2 vs 57 ± 22.1 minutes; P = .001) and the ACL plus meniscal repair cohort (84 ± 35.3 vs 67.8 ± 26.1 minutes; P = .001).
Figure 5.
PACU recovery times. The X represents the mean. The horizontal lines of each bar represent the 1st quartile, median, and 3rd quartile. The error bars represent the 4th quartile. The circles denote outliers. ACL, anterior cruciate ligament; ERAS, enhanced recovery after surgery; PACU, postanesthesia care unit.
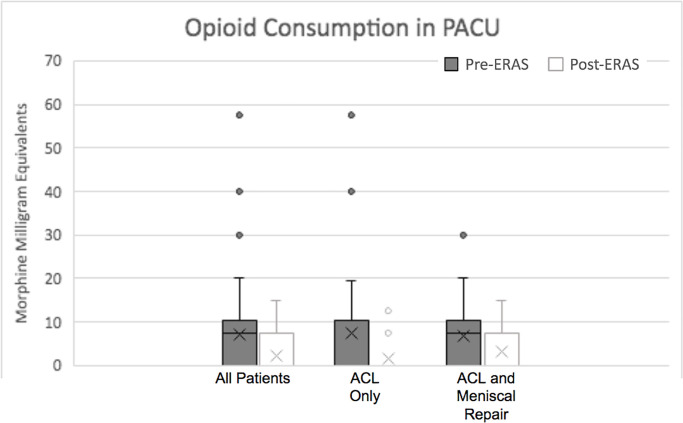
The mean dosage of oxycodone required in the PACU was also decreased significantly after ERAS implementation (pre- vs post-ERAS: 4.55 ± 4.65 vs 2.26 ± 3.9 total MMEs; P < .001). This was also the case in the ACL-only cohort (3.92 ± 4.8 vs 1.52 ± 3.43 total MMEs; P < .001) as well as the ACL plus meniscal repair cohort (5.16 ± 4.49 vs 3.3 ± 4.45 total MMEs; P = .02) (Figure 6).
Figure 6.
In-hospital opioid consumption for ACL patients. The X represents the mean. The horizontal lines of the bars represent the 1st quartile, median, and 3rd quartile. The error bars represent the 4th quartile. The circles denote outliers. ACL, anterior cruciate ligament; ERAS, enhanced recovery after surgery; PACU, postanesthesia care unit.
As seen in Figure 7, the amount of prescribed opioid medications after discharge was significantly less in the post-ERAS group in all 3 time windows evaluated (0-2, 2-6, and 0-6 weeks). In the 0- to 2-week window, post-ERAS patients were prescribed a mean of 321.3 ± 131.7 total MMEs compared with 410 ± 157 total MMEs for pre-ERAS patients (P < .001), a 21.6% decrease in opioid prescription. During the 2- to 6-week period, post-ERAS patients were prescribed a mean of 1.69 ± 13.3 total MMEs, and pre-ERAS patients were prescribed a mean of 92.6 ± 223.3 total MMEs (P < .001), a 98.2% decrease in opioid prescription post-ERAS. In the full 6 weeks after surgery, pre-ERAS patients were prescribed a mean of 494.5 ± 307.3 total MMEs and post-ERAS patients were prescribed 323 ± 1364 total MMEs (P < .001).
Figure 7.
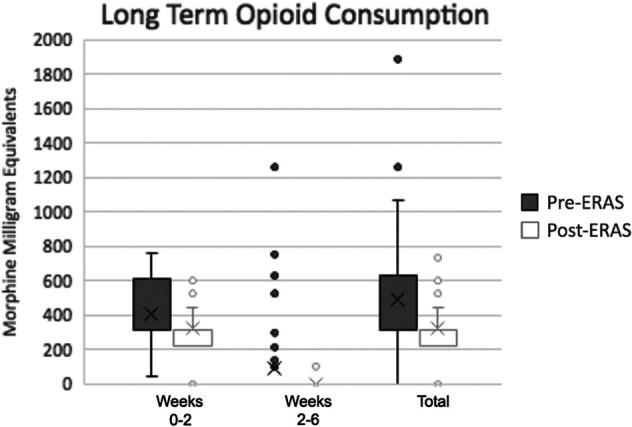
Outside hospital opioid consumption for all patients. The X represents the mean. The horizontal lines represent the 1st quartile, median, and 3rd quartile. The error bars represent the 4th quartile. The circles denote outliers. ERAS, enhanced recovery after surgery.
Patients were also evaluated based on type of autograft used during the procedure. Mean pain rating at discharge was less for hamstring recipients when compared with BTB autograft recipients with 0.95 ± 1.94 (range 0-8) compared with 2 ± 2.65 (range 0-8) (P = .001). Mean time spent recovering in the PACU was 70.7 ± 29.86 minutes for BTB patients and 78.5 ± 38.4 minutes for hamstring patients (P = .07). When comparing opioid consumption in the PACU, hamstring patients consumed a mean of 4.04 ± 3.89 total MMEs, which was significantly more than the BTB autograft patients that consumed 2.58 ± 3.84 total MMEs (P = .01).
There were no complications as a result of this intervention. After discharge, no patient returned with a postoperative infection, increased numbness, or uncontrolled pain.
Discussion
The purpose of this study was to determine whether the implementation of the ERAS protocol improved patient-reported pain scores in the immediate postoperative window, decreased opioid consumption in the PACU, and reduced recovery time in the PACU in all ACL patients and ACL-only procedures. The results of this study indicate that these interventions are effective. All categories except pain at discharge were also improved for the subgroup of patients who underwent ACL reconstruction plus meniscal repair. There was also a drastic decrease in the number of opioids prescribed after discharge at all 3 time points. In general, ERAS protocol implementation has improved ambulatory orthopaedic surgery outcomes at our institution with improved postoperative pain, earlier ambulation after surgery, and improved long-term outcomes.
Previously, pain management in ACL reconstruction has relied largely on FNB, ACB, and LIA. Recent research has indicated that periarticular anesthetics may not only be effective in conjunction with FNB but may also provide sufficient analgesia when used alone. 13 However, there are minimal data regarding the effectiveness of the novel intraoperative periarticular BKK injection in patients undergoing outpatient knee procedures. When breaking down the components of a BKK injection, bupivacaine works by increasing the threshold to elicit an action potential reducing rate of nerve excitation; ketorolac inhibits the production of prostaglandins, which are involved in the primary pain response; and ketamine inhibits sensation to pain through N-methyl-d-aspartate receptor antagonism. These 3 components work together to reduce local pain in the short-term postoperative period. As such, an ERAS protocol was implemented at our institution to create a standardized, multimodal pain management approach.
The most pronounced findings of this paper can be seen when comparing long-term opioid prescription between pre- and post-ERAS participants. After the initiation of ERAS, from 0 to 2 weeks, patients were prescribed 89 total MMEs less, on average, than historically. In the 2- to 6-week time period, post-ERAS patients were prescribed, on average, 91 total MMEs fewer opioids than pre-ERAS patients. Patients were prescribed a mean of 171.5 total MMEs less after ERAS protocol implementation over the full 6 weeks after surgery. Overprescribing of opioids in orthopaedic surgery has continued to contribute to the growing national opioid epidemic, 8 leading to a push toward forms of pain management that can reduce the need for these agents. This is a drastic reduction in prescribed opioids, dramatically reducing the risk of long-term opioid dependence.
Although modest, there was also a significant decrease among all patient opioid consumption in the PACU after implementation of ERAS, with patients consuming on average 2.29 total MMEs less in the immediate postoperative period. Patients undergoing only ACL reconstruction had a decrease of 2.4 total MMEs. Patients who completed ACL reconstruction with meniscal repair also showed a statistically significant difference in opioid consumption with a reduction of 1.86 total MMEs in the hospital. Given the risk of opioid addiction, minimizing postoperative opioid use for routine outpatient procedures is imperative. 2 One orthopaedic retrospective study showed a dose response relationship between immediate opioid use and the risk of continued long-term use. 5 This indicates that even small reductions reduce future risk for dependency. The combined use of multimodal pain management, FNB, and BKK injection reduces this risk of long-term opioid dependence.
We also found that this ERAS protocol implementation reduced pain scores at discharge from the PACU by a mean of 1.3 on a 10-point visual analog pain scale. This reduction carried over into primary ACL reconstructions with a reduction of 2 points. Although not statistically significant, the ACL reconstruction with meniscal repair cohort also had a reduction of 0.9 points. Notably, patient pain scores at discharge were quite low before protocol implementation; however, a reduction may still be clinically significant given that we demonstrated a change in opioid prescribing habits during the same time frame. Guidelines set forth by the American Pain Society indicate that increased postoperative pain is associated with increased opioid use, increased risk of opioid abuse, and higher likelihood of unexpected hospital admissions. 3 Patients with more well-controlled pain are also better able to engage in physical therapy, leading to improved outcomes.
We also demonstrate a significant reduction in PACU time by a mean of 17 minutes among all patients (a 21% decrease), with a reduction of 17 minutes for primary ACL reconstruction (a 23% decrease) and 16 minutes in ACL reconstruction with meniscal repair (a 19% decrease). There are many important clinical implications with regard to reduced PACU times. One literature review showed that reduced PACU time leads to better use of resources, improved flow of the surgical schedule, and important cost savings. 9 Reduced PACU burden was associated with improved satisfaction not only among employees, but also among patients and their families. 9
Although different than the formulation used in this study, in recent years, other LIA has been incorporated into total knee arthroplasty procedures to improve pain outcomes and increase early postoperative mobilizations. 4 This intervention has been extremely effective in total knee arthroplasty, and the findings of this study indicate that the use of LIA is also effective in ACL reconstruction. Previous studies have indicated LIA is effective in reducing postoperative pain and PACU opioid consumption. 4 Our intraoperative injection of BKK is likely the major contributing factor to the improvements in these 2 variables. However, the introduction of the IPACK is also a likely contributing factor. Decreasing the pain response in the immediate postoperative period helps decrease the secondary inflammatory response that leads to increased long-term opioid need. 6 Other aspects of multimodal pain management including scheduled acetaminophen and celecoxib likely also contribute to this continued decrease in pain response and decreased long-term opioid prescription. 6
Peripheral nerve block anesthesia, such as IPACK and FNB, have been shown to be effective in treatment of acute pain. 7 However, there are also associated risks, such as vascular injury, prolonged numbness, and possible systematic toxicity. 7 Periarticular injection anesthesia is much less likely to result in these possible negative outcomes. Although peripheral nerve block anesthesia likely affected the outcomes in this study, future research needs to be completed to determine whether patient pain can be controlled adequately without the use of the FNB or IPACK. If this is possible, these negative consequences could be avoided.
Strengths and Limitations
The strengths of this study include the use of data from 4 different sports medicine fellowship–trained orthopaedic surgeons. This creates a diverse patient population and increases generalizability of results, making them more likely to reflect the findings of other patients undergoing ACL reconstruction. We were also careful to ensure that data were extracted in the same manner for each patient to ensure intrastudy reliability. Also, different individuals extracted information from the ERAS patients and the historical patients. This helped reduce bias with data collection and interpretation. We also used 3 separate variables to measure patient outcomes, and each was shown to have significant improvement when comparing the historical data with the ERAS protocol.
Some main limitations of this study are those disadvantages inherent to retrospective study design, such as being prone to recall or misclassification bias, controls possibly not being representative of the general population, and difficulty identifying confounding variables. Another shortcoming of this study is the small sample size. Only 84 individuals were eligible for this study. This could affect the generalizability of our results; however, we aim to present promising preliminary evidence from our novel intervention, providing a framework for future studies. This study was also completed at only 1 institution, reducing the generalizability to other institutions or surgeons. Other limitations include the lack of follow-up pain scores after discharge and lack of quadriceps tendon graft use. It is difficult to discern which aspects of the ERAS protocol may have affected the outcomes of interest since several new strategies were implemented simultaneously. This study also did not evaluate other patient-related outcome measures. Moving forward, however, we seek to evaluate the relative contribution of each element of the ERAS protocol to these outcomes in a future study. Despite these limitations, this paper showed statistically significant results in all 4 domains explored.
Conclusion
We found that the combination of multimodal pain management, IPACK, and BKK (a periarticular injection) by way of implementation of a new ERAS protocol decreased time in PACU, patient pain at discharge, number of opioids consumed while in the PACU, and quantity of opioids prescribed after discharge. This study will help pave the way for future studies in this field. As a retrospective review, it serves as a pilot study for future prospective research to better evaluate the most effective components of the ERAS protocol and how it might continue to be improved.
Footnotes
Final revision submitted August 2, 2022; accepted August 12, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: J.S. has received education payments from Fortis Surgical. L.G. has received education payments from Fortis Surgical and Supreme Orthopedic Systems. R.O. has received grant support from Arthrex and education payments from Alon Medical Technology, Arthrex, Fortis Surgical, and Smith & Nephew. A.V. has received education payments from Supreme Orthopedic Systems and hospitality payments from Arthrex, RTI Surgical, and Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was waived by Virginia Commonwealth University (ref. No. HM20024246).
References:
- 1. Abdallah FW, Brull R, Joshi GP; Society for Ambulatory Anesthesia (SAMBA). Pain management for ambulatory arthroscopic anterior cruciate ligament reconstruction: evidence-based recommendations from the society for ambulatory anesthesia. Anesth Analg. 2019;128(4):631–640. [DOI] [PubMed] [Google Scholar]
- 2. Bolia IK, Haratian A, Bell JA, et al. Managing perioperative pain after anterior cruciate ligament (ACL) reconstruction: perspectives from a sports medicine surgeon. Open Access J Sports Med. 2021;12:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. [DOI] [PubMed] [Google Scholar]
- 4. Fang YY, Lee QJ, Chang EWY, Wong YC. Local infiltration analgesia in primary total knee arthroplasty. Hong Kong Med J. 2019;25(4):279–286. [DOI] [PubMed] [Google Scholar]
- 5. Ge DH, Hockley A, Vasquez-Montes D, et al. Total inpatient morphine milligram equivalents can predict long-term opioid use after transforaminal lumbar interbody fusion. Spine (Phila Pa 1976). 2019;44(20):1465–1470. [DOI] [PubMed] [Google Scholar]
- 6. Goode VM, Morgan B, Muckler VC, Cary MP, Jr, Zdeb CE, Zychowicz M. Multimodal pain management for major joint replacement surgery. Orthop Nurs. 2019;38(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. J Clin Anesth. 2016;35:524–529. [DOI] [PubMed] [Google Scholar]
- 8. Kirkham KR, Grape S, Martin R, Albrecht E. Analgesic efficacy of local infiltration analgesia vs. femoral nerve block after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Anaesthesia. 2017;72(12):1542–1553. [DOI] [PubMed] [Google Scholar]
- 9. Lalani SB, Ali F, Kanji Z. Prolonged-stay patients in the PACU: a review of the literature. J Perianesth Nurs. 2013;28(3):151–155. [DOI] [PubMed] [Google Scholar]
- 10. Lynch JR, Okoroha KR, Lizzio V, Yu CC, Jildeh TR, Moutzouros V. Adductor canal block versus femoral nerve block for pain control after anterior cruciate ligament reconstruction: a prospective randomized trial. Am J Sports Med. 2019;47(2):355–363. [DOI] [PubMed] [Google Scholar]
- 11. Marchand DK, McCormack S. Codeine for acute pain in patients undergoing orthopedic surgery: a review of clinical effectiveness. Canadian Agency for Drugs and Technologies in Health; October 29, 2019. Accessed January 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK551871/ [PubMed] [Google Scholar]
- 12. Sehmbi H, Brull R, Shah UJ, et al. Evidence basis for regional anesthesia in ambulatory arthroscopic knee surgery and anterior cruciate ligament reconstruction, part II: adductor canal nerve block––a systematic review and meta-analysis. Anesth Analg. 2019;128(2):223–238. [DOI] [PubMed] [Google Scholar]
- 13. Vorobeichik L, Brull R, Joshi GP, Abdallah FW. Evidence basis for regional anesthesia in ambulatory anterior cruciate ligament reconstruction, part I: femoral nerve block. Anesth Analg. 2019;128(3):58–65. [DOI] [PubMed] [Google Scholar]
- 14. Yung EM, Brull R, Albrecht E, Joshi GP, Abdallah FW. Evidence basis for regional anesthesia in ambulatory anterior cruciate ligament reconstruction, part III: local instillation analgesia––a systematic review and meta-analysis. Anesth Analg. 2019;128(3):426–437. [DOI] [PubMed] [Google Scholar]