Abstract
Background:
Osteoarthritis (OA) is a significant long-term concern following anterior cruciate ligament reconstruction (ACLR). Low bone mineral density (BMD), particularly of the subchondral region, has been associated with the development of OA and is evident at the knee in individuals long after ACLR. It is unknown if persistent BMD deficits are present in high-level collegiate athletes.
Hypothesis/Purpose:
Evaluate bilateral changes in BMD of the femur and tibia from preinjury to 24 months post-ACLR in collegiate athletes. We hypothesized that BMD of both the distal femur and proximal tibia would be significantly reduced within the surgical limb initially post-operatively, but return to preinjury levels by 24 months post-ACLR.
Study Design:
Analysis of routinely collected athletic performance data; Level of Evidence, 3.
Methods:
Thirty-three Division I collegiate athletes were identified between 2010 and 2021 (13 female) who had total body dual-energy x-ray absorptiometry (DXA) scans acquired prior to sustaining an ACL injury. DXA scans were repeated at 6, 12, and 24 months (6M, 12M, 24M) post-ACLR. Linear mixed effects models assessed differences in BMD of 5%, 15%, and 50% of femur length (F5, F15, F50) and 5%, 15%, and 50% of tibia length (T5, T15, T50) between post-ACLR time-points and preinjury within each limb, reported as Tukey-adjusted p-values.
Results:
Compared to preinjury, BMD at F5 of the surgical limb was reduced 0.15 (0.02) g/cm2 at 6M (p-value < 0.001). BMD at F15 of the surgical limb was reduced 0.06 (0.01), 0.09 (0.01), 0.09 (0.01) g/cm2 at 6M, 12M, and 24M, respectively (all p-values < 0.001). BMD at T5 of the non-surgical limb was reduced 0.07 (0.02) g/cm2 at 12M (p-value = 0.02) and 0.10 (0.02) g/cm2 at 24M (p-value = 0.001). BMD at T15 of the surgical limb was reduced 0.07 (0.01) g/cm2 at 6M and 0.08 (0.02) g/cm2 at 12M (p-values < 0.001).
Conclusion:
BMD deficits of the surgical limb F15 region persisted out to 24M (−7.1%) post-ACLR compared to preinjury in collegiate athletes. The surgical limb F5 and T15 regions BMD were reduced at 6M and 12M, but not at 24M compared to preinjury levels. For the non-surgical limb, no significant differences were detected except for the T5 region at 12M (−5.1%) and 24M (−7.2%). The BMD at the F50 and T50 regions of both limbs were not significantly different than preinjury levels at any time post-ACLR.
Clinical Relevance:
Following ACLR, long-term deficits in femoral BMD may be related to the early onset and progression of OA and should be further explored.
Keywords: ACL, Imaging, Bone, Knee
Introduction
Poor long-term health outcomes are a significant concern following anterior cruciate ligament reconstruction (ACLR). Individuals who have suffered an anterior cruciate ligament (ACL) injury are more likely to demonstrate decreased quality of life,12 increased body mass index,47 increased prevalence4,5,39 and earlier onset of osteoarthritis (OA),18 and higher rates of future orthopedic surgeries.48 Up to 50-90% of individuals following ACLR demonstrate signs of OA later in life compared to 12% in the general population,15,29,46 and the prevalence of total knee arthroplasty (TKA) is increased, especially at younger ages.1,35,40 Reduced subchondral bone mineral density (BMD), particularly surrounding the knee, has been associated with the development of OA in the general population.3 Therefore, bone loss following ACLR may be an important metric to consider as a precursor to the development of OA in these individuals.4,5,39 Moreover, it is plausible that such bone loss increases periprosthetic fracture risk following TKA.23
Lower extremity BMD deficits have been observed in both the short- (less than 1 year)10,26,30,34 and long-term (10-11 years) following ACLR.21 One systematic review of 10 studies found significant BMD deficits at the proximal tibia, distal femur, patella, proximal femur or hip region, and calcaneus of the involved limb following ACLR.36 More recently, longitudinal studies found significant reductions in BMD compared to pre-surgery at the distal femur and proximal tibia, which persisted 2 years post-operative.33,34 Despite being large longitudinal studies, the populations varied in physical activity levels and the findings may not generalize to high-level athletes.
To date, no studies have investigated changes in BMD following ACLR in collegiate athletes. Collegiate athletes are consistently exposed to high levels of physical activity year round, providing an excellent stimulus for bone development. In fact, healthy collegiate athletes exhibit BMD levels significantly higher than the general population norms.41 It has been postulated that reduced physical activity and bone loading following ACLR may, in part, lead to the observed reductions in BMD.21 Athletes who undergo ACLR and return to collegiate athletics are typically exposed to the same training frequency and magnitudes that they were prior to injury, which in turn, should help to restore lower extremity BMD levels post-operatively.
Therefore, the purpose of this study was to evaluate bilateral changes in BMD of the distal femur and proximal tibia from preinjury to 24 months post-ACLR in collegiate athletes. We hypothesized that BMD of both the distal femur and proximal tibia would be significantly reduced within the surgical limb compared to preinjury values throughout the first year post-ACLR, but would be restored to preinjury levels by 24 months. Additionally, we hypothesized that no differences would be detected within the non-surgical limb at any time point following ACLR.
Methods
Participants
Performance and healthcare data from NCAA Division I collegiate athletes over ten years (2010-2021) were obtained via records review from the University of Wisconsin-Madison Badger Athletic Performance database. The University’s Health Sciences Institutional Review Board approved this records review of routinely, prospectively collected data. To be included, athletes must have met all of the following criteria: 1) underwent an ACLR during the study period; 2) had no history of prior ACL injury; 3) had both a healthy, preinjury dual-energy x-ray absorptiometry (DXA) scan and at least one post-operative DXA scan near the time points of interest (6, 12, and 24 months post-ACLR); 4) had no lower extremity surgeries 12 months prior to the preinjury DXA scan; and 5) had no internal hardware beyond common ACLR fixation hardware. For athletes that sustained a second ACL injury, only scans prior to the second injury were included in this analysis. Although post-operative rehabilitation was not standardized, the majority of athletes completed rehabilitation at the same facility under similar post-operative rehabilitation protocols.
Data Collection
As part of the University Athletics’ standard performance testing protocol, DXA scans were acquired each pre-season on all collegiate athletes. For athletes following ACLR, a standard post-operative testing protocol was followed in which total body DXA scans were obtained at 6, 12, and 24 months following surgery. All BMD measures were obtained from whole-body DXA scans using a GE Healthcare Lunar iDXA densitometer and analyzed using enCORE software V.14.1 (Madison, WI, USA). International Society for Clinical Densitometry (ISCD)–certified technologists obtained and analyzed all total body scans, using standard clinical operating procedures.27,38 A physician with expertise in BMD assessments and extensive total body DXA experience reviewed all scans to validate correct acquisition and analysis.27
Data Analysis
Lower extremity BMDs were acquired using custom 2 cm tall regions of interest (ROIs) spanning the entire width of the respective lower extremity. ROIs were placed at 5%, 15%, and 50% of femur (F5, F15, F50) and tibia (T5, T15, T50) length measured from the knee joint (Figure 1). Femur length was defined as the distance from the most superior aspect of the greater trochanter to the center of the intercondylar notch. Tibia length was defined as the distance from the most superior aspect of the intercondylar eminence to the most distal aspect of the medial malleolus. Manual analysis of the DXA scans were performed by four individuals, all of whom were trained using the same standardized protocol and demonstrated excellent inter-rater reliability (Intraclass correlation coefficients = 0.97 to 0.99, Supplemental Table 1). BMD values (g/cm2) were recorded for each ROI for both the surgical and non-surgical limb at each time point of interest (preinjury, 6, 12, and 24 months), and subsequently used in the statistical analyses.
Figure 1.
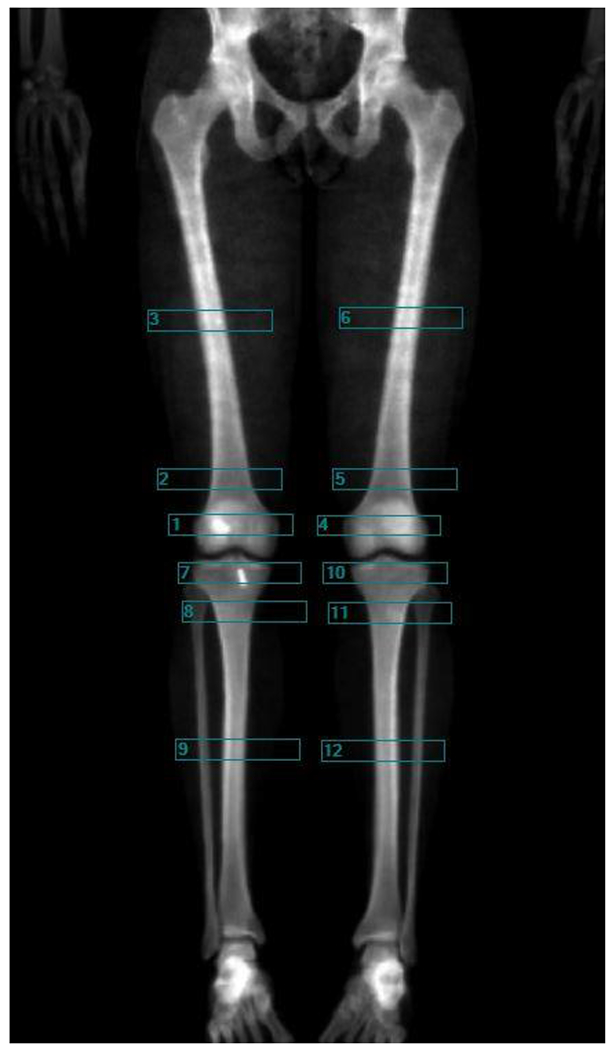
Representative image of a dual-energy x-ray absorptiometry (DXA) scan with the custom regions of interest (ROI) applied. ROIs were 2 cm tall and a width that spanned the respective lower extremity. ROIs were placed at 5% (box 1 and 4), 15% (box 2 and 5), and 50% (box 3 and 6) of femur length, measured from the distal femur, and at 5% (box 7 and 10), 15% (box 8 and 11), and 50% (box 9 and 12) of tibia length, measured from the proximal tibia. Bone mineral densities (BMD) in g/cm2 were extracted from each ROI for analysis.
Statistical Methods
Standard descriptive statistics (mean and standard deviation [SD] for continuous variables, frequency and percentage for categorical variables) were used to describe the study sample. Linear mixed effects models were constructed to assess the influence of time post-operatively, limb, and a time-limb interaction effect on each BMD ROI. Athlete and limb were assigned as random effects to account for the correlation among repeated time points and limbs. Meaningful pairwise interactions between preinjury and postoperative time points for the surgical and nonsurgical limbs were assessed. Least-square mean differences and their associated standard errors were reported alongside Tukey-adjusted p-values to account for multiple comparisons. Analyses were conducted using the R Statistical language (version 4.0.5; R Core Team, 2021).
Results
Following the records extraction process, 33 athletes were identified for inclusion in the final dataset (Figure 2), with 33, 31, 28, and 16 DXA scans included at preinjury, 6 months, 12 months, and 24 months, respectively. Athlete demographics, including: age, sex, anthropometrics, graft type, concomitant procedures, and sport are reported in Table 1.
Figure 2.
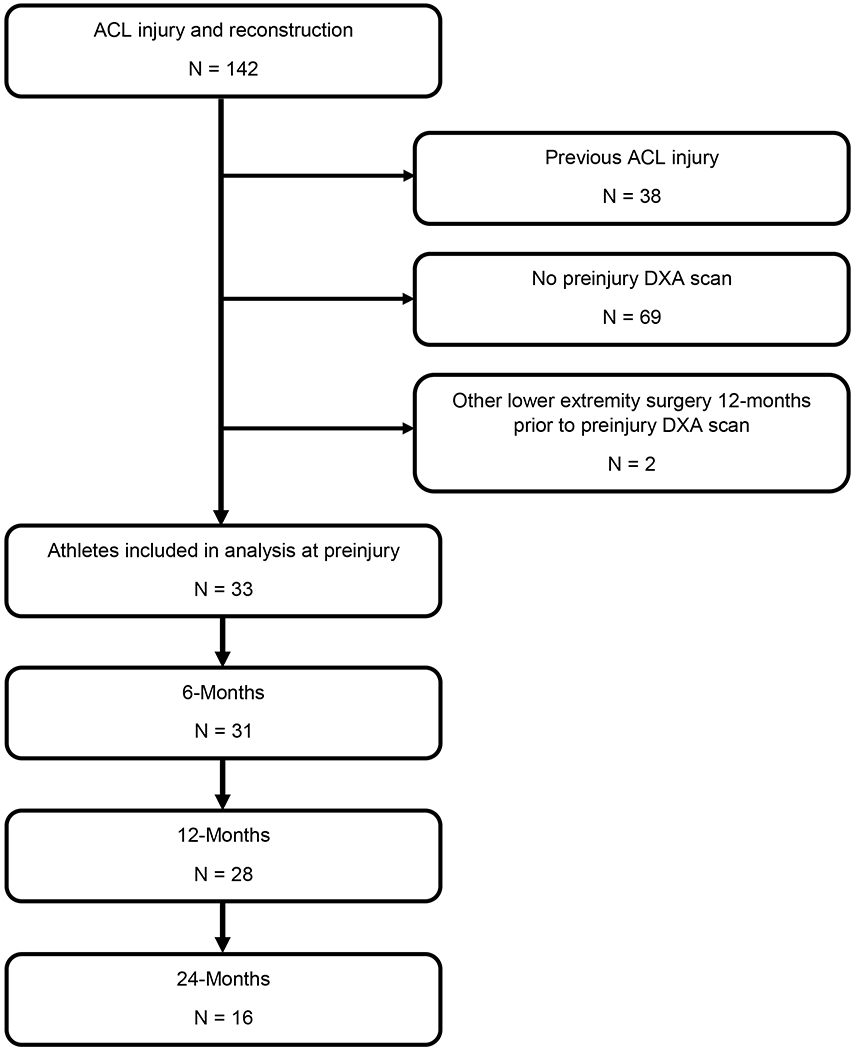
Records extraction process. All athletes included in the analysis had dual-energy x-ray absorptiometry (DXA) scans from preinjury and at least one post-operative time point. ACL, anterior cruciate ligament.
Table 1.
Summary of participant information.
| Participant Information (N = 33)a | |
|---|---|
| Values | |
| Age, y* | 21.2 (1.2) |
| Body Mass, kg* | 88.4 (20.1) |
| Height, cm* | 194.9 (44.3) |
| Body Mass Index, kg/m2* | 26.8 (4.2) |
| Females, n (% of total participants) | 13 (39%) |
| Graft type, n (% of total participants) | |
| Bone-patellar-tendon-bone | 30 (91%) |
| Hamstring tendon | 2 (6%) |
| Quadriceps tendon | 1 (3%) |
| Concomitant procedures, n (% of total participants) | |
| Meniscectomy | 10 (30%) |
| Meniscal repair | 10 (30%) |
| MPFL reconstruction | 2 (6%) |
| Time between DXA scan and ACLR, mo | |
| Preinjury | 3.0 (2.0) |
| 6-month | 6.1 (0.7) |
| 12-month | 12.1 (1.2) |
| 24-month | 24.4 (2.9) |
| Participants at each time, n (% of total participants) | |
| Preinjury | 33 (100) |
| 6-month | 31 (94) |
| 12-month | 28 (85) |
| 24-month | 16 (48) |
Values are presented as mean (SD) unless otherwise indicated.
Based on 6-month post-operative assessment.
DXA, dual-energy X-ray absorptiometry, ACLR, anterior cruciate ligament reconstruction. MPFL, medial patellar femoral ligament.
Femur
A significant time-limb interaction was detected for BMD at F5 and F15 (both p-values < 0.001), but not for BMD at F50 (p-value = 0.72). Fixed effect estimates from each linear mixed effect model can be found in Supplemental Table 2. Compared to preinjury, BMD at F5 of the surgical limb was reduced 0.15 (0.02) g/cm2 at 6-months (p-value < 0.001, Figure 3, Table 2). Compared to preinjury, BMD at F15 of the surgical limb was reduced 0.06 (0.01), 0.09 (0.01), 0.09 (0.01) g/cm2 at 6, 12, and 24-months, respectively (all p-values < 0.001, Figure 3, Table 2). A significant main effect of time was observed for F50 (p = 0.02), but this was not significant for a specific time point compared to preinjury (p = 0.08 at 24-months).
Figure 3.
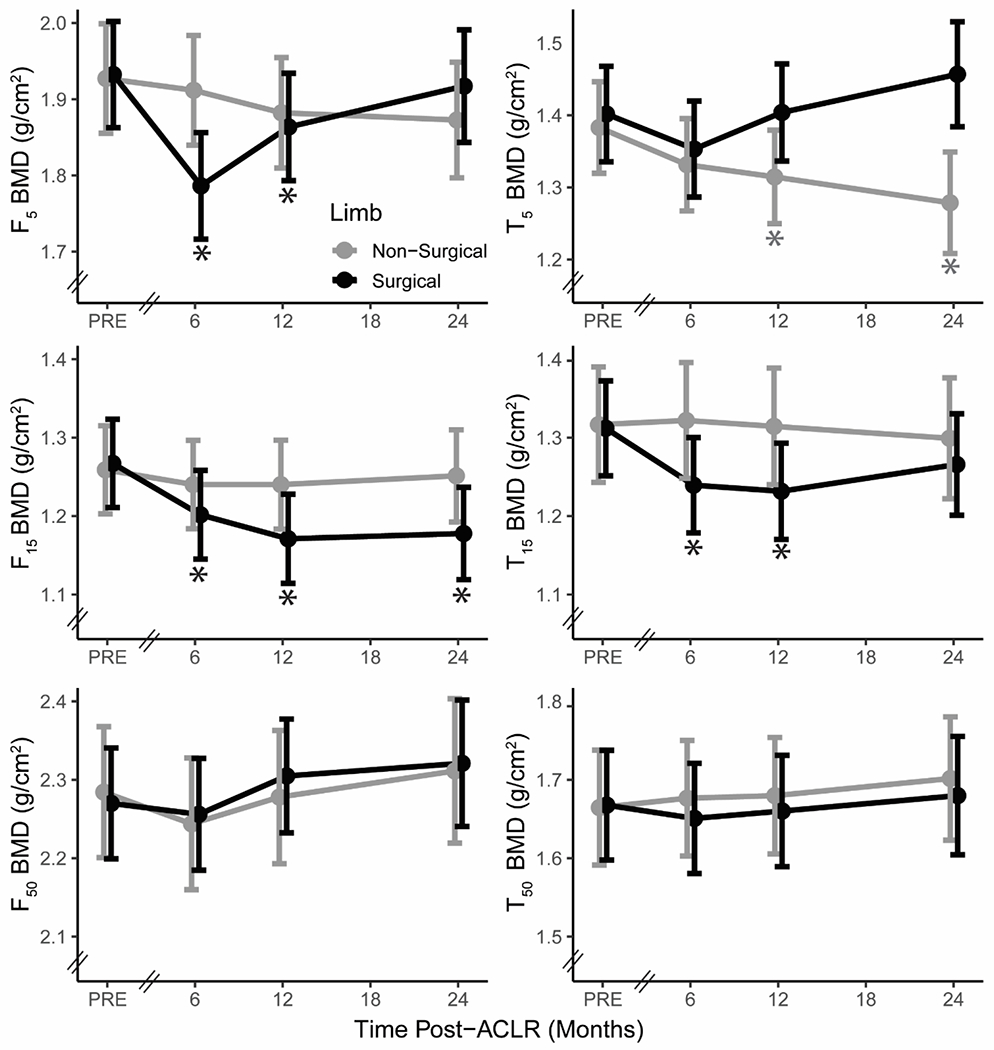
Least-square mean values of bone mineral density (BMD) at 5% (F5), 15% (F15), and 50% (F50) of femur length and 5% (T5), 15% (T15), and 50% (T50) of tibia length from preinjury (PRE) to 24-months post-anterior cruciate ligament reconstruction (ACLR) within the surgical (black) and non-surgical limb (grey). Error bars depict the 95% confidence interval of the least-square mean. The axis break between preinjury and 6-months demonstrates that the time interval between preinjury and 6-months varies between participants. * Significant within-limb difference from preinjury p < 0.002.
Table 2.
Least-square means, standard errors (SE), mean differences from preinjury, and Tukey-adjusted p-values for surgical and non-surgical limb bone mineral density (BMD) regions of interest (ROI) at all time-points. A negative value notes a deficit in the post-operative BMD compared to preinjury.
| BMD ROI | Limb | Preinjury | 6-Months Post-ACLR | 12-Months Post-ACLR | 24-Months Post-ACLR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean Difference from Preinjury (SE) [% Change] | p-value | Mean (SE) | Mean Difference from Preinjury (SE) [% Change] | p-value | Mean (SE) | Mean Difference from Preinjury (SE) [% Change] | p-value | ||
| 5% femur length (g/cm2) | Surgical | 1.94 (0.04) | 1.79 (0.04) | −0.15 (0.02) [−7.7%] |
<0.001 | 1.87 (0.04) | −0.07 (0.02) [−3.6%] |
0.002 | 1.92 (0.04) | −0.02 (0.02) [−1.0%] |
0.97 |
| Non-Surgical | 1.94 (0.04) | 1.92 (0.04) | −0.02 (0.02) [−1.0%] |
0.98 | 1.89 (0.04) | −0.05 (0.02) [−2.6%] |
0.18 | 1.89 (0.04) | −0.05 (0.02) [−2.6%] |
0.33 | |
| 15% femur length (g/cm2) | Surgical | 1.26 (0.03) | 1.20 (0.03) | −0.06 (0.01) [−4.8%] |
<0.001 | 1.17 (0.03) | −0.09 (0.01) [−7.1%] |
<0.001 | 1.17 (0.03) | −0.09 (0.01) [−7.1%] |
<0.001 |
| Non-Surgical | 1.26 (0.03) | 1.24 (0.03) | −0.02 (0.01) [−1.6%] |
0.71 | 1.24 (0.03) | −0.02 (0.01) [−1.6%] |
0.74 | 1.25 (0.03) | −0.01 (0.01) [−0.8%] |
0.99 | |
| 50% femur length (g/cm2) | Surgical | 2.27 (0.04) | 2.26 (0.04) | −0.01 (0.02) [−0.4%] |
0.99 | 2.31 (0.04) | 0.04 (0.03) [1.8%] |
0.87 | 2.32 (0.04) | 0.05 (0.02) [2.2%] |
0.84 |
| Non-Surgical | 2.28 (0.04) | 2.24 (0.04) | −0.04 (0.02) [−1.8%] |
0.73 | 2.28 (0.04) | 0.00 (0.02) [0.0%] | 0.99 | 2.32 (0.05) | 0.04 (0.02) [1.8%] |
0.97 | |
| 5% tibia length (g/cm2) | Surgical | 1.40 (0.03) | 1.35 (0.03) | −0.05 (0.02) [−3.6%] |
0.20 | 1.40 (0.03) | 0.00 (0.02) [0.0%] |
0.99 | 1.46 (0.04) | 0.06 (0.02) [4.3%] |
0.34 |
| Non-Surgical | 1.38 (0.03) | 1.33 (0.14) | −0.05 (0.02) [−3.6%] |
0.14 | 1.31 (0.03) | −0.07 (0.02) [−5.1%] |
0.02 | 1.28 (0.04) | −0.10 (0.02) [−7.2%] |
0.001 | |
| 15% tibia length (g/cm2) | Surgical | 1.31 (0.03) | 1.24 (0.03) | −0.07 (0.01) [−5.3%] |
<0.001 | 1.23 (0.03) | −0.08 (0.02) [−6.1%] |
<0.001 | 1.26 (0.03) | −0.05 (0.02) [−3.8%] |
0.21 |
| Non-Surgical | 1.31 (0.04) | 1.32 (0.04) | 0.01 (0.01) [0.8%] |
0.99 | 1.31 (0.04) | 0.00 (0.02) [0.0%] |
0.99 | 1.30 (0.04) | −0.01 (0.02) [−0.8%] |
0.98 | |
| 50% tibia length (g/cm2) | Surgical | 1.66 (0.03) | 1.65 (0.30) | −0.01 (0.01) [−0.6%] |
0.98 | 1.66 (0.03) | 0.00 (0.01) [0.0%] |
0.99 | 1.68 (0.03) | 0.02 (0.02) [1.2%] |
0.99 |
| Non-Surgical | 1.66 (0.04) | 1.67 (0.04) | 0.01 (0.02) [0.6%] |
0.99 | 1.68 (0.04) | 0.02 (0.01) [1.2%] |
0.99 | 1.70 (0.04) | 0.04 (0.02) [2.4%] |
0.75 | |
ACLR, anterior cruciate ligament reconstruction
Tibia
A significant time-limb interaction was detected for BMD at T5 and T15 (both p-values < 0.001), but not for BMD at T50 (p-value = 0.69). Fixed effect estimates from each tibia linear mixed effect model can be found in Supplemental Table 3. Compared to preinjury, BMD at T5 of the non-surgical limb was reduced 0.07 (0.02) g/cm2 at 12-months (p-value = 0.02) and 0.10 (0.02) g/cm2 at 24-months (p-value = 0.001, Figure 3, Table 2). Compared to preinjury, BMD at T15 of the surgical limb was reduced 0.07 (0.01) g/cm2 at 6-months and 0.08 (0.02) g/cm2 at 12-months (p-values < 0.001, Figure 3, Table 2).
Discussion
This study aimed to characterize changes in lower extremity BMD from preinjury to 24 months following ACLR in division I collegiate athletes. Contrary to our primary hypothesis, the F15 ROI demonstrated a significant reduction in BMD from the preinjury state that persisted to 24 months (−7.1%). However, the F5 and T15 ROIs of the surgical limb changed as hypothesized, demonstrating significantly reduced BMD at 6 and 12 months post-ACLR, which was no longer statistically different at 24 months compared to preinjury levels. Additionally, the T5 ROI did not significantly change from preinjury levels in the surgical limb at any time point. Contrary to our secondary hypothesis, a significant decrease in BMD was observed at the T5 ROI of the non-surgical limb (−7.2%). No other changes in the non-surgical limb were observed.
Interestingly, BMD deficits in the surgical limb were present in the distal femur (both F5 and F15) and the proximal tibia (T15), but not in the mid shaft regions (F50 and T50) or most proximal tibia region (T5). This may be partially explained by the fact that the distal femur and proximal tibia regions contain greater levels of trabecular bone, which experiences a higher rate of bone turnover.6 Recovery of local BMD takes roughly 4 times longer than the period of disuse based on animal models.2,43 Additionally, to maintain bone density, weight-bearing bones require a stimulus that exceeds the bones’ strain thresholds which in turn produces an osteogenic response. The strain threshold appears to be site-specific, varying between bones and location within a given bone.13,16 Strain thresholds tend to be greatest in regions with the greatest mechanical stimuli.16 In weight-bearing bones strain threshold and disuse-related bone loss tend to be greatest distally, where locomotor forces tend to be highest.9,13,16 Therefore, differences in BMD observed across sites could be due, in part, to altered movement mechanics such as reduced knee flexion angles and extensor moments during walking,8,11,44 running,22,37 jumping,24,25 and change of direction tasks,31 as well as reduced quadriceps strength.28 Site specific loss in BMD is multifactorial, likely being influenced by the initial injury, subsequent surgical procedure, local inflammation, period of immobilization, and disuse of the involved lower extremity.14,17,20,26,33 The combination of reduced lower extremity loading, altered movement mechanics, and quadriceps dysfunction following ACLR likely has an impact on bone health, as bone is particularly responsive to changes in loading.6,13
Unexpectedly, a significant decrease in the non-surgical proximal tibia BMD ROI (T5) was observed and persisted to 24 months post-ACLR. We have no explanation for this observation. Previous literature provides conflicting evidence regarding the impact ACLR has on BMD of the non-surgical tibia.26,33,49 Van Meer et al. observed a slight (3%) but significant reduction in BMD of the proximal tibia of the uninjured limb in 141 patients from three hospitals in the Netherlands that persisted to 24 months after ACL injury.33 We observed a larger (7.2%) reduction in the proximal tibia BMD of the non-surgical limb at 24 months. This discrepancy may be due to difference in the population, sample size, scan acquisition methods, surgical and rehabilitation management, reduction in physical activity post-operatively, or it may simply be an incidental finding. As this study is more exploratory in nature, future studies aimed at exploring BMD changes in the non-surgical limb are needed to confirm these findings.
This is the first study to our knowledge that highlights changes in lower extremity BMD of elite collegiate athletes following ACLR compared to the preinjury state. Prior literature has consisted of more heterogeneous samples with varying ages and levels of sports participation.33,34,36 The cohort in this study received frequent and unrestricted access to sports medicine facilities and rehabilitation. Most athletes (85%) in this cohort returned to sport. As such, this creates a homogenous group with significant exposure to both high frequency and magnitudes of loading as part of training and competition. Despite this environment for recovery following ACLR, athletes demonstrated significant reductions in BMD out to 24 months, over a year beyond when most returned to sport.
These persistent deficits in BMD observed are concerning. BMD loss early in life may predispose athletes to future bone and joint pathology such as OA and fracture. OA is a well-known long-term risk following ACLR, which can progress to the need for TKA. Individuals with a history of ACLR are at a greater risk of undergoing TKA compared to the general population and at an earlier age.1,35 As the long-term risk for OA is high in individuals who have undergone ACLR, restoring BMD may be an important factor to consider. Moreover, reduced BMD has been associated with increased risk of fracture,19,32 and cases of distal femur fractures following ACLR have been reported.7,42,45 If the reduced BMD observed persists later in life, surgical complications such as periprosthetic fractures may be a significant concern in those with a history of ACLR, which has been reported in a male that was 8 years post-TKA and 25 years post-ACLR.23
Our findings are limited by the specific nature of DXA, which provides a 2-D assessment of bone. DXA scans measure summative bone mass in a given area, and cannot differentiate between trabecular and cortical bone. BMD values for this study were acquired using full body DXA scans, which may not provide as precise estimates of local BMD as site-specific scans; however, to the authors’ knowledge, there is no published literature comparing full body to site specific scans at the knee. Moreover, we were unable to isolate only the tibia in the ROIs of the lower shank. As such, the tibia ROIs represent a cumulative BMD across both the tibia and the fibula. Similarly, the patella overlies the distal femur, providing a cumulative value for the two bones in the involved ROIs. A section of the patella falls within the F5 ROI, but does not extend into the F15 ROI. Interestingly, the F5 ROI had a significant drop in BMD at 6 months but restored to preinjury levels by 24 months post-ACLR. A portion of the patella is harvested for the bone-patellar tendon-bone (BPTB) graft during ACLR (91% of the sample). Therefore, the initial deficits and subsequent recovery in BMD at the F5 ROI may be related to the defect left from harvesting the graft and reconstitution of this defect, respectively. As the patella is a non-weight bearing joint, is disrupted when harvesting a BPTB graft, and receives its mechanical loading primarily from the quadriceps, it may be an important bone to isolate in future studies as quadriceps dysfunction is the norm following ACLR.28 Additionally, as most athletes included in this study underwent ACLR with a BPTB graft, findings may not generalize to other graft types. It is important to note that athletes activity levels were not controlled for and not all athletes underwent testing at every time point. Due to fewer collections at 24-months, a lack of significant difference from preinjury may not reflect true recovery of BMD, but may be due to reduced statistical power at that time. Linear mixed effects models were used to account for missing data. Further, findings did not change when only athletes with 24-month collections were included in the analysis.
In conclusion, NCAA Division I collegiate athletes demonstrated focal BMD deficits to the distal femur (F15) of the surgical limb that persisted to 24 months following ACLR compared to preinjury scans. Deficits in BMD were also observed in the proximal tibia (T5) of the non-surgical limb. All other regions of both the surgical and non-surgical limb remained at preinjury levels or returned to preinjury levels by 24 months post-ACLR.
Supplementary Material
References
- 1.Abram SGF, Judge A, Khan T, Beard DJ, Price AJ. Rates of knee arthroplasty in anterior cruciate ligament reconstructed patients: a longitudinal cohort study of 111,212 procedures over 20 years. Acta Orthop. 2019;90(6):568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen MR, Hogan HA, Bloomfield SA. Diferential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. J Musculoskelet Neuronal Interact. 2006;6(3):217–225. [PubMed] [Google Scholar]
- 3.Barr AJ, Campbell TM, Hopkinson D, Kingsbury SR, Bowes MA, Conaghan PG. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res Ther. 2015;17(228):1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinque ME, Dornan GJ, Chahla J, Moatshe G, LaPrade RF. High Rates of Osteoarthritis Develop After Anterior Cruciate Ligament Surgery: An Analysis of 4108 Patients. Am J Sports Med. 2018;46(8):2011–2019. [DOI] [PubMed] [Google Scholar]
- 5.Claes S, Hermie L, Verdonk R, Bellemans J, Verdonk P. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg Sport Traumatol Arthrosc. 2013;21:1967–1976. [DOI] [PubMed] [Google Scholar]
- 6.Clarke B Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3:S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coobs BR, Spiridonov SI, LaPrade RF. Intra-articular lateral femoral condyle fracture following an ACL revision reconstruction. Knee Surg Sport Traumatol Arthrosc. 2010;18:1290–1293. [DOI] [PubMed] [Google Scholar]
- 8.Davis-Wilson HC, Pfeiffer SJ, Johnston CD, et al. Bilateral Gait 6 and 12 Months Post-Anterior Cruciate Ligament Reconstruction Compared with Controls. Med Sci Sports Exerc. 2020;52(4):785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duda GN, Schneider E, Chao EYS. Internal forces and moments in the femur during walking. J Biomech. 1997;30(9):933–941. [DOI] [PubMed] [Google Scholar]
- 10.Ejerhed L, Kartus J, Nilsén R, Nilsson U, Kullenberg R, Karlsson J. The effect of anterior cruciate ligament surgery on bone mineral in the calcaneus: a prospective study with a 2-year follow-up evaluation. Arthroscopy. 2004;20(4):352–359. [DOI] [PubMed] [Google Scholar]
- 11.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Longitudinal changes in knee gait mechanics between 2 and 8 years after anterior cruciate ligament reconstruction. J Orthop Res. 2018;36(5):1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filbay SR, Ackerman IN, Russell TG, Macri EM, Crossley KM. Health-related quality of life after anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42(5):1247–1255. [DOI] [PubMed] [Google Scholar]
- 13.Giangregorio L, Blimkie CJR. Skeletal adaptations to alterations in weight-bearing activity: A comparison of models of disuse osteoporosis. Sport Med. 2002;32(7):459–476. [DOI] [PubMed] [Google Scholar]
- 14.Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris KP, Driban JB, Sitler MR, Cattano NM, Balasubramanian E, Hootman JM. Tibiofemoral osteoarthritis after surgical or nonsurgical treatment of anterior cruciate ligament rupture: A Systematic Review. J Athl Train. 2017;52(6):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo: The strain threshold for an osteogenic response varies with location. J Bone Miner Res. 2001;16(12):2291–2297. [DOI] [PubMed] [Google Scholar]
- 17.JÄRVINEN M, KANNUS P. Current Concepts Review - Injury of an Extremity as a Risk Factor for the Development of Osteoporosis*. J Bone Jt Surg. 1997;79(2):263–276. [DOI] [PubMed] [Google Scholar]
- 18.Jones MH, Oak SR, Andrish JT, et al. Predictors of Radiographic Osteoarthritis 2 to 3 Years After Anterior Cruciate Ligament Reconstruction: Data From the MOON On-site Nested Cohort. Orthop J Sport Med. 2019;7(8):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanis JA. Diagnosis of Osteoporosis and Assessment of Fracture Risk. Lancet. 2002;359:1929–1936. [DOI] [PubMed] [Google Scholar]
- 20.Kannus P, Jarvinen M, Johnson R, et al. Function of the quadriceps and hamstrings muscles in knees with chronic partial deficiency of the anterior cruciate ligament. Isometric and isokinetic evaluation. Am J Sport Med. 1992;20(2):162–168. [DOI] [PubMed] [Google Scholar]
- 21.Kannus P, Sievänen H, Järvinen M, Heinonen A, Oja P, Vuori I. A cruciate ligament injury produces considerable, permanent osteoporosis in the affected knee. J Bone Miner Res. 1992;7(12):1429–1434. [DOI] [PubMed] [Google Scholar]
- 22.Knurr KA, Kliethermes SA, Stiffler-Joachim MR, Cobian DG, Baer GS, Heiderscheit BC. Running Biomechanics Before Injury and 1 Year After Anterior Cruciate Ligament Reconstruction in Division I Collegiate Athletes. Am J Sports Med. 2021;49(10):2607–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kooner S, Gibson E, Clark M. Periprosthetic total knee fracture after remote reconstruction of the anterior cruciate ligament: A case report. J Med Case Rep. 2017;11(276). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotsifaki A, Korakakis V, Whiteley R, Van Rossom S, Jonkers I. Measuring only hop distance during single leg hop testing is insufficient to detect deficits in knee function after ACL reconstruction: A systematic review and meta-analysis. Br J Sports Med. 2020;54(3):139–153. [DOI] [PubMed] [Google Scholar]
- 25.Lepley AS, Kuenze CM. Hip and knee kinematics and kinetics during landing tasks after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. J Athl Train. 2018;53(2):144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppälä J, Kannus P, Natri A, et al. Effect of anterior cruciate ligament injury of the knee on bone mineral density of the spine and affected lower extremity: A prospective one-year follow-up study. Calcif Tissue Int. 1999;64(4):357–363. [DOI] [PubMed] [Google Scholar]
- 27.Libber J, Binkley N, Krueger D. Clinical observations in total body DXA: Technical aspects of positioning and analysis. J Clin Densitom. 2012;15(3):282–289. [DOI] [PubMed] [Google Scholar]
- 28.Lisee C, Lepley AS, Birchmeier T, O’Hagan K, Kuenze C. Quadriceps Strength and Volitional Activation After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Sports Health. 2019;11(2):163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: A systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lui PPY, Cheng YY, Yung SH, Hung ASL, Chan KM. A randomized controlled trial comparing bone mineral density changes of three different ACL reconstruction techniques. Knee. 2012;19(6):779–785. [DOI] [PubMed] [Google Scholar]
- 31.Marques JB, Paul DJ, Graham-Smith P, Read PJ. Change of Direction Assessment Following Anterior Cruciate Ligament Reconstruction: A Review of Current Practice and Considerations to Enhance Practical Application. Sport Med. 2020;50(1):55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63. [DOI] [PubMed] [Google Scholar]
- 33.Van Meer BL, Waarsing JH, van Eijsden WA, et al. Bone mineral density changes in the knee following anterior cruciate ligament rupture. Osteoarthr Cartil. 2014;22(1):154–161. [DOI] [PubMed] [Google Scholar]
- 34.Mündermann A, Payer N, Felmet G, Riehle H. Comparison of volumetric bone mineral density in the operated and contralateral knee after anterior cruciate ligament and reconstruction: A 1-year follow-up study using peripheral quantitative computed tomography. J Orthop Res. 2015;33(12):1804–1810. [DOI] [PubMed] [Google Scholar]
- 35.Murtha AS, Johnson AE, Buckwalter JA, Rivera JC. Total knee arthroplasty for posttraumatic osteoarthritis in military personnel under age 50. J Orthop Res. 2017;35(3):677–681. [DOI] [PubMed] [Google Scholar]
- 36.Nyland J, Fisher B, Brand E, Krupp R, Caborn DNM. Osseous deficits after anterior cruciate ligament injury and reconstruction: A systematic literature review with suggestions to improve osseous homeostasis. Arthroscopy. 2010;26(9):1248–1257. [DOI] [PubMed] [Google Scholar]
- 37.Pairot-de-Fontenay B, Willy RW, Elias ARC, Mizner RL, Dubé MO, Roy JS. Running Biomechanics in Individuals with Anterior Cruciate Ligament Reconstruction: A Systematic Review. Sport Med. 2019;49(9):1411–1424. [DOI] [PubMed] [Google Scholar]
- 38.Petak S, Barbu CG, Yu EW, et al. The Official Positions of the International Society for Clinical Densitometry: Body Composition Analysis Reporting. J Clin Densitom. 2013;16(4):508–519. [DOI] [PubMed] [Google Scholar]
- 39.Risberg MA, Oiestad BE, Gunderson R, et al. Changes in Knee Osteoarthritis, Symptoms, and Function after Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44(5):1215–1224. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez MJ, Garcia EJ, Dickens JF. Primary and Posttraumatic Knee Osteoarthritis in the Military. J Knee Surg. 2019;32:134–137. [DOI] [PubMed] [Google Scholar]
- 41.Sanfilippo J, Krueger D, Heiderscheit B, Binkley N. Dual-Energy X-Ray Absorptiometry Body Composition in NCAA Division I Athletes: Exploration of Mass Distribution. Sports Health. 2019;11(5):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheps DM, Reed JG, Hildebrand KA, Hiemstra LA. Supracondylar femur fracture after endoscopic anterior cruciate reconstruction using an EndoButton. Clin J Sport Med. 2006;16(5):428–429. [DOI] [PubMed] [Google Scholar]
- 43.Shirazi-Fard Y, Kupke JS, Bloomfield SA, Hogan HA. Discordant recovery of bone mass and mechanical properties during prolonged recovery from disuse. Bone. 2013;52(1):433–443. [DOI] [PubMed] [Google Scholar]
- 44.Slater LV, Hart JM, Kelly AR, Kuenze CM. Progressive Changes in Walking Kinematics and Kinetics After Anterior Cruciate Ligament Injury and Reconstruction: A Review and Meta-Analysis. J Athl Train. 2017;52(9):847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thangamani VB, Flanigan DC, Merk BR. Intra-articular distal femur fracture extending from an expanded femoral tunnel in an anterior cruciate ligament (ACL) reconstructed knee: A case report. J Trauma. 2009;67(6):E209–E212. [DOI] [PubMed] [Google Scholar]
- 46.Wang LJ, Zeng N, Yan ZP, Li JT, Ni GX. Post-traumatic osteoarthritis following ACL injury. Arthritis Res Ther. 2020;22(57). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittaker JL, Woodhouse LJ, Nettel-Aguirre A, Emery CA. Outcomes associated with early post-traumatic osteoarthritis and other negative health consequences 3-10 years following knee joint injury in youth sport. Osteoarthr Cartil. 2015;23:1122–1129. [DOI] [PubMed] [Google Scholar]
- 48.Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of Secondary Injury in Younger Athletes after Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44(7):1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zerahn B, Munk AO, Helweg J, Hovgaard C. Bone Mineral Density in the Proximal Tibia and Calcaneus Before and After Arthroscopic Reconstruction of the Anterior Cruciate Ligament. Arthroscopy. 2006;22(3):265–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


