Abstract
Background
Increased frequency of circulating double negative T (DNT, CD4−CD8−CD3+) cells with protective immune function has been observed in human immunodeficiency virus (HIV) infection and tuberculosis (TB). Here the role of circulating TCRαβ+ DNT cells was further investigated in HIV/TB co-infection.
Methods
A cross-sectional study was conducted to investigate the frequency and functional profiles of peripheral TCRαβ+ DNT cells including apoptosis, chemokine and cytokine expression among healthy individuals and patients with TB, HIV infection and HIV/TB co-infection by cell surface staining and intracellular cytokine staining combined with flow cytometry.
Results
Significantly increased frequency of TCRαβ+ DNT cells was observed in HIV/TB co-infection than that in TB (p < 0.001), HIV infection (p = 0.039) and healthy controls (p < 0.001). Compared with TB, HIV/TB co-infection had higher frequency of Fas expression (p = 0.007) and lower frequency of Annexin V expression on TCRαβ+ DNT cells (p = 0.049), and the frequency of Annexin V expression on Fas+TCRαβ+ DNT cells had no significant difference. TCRαβ+ DNT cells expressed less CCR5 in HIV/TB co-infection than that in TB (p = 0.014), and more CXCR4 in HIV/TB co-infection than that in HIV infection (p = 0.043). Compared with healthy controls, TB and HIV/TB co-infection had higher frequency of TCRαβ+ DNT cells secreting Granzyme A (p = 0.046; p = 0.005). In TB and HIV/TB co-infection, TCRαβ+ DNT cells secreted more granzyme A (p = 0.002; p = 0.002) and perforin (p < 0.001; p = 0.017) than CD4+ T cells but similar to CD8+ T cells.
Conclusions
Reduced apoptosis may take part in the mechanism of increased frequency of peripheral TCRαβ+ DNT cells in HIV/TB co-infection. TCRαβ+ DNT cells may play a cytotoxic T cells-like function in HIV/TB co-infection.
Keywords: Human immunodeficiency virus; Tuberculosis; TCRαβ+ double negative T cells; Fas, CCR5; Granzyme A
Background
Tuberculosis (TB) caused by Mycobacterium tuberculosis (MTB) is one of the most common opportunistic infections and mortality of acquired immune deficiency syndrome (AIDS). Human immunodeficiency virus (HIV) and TB co-infection influence each other to promote disease progression. HIV infection increases the susceptibility to MTB and the risk of progression to active TB, in turn, TB promotes HIV replication and viral diversity, exacerbating AIDS progression [1].
Double negative T cells (DNT cells), defined as CD3+ T cells that lack of CD4 and CD8 expression, derive either from the thymus by the escape of negative selection or from CD4 + T cells or CD8 + T cells in the periphery in response to antigenic stimulation [2–4]. According to different T cell receptor (TCR), DNT cells can be divided into TCRαβ+ DNT cells and TCRγδ+ DNT cells [5, 6]. In humans and mice, about 95% of T cells express TCRαβ, and only a few (5%) express TCRγδ [6]. DNT cells only account for a low frequency (1%-5%) of peripheral T cells in general population, while increased frequency of peripheral DNT cells have been observed in autoimmune diseases, neoplastic diseases and infectious diseases [4, 7–9].
Previous study found in AIDS patients, the frequency of DNT cells in periphery increased significantly, which is twice that of healthy individuals [7]. Both peripheral and pulmonary mucosal DNT cells were reported to be the latent HIV virus reservoirs [10], and TCRαβ+ DNT cell was the major cell type harboring productive infection in peripheral blood [11]. However, whether HIV enters and infects DNT cells via HIV co-receptors is still unclear. Moreover, DNT cells were also associated with the control of immune activation and disease progression in HIV and simian immunodeficiency virus (SIV) infection [12, 13]. Elevated frequency of peripheral TCRαβ+ DNT cells were also observed in patients with TB compared with healthy controls, with a protective immunity function by secreting more IFN-γ [14]. In vivo study found pulmonary TCRαβ+ DNT cells could reduce the growth of MTB in the lungs [15].
Currently, the frequency and functional profile of peripheral TCRαβ+ DNT cells in HIV/TB co-infection is still unknown. Based on the role of TCRαβ+ DNT cells in HIV and TB pathogenesis and their ability to harbor persistent HIV reservoirs, we aim to investigate the frequency and functional profiles of peripheral TCRαβ+ DNT cells including apoptosis, chemokine and cytokine expression among patients with HIV/TB co-infection. Our preliminary study data may enrich and expend the role of DNT cells.
Methods
Study population
Adults (≥ 18 years old) who met the following inclusion criteria of HC, TB, HIV and HT group and volunteered to participate in this study were enrolled sequentially between May, 2018 and December, 2020. Individuals enrolled in this study were divided into four groups: (1) Healthy control (HC) group: healthy people without any history of chronic inflammatory diseases and infection manifestations within 2 weeks prior to enrollment were recruited from the physical examination center in Zhongnan Hospital of Wuhan University. HIV infection was excluded by HIV antibody screening and MTB infection was excluded by chest X-ray and Interferon Gamma Release Assay (IGRA). (2) Tuberculosis (TB) group: patients with confirmed TB by etiological or histopathological methods (smear or culture positive, and/or MTB DNA test positive, and/or Xpert MTB/RIF test positive, and/or histopathological evidence supporting TB) were recruited from Wuhan Pulmonary Hospital. All patients had been excluded HIV infection and hadn’t received anti-TB therapy prior to enrollment. (3) HIV infection (HIV) group: patients with confirmed HIV infection and without MTB infection by chest X-ray and IGRA screening were recruited from AIDS Clinical Guidance and Training Center, Zhongnan Hospital of Wuhan University All patients hadn’t received antiretroviral therapy (ART) prior to enrollment. (4) HIV/TB co-infection (HT) group: patients with HIV infection and active TB were recruited from department of infectious diseases, Zhongnan Hospital of Wuhan University. All patients hadn’t received ART and anti-TB therapy prior to enrollment.
Isolation of PBMCs
EDTA coagulated peripheral blood were collected from all enrolled individuals and then peripheral blood mononuclear cells (PBMCs) were isolated with the Ficoll-Paque method. Cell pellets were treated with 5 ml RBC lysis buffer (Sigma-Aldrich) for 10 min followed by washing once with 5% FBS-PBS. PBMCs were counted and cryopreserved with fetal calf serum (FCS) containing 10% dimethyl sulfoxide (DMSO) at − 80 °C.
Cell surface molecular staining
After resuscitation and counting, 1 × 106 PBMCs were suspended with a 200μL final volume. For surface marker staining, cells were stained with specific antibodies for 30 min at 4 °C in the dark. The following antibodies were used: anti-CD3-PerCP-cy5.5 (clone UCHT1; Biolegend), anti-CD8-APC-Cy7 (clone SK1; Biolegend), anti-CD4-PE-Cy7 (clone RPA-T4; Biolegend), anti-TCRγδ-FITC (clone B1; Biolegend), anti-Fas-APC (clone DX2; BD Biosciences), Annexin V-PE (Annexin V PE Apoptosis kit, Cat#559763, BD Biosciences), anti-CCR5-PE (clone J418F1; Biolegend), anti-CXCR3-APC (clone G025H7; Biolegend), anti-CXCR4-PE (clone 12g5; Biolegend), anti-CCR7-APC (clone G043h7; Biolegend). The stained cells were fixed with 2% paraformaldehyde and then analyzed by flow cytometry.
Direct intracellular cytokine staining
This procedure was performed as described before [16]. PBMCs were incubated for one hour stimulated with anti-CD28/CD49d (1 mg/ml) in a 200μL final volume medium in round-bottom 96-well plates at 37 °C and 5% CO2. Incubation with Brefeldin A (GolgiPlug, BD Biosciences) for 5 h was performed for further intracellular cytokines staining. Use PBS to wash cells twice and then surface marker staining was performed with with specific antibodies for 30 min at 4 °C in the dark. Fixation and Permeabilization Solution (Cytofix/Cytoperm; BD Biosciences) were added for 45 min at 4 °C in the dark. For intracellular cytokines staining, the following antibodies were used: anti-Granzyme A-PE (clone CB9; Biolegend), anti-Perforin-APC (clone dG9; Biolegend), anti-IFN-γ-PE (clone 4S.B3; Biolegend) and anti-TNF-α-APC (clone MAb11; Biolegend) according to the manufacturer's instructions. The stained cells were fixed with 2% paraformaldehyde and then analyzed by flow cytometry.
Statistical analysis
SPSS 21.0 and Graphpad Prism 5.0 were used for data statistics and plotting. Variables are denoted as the median (range) or n (%). Non-parametric rank sum test was used for comparison between groups. p < 0.05 was considered statistically significant.
Results
Participants’ characteristics
A total of 218 individuals (49 in the HC group, 75 in the TB group, 45 in the HIV group and 49 in the HT group) were enrolled in this study. The basic characteristics of participants were shown in Table 1. The proportion of male in TB, HIV and HT group were higher than that in HC group, but had no significant difference between TB, HIV and HT group. Both HIV group and HT group had lower CD4+ T lymphocyte count (CD4 count) than HC group and TB group, while the CD4 count in HT group was the lowest among the four groups. The proportion of pulmonary TB and extrapulmonary TB between TB and HT group had no significant difference.
Table 1.
Characteristics of the study participants
| HC group (n = 49) | TB group (n = 75) | HIV group (n = 45) | HT group (n = 49) | p value | |
|---|---|---|---|---|---|
| Age [years, median (range)] | 29 (24–50) | 43 (25–56) | 38 (27–47) | 39 (31–49) | 0.110 |
| Male, n (%) | 11 (31) | 46 (77) | 26 (87) | 34 (85) | < 0.001 |
| CD4+ T cell counts [/ul, median (range)] | 898 (746–969) | 743 (612–924) | 400 (311–582) |
88 (49–176) |
< 0.001 |
| Pulmonary TB, n(%) | / | 57 (76) | / | 30 (61) | 0.060 |
HC healthy control, TB tuberculosis, HT HIV/TB co-infection
TCRαβ+ DNT cells frequency in patients with HIV/TB co-infection
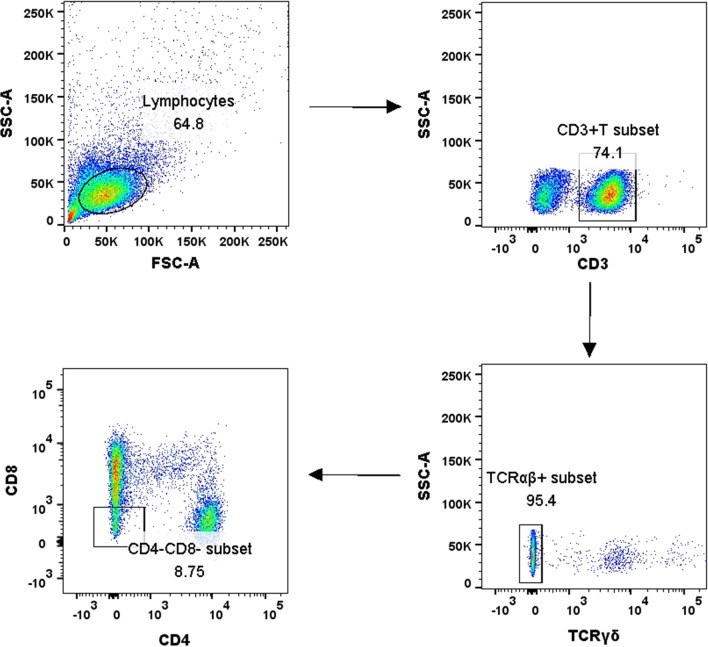
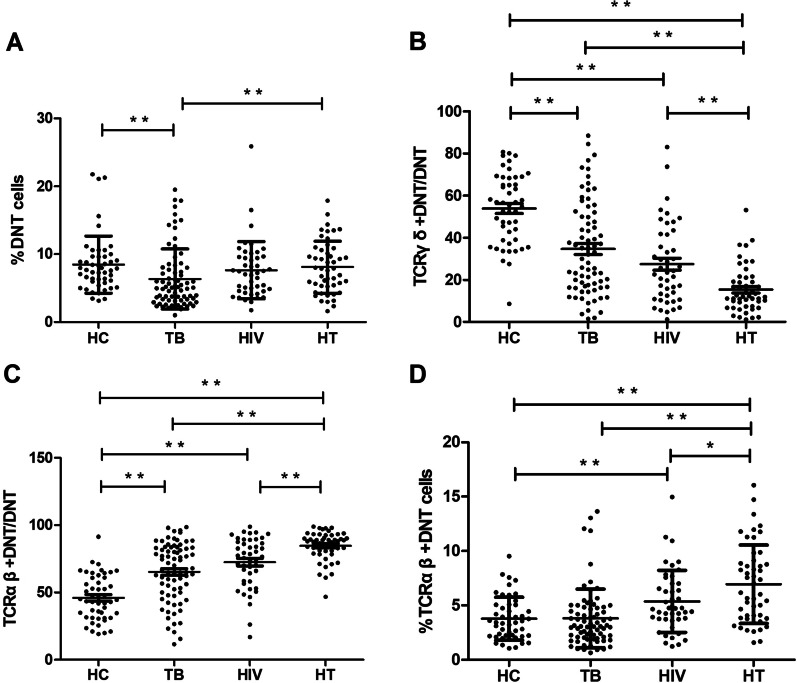
The flow cytometric gating strategy was displayed in Fig. 1. As shown in Fig. 2A, the frequency of DNT cells to CD3+ T cells in TB group was significantly lower than that in HC group (p < 0.001), and the frequency of DNT cells in HT group was significantly higher than that in TB group (p = 0.002). No significant difference of DNT cells frequency was found between HIV group and HT group.
Fig. 1.
The flow cytometric gating strategy displayed in healthy individuals, patients with TB, patients with HIV infection and patients with HIV/TB co-infection
Fig. 2.
A Comparison of the DNT cells proportion to CD3+ T cells in HC, TB, HIV and HT group. B Comparison of the TCRγδ+ DNT cells proportion to DNT cells in HC, TB, HIV and HT group. C Comparison of the TCRαβ+ DNT cells proportion to DNT cells in HC, TB, HIV and HT group. D Comparison of the TCRαβ+ DNT cells proportion to CD3+ T cells in HC, TB, HIV and HT group. *p < 0.05; **p < 0.01. HC healthy controls, TB patients with tuberculosis, HIV patients with HIV infection, HT patients with HIV/TB co-infection
Further subset analysis according to the TCR expression showed that the proportion of TCRγδ+ DNT cells to DNT cells was significantly decreased in HT group in comparison to HC, TB and HIV group (p < 0.001; p < 0.001; p = 0.001), only accounting for 12%; while the proportion of TCRαβ+ DNT cells to DNT cells was the highest in HT group in comparison to HC, TB and HIV group (p < 0.001; p < 0.001; p = 0.001), accounting for 88% (shown in Fig. 2B, C). These data suggested the expansion of TCRαβ+ DNT cells was the main contributor to the increased frequency of DNT cells in HT group, so we focused on analyzing the frequency and functional profile of peripheral TCRαβ+ DNT cells in our study. As shown in Fig. 2D, the frequency of TCRαβ+ DNT cells to CD3+T cells in HT group was significantly higher than that in TB group (p < 0.001), HIV group (p = 0.039) and HC group (p < 0.001).
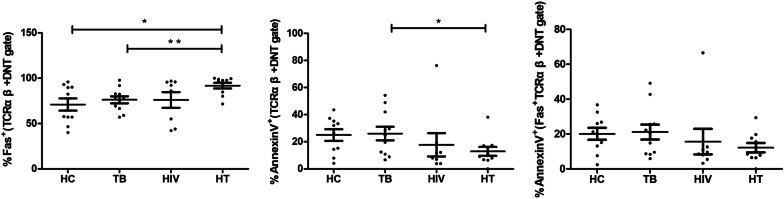
Fas expression on TCRαβ+ DNT cells in HIV/TB co-infection
The Annexin V and Fas expression of TCRαβ+ DNT cells were preliminarily analyzed to investigate whether increased TCRαβ+ DNT cells frequency was related to Fas mediated apoptosis. As shown in Fig. 3, the frequency of TCRαβ+ DNT cells with Annexin V expression in HT group was lower than that in TB group (p = 0.049). However, compared with the TB group (p = 0.007) and HC group (p = 0.010), HT group had higher frequency of TCRαβ+ DNT cells expressing Fas, and the frequency of Annexin V expression on Fas+TCRαβ+ DNT cells between HT group and TB group had no significant difference.
Fig. 3.
Comparison of TCRαβ+ DNT cells expressing Annexin V and Fas in HC, TB, HIV and HT group. *p < 0.05; **p < 0.01. HC healthy controls, TB patients with tuberculosis, HIV patients with HIV infection, HT patients with HIV/TB co-infection
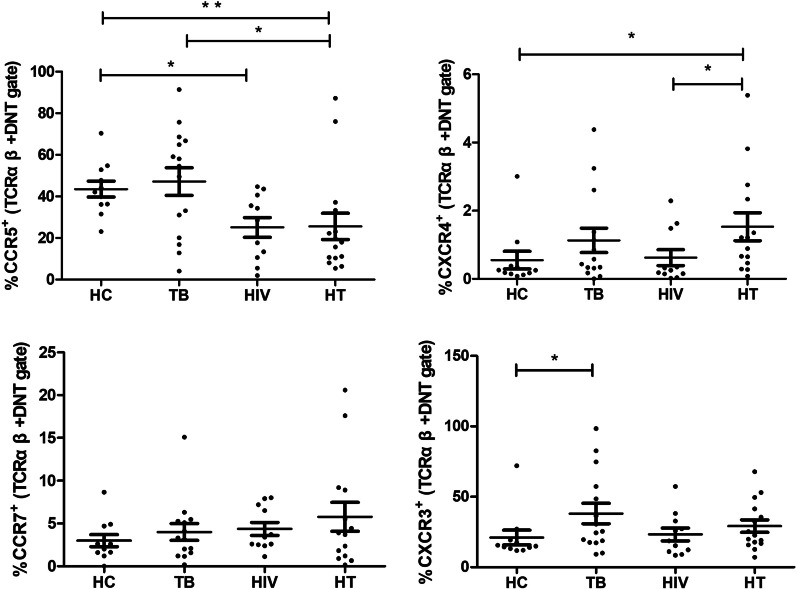
CCR5 and CXCR4 expression on TCRαβ+ DNT cells in HIV/TB co-infection
The frequencies of TCRαβ+ DNT cells expressing CC chemokine receptor and CXC chemokine receptor in each group were evaluated. As shown in Fig. 4, lower frequency of CCR5 expression on TCRαβ+ DNT cells was observed in HIV group (p = 0.014) and HT group (p = 0.005) than that in HC group, and lower frequency of CCR5 expression on TCRαβ+ DNT cells was observed in HT group than that in TB group (p = 0.036). Compared with HIV group (p = 0.043) and HC group (p = 0.013), TCRαβ+ DNT cells expressed more CXCR4 in HT group. No significant difference of TCRαβ+ DNT cells expressing CCR7 and CXCR3 were found between TB group, HIV group and HT group.
Fig. 4.
Comparison of TCRαβ+ DNT cells expressing CCR5, CXCR4, CCR7, CXCR3 in HC, TB, HIV and HT group. *p < 0.05; **p < 0.01. HC healthy controls, TB patients with tuberculosis, HIV patients with HIV infection, HT patients with HIV/TB co-infection
Cytokines secretion of TCRαβ+ DNT cells in HIV/TB co-infection
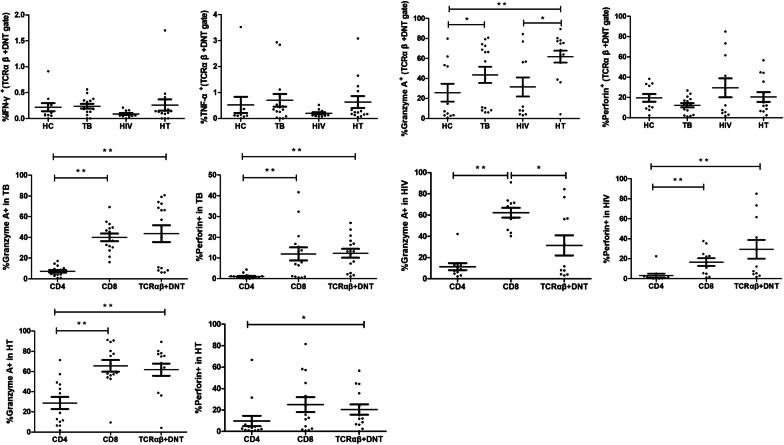
The function of cytokines secretion of TCRαβ+ DNT cells in each group were further analyzed. As shown in Fig. 5A, the frequency of TCRαβ+ DNT cells expressing granzyme A was significantly higher in HT group than that in HIV group (p = 0.025) and HC group (p = 0.005). TCRαβ+ DNT cells expressed more granzyme A in TB group than that in HC group (p = 0.046). No significant difference of TCRαβ+ DNT cells secreting perforin, IFN-γ and TNF-α were found between HC group, TB group, HIV group and HT group.
Fig. 5.
A Comparison of TCRαβ+ DNT cells secreting IFN-γ, TNF-α, granzyme A, perforin in HC, TB, HIV and HT group. B Comparison of CD4+ T, CD8+ T and TCRαβ+ DNT cells secreting granzyme A, perforin in TB, HIV and HT group. *p < 0.05; **p < 0.01. HC healthy controls, TB patients with tuberculosis, HIV patients with HIV infection, HT patients with HIV/TB co-infection
We further compared the cytokine secretion functions of CD4+ T cells, CD8+ T cells and TCRαβ+ DNT cells in TB group, HIV group and HT group, respectively. As shown in Fig. 5B, in TB group and HT group, TCRαβ+ DNT cells secreted more granzyme A (p = 0.002; p = 0.002) and perforin (p < 0.001; p = 0.017) than CD4+ T cells and the secretion of granzyme A and perforin between TCRαβ+ DNT cells and CD8 + T cells had no significant difference. In HIV group, TCRαβ+ DNT cells secreted more perforin than CD4+ T cells (p = 0.001) and less granzyme A than CD8+ T cells (p = 0.028). No significant differences of IFN-γ and TNF-α secretion between CD4+ T cells, CD8+ T cells and TCRαβ+ DNT cells were found in TB group, HIV group and HT group (Results not shown).
Discussion
To our knowledge, this is the first study to investigate the frequency and function characteristics of peripheral TCRαβ+ DNT cells, especially the Fas, CCR5, CXCR4 expression on peripheral TCRαβ+ DNT cells in HIV infection with or without TB. High frequencies of DNT cells in HIV infection and TB has been described previously [12, 14]. In our study. we found that HIV/TB co-infection could lead to an expansion of peripheral TCRαβ+ DNT cells compared with mono-TB and mono-HIV infection. In HIV infected patients, TCRαβ+ DNT cells were thought to be derived both normally from within the thymus, and also from cells originally expressing CD4, but which have undergone CD4 downregulation in response to HIV-1 infection through the cooperative action of Env, Nef, and Vpu [17–19]. Hence, co-infection with HIV may drive the CD4 internalization of HIV infected CD4+ T cells and then increase the generation of TCRαβ+ DNT cells in patients with TB. Moreover, CD4 down-regulation might change the cell signaling pathways and thus prevent CD4+ T cells from HIV gp120 induced apoptosis [20, 21]. However, whether the expansion of TCRαβ+ DNT cells in HIV/TB co-infection is related to the decreased apoptosis remains unclear.
In our study, we found the apoptosis of TCRαβ+ DNT cells in HIV/TB co-infection was lower than that in TB according to the AnnexinV expression. Fas and Fas Ligand (FasL) pathway is one of the key death receptor signaling pathways involved in the regulation of apoptotic cell death [22, 23]. Fas and FasL pathway also contributed to the T cells apoptosis in HIV infection [24, 25]. It's worth noting that our study found the frequency of TCRαβ+ DNT cells expressing Fas was higher in HIV/TB co-infection than that in TB, and the AnnexinV expression on Fas positive TCRαβ+ DNT cells had no significant difference between HIV/TB co-infection and TB. All these data suggested that the increased Fas expression didn’t mediate the higher apoptosis of TCRαβ+ DNT cells in HIV/TB co-infection. Previous studies found HIV Nef could interact with apoptosis signal-regulating kinase 1 (ASK1) and then inhibit Fas-mediated apoptosis and protect HIV-infected CD4+ T cells from CD8+ T cells killing [26, 27]. Given that TCRαβ+ DNT cells could be derived from CD4+ T cells with CD4 expression downregulated by HIV Nef [17], HIV Nef maybe also contribute to the immune evasion of TCRαβ+ DNT cells by inhibiting Fas-mediated pro-apoptotic signaling. This may be an immune evasion strategy of HIV that facilitates the survival of TCRαβ+ DNT cells (latent reservoir cells) and the persistence of intracellular viral replication.
The HIV co-receptors CXCR4 or CCR5, are well-known for their interaction with the HIV-1 gp120 to promote HIV entering into the target cells [28, 29]. Our data found that the expression of CCR5 on TCRαβ+ DNT cells was lower in HIV/TB co-infection and HIV infection than that in healthy controls, and the expression of CCR5 on TCRαβ+ DNT cells was lower in HIV/TB co-infection than that in TB. All these results suggested HIV infection might downregulate the CCR5 expression on TCRαβ+ DNT cells. In fact, previous studies found except surface CD4 expression downregulated by HIV Nef, the surface CCR5 on HIV infected CD4+ T cells could also be reduced by HIV Nef via distinct cellular mechanisms [30, 31]. Downregulation of CCR5 on TCRαβ+ DNT cells might be a viral strategy to avoid superinfection by HIV and thus protect them from premature death. More importantly, the binding of HIV gp120 to CCR5 could activate Fas/FasL pathway, thereby increase the susceptibility of T cells to Fas-mediated apoptosis [32–34]. Hence, in our study CCR5 downregulation on TCRαβ+ DNT cells may reduce the susceptibility to Fas-mediated apoptosis and thus inhibit TCRαβ+ DNT cells death in HIV infection and HIV/TB co-infection. Previous study showed high CXCR4 expression was helpful for X4 HIV entering into the target cells [35]. As AIDS progresses, there is a shift in viral coreceptor use from CCR5 to CXCR4 [36]. Our study found co-infected with TB increased the CXCR4 expression on TCRαβ+ DNT cells compared with mono-HIV infection, suggesting that HIV/TB co-infection might promote the entry of HIV into TCRαβ+ DNT cells by CXCR4 but not by CCR5.
In our study, TCRαβ+ DNT cells secreting more granzyme A in HIV/TB co-infection than that in HIV infection and healthy controls, and TCRαβ+ DNT cells secreting more granzyme A in TB than that in healthy controls, suggesting that TCRαβ+ DNT cells might exhibit cytotoxic T cells-like function in TB no matter with or without HIV infection. Consistent with this, the analysis and comparison of granzyme A and perforin secretion of CD4+ T cells, CD8+ T cells and TCRαβ+ DNT cells also showed TCRαβ+ DNT cells might display cytotoxic T cells-like function in TB and HIV/TB co-infection. Although our study found TCRαβ+ DNT cells might effect as CD8+ T cells, it is still unknown whether TCRαβ+ DNT cells derive from CD8+ T cells in HIV/TB co-infection. In autoimmune diseases, it was found TCRαβ+ DNT cells might arise from CD8+ T cells and displayed a distinct cytokine production profile by producing proinflammatory mediators that include IL-1β, IL-17, IFN-γ, CXCL3, and CXCL2. Upon activation, the CD8 downregulation on CD8 + T cells not only changed the phenotype of CD8+ T cells, but also granted them inflammatory capacity [37].
Conclusion
Our study showed that the apoptosis of TCRαβ+ DNT cells was not matched with their Fas expression. Whether it’s a strategy for viral immune evasion by inhibiting Fas-mediated pro-apoptotic signaling or reducing the susceptibility to Fas-mediated apoptosis and thus contribute to the persistent virus replication in TCRαβ+ DNT cells need further exploration. Reduced apoptosis may take part in the mechanism of increased frequency of peripheral TCRαβ+ DNT cells in HIV/TB co-infection. TCRαβ+ DNT cells may play a cytotoxic T cells-like function in HIV/TB co-infection.
Acknowledgements
We would like to thank the participants for their contributions to the study.
Abbreviations
- DNT
Double negative T
- HIV
Human immunodeficiency virus
- TB
Tuberculosis
- MTB
Mycobacterium tuberculosis
- AIDS
Acquired immune deficiency syndrome
- TCR
T cell receptor
- SIV
Simian immunodeficiency virus
- HC
Healthy control
- IGRA
Interferon gamma release assay
- ART
Antiretroviral therapy
- PBMCs
Peripheral blood mononuclear cells
- FCS
Fetal calf serum
- ASK1
Apoptosis signal-regulating kinase 1
Author contributions
LS and KL conceived and designed this investigation. YT, SZ and WG helped to design the scheme of the investigation and collected the original data. WG, YX, YD, QZ and ML performed the experiments. YT, SZ, WG and SW analyzed the data. YT, SZ and KL contributed to the writing of the paper. All authors read and approved the final manuscript.
Funding
This work was supported by Medical Science and Technology Innovation Platform Support Project of Zhongnan Hospital, Wuhan University (PTXM2020008), Science and Technology Innovation Cultivation Fund of Zhongnan Hospital, Wuhan University (cxpy2017043). Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018004).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Zhongnan Hospital of Wuhan University (2016009). All subjects have voluntarily participated in the study and signed the informed consent form. The study was performed in accordance with the guidelines of the Declaration of Helsinki and relevant regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuting Tan, Shi Zou and Wei Guo contributed equally to this work and share first authorship.
Contributor Information
Ling Shen, Email: lshen@uic.edu.
Ke Liang, Email: keliang@whu.edu.cn.
References
- 1.Bell L, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16(2):80–90. doi: 10.1038/nrmicro.2017.128. [DOI] [PubMed] [Google Scholar]
- 2.Sundaravaradan V, Mir KD, Sodora DL. Double-negative T cells during HIV/SIV infections: potential pinch hitters in the T-cell lineup. Curr Opin HIV AIDS. 2012;7(2):164–171. doi: 10.1097/COH.0b013e3283504a66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Rodríguez N, Flores-Mendoza G, Apostolidis SA, Rosetti F, Tsokos GC, Crispín JC. TCR-α/β CD4(−) CD8(−) double negative T cells arise from CD8(+) T cells. J Leukoc Biol. 2020;108(3):851–857. doi: 10.1002/JLB.1AB0120-548R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt D, Hedrich CM. TCRαβ(+)CD3(+)CD4(−)CD8(−) (double negative) T cells in autoimmunity. Autoimmun Rev. 2018;17(4):422–430. doi: 10.1016/j.autrev.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8- double-negative regulatory T cells. Blood. 2005;105(7):2828–2835. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Dong K, Fan X, Xie J, Wang M, Fu S, Li Q. DNT cell-based immunotherapy: progress and applications. J Cancer. 2020;11(13):3717–3724. doi: 10.7150/jca.39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singleterry WL, Henderson H, Cruse JM. Depletion of pro-inflammatory CD161(+) double negative (CD3(+)CD4(−)CD8(−)) T cells in AIDS patients is ameliorated by expansion of the γδ T cell population. Exp Mol Pathol. 2012;92(1):155–159. doi: 10.1016/j.yexmp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Yasukawa M, Inatsuki A, Kobayashi Y. Human double-negative (CD4-CD8-) T cells bearing alpha beta T cell receptor possess both helper and cytotoxic activities. Clin Exp Immunol. 1991;85(3):525–530. doi: 10.1111/j.1365-2249.1991.tb05761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Zheng Y, Sheng J, Han Y, Yang Y, Pan H, Yao J. CD3(+)CD4(−)CD8(−) (double-negative) T cells in inflammation, immune disorders and cancer. Front Immunol. 2022;13:816005. doi: 10.3389/fimmu.2022.816005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meziane O, Salahuddin S, Pham TNQ, Farnos O, Pagliuzza A, Olivenstein R, Thomson E, Alexandrova Y, Orlova M, Schurr E, et al. HIV infection and persistence in pulmonary mucosal double negative T cells in vivo. J Virol. 2020 doi: 10.1128/JVI.01788-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser P, Joos B, Niederöst B, Weber R, Günthard HF, Fischer M. Productive human immunodeficiency virus type 1 infection in peripheral blood predominantly takes place in CD4/CD8 double-negative T lymphocytes. J Virol. 2007;81(18):9693–9706. doi: 10.1128/JVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petitjean G, Chevalier MF, Tibaoui F, Didier C, Manea ME, Liovat AS, Campa P, Müller-Trutwin M, Girard PM, Meyer L, et al. Level of double negative T cells, which produce TGF-β and IL-10, predicts CD8 T-cell activation in primary HIV-1 infection. AIDS. 2012;26(2):139–148. doi: 10.1097/QAD.0b013e32834e1484. [DOI] [PubMed] [Google Scholar]
- 13.Vinton C, Klatt NR, Harris LD, Briant JA, Sanders-Beer BE, Herbert R, Woodward R, Silvestri G, Pandrea I, Apetrei C, et al. CD4-like immunological function by CD4- T cells in multiple natural hosts of simian immunodeficiency virus. J Virol. 2011;85(17):8702–8708. doi: 10.1128/JVI.00332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinheiro MB, Antonelli LR, Sathler-Avelar R, Vitelli-Avelar DM, Spindola-de-Miranda S, Guimarães TM, Teixeira-Carvalho A, Martins-Filho OA, Toledo VP. CD4-CD8-αβ and γδ T cells display inflammatory and regulatory potentials during human tuberculosis. PLoS ONE. 2012;7(12):e50923. doi: 10.1371/journal.pone.0050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derrick SC, Evering TH, Sambandamurthy VK, Jalapathy KV, Hsu T, Chen B, Chen M, Russell RG, Junqueira-Kipnis AP, Orme IM, et al. Characterization of the protective T-cell response generated in CD4-deficient mice by a live attenuated Mycobacterium tuberculosis vaccine. Immunology. 2007;120(2):192–206. doi: 10.1111/j.1365-2567.2006.02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao S, Huang D, Chen CY, Halliday L, Wang RC, Chen ZW. CD4+ T cells contain early extrapulmonary tuberculosis (TB) dissemination and rapid TB progression and sustain multieffector functions of CD8+ T and CD3- lymphocytes: mechanisms of CD4+ T cell immunity. J Immunol. 2014;192:2120–2132. doi: 10.4049/jimmunol.1301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMaster LK, Liu X, VanBelzen DJ, Trinité B, Zheng L, Agosto LM, Migueles SA, Connors M, Sambucetti L, Levy DN, et al. A subset of CD4/CD8 double-negative T cells expresses HIV proteins in patients on antiretroviral therapy. J Virol. 2015;90(5):2165–2179. doi: 10.1128/JVI.01913-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheney KM, Kumar R, Purins A, Mundy L, Ferguson W, Shaw D, Burrell CJ, Li P. HIV type 1 persistence in CD4- /CD8- double negative T cells from patients on antiretroviral therapy. AIDS Res Hum Retroviruses. 2006;22(1):66–75. doi: 10.1089/aid.2006.22.66. [DOI] [PubMed] [Google Scholar]
- 19.Meng Q, Canaday DH, McDonald DJ, Mayanja-Kizza H, Baseke J, Toossi Z. Productive HIV-1 infection is enriched in CD4(−)CD8(−) double negative (DN) T cells at pleural sites of dual infection with HIV and Mycobacterium tuberculosis. Arch Virol. 2016;161(1):181–187. doi: 10.1007/s00705-015-2640-7. [DOI] [PubMed] [Google Scholar]
- 20.Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76(5):853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 21.Banda NK, Bernier J, Kurahara DK, Kurrle R, Haigwood N, Sekaly RP, Finkel TH. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176(4):1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata S, Golstein P. The Fas death factor. Science. 1995;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 23.Orlinick JR, Vaishnaw AK, Elkon KB. Structure and function of Fas/Fas ligand. Int Rev Immunol. 1999;18(4):293–308. doi: 10.3109/08830189909088485. [DOI] [PubMed] [Google Scholar]
- 24.Poonia B, Pauza CD, Salvato MS. Role of the Fas/FasL pathway in HIV or SIV disease. Retrovirology. 2009;6:91. doi: 10.1186/1742-4690-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehrbod P, Ande SR, Alizadeh J, Rahimizadeh S, Shariati A, Malek H, Hashemi M, Glover KKM, Sher AA, Coombs KM, et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019;10(1):376–413. doi: 10.1080/21505594.2019.1605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geleziunas R, Xu W, Takeda K, Ichijo H, Greene WC. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature. 2001;410(6830):834–838. doi: 10.1038/35071111. [DOI] [PubMed] [Google Scholar]
- 27.Sevilya Z, Chorin E, Gal-Garber O, Zelinger E, Turner D, Avidor B, Berke G, Hassin D. Killing of latently HIV-infected CD4 T cells by autologous CD8 T cells is modulated by nef. Front Immunol. 2018;9:2068. doi: 10.3389/fimmu.2018.02068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nickoloff-Bybel EA, Festa L, Meucci O, Gaskill PJ. Co-receptor signaling in the pathogenesis of neuroHIV. Retrovirology. 2021;18(1):24. doi: 10.1186/s12977-021-00569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019;27(10):878–891. doi: 10.1016/j.tim.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel N, Allespach I, Venzke S, Fackler OT, Keppler OT. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr Biol. 2005;15(8):714–723. doi: 10.1016/j.cub.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 31.Dubey S, Khalid M, Wesley C, Khan SA, Wanchu A, Jameel S. Downregulation of CCR5 on activated CD4 T cells in HIV-infected Indians. J Clin Virol. 2008;43(1):25–31. doi: 10.1016/j.jcv.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Abbas W, Herbein G. Plasma membrane signaling in HIV-1 infection. Biochim Biophys Acta. 2014;1838(4):1132–1142. doi: 10.1016/j.bbamem.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Vlahakis SR, Algeciras-Schimnich A, Bou G, Heppelmann CJ, Villasis-Keever A, Collman RG, Paya CV. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J Clin Invest. 2001;107(2):207–215. doi: 10.1172/JCI11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Algeciras-Schimnich A, Vlahakis SR, Villasis-Keever A, Gomez T, Heppelmann CJ, Bou G, Paya CV. CCR5 mediates Fas- and caspase-8 dependent apoptosis of both uninfected and HIV infected primary human CD4 T cells. AIDS. 2002;16(11):1467–1478. doi: 10.1097/00002030-200207260-00003. [DOI] [PubMed] [Google Scholar]
- 35.Lin YL, Portales P, Segondy M, Baillat V, de Boever CM, Le Moing V, Réant B, Montes B, Clot J, Reynes J, et al. CXCR4 overexpression during the course of HIV-1 infection correlates with the emergence of X4 strains. J Acquir Immune Defic Syndr. 2005;39(5):530–536. [PubMed] [Google Scholar]
- 36.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384(6609):529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 37.Crispín JC, Tsokos GC. Human TCR-alpha beta+ CD4- CD8- T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J Immunol. 2009;183(7):4675–4681. doi: 10.4049/jimmunol.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.