Abstract
Aims
Inflammatory cytokines can destroy the immune balance and lead to adverse pregnancy outcomes. Benzo (a) pyrene (BaP) may induce premature delivery through leading inflammatory reaction. We screened out inflammatory factors related to adverse pregnancy outcomes through bioinformatics analysis. Then we verified the correlation between adverse pregnancy outcomes caused by BaP and abnormal expression of those inflammatory factors.
Main methods
The Gene Expression Omnibus (GEO) database was used to analyze by R to screen the inflammatory genes related to adverse pregnancy outcomes. Based on the established BaP exposure animal model, the expression of key cytokines in placenta was detected by immunohistochemistry.
Key findings
According to the data analysis of GEO database, the expression of IL18, IL18BP and IL18R was up-regulated, while the expression of IL1RN was down regulated in the adverse pregnancy outcome group. BaP exposure significantly increased the rate of adverse pregnancy outcome in pregnant golden hamsters, and also significantly interferes with the process of embryonic development. Meanwhile, the expression of IL18, IL18BP and IL18R in placenta was increased, while the expression of IL1RN protein was decreased, consistent with the mRNA expression level gathered by bioinformatics analysis.
Significance
BaP may induce the inflammatory reaction to cause adverse pregnancy outcome by regulating the expression of IL18, IL18BP, IL18R and IL1RN. Our findings provide experimental basis for the prevention of adverse pregnancy outcome caused by BaP.
Keywords: BaP, Uterine-placental interface, Inflammatory cytokines, Adverse pregnancy outcome
BaP; Uterine-placental interface; Inflammatory cytokines; Adverse pregnancy outcome
1. Introduction
Controllable inflammatory response plays a significant role in the process of pregnancy, but some inflammatory cytokines can cause excessive inflammatory response through related receptors, and eventually lead to adverse pregnancy outcome [1, 2]. Immune cells at the uterine-placental interface play a significant role in pregnancy [3, 4]. Local immune cells inhibit non-infectious inflammatory reaction, from immune tolerance to fetal antigen and maintain pregnancy [5]. Inflammatory cytokines can also destroy the immune balance and lead to adverse pregnancy outcomes [5]. Pro-inflammatory factors such as hypoxia, abnormal angiogenesis, trauma and oxidative stress can affect the activity of immune cells and non-immune cells in decidua, placenta, fetal membrane and myometrium, leading to adverse pregnancy outcomes such as abortion, premature delivery and fetal growth restriction [5].
Polycyclic aromatic hydrocarbons (PAHs) are the products of incomplete combustion of fossil or biofuels such as coal, petroleum, straw and others, which have the characteristics of carcinogenesis and mutagenesis. With the aggravation of PAHs exposure in the daily life environment, the impact of PAHs on pregnancy outcomes has attracted much attention. Studies have pointed out that PAHs may induce premature delivery through leading inflammatory reaction [6, 7, 8].
Inflammasomes are intracellular polymeric protein complexes that regulate the maturation and release of pro-inflammatory cytokines such as IL-1 and IL-18, resulting in inflammatory response or cell death [9]. In the process of recurrent abortion, inflammasome can cause abnormal innate immunity of endometrium, activate the innate immunity, and affect the receptivity of endometrium [9]. Chorioamnionitis and intrauterine infections are associated with most spontaneous preterm birth [9, 10]. However, most preterm births occur without any signs of infection. It has been reported that in preterm delivery patients with intact fetal membranes, inflammation in sterile amnion is more common than that in microorganism associated amnion [11]. The expression of IL-1 in serum and pregnant tissue increased in patients with high risk factors of preterm birth or history of early pregnancy [12].
In this study, we screen out inflammatory factors related to adverse pregnancy outcomes through bioinformatics analysis. Then we verify the correlation between adverse pregnancy outcomes caused by Benzo (a) pyrene (BaP) and abnormal expression of those inflammatory factors.
2. Materials and method
2.1. Microarray screening and data processing
Using the Gene Expression Omnibus (GEO) of the National Center for Biotechnology Information (NCBI) platform to retrieve the microarray containing recurrent miscarriages (RM) samples, the genome series GSE26787 was selected [13]. Recurrent miscarriages in this data refer to at least three unexplained abortions. Endometrial tissues were obtained by biopsy, and the data were analyzed by Affymetrix chips (GeneChip human genome U133 plus2.0 array).
2.2. Screening of differentially expressed genes (DEGs)
The R software package GEOquery was used to download the data, and limma package was used to correct the expression value background and normalize the expression profile data, including the transformation of the original data format, the supplement of missing value, background correction, data standardization by Quantile method, and differential expression gene screening. The standard thresholds for screening DEGs were set as adjusted P < 0.05, | log2FC | ≥ 1. The probe name was transformed into standard gene name, and the volcano map of DEGs was drawn by ggplot2 package. P values were adjusted by the Bonferroni procedure.
2.3. Gene function enrichment and annotation
The ClusterProfiler visual R package was used to analyze the gene ontology (GO) functional enrichment and Kyoto Encyclopedia of genes and genomes (KEGG) pathway of DEGs between RM and normal tissues, and ggplot2 package was used to visualize the enrichment results according to P < 0.05 screening conditions.
2.4. Construction of protein-protein interaction network
The interaction network of proteins encoded by DEGs was constructed, the number of adjacent nodes in the interaction network was counted, and the key node genes were selected using String 10 (http://www.string-db.org). The differential proteins were classified by MCL algorithm, and the first cluster protein was selected. At the same time, using the cytohubba plug-in of Cytoscape 3.8.2 (Cytoscape Consoritum, NY, USA) to analyze the correlation degree of protein-protein interaction network, the key protein clusters in protein-protein interaction network were obtained and visualized in Cytoscape.
2.5. Correlation analysis of proteins
The Corrplot 0.84 package in R (Vienna University of Economics and Business, Vienna, Austria) was utilized to analyze and visualize the correlation of key genes. Pearson correlation coefficient is realized by cor function. The single Pearson correlation coefficient was tested by cor.mtest.
2.6. Treatment of pregnant golden hamsters with BaP
Golden Syrian hamster (Mesocricetus auratus), 8-week old, (150 ± 10) g body weight were used. The golden hamsters were paired for mating on a 1:1 male: female ratio per cages overnight. Vaginal smears were made at 8 am of the next day. Sperms were seen under the microscope, which was recorded as the first day after fertilization. Dams were randomly divided into control group, BaP2.5 group and BaP5.0 group, 7 hamsters per group. The dams in the control group were given intraperitoneal injection of normal saline, and dams in the BaP2.5 and BaP5.0 groups were injected intraperitoneally at BaP (Sigma-Aldrich, B1760) 2.5 mg/kg body weight and 5 mg/kg body weight, respectively. The animals were treated once a day from the 4th day after fertilization. All dams were sacrificed after anesthesia on the 14th day after fertilization, fetuses and placenta were separated, and the number of absorbed fetuses, stillborn fetuses and live fetuses were recorded respectively. The rate of adverse pregnancy outcomes = the number of adverse pregnancy outcomes/the total number of fetal rats.
2.7. Histology and immunohistochemistry
The middle part of the placenta was cut vertically. Three placentas were randomly selected from each litter and fixed in 4% paraformaldehyde to prepare paraffin sections and section (thickness 4 μm). Some sections were subjected to Hematoxylin and eosin staining for morphological analysis. Some sections were stained by immunohistoch-emistry (IHC). The IHC used was performed using Envision IHC kit (Gene Tech, Shanghai, China, GK600505) as described previously [14]. Briefly, endogenous peroxidase was blocked by hydrogen peroxide and methanol, and the antigens were retrieved by microwave heat-induced epitope retrieval method in citrate acid buffer (pH 6.0). Then the sections were incubated at 4 °C overnight with primary antibodies IL-18, IL-18BP, IL-18R and IL-1RN, respectively. And secondary antibodies labeled with horseradish peroxidase were applied and incubated for 20 min. After washing, diaminobenzidine (DAB) was used. Then, counterstaining with hematoxylin was performed and incubated for 2 min. Subsequently, the sections were dehydrated, mounted, and observed under the microscope. Replacement of the primary antibody with PBS was used as negative control. Five placentas from different groups were randomly selected. The integrated optical density (IOD) of those protein expressions was quantified by Image Pro Plus 6.0 (Media Cybernetics, MD, USA) software.
2.8. Statistical analysis
The R and GraphPad Prism 7 software (GraphPad PRISM, La Jolla, CA, USA) was used in the statistical analysis. For the OD values, their distribution was checked for compliance with the normal distribution. We used the Shapiro-Wilk normality test to confirm that the data met assumptions of the statistical approach. Two-tail unpaired Student’s t test was used for comparing two experimental groups, and one-way ANOVA was applied if needed to compare three or more experimental groups. Categorical variables were reported as frequencies and compared with chi-square or Fisher’s exact tests, as appropriate. The test results were considered statistically significant at p < 0.05.
3. Results
3.1. To screen differentially expressed genes associated with adverse pregnancy outcomes
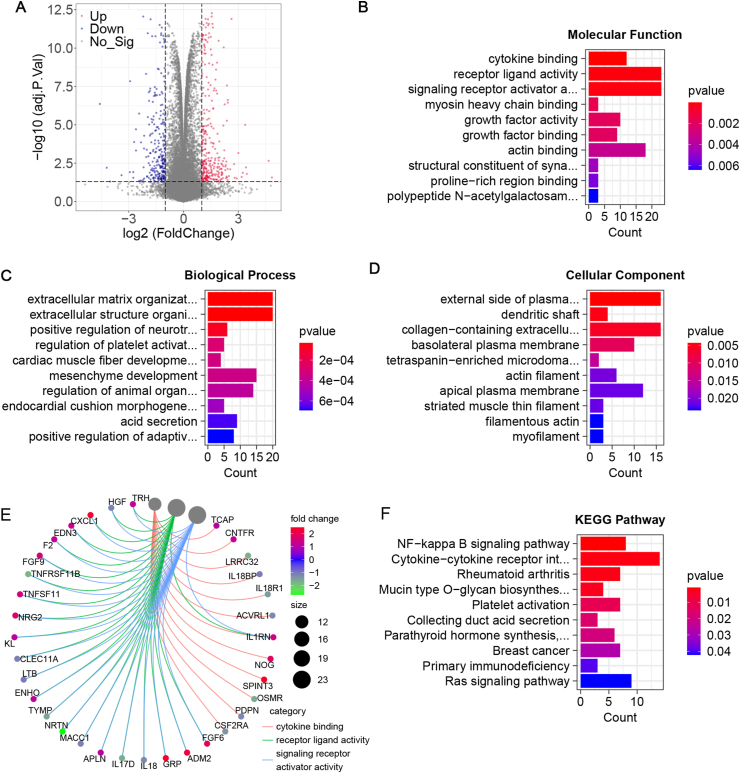
We screened 434 DEGs in the GEO series database (GSE26787), of which 239 genes were up-regulated and 195 genes were down regulated (Figure 1A). The results of GO enrichment showed that cytokine binding, receptor living activity, signaling receptor activator activity, extracellular matrix organization, extracellular structure organization, positive regulation of neurotransmitter transport, myosin heavy chain binding, growth factor activity, and growth factor binding were the significant (P < 0.05) (Figures 1B, 1C, 1D). And distribution of DEGs in GO terms was further analyzed and visualized by the R package. The results showed that DEGs were mainly enriched in cytokine binding, receiver ligand activity and signaling receiver activator activity (Figure 1E). Meanwhile, the analysis of enrichment of DEGs in KEGG pathway showed that there were significant differences in NF kappa B signaling pathway, cytokine receptor interaction, rheumatoid arthritis and other pathways (Figure 1F). These results suggest that the abnormality of cytokines may be related to the occurrence of spontaneous abortion.
Figure 1.
Screened the differentially expressed genes associated with adverse pregnancy outcomes. A. Differential gene expression data have been visualized in volcano plots. The standard thresholds for screening DEGs were set as adjusted P < 0.05, | log2FC | ≥ 1. B. Gene Ontology (GO) enrichment analysis for molecular functions. C GO enrichment analysis for biological processes. D GO enrichment analysis for cellular components. E. Distribution of candidate genes in GO term. F. KEGG pathway enrichment analysis.
3.2. To screen cytokines related spontaneous abortion
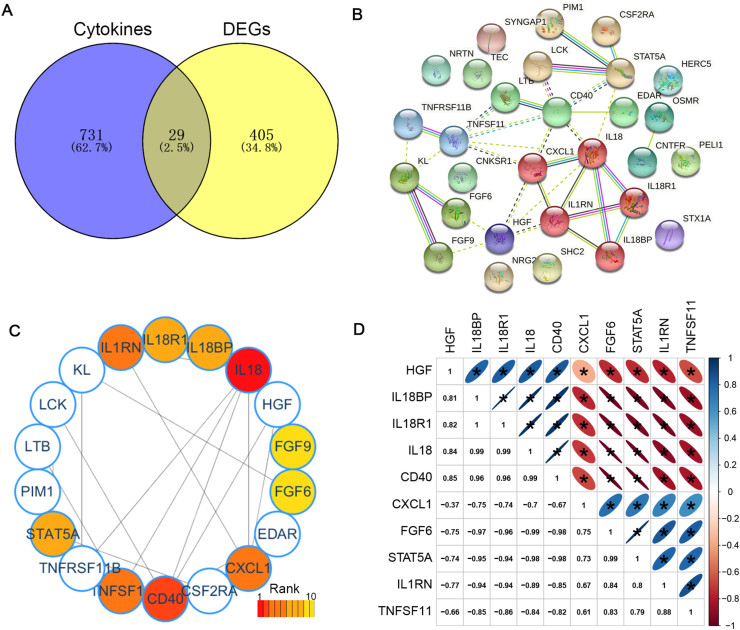
There are 760 genes in the Cytokine Signaling in Immune system (Pathcards of Genecards, https://pathcards.genecards.org). An intersection of the gene list of the cytokine signaling and the DEG list was assessed, and 29 candidate target genes were screened out (Figure 2A). The online tool String was used to analyze the interaction between 29 genes encoded proteins, and MCL algorithm was used to classify. The proteins in the first cluster included CXCL1, IL18, IL18BP, IL18R1, IL1RN (Figure 2B, red nodes). And the top 10 hub genes were analyzed by using MNC algorithm the cytohubba plug-in of the Cytoscape software. The hub genes (IL18, IL18BP, IL18R1, IL1RN, CXCL1, HGF, CD40, TNFSF11, STAT5A and FGF6) were obtained (Figure 2C). Next, we assessed the correlation of 10 hub genes. The results showed that IL18, IL18BP, IL18R1, CD40, and HGF were positively correlated with each other, CXCL1, FGF6, STAT5A, IL1RN and TNFSF11 were positively correlated with each other, besides IL18, IL18BP, IL18R1, CD40 and HGF were negatively correlated with CXCL1, FGF6, STAT5A, IL1RN and TNFSF11 (Figure 2D). The correlations among IL18, IL18BP, IL18R1 and IL1RN genes were high (R > 8.0). The results suggested that these proteins (IL18, IL18BP, IL18R1 and IL1RN) may be related to the occurrence of spontaneous abortion.
Figure 2.
Screened the cytokines related spontaneous abortion A. Venn diagrams indicating the overlap of the DEGs and cytokines. B. Protein–protein interaction (PPI) network of candidate target genes was generated using the STRING database. The color of the first cluster is red. C. Top 10 Hubba nodes by MNC algorithm of CytoHubba plug-in of the Cytoscape software. D. Heat map with Spearman correlations between the expressions of Hub genes. Red color indicates positive- and blue color negative correlation.
3.3. Effect of BaP on the embryonic development of golden hamster
It has been reported that there is a certain correlation between BaP exposure during pregnancy and adverse pregnancy outcomes. Therefore, we treated dams with different concentrations of BaP. The results showed that the number of embryos per litter in BaP 2.0 group (12.71 ± 2.29) was not significantly different from that in the control group (13.29 ± 1.97), but when the concentration of BaP reached 5.0 mg/kg, the number of embryos per litter decreased significantly (9.29 ± 2.81) (Figure 3A). The mortality of embryos in bap5.0 group (15.38%) was significantly higher than that in control group (3.45%) (Figure 3B). Next, to determine the size of the embryo we chose 5 measurement parameters, including diameter (max), diameter (min), diameter (mean), size (length), and size (width). The results showed that all measures in BaP5.0 group were significantly lower than those in control group and BaP2.0 group respectively (Figures 3C, 3D). It is suggested that exposure of dams to BaP 5.0 mg/kg can significantly affect the development of embryos.
Figure 3.
Effect of BaP on embryonic development of the golden hamster. A. The number of embryos per litter. B. The number of death and survival embryos in the control group, BaP2.5 group and BaP5.0 group. C. The size of embryos in the control group, BaP2.5 group and BaP5.0 group. The minimum diameter, maximum diameter, mean diameter, length and width of embryos were measured by Image Pro Plus. D. Representative images of embryos in the control group, BaP2.5 group and BaP5.0 group. ∗P < 0.05 compared with the control group.
3.4. Effects of BaP on placental morphology
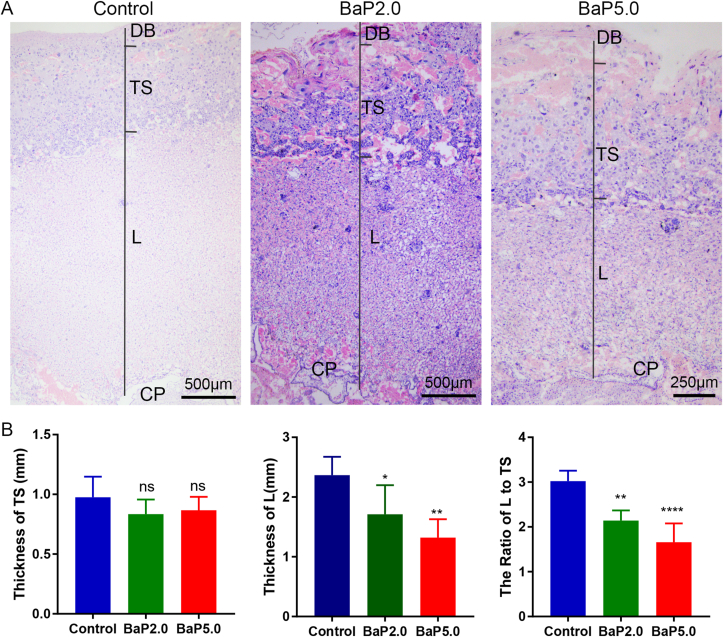
Several studies have revealed that tissue damage of the maternal-fetal interface is associated with adverse pregnancy outcomes [5, 15]. Accordingly, we assessed whether Bap exposure impairs the morphology of the maternal-fetal interface. Our histological results showed that BaP at 2.0 mg/kg and 5.0 mg/kg induced some inflammatory cells infiltrated in decidua basalis, and initiated a delay of labyrinth zone (∗P < 0.05, ∗∗∗P < 0.001) (Figure 4). Further analysis demonstrated that BaP at 2.0 mg/kg and 5.0 mg/kg decreased the ratio of the labyrinth zone to the trophospongium (∗P < 0.05, ∗∗∗P < 0.001) (Figure 4). These data suggest that the placental development in uterine exposed to BaP was delayed compared with those in the control group.
Figure 4.
Bap exposures impaired the morphology of the maternal-fetal interface. A. Representative HE staining images of placentas in control group, BaP2.5 group and BaP5.0 group. The trophospongium (TS), chorionic plate (CP), labyrinth zone (L) and decidua basalis (DB) are indicated. B. Quantification of thickness of the trophospongium and labyrinth zone. C. Ratio of the labyrinth zone to the trophospongium. ∗P < 0.05 compared with the control group (n = 5/group). Data is expressed as means ± SEM. Magnification ×40.
3.5. BaP affects the expression of IL18, IL18R1, IL18BP and IL1RN in the placenta of golden hamster
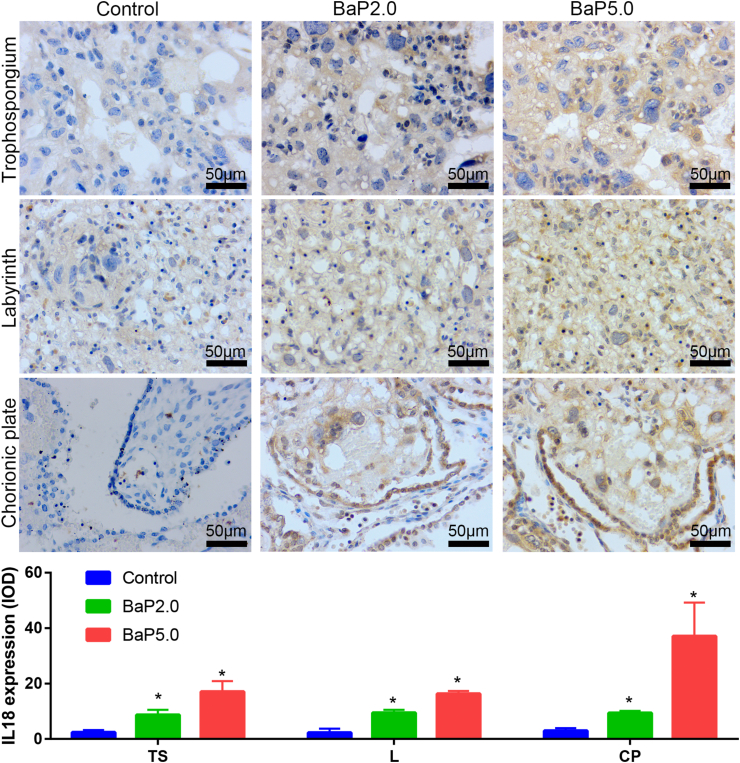
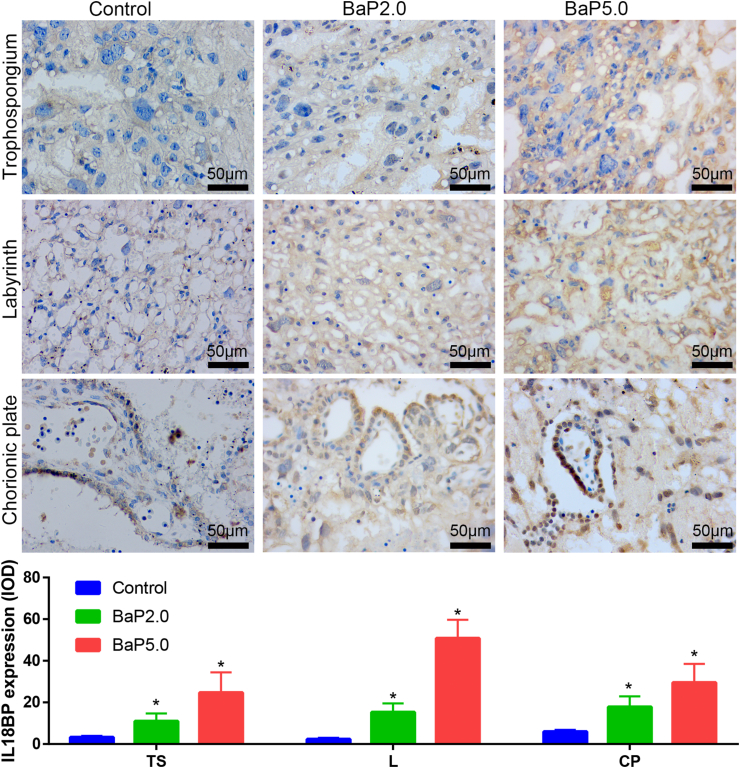
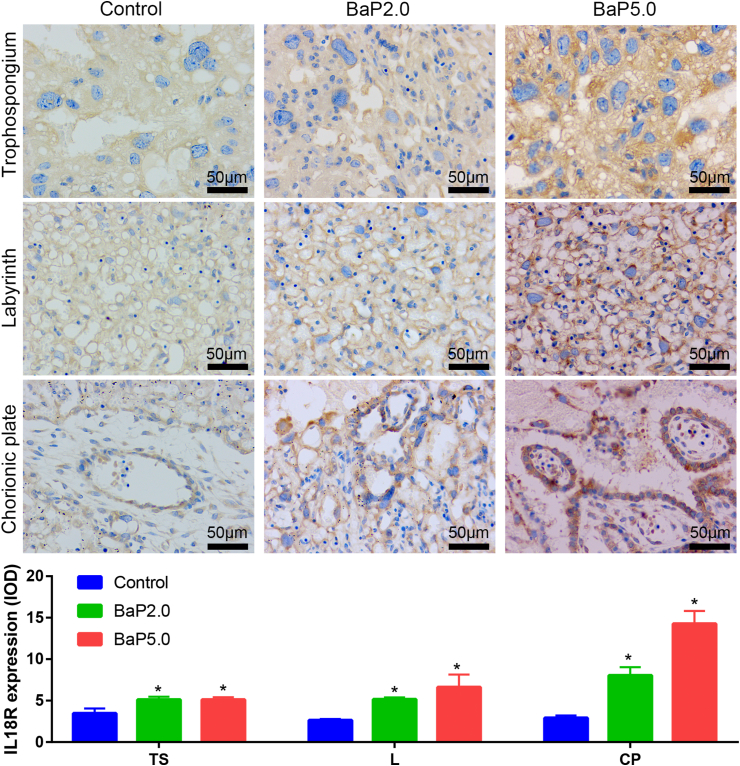
Next, in order to verify the results of bioinformatics analysis, the placental tissues of each group were taken and the expressions of IL18, IL18R1, IL18BP and IL1RN were detected by immunohistochemical technique. The results showed that the expression of IL-18, IL-18R1 and IL-18BP in Bap2.0 group and Bap5.0 group was higher than that in the control group, and the expression of IL-1RN was lower than that in the control group, which was consistent with the previous data analysis. It is suggested that BaP can increase the expression of IL18, IL-18R1 and IL-18BP, and decrease the expression of IL-1RN (Figures 5, 6, 7, and 8).
Figure 5.
BaP increased the expression of IL18 in the placenta of the golden hamster. Representative images of IL18 expression in trophospongium (TS), chorionic plate (CP), and labyrinth zone (L) are shown in the upper panel and integrated optical density (IOD) of those is shown in the lower panel. ∗P < 0.05 compared with the control group (n = 5/group). Magnification ×400.
Figure 6.
BaP increased the expression of IL18BP in the placenta of the golden hamster. Representative images of IL18BP expression in trophospongium (TS), chorionic plate (CP), and labyrinth zone (L) are shown in the upper panel and integrated optical density (IOD) of those is shown in the lower panel. ∗P < 0.05 compared with the control group (n = 5/group). Magnification ×400.
Figure 7.
BaP increased the expression of IL18R in the placenta of the golden hamster. Representative images of IL18R expression in trophospongium (TS), chorionic plate (CP), and labyrinth zone (L) are shown in the upper panel and integrated optical density (IOD) of those is shown in the lower panel. ∗P < 0.05 compared with the control group (n = 5/group). Magnification ×400.
Figure 8.
BaP decreased the expression of IL1RN in the placenta of the golden hamster. Representative images of IL18R expression in trophospongium (TS), chorionic plate (CP), and labyrinth zone (L) are shown in the upper panel and integrated optical density (IOD) of those are shown in the lower panel. ∗P < 0.05 compared with the control group (n = 5/group). Magnification ×400.
4. Discussion
Inflammatory factors are associated with different stages of embryonic development [1, 5]. The abnormal expression of inflammatory factors at the maternal fetal interface may lead to different types of adverse pregnancy outcome [7, 8, 10]. The results showed that high dose BaP exposure significantly increased the rate of adverse pregnancy outcome in pregnant golden hamsters. Meanwhile, the expressions of IL18, IL18BP and IL18R in placenta were increased, while the expression of IL1RN protein was decreased. It suggested that BaP may induce inflammatory reaction that causes adverse pregnancy outcomes.
PAHs mainly refer to hydrocarbons with two or more benzene rings, such as benzo [α] pyrene (BaP), benzo [α] anthracene, etc. PAHs may lead to inflammatory response directly or indirectly through oxidative stress [7, 16]. BaP exposure may lead to premature delivery through inflammatory reaction [6, 7]. In vivo and in vitro experiments showed that BaP may stimulate the secretion of inflammatory factors, activate IKK/NF κB signal transduction pathway, and induce the overexpression of prostaglandin cyclooxygenase II (COX-2) [17]. The increased expression of COX-2 in the placenta increases the synthesis of prostaglandins, and correspondingly increases the prostaglandins diffused to the uterine body and cervix. Prostaglandins induce uterine contraction, cervical ripening and rupture of membranes by autocrine and paracrine, which may be one reason for the initiation of labor [18]. In our experiment, pregnant golden hamsters were exposed to BaP. The results showed that the higher dose of BaP could increase the rate of adverse pregnancy outcome, and also significantly delay the process of embryonic development.
According to the data analysis of GEO database, the gene expressions of IL18, IL18BP and IL18R were up-regulated, while the expression of IL1RN was down regulated in the adverse pregnancy outcome group. IL18, a member of IL1 ligand family, is a critical regulator of innate and acquired immune response [19, 20]. Immune cells such as T cells, B cells, macrophages and monocytes express IL18, besides, many other non-immune cells such as epidermal cells and stromal cells [19].
IL-18 in the maternal-fetal interface enhances the cytotoxic activity of decidual lymphocytes [21]. Previous studies have shown that the expression level of IL-18 increased in serum and placenta of patients with recurrent abortion [22, 23], preeclampsia [24] and premature rupture of membranes [25]. IL18 plays a role in promoting inflammatory response by binding to its receptor [19]. IL-18R is expressed by using IL-12 and IFN in T cells and NK cells- α (human) stimulated or induced by signal transduction and transcriptional regulation through STAT4 (signal transduction and transcription activator 4), for effective IFN- γ Production is crucial [19]. IL-18R, which is also expressed by non-immune cells, such as epithelial cells and neurons, is involved in cell survival and differentiation [26, 27]. IL-18 can affect the expression of IL-18R in chorionic trophoblast and regulate the immune function of NK cells in decidua and peripheral blood in early pregnancy [21]. The binding affinity of IL-18BP to IL-18 is higher than IL-18R, which inhibits the biological function of IL-18 [26,28,29]. Some studies indicate that IFN-γ could up-regulate the expression level of IL-18BP by invoking a negative feedback loop for IL-18-mediated inflammation [19, 30]. IL1RN is an IL-1 receptor antagonist (IL-1RA), which has anti-inflammatory effect. We detected the expression of IL18, IL18BP, IL18R and IL1RN protein in the placenta of BaP exposed group. The results showed that the expressions of IL18, IL18BP and IL18R were increased in the placenta of BaP group, while the expression of IL1RN protein was decreased, consistent with the mRNA expression level gathered by bioinformatics analysis. The binding affinity of IL-18BP to IL-18 is higher than IL-18R, which inhibits the biological function of IL-18, however, we found that the both IL18BP and IL18R were increased in the placenta of BaP group. We speculated that BaP could cause inflammatory reaction by increasing the expression of IL18, and then affect the placental and embryonic development. Meanwhile, the over expression of IL18 might activate some mechanism to cause the increased expression of IL18BP to counteract the adverse effects of BaP. The specific feedback mechanism needs to be verified by subsequent experiments.
5. Conclusion
BaP may induce the inflammatory reaction to cause adverse pregnancy outcome by regulating the expression of IL18, IL18BP, IL18R and IL1RN. Our findings provide experimental basis for the prevention of adverse pregnancy outcome caused by BaP.
Declarations
Author contribution statement
Feibo Xu, Dong Wang: Conceived and designed the experiments; Analyzed and interpreted the data.
Heng Cai: Performed the experiments; Wrote the paper.
Hongxing Li: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the National Natural Science Foundation of China [No. 8167317].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Challis J.R., Lockwood C.J., Myatt L., Norman J.E., Strauss J.F., Petraglia F. Inflammation and pregnancy. Reprod. Sci. 2009;16(2):206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Flores V., Romero R., Miller D., Xu Y., Done B., Veerapaneni C., Leng Y., Arenas-Hernandez M., Khan N., Panaitescu B., et al. Inflammation-induced adverse pregnancy and neonatal outcomes can Be improved by the immunomodulatory peptide Exendin-4. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal K., Pierce S.H., Kozai K., Dhakal P., Scott R.L., Roby K.F., Vyhlidal C.A., Soares M.J. Evaluation of placentation and the role of the aryl hydrocarbon receptor pathway in a rat model of dioxin exposure. Environ. Health Perspect. 2021;129(11):117001. doi: 10.1289/EHP9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L., Tu J., Jiang Y., Zhou J., Yabe S., Schust D.J. Effects of lipopolysaccharide on human first trimester villous cytotrophoblast cell function in Vitro 1. Biol. Reprod. 2016;94(2):33. doi: 10.1095/biolreprod.115.134627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Zhou J., Fang M., Yu B. Pregnancy immune tolerance at the maternal-fetal interface. Int. Rev. Immunol. 2020;39(6):247–263. doi: 10.1080/08830185.2020.1777292. [DOI] [PubMed] [Google Scholar]
- 6.Polanska K., Dettbarn G., Jurewicz J., Sobala W., Magnus P., Seidel A., Hanke W. Effect of prenatal polycyclic aromatic hydrocarbons exposure on birth outcomes: the polish mother and child cohort study. BioMed Res. Int. 2014;2014:408939. doi: 10.1155/2014/408939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P., Hua R., Li K., Ma S., Wu B., Quan S., Yu Z. Polycyclic aromatic hydrocarbons exposure and early miscarriage in women undergoing in vitro fertilization-embryo transfer. Hum. Fertil(Camb). 2020;23(1):17–22. doi: 10.1080/14647273.2018.1479888. [DOI] [PubMed] [Google Scholar]
- 8.Erlandsson L., Lindgren R., Nääv Å., Krais A.M., Strandberg B., Lundh T., Boman C., Isaxon C., Hansson S.R., Malmqvist E. Exposure to wood smoke particles leads to inflammation, disrupted proliferation and damage to cellular structures in a human first trimester trophoblast cell line. Environ. Pollut. 2020;264:114790. doi: 10.1016/j.envpol.2020.114790. [DOI] [PubMed] [Google Scholar]
- 9.Alehashemi S., Goldbach-Mansky R. Human autoinflammatory diseases mediated by NLRP3-, pyrin-, NLRP1-, and NLRC4-inflammasome dysregulation updates on diagnosis, treatment, and the respective roles of IL-1 and IL-18. Front. Immunol. 2020;11:1840. doi: 10.3389/fimmu.2020.01840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrachnis N., Vitoratos N., Iliodromiti Z., Sifakis S., Deligeoroglou E., Creatsas G. Intrauterine inflammation and preterm delivery. Ann. Ny. Acad. Sci. 2010;1205(1):118–122. doi: 10.1111/j.1749-6632.2010.05684.x. [DOI] [PubMed] [Google Scholar]
- 11.Jain V.G., Willis K.A., Jobe A., Ambalavanan N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 2022;91(2):289–296. doi: 10.1038/s41390-021-01633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motomura K., Romero R., Garcia-Flores V., Leng Y., Xu Y., Galaz J., Slutsky R., Levenson D., Gomez-Lopez N. The alarmin interleukin-1α causes preterm birth through the NLRP3 inflammasome. Mol. Hum. Reprod. 2020;26(9):712–726. doi: 10.1093/molehr/gaaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lédée N., Munaut C., Aubert J., Sérazin V., Rahmati M., Chaouat G., Sandra O., Foidart J.M. Specific and extensive endometrial deregulation is present before conception in IVF/ICSI repeated implantation failures (IF) or recurrent miscarriages. J. Pathol. 2011;225(4):554–564. doi: 10.1002/path.2948. [DOI] [PubMed] [Google Scholar]
- 14.Yin C., Cai H., Yang D., Jian Y., Zhang J., Li Z., Wang D. Cigarette smoke induced neural tube defects by down-regulating noggin expression. Birth Defects Res. 2021;113(1):5–13. doi: 10.1002/bdr2.1804. [DOI] [PubMed] [Google Scholar]
- 15.Scott L.M., Bryant A.H., Rees A., Down B., Jones R.H., Thornton C.A. Production and regulation of interleukin-1 family cytokines at the materno-fetal interface. Cytokine. 2017;99:194–202. doi: 10.1016/j.cyto.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Hur D., Jeon J., Hong S. Analysis of immune gene expression modulated by benzo[a]pyrene in head kidney of olive flounder (Paralichthys olivaceus) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013;165(1):49–57. doi: 10.1016/j.cbpb.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang W., Ma Q., Li J., Zhang D., Ding J., Huang Y., Xing M., Huang C. Benzo[a]pyrene diol-epoxide (B[a]PDE) upregulates COX-2 expression through MAPKs/AP-1 and IKKβ/NF-κB in mouse epidermal Cl41 cells. Mol. Carcinog. 2007;46(1):32–41. doi: 10.1002/mc.20260. [DOI] [PubMed] [Google Scholar]
- 18.Loudon J.A.Z., Groom K.M., Bennett P.R. Prostaglandin inhibitors in preterm labour. Best Pract. Res. Cl Ob. 2003;17(5):731–744. doi: 10.1016/s1521-6934(03)00047-6. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda K., Nakanishi K., Tsutsui H. Interleukin-18 in health and disease. Int. J. Mol. Sci. 2019;20(3):649. doi: 10.3390/ijms20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou T., Damsky W., Weizman O., McGeary M.K., Hartmann K.P., Rosen C.E., Fischer S., Jackson R., Flavell R.A., Wang J., et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature. 2020;583(7817):609–614. doi: 10.1038/s41586-020-2422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokmadzic V.S., Tsuji Y., Bogovic T., Laskarin G., Cupurdija K., Strbo N., Koyama K., Okamura H., Podack E.R., Rukavina D. IL-18 is present at the maternal-fetal interface and enhances cytotoxic activity of decidual lymphocytes. Am. J. Reprod. Immunol. 2002;48(4):191–200. doi: 10.1034/j.1600-0897.2002.01132.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang F., Zhu H., Li B., Liu M., Liu D., Deng M., Wang Y., Xia X., Jiang Q., Chen D. Effects of human chorionic gonadotropin, estradiol, and progesterone on interleukin-18 expression in human decidual tissues. Gynecol. Endocrinol. 2017;33(4):265–269. doi: 10.1080/09513590.2016.1212829. [DOI] [PubMed] [Google Scholar]
- 23.Löb S., Ochmann B., Ma Z., Vilsmaier T., Kuhn C., Schmoeckel E., Herbert S., Kolben T., Wöckel A., Mahner S., et al. The role of Interleukin-18 in recurrent early pregnancy loss. J. Reprod. Immunol. 2021;148:103432. doi: 10.1016/j.jri.2021.103432. [DOI] [PubMed] [Google Scholar]
- 24.Huang X., Huang H., Dong M., Yao Q., Wang H. Serum and placental interleukin-18 are elevated in preeclampsia. J. Reprod. Immunol. 2005;65(1):77–87. doi: 10.1016/j.jri.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Menon R., Lombardi S.J., Fortunato S.J. IL-18, a product of choriodecidual cells, increases during premature rupture of membranes but fails to turn on the Fas-FasL-mediated apoptosis pathway. J. Assist. Reprod. Genet. 2001;18(5):276–284. doi: 10.1023/A:1016626620137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur D., Chachi L., Gomez E., Sylvius N., Interleukin-18 C.E. Brightling. IL-18 binding protein and IL-18 receptor expression in asthma: a hypothesis showing IL-18 promotes epithelial cell differentiation. Clin. Transl. Immunol. 2021;10(6):e1301. doi: 10.1002/cti2.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwahara-Otani S., Maeda S., Kobayashi K., Minato Y., Tanaka K., Yamanishi K., Hata M., Li W., Hayakawa T., Noguchi K., et al. Interleukin-18 and its receptor are expressed in gonadotropin-releasing hormone neurons of mouse and rat forebrain. Neurosci. Lett. 2017;650:33–37. doi: 10.1016/j.neulet.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Yoshino O., Osuga Y., Koga K., Tsutsumi O., Yano T., Fujii T., Kugu K., Momoeda M., Fujiwara T., Tomita K., et al. Evidence for the expression of interleukin (IL)-18, IL-18 receptor and IL-18 binding protein in the human endometrium. Mol. Hum. Reprod. 2001;7(7):649–654. doi: 10.1093/molehr/7.7.649. [DOI] [PubMed] [Google Scholar]
- 29.Aizawa Y., Akita K., Taniai M., Torigoe K., Mori T., Nishida Y., Ushio S., Nukada Y., Tanimoto T., Ikegami H., et al. Cloning and expression of interleukin-18 binding protein. FEBS Lett. 1999;445(2-3):338–342. doi: 10.1016/s0014-5793(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 30.Mühl H., Kämpfer H., Bosmann M., Frank S., Radeke H. J. Pfeilschifter. Interferon-γ mediates gene expression of IL-18 binding protein in nonleukocytic cells. Biochem. Biophys. Res. Commun. 2000;267(3):960–963. doi: 10.1006/bbrc.1999.2064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.