Abstract
BACKGROUND:
Cognitive impairments are related to deficits in primary auditory and visual sensory processes in schizophrenia. These impairments can be remediated by neuroscience-informed computerized cognitive trainings that target auditory and visual processes. However, it is not clear which modality results in greater improvements in cognition, symptoms and quality of life. We aimed to investigate the impact of training auditory versus visual cognitive processes in global cognition in patients with schizophrenia.
METHODS:
Seventy-nine schizophrenia participants were randomly assigned to either 40 hours of auditory or visual computerized training. Auditory and visual exercises were chosen to be dynamically equivalent and difficulties increased progressively during the training. We evaluated cognition, symptoms and quality of life before, after 20 hours, and after 40 hours of training. ClinicalTrials.gov (1R03TW009002–01).
RESULTS:
Participants who received the visual training showed significant improvements in global cognition compared to the auditory training group. The visual training significantly improved attention and reasoning and problem-solving, while the auditory training improved reasoning and problem-solving only. Schizophrenia symptoms improved after training in both groups, whereas quality of life remained unchanged. Interestingly, there was a significant and positive correlation between improvements in attention and symptoms in the visual training group.
CONCLUSIONS:
We conclude that the visual training and the auditory training are differentially efficient at remediating cognitive deficits and symptoms of clinically stable schizophrenia patients. Ongoing follow-up of participants will evaluate the durability of training effects on cognition and symptoms, as well as the potential impact on quality of life over time.
Keywords: schizophrenia, cognitive training, visual training, auditory training, neuroplasticity, computerized training
1. INTRODUCTION
Schizophrenia is a complex and highly incapacitating mental disorder that has the highest disability weight, according to the Global Burden of Disease (Salomon et al., 2012). This has a significant impact on affected individuals and their families, resulting in important societal and economic costs (Knapp et al., 2005). Schizophrenia is often characterized by cognitive impairments that are important predictors of poor quality of life, occupational and social functioning (Green, 2006; McGurk and Meltzer, 2000; Ritsner, 2007). Cognitive impairments in schizophrenia have been shown in the domains of attention, memory, executive functions, and social cognition (Bilder et al., 2002; Hutton et al., 1998; Szoke et al., 2008), and are associated with brain structural abnormalities (Crespo-Facorro et al., 2007).
Over the last decades it has been proposed that abnormalities in primary sensory brain regions in schizophrenia induce dysfunctions in basic visual and auditory processes, which may contribute to impairments in higher-order functions (Javitt, 2009). Deficits in the auditory system are apparent in basic sensory processing, such as tone encoding and detection of pitch deviation (Javitt et al., 1997, 1993; March et al., 1999). Deficits in the visual system are evident in smooth eye movements, motion detection, and contrast sensitivity (Butler et al., 2008; O’Donnell et al., 1996). Importantly, deficits in basic processing are associated with impairments in higher-order processes such as phonological processing, auditory emotion recognition, recognition of objects and facial expressions (Javitt, 2009), which may ultimately contribute to poor social outcomes (Arnott et al., 2011; Dale et al., 2010; Javitt, 2009; Kantrowitz et al., 2015; Koro et al., 2002; Martínez et al., 2015; Morrison et al., 1988; Revheim et al., 2006; Tek et al., 2002).
Over the past decade, Dr. Vinogradov’s group and others have shown that neuroscience-informed cognitive training targeting auditory processes improves global cognition, speed of processing, verbal learning and memory, as well as reasoning and problem-solving in early onset and chronic schizophrenia subjects (Fisher et al., 2009). Importantly, participants who were able to make the most progress through basic auditory training exercises also showed the most improvement in higher-level cognitive outcome measures (Adcock et al., 2009; Biagianti et al., 2016). The brain of trained schizophrenia participants recovered impaired sensory gating and oscillations in neural activity related to auditory processing (Popov et al., 2011, 2015); and restored neural patterns of response in a source monitoring task (Subramaniam et al., 2012). A significant positive relationship was evident between training-induced enhancements of prefrontal cortical activity and better ratings on social and occupational domains of the Quality of Life Scale six months after completion of training (Subramaniam et al., 2014). Cognitive improvements in schizophrenia were also associated with increases in serum levels of pro-cognitive molecules such as BDNF (Vinogradov et al., 2009) and D-serine (Panizzutti et al., 2009), as well as variations in the cognition-related gene COMT (Panizzutti et al., 2013).
On the other hand, fewer studies evaluated cognitive training targeting visual processes in schizophrenia. In one study that evaluated schizophrenia subjects undertaking different trainings (visual, auditory and cognitive control) concomitantly, improvements in the visual perceptual exercises were associated with gains in visual but not verbal memory (Surti et al., 2011). Also, a specific training on motion perception improved impairments in the visual modality, but with no transfer of effect to other cognitive measurements (Norton et al., 2011).
In sum, the evidence indicates that neuroscience-informed cognitive training targeting auditory and visual processes can remediate brain dysfunctions present in schizophrenia. However, it is not known which sensory modality (visual or auditory) of cognitive training will generalize more efficiently to the global cognition in schizophrenia. Global cognition is defined as a composite measure encompassing the Measurement And Treatment Research to Improve Cognition in Schizophrenia (MATRICS)-defined domains affected in schizophrenia: speed of processing, attention, working memory, verbal learning, visual learning, reasoning and problem-solving, and social cognition. To investigate the comparative impact of cognitive training with auditory versus visual processes in schizophrenia, we posed two questions: 1) Which modality of intensive sensory cognitive training - auditory or visual - has a larger impact on global cognition in schizophrenia participants? 2) Are the cognitive gains in participants who underwent auditory or visual cognitive training associated with changes in symptoms and quality of life?
To answer these questions, we selected two groups of cognitive training exercises that were dynamically identical, with the exception of the sensory modality that was trained (i.e. auditory or visual). We compared the effect of the auditory versus visual neuroscience-informed cognitive training exercises in schizophrenia patients, using global cognition as the primary outcome. We evaluated the effect of training on the different cognitive domains affected in schizophrenia, clinical symptoms and quality of life as secondary outcomes.
2. METHODS
2.1. Participants
Seventy-nine schizophrenia subjects were recruited from the day-hospital and outpatient clinic of the Institute of Psychiatry (IPUB) at the Federal University of Rio de Janeiro from September 2013 until December 2016.
Participants were self-referred or referred by a psychiatrist and signed a written consent form after being informed about the study procedures. We included participants between 18 and 60 years old with a schizophrenia or schizoaffective disorder diagnosis. Participants were clinically stable and had an outpatient status for at least one month before starting the training. Participants continued to take their medication as usual during the study and had no change in dosage greater than 10%. At study entry, participants received an evaluation to determine eligibility that included the WAIS–IV Information and Matrix Reasoning IQ test (Wechsler, 2008), the Mini International Neuropsychiatry Interview Plus diagnostic interview (Sheehan et al., 1998) and the Simpson-Angus Scale (Simpson and Angus, 1970) for extrapyramidal symptoms. The Positive and Negative Syndrome Scale (PANSS) (Kay SR, Fiszbein A, 1987), the Hamilton Depression and Anxiety rating scales and the World Health Organization Quality Of Life (WHO, 1998) questionnaires were administered at baseline and after 40 hours of training. Participants were excluded if they were illiterate or had any history of another psychiatric diagnosis, intellectual disability or brain damage. We also excluded participants with extrapyramidal symptom scores above 19, IQ below 80 or any serious visual or auditory impairment that precluded their participation in the study.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving patients were approved by the Brazilian National Committee of Ethics in Research (12990013.0.0000,5263) and pre-registered at ClinicalTrials.gov (1R03TW009002–01).
2.2. Study design
This study used a randomized, double-blind, parallel design, where participants performed 40 hours of auditory or visual cognitive training. Participants were randomized to either the auditory or the visual cognitive training using a hierarchical stratification by gender, age, IQ, years of education, diagnosis, PANSS scores, ethnicity, BMI, and recruitment site. Participants were blind to group assignment, and the research team performing the assessments had no access to participants’ group assignment. Participants completed cognitive assessments at baseline and after 20 and 40 hours of training. All the assessments took place in our research facilities. However, participants were given the choice to perform the online trainings in our research facilities or at home. They were asked to practice for 1 hour per day, at least 3 days a week, which enabled the training to last between 2 and 4 months. The research team monitored training compliance weekly using the electronic records available online at the training platform.
2.3. Cognitive Assessments
We assessed the seven cognitive domains defined by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), using the MATRICS Consensus Cognitive Battery recommended tests (MCCB) available in Portuguese and the Cambridge Neuropsychological Test Automated Battery (CANTAB). We administered the MCCB Category Fluency (CF) and the CANTAB Reaction time (RTI) to assess speed of processing; the CANTAB Rapid Visual Processing (RVP) to assess attention; the Wechsler Adult Intelligence Scale (WAIS) III digit backward (DB) and the CANTAB Spatial Working Memory (SWM) to assess working memory; the MCCB Hopkins Verbal Learning Task (HVLT) to assess verbal learning; the MCCB Brief Visuospatial Memory Test (BVMT) to assess visual learning; the CANTAB Stocking Of Cambridge (SOC) to assess reasoning and problem-solving; and the MCCB Mayer-Salovey-Caruso Emotional Intelligence Test – Managing emotions (MSCEIT) to assess social cognition. A detailed explanation of these tests and their outcome measures can be found in previous literature (Massardo et al., 2015; Scoriels et al., 2012). We used parallel versions of the tests that were sensitive to practice effects (i.e. HVLT, and BVMT) at 20 and 40 hours of training. Global cognition was calculated from the z-scores of speed of processing, attention, working memory, verbal learning, visual learning, reasoning and problem-solving, and social cognition.
2.4. Cognitive Training Exercises
We designed the auditory and visual computerized cognitive trainings by selecting pairs of auditory and visual exercises from Posit Science, Inc (www.brainhq.com) that had equivalent task dynamics, each in its perceptive modality. The auditory training included Sound Sweeps, Fine Tuning, Memory Grid, Auditory Ace, Syllable Stacks, and In the Know. The visual training included Visual Sweeps, Eye for Detail, Target Tracker, Card Shark, Juggle Factor, Recognition, and Face Facts. See Figure S1 from the supplementary material for details. In both trainings, participants were driven to make progressively more accurate distinctions about the spectrotemporal fine structure of auditory or visual stimuli under conditions of increasing attention, memory and difficulty load (Fisher et al., 2009). Task difficulty increased systematically as performance improved and an algorithm was used to maintain individual rates of success in the exercises at around 80%. Correct performance was rewarded through entertaining visual and auditory embellishments and the accumulation of stars. To monitor the magnitude of progress in the training exercises, we evaluated subjects improvement in performance for each individual exercise using T-scores.
2.5. Planned analyses
The main outcome, i.e. the differential effect of the auditory and visual trainings on global cognition in schizophrenia participants, was analyzed using Intent-To-Treat (ITT) Repeated-Measures Analysis of Variance (RM-ANOVA) models, with last-observation-carried forward. This type of analysis allows incorporating data from the 79 participants, even if not all of them have finished the entire protocol study. Time (baseline, 20 hours, and 40 hours) was the within-subject factor and training type was the between-subject factor. Secondary outcomes, which included the individual cognitive domains, clinical measurements and quality of life, were also analyzed using RM-ANOVA. Normality and homogeneity of data distributions were tested using the Shapiro–Wilk and Levine tests. We calculated participants’ neuropsychological tests z-scores using the formula (x – μ)/σ where x is the measure of interest, μ the mean and σ the standard deviation of the normative data of healthy participants available in the CANTAB and MCCB repository. Z-score change - or delta z-score - was also used where we wanted to show the difference between the final and initial z-scores (Delta z-score = Z-score after 40 hours of training – z-score at baseline). T-tests, Chi squared test and Mann-Whitney U test were used to assess differences in socio-demographic and cognitive data at baseline. Post hoc correlations were applied for statistically significant results from the main and secondary outcome measures’ change scores (post 40 hours minus baseline). We applied Bonferroni corrections for data with multiple dependent variables. Data were analyzed using SPSS.
3. RESULTS
3.1. Participants
Two hundred and two subjects were screened and 123 (61%) were excluded because of ineligibility or lack of interest in the study. Thus, 79 schizophrenia subjects performed the first assessment and were randomized to either training program: 40 to the auditory training and 39 to the visual training. Fifty-three participants finished the training (Figure S2). Participants were predominantly male, had an average of 42 years of age, 12 years of education and were chronically ill (all except two were taking antipsychotics over the course of the study). There were no significant socio-demographic and clinical differences between the two randomized groups (Table 1). Sixty-four participants trained in the laboratory and 15 participants trained at home, assessing the exercises on the internet. The training site did not affect the cognitive or the clinical outcomes when used as a covariate. The magnitudes of progress in the cognitive training exercises were similar between the auditory and the visual training groups.
Table 1.
Baseline demographic and clinical information
| All participants within inclusion criteria (n=79) | Visual training group (n=39) | Auditory training group (n=40) | Differences between visual and auditory training groups (p-value) | |
|---|---|---|---|---|
| Gender (men/women) | 55/24 | 29/10 | 26/14 | 0.47 |
| Age (mean (SD‡)) | 40 (12) | 39 (12) | 40 (12) | 0.68 |
| IQ (mean (SD)) | 101 (13) | 100 (14) | 100 (13) | 0.96 |
| Education (mean (SD)) | 12 (3) | 12 (3) | 12 (3) | 0.69 |
| Diagnostic (schizophrenia/schizoaffective) | 72/7 | 36/3 | 36/4 | 0.69 |
| Years of illness (mean (SD)) | 18 (11) | 18 (11) | 17 (10) | 0.99 |
| PANSS† score (mean (SD)) | 59 (14) | 59 (16) | 59 (12) | 0.85 |
| Antipsychotics generation (1st/2nd /multiple/none) | 28/43/6/2 | 14/19/5/1 | 14/24/1/1 | 0.64 |
| Anticholinergic/benzodiazepine/ mood stabilizer/antidepressant | 44/38/22/15 | 20/19/14/9 | 24/19/8/6 | 0.44 |
| Chlorpromazine equivalent (mean (SD)) | 508 (417) | 549 (371) | 454 (429) | 0.34 |
| Anticholinergic equivalent (mean (SD)) | 3 (5) | 6 (4) | 5 (5) | 0.54 |
| Global progress in the exercises (mean t-scores (SD)) | 0.78 (0.70) | 0.72 (0.59) | 0.84 (0.85) | 0.79 |
PANSS : Positive and Negative Syndrome Scale
SD: Standard deviation
3.2. Cognitive Outcomes
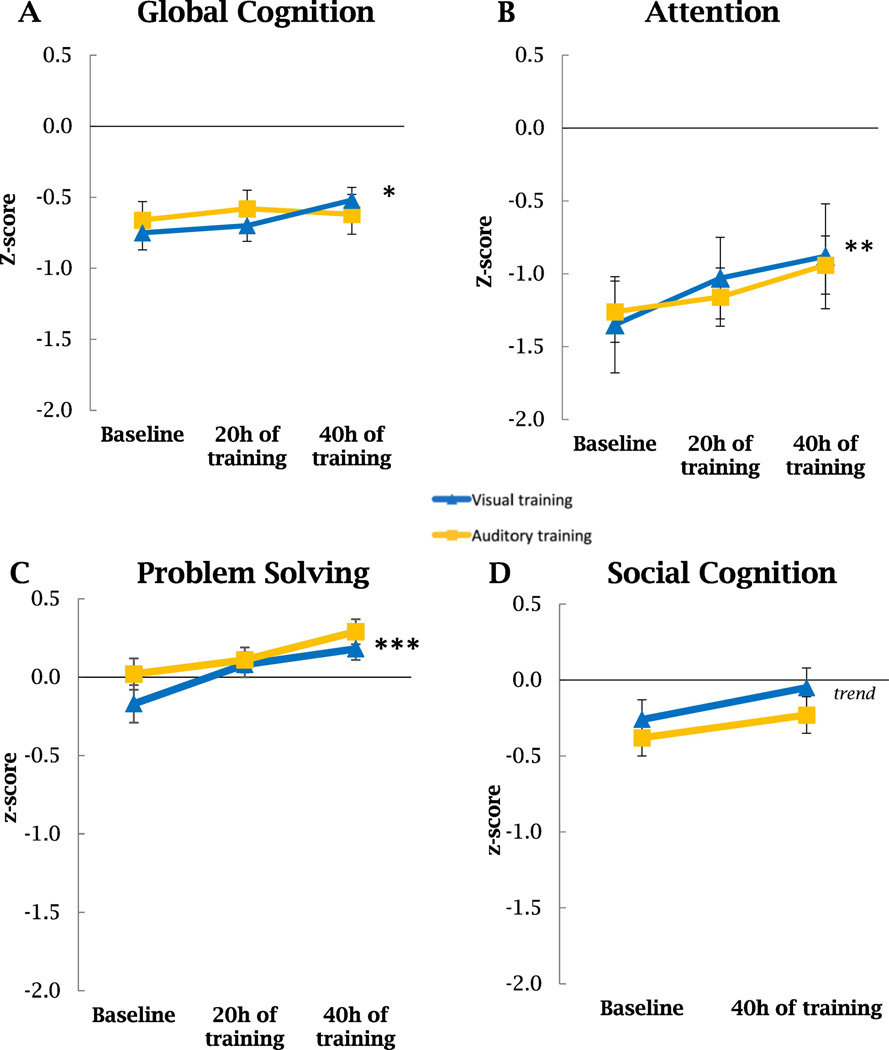
There were no statistically significant differences in baseline cognition between the auditory training and the visual training groups. The Intent-To-Treat analysis on global cognition showed a significant group by time interaction: when compared to the auditory training group, participants who received the visual training showed significant improvements in global cognition from baseline to after 40 hours of training (F(77,2)=3.35, p=0.04, d=0.42; Table 2). Furthermore, there was a significant main effect of time on global cognition (F(77,2)=4.66, p=0.01, d=0.49). Post hoc one-way RM-ANOVA for the two groups separately revealed significant improvement over time in global cognition in the visual training (F(37,2)=6.02, p=0.004, d=0.80), but not in the auditory training group (Figure 1A and Table 2).
Table 2.
Effect of visual and auditory cognitive training on global cognition and the seven cognitive domains defined by MATRICS in schizophrenia subjects.
| VISUAL TRAINING (n=39) | AUDITORY TRAINING (n=40) | ANOVA COMPARING THE 2 GROUPS | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||||||
| Baseline (mean (SD)) | 20 hours (mean (SD)) | 40 Hours (mean (SD)) | F ANOVA (time) | P-Value | Effect size (Cohen’s d*) | Baseline (mean (SD)) | 20 hours (mean (SD)) | 40 Hours (mean (SD)) | F ANOVA (time) | P-Value | Effect size (Cohen’s d*) | F ANOVA (time) | P-Value | Effect size (Cohen’s d*) | F ANOVA (group) | P-Value | Effect size (Cohen’s d*) | F ANOVA (time x group) | P-Value | Effect size (Cohen’s d*) | |
| Global cognition | −0.75(0.75) | −0.70(0.69) | −0.52(0.56) | 6.02 | 0.004 | 0.80 | −0.66(0.83) | −0.58(0.84) | −0.62(0.86) | 0.82 | 0.44 | 0.29 | 4.66 | 0.01 | 0.49 | 0.06 | 0.82 | 0.06 | 3.35 | 0.04 | 0.42 |
|
|
|
|
|||||||||||||||||||
| Speed of processing | −1.31(2.18) | −1.36(1.65) | −1.18(1.67) | 0.34 | 0.72 | 0.20 | −1.19(2.06) | −1.21(1.74) | −1.53(2.25) | 0.34 | 0.71 | 0.20 | 0.03 | 0.97 | 0.04 | 0.01 | 0.94 | 0.02 | 0.66 | 0.52 | 0.20 |
| Attention | −1.35(1.44) | −1.03(1.21) | −0.88(1.20) | 3.97 | 0.02 | 0.70 | −1.26(1.31) | −1.16(1.21) | −0.94(1.21) | 2.96 | 0.06 | 0.63 | 6.80 | 0.002 | 0.65 | 0.01 | 0.93 | 0.02 | 0.21 | 0.81 | 0.11 |
| Working memory | −0.30(0.87) | −0.33(0.99) | −0.24(0.93) | 1.63 | 0.20 | 0.46 | −0.12(1.19) | −0.05(1.07) | −0.24(0.96) | 1.28 | 0.29 | 0.41 | 0.04 | 0.97 | 0.06 | 0.34 | 0.56 | 0.04 | 2.87 | 0.06 | 0.41 |
| Verbal memory & learning | −0.21(1.16) | −0.22(1.08) | −0.21(0.94) | 0.25 | 0.78 | 0.20 | −0.05(1.05) | −0.08(1.15) | −0.06(1.12) | 0.43 | 0.66 | 0.20 | 0.05 | 0.95 | 0.41 | 0.57 | 0.46 | 0.19 | 0.60 | 0.55 | 0.20 |
| Visual memory & learning | −1.70(1.49) | −1.47(1.53) | −1.25(1.32) | 2.89 | 0.06 | 0.59 | −1.32(1.47) | −0.99(1.34) | −1.30(1.78) | 1.14 | 0.33 | 0.41 | 2.44 | 0.09 | 0.41 | 0.96 | 0.33 | 0.29 | 1.41 | 0.25 | 0.29 |
| Reasoning & problem solving | −0.17(0.73) | 0.08(0.46) | 0.18(0.44) | 5.75 | 0.005 | 0.84 | 0.02(0.58) | 0.11(0.50) | 0.29(0.47) | 12.74 | 0.00002 | 1.28 | 15.11 | 1.10−6 | 0.97 | 2.49 | 0.12 | 0.41 | 0.66 | 0.52 | 0.20 |
| Social cognition | −0.26(0.64) | N/A | −0.05(0.71) | 0.62 | 0.44 | 0.29 | −0.38(0.73) | N/A | −0.23(0.71) | 3.72 | 0.06 | 0.67 | 3.54 | 0.07 | 0.51 | 0.08 | 0.78 | 0.06 | 0.64 | 0.43 | 0.20 |
Means represent z-scores. Bold represents statistically significant results (p < 0.05). Italic represents trends for statistical significance (0.05 < p < 0.1).
Cohen’s d converted from Eta2.
Figure 1. Cognitive Performance with the Auditory and Visual training.
A. Global cognition scores were significantly higher for patients who performed the visual training compared to the auditory training (p=0.04). B. Attention scores increased significantly over time for patients who performed the visual training and at trend levels for the auditory training (p=0.02 and p=0.06 respectively). C. Reasoning and problem-solving scores increased significantly over time for patients who performed both types of trainings (p=0.005 and p=0.00002 respectively). D. Social cognition scores increased at the limit of significance over time for patients who performed the auditory training (p=0.06). Error bars represent SEM. * p < 0.05, ** p < 0.01, *** p < 0.001.
The analysis of individual cognitive domains, controlling for multiple comparisons, revealed a main effect of time for attention (F(72,2)=6.80, p=0.002, d=0.65), and reasoning and problem-solving (F(73,2)=15.11, p=0.000001, d=0.97), with no significant group by time interactions (Figure 1B, 1C and Table 2). Post hoc one-way RM-ANOVA analysis, also controlling for multiple comparisons, revealed a significant effect of time on reasoning and problem-solving in the visual and auditory training groups (F(37,2)=5.75, p=0.005, d=0.84 and F(38,2)=12.74, p=0.00002, d=1.28 respectively), and on attention in the visual training group (F(37,2)=3.97, p=0.02, d=0.70). These results showed medium to large effects in the direction of an improvement of the cognitive domains evaluated (Figure 1 and Table 2).
Additionally, we performed an exploratory analysis of individual cognitive tests separated by the sensory modality used in the neuropsychological assessment: visual or auditory (Tables S1 and S2, respectively). The visual training group improved performance over time in two visual tests: the Stockings of Cambridge (that assessed reasoning and problem-solving), and the Rapid Visual Processing (that assessed attention) (Table S1). On the other hand, the auditory training group improved performance in the visual Stockings of Cambridge test and in the auditory Digit Backward test (that assessed working memory) (Table S1 and S2).
3.3. Symptoms and Quality of Life
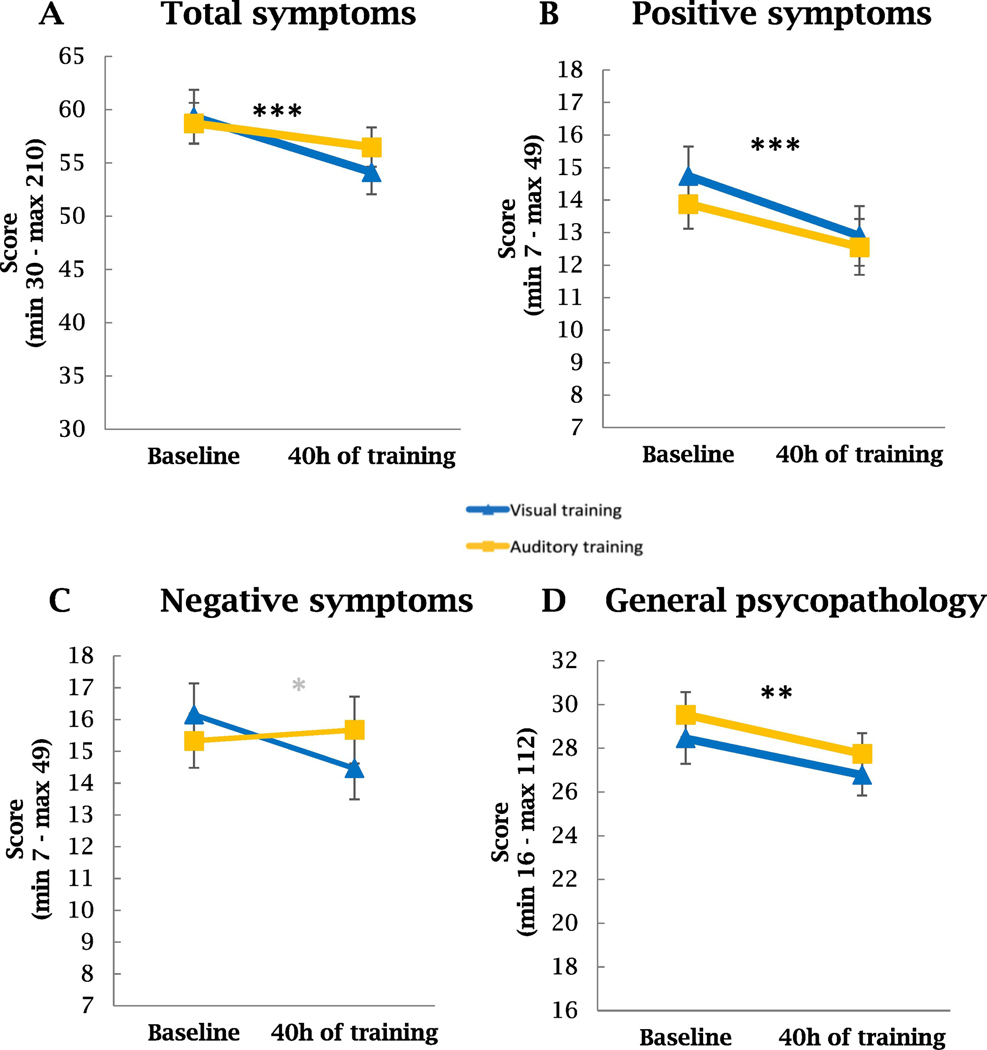
The RM-ANOVA showed an interaction of time by type of training for PANSS negative symptoms scores (F(75,1)=3.90, p=0.05, d=0.45; Table S3 and Figure 2C). Overall participants had a significant decrease of PANSS total score with time (F(75,1)=14.39, p=0.0003, d=0.87), and in particular in positive and general psychopathology symptoms (F(75,1)=18.92, p<0.0001, d=1 and F(75,1)=8.07, p=0.006, d=0.65 respectively), which reflected improvement in symptomatology (Table S3 and Figure 2A–D).
Figure 2. PANSS symptoms evolution with 40 hours of auditory or visual cognitive training in schizophrenia subjects.
A. The overall effect of time was significant for PANSS total symptoms. Post hoc analyses showed that the visual training group (but not the auditory group) improved significantly over time for PANSS total symptoms (p<0.001). B. The overall effect of time was significant for PANSS positive symptoms. Post hoc analyses showed that the visual training group (but not the auditory group) improved significantly over time for PANSS positive symptoms (p<0.001). C. The interaction between time and type of training was significant for PANSS negative symptoms (p<0.05). D. The overall effect of time was significant for PANSS general psychopathology. Post hoc analyses showed that the visual training group (but not the auditory group) improved significantly over time for general psychopathology (p<0.01).
Next, we performed a post hoc one-way RM-ANOVA of PANSS symptoms scores for the two groups separately, controlling for multiple comparisons. The visual training showed statistically significant decreases over time for total (F(37,1)=12.91, p=0.001, d=1.17), positive (F(37,1)=13.38, p=0.001, d=1.19), negative (F(37,1)=4.19, p=0.05, d=0.66), and general psychopathology (F(37,1)=7.43, p=0.01, d=0.89) symptoms, whereas the auditory training showed significant decrease in positive symptoms (F(38,1)=6.4, p=0.02, d=0.82) only (Table S3 and Figure 2A, 2B, and 2D).
We found no significant effect of time, type of training or interaction of time by type of training in the quality of life (Table S3).
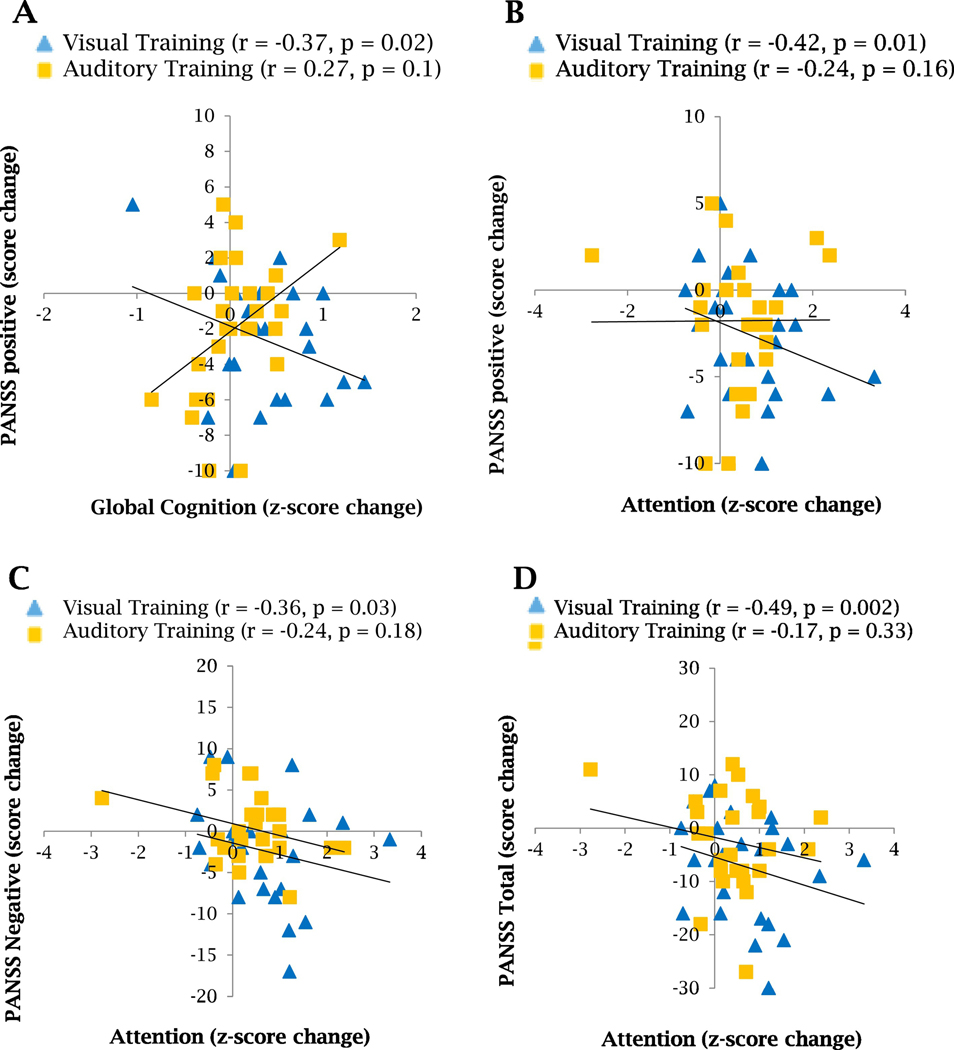
3.4. Association Between Changes in Cognition and in Clinical Symptoms
We used Pearson correlations to test the association between the post-intervention changes in global cognition, attention, and reasoning and problem-solving, and changes in PANSS symptoms scores. Interestingly, we found a statistically significant association between the improvement in global cognition and the decrease in positive symptoms (r=−0.37, p=0.02) in the schizophrenia subjects who performed 40 hours of the visual training (Figure 3A). We also found in the visual training group significant associations between the improvements in attention and the decreases in positive, negative and total symptoms (r=−0.42, p=0.01; r=−0.36, p=0.03, and r=−0.49, p=0.002 respectively, Figure 3B–D). We did not observe these associations in the auditory training group.
Figure 3. Association between PANSS symptoms and cognitive changes after 40 hours of auditory or visual cognitive training in schizophrenia subjects.
A. Pearson correlation shows a significant association between global cognition and PANSS positive symptoms improvements for subjects who performed the visual training. B. Pearson correlation shows a significant association between attention and PANSS positive symptoms improvements for subjects who performed the visual training. C. Pearson correlation shows a significant association between attention and PANSS negative symptoms improvements for subjects who performed the visual training. D. Pearson correlation shows a significant association between attention and PANSS total symptoms improvements for subjects who performed the visual training.
4. DISCUSSION
This study provides evidence that visual and auditory exercises of neuroscience-informed cognitive trainings are differentially effective at improving global cognition in schizophrenia subjects. On one hand, the visual training group notably improved in global cognition, with specific improvement observed in both low-order (attention) and high-order (reasoning and problem-solving) cognitive processes. On the other hand, the auditory training group improved over time in reasoning and problem-solving. However, only the training of visual processes was able to significantly improve global cognition.
The higher efficiency of the visual training may be explained by the predominance of the visual over the auditory processing system. Vision plays a predominant role over audition, and we are generally more prone to attend to visual stimuli over auditory stimuli (Godfroy-Cooper et al., 2015). The Mc Gurk effect is a great demonstration that the visual system takes over the auditory system, when conflicting information is observed between the two perceptual modalities (McGurk and Macdonald, 1976). Our results also suggested that the perceptive modality of the tests may be sensitive to the perceptive modality of the training. Indeed, both the visual and auditory trainings improved visual tests whereas only the auditory training improved auditory tests.
In a previous study, 50 hours of auditory exercises of neuroscience-informed cognitive training improved verbal learning and memory and cognitive control in schizophrenia subjects that lasted 6 months beyond the intervention (Fisher et al., 2010). The addition of 50 more hours of visual and cognitive control training drove enduring gains also in speed of processing and global cognition (with a z-score change of 0.4). These findings emphasize that impairments in visual processes are important targets for improving global cognition in schizophrenia.
Yet, the moderate effect of the auditory training was surprising, given that previous studies using similar auditory training exercises had found significant improvement in several cognitive domains (Fisher et al., 2015, 2009). These studies have shown that cognition is more impaired in the chronic phases than the early phases of schizophrenia, and that auditory training is more efficient in the chronic phases (Fisher et al., 2016) versus the early phases of schizophrenia (Fisher et al., 2015) or high risk for psychosis (Loewy et al., 2016). In our study, chronic schizophrenia participants in the auditory training group showed, at baseline, average performance in 4 cognitive domains, and low average performance in 3 domains, reflecting more intact cognition, and improvement in reasoning and problem solving only. This pattern of results indicates that less impairment at baseline leads to improvement in fewer cognitive domains after auditory training.
Feasibility of implementing the cognitive training program in schizophrenia subjects in different sites was verified in a multi-site, multinational study (Murthy et al., 2012). The results on cognitive outcomes were also mixed, and could be the result of the studied population, including the level of cognitive deficits, and differences in the measures used.
We found a significant decrease in symptoms of schizophrenia in both training groups. Interestingly, the visual training group showed significant improvements over time in a broad range of symptoms, which correlated significantly with improvements in attention. The literature has shown that impairments in attention are associated with both negative and positive symptoms of schizophrenia (Berman et al., 1997; Mazza et al., 2013; Sanz et al., 2012) and the correlation found in our study suggests a pathway between the improvements in attention and the decrease in the clinical symptoms of schizophrenia. This hypothesis is supported by data from a meta-analysis that found that cognitive remediation is often related to small to moderate beneficial effects in symptoms (Cella et al., 2017). Therefore, we could speculate that even small changes in cognition, and more specifically in basic cognition (i.e. attention), can make a significant difference in patients’ symptoms.
The sample size may have limited our study results, as we had a high attrition rate, with several gains in cognition very close to significance. One could also argue that the improvements we observed in cognition may be due to a test re-test effect. However, we evaluated a pilot group of schizophrenia subjects at baseline with the same battery of tests twice (one week apart), and there were no significant differences between the two sessions, demonstrating that a practice effect was improbable (unpublished data). A study by Pietrzak and colleagues also shows stable cognitive impairments in schizophrenia subjects when assessed within weeks or months (Pietrzak et al., 2009). Further, whenever possible, we used parallel versions of the same tests, and we avoided tests known to have high practice effects. Also, our study was designed such that the visual training and the auditory training would be the control of one another, thus it did not include an independent control group such as treatment as usual. However, we acknowledge that it would have been beneficial to include such a group to assess the specificity of the gains in symptomatology.
This study shows for the first time that a neuroscience-informed computerized cognitive training can be applied effectively in the Brazilian population. We conclude that the visual training and the auditory training are differentially efficient at remediating cognitive deficits and symptoms of clinically stable schizophrenia subjects. A follow-up after twelve months of training is currently in progress to evaluate the long-term effects of the training in cognition and symptoms. Should the effects persist, we would contemplate implementing this type of intervention for patients with schizophrenia in our clinical facilities.
Supplementary Material
The exercises were specifically designed to train the targeted cognitive domains from a visual and auditory perceptual modality approach.
Participants went through an initial screening to establish whether inclusion and exclusion criteria were applied. Seventy-nine of these participants were randomized to either the visual cognitive training or the auditory cognitive training. Fifty-three participants finished the training.
Acknowledgements
The authors gratefully acknowledge participants and their families; and thank Ana Claudia Rangel and Andrea Fantinatti for the excellent technical support.
Role of the funding source
This work was supported by the National Institutes of Health – Fogarty International Center (Grant R03TW009002 to SV and RP); Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ) (Grant E-26/110.305/2014 to RP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant 400455/2012–9 to RP). RP is an Atlantic Fellow of the Global Brain Health Institute. LG, LM, SK, AG, PR, FT and CN were supported by fellowships from FAPERJ and CNPq. The cognitive training software used in this study and all technical support were provided to us free of charge by Posit Science, Inc.
Footnotes
Conflict of Interest
Dr. Panizzutti is the founder of NeuroForma LTDA, a company with a financial interest in cognitive training. The remaining authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S, 2009. When top-down meets bottom-up: Auditory training enhances verbal memory in schizophrenia. Schizophr. Bull. 10.1093/schbul/sbp068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott W, Sali L, Copland D, 2011. Impaired reading comprehension in schizophrenia: Evidence for underlying phonological processing deficits. Psychiatry Res. 10.1016/j.psychres.2010.11.025 [DOI] [PubMed] [Google Scholar]
- Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI, 1997. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr. Res. 10.1016/S0920-9964(96)00098-9 [DOI] [PubMed] [Google Scholar]
- Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S, 2016. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology. 10.1037/neu0000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Kunz M, Chakos M, Cooper TB, Lieberman JA, 2002. Neurocognitive correlates of the COMT Val158Met polymorphism in chronic schizophrenia. Biol. Psychiatry 52, 701–707. 10.1016/S0006-3223(02)01416-6 [DOI] [PubMed] [Google Scholar]
- Bowie CR, Bell MD, Fiszdon JM, Johannesen JK, Lindenmayer JP, McGurk SR, Medalia AA, Penadés R, Saperstein AM, Twamley EW, Ueland T, Wykes T, 2020. Cognitive remediation for schizophrenia: An expert working group white paper on core techniques. Schizophr. Res. 10.1016/j.schres.2019.10.047 [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC, 2008. Visual Perception and Its Impairment in Schizophrenia. Biol. Psychiatry. 10.1016/j.biopsych.2008.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Preti A, Edwards C, Dow T, Wykes T, 2017. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clin. Psychol. Rev. 10.1016/j.cpr.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro PB, Barbadillo L, Pelayo-Terán JM, Rodríguez-Sánchez JM, Crespo-Facorro B, Barbadillo L, Pelayo-Terán JM, Rodríguez-Sánchez JM, 2007. Neuropsychological functioning and brain structure in schizophrenia. Int. Rev. Psychiatry 19, 325–336. 10.1080/09540260701486647 [DOI] [PubMed] [Google Scholar]
- Dale CL, Findlay AM, Adcock RA, Vertinski M, Fisher M, Genevsky A, Aldebot S, Subramaniam K, Luks TL, Simpson GV, Nagarajan SS, Vinogradov S, 2010. Timing is everything: Neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int. J. Psychophysiol. 10.1016/j.ijpsycho.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S, 2009. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatry 166, 805–11. 10.1176/appi.ajp.2009.08050757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S, 2010. Neuroplasticity-based cognitive training in schizophrenia: An interim report on the effects 6 months later. Schizophr. Bull. 36, 869–879. 10.1093/schbul/sbn170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, Schlosser D, Pham L, Miskovich T, Vinogradov S, 2015. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr. Bull. 41, 250–258. 10.1093/schbul/sbt232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Mellon SH, Wolkowitz O, Vinogradov S, 2016. Neuroscience-informed auditory training in schizophrenia: A final report of the effects on cognition and serum brain-derived neurotrophic factor. Schizophr. Res. Cogn. 3, 1–7. 10.1016/j.scog.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfroy-Cooper M, Sandor PMB, Miller JD, Welch RB, 2015. The interaction of vision and audition in two-dimensional space. Front. Neurosci. 10.3389/fnins.2015.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, 2006. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J. Clin. Psychiatry 67, 3–8. 10.4088/JCP.1006e12 [DOI] [PubMed] [Google Scholar]
- Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TR, Joyce EM, 1998. Executive function in first-episode schizophrenia. Psychol. Med. 28, 463–73. 10.1016/S0920-9964(97)88425-3 [DOI] [PubMed] [Google Scholar]
- Javitt DC, 2009. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 5, 249–75. 10.1146/annurev.clinpsy.032408.153502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG, 1993. Impairment of early cortical processing in schizophrenia: An event-related potential confirmation study. Biol. Psychiatry. 10.1016/0006-3223(93)90005-X [DOI] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N, 1997. Impaired precision, but normal retention, of auditory sensory (‘echoic’) memory information in schizophrenia. J. Abnorm. Psychol. 10.1037/0021-843X.106.2.315 [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Hoptman MJ, Leitman DI, Moreno-Ortega M, Lehrfeld JM, Dias E, Sehatpour P, Laukka P, Silipo G, Javitt DC, 2015. Neural substrates of auditory emotion recognition deficits in schizophrenia. J. Neurosci. 10.1523/JNEUROSCI.4603-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, O L, 1987. The Positive and Negative Syndrome Scale for schizophrenia. Schizophr Bull. 13, 261–276. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Knapp M, Kanavos P, King D, Yesudian HM, 2005. Economic issues in access to medications: Schizophrenia treatment in England. Int. J. Law Psychiatry. 10.1016/j.ijlp.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Koro CE, Fedder DO, L’Italien GJ, Weiss S, Magder LS, Kreyenbuhl J, Revicki D, Buchanan RW, 2002. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch. Gen. Psychiatry. 10.1001/archpsyc.59.11.1011 [DOI] [Google Scholar]
- Loewy R, Fisher M, Schlosser DA, Biagianti B, Stuart B, Mathalon DH, Vinogradov S, 2016. Intensive Auditory Cognitive Training Improves Verbal Memory in Adolescents and Young Adults at Clinical High Risk for Psychosis. Schizophr. Bull. 10.1093/schbul/sbw009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March L, Cienfuegos A, Goldbloom L, Ritter W, Cowan N, Javitt DC, 1999. Normal time course of auditory recognition in schizophrenia, despite impaired precision of the auditory sensory (‘echoic’) memory code. J. Abnorm. Psychol. 10.1037/0021-843X.108.1.69 [DOI] [PubMed] [Google Scholar]
- Martínez A, Gaspar P. a, Hillyard S. a, Bickel S, Lakatos P, Dias EC, Javitt DC, 2015. Neural oscillatory deficits in schizophrenia predict behavioral and neurocognitive impairments. Front. Hum. Neurosci. 9, 371. 10.3389/fnhum.2015.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massardo L, Bravo-Zehnder M, Calderón J, Flores P, Padilla O, Aguirre JM, Scoriels L, González a, 2015. Anti-N-methyl-D-aspartate receptor and anti-ribosomal-P autoantibodies contribute to cognitive dysfunction in systemic lupus erythematosus. Lupus 24, 558–68. [DOI] [PubMed] [Google Scholar]
- Mazza M, Tripaldi S, Pino MC, Stama R, Valchera A, Serroni N, De Berardis D, 2013. Assessment of attention network efficiency in schizophrenic patients with positive and negative symptoms. Riv. Psichiatr. 10.1708/1292.14293 [DOI] [PubMed] [Google Scholar]
- McGurk H, Macdonald J, 1976. Hearing lips and seeing voices. Nature 264, 691–811. 10.1038/264746a0 [DOI] [PubMed] [Google Scholar]
- McGurk SR, Meltzer HY, 2000. The role of cognition in vocational functioning in schizophrenia. Schizophr. Res. 45, 175–184. 10.1016/S0920-9964(99)00198-X [DOI] [PubMed] [Google Scholar]
- Morrison RL, Bellack AS, Mueser KT, 1988. Deficits in facial-affect recognition and schizophrenia. Schizophr. Bull. 10.1093/schbul/14.1.67 [DOI] [PubMed] [Google Scholar]
- Murthy NV, Mahncke H, Wexler BE, Maruff P, Inamdar A, Zucchetto M, Lund J, Shabbir S, Shergill S, Keshavan M, Kapur S, Laruelle M, Alexander R, 2012. Computerized cognitive remediation training for schizophrenia: An open label, multi-site, multinational methodology study. Schizophr. Res. 139, 87–91. 10.1016/j.schres.2012.01.042 [DOI] [PubMed] [Google Scholar]
- Norton DJ, McBain RK, Öngür D, Chen Y, 2011. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 77, 248–256. 10.1016/j.bandc.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW, 1996. Selective deficits in visual perception and recognition in schizophrenia. Am. J. Psychiatry. 10.1176/ajp.153.5.687 [DOI] [PubMed] [Google Scholar]
- Panizzutti R, Fisher M, Holland C, Vinogradov S, 2009. Increased serum levels of the NMDA receptor co-agonist d-serine correlate with cognitive gains induced by neuroplasticity-based auditory training in schizophrenia. Biol Psychiatry. [Google Scholar]
- Panizzutti R, Hamilton SP, Vinogradov S, 2013. Genetic correlate of cognitive training response in schizophrenia. Neuropharmacology 64, 264–267. 10.1016/j.neuropharm.2012.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Snyder PJ, Jackson CE, Olver J, Norman T, Piskulic D, Maruff P, 2009. Stability of cognitive impairment in chronic schizophrenia over brief and intermediate re-test intervals. Hum. Psychopharmacol. 24, 113–121. 10.1002/hup.998 [DOI] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA, 2011. Specific cognitive training normalizes auditory sensory gating in schizophrenia: A randomized trial. Biol. Psychiatry 69, 465–471. 10.1016/j.biopsych.2010.09.028 [DOI] [PubMed] [Google Scholar]
- Popov TG, Carolus A, Schubring D, Popova P, Miller GA, Rockstroh BS, 2015. Targeted training modifies oscillatory brain activity in schizophrenia patients. NeuroImage Clin. 7, 807–814. 10.1016/j.nicl.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC, 2006. Reading impairment and visual processing deficits in schizophrenia. Schizophr. Res. 10.1016/j.schres.2006.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner MS, 2007. Predicting quality of life impairment in chronic schizophrenia from cognitive variables. Qual. Life Res. 16, 929–937. 10.1007/s11136-007-9195-3 [DOI] [PubMed] [Google Scholar]
- Salomon J. a, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, Farje MR, Moncada G, Dutta A, Sazawal S, Dyer A, Seiler J, Aboyans V, Baker L, Baxter A, Benjamin EJ, Bhalla K, Bin Abdulhak A, Blyth F, Bourne R, Braithwaite T, Brooks P, Brugha TS, Bryan-Hancock C, Buchbinder R, Burney P, Calabria B, Chen H, Chugh SS, Cooley R, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, Davis A, Degenhardt L, Díaz-Torné C, Dorsey ER, Driscoll T, Edmond K, Elbaz A, Ezzati M, Feigin V, Ferri CP, Flaxman AD, Flood L, Fransen M, Fuse K, Gabbe BJ, Gillum RF, Haagsma J, Harrison JE, Havmoeller R, Hay RJ, Hel-Baqui A, Hoek HW, Hoffman H, Hogeland E, Hoy D, Jarvis D, Karthikeyan G, Knowlton LM, Lathlean T, Leasher JL, Lim SS, Lipshultz SE, Lopez AD, Lozano R, Lyons R, Malekzadeh R, Marcenes W, March L, Margolis DJ, McGill N, McGrath J, Mensah G. a, Meyer A-C, Michaud C, Moran A, Mori R, Murdoch ME, Naldi L, Newton CR, Norman R, Omer SB, Osborne R, Pearce N, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Pourmalek F, Prince M, Rehm JT, Remuzzi G, Richardson K, Room R, Saha S, Sampson U, Sanchez-Riera L, Segui-Gomez M, Shahraz S, Shibuya K, Singh D, Sliwa K, Smith E, Soerjomataram I, Steiner T, Stolk W. a, Stovner LJ, Sudfeld C, Taylor HR, Tleyjeh IM, van der Werf MJ, Watson WL, Weatherall DJ, Weintraub R, Weisskopf MG, Whiteford H, Wilkinson JD, Woolf AD, Zheng Z-J, Murray CJL, Jonas JB, 2012. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 380, 2129–43. 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JC, Gómez V, Vargas ML, Marín JJ, 2012. Dimensions of attention impairment and negative symptoms in schizophrenia: A multidimensional approach using the conners continuous performance test in a spanish population. Cogn. Behav. Neurol. 10.1097/WNN.0b013e318255feaf [DOI] [PubMed] [Google Scholar]
- Scoriels L, Barnett JH, Soma PK, Sahakian BJ, Jones PB, 2012. Effects of modafinil on cognitive functions in first episode psychosis. Psychopharmacology (Berl). 220, 249–258. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10, in: Journal of Clinical Psychiatry. pp. 22–33. 10.1016/S0924-9338(99)80239-9 [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW, 1970. A rating scale for extrapyramidal side effects. Acta Psychiatr. Scand. Suppl. 212, 11–19. 10.1111/j.1600-0447.1970.tb02066.x [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S, 2012. Computerized Cognitive Training Restores Neural Activity within the Reality Monitoring Network in Schizophrenia. Neuron 73, 842–853. 10.1016/j.neuron.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Garrett C, Chung C, Fisher M, Nagarajan S, Vinogradov S, 2014. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage. 10.1016/j.neuroimage.2014.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surti TS, Corbera S, Bell MD, Wexler BE, 2011. Successful computer-based visual training specifically predicts visual memory enhancement over verbal memory improvement in schizophrenia. Schizophr. Res. 132, 131–134. 10.1016/j.schres.2011.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoke A, Trandafir A, Dupont M-E, Meary A, Schurhoff F, Leboyer M, 2008. Longitudinal studies of cognition in schizophrenia: meta-analysis. Br. J. Psychiatry 192, 248–257. 10.1192/bjp.bp.106.029009 [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW, 2002. Visual perceptual and working memory impairments in schizophrenia. Arch. Gen. Psychiatry. 10.1001/archpsyc.59.2.146 [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH, 2009. Is Serum Brain-Derived Neurotrophic Factor a Biomarker for Cognitive Enhancement in Schizophrenia? Biol. Psychiatry. 10.1016/j.biopsych.2009.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 2008. Wechsler adult intelligence scale - Fourth Edition (WAIS-IV). San Antonio 1–3. [Google Scholar]
- WHO, 1998. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med 28, 551–558. https://doi.org/10.5.12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The exercises were specifically designed to train the targeted cognitive domains from a visual and auditory perceptual modality approach.
Participants went through an initial screening to establish whether inclusion and exclusion criteria were applied. Seventy-nine of these participants were randomized to either the visual cognitive training or the auditory cognitive training. Fifty-three participants finished the training.