Abstract
Introduction:
Despite evidence from clinical trials of favorable shifts in cancer stage and improvements in lung cancer specific mortality, the effectiveness of lung cancer screening (LCS) in clinical practice has not been clearly demonstrated.
Methods:
We performed a multicenter cohort study of patients diagnosed with a primary lung cancer between January 1, 2014, and September 30, 2019, at one of four US healthcare systems. The primary outcome variables were cancer stage distribution and annual age-adjusted lung cancer incidence. The primary exposure variable was receipt of at least one low-dose CT for LCS prior to cancer diagnosis.
Results:
3,678 individuals were diagnosed with an incident lung cancer during the study period; 404 (11%) of these patients were diagnosed after initiation of LCS. As screening volume increased, the proportion of patients diagnosed with lung cancer after LCS initiation also rose from 0% in Q1 of 2014 to 20% in Q3 of 2019. LCS did not result in a significant change in the overall incidence of lung cancer (AAPC, −0.8 [95% CI −4.7, 3.2]) between 2014 and 2018. Stage specific incidence rates increased for Stage I cancer (AAPC, 8.0 [95% CI 0.8, 15.7]) and declined for Stage IV disease (AAPC, −6.0 [95% CI −11.2, −0.5]).
Conclusions:
Implementation of LCS at four diverse healthcare systems has resulted in a favorable shift to a higher incidence of Stage I cancer with an associated decline in Stage IV disease. Overall lung cancer incidence did not increase, suggesting a limited impact of over-diagnosis.
Keywords: lung cancer, screening, stage migration
INTRODUCTION
Lung cancer represents a substantial portion of the overall burden of cancer worldwide and accounted for approximately 235,000 deaths in United States in 2021.1 Survival is strongly associated with stage of disease at time of diagnosis, but historically, the majority of lung cancers are diagnosed at late stage when curative treatment options are expensive and survival probabilities are limited.2 Five-year stage-specific survival rates range from greater than 53–69% for patients diagnosed with Stage I disease to less than 10% for patients with distant organ metastasis.3,4
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in lung cancer mortality with annual low dose CT (LDCT) among high-risk individuals.5 The Dutch–Belgian lung-cancer screening trial (Nederlands–Leuvens Longkanker Screenings Onderzoek [NELSON]) recently confirmed the mortality benefit of annual LDCT screening.6 Efforts in many countries are underway to either initiate or accelerate implementation of LCS given these recent findings. Both the NLST and NELSON trials demonstrated a significant shift in the distribution of cancer stage between LDCT and control arms, with more early stage and fewer advanced stage cancers identified with screening. The presence of this ‘stage shift’ coupled with timely evaluation, diagnosis and treatment likely represents the primary mechanism through which annual screening results in improved lung cancer specific mortality. The trial results also support our understanding of the natural history of lung cancer that suggest a progression of disease from undetectable lesions to localized tumors, followed by loco-regional involvement and later development of distant metastases. However, concerns persist regarding the detection of a large proportion of indolent cancers resulting in unnecessary diagnosis and over-treatment.7
Despite the evidence from NLST and NELSON on stage shift and lung cancer specific mortality, the effectiveness of LDCT screening in clinical practice has not been clearly demonstrated. Both trials were well-conducted, achieving high rates of adherence (>95%) and were performed largely in centers of excellence that may have resulted in optimal outcomes from diagnostic evaluation and subsequent cancer treatment. Enrolled participants were also younger, more educated, and had fewer comorbidities than the population of individuals that are potentially eligible for screening in real world settings.8,9 Concerns have also been raised that harms from screening may be greater in clinical practice have persisted and may be contributing to the slow uptake of lung cancer screening that has been observed in the United States.10,11 Emerging evidence also suggests that adherence to annual screening is considerably lower than the rates observed in the NLST and NELSON trials, which may diminish the mortality benefit observed in trial settings.12–14
Although the assessment of screening benefits, such as lung cancer mortality, are difficult to measure and may only be observed a decade after implementation of LCS in clinical practice, the distribution of cancer stage among screened individuals may allow for an early indication of the impact of screening on lung cancer outcomes. Cancer registry based analyses and individual lung cancer screening programs have started to report initial outcomes and many, though not all, have reported favorable distributions of cancer stage among their population of patients diagnosed after screening initiation.15–18 Although promising, these studies have been limited by their inability to identify the source population and meaningfully compare stage outcomes among individuals with lung cancer diagnosed among screened and unscreened groups. The aim of the present study was to evaluate the impact of LDCT-based screening on shifting the stage distribution towards earlier stage disease across a well-characterized source population, assess the effect of screening on stage-specific incidence over time, and identify factors, in addition to screening, which increase the likelihood of an early-stage diagnosis.
METHODS
Study Setting and Data Sources
The Population-based Research to Optimize the Screening Process (PROSPR) Lung Consortium is a collaboration of five diverse healthcare systems, including Henry Ford Health System (HFHS), Kaiser Permanente Colorado (KPCO), Kaiser Permanente Hawaii (KPHI), Marshfield Clinic Health System (MCHS), and the University of Pennsylvania Health System (UPHS). The current retrospective cohort analysis excluded MCHS due to incomplete cancer stage information for the full study period. PROSPR-Lung developed a standardized Common Data Model containing data on patients aged 35 to 89 who were affiliated with any of these five healthcare systems from January 1, 2010, through September 31, 2019. This Common Data Model includes harmonized data derived from administrative, electronic health record (EHR), and claims systems that is supplemented with limited chart review. Two of the healthcare systems (KPCO and KPHI) operate under an integrated care delivery model and the other two (HFHS and UPHS) limited the cohort to individuals that received primary care within their systems. Variables include patient demographics, details of lung cancer screening, procedures, diagnoses, census-based measures of socioeconomic status, and cancer registry variables collected in a manner consistent with the North American Association of Central Cancer Registries standards. Cancer registry data are obtained from manual review by certified tumor registrars and includes date of diagnosis, cancer stage, tumor characteristics, and first-course therapy. The study was reviewed and approved by the Institutional Review Board (IRB) at KPCO, the IRB of record for PROSPR-Lung, which waived the informed consent requirement because this observational study presented minimal risks to the participants whose data were analyzed.
Study Population and Variables
We restricted the cohort to adults diagnosed with primary in situ or invasive lung cancer between January 1, 2014, and September 30, 2019. Participants were excluded if they had a previous diagnosis of lung cancer, were younger than age 55 or older than age 80 or had a tobacco use history documented as “Never” or was missing. The primary outcome variable was cancer stage based on the American Joint Commission on Cancer (AJCC) system in place in the year of diagnosis and was extracted by the local cancer registry at each of institution. The primary exposure variable was the performance of at least one LDCT for lung cancer screening prior to lung cancer diagnosis. LCS-LDCT scans were identified by resulted radiology scans under one of the following CPT or HCPC codes: G0297, S8032, or 71250. We collected sociodemographic and clinical variables including age, sex, prior malignancy, comorbid conditions, chronic obstructive pulmonary disease (COPD) in the year preceding lung cancer diagnosis, and self-reported race/ethnicity, and smoking behavior. We ascertained tobacco use and body mass index (BMI) at the last date with available data in the EHR prior to lung cancer diagnosis. We used the patient’s home address mapped to census tract level information to determine the Yost Index as a measure of socioeconomic status.19
Statistical Analysis
Descriptive statistics were computed using frequencies and proportions to describe the distribution of baseline patient and tumor characteristics among individuals with and without at least one LCS-LDCT prior to diagnosis and by year of diagnosis. Age-adjusted incidence rates, including both invasive and in-situ cancers, were calculated by year between 2014 and 2018 using the age distribution of the US 2000 population as the standard. Patients of each healthcare system with a recorded encounter within the year of interest were included in the person-years-at-risk denominator calculation. Trends in incidence rates over time were analyzed using joinpoint regression analysis and summarized with average annual percentage change (AAPC) and 95% CI.20 Differences in the distribution of categorical variables between screened and unscreened groups and by year of diagnosis were compared using chi-squared tests. Risk differences (and corresponding 95% confidence intervals) were calculated as the difference in the proportion of cancer stage and histological subgroups by screening history.
For multivariable analyses, we assessed factors associated with a greater likelihood of an early-stage diagnosis. For this analysis, cancer stage was categorized as early-stage, defined as an AJCC stage 0, I, or II, and late-stage, defined as AJCC stage III or IV. For this component of the analysis, we excluded lung cancer cases with unknown stage, carcinoid histology, or a diagnosis in 2014. (Figure 1) The proportion of early and late-stage lung cancer and corresponding 95% CI intervals were calculated overall and across subgroups of a priori identified variables. Generalized estimating equations (GEE) with a generalized logit distribution and unstructured covariance structure with robust standard errors were used to estimate adjusted odds ratios (OR) and 95% confidence intervals (CIs).21 Models were clustered on healthcare system and included the following factors: age at time of lung cancer diagnosis, gender, race/ethnicity, year of diagnosis, histology (small vs non-small cell, excluding carcinoid), smoking history, receipt of prior lung cancer screening, COPD, a previous non-lung cancer malignancy, body mass index (BMI), and socio-economic status as measured by the Yost Index. Customary residual and effect statistics were examined to assess model fit and evaluate for outliers.
Figure 1. Screening Volume and Lung Cancer Diagnoses, 2014–2019.
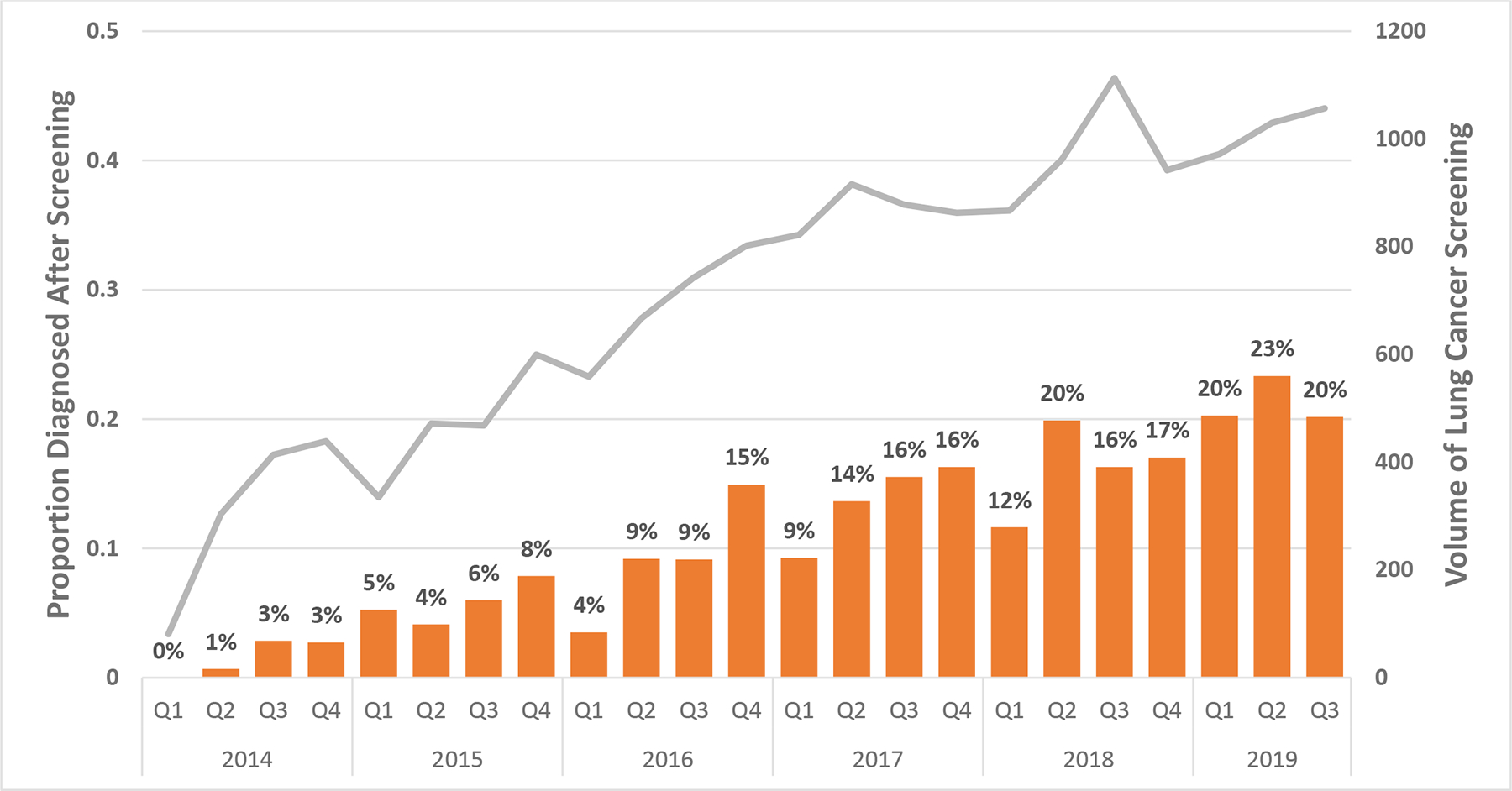
Bars demonstrates the proportion of incident lung cancer diagnoses after at least one prior LCS-LDCT by quarter between 2014 and 2019. The gray line demonstrates the total volume of LCS-LDCT scans performed in that quarter.
To address missing data for BMI (6%) and Yost Index (3%), we used multiple imputation by chained equations (MICE).22 All variables (outcome, exposure, and covariates) in the outcome model were included as independent variables in the imputation models. We performed 20 iterations of imputation and combined them.22,23 To assess the quality of our imputed data we compared distributional characteristics pre- and post-imputation. Analyses were performed using SAS® version 9.4M6 (SAS Institute Inc., Cary, North Carolina) and Joinpoint Regression Program version 4.8.0.1
RESULTS
Study Population
A total of 6012 individuals between the ages of 35 and 89 were diagnosed with a primary lung cancer during the study period. For the primary analysis, we excluded patients with a prior diagnosis of lung cancer (N=155), age < 55 or > 80 years (n=1,383), unknown/missing tobacco history (n=322), and individuals that never smoked (n=474), resulting in a cohort of 3678 individuals with an incident lung cancer; 404 (11%) individuals were diagnosed with lung cancer after screening. (Supplemental Figure 1) The median age of the cohort was 69 years (interquartile range [IQR], 64 to 74 years) and was composed of 1,922 female (52%) and 1,756 male (48%) patients. There were more previous (2,299 [63%]) than current smokers (1,379 [37%]). Most patients were non-Hispanic White (2,442 [66%]; Non-Hispanic Black (713 [19%]); or Asian, Native Hawaiian, or Pacific Islander (276 [8%]). (Table 1) Compared to patients diagnosed with lung cancer in the absence of screening, patients diagnosed after screening were more likely Non-Hispanic White, individuals who currently smoke, have greater comorbid illness, and more likely to have a diagnosis of COPD.
TABLE 1.
Baseline Characteristics of Patients Diagnosed with Lung Cancer, 2014–2019
| Variable | Lung Cancer Diagnoses, No (%) | |||
|---|---|---|---|---|
| Overall (N=3678) |
No Prior LCS (N=3274) |
LCS (N=404) |
p-value | |
| Age at Diagnosis | 0.0020 | |||
| 55 – 64 | 1025 (28) | 908 (28) | 117 (29) | |
| 65 – 74 | 1741 (47) | 1526 (47) | 215 (53) | |
| 75 – 80 | 912 (25) | 840 (26) | 72 (18) | |
| Sex | 0.0889 | |||
| Female | 1922 (52) | 1727 (53) | 195 (48) | |
| Male | 1756 (48) | 1547 (47) | 209 (52) | |
| Race and Ethnicity | 0.0098 | |||
| Non- Hispanic White | 2442 (66) | 2147 (66) | 295 (73) | |
| Non-Hispanic Black | 713 (19) | 657 (20) | 56 (14) | |
| Asian, Native Hawaiian, or Pacific Islander | 276 (8) | 254 (8) | 22 (5) | |
| Hispanic | 112 (93) | 98 (3) | 14 (3) | |
| American Indian, Other, or Unknown | 135 (4) | 118 (4) | 17 (4) | |
| Smoking History | < .0001 | |||
| Current | 1379 (37) | 1161 (35) | 218 (54) | |
| Former | 2299 (63) | 2113 (65) | 186 (46) | |
| Health System | < .0001 | |||
| Site 1 | 1368 (37) | 1227 (37) | 141 (35) | |
| Site 2 | 1031 (28) | 851 (26) | 180 (45) | |
| Site 3 | 414 (11) | 384 (12) | 30 (7) | |
| Site 4 | 865 (24) | 812 (25) | 53 (13) | |
| Modified CCI a | 0.0028 | |||
| 0 | 589 (16) | 544 (17) | 45 (11) | |
| 1 | 514 (14) | 450 (14) | 64 (16) | |
| 2 | 563 (15) | 483 (15) | 80 (20) | |
| 3+ | 2012 (55) | 1797 (55) | 215 (53) | |
| COPD b | 1683 (46) | 1438 (44) | 245 (61) | < .0001 |
| Previous Cancer | 445 (12) | 403 (12) | 42 (10) | 0.2659 |
| BMI | 0.0983 | |||
| < 25 | 1437 (39) | 1267 (39) | 170 (42) | |
| 25 – 29 | 1197 (33) | 1058 (32) | 139 (34) | |
| 30 + | 991 (27) | 903 (28) | 88 (22) | |
| Missing | 53 (1) | 46 (1) | 7 (2) | |
| Socioeconomic Status (Yost Index) | 0.4173 | |||
| Quintile 1 (lowest) | 894 (24) | 810 (25) | 84 (21) | |
| Quintile 2 | 603 (16) | 534 (16) | 69 (17) | |
| Quintile 3 | 668 (18) | 587 (18) | 81 (20) | |
| Quintile 4 | 613 (17) | 535 (16) | 78 (19) | |
| Quintile 5 (highest) | 769 (21) | 690 (21) | 79 (20) | |
| Missing | 131 (4) | 118 (4) | 13 (3) | |
Modified CCI: Excludes AIDS diagnosis
COPD diagnosis code (ICD 9/10: 491, 492, 496, J41, J42, J43, J44) in year prior to lung cancer diagnosis
Lung Cancer Screening Activity and Lung Cancer Incidence
Lung cancer screening activity, measured by the combined volume of baseline and annual scans, increased steadily over the study period, increasing from a total of 1,238 screenings in first quarter (Q1) of 2014 to 3,059 screenings in the third quarter (Q3) of 2019. As screening volume increased, the proportion of patients diagnosed with lung cancer after screening also rose from 0% in Q1 of 2014 to 20% in Q3 of 2019. (Figure 1) Initiation of LCS did not result in a significant change in the overall incidence of lung cancer (AAPC, − 0.8 [95% CI −4.7, 3.]) between 2014 and 2018. (Table 2) Stage specific incidence rates increased for Stage I (AAPC, 8.0 [95% CI 0.8, 15.7]), declined for Stage IV (AAPC, −6.0 [95% CI −11.2, −0.5]), and were not significantly altered for Stage II (AAPC, −7.5 [95% CI −28.7, 20.1]) or Stage III (AAPC, −3.3 [95% CI −14.2, 9.0]) lung cancer. (Table 2 and Figure 2) Very few Stage 0 cancers (n=11) were diagnosed during this timeframe, limiting the assessment of incidence rate for in-situ cancers.
TABLE 2.
Age-Standardized Overall and Stage-Specific Lung Cancer Incidence Rates, 2014 to 2018a
| Yearb | Overall | Stage 0 | Stage I | Stage II | Stage III | Stage IV | Unknown Stage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung Cancer Diagnoses | Lung Cancer Rate | Lung Cancer Diagnoses | Lung Cancer Rate | Lung Cancer Diagnoses | Lung Cancer Rate | Lung Cancer Diagnoses | Lung Cancer Rate | Lung Cancer Diagnoses | Lung Cancer Rate | Lung Cancer Diagnoses | Lung Cancer Rate | Lung Cancer Diagnoses | Lung Cancer Rate | |
| 2014 | 560 | 151.6 | 0 | 0.00 | 138 | 37.96 | 61 | 16.40 | 100 | 26.63 | 240 | 65.01 | 21 | 5.6 |
| 2015 | 650 | 164.4 | 4 | 1.07 | 178 | 44.91 | 59 | 15.38 | 126 | 31.40 | 271 | 68.54 | 12 | 3.1 |
| 2016 | 652 | 158.5 | 0 | 0.00 | 205 | 50.23 | 37 | 9.06 | 129 | 30.93 | 260 | 63.23 | 21 | 5.0 |
| 2017 | 690 | 153.7 | 2 | 0.48 | 211 | 47.17 | 68 | 15.30 | 132 | 28.35 | 259 | 58.06 | 18 | 4.3 |
| 2018 | 739 | 150.3 | 5 | 0.97 | 263 | 54.31 | 52 | 11.15 | 119 | 23.67 | 259 | 51.85 | 41 | 8.4 |
| AAPC | −0.8 [95%CI, −4.7, 3.2] | N/Ac | 8.0 [95%CI, 0.8–15.7] | −7.5 [95%CI, −28.7, 20.1] | −3.3 [95%CI, −14.2, 9.0] | −6.0 [95%CI, −11.2, −0.5] | 12.0 (95%CI, −22.1, 60.9) | |||||||
132 individuals that were age 80 were combined with the individuals aged 75–79 for the age adjustment calculations.
Total person-years by year: 413,511.3 (2014); 440,530.4 (2015), 468,131.5 (2016), 490,317.4 (2017), 520,511.2 (2018).
The AAPC is not reported for Stage 0 given the small number of cases which does not allow for an accurate calculation.
Abbreviations: AAPC (Average Annual Percent Change)
FIGURE 2. Stage-Specific Trends in Lung Cancer Incidence for Overall Cohort, 2014–2018.

a Stage 0 lung cancer excluded given the small number of in-situ lung cancer diagnoses (n=11)
Impact of Screening on Histology and Stage Migration
The distribution of tumor histology between screened and unscreened patients did not differ significantly between groups. (Table 3) In particular, there were no differences in the proportion of two most common histological subtypes, adenocarcinoma (47.0 vs. 42.8%; risk difference [RD], − 4.2 [95% CI, −9.3, 1.0]) and squamous cell carcinoma (23.1% vs. 26.5%, RD 3.4 [95% CI −1.1, 8.0]) between the unscreened and screened groups. Screening was, however, associated with an increase in the proportion of Stage I cancer (54.7% vs. 27.9%, RD 26.8 [95% CI 21.7, 31.9]) with a concomitant decrease in Stage IV cancer (17.6% vs. 41.7%, RD −24.1 [95% CI −28.2, −20.0]) compared to the population diagnosed with lung cancer detected without prior screening. In contrast to the observed rates with Stage I and IV, the proportion of Stage II and III lung cancer were similar between screened and unscreened groups. (Table 2)
TABLE 3.
Differences in Lung Cancer Stage by Prior LCS, 2014–2019
| No LCS (N=3274) |
LCS (N=404) |
Risk Difference (95% CI) | |
|---|---|---|---|
| Tumor Histology, N (%) | |||
| Adenocarcinoma | 1538 (47.0) | 173 (42.8) | −4.2 (−9.3, 1.0) |
| Squamous Cell | 755 (23.1) | 107 (26.5) | 3.4 (−1.1, 8.0) |
| Large Cell | 22 (0.7) | 7 (1.7) | 1.1 (−0.2, 2.4) |
| Non-Small Cell/Other | 440 (13.4) | 58 (14.4) | 0.9 (−2.7, 4.5) |
| Small cell | 456 (13.9) | 47 (11.6) | −2.3 (−5.6, 1.1) |
| Carcinoid | 63 (1.9) | 12 (3.0) | 1.1 (−0.7, 2.8) |
| AJCC Stage, N (%) | |||
| 0/I | 915 (27.9) | 221 (54.7) | 26.8 (21.7, 31.9) |
| II | 268 (8.2) | 36 (8.9) | 0.7 (−2.2, 3.7) |
| III | 601 (18.4) | 66 (16.3) | −2.0 (−5.9, 1.8) |
| IV | 1365 (41.7) | 71 (17.6) | −24.1 (−28.2, −20.0) |
| Unknown/Missing/Occult/NA | 125 (3.8) | 10 (0.5) | −1.3 (−3.0, 0.3) |
Factors Associated with a Diagnosis of Early-Stage Lung Cancer
Among those with lung cancer, we used multivariable regression to identify factors associated with a diagnosis of early-stage disease (Stage 0, I, and II) independent of lung cancer screening. Compared to patients diagnosed without prior lung cancer screening, a diagnosis of lung cancer after any screening had an increased odds of early-stage diagnosis in both unadjusted and multivariable models (aOR: 3.61, 95% CI: 2.81–4.65). (Figure 3; Supplemental Table 1) Additional factors associated with the an increased likelihood of an early-stage diagnosis included: older age (aOR1.36, 95% CI 1.08–1.70, for ages 75–80 years compared to ages 55–64 years ), female sex (aOR 1.49, 95% CI 1.27–1.75), former tobacco use (aOR1.29, 95% CI 1.08–1.54), a diagnosis of COPD (aOR 1.61, 95% CI 1.37–1.90) or prior malignancy other than lung cancer (aOR 1.82, 95% CI 1.43–2.33), and an elevated BMI (aOR 1.27, CI 1.04–1.55 for BMI >30 kg/m2 compared to BMI < 25 kg/m2). (Table 3) Patients with small cell lung cancer had a decreased odds of early-stage disease compared to those with non-small cell histology (aOR 0.12, CI 0.09–0.18). There was no association of socioeconomic status or race and ethnicity with early-stage diagnosis.
FIGURE 3. Factors Associated with a Diagnosis of Early-Stage Lung Cancer.
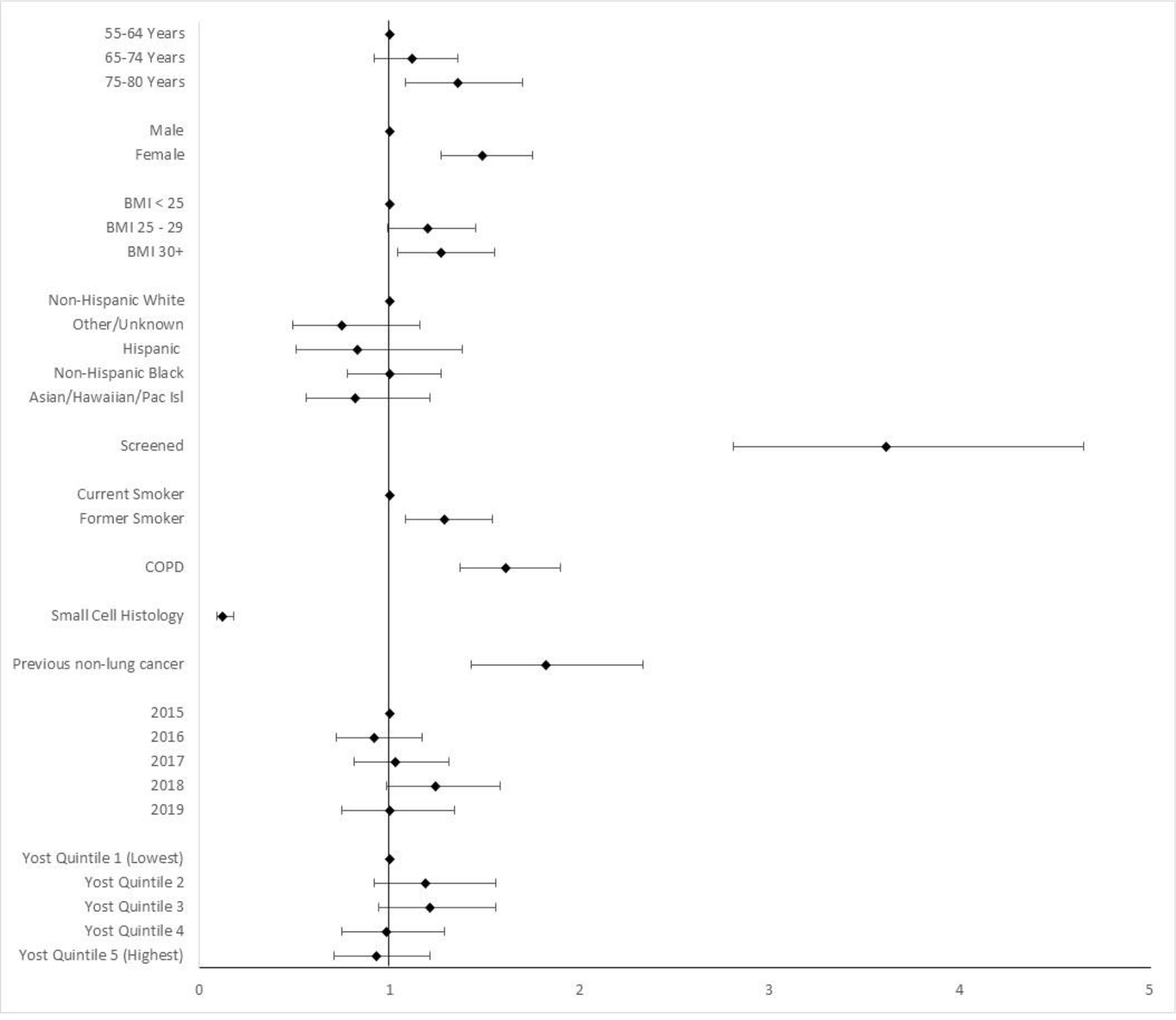
a early stage defined as Stage 0, I, and II
DISCUSSION
Although trials have shown that lung cancer screening with LDCT reduces lung cancer specific mortality, the effectiveness of LDCT in clinical practice has not been clearly demonstrated. In this multicenter cohort analysis, we examined the impact of screening on the distribution of cancer stage and lung cancer incidence and demonstrate several important findings during this relatively early phase of lung cancer screening implementation. First, among individuals who were diagnosed with lung cancer after being screened during this time period, most (64%) were identified at an early-stage, and this was accompanied by a concomitant decrease in patients with metastatic lung cancer. Second, at the population level across the four healthcare systems evaluated in this analysis, the overall annual incidence of lung cancer was relatively stable; however, there were notable changes in the incidence of stage-specific disease as the rate of screening increased over time. The annual rate of Stage I lung cancer increased by an average of 8.4% and was accompanied by an average decline of 6.6.% in Stage IV disease. By 2018, these changes in incidence resulted in a higher rate of Stage I compared to Stage IV cancers. This migration to early-stage disease with no change in the overall incidence of lung cancer suggests that implementation of screening was achieving the desired effect of identifying early-stage lung cancers that were destined to progress to more advanced stages of disease, and without resulting in a significant rate of overdiagnosis. While over-diagnosis is difficult to explicitly define, our findings stand in contrast to the evidence of overdiagnosis from LDCT screening in a largely nonsmoking population of women in Taiwan noted by Gao et.al24 who reported a 6-fold increase in the incidence of early-stage lung cancer without change in the incidence of late-stage lung cancer.
Differences between efficacy and effectiveness with respect to the benefit of lung cancer screening may exist if implementation varies in important aspects from how it was administered in the randomized trials. The NLST and NELSON trials were conducted primarily in urban academic centers and had resources and processes in place to optimize study procedures, evaluation of positive findings, including timely evaluation and minimizing harms from invasive evaluation. Whether the results observed in these trials will result in similar outcomes with screening in community-based settings as a part of standard clinical care remains unclear. For example, study procedures utilized in the NLST and NELSON trials resulted in very high adherence to annual screening, but observed rates in community settings have been considerably lower, including at the health systems included in this analysis.12,14,25
Despite lower adherence across the four systems, the distribution of cancer stage was nearly as favorable as the distribution observed in the NLST and NELSON trials. For example, when compared to NLST we observed a slightly lower rate of stage I disease (55% vs. 61%) diagnosed after screening, but this was considerably higher than the rate of stage I disease in the non-screened population (28%). Our findings build on the results of prior analyses from community and academic settings which have shown a favorable shift in stage distribution among screened populations, including a prior report from one healthcare system within our multicenter cohort.26 In the first year of screening (2013–2014) in a veterans affairs (VA) medical system, Okerke et al. reported an early-stage disease rate of 67% compared to 35% in the prescreening period.27 An 8-site VA demonstration project and a multisite program within a large integrated health system both reported a 71% rate of early-stage disease.15,28
In additional to LCS-LDCT, we identified additional factors that were associated with a diagnosis of early-stage lung cancer. A personal history of extrathoracic cancer and a history of COPD were associated with a diagnosis of early-stage lung cancer, likely reflecting higher utilization of thoracic imaging in these individuals. Patients with prior cancer generally undergo several years of surveillance which can identify lesions suspicious for lung cancer prior to clinical presentation. Patients with COPD also undergo more frequent chest imaging to establish the diagnosis or for ongoing assessment and management of symptoms and exacerbations which can similarly identify suspicious lung lesions. Individuals with an increased BMI of >30 were also more likely to be diagnosed with early-stage disease, which may also reflect greater healthcare utilization, including higher rates of imaging.
Limitations
Our study has limitations. First, our results are derived from a retrospective study of four diverse health systems in the US but may not reflect the stage distribution and stage specific rates observed in other settings or more broadly across the US. Second, our study utilizes health system level EHR data allowing accurate identification of screening events, but we could not differentiate between screen detected or interval cancers. Third, although prior studies have shown that those who elect to receive screening are healthier in ways that are difficult to measure,29 given that a 30 pack-year smoking history is an LCS eligibility requirement, we believe healthy-user bias is minimal in the PROSPR-Lung screened population. Lastly, while cancer case ascertainment relied on local cancer registry information, individuals may have sought care outside of one of the four health systems included in our analysis. We believe this was a relatively rare event given that two of the healthcare systems operate under an integrated care delivery model and the other two limited the cohort to individuals that received primary care within their systems and retain patients diagnosed with cancer given their role as comprehensive cancer centers. Furthermore, any out migration prior to lung cancer diagnosis would be anticipated to occur at similar rates for unscreened and screened individuals since there was no socioeconomic difference between the two groups.
Conclusions
To our knowledge, this is the first study to determine the impact of LCS on cancer stage migration using a population based multicenter cohort. Our results suggest that LCS results in a shift to early-stage disease coupled with a decline in the proportion diagnosed with metastatic lung cancer when compared to the unscreened population. The distribution of stage was similar to rates observed in prior clinical trials despite limitations such as lower adherence to annual screening that have been observed outside of trial settings. By the end of the study, approximately 20% of those diagnosed with lung cancer had received at least one prior LCS LDCT. While over-diagnosis remains a concern, at this rate of screening we did not observe an increase in the overall rate of lung cancer. As screening implementation progresses, future population-based studies are needed to assess the impact of screening on other effectiveness outcomes, including rates of harms related to screening and the impact on lung cancer mortality.
Supplementary Material
Funding:
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Number UM1CA221939. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosures: AV reports grants from the Moore Foundation, PCORI, Lungevity, the National Comprehensive Cancer Network, Precyte Inc., and MagArray Inc. outside of the submitted work, and personal fees as scientific advisor for the Lung Cancer Initiative at Johnson and Johnson. MJS reports personal fees from Intuitive Surgical, Gongwin Biopharm, SpinQ, and Pulmonx. MJS reports personal fees from Intuitive Surgical, Gongwin Biopharm, and PulmonX. As of February 2022, MJS is 50% employed at Intuitive Surgical. CND reports grants from PCORI through a subcontract from the University of Pennsylvania, the Moore Foundation through a subcontract from the University of Pennsylvania, NCI Connect Study, Michigan Department of Health and Human Services, and Genentech outside of the submitted work. DPR, ABH, SH, RTG report grants from the Moore Foundation through a subcontract through the University of Pennsylvania. KR reports grants from the Moore Foundation, the Lung Cancer Research Foundation of Pfizer, Lungevity, and the National Comprehensive Cancer Network outside of the submitted work.
Abbreviations:
- NLST
National Lung Screening Trial
- NELSON
Nederlands–Leuvens Longkanker Screenings Onderzoek
- LDCT
low dose CT
- LCS
lung cancer screening
- PROSPR
Population-based Research to Optimize the Screening Process
- IRB
Institutional Review Board
- AJCC
American Joint Commission on Cancer
- COPD
chronic obstructive pulmonary disease
- BMI
body mass index
- AAPC
average annual percentage change
- MICE
multiple imputation by chained equations
Footnotes
CRediT Statement
Anil Vachani: Conceptualization, Methodology, Data Curation, Formal analysis, Funding acquisition; Writing – ordinal draft, review & editing
Nikki Carroll: Conceptualization, Methodology, Data Curation, Formal analysis; Software; Writing – review & editing
Michael Simoff: Conceptualization, Writing - review & editing
Christine Neslund-Dudas: Conceptualization, Writing - review & editing
Stacey Honda: Conceptualization, Writing - review & editing
Robert T. Greenlee: Conceptualization, Writing - review & editing
Katharine A. Rendle: Conceptualization, Writing - review & editing
Andrea Burnett-Hartman: Conceptualization, Writing - review & editing
Debra P. Ritzwoller: Conceptualization, Methodology, Data Curation, Formal analysis, Funding acquisition; Writing – review & editing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–953. doi: 10.2147/CMAR.S187317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. Journal of Thoracic Oncology. 2017;12(7):1109–1121. doi: 10.1016/j.jtho.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 4.Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non–Small Cell Lung Cancer in the US. JAMA Oncol. 2021;7(12):1824. doi: 10.1001/jamaoncol.2021.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening | NEJM. Accessed December 11, 2021. 10.1056/nejmoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. New England Journal of Medicine. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 7.Brodersen J, Voss T, Martiny F, Siersma V, Barratt A, Heleno B. Overdiagnosis of lung cancer with low-dose computed tomography screening: meta-analysis of the randomised clinical trials. Breathe (Sheff). 2020;16(1):200013. doi: 10.1183/20734735.0013-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett-Hartman AN, Carroll NM, Honda SA, et al. Community-based Lung Cancer Screening Results in Relation to Patient and Radiologist Characteristics: the PROSPR Consortium. Ann Am Thorac Soc. Published online September 20, 2021. doi: 10.1513/AnnalsATS.202011-1413OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iaccarino JM, Steiling KA, Wiener RS. Lung Cancer Screening in a Safety-Net Hospital: Implications of Screening a Real-World Population versus the National Lung Screening Trial. Ann Am Thorac Soc. 2018;15(12):1493–1495. doi: 10.1513/AnnalsATS.201806-389RL [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Xu Y, Huo J, Burks AC, Ost DE, Shih YCT. Updated Analysis of Complication Rates Associated With Invasive Diagnostic Procedures After Lung Cancer Screening. JAMA Netw Open. 2020;3(12):e2029874. doi: 10.1001/jamanetworkopen.2020.29874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo J, Xu Y, Sheu T, Volk RJ, Shih YCT. Complication Rates and Downstream Medical Costs Associated With Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting. JAMA Intern Med. 2019;179(3):324. doi: 10.1001/jamainternmed.2018.6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y, Fu M, Ding R, et al. Patient Adherence to Lung CT Screening Reporting & Data System-Recommended Screening Intervals in the United States: A Systematic Review and Meta-Analysis. J Thorac Oncol. 2022;17(1):38–55. doi: 10.1016/j.jtho.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunitomo Y, Bade B, Gunderson CG, et al. Racial Differences in Adherence to Lung Cancer Screening Follow-up: A Systematic Review and Meta-analysis. Chest. 2022;161(1):266–275. doi: 10.1016/j.chest.2021.07.2172 [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Olivo MA, Maki KG, Choi NJ, et al. Patient Adherence to Screening for Lung Cancer in the US. JAMA Netw Open. 2020;3(11):e2025102. doi: 10.1001/jamanetworkopen.2020.25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handy JRJ, Skokan M, Rauch E, et al. Results of Lung Cancer Screening in the Community. Ann Fam Med. 2020;18(3):243–249. doi: 10.1370/afm.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copeland A, Criswell A, Ciupek A, King JC. Effectiveness of Lung Cancer Screening Implementation in the Community Setting in the United States. J Oncol Pract. 2019;15(7):e607–e615. doi: 10.1200/JOP.18.00788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy MPT, Cheyne L, Darby M, et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax. 2018;73(12):1128–1136. doi: 10.1136/thoraxjnl-2018-211842 [DOI] [PubMed] [Google Scholar]
- 18.Potter AL, Rosenstein AL, Kiang MV, et al. Association of computed tomography screening with lung cancer stage shift and survival in the United States: quasi-experimental study. BMJ. 2022;376:e069008. doi: 10.1136/bmj-2021-069008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. doi: 10.1007/s10552-013-0310-1 [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA, Negassa A, Edwardes MD deB, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi: 10.1093/aje/kwf215 [DOI] [PubMed] [Google Scholar]
- 22.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 24.Gao W, Wen CP, Wu A, Welch HG. Association of Computed Tomographic Screening Promotion With Lung Cancer Overdiagnosis Among Asian Women. JAMA Intern Med. 2022;182(3):283. doi: 10.1001/jamainternmed.2021.7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim R, Rendle KA, Neslund-Dudas C, et al. Community-based lung cancer screening adherence to Lung-RADS recommendations. JCO. 2021;39(15_suppl):10540–10540. doi: 10.1200/JCO.2021.39.15_suppl.10540 [DOI] [Google Scholar]
- 26.Carroll NM, Burnett-Hartman AN, Joyce CA, et al. Real-world Clinical Implementation of Lung Cancer Screening—Evaluating Processes to Improve Screening Guidelines-Concordance. J GEN INTERN MED. 2020;35(4):1143–1152. doi: 10.1007/s11606-019-05539-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okereke IC, Bates MF, Jankowich MD, et al. Effects of Implementation of Lung Cancer Screening at One Veterans Affairs Medical Center. Chest. 2016;150(5):1023–1029. doi: 10.1016/j.chest.2016.08.1431 [DOI] [PubMed] [Google Scholar]
- 28.Kinsinger LS, Anderson C, Kim J, et al. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399. doi: 10.1001/jamainternmed.2016.9022 [DOI] [PubMed] [Google Scholar]
- 29.Kramer BS, Croswell JM. Cancer screening: the clash of science and intuition. Annu Rev Med. 2009;60:125–137. doi: 10.1146/annurev.med.60.101107.134802 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


