Abstract
Background
This study was designed to provide insecticide resistance data for decision-making in terms of resistance management plans in Togo.
Methods
The susceptibility status of Anopheles gambiae sensu lato (s.l.) to insecticides used in public health was assessed using the WHO tube test protocol. Pyrethroid resistance intensity bioassays were performed following the CDC bottle test protocol. The activity of detoxification enzymes was tested using the synergists piperonyl butoxide, S.S.S-tributlyphosphorotrithioate and ethacrinic acid. Species-specific identification of An. gambiae s.l. and kdr mutation genotyping were performed using PCR techniques.
Results
Local populations of An. gambiae s.l. showed full susceptibility to pirimiphos methyl at Lomé, Kovié, Anié, and Kpèlè Toutou. At Baguida, mortality was 90%, indicating possible resistance to pirimiphos methyl. Resistance was recorded to DDT, bendiocarb, and propoxur at all sites. A high intensity of pyrethroid resistance was recorded and the detoxification enzymes contributing to resistance were oxidases, esterases, and glutathione-s-transferases based on the synergist tests. Anopheles gambiae sensu stricto (s.s.) and Anopheles coluzzii were the main species identified. High kdr L1014F and low kdr L1014S allele frequencies were detected at all localities.
Conclusion
This study suggests the need to reinforce current insecticide-based malaria control interventions (IRS and LLINs) with complementary tools.
Keywords: Malaria, Anopheles gambiae, Vector control, Intensity resistance, Togo
Background
The use of insecticides is an important component of malaria vector control programmes in Africa [1]. However, the emergence of resistance to the main classes of insecticides used in the treatment of bed nets and in indoor residual spraying (IRS) requires re-thinking the use of these tools and the management of resistance in vectors [2]. Resistance has already been reported in various West African countries including Benin, Burkina Faso, Mali [3–5] and particularly in Togo [6, 7]. Recent studies showed that the use of synergists and combination of insecticide formulationscan increase the susceptibility of malaria vectors in areas with high pyrethroid resistance [8, 9]. To support the sustainability of control strategies, it is essential to consider that resistance management should be systematically integrated into any vector control policy [2]. The implementation of resistance management plans should be supported by the confirmation of resistance in any given country [10]. According to the World Health Organization (WHO) guidelines [10], resistance management involves the implementation of a three-step protocol including (1) assessment of insecticide susceptibility status of vectors, (2) characterization of resistance intensity, and (3) assessment of physiological resistance mechanisms with a focus on the efficacy of the synergist piperonyl butoxide (PBO). In Togo, the first step (i.e., evaluation of the susceptibility status of malaria vectors to insecticides) is carried out every 2 to 3 years at the National Malaria Control Programme (NMCP) sentinel sites. The last two steps (i.e., intensity of resistance and efficacy of the synergists piperonyl butoxide (PBO), S,S,S-tributyl phosphorotrithioate (DEF), and ethacrynic acid (EA)), are not yet widely conducted.
The present study aimed to address these three aspects to provide the NMCP with reliable data for decision-making regarding resistance management in Togo.
Methods
Study area
The present study was carried out at NMCP sentinel sites selected in three health regions in Southern Togo from June to September 2021 (Fig. 1). According to geographical (different health regions) and ecological characteristics (vector abundance, permanent larval breeding sites), five NMCP surveillance sites were selected for monitoring. The sites were Lomé, Baguida, Kovié, Anié, and Kpèlè Toutou (Table 1).
Fig. 1.
Map showing the localities surveyed
Table 1.
Characteristics of monitoring sentinel sites
| Sentinel sites | Description | Latitude | Longitude | Health region | Agricultural practices | Climate | Dry Seasons | Rainy Seasons |
|---|---|---|---|---|---|---|---|---|
| Lomé | Urban | 31N0302166 | UTM 0690055 | Lomé | Capital of Togo | Sub-equatorial | April-July and October-November | December-March and August-September |
| Baguida | Peri-urban | 31N0314169 | UTM 0681627 | Maritime | Vegetables | Sub-equatorial | April-July and October-November | December-March and August-September |
| Kovié | Peri-urban | 31N0291756 | UTM 0701889 | Maritime | Rice | Sub-equatorial | April-July and October-November | December-March and August-September |
| Anié | Urban | 31N0302493 | UTM 0859340 | Plateaux | Cotton and food crop | Sub-equatorial | April-July and October-November | December-March and August-September |
| Kpele Toutou | Rural | 31N0250374 | UTM 0785118 | Plateaux | Rice | Sub-equatorial | April-July and October-November | December-March and August-September |
Mosquito collection and rearing
Anopheles larvae were collected from a minimum of 10 breeding sites located at least 150 m from each other at each sentinel site. Larvae were collected using methods described by Silver [11], pooled in 1.5 L plastic bottles and brought back to the Laboratory of Ecology and Ecotoxicology (LaEE)/Togo insectarium and reared under controlled conditions (temperature 25 ± 2 °C, Relative humidity 75 ± 2% and 12 L:12 D photoperiodicity) until adult emergence. Emerging adults were fed with 10% glucose solution.
Insecticide susceptibility tests
Insecticide susceptibility tests were assessed on 3–5-day old females morphologically identified as Anopheles gambiae sensu lato (s.l.) using four classes of insecticides following the WHO standard protocol [10]. The tests were done with papers impregnated with three pyrethroids (deltamethrin 0.05%, permethrin 0.75%, and alphacypermethrin 0.05%), one organochlorine (DDT 4%), two carbamates (bendiocarb 0.1% and propoxur 0.1%), and one organophosphate (pirimiphos-methyl 0.25%). For each insecticide paper, four replicates of 20–25 unfed females were exposed for one hour. Anopheles gambiae “Kisumu” strain was used as a reference susceptible control strain in each bioassay. Mortality was recorded 24 h after exposure.
CDC bottle intensity assays
Resistance intensity of An. gambiae to insecticides was performed using the CDC bottle bioassay protocol [12]. Three pyrethroids were tested at the following doses: deltamethrin (12.5 µg, 25 µg, 62.5 and 125 µg), permethrin (21.5 µg, 43 µg, 107.5 and 215 µg) and alphacypermethrin (12.5 µg, 25 µg, 62.5 and 125 µg). Approximately 20 unfed females aged 3 to 5 days old were introduced into a CDC bottle. The CDC bottle (250 ml) was previously impregnated with 1ml of the insecticide solution to be evaluated and dried under laboratory conditions for 24 h. Mosquitoes were exposed for 60 min. Mortality was recorded every 15 min for one hour. Four bottles were used for each concentration of insecticide. Control bottles were impregnated with ethanol.
Synergist tests
Papers impregnated with the synergist piperonyl butoxide (PBO 4%) and bottles impregnated with S.S.S-tributlyphosphorotrithioate (DEF 125 µg) and ethacrynic acid (EA 80 µg) were used for the synergist tests. For synergist testing with PBO, mosquitoes were pre-exposed to PBO papers for one hour before being exposed to pyrethroids (deltamethrin, permethrin and alphacypermethrin) using the WHO tube testing protocol [10]. Mosquitoes were exposed to DEF synergist for one hour before exposure to organophosphates (malathion 50 µg and fenitrothion 50 µg) and carbamates (bendiocarb 12.5 µg) and EA for 1 hour before exposure to organochlorines (DDT 100 µg) using the CDC bottle testing protocol [12]. After bioassay testing, mosquitoes were preserved in Eppendorf tubes containing silica gel and stored at − 20 °C for molecular analyses.
Molecular analysis
Molecular analysis was performed on a subsample of 50 females randomly selected per site at the IRSS laboratory / Burkina Faso. DNA was extracted from head-thorax of An. gambiae with 2% Cetyl Trimethyl Ammonium Bromide (2% CTAB). Members of the An. gambiae complex were identified using the PCR-SINE 200X (Short INterspersed Elements) technique of Santolamazza et al. [13].
Detection of the West (kdr-w) and East African kdr (kdr-e) mutations were performed following the protocols of Martinez-Torres et al. [14] and Ranson et al. [15], respectively.
Data analysis
Data were entered using Microsoft Office Excel 2016 software. Allelic frequencies for each kdr mutation were compared between sites for each species using the “G-test” [16] performed in Genepop 4.7 and run with R software (version 4.0.3) [17].
Resistance intensity at 5 x and 10 x diagnostic doses was interpreted according to CDC criteria whereby mosquitoes were categorized as ‘dead’ if they were immobilized by the effect of the insecticide, and unable to stand or fly. The diagnostic time for all insecticides used was 30 min except for DDT which is 45 min [10]. Mortality rates were calculated and interpreted according to the following thresholds and criteria [10] :
Mortality < 90%: Resistant.
90% ≤ mortality ≤ 97%: Probable Resistant.
Mortality ≥ 98%: Susceptible.
Results
Susceptibility status of An. gambiae to insecticides
Mortality rates of the Kisumu strain of An. gambiae was 100% with all insecticides tested (Fig. 2). Local populations of An. gambiae showed full susceptibility to pirimiphos methyl at four (4) localities (Lomé, Kovié, Anié and Kpèlè Toutou) with mortality rates of 100%. At Baguida, mortality was 90%, indicating possible resistance to pirimiphos methyl.
Fig. 2.
Susceptibility status of An. gambiae s.l. to pyrethroids (A) and to organophosphates, carbamates, and DDT (B). Alpha Alphacypermethrin, Perm Permethrin, Delta Deltamethrin, Propox Propoxur, Bendio Bendiocarb
Susceptibility tests showed resistance to DDT (mortality ranging between 2% and 20.5%), bendiocarb (15–62.5%), propoxur (19.25–51.75%), deltamethrin (16.25–59.25%), permethrin (6–46%), and alphacypermethrin (1–38.75%) across all study sites (Fig. 2).
Resistance intensity of An. gambiaes.l. to pyrethroids
Resistance intensity tests of local populations of An. gambiae exposed to pyrethroids (deltamethrin, permethrin and alphacypermethrin) showed mortality rates of less than 98% at the 5 x and 10 x diagnostic doses after 30 min. The highest mortality rates were 90.3% at Kpèlè Toutou (Table 2), 90.7% at Anié (Table 3) and 90.8% at Baguida (Table 4) for deltamethrin, permethrin and alphacypermethrin, respectively. These results reflect a high resistance intensity of An. gambiae to pyrethroids at all localities surveyed.
Table 2.
Resistance intensity of An. gambiae s.l. 30 min after exposure to deltamethrin
| Strain | Deltamethrin | Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 × (12,5 µg) | 2 × (25 µg) | 5 × (62,5 µg) | 10 × (125 µg) | ||||||
| n | % Mortality [CI] | n | % Mortality [CI] | n | % Mortality [CI] | n | % Mortality [CI] | ||
| An. gambiae « Kisumu » | 89 | 100 | 86 | 100 | 85 | 100 | 96 | 100 | Low intensity |
| Lomé | 88 | 45.64 [38.22–53.07] | 88 | 80.64 [77.27–84] | 91 | 87.03 [83.03–91.03] | 85 | 91.78 [88.19–95.37] | High intensity |
| Baguida | 79 | 79.7 [7316–86.23] | 83 | 87.97 [84.04–91.91] | 74 | 89.05 [86.80–91.3] | 80 | 91.18 [86.86–95.49] | High intensity |
| Kovié | 91 | 77.49 [67.07–87.91] | 93 | 79.38 [72.1–86.66] | 90 | 84.06 [65.48–102.6] | 97 | 91.69 [90.38–93.01] | High intensity |
| Kpèlè Toutou | 83 | 69.47 [58.86–80.08] | 83 | 79.72 [75.48–83.95] | 87 | 80.88 [70.82–90.95] | 88 | 94.28 [90.63–97.93] | High intensity |
| Anié | 83 | 75.41 [64.96–85.86] | 92 | 89.15 [85.52–92.77] | 83 | 89.22 [86.73–91.72] | 90 | 91.23 [85.97–96.48] | High intensity |
n Number of mosquitoes, CI Confidence Interval
Table 3.
Resistance intensity of An. gambiae s.l. 30 min after exposure to permethrin
| Strain | Permethrin | Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 × (21,5 µg) | 2 × (43 µg) | 5 × (107,5 µg) | 10 × (215 µg) | ||||||
| n | % Mortality [CI] | n | % Mortality [CI] | n | % Mortality [CI] | n | % Mortality [CI] | ||
| An. gambiae « Kisumu » | 89 | 100 | 68 | 100 | 79 | 100 | 81 | 100 | Low intensity |
| Lomé | 84 | 22.47 [14.49–30.44] | 82 | 35.25 [28.75–41.74] | 86 | 55.89 [48.71–63.07] | 80 | 86.09 [81.03–91.16] | High intensity |
| Baguida | 84 | 33.99 [21.48–46.5] | 91 | 68.97 [41.98–95.96] | 91 | 83.74 [79.47–88.02] | 83 | 86.72 [82.81–90.63] | High intensity |
| Kovié | 92 | 0 | 94 | 45.78 [39.62–51.95] | 76 | 69.06 [57.38–80.74] | 89 | 88.67 [84.37–92.97] | High intensity |
| Kpèlè Toutou | 83 | 5.01 [0–11.6] | 79 | 80.22 [67.44–92.99] | 97 | 77.11 [65.79–88.43] | 83 | 76.15 [63.86–88.44] | High intensity |
| Anié | 82 | 19.51 [13.68–25.34] | 83 | 42.36 [29.57–55.15] | 81 | 85.62 [77.22–94.02] | 76 | 90.68 [85.9–95.45] | High intensity |
n Number of mosquitoes, CI Confidence Interval
Table 4.
Resistance intensity of An. gambiae s.l. 30 min after exposure to alphacypermethrin
| Sites | Alphacypermethrin | Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 × (12,5 µg) | 2 × (25 µg) | 5 × (62,5 µg) | 10 × (125 µg) | ||||||
| N | % Mortality [CI] | n | % Mortality [CI] | n | % Mortality [CI] | n | % Mortality [CI] | ||
| An. gambiae « Kisumu » | 95 | 100 | 98 | 100 | 95 | 100 | 98 | 100 | Low intensity |
| Lomé | 80 | 36.81 [25.83–47.79] | 90 | 73.39 [70.18–76.59] | 86 | 87.26 [84.23–90.28] | 95 | 88.65 [85.69–91.61] | High intensity |
| Baguida | 97 | 75 [67.32–82.68] | 93 | 80.62 [77.22–84.02] | 89 | 87.61 [83.98–91.23] | 88 | 90.84 [89.48–92.2] | High intensity |
| Kovié | 82 | 2.17 [0-6.92] | 87 | 81.46 [74.15–88.78] | 91 | 84.16 [79.08–89.24] | 100 | 87.58 [76.27–98.89] | High intensity |
| Kpèlè Toutou | 89 | 2.44 [0-6.92] | 88 | 64.04 [53.56–74.51] | 90 | 80.10 [74.03–86.18] | 82 | 84.41 [80.54–88.29] | High intensity |
| Anié | 74 | 70.73 [60.86–80.61] | 72 | 74.41 [64.13–84.69] | 75 | 86.64 [83.5-89.77] | 78 | 84.56 [82.93–86.19] | High intensity |
n Number of mosquitoes, CI Confidence Interval
Efficacy of synergists in restoring susceptibility to insecticides
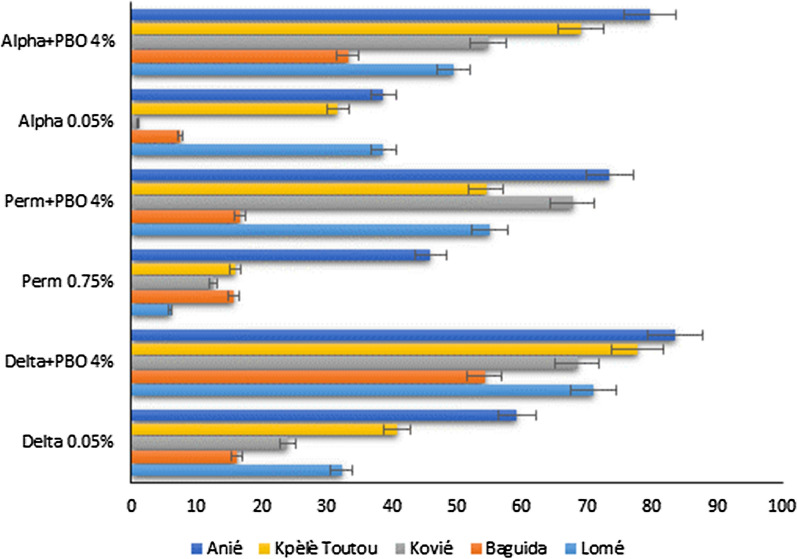
Local populations of An. gambiae were resistant to deltamethrin, permethrin and alphacypermethrin with mortality rates ranging from 1 to 60% at all sites. The use of the synergist PBO partially restored the susceptibility of An. gambiae to pyrethroids with mortality rates ranging from 16.8 to 83.5% across all sites (Fig. 3).
Fig. 3.
Susceptibility status of Anopheles gambiae s.l. to pyrethroids with pre-exposure to PBO
Delta deltamethrin, Perm permethrin, Alpha alphacypermethrin, PBO Piperonyl Butoxide
Mortality rates were less than 98% for local populations of An. gambiae across all sites for DDT, fenitrothion, malathion and bendiocarb. Using the synergist EA, partial restoration of susceptibility was obtained to DDT at all sites with mortality ranging from 8 to 40.3%. The synergist DEF partially restored the susceptibility of An. gambiae to bendiocarb in Lomé, Baguida and Kpèlè Toutou (mortality: 91–95%), but not as much at Anié and Kovié where mortality rates were 81.5% and 61.5%, respectively (Fig. 4). The synergist DEF partially restored the susceptibility of An. gambiae to organophosphates (fenitrothion and malathion) at all sites except at Anié with mortalities ranging from 70.75 to 74.25%.
Fig. 4.
Susceptibility status of An. gambiae s.l. to DDT with pre-exposure to EA and to bendiocarb, malathion, and fenitrothion with pre-exposure to DEF
Bendio bendiocarb, Mal malathion, Fen fenitrothion, EA ethacrinic acid, DEF S.S.S-tributlyphosphorotrithioate
Species composition of An. gambiae (s.l.)
A total of 250 An. gambiae (s.l.) were analysed by PCR for species identification of the An. gambiae complex. The successfully identified species were An. gambiae sensu stricto (s.s.) (26.4%, n = 64) and An. coluzzii (73.6%, n = 178) in varying proportions depending on the locality (Fig. 5). Eight (8) mosquitoes from Kovié were PCR negative (i.e., undetermined species). Anopheles coluzzii was the predominant species in Lomé, Baguida, Kovié and Kpèlè Toutou with proportions varying from 78 to 100% (Fig. 5) whereas An. gambiae s.s. predominated in Anié with a proportion of 88%.
Fig. 5.
Distribution of the sibling species of Anopheles gambiae s.l
Kdr-west (1014 F) and kdr-east (1014 S) allele frequencies
Molecular analyses detected the kdr L1014F mutation in An. gambiae s.s. and An. coluzzii (Table 5). The frequencies of this kdr mutation were relatively high (> 0.70) at all sites except Anié where a lower frequency of 0.50 was observed in An. coluzzii. In contrast, the kdr L1014S mutation was found only in An. coluzzii with low frequencies in Lomé (0.08), Kovié (0.02) and Kpèlè Toutou (0.01) (Table 5). When comparing the frequencies of the kdr L1014F mutation between the populations of An. coluzzii and An. gambiae s.s., no statistically significant difference was detected between the different study sites (G exact test; p > 0.12). The low frequencies of the kdr L1014S mutation did not allow comparison between study sites and between species.
Table 5.
Allelic frequencies of kdr L1014F and L1014S in the An. gambiae complex
| Species | Sites | n | Genotypes | f(1014F) | Genotypes | f(1014S) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SS | RS | RR | SS | RS | RR | |||||
| An. gambiae s.s. | Lomé | 2 | 0 | 1 | 1 | 0.75 | 2 | 0 | 0 | 0 |
| Baguida | 7 | 0 | 1 | 6 | 0.93 | 7 | 0 | 0 | 0 | |
| Kovié | – | – | – | – | – | – | – | – | – | |
| Kpèlè Toutou | 11 | 0 | 0 | 11 | 1 | 11 | 0 | 0 | 0 | |
| Anié | 44 | 0 | 2 | 42 | 0.98 | 44 | 0 | 0 | 0 | |
| An. coluzzii | Lomé | 48 | 0 | 22 | 26 | 0.77 | 40 | 8 | 0 | 0.08 |
| Baguida | 43 | 4 | 21 | 18 | 0.66 | 43 | 0 | 0 | 0 | |
| Kovié | 42 | 2 | 15 | 25 | 0.77 | 40 | 2 | 0 | 0.02 | |
| Kpèlè Toutou | 39 | 4 | 14 | 21 | 0.72 | 38 | 1 | 0 | 0.01 | |
| Anié | 6 | 2 | 2 | 2 | 0.5 | 6 | 0 | 0 | 0 | |
n number of mosquitoes, SS homozygote susceptible, RS heterozygote, RR homozygote resistant, f(1014 F); kdr-west mutation frequency, f(1014 S); kdr-east mutation frequency, -; not determined
Discussion
This study was conducted to update the insecticide resistance status of An. gambiae s.l. in key populations in Togo. The susceptibility tests showed that all local strains of An. gambiae s.l. were resistant to the insecticides tested except to pirimiphos methyl, where susceptibility was detected except for the strain from Baguida which showed possible resistance. The widespread use of insecticide provides a selection pressure for resistance in malaria vectors across Africa. Elsewhere in West Africa, evidence of a relationship between insecticide use in agriculture and the emergence of insecticide resistance in malaria vectors has been reported by several authors [18, 19]. These authors showed that mortality to deltamethrin was particularly low in market gardening areas [19]. In this study, the resistance was also high at Baguida, also a market gardening area. In the same area, there was probable resistance to pirimiphos methyl; this could be due to the introduction and regular use of organophosphates as an alternative to pyrethroids, the only WHO recommended class of insecticides used in market gardening. In addition, high levels of resistance to pyrethroids (deltamethrin, permethrin, and alphacypermethrin) were recorded in all localities.
Managing insecticide resistance remains the major challenge to achieving effective malaria vector control [2]. National and regional efforts through insecticide rotation and the introduction of new insecticide classes appear to be unsuccessful because of the intensity and progression of pyrethroid resistance [20]. Pyrethroids, DDT, bendiocarb, and propoxur induced low mortality rates at all localities tested, suggesting cross resistance and the presence of multiple resistance mechanisms. Resistance mechanisms, especially those involved in pyrethroid resistance, could have been selected by the large-scale use of long-lasting insecticide-treated nets (LLINs) throughout the country, as reported by Protopopoff et al. [21]. In urban areas, the use of spirals and aerosols could also contribute to insecticide resistance, as it is the case with market gardening practices [22]. Studies by Agboyi et al. [23] and Mondedji et al. [24] in Togo showed that farmers overuse synthetic pesticides for plant protection against pests. According to Agboyi et al. [23], while DDT and its derivatives are banned, they are still used by farmers in cotton and vegetable fields. Insecticide residues accumulate in the soil so that during the rainy season, breeding sites become contaminated. The exploitation of hydro-agricultural developments following the creation of agropoles could favour the introduction of new insecticide groups. In Ghana, Pwalia et al. [25] also reported a high intensity of resistance to multiple insecticides (deltamethrin, permethrin, DDT, bendiocarb, propoxur and pirimiphos methyl) using CDC bottle bioassays.
High frequencies of kdr-west alleles varying from 0.5 to 1 were detected. The L1014S allele (kdr-east) was detected at very low frequencies and was present in Lomé, Kovié and Kpèlè Toutou at proportions ranging from 0.01 to 0.08% in An. coluzzii only. Amoudji et al. [7] reported high L1014F allele frequencies (~ 0.9) at Baguida, Kovié and Kolokopé and low frequencies of L1014S allele (~ 0.02). These two alleles contribute to cross-resistance to organochlorines and pyrethroids. These results are consistent with reports by Diabaté et al. [26] and Yadouleton et al. [27] in Burkina Faso and Benin, respectively, which showed that 1014 F allele frequency in An. gambiae s.l. is higher in cash crop areas usually subjected to insecticide treatments than in rural areas where farmers only grow food crops or products for local consumption. No individual An. gambiae s.s. was homozygous SS for the kdr-west genotype. However, the proportions of heterozygous RS and homozygous RR individuals were high, suggesting strong selection pressure.
In this study, three groups of detoxification enzymes were indirectly incriminated through the testing with PBO, DEF and EA. The use of the synergists PBO with pyrethroids, EA with DDT and DEF with organophosphates and carbamates, restored partially the susceptibility of the tested mosquito populations. This is an indication of the overproduction of oxidases, glutathione-s-transferases and esterases, respectively. Similar findings were reported by Namountougou et al. [28]. It should be noted that ethacrynic acid is a synergist for GSTs with peroxidase activity specifically. This may explain the partial susceptibility restoration observed.
The use of LLINs that contain PBO can be encouraged for resistance management based on our results. Hien et al. [9] and Ahadji-Dabla et al. [29] showed that PBO partially restored the susceptibility of An. gambiae s.l. to deltamethrin, alphacypermethrin and permethrin in Burkina Faso and Togo, respectively. Moreover, recent report by Ketoh et al. [8] showed the efficacy of PBO LLINs in an area with pyrethroid resistant vector populations. Similarly, DEF and EA could be recommended as adjuvants. A study by Aïzoun et al. [30] showed that PBO, DEF and EA partially restored the susceptibility of An. gambiae s.l. to deltamethrin, permethrin and DDT in southern Benin. According to these authors, a combination of PBO and DEF better restored the susceptibility than these synergists used individually.
Two species of the An. gambiae complex (An. gambiae s.s. and An. coluzzii) were identified in different proportions according to the locality. The predominant species were An. coluzzii in Lomé, Baguida, Kpèlè Toutou and Kovié and An. gambiae s.s in Anié. This situation could be the result of the adaptability to suitable environmental conditions. In addition, the predominance of An. coluzzii in the four localities mentioned above could also be explained by the presence of irrigated perimeters and permanent water bodies in rice and vegetable-growing areas. Recently, Toglo et al. [31] reported that An. coluzzii was the only species found in Kovié.
In Anié which is primarily a cotton-growing area, most of the breeding sites were temporary and were colonized by An. gambiae s.s. Similar findings were reported by Della Torre et al. [32] and Costantini et al. [33]. In Burkina Faso, Diabaté et al. [34] found that An. coluzzii was the primary species found in areas with permanent breeding sites, while An. gambiae s.s. was found in areas with temporary breeding sites. Anopheles arabiensis, classically considered a dryland or dry season species, was not detected in this study; however, it was previously identified at low proportions at Baguida, Kovié and Kolokopé by Amoudji et al. [7]. Unfavourable environmental conditions could affect the development of this species as reported by Duvallet et al. [35].
Conclusion
This study revealed the existence of resistance to multiple key public health insecticides in the local populations of An. gambiae s.l. in southern Togo, except for pirimiphos methyl. High intensity pyrethroid resistance was observed at the study localities with probable involvement of detoxification enzymes (oxidases, esterases and glutathione-s-transferases). The kdr L1014F mutation was detected with variable but high allele frequencies (> 0.50) in the two sibling species of An. gambiae s.s. and An. coluzzii whereas the kdr L1014S mutation was present at very low frequencies and only in An. coluzzii. The synergists PBO and EA partially restored the susceptibility to pyrethroids and organochlorines, respectively, at all localities and DEF improved the susceptibility to carbamates and organophosphates at all localities except Anié. These data may help the NMCP of Togo in developing more effective strategies to control malaria vectors.
Further investigations are needed to understand the extent of resistance. It would be useful to (1) extend the study of resistance intensity to all the NMCP sentinel sites, (2) test the efficacy of newer public health insecticides such as chlorfenapyr (pyrrole) and clothianidin (neonicotinoid), (3) explore other resistance mechanisms (ace-1 and kdr N1575Y) and (4) assess the impact of resistance on the efficiency of vector control tools to prevent malaria in Togo, especially LLINs and IRS.
Acknowledgements
Authors are thankful to LaEE-UL/Togo, IRSS and Centre Muraz/Burkina Faso for technical support. Special acknowledgment to CDC Atlanta, USA for providing CDC bottle assay kit. The first author would like to thank Drs W. Nyamador, A. D. Mondedji, N. Gbényédji, M. Namountougou, Dari F. Yannick Da and Prof. A. Badolo for their help.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the CDC.
Abbreviations
- CTAB
cetyl trimethyl ammonium bromide
- DEF
S.S.S-tributlyphosphorotrithioate
- DDT
dichlorodiphenyltrichloroethane
- DNA
desoxyribonucleic acid
- EA
ethacrynic acid
- IRSS
Institut de Recherche en Sciences de la Santé
- kdr
knock down resistance
- LLIN
long-lasting insecticide-treated nets
- NMCP
National Malaria Control Programme
- PBO
Piperonyl butoxide
- PCR
polymerase chain reaction
- PNLP
Programme National de Lutte contre le Paludisme
- (RR)
resistant homozygous
- SINE 200
Short INterspersed Elements
- (SS)
susceptible homozygous
- WHO
World Health Organization.
Author contributions
YA, KMAD, DDS, RKD and GKK participated in the design of the study and data analysis. YA, KMAD, ADA, KIA and EK carried out the larval collection and bioassays. YA, DDS, RB, KLN and SM participated in the molecular and data analyses. YA, KMAD and DDS drafted the manuscript. RLVC, AL supplied reagents and critical reviewed the manuscript. RKD, AD, AMD, RTA and GKK critical reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant. Field and laboratory activities were partly supported by the CEA-ITECH-MTV/Burkina Faso and World Bank.
Availability of data and materials
All data generated or analysed during this study are included in this manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yawo Apetogbo, Email: georgesapetogbo@gmail.com.
Koffi M. Ahadji-Dabla, Email: km.ahadjidabla@gmail.com
References
- 1.WHO . Guidelines for malaria vector control; 2019. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 2.WHO . Global plan for insecticide resistance management in malaria vectors; 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 3.Dabiré RK, Namountougou M, Diabaté A, Soma DD, Bado J, Toé HK, et al. Distribution and frequency of kdr mutations within Anopheles gambiae s.l populations and first report of the Ace.1G119S mutation in Anopheles arabiensis from Burkina Faso (West Africa) PLoS ONE. 2014;9:e101484. doi: 10.1371/journal.pone.0101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yahouédo GA, Cornelie S, Djègbè I, Ahlonsou J, Aboubakar S, Soares C, et al. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large-scale implementation of vector control interventions. Parasit Vectors. 2016;9:385. doi: 10.1186/s13071-016-1661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soma DD, Zogo B, Hien DF, Hien AS, Kaboré DA, Kientega M, et al. Insecticide resistance status of malaria vectors Anopheles gambiae (s.l.) of southwest Burkina Faso and residual efficacy of indoor residual spraying with microencapsulated pirimiphos-methyl insecticide. Parasit Vectors. 2021;14:58. doi: 10.1186/s13071-020-04563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahadji-dabla KM, Nyamador WS, Amoudji AD, Apétogbo YG, Ketoh GK, Glitho IA. Susceptibility of a malaria vector Anopheles gambiae s.l (Diptera: Culicidae) to WHO recommended insecticides in Togo (West Africa) J Entomol Zool Stud. 2015;3:75–9. [Google Scholar]
- 7.Amoudji AD, Ahadji-Dabla KM, Hien AS, Apétogbo YG, Yaméogo B, Soma DD, et al. Insecticide resistance profiles of Anopheles gambiae s.l in Togo and genetic mechanisms involved, during 3-year survey: is there any need for resistance management? Malar J. 2019;18:177. doi: 10.1186/s12936-019-2813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ketoh GK, Ahadji-Dabla KM, Chabi J, Amoudji AD, Apetogbo GY, Awokou F, et al. Efficacy of two PBO long lasting insecticidal nets against natural populations of Anopheles gambiae s.l in experimental huts, Kolokopé, Togo. PLoS ONE. 2018;13:e0192492. doi: 10.1371/journal.pone.0192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hien AS, Soma DD, Maiga S, Coulibaly D, Diabaté A, Belemvire A, et al. Evidence supporting deployment of next generation insecticide treated nets in Burkina Faso: bioassays with either chlorfenapyr or piperonyl butoxide increase mortality of pyrethroid-resistant Anopheles gambiae. Malar J. 2021;20:406. doi: 10.1186/s12936-021-03936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Second edition. Geneva: World Health Organization; 2016.
- 11.Silver JB. Mosquito ecology: field sampling methods. 3rd Edn Springer Publ. 2007.
- 12.Centers for Disease Control and Prevention . Guidelines for evaluating insecticide resistance in vectors using the CDC bottle bioassay. Atlanta: CDC; 2010. [Google Scholar]
- 13.Santolamazza F, Mancini E, Simard F, Qi, Yumin Tu Zhijian, and della Torre A. insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Mol Biol. 1998;7:179–84. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 15.Ranson H, N’guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in african anophelinae mosquitoes: what are the implications for malaria control? Insect Mol Biol. 2000;9:491–7. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 16.Goudet J, Raymond M, de Meeüs T, Rousset F. Testing differentiation in diploid populations. Genetics. 1996;144:1933–40. doi: 10.1093/genetics/144.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The R Development Core Team. R: A Language and Environment for Statistical Computing. 2008; p. 1–2547. Available at: http://www.gnu.org/copyleft/gpl.html.
- 18.Hien SA, Soma DD, Hema O, Bayili B, Namountougou M, Gnankiné O, et al. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l populations from cotton growing areas in Burkina Faso, West Africa. PLoS ONE. 2017;12:e0173098. doi: 10.1371/journal.pone.0173098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chabi J, Eziefule MC, Pwalia R, Joannides J, Obuobi D, Amlalo G, et al. Impact of urban agriculture on the species distribution and insecticide resistance profile of Anopheles gambiae s.s and Anopheles coluzzii in Accra metropolis, Ghana. Adv Entomol. 2018;6:198–211. doi: 10.4236/ae.2018.63016. [DOI] [Google Scholar]
- 20.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Protopopoff N, Verhaeghen K, Bortel WV, Roelants P, Marcotty T. A significant increase in kdr in Anopheles gambiae is associated with an intensive vector control intervention in Burundi highlands. Trop Med Int Health. 2008;13:1479–87. doi: 10.1111/j.1365-3156.2008.02164.x. [DOI] [PubMed] [Google Scholar]
- 22.Klinkenberg E, McCall PJ, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra. Ghana Malar J. 2008;7:151. doi: 10.1186/1475-2875-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agboyi LK, Djade KM, Ahadji-Dabla KM, Ketoh GK, Nuto Y, Glitho IA. Vegetable production in Togo and potential impact of pesticide use practices on the environment. Int J Biol Chem Sci. 2015;9:723–36. doi: 10.4314/ijbcs.v9i2.13. [DOI] [Google Scholar]
- 24.Mondedji AD, Nyamador WS, Amevoin K, Adéoti R, Abbey GA, Ketoh GK, et al. Analyse de quelques aspects du système de production légumière et perception des producteurs de l’utilisation d’extraits botaniques dans la gestion des insectes ravageurs des cultures maraîchères au Sud du Togo. Int J Biol Chem Sci. 2015;9:98–107. doi: 10.4314/ijbcs.v9i1.10. [DOI] [Google Scholar]
- 25.Pwalia R, Joannides J, Iddrisu A, Addae C, Acquah-Baidoo D, Obuobi D, et al. High insecticide resistance intensity of Anopheles gambiae (s.l.) and low efficacy of pyrethroid LLINs in Accra, Ghana. Parasit Vectors. 2019;12:299. doi: 10.1186/s13071-019-3556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabaté A, Baldet T, Chandre F, Guiguemdé RT, Brengues C, Guillet P, et al. First report of the kdr mutation in Anopheles gambiae M form from Burkina Faso, West Africa. Parassitologia. 2002;44:157–8. [PubMed] [Google Scholar]
- 27.Yadouleton AW, Padonou G, Asidi A, Moiroux N, Bio-Banganna S, Corbel V, et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010;9:83. doi: 10.1186/1475-2875-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namountougou M, Simard F, Baldet T, Diabate A, Ouedraogo JB, Thibaud M, et al. Multiple insecticide resistance in Anopheles gambiae s.l populations from Burkina Faso, West Africa. PLoS ONE. 2012;7:e48412. doi: 10.1371/journal.pone.0048412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahadji-Dabla KM, Chabi J, Apetogbo YG, Koffi E, Hadi MP, Ketoh GK. Resistance intensity status of Anopheles gambiae s.l species at Kolokope, eastern plateau Togo: a potential site to assess new vector control tools. Heliyon. 2022;8:e09770. doi: 10.1016/j.heliyon.2022.e09770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aïzoun N, Aïkpon R, Azondekon R, Asidi A, Akogbéto M. Comparative susceptibility to permethrin of two Anopheles gambiae s.l populations from Southern Benin, regarding mosquito sex, physiological status, and mosquito age. Asian Pac J Trop Biomed. 2014;4:312–7. doi: 10.12980/APJTB.4.2014C1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toglo YAR, Ahadji-Dabla KM, Koffi E, Apétogbo GY, Ketoh GK. Mosquito (Diptera: Culicidae) diversity and malaria prevalence in Kovié, prefecture of Zio, Togo. Int J Mosq Res. 2021;8:15–9. doi: 10.22271/23487941.2021.v8.i5a.557. [DOI] [Google Scholar]
- 32.Della TA, Tu Zhijian PV. On the distribution and genetic differentiation of Anopheles gambiae s.s molecular forms. Insect Biochem Mol Biol. 2005;35:755–69. doi: 10.1016/j.ibmb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Costantini C, Ayala D, Guelbeogo WM, Pombi M, Some CY, Bassole IH, et al. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol. 2009;9:16. doi: 10.1186/1472-6785-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diabaté A, Dabiré RK, Heidenberger K, Crawford J, Lamp WO, Lauren EC, et al. Evidence for divergent selection between the molecular forms of Anopheles gambiae: role of predation. BMC Evol Biol. 2008;8:5. doi: 10.1186/1471-2148-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duvallet G, Fontenille D, Robert V. Entomologie médicale et vétérinaire. IRD Edn. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this manuscript.