Abstract
Although upfront autologous stem cell transplantation (ASCT) generally improves progression-free survival (PFS) in newly diagnosed multiple myeloma (NDMM), the overall survival (OS) benefit and optimal timing of ASCT are not well established. Patients with early response may be able to safely continue induction and avoid ASCT without compromised outcomes. We report an extended follow-up analysis of a phase 2 trial that randomized transplant-eligible patients with NDMM who responded to induction (50/65 patients) to continued induction or ASCT; median follow-up was 8.0 years. Patients had similar 8-year PFS (55% vs. 43%), 8-year OS (83% vs. 72%), and rates of at least very good partial response (72% vs. 84%) whether continuing induction of lenalidomide and dexamethasone (Ld arm) or receiving ASCT (Ld+ASCT arm) (P=0.5). Notably, over 50% of patients receiving continuous Ld had PFS of 5–10 years. These results suggest the need for prospective trials incorporating response-adapted therapeutic approaches to NDMM.
Keywords: multiple myeloma, stem cell transplantation, lenalidomide, continuous induction, response-adapted therapy
Introduction
Upfront post-induction, high-dose chemotherapy plus autologous stem cell transplantation (ASCT) is the standard treatment approach for eligible patients with newly diagnosed multiple myeloma (NDMM), following reported benefit versus conventional chemotherapy.[1–3] However, in several studies, despite improving progression-free survival (PFS), upfront ASCT demonstrated no overall survival (OS) benefit compared to delayed ASCT at relapse or continued chemotherapy using novel treatments.[4–8]
Immunomodulatory drugs and proteasome inhibitors, first introduced to treat relapsed/refractory multiple myeloma (MM),[9–11] are now established as frontline therapies.[12] In NDMM, these agents improved complete response (CR) over traditional chemotherapy in ASCT and non-ASCT settings without substantially increasing toxicity, and importantly, prolonged PFS and OS.[12] Lenalidomide, specifically, has considerable activity in NDMM, including in older patients and those ineligible for ASCT.[13–17]
The success of these agents in NDMM has brought into question the timing of ASCT, and whether continued therapy with an immunomodulator-containing regimen could be considered, for some selected patients, along with a delayed ASCT approach.[18] In a landmark analysis of a phase 1/2 trial using lenalidomide, bortezomib, and dexamethasone (RVD) among patients who had not progressed after 1 year (80%), those who continued treatment with RVD and those who proceeded to ASCT had similar 2-year PFS (P=0.38).[19] A retrospective observational study (N=290) reported that patients who received induction therapy (lenalidomide plus dexamethasone or thalidomide plus dexamethasone) had similar post-transplant time to progression (P=0.4) and 4-year OS (P=0.3), whether receiving ASCT within 12 months of diagnosis or continued therapy with ASCT later.[20]
Another phase 3 trial comparing tandem ASCT versus melphalan, prednisone, and lenalidomide (MPR) consolidation found no difference in 5-year OS (78.4% vs 70.2%, respectively) when using lenalidomide maintenance in both arms.[21] Similarly, the phase 3 Intergroupe Francophone du Myélome (IFM) 2009 trial comparing RVD induction followed by ASCT versus extended RVD, including lenalidomide maintenance for both arms, found improved PFS (50 vs 36 months, respectively; P<0.001) but no OS benefit (P=0.87) for upfront ASCT.[4] Only one published phase 3 trial has reported both improved PFS (2-year, P<0.001) and OS (5-year, P=0.004) when comparing upfront ASCT to consolidation using conventional chemotherapy plus lenalidomide.[22] Ongoing phase 3 trials are investigating the optimal timing of ASCT in NDMM (ClinicalTrials.gov Identifiers: NCT01208662 and NCT01090089); until data support a change in the treatment paradigm, upfront ASCT remains the standard of care for eligible patients.
In the aforementioned studies, patients not randomized to upfront ASCT received a fixed duration of induction and/or consolidation therapy before low-dose lenalidomide maintenance. In an updated analysis from the FIRST trial, continuous lenalidomide at the full induction dose significantly extended PFS and OS compared with melphalan, prednisone, and thalidomide. Continuous lenalidomide also significantly extended PFS compared to a fixed duration of lenalidomide induction (Rd18), with an approximately 30-month longer median time to next treatment vs Rd18 (69.5 vs 39.9 months, respectively), suggesting that continuous induction dosing of lenalidomide-based therapy may be optimal.[23]
We report the long-term follow-up of a randomized prospective phase 2 trial comparing continued induction lenalidomide plus low-dose dexamethasone (Ld) versus Ld induction followed by early ASCT in patients with NDMM whose disease is sensitive to Ld induction.
Methods
Patient population
The trial was conducted at Memorial Sloan Kettering Cancer Center (MSK) and adhered to Good Clinical Practice guidelines, local regulatory requirements, and the Declaration of Helsinki (ClinicalTrials.gov Identifier: NCT00807599). The protocol was approved by the MSK institutional review board. All patients provided written informed consent.
ASCT-eligible patients with NDMM and aged 18–75 years were enrolled. Inclusion criteria included Eastern Cooperative Oncology Group performance score ≤2 and adequate organ function as follows: left ventricular ejection fraction ≥50% by echocardiogram and diffusing capacity >50% predicted by pulmonary function testing; absolute neutrophil count ≥1,500/μL and platelet count ≥100,000/μL (unless due to MM); serum bilirubin ≤2.0 mg/dL; aspartate transaminase, alanine transaminase, and alkaline phosphatase <3.0 × upper limit of normal; and calculated creatinine clearance ≥30 mL/min (Cockroft-Gault formula). Key exclusion criteria included prior treatment for MM (apart from 1 prior dexamethasone cycle); acute renal failure or severe symptomatic bone involvement that, in the treating physician’s opinion, mandated a prompt response (ie, using triplet combinations); concurrent active malignancy; and active HIV or hepatitis B/C infection.
Treatment and trial design
Patients received 4 cycles (28 days/cycle) of Ld: lenalidomide 25 mg orally on days 1–21 plus low-dose dexamethasone 40 mg orally on days 1, 8, 15, and 22. Patients with progressive disease (PD) at any time during induction or stable disease (SD) after 4 cycles of Ld were withdrawn. Patients with a partial response (PR) or better after Ld induction underwent stem cell mobilization and collection using cyclophosphamide and granulocyte-colony stimulating factor (with optional plerixafor). Patients were stratified by risk level based on baseline cytogenetics, fluorescence in-situ hybridization, and β2 microglobulin, and then randomized (1:1 ratio via random permuted block method) to receive either: continued lenalidomide at the last tolerated dose until PD or unacceptable toxicity plus low-dose dexamethasone 20 mg weekly for 1 year from treatment initiation (continued Ld arm); or ASCT conditioned with 200 mg/m2 melphalan IV, followed by lenalidomide maintenance initiated 3 months post-ASCT at 10 mg daily and escalated after 3 months to 15 mg daily until PD or unacceptable toxicity (Ld+ASCT arm) (Fig 1). Patients in the Ld+ASCT arm who achieved less than a very good PR (VGPR) by 3 months after the first ASCT could undergo a second ASCT.
Fig 1. Treatment schema.
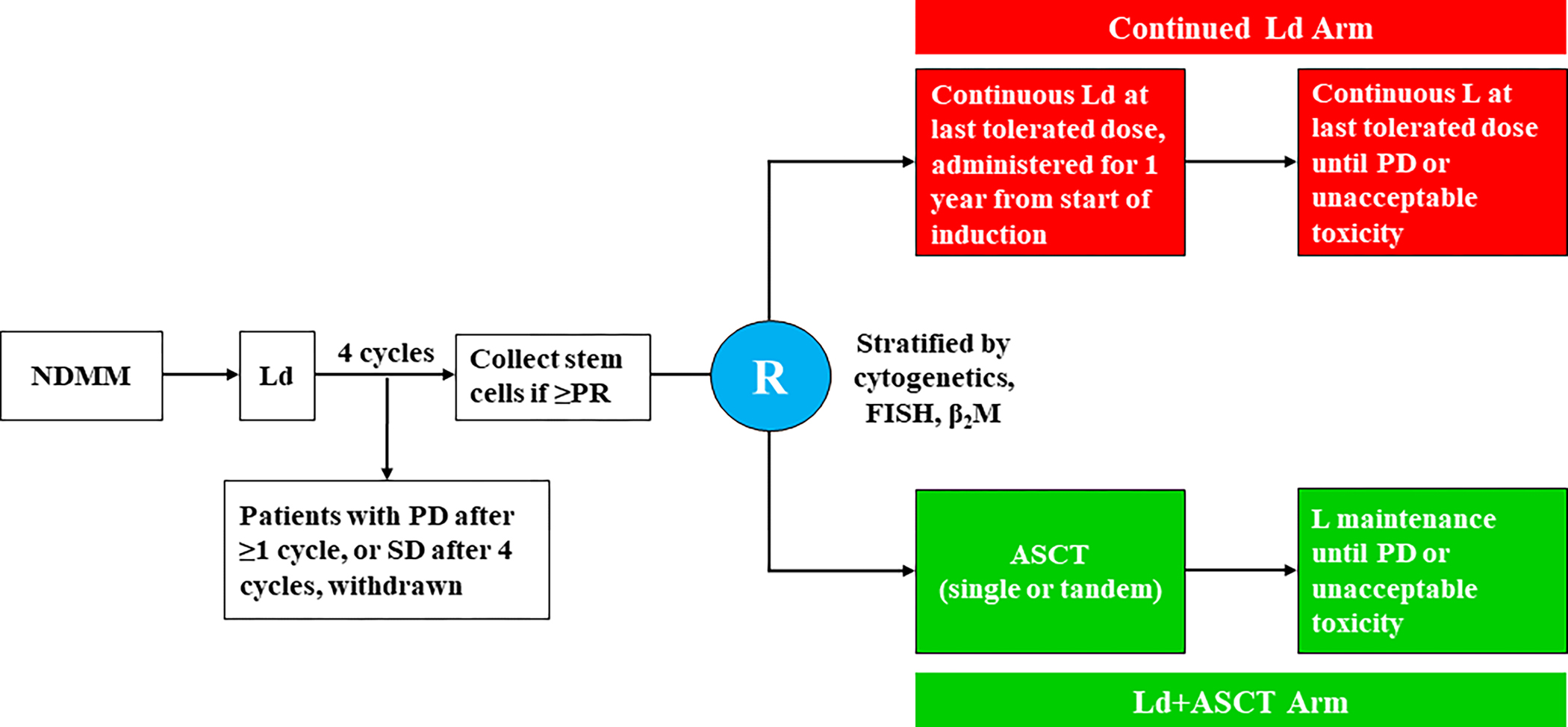
Patients received Ld induction (lenalidomide 25 mg daily on days 1–21 plus dexamethasone 40 mg once weekly during each 28-day-cycle) for 4 cycles. Patients in the Ld+ASCT arm received, starting 3 months post ASCT, L maintenance (lenalidomide 10 mg daily on days 1–28 of a 28-day cycle, escalated to 15 mg daily after 3 cycles if tolerated). Patients in the continued Ld arm received continuous Ld (continuous lenalidomide at the last tolerated dose until progression or toxicity plus dexamethasone 20 mg weekly for 1 year). ASCT, autologous stem cell transplantation; β2M, beta-2 microglobulin; FISH, fluorescence in-situ hybridization; L, lenalidomide; Ld, lenalidomide plus low-dose dexamethasone; Ld+ASCT, Ld induction followed by early ASCT; NDMM, newly diagnosed multiple myeloma; PD, progressive disease; PR, partial response; R, randomization; SD, stable disease.
Toxicity-related dose adjustments were allowed. All patients received prophylactic anticoagulation with aspirin or low-molecular-weight heparin. Patients were withdrawn in the event of PD, unacceptable toxicity, pregnancy, consent withdrawal, or non-compliance. Patients withdrawn from the continued Ld arm for PD were offered ASCT.
Response assessments
Responses were evaluated according to the 2011 Myeloma Workshop Consensus Panel 1 guidelines[24] on day 1 of every cycle during induction, at the end of induction, 3 months post-ASCT in the Ld+ASCT arm, and every 3 months during continued Ld treatment (Ld arm) and the maintenance phase (Ld+ASCT arm). Treatment responses were formally scored by an MSK response review group and monitored by the MSK Therapeutic Response Review Committee. Adverse events (AEs) were monitored throughout treatment and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.[25]
Statistical analyses
The primary objective was to estimate PFS as a binary endpoint in each treatment arm at 2 years post-randomization. Secondary objectives were to assess best response (including stringent CR [sCR], CR, VGPR, and PR rates), OS, and AEs, including second primary malignancies (SPMs). Under a pick-the-winner approach, two parallel single-stage designs were implemented separately in each arm to distinguish unpromising (<65%) from promising (≥87%) PFS rates. Type I and II errors were both set at 0.10. Treatment in either arm was considered successful if at least 20 of 25 patients randomized to each arm at two years were alive and progression-free. Kaplan-Meier methods were used to estimate OS and PFS following randomization. Fisher’s exact test was used to compare response by treatment arm. All randomized patients were included in the efficacy and safety analyses. The length of follow-up and all treatment outcomes were evaluated from the time of randomization. Follow-up times were measured in surviving patients only. Statistical analyses were performed using R version 3.6.2.
Results
Patient characteristics and disposition
Of the 69 patients enrolled four did not initiate therapy on trial, after diagnosis of concurrent amyloidosis (n=1), inpatient hospitalization for development of acute renal failure and initiation of bortezomib (n=1), inpatient hospitalization for bone fracture and initiation of bortezomib/cyclophosphamide (n=1), and an unknown cause (n=1). Sixty-five patients started treatment, and 50 achieved PR or better following 4 cycles of Ld induction and were randomized (25 patients to each treatment arm: continued Ld or Ld+ASCT) (Fig 2). Fifteen patients were withdrawn during or after induction due to SD after 4 cycles of induction (n=7), PD during or shortly after 4 cycles of induction (n=2), toxicity (n=4), patient withdrawal (n=1), and physician discretion (n=1).
Fig 2. CONSORT diagram of clinical trial enrollment and treatment.
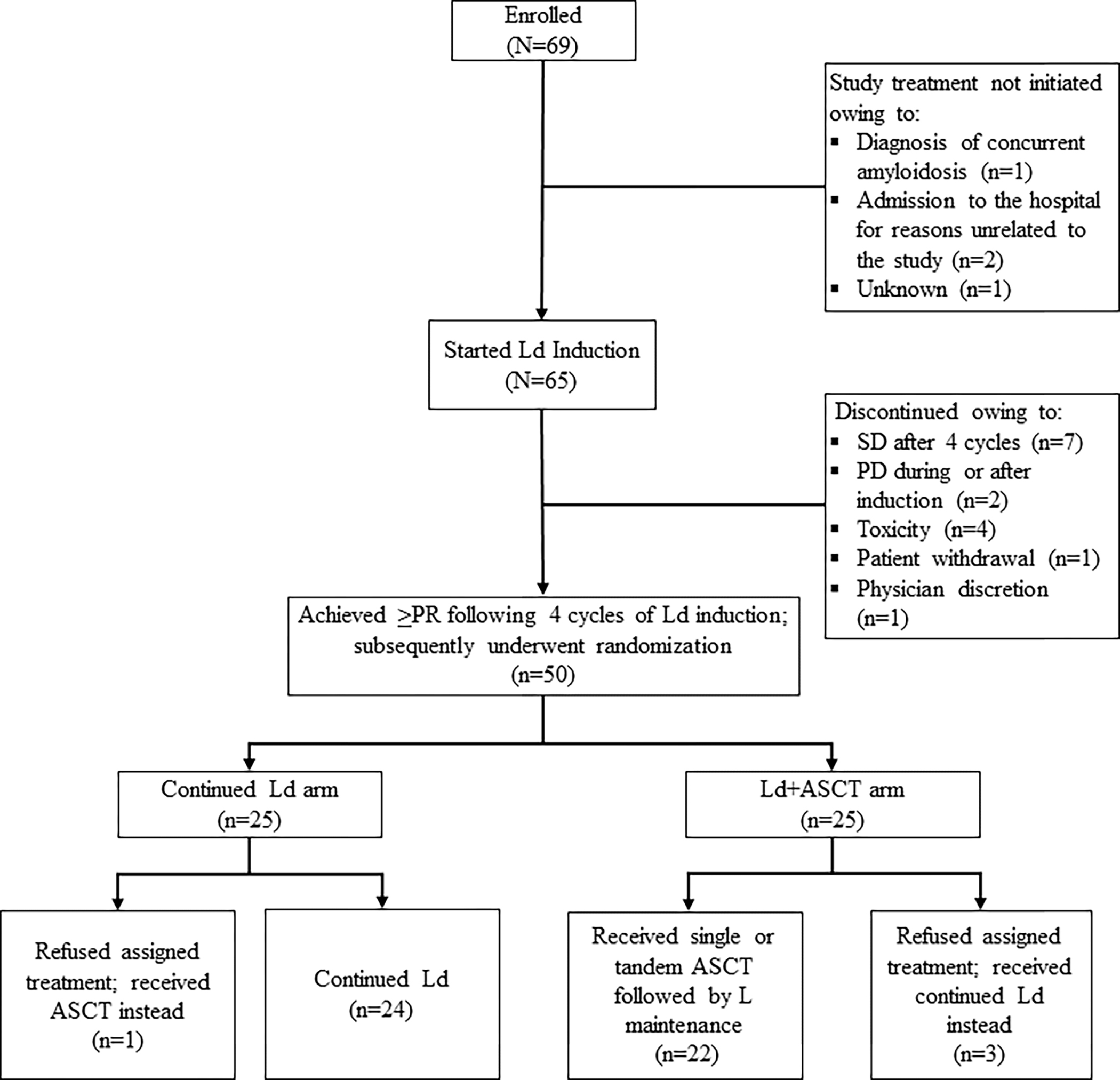
ASCT, autologous stem cell transplantation; L, lenalidomide; Ld, lenalidomide plus low-dose dexamethasone; Ld+ASCT, Ld induction followed by early ASCT; PD, progressive disease; PR, partial response; SD, stable disease.
Among randomized patients, 4 refused the assigned treatment and crossed over to the alternative treatment arm (3 randomized to Ld+ASCT received continued Ld; 1 randomized to continued Ld received ASCT). These patients were included in the intention-to-treat analysis in the arms to which they had been randomized. The 15 non-randomized patients were immediately offered alternative induction after withdrawal from trial, followed by upfront ASCT. Among these 15 patients, 11 proceeded to ASCT after alternative induction, 3 refused ASCT and remained on Ld after toxicity was controlled, and 1 was lost to follow-up.
Baseline and demographic characteristics for the 65 patients who started Ld induction were generally well-matched between the two randomized treatment arms (Table 1). However, we note that the group of 15 patients who did not undergo randomization (mostly because of suboptimal response to induction as shown below) was enriched in high risk disease, as shown in Table 1.
Table 1.
Baseline patient characteristics.
| Characteristic* | Ld+ASCT (n=25) | Continued Ld (n=25) | Not Randomized (n=15) | Overall (N=65) |
|---|---|---|---|---|
|
| ||||
| Median age (range), years | 60 (58–67) | 62 (58–66) | 61 (56–63) | 61 (56–67) |
| Male | 16 (64) | 13 (52) | 6 (40) | 35 (54) |
| Race | ||||
| African American | 3 (12) | 6 (24) | 2 (13) | 11 (17) |
| White | 21 (84) | 18 (72) | 13 (87) | 52 (80) |
| Other | 1 (4) | 1 (4) | 0 (0) | 2 (3) |
| Intact Ig vs FLC only | ||||
| FLC | 5 (20) | 5 (20) | 4 (27) | 14 (22) |
| Ig | 20 (80) | 20 (80) | 11 (73) | 51 (78) |
| M protein subtype | ||||
| IgA Kappa | 1 (4) | 2 (8) | 0 (0) | 3 (5) |
| IgA Lambda | 3 (12) | 2 (8) | 2 (13) | 7 (11) |
| IgG Kappa | 10 (40) | 14 (56) | 6 (40) | 30 (46) |
| IgG Lambda | 6 (24) | 2 (8) | 2 (13) | 10 (15) |
| Kappa | 4 (16) | 4 (16) | 4 (27) | 12 (18) |
| Lambda | 1 (4) | 1 (4) | 1 (7) | 3 (5) |
| Mean M protein level (SD), g/dL | 1.9 (1.8) | 1.8 (1.6) | 2.1 (2.2) | 1.9 (1.8) |
| Mean involved vs uninvolved ratio (SD) | 758.7 (1519.7) | 818.2 (2316.9) | 698.2 (821.9) | 767.6 (1680) |
| Mean β2M (SD), g/dL | 3.3 (1.5) | 3.5 (1.6) | 4.1 (1.6) | 3.6 (1.6) |
| Mean albumin (SD), g/dL | 3.8 (0.7) | 3.9 (0.4) | 3.9 (0.6) | 3.9 (0.6) |
| ISS Stage | ||||
| I | 15 (60) | 15 (60) | 3 (20) | 33 (51) |
| II | 6 (24) | 6 (24) | 8 (53) | 20 (31) |
| III | 4 (16) | 4 (16) | 3 (20) | 11 (17) |
| NA | 0 (0) | 0 (0) | 1 (7) | 1 (2) |
| Mean LDH (SD), U/L | 182.4 (51.6) | 212.9 (80.3) | 169.5 (40.5) | 191.2 (64) |
| Cytogenetic Groups | ||||
| High | 6 (24) | 4 (16) | 6 (40) | 16 (25) |
| Standard | 17 (68) | 19 (76) | 8 (53) | 44 (68 |
| NA | 2 (8) | 2 (8) | 1 (7) | 5 (8) |
Ld, lenalidomide plus low-dose dexamethasone; Ld+ASCT, Ld induction followed by early ASCT; Ig, immunoglobulin; FLC, free light chain; β2M, beta-2 microglobulin; ISS, International Staging System; LDH, lactate dehydrogenase; M protein, monoclonal protein; SD, standard deviation; vs, versus.
Data are shown as n (% of in-group total) unless otherwise noted.
As of the cutoff date of August 9, 2020, all patients continued treatment on lenalidomide as planned until progression of disease (n=20), development of a new SPM (n=7), toxicity (n=1), or unknown reason (n=1). All other 21 patients remained on treatment as planned per protocol. The median times on lenalidomide since initiation of therapy were not significantly different between the two groups (4.32 years [95% CI, 2.07–6.46] and 4.68 years [95% CI, 2.39–6.62] for the continued Ld and Ld+ASCT arms, respectively).
Response
Among patients who started Ld induction, the best responses during induction were PD (n=1), SD (n=6), PR (n=36), VGPR (n=15), and sCR (n=7). Fifty patients who remained in ≥PR by the end of Ld induction and had acceptable toxicity were randomized. The ≥VGPR rates at any time during first-line therapy was not statistically different in the two arms (P=0.5), although more patients achieved ≥VGPR in the Ld+ASCT arm (84%; 21/25) compared with the continued Ld arm (72%; 18/25) (Table 2). Eleven patients in the continued Ld arm and 12 in the Ld+ASCT arm achieved sCR. Among the 15 non-randomized patients offered alternative induction followed by ASCT, 1 was lost to follow-up, and 79% of the remaining patients achieved ≥VGPR as their best response to alternative induction followed by ASCT, including sCR (n=2), CR (n=6), VGPR (n=3), while 3 achieved PR.
Table 2.
Best treatment response rates in randomized patients (n=50).
| Best response, No. (%) | Ld+ASCT (n=25) | Continued Ld (n=25) | P value |
|---|---|---|---|
|
| |||
| sCR | 12 (48) | 11 (44) | >0.99 |
| CR | 3 (12) | 1 (4) | |
| VGPR | 6 (24) | 6 (24) | |
| PR | 4 (16) | 7 (28) | |
|
| |||
| ≥VGPR | 21 (84) | 18 (72) | 0.5 |
No., number; Ld, lenalidomide plus low-dose dexamethasone; Ld+ASCT, Ld induction followed by early ASCT; sCR, stringent complete response; CR, complete response; VGPR, very good partial response; PR, partial response; ≥VGPR, VGPR or better.
For patients achieving ≥CR, median time to achieve best response was 12.5 (range, 3.8–80.4) months versus 10.1 (range, 3.5–61.1) months in the Ld+ASCT and Ld groups, respectively. Interestingly, 12% (3/25) of patients in the Ld arm and 12% (3/25) in the Ld+ASCT arm achieved their best responses beyond 24 months from initiation of therapy: 1 converting to VGPR and 5 to CR as best response after 2 years.
Progression-free and overall survival
By intention-to-treat analysis (ITT), median follow-up was 8.0 (range, 0.5–11.1) years, 7.9 (range, 3.1–11.1) years, and 8.3 (range, 0.5–11.0) years for the entire randomized population (n=50), continued Ld arm, and Ld+ASCT arm, respectively. Median PFS and OS from treatment initiation for the entire population (N=65) from the time of enrollment was 4.7 years (95% CI, 3.2-Not Reached [NR]) and 10.1 years (95% CI, 8.7-NR), respectively. PFS from randomization did not differ between treatment arms. In the continued Ld and Ld+ASCT arms, 2-year PFS rates were 80% (95% CI, 66%–97%) and 84% (95% CI, 71%–100%), respectively (Fig 3A); corresponding 8-year PFS were 55% (95% CI, 39%–79%) and 43% (95% CI, 27%–68%), respectively. Similarly, OS rates did not differ. In the continued Ld and Ld+ASCT arms, 4-year OS rates were 96% (95% CI, 89%–100%) and 92% (95% CI, 81%–100%), respectively (Fig 3B); corresponding 8-year OS were 83% (95% CI, 67%–100%) and 72% (95% CI, 55%–95%), respectively (Fig 3C). Note that rates of 4-year second progression-free survival (PFS2) were 0.85 (0.73->0.99) and 0.87 (0.74->0.99), while rates of 8-year PFS2 were 0.44 (0.27–0.72) and 0.54 (0.36–0.8) for the Ld and Ld+ASCT arms, respectively (P value NS).
Fig 3. Survival analyses.
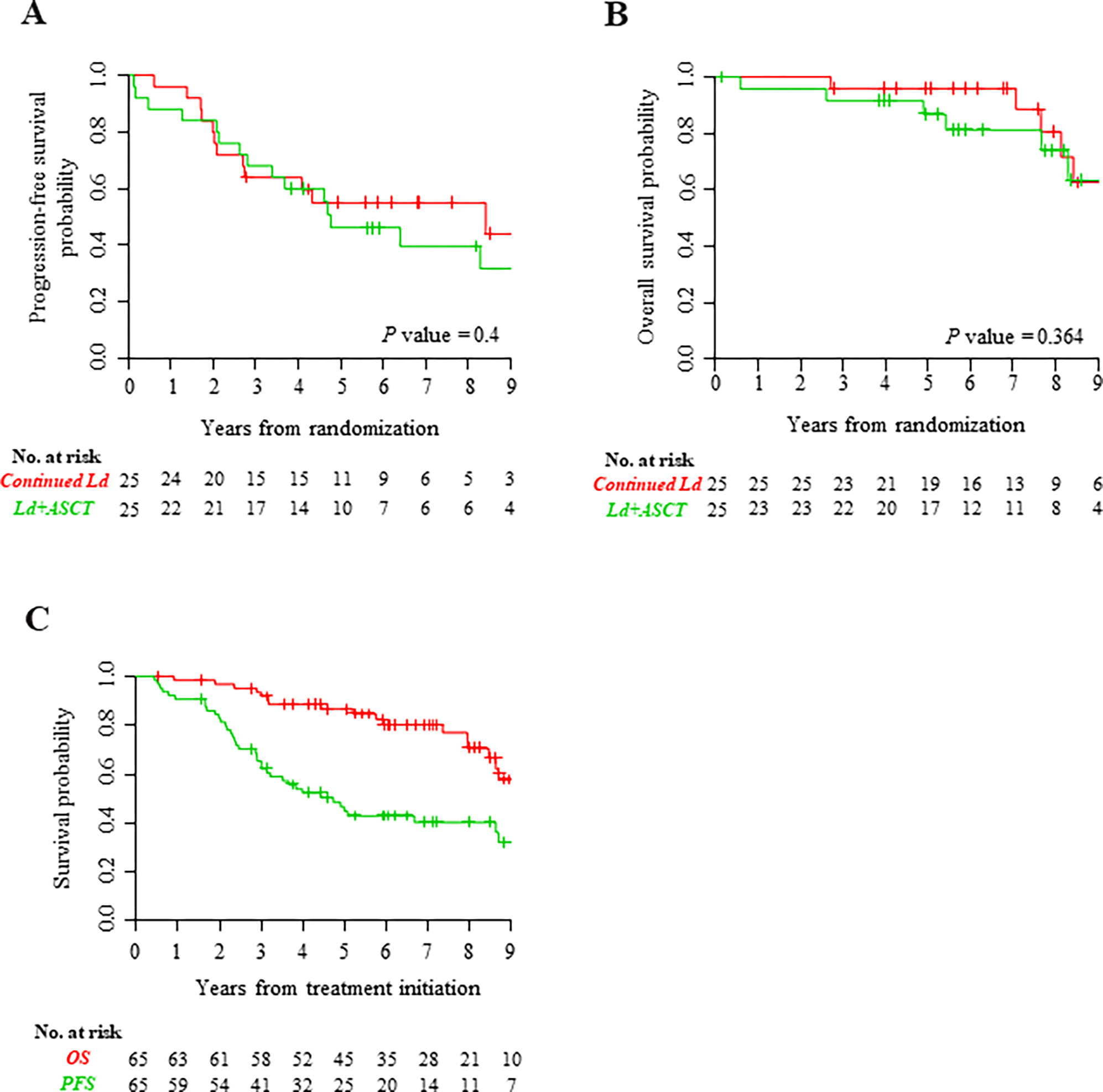
(A) Progression-free survival and (B) overall survival (Kaplan-Meier analysis) of all randomized patients, the intention-to-treat population (n = 50). (C) Overall survival and progression-free survival from the start of treatment of all patients who initiated Ld induction (N = 65). Ld, lenalidomide plus low-dose dexamethasone; Ld+ASCT, Ld induction followed by early ASCT; No., number; OS, overall survival; PFS, progression-free survival.
We have also analyzed PFS and OS based on treatment received rather than ITT. The results are very similar: rates of 8-year PFS were 51% (95% CI, 35%–74%) and 47% (95% CI, 30%–73%) for Ld and Ld+ASCT, respectively. Similarly, OS rates did not differ: rates of 8-year OS were 80% (95% CI, 64%–99%) and 74% (95% CI, 57%–97%), respectively.
Among 27 patients who actually received only continuous Ld (accounting for those patients who crossed over), 15 (56%) were progression-free as of the cut-off date (August 9, 2020); 14 (52%) of these patients had been progression-free for over 5 years of follow-up. One of 15 patients was censored at 2 years. More than 50% of patients who responded to Ld induction and continued that treatment have received only a single drug for over 5 years and up to 10 years and beyond without PD.
Safety
Excluding the 3-month post-ASCT period, the most common grade 3 and 4 AEs were lymphopenia, leukopenia, neutropenia, thrombocytopenia, and infections, as expected. Incidences of grade 3 and 4 hematologic and non-hematologic AEs were similar in both arms (Table 3).
Table 3.
Most common grade 3 and 4 adverse events.
| Adverse events, No. (%) | Ld+ASCT (n=25) | Continued Ld (n=25) |
|---|---|---|
|
| ||
| Hematologic | ||
| Lymphopenia | 19 (76) | 22 (88) |
| Leukopenia | 17 (68) | 16 (64) |
| Neutropenia | 14 (56) | 16 (64) |
| Thrombocytopenia | 7 (28) | 8 (32) |
| Anemia | 7 (28) | 8 (32) |
| Abnormal INR | 2 (8) | 4 (16) |
| Second primary malignancy | 6 (24) | 3 (12) |
|
| ||
| Non-hematologic | ||
| Infection* | 13 (52) | 5 (20) |
| Hypophosphatemia | 7 (28) | 9 (36) |
| Hyperglycemia | 2 (8) | 8 (32) |
| Hypokalemia | 6 (24) | 1 (4) |
| Febrile neutropenia | 3 (12) | 3 (12) |
| Hyponatremia | 2 (8) | 3 (12) |
| Diarrhea | 1 (4) | 0 (0) |
No., number; Ld, lenalidomide plus low-dose dexamethasone; Ld+ASCT, Ld induction followed by early ASCT; INR, international normalized ratio.
Includes any type of infection or infestation.
The percentage of patients with SPMs was slightly higher than those reported in other trials: 9 (18%) SPMs were reported among randomized patients, including 3 in the continued Ld arm (1 acute lymphocytic leukemia, 1 myelodysplastic syndrome, and 1 acute myelogenous leukemia) and 6 in the Ld+ASCT arm (2 acute myeloid leukemia, 1 myelodysplastic syndrome, 1 melanoma, 1 metastatic colon carcinoma, and 1 rectal carcinoma). Importantly, 3 out of 25 patients crossed over from ASCT to continuous Ld while 1 out of 25 crossed over from continuous Ld to ASCT, so that 3 of 27 (11%) patients who actually received continuous Ld and 6 of 23 (26%) patients in the ASCT arm developed an SPM (P=0.156). The median time from start of treatment until the diagnosis of SPM was 70 months for these 9 patients overall, and 70 vs 64 months for those in the continuous Ld and ASCT arms, respectively. Only 1 out of 15 patients in the non-randomized group developed an SPM although almost 80% underwent ASCT.
Discussion
In this trial, patients with NDMM who were responsive to Ld induction had excellent outcomes whether randomized to continue Ld induction dosing or to receive ASCT. Although the sample size is small and not powered for conclusive comparison, neither PFS nor OS differed by treatment strategy despite a very long median follow-up of 8.0 (0.5–11.1) years. For the interpretation of these results, only patients who responded to Ld induction were randomized. Patients in the continued Ld arm received relatively more-intensive lenalidomide and dexamethasone continuous induction dosing compared with the conventional Ld+ASCT arm.
Other studies comparing ASCT with standard chemotherapy in NDMM have had similar results for OS. Before novel therapies were available, most studies found a PFS or event-free survival benefit[1,2,5,20,26–28] but only two showed an OS benefit for upfront ASCT.[1,2] A meta-analysis of all randomized controlled trials including 2411 patients, a large majority treated with novel agents, indicated a PFS benefit but no OS benefit for upfront ASCT while noting considerable heterogeneity among the studies.[7] More recently, retrospective studies reported comparable outcomes using novel agent combinations followed by early or late ASCT, including no OS benefit for early ASCT.[20,29,30] However, crucially and inherent to the design of these retrospective studies, all patients in the delayed-transplant arms received transplant as salvage treatment.
Recent phase 3 trials that compared lenalidomide-based regimens to ASCT in NDMM favored ASCT.[8,21,22] Palumbo et al reported significantly increased PFS and OS using ASCT versus MPR consolidation following Ld induction (respectively, median PFS, 43.0 vs 22.4 months [P<0.001]; 4-year OS, 81.6% vs 65.3% [P=0.02]).[21] Similarly, Gay et al reported both prolonged PFS and OS with ASCT versus cyclophosphamide, lenalidomide, and dexamethasone consolidation following Ld induction (respectively, median PFS, 43.3 vs 28.6 months [P<0.0001]; 4-year OS, 86% vs 73% [P=0.004]).[22] In a similar study, Cavo et al reported an increased PFS when comparing ASCT versus VMP (bortezomib, melphalan and dexamethasone) consolidation, following CVD (cyclophosphamide, bortezomib and dexamethasone) induction (respectively, median PFS, 56.7 vs 41.9 months [P< 0.0001]); 5-year OS was not different between the two groups (respectively 75.1% vs 71.6% [P=0.35])[8].
While we do not suggest comparing the current randomized phase 2 trial with these sizable phase 3 trials, we hypothesize that continued higher lenalidomide dose administered during consolidation in the current trial could explain the enhanced PFS and OS results seen in the continuous Ld arm. Moreover, melphalan or cyclophosphamide used during consolidation in those three phase 3 trials may have lowered patients’ tolerance of full-dose lenalidomide and bortezomib and increased the toxicity of the regimen, necessitating lenalidomide dose reductions, and ultimately reduced treatment efficacy. In these three trials, the rates of discontinuation of treatment during consolidation therapy were strikingly higher in the non-transplant versus transplant arms (non-transplant: 12%, 18% and 38%; transplant: 4%, 8% and 19%, respectively). Additionally, in these three phase 3 trials, respectively, only 63%, 43%, and 27% of patients in the non-transplant arms received salvage ASCT; while in the current trial, 80% who progressed in the Ld arm did, which may account for the favorable OS.
Recently, the large, randomized IFM study found significantly improved rates of response and PFS but no OS advantage for ASCT compared to RVD consolidation, following RVD induction.[4] However, maintenance lenalidomide, although administered in both arms, was limited to 1 year. The similar US study (ClinicalTrials.gov Identifier: NCT01208662) will report its findings in the coming months. Importantly, maintenance lenalidomide administration is not limited to 1 year in the US trial but continues until PD in both arms, an approach that may yield a substantially different outcome than the IFM trial by blunting the PFS advantage of transplant. Interestingly, in the initial IFM report, PFS and OS for patients achieving negative minimal residual disease (MRD) was not statistically different, whether they were treated with ASCT or RVD consolidation. This prompted the authors to propose trials investigating ASCT use according to response to induction (or MRD status) as one particular approach to ‘tailoring therapy and further improving clinical benefit’”[4].
In our analysis, grade 3 and 4 infections occurred at a higher rate in the Ld+ASCT arm despite exclusion of transplant-related AEs occurring within 3 months post-transplant, and despite higher doses of lenalidomide and prolonged dexamethasone administration in the Ld group. The overall percentage of patients with SPMs in this trial (18%) is slightly higher than reported in the 2018 updated CALGB100104 trial for patients treated with lenalidomide maintenance (14%), although it should be noted that in the current, single-institution study with available close follow-up on all patients, the median follow-up is slightly longer and all SPMs have been captured.[31] Similarly, we noted a higher proportion of SPMs in the continuous Ld arm (12%) compared to the continuous Ld arm from the final analysis of the FIRST study (7%),[23] which may be attributable, at least partially, to the longer follow-up in the present study (96 vs 67 months). Interestingly, when considering treatment actually received, the incidence of SPMs was higher in the Ld+ASCT arm than in the continued Ld arm (26% vs.11%, p=0.156), consistent with the increased frequency of SPMs when combining lenalidomide with melphalan, which was found in a 2014 meta-analysis of patients with NDMM.[32] In contrast, we noted a lower rate of SPMs in the 15 patients who were not randomized (1 out 15 patients), although 80% underwent ASCT. This low rate of SPM may be explained by (1) the shorter overall survival of these patients, with 6 out of 14 patients (1 unknown) deceased by the cutoff date and (2) the shorter length of lenalidomide therapy for these patients, who proceeded to alternative therapy due to poor response or intolerance to lenalidomide.
This small but extended follow-up randomized phase 2 trial suggests that, in patients with NDMM who are responsive to Ld induction, continued Ld as administered—higher dosing, avoiding other myelotoxic drugs, such as alkylating agents, and treatment until progression—may result in excellent outcomes. The continuous treatment combination used in this trial likely allowed patients to have maximal exposure to lenalidomide and its beneficial effects, blunting the advantage conferred by ASCT. Notably, among 27 patients who received only continuous Ld, >50% have remained progression-free for at least 5 years and up to 10 years and beyond. Early response and tolerance to Ld induction appears to allow the selection of patients who could benefit from continued induction. The population selected for randomization in this trial is somewhat enriched in lower risk patients when compared to all-comers, since the 15 patients excluded from randomization after induction (mainly due to poor response or toxicity) had higher risk or other poor prognostic features (i.e., poor response or susceptibility to toxicity). This response-adapted approach may thus select for patients who can safely be administered prolonged lower-intensity treatment while preserving excellent long-term outcomes.
We acknowledge that the limited sample size is not powered to draw definitive conclusions when comparing both treatment arms. We also acknowledge the use in this trial of induction regimens currently considered suboptimal (ie, doublets vs triplets or quadruplets), and do not endorse the use of doublets in transplant-eligible patients; however, and as proposed by the IFM study quoted above, the results further support investigating prospectively therapeutic strategies tailored to individual patients’ response after induction, possibly optimizing outcomes. Such a response-adapted-approach, along with pre-treatment analysis of risk factors, may allow the selection of patients whose upfront ASCT could be delayed, and guide the judicious use of treatment intensification, including ASCT.
Statement of prior presentation:
Presented in abstract form (interim analysis) at the 56th annual meeting of the American Society of Hematology (San Francisco, CA, 6 December 2014) and at the 57th annual meeting of the American Society of Hematology (Orlando, FL, 3 December 2015).
Acknowledgments
The reported research was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748). Editorial support in the preparation of the manuscript was provided by the Memorial Sloan Kettering Cancer Center Editorial Service, Hannah Rice, BA, ELS, and Crystal Tran, BS. Medical writing support in the preparation of this manuscript was provided by Sandralee Lewis, PhD, on behalf of the Investigator Initiated Research Writing Group (an initiative from Ashfield Healthcare, a part of UDG Healthcare plc), and was funded by Celgene Corporation.
Footnotes
Potential Competing Interests: H.H. received consultancy fees and honoraria from Novartis; received research funding from Celgene and Takeda Pharmaceuticals; and served on the advisory committee for Takeda Pharmaceuticals. O.L. served on the advisory board for MorphoSys AG. H.L. served on the advisory committee for Takeda Pharmaceuticals, Celgene, Janssen Pharmaceuticals, Sanofi, and Caelum Biosciences; received honorarium from Sanofi; and received research funding from Takeda Pharmaceuticals. T.K. received consultancy fees from Juno Pharmaceuticals Inc., Gilead Sciences, Inc., Concert Pharmaceuticals Inc., and AbbVie, Inc. C.O.L. received consultancy fees from Amgen Inc., Merck & Co., Inc., Millennium Pharmaceuticals, Inc. (acquired by Takeda Pharmaceuticals in 2008), Onyx Pharmaceuticals, Inc. (acquired by Amgen in 2013), and Takeda Pharmaceuticals. The remaining authors declare no competing financial interests that might benefit from this publication.
References
- 1.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996. Jul 11;335(2):91–7. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003. May 8;348(19):1875–83. [DOI] [PubMed] [Google Scholar]
- 3.Lenhoff S, Hjorth M, Holmberg E, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000. Jan 1;95(1):7–11. [PubMed] [Google Scholar]
- 4.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017. Apr 6;376(14):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998. Nov 1;92(9):3131–6. [PubMed] [Google Scholar]
- 6.Gertz MA, Lacy MQ, Inwards DJ, et al. Early harvest and late transplantation as an effective therapeutic strategy in multiple myeloma. Bone Marrow Transplant. 1999. Feb;23(3):221–6. [DOI] [PubMed] [Google Scholar]
- 7.Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007. Feb;13(2):183–96. [DOI] [PubMed] [Google Scholar]
- 8.Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020. Jun;7(6):e456–e468. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009. Nov;23(11):2147–52. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005. Jun 16;352(24):2487–98. [DOI] [PubMed] [Google Scholar]
- 11.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999. Nov 18;341(21):1565–71. [DOI] [PubMed] [Google Scholar]
- 12.Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015. May 14;125(20):3076–84. [DOI] [PubMed] [Google Scholar]
- 13.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007. Oct;82(10):1179–84. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA--Italian Multiple Myeloma Network. J Clin Oncol. 2007. Oct 1;25(28):4459–65. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012. May 10;366(19):1759–69. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005. Dec 15;106(13):4050–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010. Jan;11(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson PG, Laubach JP, Munshi NC, et al. Early or delayed transplantation for multiple myeloma in the era of novel therapy: does one size fit all? Hematology Am Soc Hematol Educ Program. 2014. Dec 5;2014(1):255–61. [DOI] [PubMed] [Google Scholar]
- 19.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010. Aug 5;116(5):679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar SK, Lacy MQ, Dispenzieri A, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012. Mar 15;118(6):1585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014. Sep 4;371(10):895–905. [DOI] [PubMed] [Google Scholar]
- 22.Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015. Dec;16(16):1617–29. [DOI] [PubMed] [Google Scholar]
- 23.Facon T, Dimopoulos MA, Dispenzieri A, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018. Jan 18;131(3):301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011. May 5;117(18):4691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003. Jul;13(3):176–81. [DOI] [PubMed] [Google Scholar]
- 26.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006. Feb 20;24(6):929–36. [DOI] [PubMed] [Google Scholar]
- 27.Blade J, Esteve J, Rives S, et al. High-dose therapy autotransplantation/intensification vs continued standard chemotherapy in multiple myeloma in first remission. Results of a non-randomized study from a single institution. Bone Marrow Transplant. 2000. Oct;26(8):845–9. [DOI] [PubMed] [Google Scholar]
- 28.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005. Dec 20;23(36):9227–33. [DOI] [PubMed] [Google Scholar]
- 29.Richardson P, Mitsiades C, Laubach J, et al. Lenalidomide in multiple myeloma: an evidence-based review of its role in therapy. Core Evid. 2010;4:215–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunavin NC, Wei L, Elder P, et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma. 2013. Aug;54(8):1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holstein SA, Jung SH, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol. 2017. Sep;4(9):e431–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palumbo A, Bringhen S, Kumar SK, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014. Mar;15(3):333–42. [DOI] [PubMed] [Google Scholar]


