Abstract
Background
Irritable bowel syndrome patients report reduced disease‐specific quality of life (IBSQOL). Factors of potential relevance for QOL include gastrointestinal (GI), psychological, and somatic symptoms, demographics, and GI motor and sensory abnormalities.
Objective
The aim of our study was to evaluate the relative importance of these factors on the different IBSQOL dimensions.
Methods
We included irritable bowel syndrome (IBS) patients who completed validated questionnaires to assess QOL, stool form and frequency, GI symptom severity, psychological distress, GI‐specific anxiety, sense of coherence, and overall somatic symptom severity. Patients also underwent tests for oroanal transit time and rectal sensitivity. The nine dimensions of IBSQOL and their average (overall IBSQOL) were used as outcome variables, and factors associated with these were assessed using general linear models.
Results
We included 314 IBS patients (74% female, mean age 36.3 ± 12.2 years). Higher stool frequency, GI and overall somatic symptom severity, psychological distress, and GI‐specific anxiety were independently associated with reduced overall IBSQOL, with the model explaining 60% of the variance (p < 0.001). In models using each of the nine dimensions as outcomes, different association of demographic factors, GI symptoms, overall somatic symptom severity, psychological factors and sense of coherence were associated with reduced IBSQOL, explaining 20%–60% of the variance, with GI‐specific anxiety being the factor that contributed most frequently. Rectal sensitivity or oroanal transit time were not independently associated with any of the dimensions.
Conclusion
Different combinations of demographic factors, GI and somatic symptoms, and psychological factors are of importance for the nine IBSQOL dimensions. Gastrointestinal‐specific anxiety was the most important factor contributing to the majority of those dimensions in patients with IBS.
Keywords: disease‐specific QOL, disease‐specific quality of life, factors, IBS, IBSQOL, irritable bowel syndrome, QOL, quality of life
Key summary.
Established knowledge on this subject
Patients with irritable bowel syndrome (IBS) report reduced disease‐specific quality of life (QOL).
Factors of potential relevance for QOL include gastrointestinal (GI), non‐GI and psychological symptoms, demographic factors and GI motor and sensory abnormalities.
New findings
Demographic factors, GI, non‐GI symptoms, and psychological factors were of importance for the different dimensions of IBSQOL with different combinations for each individual dimension.
GI‐specific anxiety was the factor that most consistently explained reduced IBSQOL.
These findings can inform clinicians about factors to address in order to improve affected dimensions of IBSQOL.
Psychological interventions aiming at improving GI‐specific anxiety may have the potential to improve most of the dimensions of IBSQOL.
INTRODUCTION
Patients with IBS experience significant impairments in quality of life (QOL) compared to the general population. 1 Disease‐specific QOL in IBS (IBSQOL) is associated with the perceived IBS severity, underlying the importance of the limitations the disease imposes, rather than by the symptoms themselves. 2 Therefore, it is easily understandable that IBSQOL might be affected by several factors.
Demographic characteristics (female sex, younger age and overweight),3, 4, 5, 6 IBS‐specific variables (IBS duration, abdominal pain, diarrhea, and abdominal discomfort),3, 7, 8 somatic symptom severity,7, 9 psychological distress,7, 9 GI specific anxiety 10 and coping strategies,11, 12 have been found to be associated with reduced QOL in IBS. Moreover, some studies have shown that IBSQOL is associated with visceral hypersensitivity, psychological distress and abnormal colonic transit time, with a negative cumulative effect.11, 13, 14 However, most of the studies published so far have assessed the impact of some of the factors mentioned above on QOL, but have not considered all of the factors concomitantly. Hence, it is not completely understood how these different biopsychosocial factors interact and influence IBSQOL and its different dimensions, and their relative importance.
Therefore, taking the available literature into account, the aims of our study were (1) to assess disease‐specific QOL in IBS using IBSQOL, and (2) to evaluate preselected factors considered to be of potential importance, such as demographic factors, GI, non‐GI and psychological symptoms, GI motor and sensory abnormalities, regarding their association to IBSQOL and its nine dimensions, in order to develop comprehensive models for disease‐specific QOL in IBS. We hypothesized that IBSQOL would be reduced in our cohort, and that all the cited factors would be involved in reduced IBSQOL, with different associations for each dimension.
METHODS
Patients
We included patients with IBS from two prospective cohort studies that were conducted at the University of Gothenburg, Sweden: one large study focussing on the link between different pathophysiological factors and symptoms, and one intervention trial (NCT01252550), from which baseline data before the intervention was used. All patients were referred to our unit from primary care or via self‐referral, or responded to advertisements regarding participation in research studies, and met the Rome criteria for IBS in use at the time of inclusion (Rome II or III).15, 16 For the present study, we included patients who had completed the IBSQOL questionnaire, self‐report symptom questionnaires and tests for measurement of oroanal transit time and rectal sensitivity (see below for detailed descriptions).
All the studies were approved by the Regional Ethical Review Board in Gothenburg (731/09 and 489/02 approved in 2009 and 2002). All patients gave verbal and written informed consent before any study‐related procedures were initiated, according to the declaration of Helsinki.
Self‐report questionnaires
All patients completed the following validated self‐report questionnaires.
-
‐
The IBSQOL is a self‐administered disease‐specific QOL questionnaire for IBS. 17 Thirty items measure nine dimensions of QOL referring to the previous 4 weeks, including emotional functioning, mental health, sleep, energy, physical functioning, food, social role, physical role, and sexual relations. Each question has five or six response options (supplementary 1). table For each dimension, the score is transformed into a 0–100 scale, with a higher score representing better QOL.
-
‐
The GI symptom rating scale‐IBS (GSRS‐IBS) 18 is a questionnaire that assesses IBS symptom severity. The total score ranges from 7 to 91, with a higher score representing more severe GI symptoms.
-
‐
Stool frequency and consistency were assessed using a prospective stool diary for 14 days based on the Bristol Stool Form 19 scale.
-
‐
Psychological distress was assessed using the Hospital Anxiety and Depression Scale (HADS). 20 An overall psychological distress score range from 0 to 42. For this study we used the total HADS score (because of expected multicollinearity between HADS anxiety and depression scores) with higher scores being indicative of more severe psychological distress.
-
‐
GI‐specific anxiety was assessed using the Visceral Sensitivity Index. 21 The total scores vary from 0 to 75, with higher scores indicating higher levels of GI‐specific anxiety.
-
‐
Overall somatic symptom severity was assessed using the Patient Health Questionnaire (PHQ)‐15 22 and the Symptom Checklist (SCL)‐90‐Revised. 23 PHQ‐15 comprises 15 questions about the extent to which the subject has been bothered by somatic symptoms, each scored from 0 (not bothered at all) to 2 (bothered a lot). The total PHQ‐15 score ranges from 0 to 30 for women (due to an additional question regarding menstrual pain) and 0–28 for men. The SCL‐90 measures a broad range of psychological problems and psychopathology symptoms (90 items). In the current study, only the mean score of the somatization dimension, which includes 12 somatic symptoms, was used as a measure of overall somatic symptom severity. This score ranges from 0 to 4. In both, a higher score indicates more severe somatic symptoms.
-
‐
The Sense of Coherence scale measures the ability to respond to and endure stressful situations, 24 that is, it reflects the coping capacity to deal with everyday life stressors. The total score, which was used in this study, ranges from 29 to 203. A higher score expresses a stronger sense of coherence, that is, better ability to mobilize resources to face demanding situations.
Physiological measurements
Rectal pain threshold
In our two cohorts, two different rectal barostat protocols were used to assess rectal sensitivity. In the first cohort we used phasic isobaric distensions with stepwise increments in pressure, while in the second cohort, we used ramp inflations, as previously described. 14 We used the rectal sensory thresholds for pain as our measure of rectal sensitivity.
Oroanal transit time
The oroanal transit time was measured by using radiopaque markers, as described in greter detail elsewhere. 25
Statistical analysis
First, we aimed to assess the factors of importance for overall IBSQOL. The average of the nine dimensions of the IBSQOL was used as outcome variable, hereafter named overall IBSQOL (range 0–100). In order to identify potential differences in overall IBSQOL between IBS subtypes, we used One‐way Analysis of variance (ANOVA) and post‐hoc pairwise Tukey comparisons.
Secondly, bivariate associations between overall IBSQOL and the preselected factors of potential importance in disease‐specific QOL including gender, age, body mass index (BMI), average stool consistency and frequency, GI symptom severity, psychological distress, GI‐specific anxiety, sense of coherence, overall somatic symptom severity, rectal pain threshold, and oroanal transit time, were assessed using Pearson correlations for all variables, except for gender, where a student’ t‐test was used. HAD anxiety and depression were combined in one single sum score referring to psychological distress, since anxiety and depression demonstrated multicollinearity (r = 0.6). These analyses were considered exploratory and formed the basis for the general liner models (GLM), with the aim to identify factors independently associated with IBSQOL. Hence, the variables that presented significant bivariate associations with overall IBSQOL at p < 0.05 were used as independent variables in one step in GLM with overall IBSQOL as the dependent variable. Thereafter, the same procedure was repeated for each of the nine IBSQOL dimensions, with each of the IBSQOL dimensions as the dependent variable in the GLM.
To be able to use a single measure for overall somatic symptom severity (SCL‐90 somatization and PHQ‐15) and rectal pain threshold (from the two different protocols used in cohort 1 and 2) across our study sample, z scores were created, and used as the overall somatic symptom severity variable and the overall rectal pain threshold variable for all patients. Multicollinearity was analysed within the GLM, using variance inflation factor. No multicollinearity was found (all VIFs <3). All statistical analyses were performed using the IBM SPSS Statistics 27 software (SPSS Inc., Chicago, IL, USA). Data are presented as number (percentage) for categorical variables and mean ± standard deviation (SD) for continuous variables, with the significance level set at p < 0.05.
RESULTS
Study sample
We included 314 IBS patients, (56% and 44% according to the Rome II and III criteria respectively). Irritable bowel syndrome with diarrhea (IBS‐D) was seen in 42% of the patients, IBS with constipation (IBS‐C) in 22%, IBS mixed (IBS‐M) in 12% and IBS unsubtyped (IBS‐U) in 24%. The other characteristics of the complete study sample can be seen in Table 1. Irritable bowel syndrome patients report reduced disease‐specific quality of life in our sample can be found in Figure 1 and in the IBS subtypes as supplementary material and in table supplementary 2.
TABLE 1.
Demographic and clinical characteristics of the sample
| Study sample (n = 314) | |
|---|---|
| Age (years) (n = 314) | 36.3 ± 12.2 |
| Female gender (n = 314) | 231 (73.6) |
| BMI (kg/m2) (n = 296) | 23.4 ± 3.8 |
| IBS duration (years) (n = 289) | 11.6 ± 11.3 |
| Average stool consistency (BSF 1–7) (n = 227) | 4.2 ± 1.2 |
| Average stool frequency (stools/day) (n = 228) | 2.0 ± 1.3 |
| GI symptom severity (GSRS‐IBS) (n = 309) | 47.1 ± 11.8 |
| Psychological distress (HADS) (n = 312) | 12.8 ± 7.4 |
| GI‐specific anxiety (VSI) (n = 310) | 39.0 ± 17.2 |
| Overall somatic symptom severity (z‐score) (n = 267) | 0.0 ± 1.0 |
| Sense of coherence (n = 265) | 135.8 ± 26.4 |
| Rectal pain threshold (z‐score) (n = 314) | 1.0 ± 1.0 |
| Oroanal transit time (days) (n = 262) | 1.6 ± 1.1 |
Note: Results are presented as mean ± SD and n (%).
Abbreviations: BMI, Body Mass Index; BSF, Bristol Stool Form; GI, gastrointestinal; GSRS, Gastrointestinal Symptom Rating Scale; HADS, Hospital Anxiety and Depression Scale; IBS, Irritable Bowel Syndrome; IBSQOL, Irritable Bowel Syndrome Quality of Life questionnaire; VSI, Visceral Sensitivity Index.
FIGURE 1.
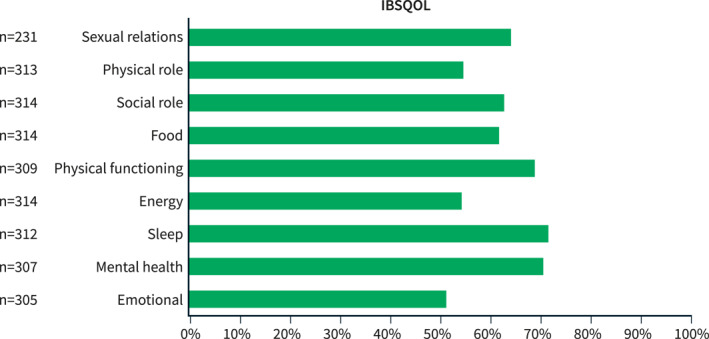
The disease‐specific quality of life (IBSQOL) dimensions. This graph presents the result of the nine dimensions of IBSQOL in our study sample, with a higher score representing better quality of life (QOL). IBSQOL: irritable bowel syndrome (IBS) QOL questionnaire. Data are presented for the mean of each IBSQOL dimension
Factors of importance for overall disease‐specific quality of life
Gender, average stool frequency, GI symptom severity, psychological distress, GI‐specific anxiety, overall somatic symptom severity, sense of coherence and rectal pain threshold were all bivariately associated with overall IBSQOL (Table 2).
TABLE 2.
Bivariate associations between study variables
| Overall IBSQOL | Emotional | Mental health | Sleep | Energy | Physical functioning | Food/diet | Social role | Physical role | Sexual relations | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.054 (0.342) | 0.165 (0.004) | 0.159 (0.005) | −0.141 (0.013) | −0.022 (0.692) | −0.083 (0.147) | 0.186 (0.001) | −0.009 (0.880) | 0.075 (0.186) | −0.019 (0.770) |
| Gender* | <0.001 | <0.001 | 0.014 | 0.129 | 0.005 | 0.017 | <0.001 | 0.452 | 0.372 | <0.001 |
| BMI | 0.077 (0.185) | 0.097 (0.101) | 0.094 (0.110) | 0.006 (0.920) | 0.041 (0.484) | −0.081 (0.165) | 0.197 (0.001) | 0.009 (0.873) | 0.026 (0.653) | 0.104 (0.123) |
| IBS duration | 0.003 (0.961) | 0.023 (0.707) | 0.097 (0.103) | −0.050 (0.399) | −0.066 (0.262) | −0.032 (0.595) | −0.043 (0.465) | −0.003 (0.965) | 0.028 (0.630) | 0.033 (0.634) |
| Average stool consistency (BSF) | −0.090 (0.175) | −0.086 (0.203) | −0.069 (0.303) | −0.016 (0.813) | −0.054 (0.421) | −0.043 (0.524) | −0.015 (0.826) | −0.156 (0.019) | −0.128 (0.054) | 0.030 (0.699) |
| Average stool frequency (stools/day) | −0.143 (0.031) | −0.055 (0.413) | 0.047 (0.482) | −0.270 (<0.001) | −0.051 (0.447) | −0.038 (0.575) | −0.085 (0.202) | −0.174 (0.009) | −0.194 (0.003) | −0.083 (0.286) |
| GI symptom severity (GSRS‐IBS) | −0.568 (p < 0.001) | −0.493 (<0.001) | −0.412 (<0.001) | −0.385 (<0.001) | −0.443 (<0.001) | −0.274 (<0.001) | −0.490 (<0.001) | −0.409 (<0.001) | −0.360 (<0.001) | −0.422 (<0.001) |
| Psychological distress (HADS) | −0.586 (<0.001) | −0.589 (<0.001) | −0.703 (<0.001) | −0.268 (0.004) | −0.473 (<0.001) | −0.316 (<0.001) | −0.271 (<0.001) | −0.425 (<0.001) | −0.390 (<0.001) | −0.321 (<0.001) |
| GI‐specific anxiety (VSI) | −0.664 (<0.001) | −0.627 (<0.001) | −0.704 (<0.001) | −0.269 (<0.001) | −0.500 (<0.001) | −0.302 (<0.001) | −0.402 (<0.001) | −0.650 (<0.001) | −0.462 (<0.001) | −0.308 (<0.001) |
| Overall somatic symptom severity (z‐score) | −0.548 (<0.001) | −0.453 (<0.001) | −0.457 (<0.001) | −0.334 (<0.001) | −0.540 (<0.001) | −0.448 (<0.001) | −0.370 (<0.001) | −0.261 (<0.001) | −0.322 (<0.001) | −0.390 (<0.001) |
| Sense of coherence | 0.526 (<0.001) | 0.567 (<0.001) | 0.592 (<0.001) | 0.185 (0.003) | 0.416 (<0.001) | 0.300 (<0.001) | 0.263 (<0.001) | 0.399 (<0.001) | 0.326 (<0.001) | 0.292 (<0.001) |
| Rectal pain threshold (z‐score) | 0.286 (<0.001) | 0.262 (<0.001) | 0.229 (<0.001) | 0.091 (0.110) | 0.207 (<0.001) | 0.175 (0.002) | 0.266 (<0.001) | 0.157 (0.005) | 0.216 (<0.001) | 0.237 (<0.001) |
| Oroanal transit time (days) | −0.024 (0.698) | −0.28 (0.653) | 0.003 (0.966) | −0.066 (0.288) | −0.057 (0.358) | −0.084 (0.178) | −0.020 (0.753) | 0.121 (0.51) | 0.065 (0.293) | −0.149 (0.040) |
Note: Pearson correlations are presented as R (p‐value). Significant associations/differences are highlighted in bold style.
Abbreviations: BMI, Body Mass Index; BSF, Bristol Stool Form; GI, gastrointestinal; GSRS, Gastrointestinal Symptom Rating Scale; HADS, Hospital Anxiety and Depression Scale; IBS, Irritable Bowel Syndrome; IBSQOL, Irritable Bowel Syndrome Quality of Life questionnaire; VSI, Visceral Sensitivity Index.
*Student‐t test are presented as p‐value.
These variables were then included in the GLM analysis (F(8,194) = 38.666, p < 0.001, R 2 = 0.599), in which higher stool frequency, GI and overall somatic symptom severity, psychological distress, and GI‐specific anxiety were independently associated with reduced overall IBSQOL. Among the included factors in the final model, GI‐specific anxiety made the strongest individual contribution to the overall IBSQOL (Table 3 and Figure 2).
TABLE 3.
General linear model for overall disease‐specific quality of life (IBSQOL)
| R 2 = 0.599, n = 203 | Overall IBSQOL | ||
|---|---|---|---|
| β | p‐value | 95% CI | |
| Demographic data | |||
| Female | 0.012 | 0.804 | −3.593; 4.630 |
| GI and somatic symptoms | |||
| Average stool frequency (stools/day) | −0.109 | 0.022 | −2.860; −0.222 |
| GI symptom severity (GSRS‐IBS) | −0.160 | 0.009 | −0.432; −0.062 |
| Overall somatic symptom severity (z‐score) | −0.171 | 0.005 | −5.362; −0.948 |
| Psychological symptoms | |||
| Psychological distress (HADS) | −0.194 | 0.011 | −0.858; −0.114 |
| GI‐specific anxiety (VSI) | −0.330 | <0.001 | −0.499; −0.231 |
| Sense of coherence | 0.092 | 0.205 | −0.036; 0.165 |
| GI sensorimotor function | |||
| Rectal pain threshold (z‐score) | 0.090 | 0.061 | −0.081; 3.459 |
Significant differences are highlighted in bold style.
Abbreviations: CI, Confidence Interval; GI, gastrointestinal; GSRS, Gastrointestinal Symptom Rating Scale; HADS, Hospital Anxiety and Depression Scale; IBS, Irritable Bowel Syndrome; IBSQOL, Irritable Bowel Syndrome Quality of Life questionnaire; VSI, Visceral Sensitivity Index.
FIGURE 2.
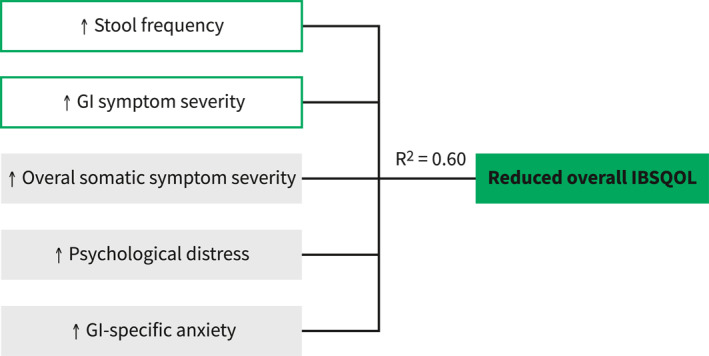
Factors of importance in overall disease‐specific quality of life (IBSQOL). Higher stool frequency, gastrointestinal (GI) symptom severity, overall somatic symptom severity, psychological distress, and GI‐specific anxiety were the factors explaining 60% of the variance of reduced overall IBSQOL. GI: gastrointestinal, IBSQOL: irritable bowel syndrome (IBS) quality of life (QOL) questionnaire
Factors of importance for the nine dimensions of the disease‐specific quality of life
For each of the nine IBSQOL dimensions, only the variables bivariately associated with the respective IBSQOL dimension (Table 2) were included in the GLM (Table 4). A summary of the variables (grouped into main categories) independently associated with the IBSQOL dimensions is displayed in Figure 3.
TABLE 4.
General linear model for disease‐specific quality of life (IBSQOL) dimensions
| Emotional functioning (n = 250) | Mental health (n = 251) | Sleep (n = 204) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p‐value | 95% CI | β | p‐value | 95% CI | β | p‐value | 95% CI | |
| Demographic data | |||||||||
| Gender a | −0.046 | 0.340 | −7.454; 2.581 | −0.014 | 0.752 | −5.558; 4.020 | ‐ | ‐ | ‐ |
| Age | 0.008 | 0.865 | −0.165; 0.196 | 0.049 | 0.248 | −0.071; 0.274 | −0.222 | <0.001 | −0.696; −0.213 |
| BMI | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| GI and somatic symptoms | |||||||||
| Average stool consistency | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Average stool frequency | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | −0.256 | <0.001 | −6.941; −2.526 |
| GI symptom severity | −0.125 | 0.033 | −0.472; −0.020 | −0.004 | 0.946 | −0.223; 0.208 | −0.289 | <0.001 | −0.898; −0.267 |
| Overall somatic symptom severity | −0.074 | 0.206 | −4.416; 0.959 | −0.059 | 0.267 | −4.018; 1.116 | −0.153 | 0.045 | −7.293; −0.090 |
| Psychological symptoms | |||||||||
| Psychological distress | −0.142 | 0.055 | −0.920; 0.009 | −0.290 | <0.001 | −1.417; −0.533 | −0.108 | 0.278 | −0.995; 0.287 |
| GI‐specific anxiety | −0.328 | <0.001 | −0.621; −0.288 | −0.431 | <0.001 | −0.785; −0.468 | −0.008 | 0.917 | −0.242; 0.217 |
| Sense of coherence | 0.226 | 0.001 | 0.080; 0.317 | 0.132 | 0.034 | 0.009; 0.234 | −0.017 | 0.861 | −0.188; 0.157 |
| GI sensorimotor function | |||||||||
| Rectal pain threshold | 0.044 | 0.359 | −1.180; 3.248 | 0.028 | 0.514 | −1.407; 2.802 | ‐ | ‐ | ‐ |
| Oroanal transit time | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Energy (n = 257) | Physical functioning (n = 254) | Food (n = 250) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p‐value | 95% CI | β | p‐value | 95% CI | β | p‐value | ||
| Demographic data | |||||||||
| Gender a | 0.007 | 0.888 | −6.130; 7.078 | 0.048 | 0.431 | −3.502; 8.183 | −0.093 | 0.110 | −10.236; 1.052 |
| Age | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 0.026 | 0.652 | −0.162; 0.258 |
| BMI | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 0.132 | 0.020 | 0.123; 1.398 |
| GI and somatic symptoms | |||||||||
| Average stool consistency | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Average stool frequency | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| GI symptom severity | −0.089 | 0.163 | −0.513; 0.087 | 0.015 | 0.841 | −0.238; 0.292 | −0.280 | <0.001 | −0.772; −0.266 |
| Overall somatic symptom severity | −0.311 | <0.001 | −12.353; −5.221 | −0.344 | <0.001 | −10.545; −4.249 | −0.098 | 0.163 | −5.160; 0.872 |
| Psychological symptoms | |||||||||
| Psychological distress | −0.159 | 0.044 | −1.208; −0.017 | −0.062 | 0.504 | −0.722; 0.356 | 0.019 | 0.220 | −0.456; 0.570 |
| GI‐specific anxiety | −0.233 | 0.001 | −0.611; −0.169 | −0.100 | 0.201 | −0.324; 0.069 | −0.204 | 0.005 | −0.455; −0.081 |
| Sense of coherence | 0.031 | 0.670 | −0.120; 0.186 | 0.058 | 0.498 | −0.090; 0.184 | 0.048 | 0.553 | −0.091; 0.169 |
| GI sensorimotor function | |||||||||
| Rectal pain threshold | 0.013 | 0.796 | −2.470; 3.218 | −0.094 | 0.114 | −0.811; 4.239 | 0.064 | 0.263 | −1.058; 3.852 |
| Oroanal transit time | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Social role (n = 202) | Physical role (n = 203) | Sexual relations (n = 178) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p‐value | 95% CI | β | p‐value | 95% CI | β | p‐value | 95% CI | |
| Demographic data | |||||||||
| Gender a | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | −0.200 | 0.009 | −20.530; −3.029 |
| Age | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| BMI | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| GI and somatic symptoms | |||||||||
| Average stool consistency | −0.122 | 0.023 | −4.932; −0.373 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Average stool frequency | −0.108 | 0.051 | −4.166; 0.008 | −0.183 | 0.004 | −7.322; −1.444 | ‐ | ‐ | ‐ |
| GI symptom severity | −0.079 | 0.250 | −0.440; 0.115 | −0.104 | 0.204 | −0.689; 0.148 | −0.263 | 0.004 | −1.001; −0.189 |
| Overall somatic symptom severity | 0.102 | 0.132 | −0.767; 5.792 | −0.038 | 0.633 | −6.044; 3.687 | −0.030 | 0.741 | −5.429; 3.867 |
| Psychological symptoms | |||||||||
| Psychological distress | −0.037 | 0.663 | −0.682; 0.435 | −0.081 | 0.423 | −1.189; 0.501 | −0.100 | 0.321 | −1.184; 0.391 |
| GI‐specific anxiety | −0.585 | <0.001 | −1.065; −0.662 | −0.231 | 0.006 | −0.735; −0.127 | −0.029 | 0.746 | −0.328; 0.235 |
| Sense of coherence | 0.112 | 0.172 | −0.046; 0.257 | 0.143 | 0.144 | −0.058; 0.398 | 0.061 | 0.511 | −0.134; 0.267 |
| GI sensorimotor function | |||||||||
| Rectal pain threshold | 0.024 | 0.659 | −2.094; 3.302 | 0.081 | 0.209 | −1.436; 6.536 | 0.105 | 0.136 | −0.866; 6.342 |
| Oroanal transit time | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | −0.055 | 0.424 | −4.503; 1.903 |
Significant results are highlighted in bold style.
Abbreviations: BMI, Body Mass Index; GI, gastrointestinal; IBS, Irritable Bowel Syndrome.
: 0: male and 1: female.
FIGURE 3.
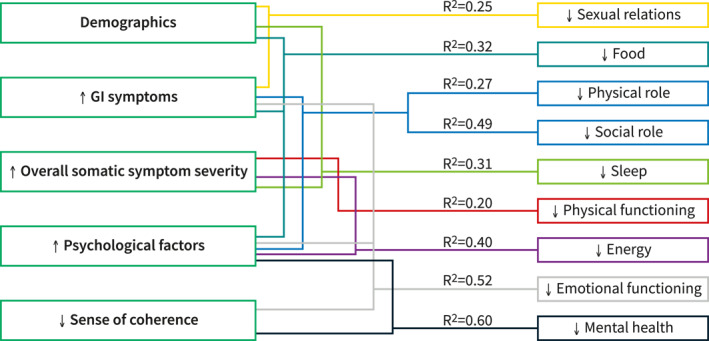
Factors of importance in the different disease‐specific quality of life (IBSQOL) dimensions. Figure 3 summarizes the variables (grouped into main categories) independently associated with the IBSQOL dimensions, including the proportion of the variance explained by each model (R 2 values). The R 2 values for the models varied between 0.20 and 0.60, with different combinations of factors independently associated with the IBSQOL dimensions. GI: gastrointestinal, IBSQOL: irritable bowel syndrome (IBS) quality of life (QOL) questionnaire
The proportion of the variance explained by the models varied between 0.20 and 0.60, with different combinations of factors being independently associated with the IBSQOL dimensions. Among the individual variables included in the GLMs, all but rectal sensitivity and oroanal transit time made a unique statistically significant contribution to at least one model for the IBSQOL dimensions, with GI‐specific anxiety being included in the largest number of models (Table 4). The main findings of the GLM for the IBSQOL dimensions, with details presented in Table 4, are:
-
*
The GLM for emotional functioning explained 52% of the variance (F(8,241) = 34.657, p < 0.001, R 2 = 0.52), with higher GI symptom severity, GI‐specific anxiety, and lower sense of coherence being independently associated with reduced emotional QOL.
-
*
The GLM for mental health explained 60% of the variance (F(8,242) = 48.439, p < 0.001, R 2 = 0.60), and revealed that higher levels of psychological distress, and GI‐specific anxiety, and lower levels of sense of coherence were independently associated with reduced mental health QOL.
-
*
The GLM for sleep explained 31% of the variance (F(7,195) = 13.808, p < 0.001, R 2 = 0.31), and being older and reporting higher stool frequency, GI and overall somatic symptom severity were independently associated with reduced sleep QOL.
-
*
The GLM for energy explained 40% of the variance (F(7,249) = 25.370, p < 0.001, R 2 = 0.40), with higher psychological distress, GI‐specific anxiety and overall somatic symptom severity being independently associated with reduced energy QOL.
-
*
The GLM for physical functioning explained 20% of the variance (F(7,246) = 10.021, p < 0.001, R 2 = 0.20), with higher overall somatic symptom severity being independently associated with reduced physical functioning QOL.
-
*
The GLM for food explained 32% of the variance (F(9,240) = 14.292, p < 0.001, R 2 = 0.32), with higher GI symptom severity and GI‐specific anxiety, and lower BMI being independently associated with reduced food QOL.
-
*
The GLM for social role explained 49% of the variance (F(8,193) = 25.521, p < 0.001, R 2 = 0.49), with higher GI‐specific anxiety and looser stools being independently associated with reduced social role QOL.
-
*
The GLM for physical role explained 27% of the variance (F(7,195) = 11.743, p < 0.001, R 2 = 0.27), and higher GI‐specific anxiety and stool frequency were independently associated with reduced physical role QOL.
-
*
The GLM for sexual relations explained 25% of the variance (F(8,169) = 8.570, p < 0.001, R 2 = 0.25), with higher GI symptom severity and female gender being independently associated with reduced sexual relations QOL.
DISCUSSION
Our study is the first to analyse the importance of different biopsychosocial factors in a comprehensive model of the different dimensions of disease‐specific QOL in IBS patients. The factors of importance explaining reduced overall IBSQOL included higher stool frequency, more severe GI and overall somatic symptoms, psychological distress, and GI‐specific anxiety. Each IBSQOL dimension was explained by a different set of factors and not all factors were involved in all the different dimensions, highlighting that different aspects related to the patients' and their health can influence individual QOL dimensions differently.
The patients included in this study is representative of what we report in the larger Swedish IBS group, and clearly demonstrates reduced disease‐specific QOL. 26 However, the IBSQOL scores of our sample deviate somewhat from previously published IBS samples from the US and the UK. Emotional, physical and social roles were higher in our sample while physical functioning was lower. These differences could potentially be explained by differences in recruitment periods and by differences in form of recruitment. 17 In our study, patients were included in a secondary/tertiary care centre through primary care referral, self‐referral or via advertisements, while in Hahn et al. the IBS patients were selected from a random sample from US and UK patient organizations. 17 However, the general feature of IBS having a significant negative impact on QOL is shared among the studies.
Our second aim was to identify the factors involved in reduced overall IBSQOL. All the factors independently involved, including higher stool frequency, GI symptom severity, overall somatic symptom severity, psychological distress and GI‐specific anxiety, have been previously identified as factors associated with disease‐specific QOL in IBS in individual studies.3, 7, 8, 9, 11, 13, 14 The other important point was to assess the overall and relative importance of these factors. Together, they explained a substantial degree of the variance of overall IBSQOL, that is, 60%. The most important factor involved in explaining overall IBSQOL was GI‐specific anxiety. This result is congruent with previous research, as GI‐specific anxiety has been identified as an important factor for GI symptom severity and QOL in IBS patients. 10 The physiological variables included in this study, that is, oroanal transit time and rectal sensitivity, were not independently associated with overall IBSQOL, or with any of the IBSQOL dimensions. These pathophysiological variables have previously been found to be moderately associated with different GI symptoms in IBS, including an association between stool frequency and consistency and oroanal transit time, and between overall GI symptom severity and rectal sensitivity.25, 27 Both higher stool frequency and overall GI symptom severity were independently associated with overall IBSQOL and beyond that, no unique contributions of the physiological variables on disease‐specific QOL in IBS could be identified in our study.
We also aimed to identify factors that made unique contributions to each of the nine dimensions of IBSQOL. Most of the factors involved can be expected from and are congruent with previous literature, or by the nature of the questions included in the IBSQOL dimensions. In contrast with overall IBSQOL, demographics were relevant for three individual dimensions of IBSQOL. Older age was associated with reduced sleep QOL, which is in accordance with findings in the general population. 28 A lower BMI was linked to reduced food QOL and could potentially be explained by associated eating disorders 29 or by food avoidance and restriction that is commonly seen in IBS and other disorders of gut‐brain interaction. 30 Furthermore, female gender was associated with reduced sexual QOL, which is in line with published results in the young general population. 31 However, the absence of an independent association between gender and social role QOL was surprising, as men are often expected to have better social role scores. 32 Different measures of the GI symptoms were independently associated with most of the IBSQOL dimensions, except for energy, physical functioning, and mental health. However, in the existing literature, the effect of GI symptoms per se is limited in explaining large parts of the reduced QOL, and other factors may be of greater importance. 33 In our study, overall GI symptom severity only contributed uniquely to three IBSQOL dimensions, food, sexual relations, and sleep. The importance of GI symptom severity has already been highlighted in the literature for food 26 and for sexual dysfunction. 34 Regarding sleep, both GI symptom severity and stool frequency were found to be of importance. Sleep disturbances are already known to correlate with GI pain, 35 which is an important part of overall GI symptom severity in IBS. Regarding stool frequency, we hypothesize that frequent stools during the night or early in the morning may have a negative impact on sleep. Another potential explanation could involve the role of circadian rhythms in symptom generation. 36 Overall somatic symptom severity was the most important factor in explaining energy and physical functioning QOL, which is expected, as these dimensions mostly assess physical activity and overall somatic symptom severity includes symptoms influencing the overall activity level, including fatigue. In line with this, overall somatic symptoms also made a unique contribution to sleep, but to a lesser degree than previously reported. 35 In the existing literature, psychological factors have consistently been found to be of importance for QOL.7, 9, 14 Our study confirmed the importance of psychological factors for IBSQOL, since they were involved in most of the models explaining the IBSQOL dimensions, except for sleep, physical functioning and sexual relations. Among the psychological factors included in our study, GI‐specific anxiety was the factor that was included in the largest number of models explaining the individual IBSQOL dimensions, supporting the relevance of this construct in IBS. GI‐specific anxiety has also recently been found to be an important mediator of the effect of GI symptom severity on generic mental QOL in IBS. 37 In addition, psychological distress, including general anxiety and depression, was associated with mental health and energy, which could be expected, considering the existing literature. 7 Based on its definition, we expected sense of coherence to be more strongly associated with disease‐specific QOL in IBS, but unexpectedly, it was only independently associated with emotional functioning and mental health. Sense of coherence may also serve as a mediator of the effects of GI symptom severity on mental and other aspects of QOL, 12 but this was not specifically addressed in our study.
There are strengths and limitations to our study. The strengths of our study include the high number of well characterized subjects, and the use of validated questionnaires. Additionally, we considered a combination of a wide range of symptoms and also measurements of GI function that could explain disease‐specific QOL in our IBS sample. Furthermore, we have not only analysed the factors of importance for overall IBSQOL but also for its nine dimensions in comprehensive integrative models. Limitations include the recruitment in a secondary/tertiary care centre, potentially limiting generalizability to subjects with IBS in primary care and those who do not seek health care for their symptoms. There are also factors of potential importance for disease‐specific QOL in IBS that were not analysed in this study, including for example, other diseases, medication use, physical conditions and disabilities, country, environment, financial aspects, culture, or stress. We also have a proportion of subjects with missing data for the stool diaries but also for sexual QOL (available in 231 of 314 patients). The missing data on the latter may be explained by the construction of the questionnaire, with subjects reporting absence of sexual activity during the previous 4 weeks by definition having missing data in this dimension. The different proportion of men and women responding to this dimension in our sample should also be considered as a possible source of bias (76% vs. 56% of men vs. women).
In conclusion, in this study we have demonstrated that combinations of factors, including demographics, GI and overall somatic symptoms, and psychological factors are of importance for disease‐specific QOL in IBS. Different combinations of these factors differently influenced the nine individual dimensions of IBSQOL. These combinations can be used as a source of information to guide clinicians regarding factors of importance to influence in order to improve various aspects of disease‐specific QOL in IBS. The factor that contributed strongest and most consistently to IBSQOL was GI‐specific anxiety. Hence, in line with suggestions from longitudinal studies in IBS, this factor seems to be important to assess and target in order to improve the overall disease‐specific QOL in IBS. 38
CONFLICT OF INTEREST
Chloé Melchior has served as a consultant/advisory board member for Kyowa Kirin, Norgine, Biocodex, Mayoly Spindler, Tillots, and Ipsen. Magnus Simrén has received unrestricted research grants from Danone Nutricia Research, and Glycom; served as a consultant/advisory board member for Danone Nutricia Research, Ironwood, Menarini, Biocodex, GeneticAnalysis AS, Glycom, Arena, and Adnovate and been on the Speakers' bureau for Tillotts, Menarini, Kyowa Kirin, Takeda, Shire, Biocodex, Alimentary Health, Alfasigma, and Falk Foundation. IT has served as a consultant for Pfizer Inc. Esther Colomier, Johann P. Hreinsson and Hans Törnblom have no conflicts of interest to declare.
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
The study was funded by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF‐agreement (ALFGBG‐726561, 722331, 875581), the Swedish Research Council (2018–02566), and the Faculty of Medicine at the University of Gothenburg. CM has been awarded the UEG Research Award 2020 for her stay at The University of Gothenburg. Mahrukh Khadija has been awarded Mary von Sydow research award 2021.
Melchior C, Colomier E, Trindade IA, Khadija M, Hreinsson JP, Törnblom H, et al. Irritable bowel syndrome: factors of importance for disease‐specific quality of life. United European Gastroenterol J. 2022;10(7):754–64. 10.1002/ueg2.12277
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health‐related quality of life. Gastroenterology. 2000;119(3):654–60. 10.1053/gast.2000.16484 [DOI] [PubMed] [Google Scholar]
- 2. Hahn BA, Kirchdoerfer LJ, Fullerton S, Mayer E. Patient‐perceived severity of irritable bowel syndrome in relation to symptoms, health resource utilization and quality of life. Aliment Pharmacol Ther. 1997;11(3):553–9. 10.1046/j.1365-2036.1997.00160.x [DOI] [PubMed] [Google Scholar]
- 3. Amouretti M, Le Pen C, Gaudin AF, Bommelaer G, Frexinos J, Ruszniewski P, et al. Impact of irritable bowel syndrome (IBS) on health‐related quality of life (HRQOL). Gastroenterol Clin Biol. 2006;30(2):241–6. 10.1016/s0399-8320(06)73160-8 [DOI] [PubMed] [Google Scholar]
- 4. Bjorkman I, Jakobsson Ung E, Ringstrom G, Tornblom H, Simren M. More similarities than differences between men and women with irritable bowel syndrome. Neuro Gastroenterol Motil. 2015;27(6):796–804. 10.1111/nmo.12551 [DOI] [PubMed] [Google Scholar]
- 5. Dong Y, Berens S, Eich W, Schaefert R, Tesarz J. Is body mass index associated with symptom severity and health‐related quality of life in irritable bowel syndrome? A cross‐sectional study. BMJ Open. 2018;8(10):e019453. 10.1136/bmjopen-2017-019453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minocha A, Johnson WD, Abell TL, Wigington WC. Prevalence, sociodemography, and quality of life of older versus younger patients with irritable bowel syndrome: a population‐based study. Dig Dis Sci. 2006;51(3):446–53. 10.1007/s10620-006-3153-8 [DOI] [PubMed] [Google Scholar]
- 7. Seres G, Kovacs Z, Kovacs A, Kerekgyarto O, Sardi K, Demeter P, et al. Different associations of health related quality of life with pain, psychological distress and coping strategies in patients with irritable bowel syndrome and inflammatory bowel disorder. J Clin Psychol Med Settings. 2008;15(4):287–95. 10.1007/s10880-008-9132-9 [DOI] [PubMed] [Google Scholar]
- 8. Singh P, Staller K, Barshop K, Dai E, Newman J, Yoon S, et al. Patients with irritable bowel syndrome‐diarrhea have lower disease‐specific quality of life than irritable bowel syndrome‐constipation. World J Gastroenterol. 2015;21(26):8103–9. 10.3748/wjg.v21.i26.8103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lackner JM, Gudleski GD, Thakur ER, Stewart TJ, Iacobucci GJ, Spiegel BM. The impact of physical complaints, social environment, and psychological functioning on IBS patients' health perceptions: looking beyond GI symptom severity. Am J Gastroenterol. 2014;109(2):224–33. 10.1038/ajg.2013.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jerndal P, Ringstrom G, Agerforz P, Karpefors M, Akkermans LM, Bayati A, et al. Gastrointestinal‐specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neuro Gastroenterol Motil. 2010;22(6):646–e179. 10.1111/j.1365-2982.2010.01493.x [DOI] [PubMed] [Google Scholar]
- 11. Knowles SR, Austin DW, Sivanesan S, Tye‐Din J, Leung C, Wilson J, et al. Relations between symptom severity, illness perceptions, visceral sensitivity, coping strategies and well‐being in irritable bowel syndrome guided by the common sense model of illness. Psychol Health Med. 2017;22(5):524–34. 10.1080/13548506.2016.1168932 [DOI] [PubMed] [Google Scholar]
- 12. Stanculete MF, Matu S, Pojoga C, Dumitrascu DL. Coping strategies and irrational beliefs as mediators of the health‐related quality of life impairments in irritable bowel syndrome. J Gastrointestin Liver Dis. 2015;24(2):159–64. 10.15403/jgld.2014.1121.242.strt [DOI] [PubMed] [Google Scholar]
- 13. Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, et al. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28(4):484–90. 10.1111/j.1365-2036.2008.03759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simren M, Tornblom H, Palsson OS, Van Oudenhove L, Whitehead WE, Tack J. Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient‐reported outcomes in irritable bowel syndrome. Gastroenterology. 2019;157(2):391–402.e2. 10.1053/j.gastro.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 15. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. 10.1053/j.gastro.2005.11.061 [DOI] [PubMed] [Google Scholar]
- 16. Thompson WGLG, Drossman DA, Heaton KW, Irvine EJ, Müller‐Lissner SA, Corazziari E, et al. Rome II: the functional gastrointestinal disorders diagnosis, pathophysiology and treatment: a multinational consensus. Degnon Associates. 2000:351–432. [Google Scholar]
- 17. Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion. 1999;60(1):77–81. 10.1159/000007593 [DOI] [PubMed] [Google Scholar]
- 18. Wiklund IK, Fullerton S, Hawkey CJ, Jones RH, Longstreth GF, Mayer EA, et al. An irritable bowel syndrome‐specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38(9):947–54. 10.1080/00365520310004209 [DOI] [PubMed] [Google Scholar]
- 19. O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300(6722):439–40. 10.1136/bmj.300.6722.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 21. Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom‐specific anxiety scale. Aliment Pharmacol Ther. 2004;20(1):89–97. 10.1111/j.1365-2036.2004.02007.x [DOI] [PubMed] [Google Scholar]
- 22. Kroenke K, Spitzer RL, Williams JB. The PHQ‐15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–66. 10.1097/00006842-200203000-00008 [DOI] [PubMed] [Google Scholar]
- 23. Derogatis LR, Rickels K, Rock AF. The SCL‐90 and the MMPI: a step in the validation of a new self‐report scale. Br J Psychiatry. 1976;128(3):280–9. 10.1192/bjp.128.3.280 [DOI] [PubMed] [Google Scholar]
- 24. Antonovsky A. Unraveling the mystery of health: how people manage stress and stay well. Jossey‐Bass; 1987. [Google Scholar]
- 25. Tornblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simren M. Colonic transit time and IBS symptoms: what's the link? Am J Gastroenterol. 2012;107(5):754–60. 10.1038/ajg.2012.5 [DOI] [PubMed] [Google Scholar]
- 26. Melchior C, Algera J, Colomier E, Tornblom H, Simren M, Storsrud S. Food avoidance and restriction in irritable bowel syndrome: relevance for symptoms, quality of life and nutrient intake. Clin Gastroenterol Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 27. Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133(4):1113–23. 10.1053/j.gastro.2007.07.024 [DOI] [PubMed] [Google Scholar]
- 28. Vitiello MV, Larsen LH, Moe KE. Age‐related sleep change: gender and estrogen effects on the subjective‐objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res. 2004;56(5):503–10. 10.1016/s0022-3999(04)00023-6 [DOI] [PubMed] [Google Scholar]
- 29. Melchior C, Desprez C, Riachi G, Leroi AM, Dechelotte P, Achamrah N, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatr. 2019;10:928. 10.3389/fpsyt.2019.00928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray HB, Kuo B, Eddy KT, Breithaupt L, Becker KR, Dreier MJ, et al. Disorders of gut‐brain interaction common among outpatients with eating disorders including avoidant/restrictive food intake disorder. Int J Eat Disord. 2021;54(6):952–8. 10.1002/eat.23414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ljungman L, Lampic C, Wettergren L. Sexual dysfunction among young adults in Sweden‐A population‐based observational study. Sex Med. 2020;8(4):631–42. 10.1016/j.esxm.2020.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sullivan M, Karlsson J. The Swedish SF‐36 Health Survey III. Evaluation of criterion‐based validity: results from normative population. J Clin Epidemiol. 1998;51(11):1105–13. 10.1016/s0895-4356(98)00102-4 [DOI] [PubMed] [Google Scholar]
- 33. Michalsen VL, Vandvik PO, Farup PG. Predictors of health‐related quality of life in patients with irritable bowel syndrome. A cross‐sectional study in Norway. Health Qual Life Outcome. 2015;13(1):113. 10.1186/s12955-015-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fass R, Fullerton S, Naliboff B, Hirsh T, Mayer EA. Sexual dysfunction in patients with irritable bowel syndrome and non‐ulcer dyspepsia. Digestion. 1998;59(1):79–85. 10.1159/000007471 [DOI] [PubMed] [Google Scholar]
- 35. Patel A, Hasak S, Cassell B, Ciorba MA, Vivio EE, Kumar M, et al. Effects of disturbed sleep on gastrointestinal and somatic pain symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(3):246–58. 10.1111/apt.13677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orr WC, Fass R, Sundaram SS, Scheimann AO. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(6):616–24. 10.1016/s2468-1253(19)30412-1 [DOI] [PubMed] [Google Scholar]
- 37. Trindade IA, Melchior C, Tornblom H, Simren M. Quality of life in irritable bowel syndrome: exploring mediating factors through structural equation modelling. J Psychosom Res. 2022;159:110809. 10.1016/j.jpsychores.2022.110809 [DOI] [PubMed] [Google Scholar]
- 38. Clevers E, Tack J, Tornblom H, Ringstrom G, Luyckx K, Simren M, et al. Development of irritable bowel syndrome features over a 5‐year period. Clin Gastroenterol Hepatol. 2018;16(8):1244–51.e1. 10.1016/j.cgh.2018.02.043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data available on request from the authors.


