Abstract
Background
Adipose tissue macrophages (ATMs) are a well characterized regulator of adipose tissue inflammatory tone. Previously defined by the M1 vs M2 classification, we now have a better understanding of ATM diversity that departs from the old paradigm and reports a spectrum of ATM function and phenotypes in both brown and white adipose tissue.
Scope of Review
This review provides an updated overview of ATM activation and function, ATM diversity in humans and rodents, and novel ATM functions that contribute to metabolic homeostasis and disease.
Major Conclusions
While the paradigm that resident ATMs predominate in the lean state and obesity leads to the accumulation of lipid-associated and inflammatory ATMs still broadly remains rigorously supported, the details of this model continue to be refined and single cell data provide new insight into ATM subtypes and states.
Keywords: Adipose tissue, Macrophage, Obesity, Inflammation, Lipids
Abbreviations: AT, Adipose tissue; ATM, Adipose tissue macrophage; MMe, Metabolically activated macrophage; CLS, Crown-like structures; LAM, Lipid-associated macrophage; HFD, High-fat diet; ND, Normal diet; VAT, Visceral adipose tissue; BAT, Brown adipose tissue; WAT, White adipose tissue; eWAT, Epididymal white adipose tissue; iWAT, Inguinal white adipose tissue; T2D, Type 2 Diabetes
Highlights
-
•
Lipid Associated Macrophage (LAM) adipose tissue macrophages (ATMs) are the dominant subtype induced by obesity in mice.
-
•
LAM ATMs in humans can be found in lean adipose tissue and their induction with obesity or metabolic disease is not prominent.
-
•
Regulatory functions of ATMs include phagocytosis, intercellular communication, and responses to adipose tissue activation.
-
•
Multiomic single cell studies identify ATM subtypes that extend beyond the classic M1/M2 paradigm of ATM activation.
1. Introduction
It has been almost 20 years since the investigation of adipose tissue (AT) biology and metabolism collided with immunology research as studies highlighted the presence of resident adipose tissue leukocytes such as adipose tissue macrophages (ATMs) [1]. These discoveries led to a reassessment of the structure, composition, and regulation of adipose tissue function. We now know that a network of leukocytes exists in adipose tissue that includes components of both the innate and adaptive immune systems. Innate immune components such as ATMs, adipose tissue dendritic cells (ATDC), natural killer (NK) cells, and innate lymphoid cells (ILC) dominate the resident leukocyte population in adipose tissue [[2], [3], [4]]. Recent studies improve the delineation of resident adipose tissue leukocytes distinct from blood-derived leukocytes found in adipose tissue and support the presence of a distinct pool of leukocytes sequestered within lean and obese adipose tissue [[5], [6], [7]].
The next challenges in the field of AT immunometabolism center on expanding our understanding of AT inflammation in the context of how the wide range of AT leukocytes communicate with each other and separate from how each cell type stands alone. In this light, ATMs serve as a key inflammatory node in the coordination of immune responses between innate and adaptive immune cells. The capacity of macrophages to function in antigen presentation, cytokine production, host defense, and patrolling the local microenvironment to maintain homeostasis has drawn attention to the diversity of ATM subtypes or states revealed by recent single cell transcriptomic studies [8]. ATM diversity aligns with the diversity of AT, which we now know encompasses multiple types (e.g., brown, beige/brite, white) that span a range of functions related to their anatomic location and role in metabolism [9]. This diversity provides a future challenge and opportunity to understand new aspects of macrophage biology in AT.
Here we provide a review of recent insights and advances in our understanding of the function of ATMs and their role as regulators of AT immunometabolism presented in the context of their diversity. The initial model of “M1 vs M2” ATMs proposed by our group many years ago is outdated and overly simplistic in relation to our current understanding of macrophage diversity [10]. We hope to highlight novel concepts in ATM regulation that can set the stage for future discoveries and expansion. In addition, we highlight future areas for development and investigation that will move the field toward a more comprehensive understanding of ATMs in health and disease.
2. ATM diversity
ATMs were initially described along the lines of two classical macrophage phenotypes: proinflammatory M1 and anti-inflammatory M2 based on limited markers. We now understand that this is a vast oversimplification and macrophage activation states can be defined along a spectrum as opposed to a simple two-dimensional axis [2,7]. Treatment of human monocyte derived macrophages with numerous stimuli identified >40 gene modules that uniquely respond to stimuli that can be used to better define co-expressed gene networks in response to stimuli outside the M1/M2 axis. In addition, features of macrophages such as developmental origin (e.g., yolk sac versus monocyte derived), proliferative capacity, and tissue location generate a broad range of macrophage phenotypes [11,12]. Our current understanding of ATM diversity is primarily derived from mouse models where tools for tracking and differentiating the developmental ontogeny of macrophage subtypes have been powerful tools. The expansion of single cell and single nuclear RNAseq datasets on ATMs has also revealed a greater diversity of ATMs than was previously known.
2.1. Resident ATMs in lean states have multiple developmental origins
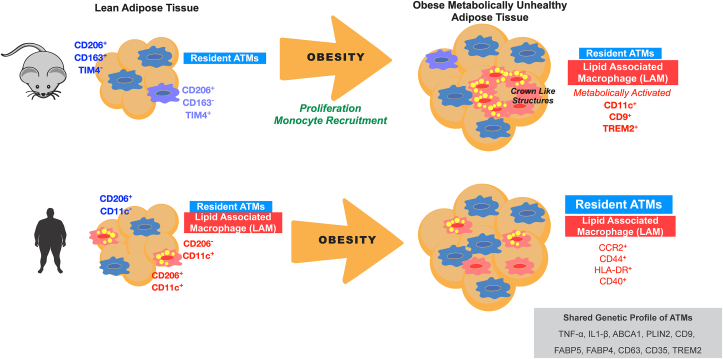
In lean mice, the majority of resident ATMs express markers of alternatively activated M2 macrophages such as CD206 and CD163 that facilitate their identification and experimental separation [[13], [14], [15]]. Experimental ablation of CD206+ ATMs leads to a remodeling response in adipose tissue characterized by smaller adipocytes and the proliferation of adipocyte progenitor cells [16]. Recent use of single cell mass cytometry (CyTOF) further subdivided the lean ATMs based on markers TIM4, CD163, and MHC II (Figure 1). CD163+ and TIM4- populations are dependent on Ccr2 in mice supporting their origin from bone marrow derived monocytes [8]. TIM4+ ATMs are of embryonic origin based on lineage tracing and inducible labeling [8,17]. These observations align with data defining TIM4+ ATMs as a source of PDGFcc and regulator of adipose tissue energy storage in mice [18]. The regulation of resident ATM content is controlled independent from monocyte derived macrophages and appears to involve mechanisms related to in situ proliferation. For example, early adipose tissue remodeling by HFD feeding triggers rapid ATM proliferation that expands the ATM pool in concert with adipocyte enlargement and hypertrophy (Figure 1) [12,19].
Figure 1.
Comparison of mouse and human ATM diversity during obesity. For mice, lean states are dominated by a resident ATM pool that can be divided into at least two subtypes. With diet induced obesity, lipid associated ATMs (LAM) accumulate in crown like structures (CLS) and are maintained via proliferative and recruitment mechanisms. In humans, LAM ATMs are part of the resident tissue pool along with CD206+ ATMs. With obesity and metabolic disease, CD206+ ATMs expanded in adipose tissue in contrast with mouse models of obesity. In addition, CLS are much less abundant in human adipose tissue.
2.2. Obesity triggers the accumulation of lipid associated macrophages (LAMs)
In mice, obesity induces a prominent population of ATMs expressing CD11c, CD9, and TREM2 [10,20,21]. Such recruited ATMs were shown to be monocyte derived and dependent on chemokine pathways such as the CCR2/CCL2 axis for accumulation in adipose tissue in mice [22]. Over the years, CD11c+ ATMs induced by obesity have been described as having metabolically activated phenotypes and lysosomally activated phenotypes that are distinct from the classic M1/M2 paradigm [7,23]. Metabolically activated macrophages (MMes) are a phenotype induced by metabolites such as free-fatty acids (FFAs), high glucose, and insulin to produce a unique inflammatory profile and express lysosomal surface proteins [24], differentiating them from classically activated M1 ATMs [7]. Cytokine expression in MMes is regulated by toll-like receptor 2 (TLR2), NADPH oxidase 2 (NOX2), and MYD88 [24]. More recently, single cell RNAseq studies have coalesced around defining the lipid associated macrophage (LAM) phenotype of CD11c+ ATMs as being associated with TREM2 expression and with a lipid activated profile that appears to be a common activation pathway for many types of tissue macrophages including in adipose tissue, liver, and the brain [21,25,26].
Surprisingly, the capacity of weight loss or calorie restriction to reverse the accumulation of LAMs in adipose tissue appears to be limited [[26], [27], [28]]. Weight loss interventions in mice do not restore AT immune cells to the lean state which may have implications for health and metabolic risk for individuals who were formerly obese.
3. ATM activation and function
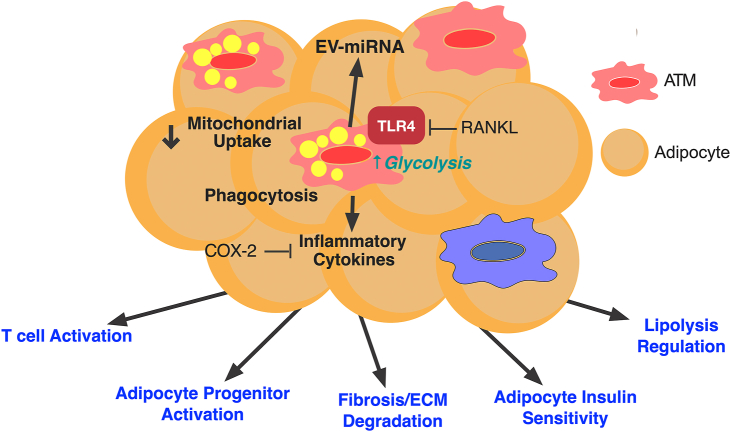
The range of functions of ATMs is vast and includes their capacity to proliferate in response to adipose tissue remodeling, control of fibrotic responses and ECM production, activate adaptive immune cells such as T cells by their function as antigen presenting cells, production of metabolic enzymes and factors that control intercellular communication in adipose tissue, modulation of adipocyte insulin sensitivity, and regulation of adipose tissue progenitor activation. (Figure 2) We will highlight some of the more recent advances in identifying novel ATM functions and mechanisms of action.
Figure 2.
Broad functions for ATMs in the regulation of adipose tissue homeostasis. ATMs have a broad range of functions in different aspects of adipose tissue remodeling. Novel functions for ATMs include mitochondrial uptake which is a protective mechanisms that is suppressed with obesity, induction of glycolysis, induction of phagocytic capacity, generation and reception of extracellular vesicles (EV), and generation of inflammatory cytokines.
3.1. ATM cytokine production and TLR4 signaling
The capacity of ATMs to generate pro-inflammatory cytokines such as TNFα and IL-6 has been reproducibly shown to be a key feature of ATM function. TLR4 activation and its downstream signaling pathways have been shown to be essential for adipose tissue immune cell crosstalk and ATM activation and cytokine secretion [12,29,30]. Recent studies suggest inhibitory pathways in ATMs may play an important role in attempting to restrain inflammatory signals. The receptor activator of NF-κB ligand (RANKL) inhibits TLR4 activation in ATMs, subsequently crippling ATM cytokine production [31]. RANKL promotes the interaction of its receptor RANK with TRAF6, preventing TLR4 activation, and inhibiting iNOS, TNFɑ, and IL1β production in ATMs [31]. This data suggests that RANKL may be a future target to limit the inflammatory response in ATMs. TLR4 signaling also regulates COX-2 expression [32], which is induced in ATMs during obesity and has been shown to limit AT inflammation [33]. Myeloid-specific deletion of COX-2 in obese mice resulted in worsened inflammation compared to WT obese mice. COX-2 deficient mice had increased ATM proliferation, pro-inflammatory cytokine production, and crown like structures (CLS) in adipose tissue.
3.2. ATM phagocytic capacity as a regulator of the adipose tissue environment
One of the earliest observations is the clustering of CD11c+ LAM ATMs around dead and dying adipocytes presumable to clear debris and lipid droplets. Analysis of the major subset of resident ATMs from murine epididymal white adipose tissue (eWAT) defined by TIM4 and CD163 showed that all subsets have phagocytic capacity with the TIM4+ populations having the greatest endocytic potential in vitro and in vivo [8]. The phagocytic capacity of TIM4+ ATMs decreases with age but does not change with HFD. In obesity, ATMs contribute to the formation of multinucleated giant cells with high phagocytic function and the capacity to digest large particles in obese adipose tissue [34]. However, the true nature of the type of phagocytosis ATMs participate in within CLS is unclear. Studies suggest that a unique type of phagocytosis termed exophagy is the primary mechanism of CLS formation, where lysosomal enzymes are delivered to the extracellular space to facilitate the digestion of adipocyte fragments [3]. Weight loss by calorie restriction appears to activate ATM phagocytic capacity suggesting a distinct remodeling function of ATMs in settings of adipose tissue contraction [26].
Beyond the digestion of adipocytes, ATMs may participate in the uptake of other byproducts of adipocyte remodeling. Transfer of mitochondria from stressed or activated adipocytes has been shown to occur between adipocytes and macrophages in WAT and brown adipose tissue (BAT). In BAT, ATMs are responsible for removing extracellular vesicles carrying damaged mitochondria released from brown adipocytes during thermogenic activation [35]. Adipocyte to ATM mitochondrial transfer was observed to be decreased in eWAT with obesity [36]. Blockade of ATM mitochondrial transfer by inhibition of heparan sulfate biosynthesis led to increased adipose tissue mass and insulin resistance, suggesting mitochondrial clearance as a novel homeostatic function of ATMs in maintaining metabolic homeostasis. Dietary factors appear to regulate the amount of mitochondrial transfer between adipocytes and ATMs. Long-chain fatty acids suppress ATM mitochondrial capture increasing circulating mitochondrial load from adipocytes for delivery to other organs [37]. Obese mice with myeloid-specific deletion of COX-2 had decreased expression of LAMP2, CD36, and Gas6 expression in adipose tissue, indicative of decreased phagocytotic capacity [33], suggesting that COX-2 is essential for phagocytosis by ATMs.
3.3. ATM extracellular vesicles and intercellular communication
Another form of intercellular communication in adipose tissue that involves ATMs is the production and uptake of extracellular vesicles (EV). Adipose tissue derived EV were shown to be taken up by monocytes and promote pro-inflammatory cytokine production by monocyte-derived macrophages [38]. Such EV were shown to promote insulin resistance when injected into mice. Adipocyte derived EV carrying miR-34a has been shown to be induced by obesity and transferred to ATMs where it suppressed M2 polarization pathways and promote adipose tissue inflammation and insulin resistance [39]. Beyond receiving signals through EV, ATMs have been shown to potentially modulate metabolic inflammation via exosomes they generate. miR-690 is enriched in M2 polarized macrophage EV that are sufficient to improve insulin sensitivity and glucose tolerance in obese mice [40]. Insulin sensitivity has also been shown to be suppressed by the delivery of miR-155 containing EVs from ATMs to adipocytes which target PPARγ. While body weight was not different between mice treated with obese ATM EV in lean mice, treated mice had impaired insulin signaling and −61attenuated glucose uptake in adipocytes, myocytes, and hepatocytes. Treating mice with ATM EV from lean mice repaired insulin resistance [41].
3.4. Metabolic regulation of ATM function
While much of the focus of immunometabolism research has centered on how ATMs alter adipose tissue and systemic metabolism, metabolic regulation of ATM function may also play a role in the response to obesity. ATMs from obese mice express gene pathways involved in glycolysis and oxidative phosphorylation, making them distinct from LPS-activated ATMs [42]. In mice, ATM activation and cytokine production are highly dependent on the bioenergetic profile of ATMs. ATMs isolated from obese have high glycolytic and oxidative capacity [43].
Mitochondrial function and biogenesis in adipose tissue have been shown to be impaired with obesity and Type 2 Diabetes [44,45]. The activation state of macrophages is known to significantly alter mitochondrial function in macrophages with classical M1 activation making macrophages more reliant on glycolysis than oxidative phosphorylation (OXPHOS) [46]. Gaps remain in our understanding of specific mitochondrial activity in ATMs in mice or humans. However, mice with reduced mitochondrial OXPHOS due to myeloid-specific deletion of CR6-interacting factor 1 (Crif1) had increased M1 polarization of macrophages, AT inflammation, and insulin resistance, suggesting a role for ATM mitochondrial function in metabolic dysfunction [47]. In addition, adenine nucleotide translocase 2 (ANT2) is induced in obese ATMs leading to increased mitochondrial reactive oxygen species production and damage due to modulation of free fatty acid-induced mitochondrial permeability alterations [40].
Efforts to understand the role of ATM mitochondria in cytokine production led to the testing of the near-infrared dye, IR-61, as a potential candidate to treat AT inflammation. IR-61 is a small molecule that localizes to the mitochondria of ATMs, suppressing inflammatory genes in macrophages [36]. Bone marrow-derived macrophages (BMDM) treated with IR-61 had decreased TNF, IL-6, and IL1β mRNA and protein expression upon LPS activation. This was paired with increased oxygen consumption rate and mitochondrial ATP production. Treatment of obese mice with IR-61 suppressed pro-inflammatory gene expression and reduced crown like structures in adipose tissue accompanied by weight loss and improvements in insulin sensitivity.
Direct action of hormones altered in metabolic disease such as insulin can also shape ATM function. Insulin receptor (IR) signaling in ATMs has been shown in several studies to contribute to insulin resistance. Deletion of IR in myeloid cells led to an improvement in insulin sensitivity with obesity related to preservation of IRS2/IL-4 signaling that preserves M2 activation profiles [48]. Aligned with this observation are studies showing that activation of the IR in ATMs promotes the secretion of IL-10, which has been shown to regulate hepatic glucose metabolism, suppressing gluconeogenic genes G6pc and Pck and significantly reducing glucose concentration [49]. Insulin signaling and IL-10 production in ATMs are both stunted under obese conditions [49,50] which can promote hyperglycemia. Myeloid IR signaling may also play a role in the accumulation of ATMs and adipose tissue dysfunction with obesity. Myeloid IR deletion in obese mice decreased basal hepatic glucose production, increased insulin-stimulated glucose disposal in skeletal muscle, and blocked the accumulation of ATMs in adipose tissue with obesity [51].
A primary response to obesity and a differentiator of ATM subtypes is their accumulation and metabolism of lipids. Lipid uptake by ATMs increases with obesity in mice, with ATMs from obese mice had increased levels of triglycerides and expression of lipoprotein lipase (LPL) [19,52]. Silencing LPL in ATMs from VAT of obese mice inhibited lipid accumulation in ATMs and increased serum FFA levels but did not effect serum triglyceride levels [53]. ATMs also express hypoxia inducible lipid droplet associated (Hilpda), a protein that mediates lipid accumulation in adipose tissue and whose expression is increased with obesity and FFA exposure [54]. Overexpression of acyl CoA: diacylglycerol acyltransferase 1 (DGAT1) protects mice from adipose tissue inflammation suggesting that inflammatory activation and lipid storage may be decoupled in some situations [55].
TREM2+ LAM ATMs have been shown to play a functional role in adipose tissue lipid metabolism in both mouse and human VAT [21]. LAMs from Trem2 −/− mice on HFD effectively store lipids and failed to accumulate in adipose tissue suggesting a requirement for Trem2 in LAM ATM accumulation [21]. We note that studies on the metabolic role of TREM2 in mice have been somewhat mixed. Trem2 −/− mice have been shown to have worse insulin resistance and hepatic steatosis related to increased serum ceramide levels [56]. Bone marrow transplantation suggested that these effects of Trem2 deficiency were not related to immune cell TREM2 function. Overall, while TREM2 appears to be a strong marker of LAM ATMs, the functional role of TREM2 in metabolic inflammation remains not fully understood.
4. BAT ATMs and remodeling with activation
The role of resident ATMs in brown adipose tissue (BAT) is a prominent feature of BAT remodeling upon activation. Single cell RNA sequencing identifies dramatic remodeling of BAT macrophages and dendritic cells upon activation [57,58]. BAT ATMs include populations of TREM2+ LAMs and localize to regions of new adipocyte formation suggesting a critical role for ATMs in remodeling BAT upon activation. Although the percentage of ATMs from BAT decreases with obesity [59], they have increased expression of pro-inflammatory cytokines induced by the activation of toll-like receptors [60]. Upon cold exposure, inflammatory monocytes differentiate into CD45+CD11b+F4/80+ ATMs in BAT, expressing markers of M2-like or anti-inflammatory ATMs [35].
Recent studies identified methyl-CpG binding protein 2 (Mecp2) as a potential regulator of BAT ATM activation. Mice with mutated nuclear transcription regulator Mecp2 in BAT macrophages had increased weight gain, increased fat mass, and increased leptin secretion compared to controls. Mecp2 knockout mice also exhibited decreased heat production evidenced by decreased expression of UCP-1 in BAT [61]. These findings indicate a role for ATMs from BAT in regulating adiposity and energy expenditure.
5. Human ATMs versus mouse ATMs
There remain many gaps in our understanding of human ATM diversity and its relationship with obesity and metabolic disease. Recent RNAseq studies identify analogous ATM populations between humans and mice, however, the proportions of these cells are dramatically different in obese humans and mouse models [33,62] (Figure 1). In general, the profound induction of LAMs in obese mice is not strongly recapitulated in human obesity. While CD11c+ LAM ATMs dominate in obese and insulin resistant mice, this does not appear to be a prominent feature in human adipose tissue. Initial studies suggested that CD206+CD11c+ ATMs were increased in subcutaneous compared to visceral/omental adipose tissue depots in humans [14]. The finding of double positive CD206+CD11c+ ATMs, a population that is less prominent in mice has been a consistent finding in human ATMs in several studies as noted below.
Unlike mice where metabolic responses to high fat diet induced obesity are relatively uniform, challenges remain in characterizing ATMs in the context of variation in metabolic dysfunction in obese humans [63]. CD206+CD11c+ inflammatory ATMs in subcutaneous adipose tissue (SAT) were reported to be increased in obese individuals with non-alcoholic fatty liver disease (NAFLD) compared to lean and obese individuals without NAFLD [64]. Macrophage concentration and Plasminogen activator inhibitor-1 (PAI-1) in SAT were inversely correlated with hepatic and systemic insulin sensitivity. Metabolic activation of ATMs was more robust in ATMs from obese, diabetic humans compared to obese, non-diabetic controls [42].
Using similar markers from mouse studies (CD206 and CD11c), flow cytometry analysis of human adipose tissue from obese subjects showed that visceral adipose tissue (VAT) has significantly more M1-like (CD206+CD11c+) than M2-like (CD206+CD11c−) ATMs compared to subcutaneous adipose tissue (SAT) [65]. These M1-like ATMs were differentiated from M2-like ATMs by cell-surface markers CCR2, CD44, HLA-DR, and CD40, reinforcing their pro-inflammatory phenotype (Figure 1). This study observed a correlation between M1-like ATMs and the ratio of M1/M2-like ATMs in VAT with measures of insulin resistance. Additionally, human M1-like macrophages are identified by cell surface markers CD38, CD274, and CD319 [7]. While traditionally we understood that ATMs undergo a phenotypic switch from resident M2 to pro-inflammatory M1 ATMs during obesity, there is evidence that obesity produces a distinct subset of metabolically activated ATMs (MMes), ATMs isolated from obese human SAT and VAT did not express markers of classical M1 activation as expected, but expressed markers for a unique subset of MMes. While both M1 ATMs and MMes produce pro-inflammatory cytokines, MMes are differentiated from M1 ATMs by cell-surface markers ABCA1, CD36, and PLIN2, regulated by PPARγ [7].
However, many early studies did not delineate or differentiate ATMs from other myeloid cells such as adipose tissue dendritic cells which are prominent in adipose tissue and express many of the same markers such as CD11c and HLA-DR [66]. Using a more stringent separation of ATMs from dendritic cells, our group identified three primary ATM populations in human adipose tissue in both SAT and VAT depots - CD206+CD11c−, CD206+CD11c+, and CD206−CD11c+ [15]. CD206−CD11c+ dendritic cells and CD206+CD11c+ ATMs had gene expression signatures that overlapped with LAMs and were more lipid laden. In contrast, CD206+CD11c− resident ATMs had low lipid staining and were enriched for scavenger receptor genes. Importantly, all three populations were found in lean and obese individuals indicating that the LAMs are not dependent on obesity to reside in adipose tissue. In addition, LAM ATMs were increased in SAT compared to VAT - again a contrast from mouse models. Surprisingly, there was no significant difference in LAM-like CD11c+ ATMs between obese and lean subjects. Instead, the quantity of CD206+CD11c− resident ATMs were found to be increased in VAT in obese diabetic compared to lean and obese non-diabetic individuals. It is unclear if these quantitative differences contribute to metabolically unhealthy obese phenotypes or a homeostatic reaction to the obese-diabetic state.
Both human and mouse ATMs from obese adipose tissue significantly express TNFɑ, IL1β, ABCA1, and PLIN2. In addition, ATMs from obese VAT have greater expression of scavenger receptor CD36 compared to obese SAT in humans. A positive correlation between CD36 expression and BMI has been reported [7]. scRNA-seq of human AT identified an uncharacterized subset of inflammatory ATMs, named IM, expressing CCL3L1, TNF, and CXCL3 that accumulate in AT of obese subjects [67]. In this report, ∼30% of CD11b+CD14+ ATMs from obese WAT are IMs, which are presumably derived from a specific subset of monocytes. A wider diversity of human ATM types was reported in other single cell studies but has not been experimentally validated [62].
6. Conclusion
As the most prominent resident immune cell in adipose tissue, ATMs helped shine a spotlight on immunometabolic regulation of obesity and metabolic disease. Future needs in the field are to close many of the gaps in our understanding of ATM biology in mouse models and human physiology. The vast diversity of adipose tissue depots and their local influences and effects makes human research on ATMs a continued challenge. The possibility that ATMs may be directly targeted for pharmacologic manipulation is still an outstanding question and there is evidence that many existing treatments for insulin resistance such as thiazolidinediones mediate their effects via anti-inflammatory influences on macrophage activation [68]. In addition, recent advances in identifying phenotypic subtypes of type 2 diabetes provide an increased diversity in human metabolic disease that future studies on ATM biology need to take into account [69].
Funding
The authors are supported by grants from the National Institutes of Health F31DK122724, R01DK090262, R03DK129636.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haka A.S., Barbosa-Lorenzi V.C., Lee H.J., Falcone D.J., Hudis C.A., Dannenberg A.J., et al. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res. 2016;57:980–992. doi: 10.1194/jlr.M064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Sullivan T.E., Rapp M., Fan X., Weizman O.E., Bhardwaj P., Adams N.M., et al. Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity. 2016;45:428–441. doi: 10.1016/j.immuni.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva H.M., Bafica A., Rodrigues-Luiz G.F., Chi J., Santos P.D.A., Reis B.S., et al. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med. 2019;216:786–806. doi: 10.1084/jem.20181049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg E.L., Shchukina I., Asher J.L., Sidorov S., Artyomov M.N., Dixit V.D. Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat Metab. 2020;2:50–61. doi: 10.1038/s42255-019-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E., et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metabol. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felix I., Jokela H., Karhula J., Kotaja N., Savontaus E., Salmi M., et al. Single-cell proteomics reveals the defined heterogeneity of resident macrophages in white adipose tissue. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.719979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakers A., De Siqueira M.K., Seale P., Villanueva C.J. Adipose-tissue plasticity in health and disease. Cell. 2022;185:419–446. doi: 10.1016/j.cell.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trzebanski S., Jung S. Plasticity of monocyte development and monocyte fates. Immunol Lett. 2020;227:66–78. doi: 10.1016/j.imlet.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Griffin C., Eter L., Lanzetta N., Abrishami S., Varghese M., McKernan K., et al. TLR4, TRIF, and MyD88 are essential for myelopoiesis and CD11c(+) adipose tissue macrophage production in obese mice. J Biol Chem. 2018;293:8775–8786. doi: 10.1074/jbc.RA117.001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourlier V., Zakaroff-Girard A., Miranville A., De Barros S., Maumus M., Sengenes C., et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 14.Wentworth J.M., Naselli G., Brown W.A., Doyle L., Phipson B., Smyth G.K., et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir L.A., Cho K.W., Geletka L.M., Baker N.A., Flesher C.G., Ehlers A.P., et al. Human CD206+ macrophages associate with diabetes and adipose tissue lymphoid clusters. JCI Insight. 2022;7 doi: 10.1172/jci.insight.146563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawaz A., Aminuddin A., Kado T., Takikawa A., Yamamoto S., Tsuneyama K., et al. CD206(+) M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun. 2017;8:286. doi: 10.1038/s41467-017-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etzerodt A., Moulin M., Doktor T.K., Delfini M., Mossadegh-Keller N., Bajenoff M., et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med. 2020;217 doi: 10.1084/jem.20191869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox N., Crozet L., Holtman I.R., Loyher P.L., Lazarov T., White J.B., et al. Diet-regulated production of PDGFcc by macrophages controls energy storage. Science. 2021;373 doi: 10.1126/science.abe9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano S.U., Cohen J.L., Vangala P., Tencerova M., Nicoloro S.M., Yawe J.C., et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metabol. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill D.A., Lim H.W., Kim Y.H., Ho W.Y., Foong Y.H., Nelson V.L., et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E5096–E5105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaitin D.A., Adlung L., Thaiss C.A., Weiner A., Li B., Descamps H., et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178 doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg S.P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X., Grijalva A., Skowronski A., van Eijk M., Serlie M.J., Ferrante A.W., Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metabol. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coats B.R., Schoenfelt K.Q., Barbosa-Lorenzi V.C., Peris E., Cui C., Hoffman A., et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20:3149–3161. doi: 10.1016/j.celrep.2017.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong X., Kuang H., Ansari S., Liu T., Gong J., Wang S., et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell. 2019;75 doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstock A., Brown E.J., Garabedian M.L., Pena S., Sharma M., Lafaille J., et al. Single-cell RNA sequencing of visceral adipose tissue leukocytes reveals that caloric restriction following obesity promotes the accumulation of a distinct macrophage population with features of phagocytic cells. Immunometabolism. 2019;1 doi: 10.20900/immunometab20190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cottam M.A., Caslin H.L., Winn N.C., Hasty A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat Commun. 2022;13:2950. doi: 10.1038/s41467-022-30646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamarron B.F., Mergian T.A., Cho K.W., Martinez-Santibanez G., Luan D., Singer K., et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes. 2017;66:392–406. doi: 10.2337/db16-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKernan K., Varghese M., Patel R., Singer K. Role of TLR4 in the induction of inflammatory changes in adipocytes and macrophages. Adipocyte. 2020;9:212–222. doi: 10.1080/21623945.2020.1760674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q., Cherayil B.J. Role of Toll-like receptor 4 in macrophage activation and tolerance during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2003;71:4873–4882. doi: 10.1128/IAI.71.9.4873-4882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mota R.F., Cavalcanti de Araujo P.H., Cezine M.E.R., Matsuo F.S., Metzner R.J.M., Oliveira de Biagi Junior C.A., et al. RANKL impairs the TLR4 pathway by increasing TRAF6 and RANK interaction in macrophages. BioMed Res Int. 2022;2022 doi: 10.1155/2022/7740079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukata M., Chen A., Klepper A., Krishnareddy S., Vamadevan A.S., Thomas L.S., et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emont M.P., Jacobs C., Essene A.L., Pant D., Tenen D., Colleluori G., et al. A single-cell atlas of human and mouse white adipose tissue. Nature. 2022;603:926–933. doi: 10.1038/s41586-022-04518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braune J., Lindhorst A., Froba J., Hobusch C., Kovacs P., Bluher M., et al. Multinucleated giant cells in adipose tissue are specialized in adipocyte degradation. Diabetes. 2021;70:538–548. doi: 10.2337/db20-0293. [DOI] [PubMed] [Google Scholar]
- 35.Rosina M., Ceci V., Turchi R., Chuan L., Borcherding N., Sciarretta F., et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metabol. 2022;34 doi: 10.1016/j.cmet.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brestoff J.R., Wilen C.B., Moley J.R., Li Y., Zou W., Malvin N.P., et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metabol. 2021;33 doi: 10.1016/j.cmet.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borcherding N., Jia W., Giwa R., Field R.L., Moley J.R., Kopecky B.J., et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metabol. 2022;34(10):1499–1513. doi: 10.1016/j.cmet.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Z.B., Poliakov A., Hardy R.W., Clements R., Liu C., Liu Y., et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan Y., Hui X., Hoo R.L.C., Ye D., Chan C.Y.C., Feng T., et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834–849. doi: 10.1172/JCI123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon J.S., da Cunha F.F., Huh J.Y., Andreyev A.Y., Lee J., Mahata S.K., et al. ANT2 drives proinflammatory macrophage activation in obesity. JCI Insight. 2021;6 doi: 10.1172/jci.insight.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J.B., et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171 doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Boutens L., Hooiveld G.J., Dhingra S., Cramer R.A., Netea M.G., Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–953. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serbulea V., Upchurch C.M., Schappe M.S., Voigt P., DeWeese D.E., Desai B.N., et al. Macrophage phenotype and bioenergetics are controlled by oxidized phospholipids identified in lean and obese adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E6254–E6263. doi: 10.1073/pnas.1800544115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter C.A., Kartal F., Koc Z.C., Murphy T., Kim J.H., Denvir J., et al. Mitochondrial oxidative phosphorylation is impaired in TALLYHO mice, a new obesity and type 2 diabetes animal model. Int J Biochem Cell Biol. 2019;116:105616. doi: 10.1016/j.biocel.2019.105616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinonen S., Buzkova J., Muniandy M., Kaksonen R., Ollikainen M., Ismail K., et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64:3135–3145. doi: 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- 46.Kelly B., O'Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung S.B., Choi M.J., Ryu D., Yi H.S., Lee S.E., Chang J.Y., et al. Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nat Commun. 2018;9:1551. doi: 10.1038/s41467-018-03998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubota T., Inoue M., Kubota N., Takamoto I., Mineyama T., Iwayama K., et al. Downregulation of macrophage Irs2 by hyperinsulinemia impairs IL-4-indeuced M2a-subtype macrophage activation in obesity. Nat Commun. 2018;9:4863. doi: 10.1038/s41467-018-07358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toda G., Soeda K., Okazaki Y., Kobayashi N., Masuda Y., Arakawa N., et al. Insulin- and lipopolysaccharide-mediated signaling in adipose tissue macrophages regulates postprandial glycemia through akt-mTOR activation. Mol Cell. 2020;79 doi: 10.1016/j.molcel.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawano Y., Nakae J., Watanabe N., Fujisaka S., Iskandar K., Sekioka R., et al. Loss of Pdk1-Foxo1 signaling in myeloid cells predisposes to adipose tissue inflammation and insulin resistance. Diabetes. 2012;61:1935–1948. doi: 10.2337/db11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauer J., Chaurasia B., Plum L., Quast T., Hampel B., Bluher M., et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prieur X., Mok C.Y., Velagapudi V.R., Nunez V., Fuentes L., Montaner D., et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aouadi M., Vangala P., Yawe J.C., Tencerova M., Nicoloro S.M., Cohen J.L., et al. Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab. 2014;307:E374–E383. doi: 10.1152/ajpendo.00187.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Dierendonck X., de la Rosa Rodriguez M.A., Georgiadi A., Mattijssen F., Dijk W., van Weeghel M., et al. HILPDA uncouples lipid droplet accumulation in adipose tissue macrophages from inflammation and metabolic dysregulation. Cell Rep. 2020;30 doi: 10.1016/j.celrep.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 55.Koliwad S.K., Streeper R.S., Monetti M., Cornelissen I., Chan L., Terayama K., et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120:756–767. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharif O., Brunner J.S., Korosec A., Martins R., Jais A., Snijder B., et al. Beneficial metabolic effects of TREM2 in obesity are uncoupled from its expression on macrophages. Diabetes. 2021;70:2042–2057. doi: 10.2337/db20-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burl R.B., Rondini E.A., Wei H., Pique-Regi R., Granneman J.G. Deconstructing cold-induced brown adipocyte neogenesis in mice. Elife. 2022;11 doi: 10.7554/eLife.80167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burl R.B., Ramseyer V.D., Rondini E.A., Pique-Regi R., Lee Y.H., Granneman J.G. Deconstructing adipogenesis induced by beta3-adrenergic receptor activation with single-cell expression profiling. Cell Metabol. 2018;28 doi: 10.1016/j.cmet.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson K.R., Flaherty D.K., Hasty A.H. Obesity alters B cell and macrophage populations in Brown adipose tissue. Obesity. 2017;25:1881–1884. doi: 10.1002/oby.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae J., Ricciardi C.J., Esposito D., Komarnytsky S., Hu P., Curry B.J., et al. Activation of pattern recognition receptors in brown adipocytes induces inflammation and suppresses uncoupling protein 1 expression and mitochondrial respiration. Am J Physiol Cell Physiol. 2014;306:C918–C930. doi: 10.1152/ajpcell.00249.2013. [DOI] [PubMed] [Google Scholar]
- 61.Wolf Y., Boura-Halfon S., Cortese N., Haimon Z., Sar Shalom H., Kuperman Y., et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat Immunol. 2017;18:665–674. doi: 10.1038/ni.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vijay J., Gauthier M.F., Biswell R.L., Louiselle D.A., Johnston J.J., Cheung W.A., et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2:97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samocha-Bonet D., Dixit V.D., Kahn C.R., Leibel R.L., Lin X., Nieuwdorp M., et al. Metabolically healthy and unhealthy obese--the 2013 Stock Conference report. Obes Rev. 2014;15:697–708. doi: 10.1111/obr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuchs A., Samovski D., Smith G.I., Cifarelli V., Farabi S.S., Yoshino J., et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology. 2021;161 doi: 10.1053/j.gastro.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strand K., Stiglund N., Haugstoyl M.E., Kamyab Z., Langhelle V., Lawrence-Archer L., et al. Subtype-specific surface proteins on adipose tissue macrophages and their association to obesity-induced insulin resistance. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.856530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho K.W., Zamarron B.F., Muir L.A., Singer K., Porsche C.E., DelProposto J.B., et al. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol. 2016;197:3650–3661. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hildreth A.D., Ma F., Wong Y.Y., Sun R., Pellegrini M., O'Sullivan T.E. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat Immunol. 2021;22:639–653. doi: 10.1038/s41590-021-00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hevener A.L., Olefsky J.M., Reichart D., Nguyen M.T., Bandyopadyhay G., Leung H.Y., et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahlqvist E., Prasad R.B., Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020;69:2086–2093. doi: 10.2337/dbi20-0001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.