Abstract
Purpose
Advanced, unresectable pancreatic cancer is often treated with either gemcitabine plus nab-paclitaxel (Gem/NabP) or FOLFIRINOX, although these regimens have never been compared in a head to head trial. In this study we compared these two regimens using Veterans Administration (VA) data and evaluated the use of a novel tumor growth formula to predict outcomes.
Methods
We identified 670 Veterans from national VA data with unresected stage II-IV pancreatic adenocarcinoma diagnosed between 2003–2016 who were treated with either first-line Gem/NabP or FOLFIRINOX. We compared overall survival (OS) and adverse events by treatment using propensity scores (PS) to account for allocation bias. Using longitudinal CA19–9 biomarker information we then fit the data to a novel tumor growth equation, comparing growth with OS.
Results
We found no difference in PS-adjusted (hazard ratio [HR] 1.00; 95% confidence interval [95% CI] 0.84–1.20) or PS-matched (HR: 0.93; 95% CI: 0.76–1.13) OS between the two treatment groups. Tumor growth analysis revealed similar growth parameter values for Gem/NabP and FOLFIRINOX (p=0.074 for difference).
Conclusions
Gem/NabP appeared non-inferior to FOLFIRINOX for survival outcomes for advanced pancreatic adenocarcinoma based on national VA data. Biomarker-based growth equations may be useful for monitoring treatment response and predicting prognosis for pancreatic cancer.
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer death in the United States and is expected to be the second leading cause by 2030.1 The majority of pancreatic cancers are ductal adenocarcinomas, which are associated with a 5-year survival of less than 10%. Contributing to this poor prognosis is that most patients with pancreatic cancer are diagnosed at advanced stage, where surgical resection is not possible and chemotherapy (in combination with radiotherapy in some circumstances) is the standard of care. Long known as a highly drug resistant cancer, chemotherapy regimens have been developed that show convincing tumor shrinkage and improved survival. However, drug resistance inevitably emerges.
Despite many attempts to improve on gemcitabine alone, it was the mainstay of chemotherapy for advanced pancreatic cancer prior to trials showing improved survival with the gemcitabine/nab-paclitaxel (Gem/NabP) and FOLFIRINOX regimens (folinic acid, fluorouracil, irinotecan and oxaliplatin). The superiority of FOLFIRINOX to gemcitabine monotherapy was established by a UNICANCER trial, which demonstrated 11.1 months of median overall survival compared to 6.8 months, respectively.2 The use of first-line Gem/NabP was supported in the MPACT trial, which found that this regimen improved survival by 1.8 months compared to single agent gemcitabine, with overall survival 8.5 and 6.7 months, respectively.3 Comparison of these trial results led to the conclusion that in patients with good performance status FOLFIRINOX was frequently optimal.4 However, Gem/NabP and FOLFIRINOX have not been compared head to head in a clinical trial for metastatic disease although several observational studies have been conducted that directly compare outcome differences.5, 6
Given the aggressiveness of pancreatic cancer, time to disease progression and overall survival are appropriate outcome measures for the comparison of treatments. Previous data from a clinical trial of advanced pancreatic cancer suggested that CA19–9 measurements provided an early measure of treatment effectiveness and eventual prognosis.7 This concept may be further expanded by fitting novel growth analyses to longitudinal biomarker measurements (such as CA19–9) for the evaluation of treatment response. These methods may expedite clinical trials and/or provide additional information for guiding therapeutic decisions. We have extensively validated a method of assessing tumor growth based on mathematical equations describing the exponential growth of different cancer types.8–10 Both decay and growth rates are derived from equations fit to tumor measurement data, including tumor marker data such as that provided by CA19–9.
In this study we used national data from a large cohort of Veterans with unresectable, advanced pancreatic cancer and evaluated long-term outcomes treated with either Gem/NabP or FOLFIRINOX. Our study had two aims: 1) to use comparative effectiveness techniques to evaluate the relative efficacies of these regimens and to evaluate the risk of chemotherapy toxicity and; 2) to use mathematical models of tumor burden fitted to longitudinal biomarker data, previously unevaluated in pancreatic cancer, from our real-world cohort data to separately measure treatment response. We also compared these tumor growth model results to data from the pivotal UNICANCER and MPACT trials.
METHODS
Patient Selection
For this project we used national Veterans Administration Health System (VA) clinical and administrative data present in the Corporate Data Warehouse (CDW). Using the CDW, we identified patients with pathologically confirmed, unresected stage II-IV pancreatic adenocarcinoma using cancer registry data. We then limited the cohort to Veterans treated with first line chemotherapy either Gem/NabP or FOLFIRINOX from 2003 through 2016, as identified using either pharmacy data or relevant procedure codes.
Study Variables
We identified baseline sociodemographic variables from administrative files and cancer registry information. Using outpatient and inpatient VA diagnostic codes we identified comorbid illnesses registered in the 12 months prior to cancer diagnosis. We then calculated Charlson-Deyo scores for the cohort to estimate the overall burden of comorbidities.11–13 Marital status and alcohol use were classified using data from VA oncology files. Smoking status was determined using a published algorithm employing VA health factors data with additional information added from the cancer registry files.14 VA laboratory data was used to calculate glomerular filtration rate estimates (eGFR) from creatinine values. Laboratory files were also used to collect serum bilirubin values as well as all available CA19–9 measures for each patient. We then used VA oncology files to collect cancer data including stage and diagnosis date.
Lack of surgery in the initial treatment course was confirmed by reviewing registry data and procedure claims from administrative files. Pharmacy files and procedure codes for chemotherapy agents were reviewed to identify either concurrent use of Gem/NabP or FOLFIRINOX agents with treatment start and stop dates. An intent-to-treat approach was implemented for treatment assignment in the analysis and patients were assigned the first regimen administered.
Overall survival was the primary study outcome. We also identified the occurrence of major treatment toxicities using primary diagnostic codes from inpatient hospitalizations as a proxy for grade 3–4 toxicity (for all complications except neuropathy, for which outpatient codes were also used) during the six-month period after the initiation of chemotherapy. Survival times were calculated using the date of first chemotherapy administration until the date of death or last known date alive.
Statistical Analysis
We first tested for differences in baseline and clinical characteristic between Gem/NabP and FOLFIRINOX treatment groups using the t-test or Wilcoxon test for normal or non-normal continuous variables and the chi square test for categorical variables, respectively.
In unadjusted analyses we then estimated overall survival (OS) by treatment group using Kaplan-Meier methods and compared curves using a log-rank test. To minimize allocation bias associated with the use of either treatment we used propensity score (PS) analysis.15 We calculated PS for each patient by determining the probability of the use of Gem/NabP using logistic regression. Variables included in the PS were determined a priori and included sociodemographic characteristics (age, race, sex, and marital status), smoking and alcohol use, comorbidity score, eGFR, baseline neuropathy, significant liver disease, tumor characteristics (overall stage, T stage and N stage), year of diagnosis, CA19–9 and bilirubin levels. Smoking status, T-stage, N-stage, alcohol use, CA19–9, bilirubin, and creatinine all had missing values (3%, 10%, %, 9%, 10%, 8%, 0.3%, and 4%, respectively); we used multiple imputation methods to estimate missing values in our PS calculation. We first fitted Cox regression models comparing OS adjusted for PS between treatment groups and then stratified this comparison by cancer stage. We then matched subjects in the two treatment arms by PS using a caliper value of 0.05 (based on PS standard deviation16) to perform similar OS comparisons (using robust standard error methods) and then to compare the incidence of toxicity (see Table 3 for toxicity types) using logistic regression models. For toxicities where no events were observed in the Gem/NabP group we tested for differences in prevalence using the chi-square test.
Table 3.
Major toxicity events by study arm for propensity score-matched cohort
| Toxicity | Odds Ratio for Toxicity with Gemcitabine/Nab-Paclitaxel Vs. FOLFIRINOX | 95% CI |
|---|---|---|
| Acute # | ||
| Renal Failure | * | |
| Neutropenia | † | |
| Anemia | 0.21 | 0.05–1.00 |
| Nausea/Vomiting | 0.21 | 0.05–1.00 |
| Dehydration | 0.10 | 0.01–0.75 |
| Cellulitis | ‡ | |
| Diarrhea | § | |
| Sepsis | | | |
| Pneumonia | 0.50 | 0.04–5.53 |
| Chronic | ||
| Neuropathy | 0.48 | 0.19–1.22 |
events associated with hospital admission, as a proxy for grade 3–4 toxicity.
no renal failure in Gem/NabP versus 3.1% in FOLFIRINOX (p=0.01)
no neutropenia in Gem/NabP versus 2.6% in FOLFIRINOX (p=0.02)
no cellulitis in Gem/NabP versus 1.0% in FOLFIRINOX (p=0.16)
no diarrhea in Gem/NabP versus 3.7% in FOLFIRINOX (p=0.008)
no sepsis in Gem/NabP versus 2.1% in FOLFIRINOX (p=0.04)
Tumor growth models employed longitudinal CA19–9 measured during chemotherapy; the first value was identified in the 30 days prior to the initiation of chemotherapy and the final value up to 30 days after the last dose of chemotherapy. Using the TUMGr package for R, a novel algorithm validated in >10 000 patients, cohort subject data was evaluated for optimal fit to a set of four related tumor kinetics models, each based on the exponential growth and regression pattern of cancers. 12–18 The first model, gd, assumes that tumor quantity changes during therapy due to the simultaneous occurrence of exponential decay/regression, termed d, and exponential growth/regrowth of the tumor, termed g. In cases where the data show a continuous tumor burden decrease from the start of treatment, g is zero and the decay rate estimated using only the d parameter from the dx equation. Similarly, in cases where the data show continuous growth from the start of treatment, d is eliminated and the growth rate estimated using the gx equation, containing only a growth term. A fourth model, gdØ, contains the parameter Ø representing the proportion of tumor cells sensitive to a therapy that influences the g term. P-values were calculated for each model fit and models were retained under a threshold value of 0.1. If multiple model types fit for a subject, then the model that minimized the Akaike Information Criterion was selected for final fit in each case. Models could not be fit if there were <3 CA19–9 values or if convergence could not be achieved. For comparison, we also obtained pivotal trial data with similar available information for fitting of growth models from the UNICANCER PRODIGE 4/ACCORD-11 and Celgene MPACT trials of Gem/NabP and FOLFIRINOX, respectively, and data are summarized in Table 4.2, 3 We then used the Wilcoxon test to compare the growth rates associated with the two treatment arms. Parameters g and d were then categorized into tertiles and overall survival was compared by tertiles using Kaplan-Meier methods. Analyses were performed using SAS 9.2, STATA 15 and R statistical packages.
Table 4.
Growth model data for current study and pivotal clinical trials
| Celgene (Gem/NabP) | VA Gem/NabP | VA FOLFIRINOX | UNICANCER (FOLFIRINOX) | ||
|---|---|---|---|---|---|
| Patients, n* | 431 | 282 | 321 | 171 | |
| Patients with valid data, n (%)** | 229 (53%) | 163 (58%) | 207 (64%) | 75 (44%) | |
|
| |||||
| Model Fit Information | |||||
|
| |||||
| Fit | dx | 100 | 54 | 65 | 9 |
| gdphi | 23 | 16 | 11 | 17 | |
| gd | 41 | 30 | 29 | 16 | |
| gx | 18 | 34 | 60 | 12 | |
|
| |||||
|
| |||||
| Not Fit | In Normal Range | 31 | 18 | 15 | 14 |
| Not Fit | 16 | 11 | 27 | 7 | |
|
| |||||
| Patients with data that fit growth model (%) | 182 (79%) | 134 (82%) | 165 (80%) | 54 (72%) | |
|
| |||||
| Data Points and Parameters | |||||
|
| |||||
| Median data points, n | 5 (3–6) | 6 (4–9) | 6 (4–8) | 4 (3–5) | |
| Time on Therapy, days, (IQR) | 181 (115–245) | 141 (83–265) | 133 (65–251) | 291 (221–338) | |
| Initial CA19–9, units/ml, (IQR) | 3 311 (560–15 854) | 913 (167–4 095) | 1 809 (269–6995) | 1 033 (200–9774) | |
| Estimated d, (IQR) | 0.0280 (0.0187–0.0444) | 0.0152 (0.0080–0.0306) | 0.0158 (0.0085–0.0301) | 0.0166 (0.0115–0.0289) | |
| Estimated g, (IQR) | 0.0012 (0.0003–0.0059) | 0.0029 (0.0011–0.0107) | 0.0066 (0.0014–0.0147) | 0.0021 (0.0008–0.0048) | |
| Weighted g | 0.0016 | 0.0027 | 0.0037 | 0.0026 | |
| Doubling time, days (weighted g) | 433 | 257 | 187 | 267 | |
| Difference in g VA vs pivotal trial, p value | 0.0041 | 0.0006 | |||
| VA Gem/NabP vs. VA FFX, p value | 0.0736 | ||||
Excluded: patients with insufficient data defined as 2 or fewer datapoints, or CA19–9 within normal range.
Across all datasets (N=1,205 patients), 44% of patients had insufficient data for analysis. In the VA data, 39% of patients had insufficient data for analysis.
Gem/NabP: Gemcitabine plus Nab-Paclitaxel; FFX: FOLFIRINOX; IQR: Interquartile range
RESULTS
We identified 670 Veterans who received either Gem/NabP or FOLFIRINOX for unresectable pancreatic cancer as first-line treatment (Table 1). The median age for cohort subjects was 64, nearly all subjects were men (96%) and the majority were Caucasian/Hispanic (71%), although there was substantial representation of African American patients (24%). Patients who were treated with Gem/NabP were older, more likely to be men, had higher comorbidity scores and had lower eGFR values compared to patients treated with FOLFIRINOX.
Table 1.
Baseline characteristics of patients treated with gemcitabine/nab-paclitaxel versus FOLFIRINOX
| Gemcitabine/Nab-Paclitaxel |
FOLFIRINOX |
|||
|---|---|---|---|---|
| Characteristic | N=317 | N=353 | p-value | PS adjusted p-value |
| Age, Median, Years (IQR) | 66 (62–71) | 61 (57–66) | <0.001 | 0.98 |
| Men, N (%) | 312 (98) | 333 (94) | 0.005 | 0.90 |
| Ethnic group, N (%) | 0.80 | 0.99 | ||
| Caucasian | 219 (69) | 255 (72) | ||
| African American | 81 (26) | 77 (22) | ||
| Hispanic | 11 (3.5) | 12 (3) | ||
| Other | 3 (1) | 6 (2) | ||
| Missing | 3 (1) | 3 (1) | ||
| Year of diagnosis, N (%) | <0.001 | 0.84 | ||
| 2003–2010 | 14 (4) | 37 (11) | ||
| 2011–2013 | 78 (25) | 192 (54) | ||
| 2014–2015 | 225 (71) | 124 (35) | ||
| Smoking Status, N (%) | 0.2 | 0.97 | ||
| Current | 127 (40) | 156 (44) | ||
| Former | 125 (39) | 114 (32) | ||
| Never | 55 (17) | 74 (21) | ||
| Missing | 10 (3) | 9 (3) | ||
| Alcohol Use, N (%)* | 206 (65) | 230 (65) | 0.9 | 0.99 |
| Stage at Diagnosis, N | 0.05 | 0.98 | ||
| II | 61 (19) | 52 (15) | ||
| III | 41 (13) | 68 (19) | ||
| IV | 215 (68) | 233 (66) | ||
| T stage, N (%) | 0.6 | 0.85 | ||
| 1 | 12 (3) | 12 (4) | ||
| 2 | 77 (24) | 71 (20) | ||
| 3 | 92 (29) | 113 (32) | ||
| 4 | 100 (32) | 122 (35) | ||
| Missing | 36 (11) | 35 (10) | ||
| N stage, N (%) | 0.08 | 0.16 | ||
| 0 | 164 (52) | 170 (48) | ||
| 1 | 117 (37) | 156 (44) | ||
| Missing | 36 (11) | 27 (8) | ||
| Charlson Comorbidity Score, N (%) | 0.01 | 0.90 | ||
| 0 | 119 (38) | 168 (48) | ||
| 1 | 68 (22) | 76 (22) | ||
| 1+ | 130 (41) | 109 (31) | ||
| Diabetes, N (%) | 37 (12) | 38 (11) | 0.7 | 0.97 |
| Bilirubin, mg/dl, median | 0.7 (0.5–2.7)* | 0.8 (0.5–3.0)§ | 0.4 | 0.98 |
| eGFR, ml/min, median | 89 (74–110)† | 96 (80–116)‡ | <0.001 | 0.94 |
| CA19–9, units/ml, median | 594 (85–4,064)¶ | 900 (104–5,248)| | 0.1 | 0.99 |
missing for 9% of both groups
missing 13 values
missing 11 values
missing 2 values
missing 29 values
missing 22 values
In unadjusted analyses of OS, we calculated a median survival for patients receiving Gem/NabP of 7.0 months (Table 2; 95% confidence interval [CI]: 6.1–8.2 months), not significantly different from the median overall survival for FOLFIRINOX of 8.1 months (95% CI: 7.5–9.2 months; p=0.22 for difference). There were also no differences by treatment regimen in unadjusted median survival times when stratifying by stage. Gem/NabP patients with stage II cancer had a median survival of 10.9 months, those with stage III cancer had median survival of 13.4 months and those with stage IV disease had a median overall survival of 5.6 months, which did not differ from median overall survivals of 13.2, 13.0 and 6.5 months respectively for stages II, III, and IV patients receiving FOLFIRINOX. In propensity score adjusted analyses we still found no difference in overall survival between the two treatment groups (hazard ratio [HR] 1.00; 95% CI: 0.84–1.20) for the overall cohort or after stratifying by cancer stage. There were also no differences between survival outcomes for Gem/NabP vs. FOLFIRINOX in the smaller (n=386) PS matched cohort.
Table 2.
Overall survival by study arm.
| Outcome | Median Survival, Months (95% CI) |
Unadjusted HR* for OS | 95% CI | PS Adjusted HR* for OS | 95% CI | PS Matched HR* for OS** | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Gemcitabine/Nab-Paclitaxel | FOLFIRINOX | |||||||
| Full Cohort (n=670) | 7.0 (6.1–8.2) | 8.1 (7.5–9.2) | 1.10 | 0.94–1.28 | 1.00 | 0.84–1.20 | 0.93 | 0.76–1.13 |
| Stage II | 10.9 (9.4–12.9) | 13.2 (9.2–16.5) | 1.13 | 0.77–1.65 | 1.11 | 0.74–1.66 | 1.09 | 0.67–1.78 |
| Stage III | 13.4 (8.8–18.5) | 13.0 (8.8–16.5) | 0.94 | 0.63–1.38 | 0.83 | 0.51–1.36 | 0.79 | 0.47–1.33 |
| Stage IV | 5.6 (5.0–6.2) | 6.5 (5.2–7.9) | 1.13 | 0.94–1.36 | 1.02 | 0.82–1.27 | 0.92 | 0.71–1.17 |
Hazard ratio for Gemcitabine/Nab-Paclitaxel versus FOLFIRINOX;
Matched cohort n=386.
Major toxicity events were less frequent for patients who were treated with Gem/NabP compared to PS matched patients receiving FOLFIRINOX (Table 3). No renal failure, neutropenia, cellulitis, diarrhea or sepsis were found in Gem/NabP patients compared to 3.1%, 2.6%, 1.0%, 3.7%, 2.1% of FOLFIRINOX patients respectively (corresponding unadjusted p-values for comparisons: 0.01, 0.02, 0.16, 0.008, and 0.04). In propensity-matched analyses, Gem/NabP patients were also significantly less likely to experience dehydration (OR: 0.10; 95% CI: 0.01–0.75).
In analyses of tumor growth measured by fitting the tumor growth algorithm, 39% of patients had insufficient or missing data for analysis. There were 134 and 165 patients receiving Gem/NabP or FOLFIRINOX, respectively, with data that could be fit to growth equations. Data could not be fit to an equation when levels were entirely within the normal range (11%). Figure 1A shows examples of equation curve fits from VA patients with pancreatic cancer. Figure 1B shows the distribution of best fits to the equations in the VA and pivotal datasets. This analysis shows that comparable numbers of patients fit the dx equation following Gem/NabP and FOLFIRINOX among the VA patients. Overall, more patient data fit the gx equation in the VA data set than in the pivotal trial data. Figure 1C shows the Kaplan-Meier analysis of survival in the Veterans with pancreatic cancer, based on g value. Those with slowest g have the longest overall survival. Those in whom a g could not be detected, the dx group, had a survival similar to the median tertile. This suggests that patients whose data fit the dx equation developed disease progression soon after discontinuing therapy. Figure 1D shows dot plots of the g values from VA data for Gem/NabP vs. FOLFIRINOX, compared to data sets obtained from the pivotal UNICANCER and MPACT studies. Better control of g is observed in patients treated with Gem/NabP than in those treated with FOLFIRINOX. Median g values for Gem/NabP and FOLFIRINOX were 0.0027 and 0.0037 (Table 4; p=0.074), equivalent to a doubling time of 257 and 187 days, respectively. A median 6 data points were available for these analyses. Growth values were higher for both treatment groups among Veterans compared to respective clinical trial arms (both p<0.005).
Figure 1.
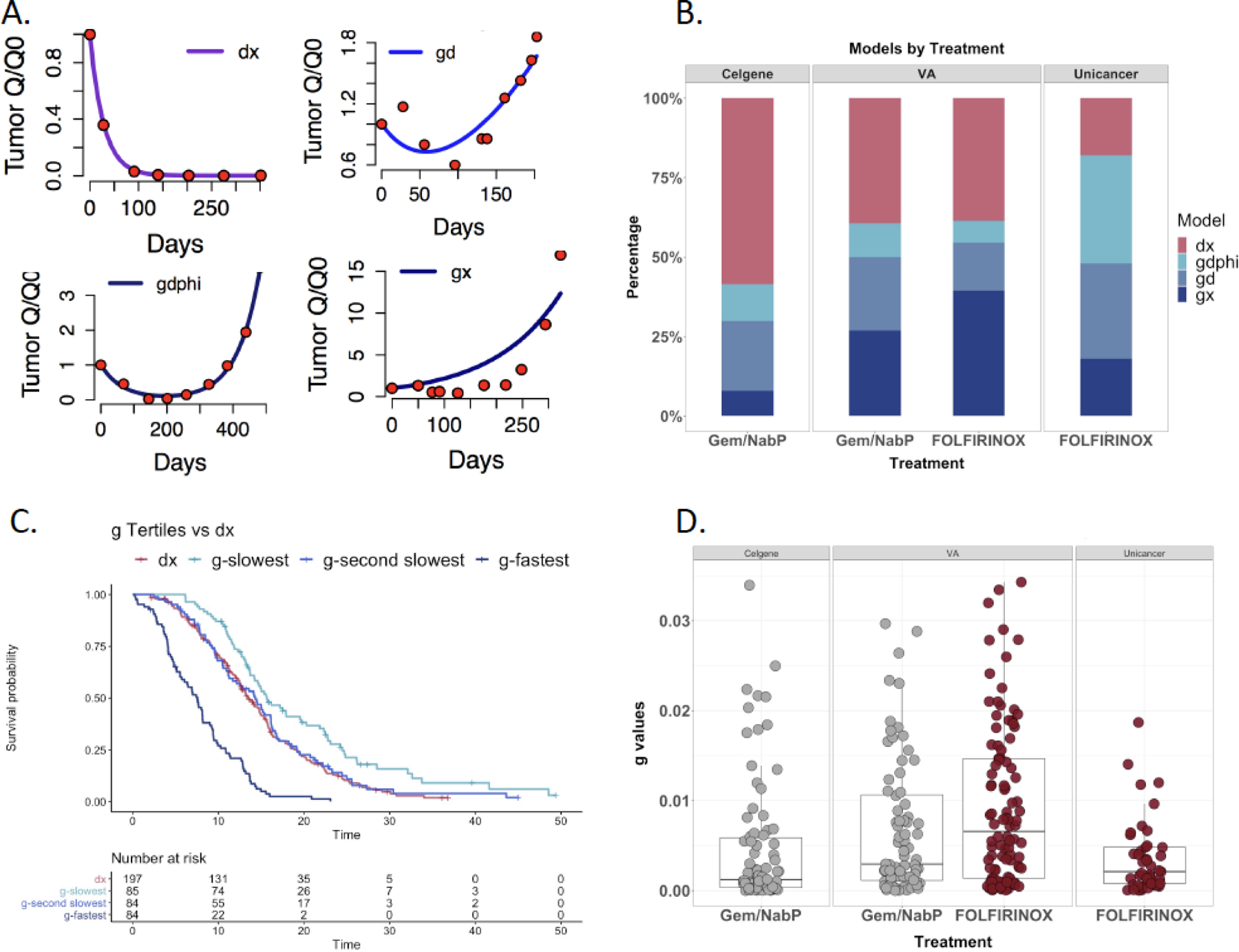
Tumor growth rate analysis in VA and pivotal trial cohorts based on CA19–9 data.
A. Examples of individual patient data fit to the growth rate equation noted in the panels, from left to right: dx (decay without growth), gd (initial decay then growth), gdphi (decay with subsequent growth reflecting heterogeneous response to chemotherapy by tumor cells) and gx (growth only).
B. Stacked bar graph indicating the proportion of patients whose CA19–9 data were best fit to each of the four models. This showed that there were more patients fitting the gx growth only equation in the VA data than in the pivotal studies.
C. Kaplan-Meier curves of growth rates from Veterans divided according to tertile, with those with the fastest g having the shortest overall survival, while those with slowest g have the longest survival. The Kaplan-Meier curve derived from patients whose data fit only the dx model is shown in red, overlying the middle tertile, indicating that those patients did not have a biology different from others in whom a g could be estimated.
D. Dot plot of g values estimated from the models, with patients whose data fit only the dx model excluded. The lowest median g is observed in the gemcitabine/nab-paclitaxel pivotal trial data.
DISCUSSION
In this study we collected data from a real-world cohort of Veterans with advanced, unresected pancreatic cancer treated with either Gem/NabP or FOLFIRINOX and compared survival outcomes accounting for allocation bias, assessed risk of toxicity and fit these data to a novel tumor growth equation using CA19–9 values. We found no difference in survival outcomes when comparing these regimens, suggesting potential non-inferiority of Gem/NabP compared to FOLFIRINOX, a regimen associated with more toxicity in our cohort. We also found that CA19–9 measurements could inform a novel tumor growth equation, and were directly associated with overall survival, suggesting that these methods might be useful for evaluating treatment response.
FOLFIRINOX was established as a standard-of-care for patients with advanced pancreatic cancer with good performance status by the results of the PRODIGE 4/ACCORD 11 trial which randomized patients to either FOLFIRINOX or single agent gemcitabine.2 That trial found prolonged survival (11.1 months versus 6.8 months) for FOLFIRINOX, although the regimen was also associated with substantial toxicity. Subsequently, a large phase III RCT (MPACT) comparing Gem/NabP to single agent gemcitabine found a significant, albeit less robust difference, in survival (8.5 months versus 6.7 months) associated with combination therapy, in a patient group with generally worse performance status than PRODIGE 4/ACCORD 11.3 These trials have raised the issue as to which regimen is optimal for advanced pancreatic cancer; no head-to-head RCTs have been performed comparing Gem/NabP to FOLFIRINOX. However, Gem/NabP has been seen as an alternative regimen, with a more favorable toxicity profile and greater suitability in patients who are older or who have worse performance status.17 As seen in this study of Veterans, patients who are older or with more comorbidities were treated with Gem/NabP, while younger and healthier patients were treated with FOLFIRINOX. This difference was also reflected in the eligibility criteria in the pivotal trials, creating a bias limiting direct comparison between those studies.
Several analyses have directly compared survival using non-randomized methods. A Bayesian meta-analysis utilized a network approach to compare randomized clinical trials to estimate the efficacy of Gem/NabP versus FOLFIRINOX. Using this evidence synthesis approach the authors found similar effects on overall survival for both regimens and no difference in major toxicities.18 Additionally, multiple retrospective “real-world” cohorts with fewer patients than the current analysis have found generally similar survival results to our analysis19; one multisite cohort of 414 patients with advanced pancreatic cancer (255 Gem/NabP versus 159 FOLFIRINOX) found no difference in overall survival by study arm.5 A larger study of 654 patients (337 Gem/NabP versus 317 FOLFIRINOX) demonstrated no difference in overall survival by comparison arm and similar to our study found higher toxicity in FOLFIRINOX-treated patients.6 Previous analyses of observational data comparing Gem/NabP to FOLFIRINOX have found a greater burden of comorbidities in Gem/NabP treated patients, although our analysis is the first to apply causal inference methods to minimize allocation bias related to those factors in the assignment of therapy.
Overall survival rates for patients with unresected pancreatic cancer were worse for the Veterans in our study population than patients with similar cancer stages from clinical trials. However, it is likely that cases from regulatory trials represented healthier patients, as median survival rates from the Surveillance, Epidemiology and End-Results (SEER) database are much worse. A study of advanced pancreatic cancer cases (stage III-IV) comparing >55,000 SEER cases to >20,000 cases from international cancer databases found median survival rates of 5 months for patients younger than 60 and 4 months for patients ages 60–69 during 2003–2013.20 Another SEER study describing data from unresected stage II-III pancreatic cancer cases reported median survival of approximately 6 months, which was less than what we observed.21
We evaluated the use of a novel tumor growth algorithm (previously unapplied to pancreatic cancer) for modeling treatment response and potentially serving as an early measure of outcomes. The majority of cases with CA19–9 measures were fitted to the growth equations and these equations demonstrated that parameter g, representing the rate of tumor growth, was not controlled as well in Veterans as in the pivotal studies. For example, there were more patients whose CA19–9 values a bet fit to the growth only equation (the gx equation). For Gem/NabP, there were fewer patients with data fitting the regression only equation (the dx equation) than in the pivotal trial. Although the dx cohort implies no tumor growth, it is important to note that CA19–9 values were obtained only during therapy, and some patients discontinued therapy at 6 months. This cohort also had a similar survival to the entire population, suggesting that tumor growth would likely have occurred soon after discontinuing therapy. Last, g values in the VA were inferior compared to the pivotal trials, particularly for FOLFIRINOX. Poorer control of tumor growth relative to the pivotal studies suggests that the Veterans may have required more dose reductions due to comorbidities and is consistent with our finding of more severe toxicities in FOLFIRINOX patients.
Our study benefited from a large, national sample of patients from a single health system. We had access to detailed information regarding patient and cancer characteristics, chemotherapy data, longitudinal laboratory measures and survival information. This allowed us to fit a propensity score to statistically balance the treatment groups and minimize potential confounding. Our study was limited, however, in several ways. First, despite using methods to limit confounding, these data are still inferior to those from a randomized, blinded, comparative trial. Next, we did not have chemotherapy dosing information, nor were we able to determine whether subjects completed their planned chemotherapy courses or whether regimens were modified. Given the co-morbidities prevalent in the VA patient population, it is likely that dose reduction occurred and this will be the subject of future investigations. Our intention-to-treat analysis, however, can be seen as conservative, as all subjects were administered at least an initial cycle of each chemotherapy regimen. Second, we did not have information on cancer progression, as this is not routinely recorded in the VA cancer data. It is likely, however, that most patients died of pancreatic cancer, and therefore overall survival is an appropriate outcome measure for evaluating therapies. In addition, we did not have performance status data. As patients treated with Gem/NabP may have had worse performance status, this may have been an important variable, although as these patients did not have worse outcomes than those treated with FOLFIRINOX, it further suggests that there may be similarity in benefits for these two regimens. Next, there were temporal differences in the use of the two regimens (FOLFIRINOX was used more in the early study years while Gem/NabP was used more in later years) that may have impacted outcomes. To address this issue we included treatment year in our propensity score. In addition, we included a broad group of tumor stages, which may have impacted the validity of our findings; however, stage stratified analyses were internally consistent. Last, our study cohort was predominately men; this limits the representativeness of our findings to women who may have different responses to either therapy.
In our study comparing Gem/NabP to FOLFIRINOX for a national cohort of Veterans with unresectable pancreatic cancer, we found no difference in survival for either regimen, and evidence of greater toxicity for patients treated with FOLFIRINOX. These findings are likely to explain a shift towards a higher proportion of patients with unresected pancreatic cancer being treated with Gem/NabP over time. In patients with comorbidities, limited performance status and/or with limited capacity to tolerate chemotherapy toxicity the use of Gem/NabP may optimize benefits and harms compared to FOLFIRINOX. We also found that a novel tumor growth equation fitted to data collected during chemotherapy treatment reliably predicted survival. Further exploration of this method as a biomarker for evaluating chemotherapy efficacy is warranted.
Acknowledgements
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. This publication is based on research using data from data contributors Celgene Corporation, that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study has not been previously presented.
Conflicts of Interest
Dr. Wisnivesky has received consulting honorarium from GSK, Sanofi and Banook and a research grant from Sanofi. Dr. Bates has received consulting honoraria from Pegascy, Inc., and research funding from Pancreatic Cancer Action Network. Dr. Nadkarni receives financial compensation as consultant and advisory board member for RenalytixAI, and owns equity in RenalytixAI and Pensieve Health. He is a scientific co-founder of RenalytixAI and Pensieve Health. Dr. Nadkarni has also received operational funding from Goldfinch Bio and consulting fees from BioVie Inc, AstraZeneca, Reata, Variant Bio and GLG consulting in the past three years.
Ethics Approval
This study was approved by the institutional review board of the James J. Peters VA Medical Center. This ethics board provided a waiver of informed consent. This was a retrospective study and was performed in accordance with the Declaration of Helsinki.
Data Availability
The primary data used in this study is from the United States Veterans Health Administration and are not publicly available.
References
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Research 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert A, Gavoille C, Conroy T. Current status on the place of FOLFIRINOX in metastatic pancreatic cancer and future directions. Therap Adv Gastroenterol 2017;10:631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartwright TH, Parisi M, Espirito JL, et al. Clinical Outcomes with First-Line Chemotherapy in a Large Retrospective Study of Patients with Metastatic Pancreatic Cancer Treated in a US Community Oncology Setting. Drugs Real World Outcomes 2018;5:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Signorovitch JE, Yang H, et al. Comparative Effectiveness of nab-Paclitaxel Plus Gemcitabine vs FOLFIRINOX in Metastatic Pancreatic Cancer: A Retrospective Nationwide Chart Review in the United States. Adv Ther 2018. [DOI] [PMC free article] [PubMed]
- 7.Chiorean EG, Von Hoff DD, Reni M, et al. CA19–9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 2016;27:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leuva H, Sigel K, Zhou M, et al. A novel approach to assess real-world efficacy of cancer therapy in metastatic prostate cancer. Analysis of national data on Veterans treated with abiraterone and enzalutamide. Semin Oncol 2019;46:351–361. [DOI] [PubMed] [Google Scholar]
- 9.Stein WD, Wilkerson J, Kim ST, et al. Analyzing the pivotal trial that compared sunitinib and IFN-alpha in renal cell carcinoma, using a method that assesses tumor regression and growth. Clin Cancer Res 2012;18:2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shustov A, Coiffier B, Horwitz S, et al. Romidepsin is effective and well tolerated in older patients with peripheral T-cell lymphoma: analysis of two phase II trials. Leuk Lymphoma 2017;58:2335–2341. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Keating NL, Potosky AL, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. JNCI Journal of the National Cancer Institute 2011;103:384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santana-Davila R, Devisetty K, Szabo A, et al. Cisplatin and Etoposide Versus Carboplatin and Paclitaxel With Concurrent Radiotherapy for Stage III Non-Small-Cell Lung Cancer: An Analysis of Veterans Health Administration Data. Journal of Clinical Oncology 2014. [DOI] [PMC free article] [PubMed]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 14.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res 2011;13:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Cai H, Li C, et al. Optimal caliper width for propensity score matching of three treatment groups: a Monte Carlo study. PLoS One 2013;8:e81045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macarulla T, Pazo-Cid R, Guillen-Ponce C, et al. Phase I/II Trial to Evaluate the Efficacy and Safety of Nanoparticle Albumin-Bound Paclitaxel in Combination With Gemcitabine in Patients With Pancreatic Cancer and an ECOG Performance Status of 2. J Clin Oncol 2018:JCO1800089. [DOI] [PubMed]
- 18.Chan K, Shah K, Lien K, et al. A Bayesian meta-analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer. PLoS One 2014;9:e108749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh et al. from ths issue - Yeh C and Bates SE - Two decades of research toward the treatment of locally advanced and metastatic pancreatic cancer: remarkable effort and limited gain. Seminars in Oncology in this issue [DOI] [PubMed]
- 20.Huang L, Jansen L, Balavarca Y, et al. Stratified survival of resected and overall pancreatic cancer patients in Europe and the USA in the early twenty-first century: a large, international population-based study. BMC Med 2018;16:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz MH, Hu CY, Fleming JB, et al. Clinical calculator of conditional survival estimates for resected and unresected survivors of pancreatic cancer. Arch Surg 2012;147:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]


