Abstract
Background
This study aimed to investigate the effects of maternal exposure to external radiation on perinatal outcomes among women who experienced the Fukushima Daiichi Nuclear Disaster (FDND) using the Fukushima Health Management Survey (FHMS).
Methods
Data from the Pregnancy and Birth Survey and Basic Survey in the FHMS were combined to analyze external maternal radiation exposure following the FDND, and the relationship between radiation dose and perinatal outcomes was analyzed using binomial logistic regression analysis. Missing dose data were supplemented using multiple imputation.
Results
A total of 6,875 individuals responded to the survey. Congenital anomalies occurred in 2.9% of patients, low birth weight (LBW) in 7.6%, small for gestation age (SGA; <10th percentile) in 8.9%, and preterm birth in 4.1%. The median maternal external radiation dose was 0.5 mSv (maximum, 5.2 mSv). Doses were classified as follows: <1 mSv (reference), 1 to <2 mSv, and ≥2 mSv. For congenital anomalies, the crude odds ratio for 1 to <2 mSv was 0.81 (95% confidence interval [CI], 0.56–1.17) (no participants with congenital anomaly were exposed to ≥2 mSv). At 1 to <2 mSv and ≥2 mSv, the respective adjusted odds ratios were 0.91 (95% CI, 0.71–1.18) and 1.21 (95% CI, 0.53–2.79) for LBW, 1.14 (95% CI, 0.92–1.42) and 0.84 (95% CI, 0.30–2.37) for SGA, and 0.91 (95% CI, 0.65–1.29) and 1.05 (95% CI, 0.22–4.87) for preterm birth.
Conclusion
External radiation dose due to the FDND was not associated with congenital anomalies, LBW, SGA, or preterm birth.
Key words: nuclear accident, radiation dose, maternal exposure, pregnancy, perinatal outcomes
INTRODUCTION
The tsunami caused by the Great East Japan Earthquake of March 11, 2011 directly hit the Fukushima Daiichi Nuclear Power Plant, causing the Fukushima Daiichi Nuclear Disaster (FDND), which resulted in fear of fetal radiation exposure and subsequent evacuation, forcing pregnant women to change medical institutions for prenatal check-ups and resulting in considerable physical and mental stress.1
Fukushima Prefecture launched the Fukushima Health Management Survey (FHMS) in July 2011 to monitor the future health of prefectural residents by assessing the direct damage caused by the disaster and the FDND radiation effects.2,3
FHMS comprises a detailed and Basic Survey (BS). The BS covered all residents of Fukushima Prefecture between March 11 and July 1, 2011 and estimated the external radiation doses in the 4 months following FDND, obtaining baseline data for health monitoring and protection. The Pregnancy and Birth Survey (PBS), a questionnaire-based survey, was commissioned by Fukushima Prefecture and conducted by Fukushima Medical University to assess the health status of pregnant and nursing mothers in Fukushima Prefecture who may have been forced to evacuate or change medical facilities.4
The PBS provides data on perinatal outcomes, including preterm birth, low birth weight (LBW), small for gestational age (SGA), congenital anomalies,5–9 postpartum depression,10,11 hypertensive disorders of pregnancy (HDP),12 and the impact of the earthquake on newborns.13 Rates of stillbirth, preterm birth, LBW, and congenital anomalies did not deviate from the Japanese standard frequency,1,5 and SGA incidence was not elevated by the earthquake or its aftermath.8 Depressive symptoms were observed in some new mothers in Fukushima Prefecture,10 particularly those living near the power plant or who experienced miscarriage or stillbirth.11 Pregnant women living near the earthquake epicenter during their third trimester were at a higher risk for HDP, suggesting an association with psychological stress.12 However, no regional differences in the mothers’ and infants’ conditions were observed at 1-month postnatal check-ups.13
The negative effects of fetal radiation exposure, transgenerational effects of radiation exposure, and effects on germline de novo mutation have been elucidated.14–18 Radiation exposure following the Chernobyl accident did not change the frequency of fetal malformations,14 and no fetal effects or association with maternal exposure were observed among those who survived the Hiroshima and Nagasaki bombings.16 Similarly, low-dose exposure following FDND is considered free from effects, but no studies have examined associations with actual exposure dose.
Here, we investigated the relationship between maternal external radiation dose and major PBS outcomes—congenital anomalies, LBW, SGA, and preterm birth—based on individuals’ external radiation doses. Few reports have examined these relationships, and our results will impact disaster medicine greatly.
METHODS
Study population and design
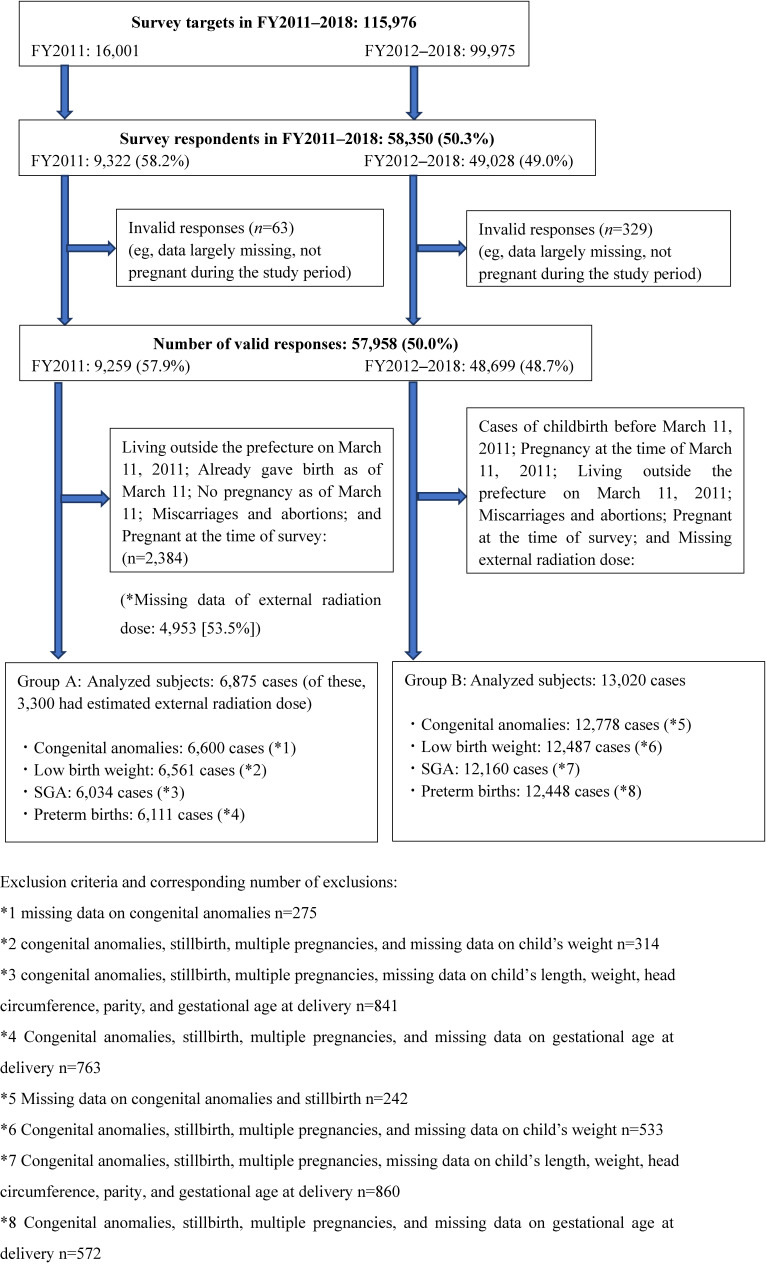
In Japan, pregnant women receive a Maternal and Child Health Handbook from their municipality when they register their pregnancy and receive free antenatal and infant health check-ups. The PBS, a cohort study and a division of FHMS, was conducted as follows: fiscal year (FY) 2011 PBS questionnaires were sent to women who received a handbook from municipalities in Fukushima Prefecture between August 1, 2010, and July 31, 2011, and were completed voluntarily and returned by mail. This practice continued subsequently, with online responses introduced in 2016. Subjects in the FY2011 survey were defined as Group A if March 11, 2011, fell between 2w0d of pregnancy, which coincides with the time of fertilization, and the delivery date. Respondents from FY2011 whose 2w0d of pregnancy fell after March 11, 2011, and respondents between FY2012 and FY2018 were designated as Group B. Participants living outside the prefecture when the disaster occurred were excluded, as were those with multiple pregnancies and pregnancy termination at <22 weeks’ gestation. Infants with congenital anomalies, stillbirth, or missing birth weight and maternal parity data were excluded.
Primary outcomes
The primary outcomes of this study were perinatal outcomes, namely congenital anomalies, LBW, SGA, and preterm birth. A congenital anomaly was defined as any indication of congenital anomaly in the questionnaire. We excluded cases with missing data on the following congenital anomalies: cataract, heart, kidney, or urinary tract anomaly, neural tube defects, microcephaly, hydrocephaly, cleft lip or palate, digestive tract atresia, imperforate anus, polydactyly, syndactyly, or other (with free response). Stillbirths and cases with missing data regarding gestational age at delivery were also excluded. LBW was defined as birth weight <2,500 g, excluding infants with congenital anomalies, stillbirth, or missing birth weight data. SGA was defined as birth weight <10th percentile for the gestational age based on the child’ sex and mother’s parity.19 Preterm birth was defined as delivery between 22 and 37 weeks’ gestation.
Factors associated with the primary outcomes
The primary variable was maternal external radiation dose. In the BS, we implemented a precise system to estimate external radiation dose using technical support from the National Institute of Radiological Sciences. Self-administered questionnaires were mailed to 2,055,257 residents living in Fukushima Prefecture at the time of FDND to obtain information regarding their residence, places visited, time spent indoors and outdoors, and travel time in the 4-month period between March 11 and July 11, 2011, when atmospheric radiation levels peaked.20,21 Returning the survey was optional. Respondents’ behavior and location (“trail”) were used to estimate their external radiation dose in a manner similar to studies on the Chernobyl accident.22 The BS response rate was 27.7%, and the maternal external radiation dose distribution was confirmed using BS results; these values were then categorized. External radiation dose data were supplemented for PBS respondents who did not respond to the BS. We used multiple imputation by chained equation with predictive mean-matching methods under fully conditional specification to generate 10 datasets with imputation for the missing estimated external radiation dose.23–27
Other explanatory variables included maternal parity and child’s sex, height, and weight. Maternal age was defined as that on April 1 of the year following the survey. Stillbirth was defined as delivery of a dead fetus after 22 weeks’ gestation. Options for mode of pregnancy were “natural pregnancy,” “induced ovulation,” “artificial insemination with husband’s semen,” and “assisted reproductive technology.” All options, except “natural pregnancy,” were considered infertility treatments. Trimester of pregnancy at the time of the earthquake (first, second, or third) was regarded as a variable. As pregnancy complications, HDP and placenta previa were entered as outcome-related variables. HDP was defined as indicating pre-pregnancy hypertension and the development of hypertensive disease after pregnancy in the questionnaire. Placenta previa was considered present in participants who stated that they had the disease. Mental disorders were considered pregnancy complications and defined as a pre-pregnancy history of mental illness or insomnia, anxiety, and other mental disorders during pregnancy.
After FDND, the government ordered residents of 12 municipalities to evacuate.2 “Forced to change health check-up facility or intervals between visits due to disaster” was defined as responding “No” to the questions “Did you continue to have your antenatal check-ups and delivery at the facility where you had originally planned?” and “Were you able to receive a prenatal check-up as scheduled?” or the answer “I had to change to a facility outside of the prefecture by myself (not on the doctor’s instruction)”.
Statistical analysis
For each major outcome, two-group comparisons were performed using the t-test for continuous variables and the chi-square or Fisher’s exact probability test for categorical variables (proportion and frequency). Significance was set at 5% was set using two-sided probability. Statistically significant variables were used in univariate logistic regression analysis. Fetal height was excluded as it associated with fetal weight and duration of pregnancy. To examine the association between primary outcomes and external radiation, variables found to differ significantly in the univariate analysis were employed in the multivariate binomial logistic regression analysis except as follows: Only univariate analysis was performed during congenital anomaly analysis as no covariates were suitable for multivariate analysis. Congenital anomalies include various diseases often accompanied by smaller body size, shorter gestation period, and stillbirth; therefore, gestational days at delivery, LBW, SGA, and preterm birth were not included in the multivariable analysis. Items not biologically associated with the occurrence of congenital anomalies, such as changes in health check-up facility and mental disorders during pregnancy, were also excluded. Regarding other multivariate analysis models, birth weight was strongly associated with pregnancy duration, and these items define the outcomes; therefore, we did not employ them as independent variables. The crude odds ratio and 95% confidence interval (CI) are shown for univariate analysis, and the adjusted odds ratio (aOR) and 95% CI are shown for the multivariate analysis.
Statistical analyses were performed using SAS (ver. 9.4; SAS Institute, Cary, NC, USA).
Ethical considerations
The ethics committee of Fukushima Medical University approved this study (No. 1317, 2020-203), which was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The survey aims were detailed in a cover letter attached to the questionnaires sent to the participants. Participants were considered to provide informed consent by responding to the survey, as it was optional.
RESULTS
Figure 1 shows the flowchart of the process by which the survey targets of FY2011 and FY2012–2018 were selected for Group A and Group B analysis. A total of 16,001 questionnaires were mailed during FY2011. Of the 9,322 responses, 9,259 were valid (57.9%). External radiation dose data were missing in 4,953 (53.5%). After exclusion, 6,875 participants were eligible for imputation and analysis. Of these, 6,600, 6,561, 6,034, and 6,111 participants were eligible for analysis due to congenital anomalies, LBW, SGA, and preterm birth, respectively.
Figure 1. Participants of the pregnancy and birth and basic surveys.
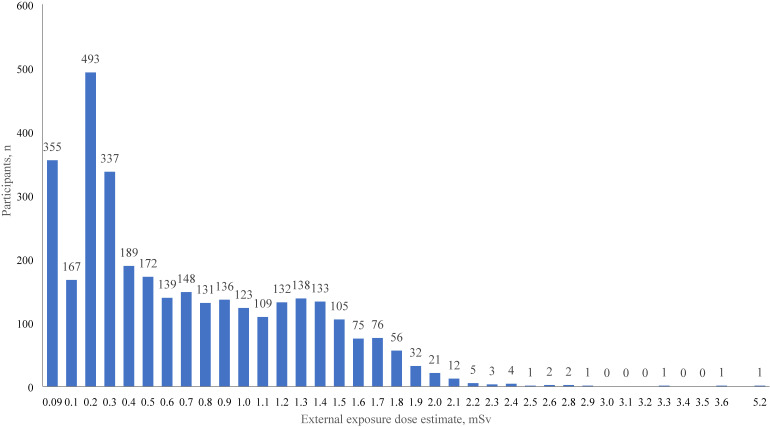
The distribution of the external exposure dose estimates for 3,300 participants before imputation (∼48%) is shown in Figure 2. The median and mean doses were 0.5 mSv and 0.7 mSv. The highest recorded dose was 5.2 mSv. Only 1.6% of participants were exposed to ≥2 mSv radiation. The participants were therefore grouped as follows: <1 mSv (0 to <1 mSv), 1 to <2 mSv, and ≥2 mSv.
Figure 2. Distribution of external exposure dose estimates (n = 3,300).
Table 1 shows the participants’ characteristics and their external radiation dose. The external exposure doses were as follows: <1 mSv, 2,267 participants (33.0%); 1 to <2 mSv, 979 (14.2%); ≥2 mSv, 54 (0.8%). Participants with missing dose data (3,575 participants, 52.0%) were classified into a single category. Rates of congenital anomaly, LBW, SGA, and preterm birth following maternal external radiation dose ≥2 mSv were 0%, 9.3%, 4.4%, and 4.3%, respectively. The relationship between maternal external radiation dose and the presence or absence of congenital anomalies, LBW, SGA, or preterm birth remains unclear (Table 2).
Table 1. Characteristics of 6,875 cases according to external radiation dose.
| External radiation dose, mSv | ||||||
| Total | (missing) | <1 mSv | 1 to <2 mSv | ≥2 mSv | ||
| Ñ | 6,875 (100.0) | 3,575 (52.0) | 2,267 (33.0) | 979 (14.2) | 54 (0.8) | |
| Maternal age, years | 6,875 | 30.9 (5.0) | 30.3 (5.2) | 31.5 (4.6) | 31.6 (4.7) | 30.5 (5.4) |
| Child’s sex (male), % | 6,814 | 51.3 | 51.8 | 50.6 | 51.8 | 46.3 |
| Child’s length, cm | 6,783 | 49.1 (2.2) | 49.1 (2.3) | 49.1 (2.2) | 49.2 (2.2) | 49.1 (2.1) |
| Child’s weight, g | 6,815 | 3,029.3 (403.1) | 3,025.6 (411.8) | 3,036.1 (394.9) | 3,028.0 (391.1) | 3,006.2 (382.4) |
| Gestational days at delivery, days | 6,348 | 276 (11.0) | 275.2 (11.4) | 275.7 (10.6) | 276.1 (10.0) | 275.3 (10.7) |
| Low birth weight (<2,500 g), % | 6,815 | 7.6 | 7.8 | 7.3 | 7.3 | 9.3 |
| SGA (<−10%), % | 6,270 | 8.9 | 8.5 | 8.8 | 10.7 | 4.4 |
| Congenital anomalies, % | 6,600 | 2.9 | 3.1 | 2.9 | 2.0 | 0.0 |
| Stillbirth, % | 6,875 | 0.2 | 0.3 | 0.2 | 0.1 | 0.0 |
| Preterm birth (<37 weeks), % | 6,348 | 4.1 | 4.5 | 3.6 | 3.5 | 4.3 |
| Multiple pregnancies, % | 6,872 | 0.9 | 1.1 | 0.6 | 0.8 | 0.0 |
| Primiparous, % | 6,840 | 25.4 | 24.8 | 25.3 | 27.4 | 37.0 |
| Infertility treatment, % | 6,875 | 4.9 | 4.2 | 5.6 | 6.0 | 3.7 |
| Placenta previa, % | 6,875 | 1.4 | 1.3 | 1.3 | 2.0 | 1.9 |
| Forced to change health check-up facility, % | 6,809 | 35.4 | 32.4 | 45.6 | 23.7 | 20.4 |
| Trimester of pregnancy at earthquake | 6,259 | |||||
| First (2–14 weeks), % | 32.7 | 35.0 | 30.2 | 30.5 | 23.4 | |
| Second (14–28 weeks), % | 40.0 | 40.3 | 40.5 | 37.1 | 53.2 | |
| Third (≥28 weeks), % | 27.3 | 24.7 | 29.3 | 32.4 | 23.4 | |
| Hypertensive disorders of pregnancy, % | 6,875 | 3.3 | 3.3 | 3.4 | 2.8 | 5.6 |
| Mental disorders before birth, % | 6,875 | 6.0 | 5.8 | 6.1 | 6.7 | 3.7 |
| Evacuation area, % | 6,875 | 10.8 | 9.0 | 15.5 | 6.5 | 5.6 |
SGA, small for gestational age.
Table 2. Factors associated with preterm birth, low birth weight, SGA, and congenital anomalies.
| Congenital anomaly (n = 6,600) | Low birth weight (n = 6,561) | |||||
| No | Yes | P * | ≥2,500 g | <2,500 g | P * | |
| N (%) or mean (SD) | N (%) or mean (SD) | |||||
| 6,411 (97.1) | 189 (2.9) | 6,116 (93.2) | 445 (6.8) | |||
|
| ||||||
| External radiation dose | 0.188 | 0.811 | ||||
|
| ||||||
| Missing | 3,308 (51.6) | 106 (56.1) | 3,150 (51.5) | 235 (52.8) | ||
| <1 mSv, % | 2,124 (33.1) | 64 (33.9) | 2,032 (33.2) | 141 (31.7) | ||
| 1 to <2 mSv, % | 925 (14.4) | 19 (10.1) | 885 (14.5) | 64 (14.4) | ||
| ≥2 mSv, % | 54 (0.8) | 0 (0.0) | 49 (0.8) | 5 (1.1) | ||
| Maternal age, years | 30.9 (5.0) | 30.6 (5.2) | 0.456 | 30.9 (5.0) | 31.0 (5.2) | 0.458 |
| Child’s sex (male), % | 3,284 (51.3) | 105 (55.9) | 0.221 | 3,168 (51.9) | 183 (41.1) | <0.001 |
| Child’s length, cm | 49.2 (2.1) | 48.5 (3.4) | 0.007 | 49.5 (1.8) | 45.5 (2.8) | <0.001 |
| Child’s weight, g | 3,036 (392.5) | 2,904 (556.8) | 0.002 | 3,097 (327.5) | 2,251 (318.2) | <0.001 |
| Gestational days at delivery, days | 275.7 (10.3) | 272.2 (18.4) | 0.013 | 277.0 (8.3) | 260.6 (18.9) | <0.001 |
| Primiparous, % | 1,628 (25.5) | 44 (23.4) | 0.511 | 1,537 (25.3) | 129 (29.1) | 0.077 |
| Low birth weight (<2,500 g), % | 459 (7.2) | 33 (17.7) | <0.001 | — | — | |
| SGA, % | 520 (8.8) | 24 (13.9) | 0.022 | 290 (5.2) | 228 (54.0) | <0.001 |
| Stillbirth, % | 5 (0.1) | 3 (1.6) | 0.001** | — | — | |
| Preterm birth (<37 weeks), % | 217 (3.6) | 22 (12.6) | <0.001 | 81 (1.4) | 130 (30.7) | <0.001 |
| Forced to change health check-up facility, % | 2,234 (35.2) | 80 (42.6) | 0.038 | 2,117 (35.0) | 156 (35.3) | 0.831 |
| Trimester of pregnancy at earthquake | 0.550 | 0.656 | ||||
| First (2–14 weeks), % | 1,900 (32.3) | 61 (36.3) | 1,830 (32.7) | 128 (30.8) | ||
| Second (14–28 weeks), % | 2,355 (40.1) | 63 (37.5) | 2,229 (39.8) | 174 (41.9) | ||
| Third (≥28 weeks), % | 1,625 (27.6) | 44 (26.2) | 1,537 (27.5) | 113 (27.2) | ||
| Infertility treatment, % | 316 (4.9) | 12 (6.4) | 0.376 | 275 (4.5) | 30 (6.7) | 0.030 |
| Placenta previa, % | 94 (1.5) | 1 (0.5) | 0.286 | 85 (1.4) | 11 (2.5) | 0.066 |
| Hypertensive disorders of pregnancy, % | 202 (3.2) | 5 (2.7) | 0.694 | 164 (2.7) | 46 (10.3) | <0.001 |
| Mental disorders before birth, % | 382 (6.0) | 18 (9.5) | 0.043 | 368 (6.0) | 24 (5.4) | 0.592 |
| Evacuation area, % | 686 (10.7) | 20 (10.6) | 0.959 | 657 (10.7) | 44 (9.8) | 0.573 |
|
| ||||||
| SGA (n = 6,034) | Preterm birth (n = 6,111) | |||||
| ≥−10% | <−10% | P * | ≥37 weeks | <37 weeks | P * | |
| 5,516 (91.4) | 518 (8.6) | 5,899 (96.5) | 212 (3.5) | |||
|
| ||||||
| External radiation dose | 0.103 | 0.335 | ||||
|
| ||||||
| Missing | 2,854 (51.7) | 252 (48.7) | 3,029 (51.4) | 122 (57.6) | ||
| <1 mSv, % | 1,818 (33.0) | 170 (32.8) | 1,954 (33.1) | 60 (28.3) | ||
| 1 to <2 mSv, % | 800 (14.5) | 94 (18.2) | 871 (14.8) | 28 (13.2) | ||
| ≥2 mSv, % | 44 (0.8) | 2 (0.4) | 45 (0.8) | 2 (0.9) | ||
| Maternal age, years | 30.9 (5.0) | 30.5 (5.2) | 0.105 | 30.8 (5.0) | 31.6 (5.4) | 0.040 |
| Child’s sex (male), % | 2,831 (51.3) | 248 (47.9) | 0.134 | 2,983 (50.7) | 120 (56.9) | 0.080 |
| Child’s length, cm | 49.4 (2.0) | 47.1 (2.3) | <0.001 | 49.3 (1.9) | 45.2 (3.7) | <0.001 |
| Child’s weight, g | 3,091 (359.1) | 2,481 (281.6) | <0.001 | 3,065 (359.9) | 2,329 (561.8) | <0.001 |
| Gestational days at delivery, days | 275.7 (10.1) | 276.4 (11.3) | 0.189 | 276.9 (7.9) | 244.5 (18.3) | <0.001 |
| Primiparous, % | 1,465 (26.6) | 107 (20.7) | 0.003 | 1,522 (25.9) | 57 (26.9) | 0.753 |
| Low birth weight (<2,500 g), % (%) | 194 (3.5) | 228 (44.0) | <0.001 | 294 (5.0) | 130 (61.6) | <0.001 |
| SGA, % | 495 (8.5) | 23 (10.9) | 0.222 | |||
| Stillbirth, % | — | — | ||||
| Preterm birth (<37 weeks), % | 188 (3.4) | 23 (4.4) | 0.222 | — | — | |
| Forced to change health check-up facility, % | 1,927 (35.3) | 174 (33.8) | 0.496 | 2,059 (35.2) | 71 (34.5) | 0.822 |
| Trimester of pregnancy at earthquake | 0.360 | 0.179 | ||||
| First (2–14 weeks), % | 1,765 (32.5) | 166 (32.7) | 1,888 (32.5) | 69 (33.7) | ||
| Second (14–28 weeks), % | 2,181 (40.2) | 190 (37.4) | 2,313 (39.8) | 91 (44.4) | ||
| Third (≥28 weeks), % | 1,483 (27.3) | 152 (29.9) | 1,605 (27.6) | 45 (22.0) | ||
| Infertility treatment, % | 259 (4.7) | 27 (5.2) | 0.597 | 277 (4.7) | 13 (6.1) | 0.334 |
| Placenta previa, % | 85 (1.5) | 4 (0.8) | 0.165 | 76 (1.3) | 13 (6.1) | <0.001** |
| Hypertensive disorders of pregnancy, % | 156 (2.8) | 40 (7.7) | <0.001 | 173 (2.9) | 26 (12.3) | <0.001 |
| Mental disorders before birth, % | 344 (6.2) | 24 (4.6) | 0.145 | 360 (6.1) | 12 (5.7) | 0.791 |
| Evacuation area, % | 582 (10.6) | 49 (9.5) | 0.438 | 624 (10.6) | 18 (8.5) | 0.330 |
SGA, small for gestational age.
*t-test was used for continuous variables and chi-square tests was used for other categorical variables.
**Fisher’s exact test was used for other categorical variables.
Congenital anomalies were observed in 189 of 6,600 participants (2.9%). Infants with a congenital anomaly were smaller (mean 48.5; standard deviation [SD], 3.4 cm, vs mean 49.2; SD, 2.1 cm, P = 0.007), weighed less (mean 2,904; SD, 556.8 g vs mean 3,036; SD, 392.5 g, P = 0.002), had a shorter gestation period (mean 272.2; SD, 18.4 days vs mean 275.7; SD, 10.3 days, P = 0.013), and higher frequencies of preterm birth (12.6% vs 3.6%, P < 0.001), LBW (17.7% vs 7.2%, P < 0.001), and stillbirth (1.6% vs 0.1%, P = 0.001). These values increased among individuals “forced to change health check-up facility or intervals between visits due to disaster” (42.6% vs 35.2%, P = 0.038) and those with maternal mental disorders (9.5% vs 6.0%, P = 0.043).
LBW was observed in 445 of 6,561 participants (6.8%). LBW was less common in males (41.1% vs 51.9%, P < 0.001) and infants with LBW were shorter (mean 45.5; SD, 2.8 cm vs mean 49.5; SD, 1.8 cm, P < 0.001) and were born earlier (mean 260.6; SD, 18.9 days vs mean 277.0; SD, 8.3 days, P < 0.001) and was therefore more common in preterm births (30.7% vs 1.4%, P < 0.001). LBW also occurred more commonly in those treated for infertility (6.7% vs 4.5%, P = 0.030) and those with HDP (10.3% vs 2.7%, P < 0.001).
SGA was observed in 518 of 6,034 participants (8.6%). Infants with SGA were shorter (mean 47.1; SD, 2.3 cm vs mean 49.4; SD, 2.0 cm, P < 0.001), less commonly born to first-time mothers (20.7% vs 26.6%, P = 0.003), and more common among those with HDP (7.7% vs 2.8%, P < 0.001). Infant sex, gestational age at delivery, and preterm birth and infertility treatment rates did not differ significantly, whereas these values differed among those with LBW.
Preterm birth was observed in 212 of 6,111 participants (3.5%), and tended to occur in those with higher maternal ages compared to those of term births (mean 31.6; SD, 5.4 years vs mean 30.8; SD, 5.0 years, P = 0.040), and was associated with smaller infant height (mean 45.2, SD, 3.7 cm vs mean 49.3; SD, 1.9 cm, P < 0.001), lower birth weight (mean 2,329; SD, 561.8 g vs mean 3,065; SD, 359.9 g, P < 0.001), and increased LBW incidence (61.6% vs 5.0%, P < 0.001). Preterm birth was also more common in cases of placenta previa (6.1% vs 1.3%, P < 0.001).
Table 3 shows the results of binomial logistic regression analysis that determined the association between the occurrence of the primary outcomes of congenital anomalies, LBW, SGA, preterm birth, and maternal external radiation dose and factors associated with other outcomes.
Table 3. Associations between obstetric outcomes and external radiation dose (imputation of missing doses).
| Congenital anomaly | Low birth weight | ||||||||||||
| Crude | Multivariate adjusted | Crude | Multivariate adjusted | ||||||||||
| Ref. | OR | (95% CI) | P * | OR | (95% CI) | P ** | OR | (95% CI) | P * | OR | (95% CI) | P ** | |
| External radiation dose | |||||||||||||
| 1 to <2 mSv | <1 mSv | 0.81 | (0.56–1.17) | 0.253 | 0.91 | (0.71–1.17) | 0.448 | 0.91 | (0.71–1.18) | 0.472 | |||
| ≥2 mSv | <1 mSv | — | — | 1.26 | (0.56–2.83) | 0.581 | 1.21 | (0.53–2.79) | 0.649 | ||||
| Maternal age | 1SD | 0.95 | (0.82–1.10) | 0.456 | 1.04 | (0.94–1.14) | 0.458 | ||||||
| Child’s sex | Female | 1.20 | (0.90–1.61) | 0.221 | 0.65 | (0.53–0.79) | <0.001 | 0.65 | (0.53–0.79) | <0.001 | |||
| Gestational days at delivery | 1SD | 0.79 | (0.71–0.88) | <0.001 | 0.26 | (0.23–0.29) | <0.001 | ||||||
| Primiparous | Multiparous | 0.89 | (0.63–1.26) | 0.511 | 1.21 | (0.98–1.50) | 0.078 | ||||||
| LBW | ≥2,500 g | 2.78 | (1.88–4.09) | <0.001 | — | — | |||||||
| SGA | ≥−10% | 1.67 | (1.07–2.59) | 0.023 | 21.6 | (17.2–27.0) | <0.001 | ||||||
| Preterm birth | ≥37 weeks | 3.83 | (2.40–6.11) | <0.001 | 30.5 | (22.6–41.2) | <0.001 | ||||||
| Placenta previa | No | — | — | 1.80 | (0.95–3.40) | 0.070 | |||||||
| Forced to change health check-up facility | No | 1.36 | (1.02–1.83) | 0.038 | 1.02 | (0.84–1.25) | 0.830 | ||||||
| Trimester of pregnancy at earthquake | |||||||||||||
| Second (14–28 weeks) | First | 0.78 | (0.46–1.30) | 0.335 | 1.12 | (0.88–1.41) | 0.363 | ||||||
| Third (≥28 weeks) | First | 0.57 | (0.31–1.05) | 0.069 | 1.05 | (0.81–1.37) | 0.709 | ||||||
| Infertility treatment | No | 1.31 | (0.72–2.37) | 0.376 | 1.54 | (1.04–2.27) | 0.031 | 1.49 | (1.01–2.21) | 0.046 | |||
| Hypertensive disorders of pregnancy | No | 0.84 | (0.34–2.05) | 0.695 | 4.18 | (2.97–5.89) | <0.001 | 4.14 | (2.93–5.84) | <0.001 | |||
| Mental disorders before birth | No | 1.66 | (1.01–2.73) | 0.045 | 0.89 | (0.58–1.36) | 0.592 | ||||||
| Evacuation area | No | 0.99 | (0.62–1.58) | 0.959 | 0.91 | (0.66–1.26) | 0.573 | ||||||
|
| |||||||||||||
| SGA | Preterm birth | ||||||||||||
| Crude | Multivariate adjusted | Crude | Multivariate adjusted | ||||||||||
| Ref. | OR | (95% CI) | P * | OR | (95% CI) | P ** | OR | (95% CI) | P * | OR | (95% CI) | P ** | |
|
| |||||||||||||
| External radiation dose | |||||||||||||
| 1 to <2 mSv | <1 mSv | 1.12 | (0.91–1.40) | 0.286 | 1.14 | (0.92–1.42) | 0.229 | 0.92 | (0.65–1.30) | 0.638 | 0.91 | (0.65–1.29) | 0.602 |
| ≥2 mSv | <1 mSv | 0.84 | (0.30–2.39) | 0.744 | 0.84 | (0.30–2.37) | 0.735 | 1.08 | (0.24–4.84) | 0.919 | 1.05 | (0.22–4.87) | 0.955 |
| Maternal age | 1SD | 0.93 | (0.85–1.02) | 0.105 | 1.17 | (1.02–1.34) | 0.025 | 1.13 | (0.98–1.29) | 0.084 | |||
| Child’s sex | Female | 0.87 | (0.73–1.04) | 0.134 | 1.28 | (0.97–1.69) | 0.081 | ||||||
| Gestational days at delivery | 1SD | 1.07 | (0.98–1.18) | 0.150 | — | — | |||||||
| Primiparous | Multiparous | 0.72 | (0.58–0.90) | 0.004 | 0.69 | (0.56–0.87) | 0.001 | 1.05 | (0.77–1.43) | 0.753 | |||
| LBW | ≥2,500 g | 21.6 | (17.2–27.0) | <0.001 | 30.5 | (22.6–41.2) | <0.001 | ||||||
| SGA | ≥−10% | — | — | 1.32 | (0.85–2.05) | 0.222 | |||||||
| Preterm birth | ≥37 weeks | 1.32 | (0.85–2.05) | 0.222 | |||||||||
| Placenta previa | No | 0.50 | (0.18–1.36) | 0.174 | 5.01 | (2.73–9.17) | <0.001 | 4.81 | (2.60–8.89) | <0.001 | |||
| Forced to change health check-up facility | No | 0.94 | (0.77–1.13) | 0.496 | 0.97 | (0.72–1.30) | 0.823 | ||||||
| Trimester of pregnancy at earthquake | |||||||||||||
| Second (14–28 weeks) | First | 0.93 | (0.75–1.15) | 0.490 | 1.08 | (0.78–1.48) | 0.650 | ||||||
| Third (≥28 weeks) | First | 1.09 | (0.87–1.37) | 0.465 | 0.77 | (0.52–1.12) | 0.173 | ||||||
| Infertility treatment | No | 1.12 | (0.74–1.68) | 0.597 | 1.33 | (0.75–2.36) | 0.334 | ||||||
| Hypertensive disorders of pregnancy | No | 2.88 | (2.01–4.12) | <0.001 | 3.01 | (2.10–4.32) | <0.001 | 4.63 | (2.99–7.17) | <0.001 | 4.50 | (2.89–7.00) | <0.001 |
| Mental disorders before birth | No | 0.73 | (0.48–1.12) | 0.147 | 0.92 | (0.51–1.67) | 0.791 | ||||||
| Evacuation area | No | 0.89 | (0.65–1.20) | 0.438 | 0.79 | (0.48–1.28) | 0.332 | ||||||
CI, confidence interval; LBW, low birth weight; OR, odds ratio; SGA, small for gestational age.
*Univariate logistic regression analysis by forced entry method.
**Factors significant in univariate analysis were entered into multivariate analysis using binominal logistic regression.
Maternal external radiation dose (1 to <2 mSv) was not associated with congenital anomalies (OR, 0.81; 95% CI, 0.56–1.17, P = 0.253). Congenital anomalies, usually identified prenatally, may have necessitated a change of facilities after the disaster or caused mental disorders. Accordingly, post-disaster change of facilities and mental disorders were excluded from the binomial logistic regression analysis, despite being significant in univariate analysis.
Multivariate analysis of LBW was adjusted for dose (1 to <2 mSv, ≥2 mSv), sex (reference: female), infertility treatment, and HDP. The doses did not cause significant differences, with aORs of 0.91 (95% CI, 0.71–1.18, P = 0.472) for 1 to <2 mSv and 1.21 (95% CI, 0.53–2.79, P = 0.649) for ≥2 mSv. Male sex was independently associated with reduced LBW incidence (aOR 0.65; 95% CI, 0.53–0.79, P < 0.001), whereas infertility treatment (aOR 1.49; 95% CI, 1.01–2.21, P = 0.046) and HDP (aOR 4.14; 95% CI, 2.93–5.84, P < 0.001) were independently associated with increased LBW.
Multivariate analysis of SGA was adjusted for maternal radiation dose (1 to <2 mSv, ≥2 mSv), primiparity, and HDP. Different doses did not cause significant differences, and aORs of 1.14 (95% CI, 0.92–1.42, P = 0.229) and 0.84 (95% CI, 0.30–2.37, P = 0.735) for 1 to <2 mSv and ≥2 mSv, respectively, were observed. Primiparity was independently associated with reduced SGA (aOR 0.69; 95% CI, 0.56–0.87, P = 0.001), whereas HDP was independently associated with increased SGA (aOR 3.01; 95% CI, 2.10–4.32, P < 0.001).
Multivariate analysis for preterm birth was adjusted for dose (1 to <2 mSv, ≥2 mSv), maternal age, placenta previa, and HDP. The results did not differ significantly based on dose, and aORs of 0.91 (95% CI, 0.65–1.29, P = 0.602) and 1.05 (95% CI, 0.22–4.87, P = 0.955) for 1 to <2 mSv and ≥2 mSv, respectively, were observed. Placenta previa (aOR 4.81; 95% CI, 2.60–8.89, P < 0.001) and HDP (aOR 4.50; 95% CI, 2.89–7.00, P < 0.001) were independently associated with increased preterm birth.
Table 4 displays congenital anomaly types, of which the most common was cardiac malformation (0.86%). No congenital anomalies were found among the children born to mothers exposed to ≥2 mSv.
Table 4. Congenital anomalies (n = 6,600).
| Total | <1 mSv | 1 to <2 mSv | ≥2 mSv | (missing) | |
| n = 6,600 | n = 2,188 | n = 944 | n = 54 | n = 3,414 | |
| Total* | 189 (2.86) | 64 | 19 | 0 | 106 |
| Cataract | 1 (0.02) | 0 | 1 | 0 | 0 |
| Neural tube defects | 3 (0.05) | 1 | 2 | 0 | 0 |
| Microcephaly | 0 (0.00) | 0 | 0 | 0 | 0 |
| Cardiac malformation | 57 (0.86) | 20 | 4 | 0 | 33 |
| Kidney/urinary tract malformation | 19 (0.29) | 5 | 3 | 0 | 11 |
| Hydrocephaly | 1 (0.02) | 1 | 0 | 0 | 0 |
| Cleft lip/palate | 12 (0.18) | 1 | 3 | 0 | 8 |
| Digestive tract atresia | 5 (0.08) | 3 | 0 | 0 | 2 |
| Imperforate anus | 4 (0.06) | 1 | 0 | 0 | 3 |
| Poly/syndactyly | 18 (0.27) | 7 | 1 | 0 | 10 |
| Others | 83 (1.26) | 28 | 6 | 0 | 49 |
*Multiple answers were allowed.
No outcomes were associated with external radiation exposure following analysis of missing external radiation dose information following multiple imputation. Similarly, we found no associated outcomes across all significant risk factors (Table 5).
Table 5. Association between obstetric outcomes and external radiation dose (no deficit in external radiation dose).
| Congenital anomaly | Low birth weight | ||||||||||||
| Crude | Multivariate adjusted | Crude | Multivariate adjusted | ||||||||||
| Ref. | OR | (95% CI) | P * | OR | (95% CI) | P ** | OR | (95% CI) | P * | OR | (95% CI) | P ** | |
| External radiation dose | |||||||||||||
| 1 to <2 mSv | <1 | 0.64 | (0.38–1.08) | 0.096 | 0.64 | (0.38–1.08) | 0.096 | 1.04 | (0.77–1.42) | 0.791 | 1.06 | (0.78–1.44) | 0.720 |
| ≥2 mSv | <1 | — | — | 1.47 | (0.58–3.75) | 0.416 | 1.38 | (0.53–3.55) | 0.507 | ||||
| Maternal age | 1SD | 1.04 | (0.84–1.29) | 0.723 | 1.15 | (1.00–1.32) | 0.053 | ||||||
| Child’s sex | Female | 1.14 | (0.74–1.77) | 0.558 | 0.67 | (0.50–0.89) | 0.005 | 0.67 | (0.51–0.90) | 0.007 | |||
| Gestational days at delivery | 1SD | 0.73 | (0.63–0.84) | <0.001 | 0.30 | (0.25–0.35) | <0.001 | ||||||
| Primiparous, % | Multiparous | 1.03 | (0.63–1.69) | 0.903 | 1.30 | (0.96–1.76) | 0.095 | ||||||
| LBW | ≥2,500 g | 3.22 | (1.84–5.65) | <0.001 | — | — | |||||||
| SGA | ≥−10% | 1.30 | (0.64–2.63) | 0.469 | 26.3 | (18.9–36.4) | <0.001 | ||||||
| Preterm birth | ≥37 weeks | 6.32 | (3.36–11.88) | <0.001 | 25.1 | (16.0–39.4) | <0.001 | ||||||
| Placenta previa | No | — | — | 0.92 | (0.28–2.98) | 0.890 | — | ||||||
| Forced to change health check-up facility | No | 1.74 | (1.13–2.70) | 0.013 | 1.08 | (0.81–1.44) | 0.620 | ||||||
| Trimester of pregnancy at earthquake | |||||||||||||
| Second (14–28 weeks) | First | 0.78 | (0.46–1.30) | 0.335 | 1.01 | (0.71–1.43) | 0.974 | ||||||
| Third (≥28 weeks) | First | 0.57 | (0.31–1.05) | 0.069 | 1.10 | (0.76–1.58) | 0.628 | ||||||
| Infertility treatment | No | 1.28 | (0.55–2.98) | 0.566 | 1.59 | (0.94–2.67) | 0.083 | ||||||
| Hypertensive disorders of pregnancy | No | 0.39 | (0.05–2.80) | 0.347 | 4.17 | (2.54–6.83) | <0.001 | 4.11 | (2.50–6.74) | <0.001 | |||
| Mental disorders before birth | No | 2.08 | (1.06–4.09) | 0.034 | 0.93 | (0.51–1.69) | 0.805 | ||||||
| Evacuation area | No | 0.96 | (0.49–1.87) | 0.895 | 0.99 | (0.64–1.51) | 0.946 | ||||||
|
| |||||||||||||
| SGA | Preterm birth | ||||||||||||
| Crude | Multivariate adjusted | Crude | Multivariate adjusted | ||||||||||
| Ref. | OR | (95% CI) | P * | OR | (95% CI) | P ** | OR | (95% CI) | P * | OR | (95% CI) | P ** | |
|
| |||||||||||||
| External radiation dose | |||||||||||||
| 1 to <2 mSv | <1 | 1.26 | (0.96–1.64) | 0.092 | 1.27 | (0.97–1.65) | 0.083 | 1.05 | (0.66–1.65) | 0.844 | 1.04 | (0.65–1.65) | 0.875 |
| ≥2 mSv | <1 | 0.49 | (0.12–2.02) | 0.322 | 0.46 | (0.11–1.92) | 0.286 | 1.45 | (0.34–6.11) | 0.615 | 1.32 | (0.30–5.77) | 0.710 |
| Maternal age | 1SD | 0.96 | (0.85–1.09) | 0.520 | 1.37 | (1.11–1.70) | 0.004 | 1.31 | (1.06–1.62) | 0.014 | |||
| Child’s sex | Female | 1.03 | (0.80–1.32) | 0.833 | 1.33 | (0.87–2.04) | 0.190 | ||||||
| Gestational days at delivery | 1SD | 0.99 | (0.87–1.12) | 0.827 | — | — | |||||||
| Primiparous, % | Multiparous | 0.78 | (0.58–1.05) | 0.101 | 1.05 | (0.66–1.68) | 0.827 | ||||||
| LBW | ≥2,500 g | 26.26 | (18.94–36.39) | <0.001 | 25.06 | (15.95–39.39) | <0.001 | ||||||
| SGA | ≥−10% | — | — | 1.59 | (0.85–2.95) | 0.146 | |||||||
| Preterm birth | ≥37 weeks | 1.59 | (0.85–2.95) | 0.146 | — | — | |||||||
| Placenta previa | No | 0.23 | (0.03–1.64) | 0.141 | 3.21 | (1.13–9.16) | 0.029 | 3.20 | (1.10–9.33) | 0.033 | |||
| Forced to change health check-up facility | No | 0.83 | (0.64–1.09) | 0.178 | 0.97 | (0.62–1.51) | 0.886 | ||||||
| Trimester of pregnancy at earthquake | |||||||||||||
| Second (14–28 weeks) | First | 0.94 | (0.69–1.28) | 0.679 | 1.08 | (0.66–1.75) | 0.768 | ||||||
| Third (≥28 weeks) | First | 1.05 | (0.76–1.46) | 0.750 | 0.61 | (0.34–1.10) | 0.101 | ||||||
| Infertility treatment | No | 1.40 | (0.85–2.30) | 0.187 | 1.25 | (0.54–2.91) | 0.602 | ||||||
| Hypertensive disorders of pregnancy | No | 2.92 | (1.77–4.81) | <0.001 | 2.97 | (1.80–4.91) | <0.001 | 6.11 | (3.32–11.25) | <0.001 | 5.73 | (3.09–10.63) | <0.001 |
| Mental disorders before birth | No | 0.57 | (0.30–1.09) | 0.089 | 0.70 | (0.25–1.93) | 0.489 | ||||||
| Evacuation area | No | 0.90 | (0.60–1.34) | 0.597 | 0.68 | (0.33–1.42) | 0.304 | ||||||
CI, confidence interval; LBW, low birth weight; OR, odds ratio; SGA, small for gestational age.
*Univariate logistic regression analysis by forced entry method.
**Factors significant in univariate analysis were entered into multivariate analysis using binominal logistic regression.
Although our analysis primarily included women who were pregnant at the time of the earthquake, Group B (13,020 individuals, excluding missing data on radiation dose [70.8%]; Figure 1) was subjected to the similar analysis. These results are presented in eTable 1.
DISCUSSION
Our results revealed that 98.4% of participants were exposed to <2 mSv of radiation in the 4 months following the Great East Japan Earthquake, with a mean and maximum of 0.7 and 5.2 mSv, respectively. Major perinatal outcomes—LBW, SGA, preterm birth, and congenital anomalies—were not affected. LBW incidence decreased in males and increased in cases of infertility treatment and HDP. The frequency of preterm birth was elevated in cases of placenta previa and HDP.
Exposure was low, especially as evacuees of the Chernobyl accident exhibited an average internal and external exposure of 31 mSv.28 No effects on perinatal outcomes at low exposures were observed in surrounding countries following the incident.29 Associations with perinatal outcomes have been observed during ecological studies on FDND, but no direct association with actual exposure doses has been observed.30–32 These studies reported annual trends in the number of births, and possible worsening of perinatal outcomes, reduction in live birth rates, and a possible association between average prefecture-specific Cs-137 deposition and LBW were observed in radiation-contaminated prefectures. After the Chernobyl incident, residents experienced considerable internal exposure.33 However, food was strictly inspected for radioactive material following FDND, making significant internal exposure unlikely. A whole-body counter study found Cs-134 and Cs-137 internal exposure of residents of Fukushima Prefecture <1 mSv.2 The source of radiation exposure following FDND was γ-rays emitting from radioactive cesium in the environment. Current guideline recommends that fetuses not be exposed to >5 mSv.34 Most participants met this criterion. The incidence of perinatal outcomes in Fukushima Prefecture is comparable to that observed during the Japan Environment and Children’s Study (JECS), a nationwide birth cohort study encompassing the same period as this survey.35 The JECS lacks information on radiation exposure but surveyed 12,804 pregnant women during the same period in Fukushima Prefecture.
In our study, congenital anomalies were observed in 2.9% of cases, but only in 1.7% of participants in the JECS survey. The International Clearing House for Birth Defects Monitoring Systems Japan Center, which collects congenital anomaly statistics from all over Japan, reported an incidence rate of 2.4% nationwide in 2011.36 Our study was based on surveys completed by the mothers themselves, while the latter two studies were conducted using medical records. Fetal radiation exposure causes congenital anomalies at an estimated threshold of 100 mGy.37–39 The threshold for the development of microcephaly is 120 mGy or more.40 No participants reporting congenital anomalies were exposed to radiation doses >2 mSv. Radiation is evenly distributed over the body during maternal exposure, and the effective dose (mSv) can be directly replaced by the equivalent dose (mGy) for the fetus.41 Causes of congenital anomaly other than external exposure should be considered.
LBW was less common among males, while SGA was more common among first-time mothers, as previously reported.42,43 HDP was also an independent risk factor for SGA (<−1.5 SD) in the FHMS in FY2011.8 We adopted the standard international definition of weight <10th percentile for gestational age as SGA.44 Assisted reproductive technology increases the risk of LBW and SGA and is associated with preterm births.45–47 We defined fertility treatment as any mode of pregnancy besides spontaneous. We could not investigate the details of assisted reproductive technology in this study, which may have influenced the results.
HDP was observed in 3.3% of cases in this study but was observable in 2.5% of 12,804 cases evaluated during the JECS in Fukushima Prefecture (early onset, 0.3%; late onset, 2.1%),35 and is strongly associated to preterm birth.48
Placenta previa, which may require cesarean section (CS) due to sudden genital bleeding or causes massive bleeding49 during CS, is an acceptable cause of CS at <37 weeks gestation50 and was associated with preterm birth.
Indicators of physical and mental stress (“forced to change health check-up facility or intervals between visits due to disaster” and “mental disorders”) were not associated with any outcomes. Prenatal maternal stress in the fifth and sixth months of pregnancy increases the risk of LBW, SGA, and preterm birth.51 As we observed previously,8 no trimester was associated with perinatal outcomes for LBW or preterm birth, possibly due to the small sample size (versus 2.6 million in the literature).
This is one of the few epidemiological studies of pregnant women conducted over several years in a single region with support from a public institution. The strengths include the use of multiple imputation to clarify the results. Similar results were obtained through sensitivity analysis without multiple imputation conducted with information of those with maternal external radiation dose data. However, the results regarding the presence or absence of the main outcomes and survey variables may be inaccurate, as this survey was questionnaire-based. Another limitation is the lack of data on maternal body size or lifestyle (eg, smoking), which cannot be addressed in a survey of perinatal outcomes. The response rate of 60% may raise concerns about the representativeness of the target population; however, the factors associated with perinatal outcomes in this study were similar to those observed previously, so the results can be considered reliable. This survey confirms that maternal external radiation dose was low and not associated with the frequency of perinatal outcomes, which was a major concern of the people in Fukushima Prefecture. The results must be explained to pregnant and nursing mothers in Fukushima Prefecture. The impact of FDND on the perinatal outcomes remains a concern. The PBS aims to elucidate these outcomes and will continue to be conducted.
Maternal external radiation exposure following FDND was not associated with congenital anomalies, LBW, SGA, or preterm birth.
ACKNOWLEDGMENTS
The survey was commissioned by the Fukushima Prefectural Government and carried out by the Pregnancy and Birth Survey Group conducted by the Radiation Medical Science Center for the Fukushima Health Management Survey, Fukushima Medical University. The work was supported by part of the funding provided for the Fukushima Health Management Survey. The opinions expressed in this paper are those of the authors and not of the Fukushima Prefectural Government. ThinkSCIENCE, Inc. (Tokyo, Japan) provided language editing assistance.
Funding: This survey was conducted as part of Fukushima Prefecture’s post-disaster recovery plans and was supported by the national “Health Fund for Children and Adults Affected by the Nuclear Incident” The funder had no role in study design; the collection, analysis or interpretation of the data; in the writing of the report; or in the decision to submit the article for publication.
Data availability statement: The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of interest: None declared.
Authors’ affiliations and their roles in the activities of the Fukushima Health Management Survey are listed on pages S116–S119.
SUPPLEMENTARY MATERIAL
The following is the supplementary data related to this article:
eTable 1. Association between obstetric outcomes and external radiation dose in Group B
REFERENCES
- 1.Fujimori K, Nomura Y, Yasuda S, et al. Obstetrical care in Fukushima Prefecture: obstetrical care and pregnancy trends immediately after the disaster. Shusanki Igaku. 2012;42:303–306 [in Japanese]. [Google Scholar]
- 2.Yasumura S, Hosoya M, Yamashita S, et al. ; Fukushima Health Management Survey Group . Study protocol for the Fukushima Health Management Survey. J Epidemiol. 2012;22(5):375–383. 10.2188/jea.JE20120105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasumura S, Ohira T, Ishikawa T, et al. Achievements and current status of the Fukushima Health Management Survey. J Epidemiol. 2022;32(Suppl 12):S3–S10. 10.2188/jea.JE20210390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyozuka H, Murata T, Yasuda S, et al. The effects of the Great East Japan Earthquake on perinatal outcomes: results of the pregnancy and birth survey in the Fukushima Health Management Survey. J Epidemiol. 2022;32(Suppl 12):S57–S63. 10.2188/jea.JE20210444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimori K, Kyozuka H, Yasuda S, et al. ; Pregnancy and Birth Survey Group of the Fukushima Health Management Survey . Pregnancy and birth survey after the Great East Japan Earthquake and Fukushima Daiichi Nuclear Power Plant accident in Fukushima prefecture. Fukushima J Med Sci. 2014;60(1):75–81. 10.5387/fms.2014-9 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Fujimori K, Yasumura S, Nakai A; Pregnancy and Birth Survey Group of the Fukushima Health Management Survey . Impact of the Great East Japan Earthquake and Fukushima nuclear power plant accident on assisted reproductive technology in Fukushima prefecture: the Fukushima Health Management Survey. J Clin Med Res. 2017;9:776–781. 10.14740/jocmr3105w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii K, Goto A, Ota M, Yasumura S, Fujimori K. Pregnancy and birth survey of the Fukushima Health Management Survey. Asia Pac J Public Health. 2017;29(2_suppl):56S–62S. 10.1177/1010539516684534 [DOI] [PubMed] [Google Scholar]
- 8.Yasuda S, Kyozuka H, Nomura Y, et al. Influence of the Great East Japan Earthquake and the Fukushima Daiichi nuclear disaster on the birth weight of newborns in Fukushima prefecture: Fukushima Health Management Survey. J Matern Fetal Neonatal Med. 2017;30:2900–2904. 10.1080/14767058.2016.1245718 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Goto A, Fujimori K. Effect of medical institution change on gestational duration after the Great East Japan Earthquake: the Fukushima Health Management Survey. J Obstet Gynaecol Res. 2016;42:1704–1711. 10.1111/jog.13102 [DOI] [PubMed] [Google Scholar]
- 10.Goto A, Bromet EJ, Fujimori K; Pregnancy and Birth Survey Group of Fukushima Health Management Survey . Immediate effects of the Fukushima nuclear power plant disaster on depressive symptoms among mothers with infants: a prefectural-wide cross-sectional study from the Fukushima Health Management Survey. BMC Psychiatry. 2015;15:59. 10.1186/s12888-015-0443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida-Komiya H, Goto A, Yasumura S, Fujimori K, Abe M; Pregnancy and Birth Survey Group of Fukushima Health Management Survey . Immediate mental consequences of the Great East Japan Earthquake and Fukushima Nuclear Power Plant accident on mothers experiencing miscarriage, abortion, and stillbirth: the Fukushima Health Management Survey. Fukushima J Med Sci. 2015;61(1):66–71. 10.5387/fms.2014-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyozuka H, Murata T, Yasuda S, et al. ; Pregnancy and Birth Survey Group of the Fukushima Health Management Survey . The effect of the Great East Japan Earthquake on hypertensive disorders during pregnancy: a study from the Fukushima Health Management Survey. J Matern Fetal Neonatal Med. 2020;33(24):4043–4048. 10.1080/14767058.2019.1594763 [DOI] [PubMed] [Google Scholar]
- 13.Kyozuka H, Yasuda S, Kawamura M, et al. Impact of the Great East Japan Earthquake on feeding methods and newborn growth at 1 month postpartum: Results from the Fukushima Health Management Survey. Radiat Environ Biophys. 2016;55:139–146. 10.1007/s00411-016-0636-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolominsky Y, Igumnov S, Drozdovitch V. The psychological development of children from Belarus exposed in the prenatal period to radiation from the Chernobyl atomic power plant. J Child Psychol Psychiatry. 1999;40:299–305. 10.1111/1469-7610.00444 [DOI] [PubMed] [Google Scholar]
- 15.Yeager M, Machiela MJ, Kothiyal P, et al. Lack of transgenerational effects of ionizing radiation exposure from the Chernobyl accident. Science. 2021;372:725–729. 10.1126/science.abg2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada M, Furukawa K, Tatsukawa Y, et al. Congenital malformations and perinatal deaths among the children of atomic bomb survivors: a reappraisal. Am J Epidemiol. 2021;190(11):2323–2333. 10.1093/aje/kwab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otake M, Schull WJ, Neel JV. Congenital malformations, stillbirths, and early mortality among the children of atomic bomb survivors: a reanalysis. Radiat Res. 1990;122:1–11. 10.2307/3577576 [DOI] [PubMed] [Google Scholar]
- 18.Green DM, Sklar CA, Boice JD Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–2381. 10.1200/JCO.2008.21.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itabashi K, Miura F, Uehara R, Nakamura Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int. 2014. Oct;56(5):702–708. 10.1111/ped.12331 [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Yasumura S, Akahane K, et al. External doses available for epidemiological studies related to the Fukushima Health Management Survey: first 4-month individual doses and municipality-average doses for the first year. J Epidemiol. 2022;32(Suppl 12):S11–S22. 10.2188/jea.JE20210166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa T, Yasumura S, Ozasa K, et al. The Fukushima Health Management Survey: estimation of external doses to residents in Fukushima Prefecture. Sci Rep. 2015;5:12712. 10.1038/srep12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Likhtarev IA, Chumack VV, Repin VS. Retrospective reconstruction of individual and collective external gamma doses of population evacuated after the Chernobyl accident. Health Phys. 1994;66:643–652. 10.1097/00004032-199406000-00004 [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 24.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 26.Sakai A, Nagao M, Nakano H, et al. Effects of external radiation exposure resulting from the Fukushima Daiichi Nuclear Power Plant accident on the health of residents in the evacuation zones: the Fukushima Health Management Survey. J Epidemiol. 2022;32(Suppl 12):S84–S94. 10.2188/jea.JE20210286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura I, Nagao M, Nakano H, et al. Associations between external radiation doses and the risk of psychological distress or post-traumatic stress after the Fukushima Daiichi Nuclear Power Plant accident: the Fukushima Health Management Survey. J Epidemiol. 2022;32(Suppl 12):S95–S103. 10.2188/jea.JE20210226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United Nations. Sources and effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation 2008 Report. https://www.unscear.org/docs/publications/2008/UNSCEAR_2008_Report_Vol.II.pdf; 2011 Accessed 17/04/2021.
- 29.Hoffmann W. Fallout from the Chernobyl nuclear disaster and congenital malformations in Europe. Arch Environ Health. 2001;56:478–484. 10.1080/00039890109602895 [DOI] [PubMed] [Google Scholar]
- 30.Scherb HH, Mori K, Hayashi K. Increases in perinatal mortality in prefectures contaminated by the Fukushima nuclear power plant accident in Japan: a spatially stratified longitudinal study. Medicine (Baltimore). 2016;95:e4958. 10.1097/MD.0000000000004958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Körblein A. Reduction in live births in Japan nine months after the Fukushima nuclear accident: an observational study. PLoS One. 2021;16:e0242938. 10.1371/journal.pone.0242938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherb H, Hayashi K. Spatiotemporal association of low birth weight with Cs-137 deposition at the prefecture level in Japan after the Fukushima nuclear power plant accidents: an analytical-ecologic epidemiological study. Environ Health. 2020;19:82. 10.1186/s12940-020-00630-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United Nations. United Nations Scientific Committee on the Effects of Atomic Radiation, Source and Effects of Ionization Radiation, Annex J: Exposure and effects of the Chernobyl accident, UNSCEAR 2000 Report Vol. II. https://www.unscear.org/docs/publications/2000/UNSCEAR_2000_Annex-J.pdf; 2000 Accessed 18/04/2021.
- 34.United States Nuclear Regulatory Commission (USNRC). Dose equivalent to an embryo/fetus. https://www.nrc.gov/reading-rm/doc-collections/cfr/part020/part020-1208.html; 2021 Accessed 18/04/2021.
- 35.Kyozuka H, Fujimori K, Hosoya M, et al. The Japan Environment and Children’s Study (JECS) in Fukushima prefecture: pregnancy outcome after the Great East Japan Earthquake. Tohoku J Exp Med. 2018;246:27–33. 10.1620/tjem.246.27 [DOI] [PubMed] [Google Scholar]
- 36.International Commission on Radiological Protection (ICRP). Annals of the ICRP: Publication 84: Pregnancy and Medical Radiation. https://journals.sagepub.com/doi/pdf/10.1177/ANIB_30_1; 2000 Accessed 18/04/2021.
- 37.International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). Annual Report 2013. http://www.icbdsr.org/wp-content/annual_report/Report2013.pdf Accessed 19/04/2021.
- 38.Kumar R, De Jesus O. Radiation effects on the fetus. 2021 Feb 7. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 39.De Santis M, Di Gianantonio E, Straface G, et al. Ionizing radiations in pregnancy and teratogenesis: a review of literature. Reprod Toxicol. 2005;20:323–329. 10.1016/j.reprotox.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 40.Otake M, Schull WJ, Yoshimaru H. A review of forty-five years study of Hiroshima and Nagasaki atomic bomb survivors. Brain damage among the prenatally exposed. J Radiat Res. 1991;32(Suppl):249–264. 10.1269/jrr.32.SUPPLEMENT_249 [DOI] [PubMed] [Google Scholar]
- 41.Ministry of the Environment, Government of Japan. Booklet to provide basic information regarding health effects of radiation. 1st ed. https://www.env.go.jp/en/chemi/rhm/basic-info/1st/02-03-06.html; 2013 Accessed 18/04/2021.
- 42.Morisaki N, Urayama KY, Yoshii K, Subramanian SV, Yokoya S. Ecological analysis of secular trends in low birth weight births and adult height in Japan. J Epidemiol Community Health. 2017;71:1014–1018. 10.1136/jech-2017-209266 [DOI] [PubMed] [Google Scholar]
- 43.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–793. 10.1016/j.bpobgyn.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 44.Lee PA, Chernausek SD, Hokken-Koelega ACS, Czernichow P; International Small for Gestational Age Advisory Board . International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24–October 1, 2001. Pediatrics. 2003;111:1253–1261. 10.1542/peds.111.6.1253 [DOI] [PubMed] [Google Scholar]
- 45.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–737. 10.1056/NEJMoa010806 [DOI] [PubMed] [Google Scholar]
- 46.Schieve LA, Ferre C, Peterson HB, Macaluso M, Reynolds MA, Wright VC. Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States. Obstet Gynecol. 2004;103:1144–1153. 10.1097/01.AOG.0000127037.12652.76 [DOI] [PubMed] [Google Scholar]
- 47.Wennerholm UB, Henningsen AKA, Romundstad LB, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28:2545–2553. 10.1093/humrep/det272 [DOI] [PubMed] [Google Scholar]
- 48.Gillon TER, Pels A, von Dadelszen P, MacDonell K, Magee LA. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One. 2014;9:e113715. 10.1371/journal.pone.0113715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kyozuka H, Yamaguchi A, Suzuki D, et al. ; Japan Environment and Children’s Study (JECS) Group . Risk factors for placenta accreta spectrum: findings from the Japan environment and Children’s study. BMC Pregnancy Childbirth. 2019;19(1):447. 10.1186/s12884-019-2608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126:654–668. 10.1097/AOG.0000000000001005 [DOI] [PubMed] [Google Scholar]
- 51.Class QA, Lichtenstein P, Långström N, D’Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosom Med. 2011;73:234–241. 10.1097/PSY.0b013e31820a62ce [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.