Abstract
The lipooligosaccharide (LOS) of Haemophilus ducreyi, the etiologic agent of chancroid, chemically and immunologically resembles human glycosphingolipid antigens. To test whether LOS that contains paragloboside-like structures was required for pustule formation, an isogenic mutant (35000HP-RSM2) was constructed in losB, which encodes d-glycero-d-manno-heptosyltransferase. 35000HP-RSM2 produces a truncated LOS whose major glycoform terminates in a single glucose attached to a heptose trisaccharide core and 2-keto-3-deoxyoctulosonic acid. Five human subjects were inoculated with 35000HP and 35000HP-RSM2 in a dose-response trial. For estimated delivered doses (EDDs) of ≥25 CFU, the pustule formation rates were 80% for 35000HP and 58% for 35000HP-RSM2. Preliminary data indicated that a previously described Tn916 losB mutant made a minor glycoform that does not require dd-heptose to form the terminal N-acetyllactosamine. If 35000HP-RSM2 made this glycoform, then 35000HP-RSM2 could theoretically make a sialylated glycoform. To test whether sialylated LOS was required for pustule formation, a second trial comparing an isogenic sialyltransferase mutant (35000HP-RSM203) to 35000HP was performed in five additional subjects. For EDDs of ≥25 CFU, the pustule formation rates were 30% for both 35000HP and 35000HP-RSM203. The histopathology and recovery rates of H. ducreyi from surface cultures and biopsies obtained from mutant and parent sites in both trials were similar. These results indicate that neither the expression of a major glycoform resembling paragloboside nor sialylated LOS is required for pustule formation by H. ducreyi in humans.
The lipooligosaccharide (LOS) of Haemophilus ducreyi, the etiologic agent of the genital ulcer disease chancroid, chemically and immunologically resembles human glycosphingolipid antigens (7, 9, 14). The oligosaccharide of the major glycoform of most H. ducreyi isolates is Galβ1-4GlcNacβ1-3Galβ1-4Hepα1-6Glcβ1-4Hepα1-KDO (2-keto-3-deoxyoctulosonic acid). This oligosaccharide is similar in structure to paragloboside, a precursor of the major human blood group antigens, I and i. Like the mature human I and i antigen, the terminal N-acetyllactosamine of the major glycoform of LOS is substituted with sialic acid (14). This LOS structure may help H. ducreyi evade immune responses by resembling the human host or may facilitate adherence to and/or invasion of human cells by binding to host receptors for glycosphingolipids or sialic acid.
We recently reported the characterization of a Tn916 derivative of H. ducreyi 35000 in which losB, which encodes d-glycero-d-manno-heptosyltransferase, was insertionally inactivated (9). The mutant, designated 1381, produces a truncated LOS whose major glycoform terminates in a single glucose attached to a heptose trisaccharide core and KDO. In vitro, H. ducreyi 1381 adheres to and invades human keratinocytes less well than its isogenic parent. Complementation of the mutant with a plasmid containing losB restores production of parental LOS and adherence and invasion of keratinocytes to parental levels (9).
The most complex major glycoform produced by strain 1381 in vitro lacks the terminal N-acetyllactosamine and thus will not be sialylated. However, an alternative glycoform (Galβ1-4Glcβ1-4Hepα1-KDO) which lacks the dd-heptose has been identified in strains ITM 4747 and 33921 (14, 21). This glycoform is synthesized by an alternative pathway that does not require dd-heptose. Preliminary data suggested that a minor dd-heptose-deficient glycoform (Galβ1-4GlcNacβ1-3Galβ1-4Glcβ1-4Hepα1-KDO) may be produced by strain 1381 (9). This glycoform would likely be a substrate for sialylation. Although we have not observed a significant quantity of this alternative glycoform in vitro, it is possible that this alternative glycoform is produced in substantial quantities in vivo.
To study early events in H. ducreyi pathogenesis, we developed an experimental model of infection in human subjects (2, 22, 23). Human volunteers are inoculated on the skin of the upper arm with H. ducreyi via puncture wounds made with an allergy testing device. The clinical course and histopathology of experimental infection mimics natural infection up to the pustular stage of disease. Lesion outcome (papule, pustule, and resolved) at multiple sites inoculated with the same suspension of one isolate in an individual subject is independent (2, 22). In virulence testing, subjects are inoculated at multiple sites with an isogenic mutant and its parent, and site rather than subject is used as the unit of comparison. In previous trials, the pustule formation rates for several isogenic mutants were similar to those caused by the parent (2a, 18). However, a hemoglobin receptor (HgbA) deficient mutant was unable to form pustules even at doses ten fold that of the parent (2b). Thus, the model can differentiate between an isogenic mutant and its parent in their ability to form pustules.
We tested here the hypotheses that expression of the major glycoform containing terminal N-acetyllactosamine or expression of sialylated LOS is required for the virulence of H. ducreyi in humans. We constructed a new isogenic mutant (35000HP-RSM2) in a human-passaged isolate of 35000 (35000HP) by insertion of a nonmobilizable element, the ΩKm-2 cassette, into losB. The virulence of the isogenic pair of isolates was tested in a double-blinded, escalating-dose-response study. Since 35000HP-RSM2 might express an alternative glycoform which could be sialylated, we also compared an isogenic sialyltransferase mutant (35000HP-RSM203) and its parent in a second trial. We compared the papule and pustule formation rates, the cellular infiltrate, and the recovery of bacteria from lesions inoculated with the mutant and the parent in each trial.
MATERIALS AND METHODS
Bacteria and plasmids.
H. ducreyi 35000HP is a human-passaged variant of 35000, which was isolated from a volunteer's lesion 13 days after inoculation with 35000 (2, 22). H. ducreyi 35000HP-RSM203, an isogenic sialyltransferase mutant that contains an ΩKm-2 insertion in lst, was described previously (5).
MAbs.
Monoclonal antibody (MAb) 1B2-1B7 was purchased from the American Type Culture Collection, Rockville, Md. MAb 1B2-1B7 has the same specificity as MAb 3F11 (6) and binds to the terminal tetrasaccharide of human paragloboside and H. ducreyi LOS. MAb 4C10 was developed to a H. ducreyi pyocin-resistant LOS variant strain 188-2 (6). This antibody was developed by using a modification of a previously described method (7).
Construction of a losB mutant.
The cloning and characterization of dd-heptosyltransferase genes, designated losB or lbgB, was reported earlier (9, 24). A unique SrfI site was introduced into the losB gene in pRSM1494 (9) by utilizing the Chameleon double-stranded, Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer's instructions. The mutagenic oligonucleotide was 5′-AAGAAAATTACGTATAGCCCGGGCTAAATTGGTTTTAGATAG, where the SrfI site is underlined. The sequence flanking the SrfI site corresponds to nucleotides 1161 to 1202 of GenBank entry AF004712. In this sequence, the translational start of losB is nucleotide 1141. A plasmid with the correct restriction map was identified and designated pRSM1675. DNA sequence was determined to verify the sequence surrounding the engineered SrfI site. The ΩKm-2 cassette, which had been isolated from pJRS102.0 (19) as an EcoRI fragment, was blunt ended with Klenow and ligated to pRSM1675 that was linearized with SrfI. The ligation mixture was transformed into E. coli DH5α, and clones resistant to kanamycin and ampicillin were identified. A plasmid with the appropriate restriction map was identified and saved as pRSM1694. In order to construct the isogenic mutant, pRSM1694 was linearized with PstI and electroporated into 35000HP as described earlier (18). Transformants were identified on chocolate agar containing kanamycin (20 μg/ml). Kanr clones were screened for a loss of reactivity to MAb 3F11, and the genotype was verified by Southern hybridization. A non-3F11 reactive transformant that had undergone allelic replacement in losB was designated 35000HP-RSM2.
Outer membrane, LOS, and Southern blot analysis.
LOS and outer membranes were prepared from 35000HP, 35000HP-RSM203, and 35000HP-RSM2 and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8, 16). Southern blot analysis was done on 35000HP-RSM2 (16).
Human challenge protocol.
Healthy adult male and female volunteers over 18 years of age were recruited for the study. Informed consent was obtained from the subjects for participation and for human immunodeficiency virus serology. Enrollment procedures, exclusion criteria, preparation of the bacteria, and clinical observations and surface cultures were done as described in detail elsewhere (2, 22, 23).
To infect the subjects, bacterial suspensions were loaded onto a Multi-Test applicator (Lincoln Diagnostics, Decatur, Ill.) and pressed into the skin of the upper arm. The CFU contained in each suspension were determined immediately before and immediately after inoculation of each group of subjects and then averaged. This device delivers approximately 1/1,000 of the volume of solutions of antigens loaded onto its tines into the epidermis and dermis (12, 22). The delivery characteristics of the device for bacterial suspensions is unknown. However, no disease occurs when less than 1,000 CFU of H. ducreyi are loaded onto the tines (22). Although we did not experimentally determine the actual delivered dose, throughout the text we will refer to the estimated delivered dose (EDD) as being 1,000-fold less than the average CFU loaded on the tines.
A modification of an escalating dose-response study was used to compare the virulence of 35000HP, 35000HP-RSM2, and 35000HP-RSM203 as described previously (2b, 17, 18). The rationale for the design is described in detail elsewhere (1, 2b, 18). Briefly, we infected groups of three subjects at six sites that were 3 cm apart and spaced in two rows on one arm. Three sites were inoculated with twofold serial dilutions of one of the mutants. Two of the sites were inoculated with the identical suspensions of the parent, and one was inoculated with the highest dose of the heat-killed mutant. To blind the study, the five suspensions containing live bacteria were randomized, given a code number, and inoculated at identical sites on each subject. The clinicians who evaluated the subjects were unaware of the identity of the suspensions. To provide a reference for clinical evaluation, the heat-killed control was inoculated at a known site on each subject. Subjects were observed until they reached a clinical endpoint, defined as either 14 days after inoculation, the development of a painful pustule, or the resolution of infection at all sites. When a clinical endpoint was achieved, the code was broken and up to two sites with active disease (one inoculated with the parent and one with the mutant), if present, were biopsied with a punch forceps. The subjects were then treated with two doses of oral ciprofloxacin (500 mg), given 12 h apart.
Semiquantitative culture and histological analysis.
Specimens were cut into sections. One section was cultured semiquantitatively (22, 23). Another section was fixed in formalin and used for immunohistological studies (22, 23). The slides were coded and interpreted by a dermatopathologist who was unaware of the code.
Phenotypes of recovered bacteria.
Individual colonies from the inocula, surface cultures, and biopsies were picked, suspended in freezing medium, and frozen in 96-well plates. All colonies were scored for susceptibility to kanamycin on agar plates as described above. Colonies recovered in the losB mutant trial were also scored for reactivity to MAbs 1B2-1B7 and 4C10 in a modification of a colony blot assay (9, 11). Antigen-antibody complexes were detected with peroxidase-labeled goat anti-mouse immunoglobulin M (for 1B2-1B7) or with peroxidase-labeled protein A (for 4C10) (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.) and horseradish peroxidase color development reagent (Bio-Rad Laboratories, Richmond, Calif.). If available, a minimum of 30 individual colonies were analyzed per specimen.
RESULTS
Characterization of the isogenic LOS mutants.
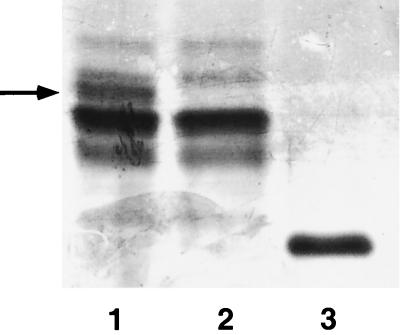
H. ducreyi 35000HP-RSM2 was constructed by disrupting the cloned losB gene in E. coli, followed by exchange of the disrupted allele into 35000HP. Briefly, a unique restriction site was inserted near the 5′ end of the heptosyltransferase gene, and the ΩKm-2 cassette was cloned into the site. H. ducreyi 35000HP was transformed with this construct. Kanr transformants were identified and analyzed for loss of reactivity to MAb 3F11, which binds to the terminal N-acetyllactosamine of the strain 35000HP LOS (6). Southern blot analysis of a Kanr and 3F11 nonreactive mutant, designated 35000HP-RSM2, confirmed that the losB gene had been replaced with the insertionally inactivated allele containing the ΩKm-2 cassette (data not shown). The major glycoform of the LOS of 35000HP-RSM2 migrated more rapidly in SDS-PAGE then the major glycoforms of the LOS produced by the parent (Fig. 1). This glycoform had a mobility identical to that of the major glycoform produced by strain 1381, the Tn916 mutant previously described. The growth rates of 35000HP and 35000HP-RSM2 in broth and the outer membrane protein profiles of the two strains were identical (data not shown).
FIG. 1.
Silver stain of an SDS–16% PAGE gel of LOS preparations from the parent and mutant strains of H. ducreyi. Lane 1, LOS from strain 35000HP; lane 2, LOS from the sialyltransferase mutant 35000HP-RSM203; lane 3, LOS from the dd-heptosyltransferase mutant 35000HP-RSM2. The sialic acid-containing glycoform in the parent strain is designated by the arrow.
The construction and characterization of the sialyltransferase mutant 35000HP-RSM203 has been reported (5). The glycoforms produced by the sialyltransferase mutant are shown in Fig. 1.
Evaluation of the losB mutant in human subjects.
Two men and five women (one Hispanic, two black, four white; age range, 25 to 54 years; mean age ± standard deviation [SD], 37.1 ± 10.5 years) with no history of chancroid enrolled in the study. One female subject was excluded because she developed sinusitis and needed to take an antibiotic. A second female subject withdrew prior to inoculation. Three subjects (86 to 88) were challenged in one iteration, and two subjects (91 and 92) were challenged in a second iteration (Table 1).
TABLE 1.
Response to inoculation of live H. ducreyi strainsa
| Subject no. | Days of observation | Isolate | No. of initial papule(s) | Final outcome (no.) of initial papule(s)
|
||
|---|---|---|---|---|---|---|
| Papule | Pustule | Resolved | ||||
| 86 | 7 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-RSM2 | 3 | 2 | 1 | |||
| 87 | 14 | |||||
| 35000HP | 2 | 1 | 1 | |||
| 35000HP-RSM2 | 3 | 3 | ||||
| 88 | 5 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-RSM2 | 3 | 3 | ||||
| 91 | 6 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-RSM2 | 3 | 3 | ||||
| 92 | 14 | |||||
| 35000HP | 2 | 1 | 1 | |||
| 35000HP-RSM2 | 3 | 3 | ||||
| 100 | 7 | |||||
| 35000HP | 1 | 1 | ||||
| 35000HP-RSM203 | 3 | 3 | ||||
| 117 | 14 | |||||
| 35000HP | 2 | 1 | 1 | |||
| 35000HP-RSM203 | 3 | 3 | ||||
| 118 | 7 | |||||
| 35000HP | 1 | 1 | ||||
| 35000HP-RSM203 | 3 | 3 | ||||
| 119 | 7 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-RSM203 | 3 | 1 | 2 | |||
| 120 | 7 | |||||
| 35000HP | 1 | 1 | ||||
| 35000HP-RSM203 | 1 | 1 | ||||
Each volunteer was inoculated at two sites with the parent (35000HP) and at three sites with the mutants (35000HP-RSM2 or 35000HP-RSM203).
In the first iteration, we attempted to inoculate the subjects at three sites with EDDs of 20, 40, and 80 CFU of the mutant and at two sites with an EDD of 30 CFU of the parent. A sixth site was inoculated with a heat-killed control. The actual EDD in the first iteration was 40 CFU for 35000HP and 60, 30, and 15 CFU for 35000HP-RSM2. No lesions developed at sites inoculated with the heat-killed control. Papules developed at all sites inoculated with live bacteria (Table 1). At the endpoint, five of six lesions resulting from inoculation of the parent (parent site) and five of nine lesions resulting from inoculation of the mutant (mutant site) were pustules.
Since inoculation of both the mutant and the parent caused pustules, we repeated the experiment with the same target doses. In the second iteration, two subjects were inoculated with an EDD of 30 CFU of 35000HP and 100, 50, and 25 CFU of 35000HP-RSM2. No lesions developed at sites inoculated with the heat-killed control, and papules developed at all sites inoculated with live bacteria (Table 1). At endpoint, three of four parent sites and three of six mutant sites contained pustules.
For EDDs in the range (25 to 100 CFU) where historical controls (1, 2, 22) have shown that ≥50% of sites inoculated with the parent evolve into pustules, 8 of 10 parent sites and 7 of 12 mutant sites developed pustules. For EDDs of <25 CFU, where papules frequently resolve, a pustule formed at one of three mutant sites. Thus, expression by H. ducreyi of a major glycoform whose structure is similar to human paragloboside was not required for pustule formation.
Evaluation of the sialyltransferase mutant in human subjects.
As discussed in the introduction, strain 35000HP-RSM2 could produce a sialic acid-containing glycoform. In order to exclude the possibility that sialylated LOS was necessary for the virulence of H. ducreyi in the human challenge model, we also evaluated a sialyltransferase mutant in a second trial. Three women and five men (seven white and one Asian; age range, 19 to 48 years; mean age ± SD, 32.5 ± 9.1 years) enrolled in the study. Two males and one female withdrew from the study prior to inoculation. Three subjects (100, 117, and 118) were challenged in the first iteration, and two subjects (119 and 120) were challenged in the second iteration.
The EDD in the first iteration was 47 CFU for 35000HP and 70, 35, and 18 CFU for 35000HP-RSM203. No lesions developed at sites inoculated with the heat-killed control. Papules developed at four of six parent sites and nine of nine mutant sites (Table 1). Papules resolved at two of four parent sites and six of nine mutant sites. One subject (number 117) had a parent site that remained at the papular stage at endpoint. At endpoint, one of six parent sites and three of nine mutant sites contained pustules.
In the second iteration, two subjects were inoculated with an EDD of 81 CFU of 35000HP and 60, 30, and 15 CFU of 35000HP-RSM203. No lesions developed at heat-killed control sites. Papules developed at three of four parent sites and four of six mutant sites (Table 1). Papules resolved at one of three parent sites and three of four mutant sites. At the endpoint, pustules were present at two of four parent sites and one of six mutant sites.
For EDDs of >25 CFU, pustules formed at three of ten sites for both the parent and mutant strains. For EDDs of <25 CFU, a pustule formed at one of five mutant sites. Thus, sialylation of H. ducreyi LOS was not required for pustule formation.
Cellular infiltrate of mutant and parent lesions.
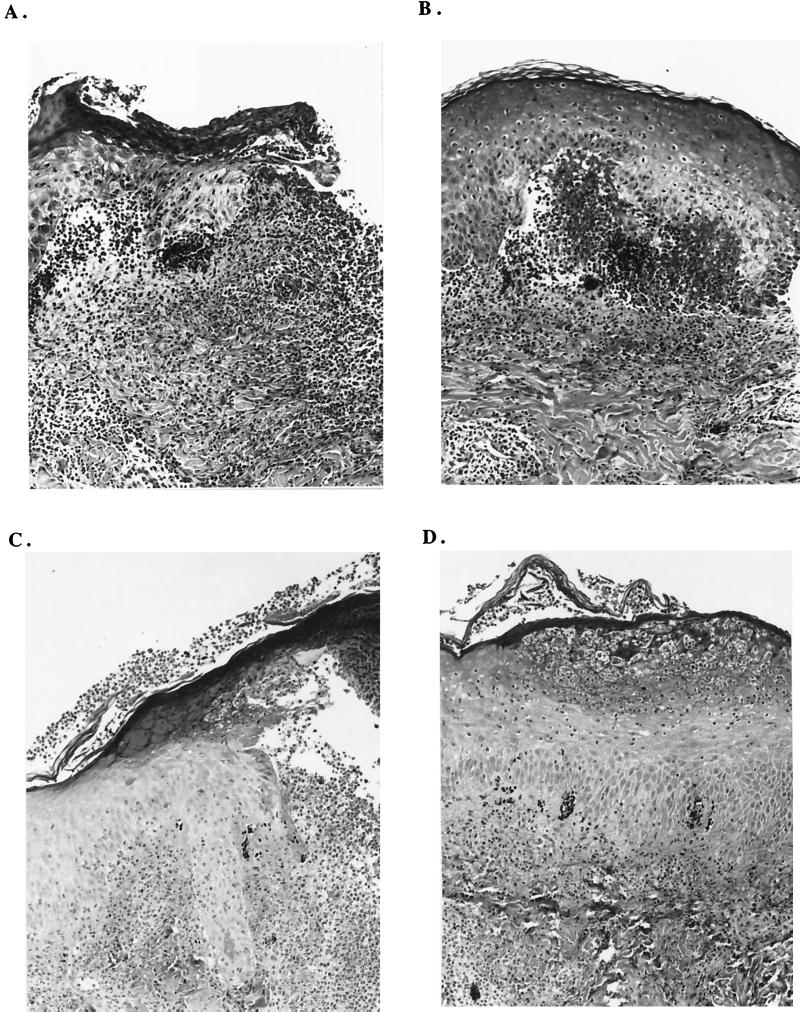
We compared the cellular infiltrates from paired mutant and parent lesions. The histology of mutant and parent lesions obtained from subjects 86, 88, and 91 (in trial 1) and subjects 117 and 119 (in trial 2) was similar. Subepidermal or intraepidermal micropustules with polymorphonuclear leukocytes were present. The dermis contained a perivascular infiltrate of mononuclear cells and some polymorphonuclear leukocytes, and the venules were lined with reactive endothelial cells (Fig. 2). Most of the mononuclear cells were CD3 cells in both mutant and parent lesions (data not shown). The parent pustules obtained from subject 87 and from subject 92 were similar to the pustules obtained from the other subjects.
FIG. 2.
Hematoxylin-eosin-stained sections (×25) obtained from sites inoculated with 35000HP (A), 35000HP-RSM2 (B), 35000HP (C), and 35000HP-RSM203 (D). Tissues shown in panels A and B were obtained from subject 88, and those in panels C and D were from subject 119. Note that all specimens contain subepidermal or intraepidermal pustules.
Recovery of bacteria from lesions.
Surface cultures were obtained from all inoculation sites at each follow-up visit. No bacteria were recovered from sites inoculated with the heat-killed control. In trial 1, H. ducreyi was recovered intermittently from parent and mutant sites in two of five subjects. The recovery rate was 8% from sites inoculated with the parent (n = 78) and 9% from sites inoculated with the mutant (n = 117). In trial 2, bacteria were isolated from two of five subjects, and the recovery rate was 2% from the parent (n = 42) and 3% from the mutant (n = 74) sites. All biopsies were semiquantitatively cultured. Bacteria were recovered from four of seven parent sites and five of five mutant sites, and the yield ranged from 1.0 × 103 to 3.9 × 105 CFU/g of tissue. Thus, bacteria were intermittently shed from both mutant and parent sites in some of the subjects, and bacterial recovery rates from biopsies of parent and mutant sites were similar.
Confirmation of the phenotype of the recovered bacteria.
To confirm that the inocula were correct and that no phenotypic changes occurred during infection, individual colonies from each of the broth cultures used to prepare the inocula, from surface cultures and from biopsy specimens, were analyzed for kanamycin susceptibility. For the losB mutant, reactivity to MAbs 4C10 and 1B2-1B7 was also tested. If available, we analyzed a minimum of 30 individual colonies from each specimen. If 30 colonies had the correct phenotype (mutant, Kanr, 1B2-1B7 nonreactive, 4C10 reactive; parent, Kans, 1B2-1B7 reactive, 4C10 nonreactive), then there was a 95% probability that, at most, 11% of the colonies could have the incorrect phenotype in an individual specimen.
For the eight cultures used to prepare the inocula in both trials, all 146 parent colonies and 147 mutant colonies tested were phenotypically correct. Positive surface cultures were obtained from subjects 88, 91, 117, and 119. For subjects 88, 117, and 119, all 18 colonies obtained from parent sites and 54 colonies obtained from mutant sites had the expected phenotypes. Of four parent biopsies and five mutant biopsies that were culture positive, all 146 parent colonies and 143 mutant colonies tested were correct. Thus, all colonies tested from the inocula and biopsies had the expected phenotype.
For subject 91, heavy bacterial growth was obtained from all five sites inoculated with live bacteria on day 5. Individual colonies could only be picked from the edge of each section of the inoculated plate. All 115 colonies picked from two mutant sites were correct. Cultures of the remaining three sites were mixed, with 66 of 90 colonies correct. However, two of the sites that had mixed growth were biopsied the following day and yielded only phenotypically correct colonies. The mixed growth observed on one occasion most likely was due to cross-contamination of the plate rather than to cross-contamination of the lesions.
DISCUSSION
H. ducreyi LOS contains a major glycoform which is immunochemically identical to paragloboside, a major component of human blood group antigens (5, 7, 10, 13–15, 21, 25). The major glycoform terminates in galactose and is modified with sialic acid. Approximately one-third of the galactose residues are substituted with sialic acid (5). Thus, H. ducreyi LOS has been postulated to be a major virulence determinant in that it mimics sialylated human antigens and may allow the organism to utilize sialic acid or glycosphingolipid receptors on host cells (13, 15). H. ducreyi mutant 1381, with a transposon insertion in the losB gene, produces a truncated LOS and exhibits reduced adherence to and invasion of primary human keratinocytes (9). To our surprise, a losB mutant and a sialyltransferase mutant caused papule and pustule formation rates that were indistinguishable from their isogenic parent in human volunteers.
In the losB mutant parent comparison, pustules formed at 80% of the parent sites and 58% of the mutant sites inoculated with EDDs of ≥25 CFU. To definitively determine whether sialic acid-containing glycoforms are necessary for lesion formation, a sialyltransferase mutant, 35000HP-RSM203, was studied in a second trial. In this trial, the pustule formation rate was 30% for both the parent and the mutant at EDDs of >25 CFU. In the sialyltransferase trial, lesions resolved at all sites in three of the five subjects, suggesting that there was subject-to-subject variation in susceptibility to pustule formation. These data emphasize the importance of including concurrent parental controls in our mutant-parent trials. Our studies conclusively show that neither the N-acetyllactosamine-containing major glycoform of the LOS nor sialic acid-containing glycoforms of the LOS are required for pustule formation in humans.
The kinetics of papule and pustule formation in the human model resemble the course of natural infection, and the histopathology of experimental lesions is similar to that found in naturally occurring ulcers (2, 17, 22). In naturally infected patients, the presumed portals of entry are microabrasions that occur during sexual intercourse. We have shown that placement of H. ducreyi on intact skin does not cause disease (23). One limitation of the model is that the route of inoculation is artificial. The tines of the Multi-Test applicator penetrate the skin up to 1.9 mm and probably deliver the inoculum to both the epidermis and dermis. By introducing the bacteria into both compartments of the skin, interactions that may be important for the establishment of natural disease, such as those between H. ducreyi and keratinocytes, may be minimized. However, the early disease seen in experimental infection (papules) resembles papular lesions found in natural infection. A second limitation of the model is that we only infected subjects until they developed painful pustules. We doubt subjects would tolerate the pain associated with ulcers and do not want them to develop ulcerative lesions that may become superinfected with other bacteria. Thus, we cannot exclude the possibility that expression of a major glycoform containing paragloboside or sialylated LOS are important for ulcer formation.
The LOS of N. gonorrhoeae and H. ducreyi are immunochemically similar. In experimental gonococcal infection in men, volunteers inoculated with a low-molecular-weight LOS variant shed bacteria that expressed a higher-molecular-weight LOS that contained paragloboside (20). A similar LOS variant was shed by men who were naturally infected (20). Isogenic gonococcal LOS mutants have not been evaluated in human volunteers. However, the data suggest that gonococci that express paragloboside are selected in vivo. Thus, expression of LOS that resembles paragloboside may be important for bacterial infection at the mucosal surfaces.
In vitro studies suggest that a full-length LOS and sialylated LOS may be important virulence factors for H. ducreyi (9). In animals, injection of full-length H. ducreyi LOS results in the formation of an intradermal abscess (8). However, in the temperature-dependent rabbit model, a lbgB (losB) mutant was as virulent as the parent strain, a result similar to our findings in humans (24). In the rabbit model, a gmhA mutant and a waaF mutant were less virulent than their isogenic parent (3, 4). Although their LOS structures were not determined, the gmhA mutant probably produces an LOS composed of lipid A and KDO, while the waaF mutant makes lipid A-KDO-heptose. However, these truncated LOS mutants also exhibited changes in their outer membrane protein profiles relative to the parent, as has been reported for enteric deep rough lipopolysaccharide mutants (3, 4).
In summary, we have conclusively shown that neither full-length nor sialylated LOS is required for papule or pustule formation in human volunteers. Future studies will examine whether mutations that truncate the major oligosaccharide proximal to the terminal glucose of the losB mutant or alter the lipid A component of the LOS affects the ability of the organism to cause disease.
ACKNOWLEDGMENTS
This work was supported by grants AI27863, AI31494, and AI75329 (to S.M.S.), AI30006 (to A.A.C.), AI38444 (to R.S.M.), and AI09813 (to J.A.B.) from the National Institutes of Health (NIH). The human challenge trials were also supported by NIH grant MO1RR00750 to the GCRC at Indiana University. The DNA sequence was determined by the Core Facility at the Children's Research Institute, which was supported in part by NIH grant HD34615.
We thank Huachun Zhong for excellent technical assistance and Byron Batteiger and Margaret Bauer for advice and assistance with the manuscript.
REFERENCES
- 1.Al-Tawfiq, J., J. Harezlak, B. P. Katz, and S. M. Spinola. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sexually Trans. Dis., in press. [DOI] [PubMed]
- 2.Al-Tawfiq J A, Thornton A C, Katz B P, Fortney K R, Todd K D, Hood A F, Spinola S M. Standardization of the experimental model of Haemophilus ducreyiinfection in human subjects. J Infect Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 2a.Al-Tawfiq, J. A., M. E. Bauer, K. R. Fortney, B. P. Katz, A. F. Hood, M. Ketterer, M. A. Apicella, and S. M. Spinola. Submitted for publication.
- 2b.Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, C. Elkins, and S. M. Spinola. Submitted for publication.
- 3.Bauer B A, Lumbley S R, Hansen E J. Characterization of a waaF (RfaF) homolog expressed by Haemophilus ducreyi. Infect Immun. 1999;67:899–907. doi: 10.1128/iai.67.2.899-907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer B A, Stevens M K, Hansen E J. Involvement of the Haemophilus ducreyi gmhAgene product in lipooligosaccharide expression and virulence. Infect Immun. 1998;66:4290–4298. doi: 10.1128/iai.66.9.4290-4298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozue J A, Tullius M V, Wang J, Gibson B W, Munson R S., Jr Haemophilus ducreyiproduces a novel sialyltransferase: identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J Biol Chem. 1999;274:4106–4114. doi: 10.1074/jbc.274.7.4106. [DOI] [PubMed] [Google Scholar]
- 6.Campagnari A A, Karalus R, Apicella M, Melaugh W, Lesse A J, Gibson B W. Use of pyocin to select a Haemophilus ducreyivariant defective in lipooligosaccharide biosynthesis. Infect Immun. 1994;62:2379–2386. doi: 10.1128/iai.62.6.2379-2386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campagnari A A, Spinola S M, Lesse A J, Abu Kwaik Y, Mandrell R E, Apicella M A. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990;8:353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 8.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson B W, Campagnari A A, Melaugh W, Phillips N J, Apicella M A, Grass S, Wang J, Palmer K L, Munson R S., Jr Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi35000 defective in lipooligosaccharide biosynthesis. J Bacteriol. 1997;179:5062–5071. doi: 10.1128/jb.179.16.5062-5071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson B W, Melaugh W, Phillips N J, Apicella M A, Campagnari A A, Griffiss J M. Investigation of the structural heterogeneity of lipooligosaccharides from pathogenic Haemophilus and Neisseria species and of R-type lipopolysacchardies from Salmonella typhimuriumby electrospray mass spectrometry. J Bacteriol. 1993;175:2702–2712. doi: 10.1128/jb.175.9.2702-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiltke T J, Campagnari A A, Spinola S M. Characterization of a novel lipoprotein expressed by Haemophilus ducreyi. Infect Immun. 1996;64:5047–5052. doi: 10.1128/iai.64.12.5047-5052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs M M, SanMateo L R, Orndorff P E, Almond G, Kawula T H. Swine model of Haemophilus ducreyiinfection. Infect Immun. 1995;63:3094–3100. doi: 10.1128/iai.63.8.3094-3100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandrell R E, Griffiss J M, Macher B A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidishave components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melaugh W, Campagnari A A, Gibson B W. The lipooligosaccharides of Haemophilus ducreyiare highly sialylated. J Bacteriol. 1996;178:564–570. doi: 10.1128/jb.178.2.564-570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melaugh W, Phillips N J, Campagnari A A, Tullius M V, Gibson B W. Structure of the major oligosaccharide from the lipooligosaccharide of Haemophilus ducreyistrain 35000 and evidence for additional glycoforms. Biochemistry. 1994;33:13070–13078. doi: 10.1021/bi00248a016. [DOI] [PubMed] [Google Scholar]
- 16.Palmer K L, Goldman W E, Munson R S., Jr An isogenic hemolysin-deficient mutant of Haemophilus ducreyilacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 17.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C-Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyiresembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 18.Palmer K L, Thornton A C, Fortney K A, Hood A F, Munson R S, Jr, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyiinfection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Casal J, Caparon M G, Scott J R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogeneswith similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider H, Griffiss J M, Boslego J W, Hitchcock P J, Zahos K M, Apicella M A. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J Exp Med. 1991;174:1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schweda E K H, Sundstrom A C, Eriksson L M, Jonasson J A, Lindberg A A. Structural studies of the cell envelope lipopolysaccharides from Haemophilus ducreyistrains ITM 2665 and ITM 4747. J Biol Chem. 1994;269:12040–12048. [PubMed] [Google Scholar]
- 22.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyielicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 23.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 24.Stevens M K, Klesney-Tait J, Lumbley S, Walters K A, Joffe A M, Radolf J D, Hansen E J. Identification of tandem genes involved in lipooligosaccharide expression by Haemophilus ducreyi. Infect Immun. 1997;65:651–660. doi: 10.1128/iai.65.2.651-660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tullius M V, Munson R S, Jr, Wang J, Gibson B W. Purification, cloning, and expression of a cytidine 5′-monophosphate N-acetylneuraminic acid synthetase from Haemophilus ducreyi. J Biol Chem. 1996;271:15373–15380. doi: 10.1074/jbc.271.26.15373. [DOI] [PubMed] [Google Scholar]