Abstract
In cancer metastasis, extravasation refers to the process where tumor cells exit the bloodstream by crossing the endothelium and invade the surrounding tissue. Tumor cells engage in complex crosstalk with other active players such as the endothelium leading to changes in functional behavior that exert pro-extravasation effects. Most in vitro studies to date have only focused on the independent effects of molecular targets on the functional changes of cancer cell extravasation behavior. However, singular targets cannot combat complex interactions involved in tumor cell extravasation that affects multiple cell types and signaling pathways. In this study, we employ an organotypic microfluidic model of human vasculature to investigate the independent and combined role of multiple upregulated secreted factors resulting from cancer-vascular interactions during cancer cell extravasation. The device consists of a tubular endothelial vessel generated from induced pluripotent stem cell derived endothelial cells within a collagen-fibrinogen matrix with breast cancer cells injected through and cultured along the lumen of the vessel. Our system identified cancer-vascular crosstalk, involving invasive breast cancer cells, that results in increased levels of secreted IL-6, IL-8 and MMP-3. Our model also showed that upregulation of these secreted factors correlates with invasive/metastatic potential of breast cancer cells. We also used therapeutic inhibitors to assess the independent and combined role of multiple signaling factors on the overall changes in functional behavior of both the cancer cells and the endothelium that promote extravasation. Taken together, these results demonstrate the potential of our organotypic model in elucidating mechanisms through which cancer-vascular interactions can promote extravasation, and in conducting functional assessment of therapeutic drugs that prevent extravasation in cancer metastasis.
Keywords: Cancer cell extravasation, Induced pluripotent stem cell-derived endothelial cells, Microfluidic in vitro model, Therapeutic drug testing platform, Endothelial vessels
Introduction
Breast cancer is the second leading cause of cancer-related death in women1. Most patients who experience relapses display disseminated metastases rather than isolated local recurrences2. Thus, targeting metastasis, especially in the later stages of cancer progression, is critical to improving patient outcomes.
One key event in the metastatic cascade is the extravasation of circulating tumor cells (CTCs) out of blood vessels. Extravasation of tumor cells involves two critical steps: 1) arrest of CTCs on the blood vessel endothelium and 2) migration of CTCs across the endothelium through a process called transendothelial migration3. Recent findings suggest that multiple routes exist leading to each event in the extravasation process. For instance, CTCs can be arrested not only by entrapment within capillary vessels/capillary bifurcations where the capillary diameter is less than the diameter of CTCs, but also by attachment to the endothelia of larger vessels where the shear flow is relatively low, via strong adhesion4. Entry into the perivascular space can also involve several mechanisms including CTC migration across an intact endothelium via a paracellular route or transcellular migration by disruption of the endothelium5. Understanding the molecular signaling that governs tumor cell behavior (i.e., adhesion, proliferation and migration) and its interactions with surrounding tissue (i.e., the endothelium) during extravasation are crucial to identifying potential therapeutic targets for metastatic spread.
Currently, breast cancer metastasis can be studied in mice by tail vein, intracardial or orthotopic injections of breast cancer cells6. However, in addition to low throughput due to practical constraints on the use of animal models (e.g., cost and time), there are important differences between human and mouse biology that hinder the identification of actionable targets in the clinic. In vitro approaches, on the other hand, often lack the three-dimensional (3D) complexity, spatial organization and relevant cell-to-cell and cell-extracellular matrix (ECM) interactions for modeling in vivo responses. Thus there is a compelling need for human-relevant in vitro models that can reflect relevant 3D architectures of the in vivo microenvironment and capture the complex process of cancer metastasis.
Tumor cell extravasation into intact organs has been observed in animal models, but these events are rare and transient in nature, making it a difficult process to study. Organ-on-a-chip modeling techniques enable us to create multi-organ interactions and generate organ-specific tissue functions by providing precise control over 3D environmental features (e.g., tissue architectures and biomechanical cues), and microenvironmental interactions (e.g., cell-to-cell, cell-matrix, and soluble factor signaling)7,8. In recent years, several microengineered platforms have been reported that can better recapitulate the interactions between human-relevant cells sources (e.g., cancer cells and endothelial cells) and the ECM observed in vivo to gain mechanistic insights into the process of cancer metastasis9–12. Moreover, microfluidic in vitro models of tumor cell extravasation are beginning to emerge, and these models are specifically well-suited to investigate cancer-specific molecular mechanisms resulting from biophysical and biomolecular cues that would otherwise be challenging in conventional in vitro systems13,14. These microfluidic devices have been designed to model tissue invasion15, systemic flow of tumor cells in circulation and their endothelial capture from flow, and subsequent extravasation through the endothelial monolayer16 or microvascular networks17 towards a chemotactic gradient generated by organ-specific cells18,19. As tumor cells are not independent players in metastatic spread, several studies using these models have shed light on the impact of cancer-associated interactions with immune cells20,21, the vasculature22,23, and the underlying extracellular matrix containing components of secondary metastatic sites18,19,24 (e.g., bone and muscle) in generating pro-extravasation effects. These findings underscore the importance of exploring cancer-associated molecular signaling and cancer cell-mediated changes to the microenvironment, such as matrix remodeling, endothelial activation, and impairment to endothelial barrier function as potential therapeutic targets in metastatic progression. However, most investigations of tumor cell extravasation have been limited to examining the independent effects of molecular targets on cancer cell behavior, and often lack insight into cancer-associated molecular and functional changes to the microenvironment. Moreover, these microscale models have not been exploited to study the direct involvement of multiple signaling interactions and their additive influence on the creation of microenvironments facilitating cancer cell extravasation. In this work, we investigate the role that several cancer-vascular signaling factors play, individually and in combination, in altering cellular function and the components of the microenvironment to promote tumor cell extravasation.
In this study, we employ a human organotypic vascularized model to study breast cancer cell (BCC) extravasation and cancer-vascular interactions that facilitate modulation of endothelial properties and increase events of extravasation. One of the advantages of our approach is that the arrayable microfluidic platform allows us to directly investigate different combinations of therapeutics to probe specific molecular signaling factors in a robust and controlled manner. In particular, we identify multiple molecular targets and examine their influence on the functional behavior of cancer cells and the endothelium during extravasation. Our results show that BCCs can activate endothelial cells (ECs) through paracrine signaling and modulate components of the endothelial basement membrane during extravasation and, moreover, that paracrine signaling alone has significant impact on vascular barrier function. We examine the role of cancer-mediated IL-6, MMP-3 and IL-8 paracrine signaling in vascular disruption, basement membrane degradation and increased extravasation events using clinically active therapeutics. Finally and most importantly, we demonstrate the individual and combined effects of these molecular targets on the overall molecular secretion profiling and functional responses of both cancer cells and the endothelium. Our findings provide a strong rationale for exploring additive and synergistic effects of multiple signaling interactions as therapeutic approaches for preventing cancer cell metastatic progression.
Materials and Methods
Organotypic vascular device fabrication and assembly
The organotypic model of cancer cell extravasation presented here is fabricated using the LumeNEXT platform25. Details of device fabrication and vasculature model formation can be found elsewhere25,26. This approach allows for fabrication of one or more lumen structures with variable sizes, configuration and lumen spacing controlled by micromold design in soft lithography. Here, a three-lumen device was designed, with each lumen having a separate inlet and outlet port oriented in parallel to one another. All lumens were designed to be within a single chamber accompanied by perpendicularly oriented side ports. Two stacked PDMS layers, with microscale features patterned into them, formed the chamber with three lumen structures in which ECM gels can be loaded, (Figure 1a). Once the gel is polymerized, removable PDMS rods form the hollow lumen structures surrounded by an ECM gel. PDMS rods used this study were approximately ∼260 μm - ∼337 μm in diameter and ∼500 μm apart. The gel was ∼ 3 mm in length, ∼ 5 mm in width and 1.25 mm high, (Supplementary Figure S1). Once assembled, the PDMS layers were oxygen-plasma bonded onto a glass-bottom culture dish (P50G-1.5–30-F, MatTek Corporation, Ashland, MA) using a Diener Electronic Femto Plasma Surface System.
Figure 1. 3D organotypic BCC extravasation model.

(a) Schematic representation of device layers and assembly. The top layer forms the cover of the devices and consists of features that make up the top-half of ECM gel chamber and ports for fluid handling / cell seeding. The bottom layer consists of features making up the bottom-half of the ECM gel chamber and channels that support the three lumen rods suspended across the gel chamber. The assembled layers are plasma-bonded to a glass cover-slip. (b) Schematic isometric and cross-section view of the BCC extravasation model that mimics the structure of blood vessels surrounded by ECM and cancer cell extravasation across blood vessels. Top inset shows a photograph of the assembled microfluidic device. (c) Experimental workflow and cell seeding configuration of the BCC extravasation model. (d) Confocal projection of center lumen lined with iPSC-ECs that form an endothelialized vessel as characterized by expression of endothelial marker CD31 (i) and VE-cadherin (ii), scale bars indicate 150 µm (e) Confocal microscopy of human BCC line MDA-MB-231 extravasating across iPSC-EC vessels and COL IV (top inset) deposited by iPSC-ECs. Cross-sectional 3D reconstruction of COL IV deposition and extravasated MDA-MB-231 cells (bottom inset), scale bar indicates 150 µm.
ECM gel preparation and loading
The bonded devices were UV sterilized for 20 min and moved to the biosafety cabinet prior to ECM gel loading. To promote matrix adhesion to PDMS, the device chambers were treated with 2% polyethylenimine (03880, Sigma-Aldrich, Saint Louis, Missouri) in deionized (DI) water solution for 10 min, followed by a 30 min treatment of 0.1% glutaraldehyde (G6357, Sigma-Aldrich, Saint Louis, Missouri) in DI water at room temperature. Devices were then flushed with DI water solution 5 times to remove excess glutaraldehyde. A mix of rat-tail collagen type I solution (Col-I) (354249, BD Biosciences, San Jose, CA) neutralized with 0.5N sodium hydroxide (1310-73-2, Fisher Scientific, Pittsburgh, PA), fibrinogen (9001-32-5, Sigma-aldrich, St. Louis, MO), 7.5 pH 5X phosphate buffered saline (PBS) and Induced pluripotent stem cell-derived endothelial cells (iPSC-EC) complete growth medium was prepared at a final concentration of 3 mg/mL Col-I and 1 mg/mL fibrinogen to form the ECM gel. The pH of the ECM mix was adjusted to 7.2 pH prior to loading the mix into the gel-chamber of the device. 15µL of prepared gel solution was loaded into each device and incubated at room temperature for 20 min then moved to an incubator at 37°C for 1 h.
Cell culture
iPSC-ECs (Cellular Dynamics International, Madison, WI) were maintained in Vasculife Basal maintenance media (LM-0002, LifeLine Cell Technologies Frederick, MD) supplemented with iCell Endothelial Cell Medium Supplement (Cellular Dynamics International) and used up to passage 6. GFP expressing MCF-7 cells and MDA-MB-231 cells were generated by infecting cells with lentiviruses expressing turbo-GFP (pGIPz-turboGFP vector, purchased from Addgene) and selecting for stable expression in 5µg/ml puromycin for 5–7 days. MDA-MB-231 triple negative BBCs and MCF-7 HER2 negative BCCs were cultured in DMEM (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (FBS, 35-010-CV, Corning-cellgro, Manassas, VA) and 1% Penicillin-Streptomycin (Pen/Strep, 15070063, ThermoFisher Scientific, Waltham, MA,) up to passage 20. Hs-578T triple negative BCCs were maintained in DMEM (ATCC) supplemented with 10% FBS (Corning-cellgro), 0.01 mg/ml bovine insulin (ThermoFisher Scientific) and 1% Pen/Strep (ThermoFisher Scientific) up to passage 12. Prior to cell seeding within the device, Hs-578T cells were stained with CellTracker Green CMFDA (C7–25, ThermoFisher Scientific) in PBS for 10 min.
Cell seeding and lumen formation
To generate lumen structures, a droplet of 30 µg/mL fibronectin solution (Sigma-Aldrich) in iPSC-EC complete growth media was added to the outlet port of the center lumens and PDMS rods was pulled out from the inlet or outlet ports with a fine-tip tweezer, leaving behind a fluid-filled lumen within the ECM gel. The fibronectin solution in the center lumens was then incubated at 37°C for 20 min. Following incubation, the fibronectin solution was aspirated out of the center lumens prior to iPSC-EC seeding. iPSC-ECs were trypsinized with 0.05% Trypsin-EDTA (25300062, Thermo Fisher, Waltham, Massachusetts), suspended in iPSC-EC complete growth media and seeded into the outlet ports of the center lumens at 15,000 cells/µL. The MatTek dish with the bonded devices were then incubated at 37°C and flipped (upside-down then right-side-up) every 30 min. After 2 hours of flipping and incubation, unadhered cells were aspirated out and the center lumen was filled with fresh media. Media was replaced every 24 h for two days before coating the center lumen with BCCs for direct-contact co-culture or side lumen for indirect-contact co-culture (paracrine signaling alone). All cultures were maintained in a humidified incubator at 37°C with 5% CO₂.
Direct-contact and indirect-contact co-culture of BCCs
For direct-contact co-culture, BCCs (i.e., MDA-MB-231, MCF-7 and Hs-578T cells) were trypsinized, resuspended in iPSC-EC complete growth media at 1800 cells/µL and loaded into the center or side lumen (2µL per lumen) via passive pumping. For co-culture involving paracrine signaling alone (indirect-contactco-culture), BCCs were seeded into a lumen adjacent to the iPSC-EC vessel. The MatTek dishes were then flipped and incubated, as described before, to coat the lumens with BCCs. Unadhered cells were flushed and replaced with 30 µL of iPSC-EC complete growth media. Media was replaced every 24 h for the entire duration of culture.
Blocking and neutralization
Anti-IL-6R mAb, Tocilizumab, (Actemra, Genentech/Roche, South San Francisco, CA), allosteric inhibitor of chemokine receptors 1 and 2 (CXCR1/2) or IL-8R, Reparixin ( Cayman Chemical, Ann Arbor, MI, USA) and MMP-3 inhibitor, UK-356618 (Santa Cruz Biotechnology, Dallas, TX) were used for IL-6R, IL-8R and MMP-3 inhibition experiments. Dosing was determined by independent and combined treatment of iPSC-ECs cultured in a 96-well plate with tocilizumab, reparixin and UK-356618 at varying concentrations. Concurrently, a control set of iPSC-EC wells were treated with the vehicle (DMSO), (Supplementary Figure S9). To inhibit IL-6, IL-8 and MMP-3 signaling in our system, MDA-MB-231 cells were co-cultured (direct-contact) with iPSC-EC vessels for 2 h followed by independent or combined treatment with media containing tocilizumab (100 µg/mL), reparixin (10 µM), UK-356618 (0.3 µM) and vehicle control. After 24 h of treatment, media were collected for protein secretion analysis, and vessels were used for quantifying extravasation events and dextran diffusion analysis.
Secreted factor quantification
Protein secretion analysis was performed on media collected from monocultures and co-cultures of iPSC-EC vessels and three different BCC types on day 4. For conditioned media experiments, conditioned media collected from monoculture systems after 48 h were diluted with iPSC-EC complete growth media (50:50) and subsequently added to separate monocultures of iPSC-EC vessels or MDA-MB-231 cells. Media were then collected from conditioned media treated systems after 48 h for analysis. For therapeutic inhibition experiments, media were collected from treated or vehicle control systems after 24 h. 25 µL of maintenance media were obtained in all cases from 4 separate systems on the final day of culture and pooled to generate one biological replicate. Four biological replicates were performed over three separate experiments. A custom selection of analytes (Angiopoietin-2, ANGPTL4, HB-EGF, IL-6, IL-8, MCP-1, MMP-1, MMP-3 and VEGF-C) from an oncology multiplex assay panel (R&D Systems, Minneapolis, MN) were selected for this analysis. Sample preparation was performed as per manufacturer’s protocol and measured using MAGPIX Luminex Xmap (Luminex Corporation, Austin, Texas). Luminex xPonent software was used for data collection.
Immunofluorescence Staining
For immunostaining, lumens were fixed with 4% (v/v) paraformaldehyde (Alfa Aesar, Haverhill, MA) for 20 min and permeabilized with 0.2% (v/v) Triton-X 100 (MP Biomedicals, Santa Ana, CA) for 30 min at room temperature. To reduce non-specific background fluorescence from collagen, systems were incubated in 0.1 M glycine (Fisher Scientific, Pittsburgh, PA) for 30 min. Systems were washed with sterile PBS between each step. Cells were then blocked with 3% (wt/v) BSA (Sigma-Aldrich, St. Louis, MO) and 0.1% (v/v) Tween-20 (Fisher Scientific, Pittsburgh, PA) overnight at 4 °C. For antibody-based staining, primary antibodies diluted at desired concentrations (Supplementary Table S1) were added to the devices and incubated at 4 °C overnight. Excess primary antibodies were removed by washing the devices multiple times. Secondary antibodies diluted at 1:100 along with Hoechst 33342 (Thermo Fisher, Waltham, Massachusetts) in blocking-buffer were added to the devices and incubated for at 4 °C overnight. Stained vessels were washed multiple times with sterile PBS over a period of 24 h prior to imaging. For live cell imaging of endothelial vessels, iPSC-ECs were stained in red with the fluorescent lipid Dil (V22889, Thermo Fisher) in iPSC-EC complete growth media for 10 min prior to cell seeding within lumen.
Image Acquisition
Fluorescent images used to analyze extravasation events and vascular permeability were acquired using a Nikon TI Eclipse inverted microscope (Melville, NY) and analyzed using the National Institutes of Health ImageJ software. Confocal images were acquired at University of Wisconsin-Madison Optical Imaging Core using a Nikon A1RS confocal microscope (Melville, NY).
Image Analysis (Extravasation events, ICAM-1 expression, and COL IV deposition)
Extravasation events were measured by capturing images across the z-plane of the lumen at 6x magnification. Images were processed using ImageJ software where z-plane images were maximally projected to create a 2D image. A smoothing filter and background subtraction was used. Extravasated cells were considered to be those present outside of the lumen-edge. GFP-expression and nuclear stain were used to count the number of extravasated cells. To characterize intercellular adhesion molecule-1 (ICAM-1) expression and collagen type IV (COL) IV deposition confocal images were analyzed using ImageJ software. Briefly, raw images were prepared by enhancing contrast and removing noise. Same thresholding parameters and area of region of interest were applied to all images compared. Sequences of instructions for calculating mean intensities of ICAM-1 expression and expression profiles of COL IV deposition are shown in supplementary section (Supplemental Figures S2 and S3).
Statistical Analysis
GraphPad Prism 7 (GraphPad Software, La Jolla, CA) was used for statistical analysis. One-way ANOVA with Tukey post hoc test for pairwise comparison was used for significance testing on three or more data-sets. Non-paired student’s t-test was performed for determining statistical significance between two conditions. Tests were considered significant for p ≤ 0.05. The number of replicates ranged from n = 4 to n = 6 for each experimental condition.
Results
Organotypic breast cancer cell extravasation model
We engineered a microscale organotypic in vitro model that allows the study of cancer cell extravasation. The device consists of two thin PDMS layers with patterned microscale features bonded to a coverslip glass (Figure 1a). To generate lumen structures, three PDMS rods are embedded between the PDMS layers of the device. This method enables robust control over vasculature size, number, configuration and allows for fabrication of arrayed devices for assaying multiple conditions in parallel. The lumens can be lined with ECs to model blood vessels. Cancer cells can be introduced to the EC lined lumen and co-cultured to investigate extravasation behavior, (Figure 1b).
To generate EC lined lumen structures, we used a gel consisting of 3 mg/mL type I collagen and 1 mg/mL fibrinogen, (Figure 1c). iPSC-ECs line the center lumen of the device while the side lumens are used as additional media source to feed all cell types within the system. Within 48 hours of culture, iPSC-ECs form a continuous monolayer with a hollow lumen, similar to in vivo vasculature. The separate inlet and outlet ports of each lumen in the device are used for cell seeding and media exchange. In vivo, ECs form monolayer barriers that regulate the passage of fluids, compounds and cells via the use of cell-cell junction proteins27. In our model, iPSC-ECs expressed endothelial cell marker CD31 and junctional marker VE-cadherin, which were localized to the periphery of endothelial cell-cell contacts, indicating the presence of adherens junctions, (Figure 1d). The establishment of a continuous monolayer resulted in the deposition of COL IV, a major component of the basement membrane.
To study cancer cell extravasation, human BCCs were seeded into the iPSC-EC-lined lumen through the pipette-accessible ports. The direct-contact co-culture of cancer cells on the endothelium mimics cancer cell-EC interactions, in larger vessels, after adhesion but prior to transendothelial migration. Following direct-contact co-culture within our model, MDA-MB-231 cells were found to be both adhered to the inner surface of the endothelial lumen and in the matrix space surrounding the vessel. 3D reconstruction of vessel cross-section showed extravasated MDA-MB-231 cells outside of the iPSC-EC lumen, (Figure 1e). The ability to visualize co-localization of invasive BCCs during extravasation enables the study of cancer cell-mediated modulation of the endothelium.
BCCs modulate endothelial properties and basement membrane components
To study BCC extravasation, we seeded MDA-MB-231 cells at a density high enough to observe at least one extravasation event within 24 hours of co-culture. Following, cell seeding, MDA-MB-231 cells appeared to extravasate out of iPSC-EC vessels within 8 hours, (Figure 2a). Fluorescent microscopy images demonstrate the capability of our system to capture morphological dynamics of cancer-vascular interactions and single-cell extravasation events in high detail. Following co-culture, MDA-MB-231 cells appeared to take on spherical and elongated shapes both within the vasculature and in the matrix. Cells can be seen adhered to the inner surface of the lumen vasculature, partially protruding outside of the lumen barrier into the matrix, and fully transmigrated into the matrix, (Figure 2b). Confocal microscopy also revealed exchange of membrane components between ECs and BCCs. Fragments of iPSC-EC membranes, labeled with a lipophilic membrane stain, were observed within GFP expressing MDA-MB-231 cells during direct-contact co-culture, suggesting intercellular crosstalk (Supplementary Figure S4).
Figure 2. Breast cancer cell extravasation behavior and cancer-vascular interactions.
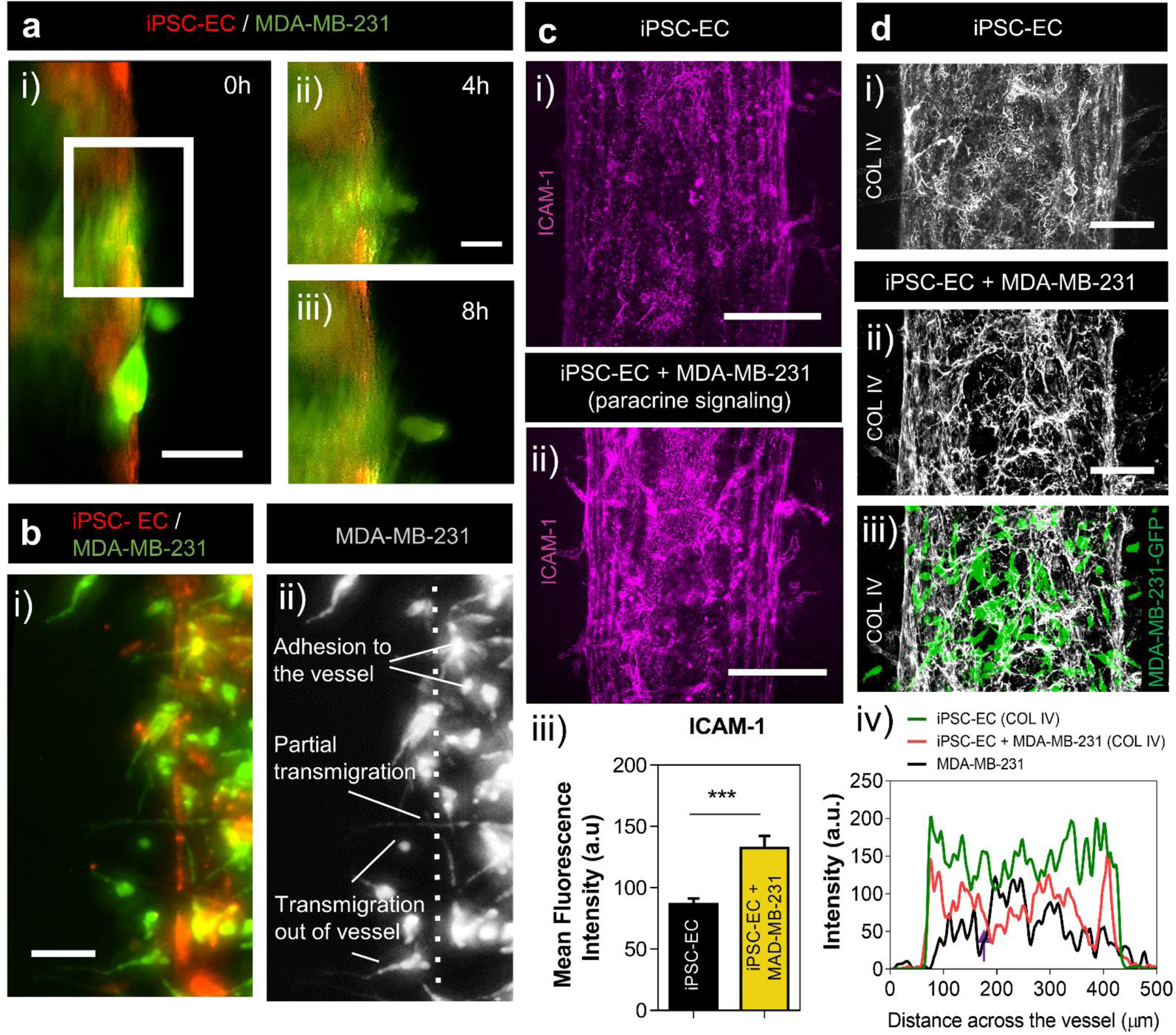
(a) Fluorescent images of MDA-MB-231 cells extravasating out of iPSC-EC vessels within 8h of co-culture, scale bar indicates 50 µm. Protrusions of MDA-MB-231 cells out of iPSC-EC vessel at 4h (i) and 8h (ii), scale bar indicates 20 µm. (b) MDA-MB-231 cells take on variety of morphologies during extravasation out of an iPSC-EC vessel, including adhesion to the internal surfaces of the vessel, partial transmigration and full transmigration out of the vessels (i-ii), scale bar indicates 50 µm. (c) Confocal projections of ICAM-1 expression on iPSC-EC vessels (i) and on iPSC-EC vessels in co-culture with MDA-MB-231 cells in an adjacent lumen (ii), scale bar indicates 150 µm. Image intensity analysis of ICAM-1 expression on iPSC-EC vessels alone and in co-culture with MDA-MB-231 cells (iii). Plot: mean fluorescence intensity + SD, statistical analysis: Student-test, ***p ≤ 0.001. Data represents average of at least 6 different region of interests (ROIs) across 6 different systems. (d) COL IV deposition by iPSC-EC vessel alone (i) and after MDA-MB-231 extravasation (ii-iii, normalized thresholding applied for improved visualization), scale bars indicate 100 µm. Image intensity analysis of COL IV deposition by iPSC-EC vessels in monoculture and after MDA-MB-231 cells extravasation (iv). Plot: intensity profiles across vessels.
Cancer cells are known to prime the endothelium and facilitate their own transmigration across the endothelial barrier28. Attachment of cancer cells to the endothelium is mediated by adhesion molecules, which are absent or minimally expressed in un-activated endothelium29. To investigate the effects of BCCs on EC modification, we assessed the expression of ICAM-1 on the vasculature in response to co-culture with MDA-MB-231 cells (Supplementary Figure S5). Recent reports show that BCC lines express ICAM-1, and it is relatively overexpressed in triple-negative BCC lines including MDA-MB-231 cells30. To assess crosstalk-associated upregulation of ICAM-1 on the endothelium alone without addition of ICAM-1 signals from cancer cells, we examined ICAM-1 expression on iPSC-EC vessels in response to soluble factor signaling, (Figure 2c i–ii). To achieve this, MDA-MB-231 cells were co-cultured in an adjacent lumen to the iPSC-EC lumen to determine their effect on ICAM-1 expression via secreted factor signaling. Mean fluorescence intensity values of ICAM-1 staining on iPSC-EC vessels increased in response to paracrine signaling from MDA-MB-231 cells relative to control monoculture conditions, (Figure 2c iii). Details of intensity profile analysis can be found in (Supplementary Figure S2). This result indicates that BCC secreted factors precondition the endothelium, increasing the expression of cancer associated adhesion molecules.
Modification of the iPSC-EC derived basement membrane by extravasating BCCs was also observed. COL IV is the most abundant component of basement membranes making up 50% of basement membrane composition31,32. Confocal microscopy showed COL IV deposition by ECs in our device, (Figure 2d i), which were consistent with previously reported basement membrane distributions of vascular tissues33. Following extravasation of MDA-MB-231 cells, large areas devoid of COL IV were observed surrounding the endothelium, (Figure 2d ii–iii). Intensity profile analysis of COL IV deposition across iPSC-EC vessels exhibited an overall decrease in COL IV expression resulting from direct-contact co-culture with MDA-MB-231. Moreover, intensity profiles showed an intensity decrease (Figure 2d iv, purple arrow) at the position of interaction/overlap between MDA-MB-231 cells and the endothelium. Interestingly, our results suggest more COL IV remodeling and degradation resulting from direct-contact interactions between ECs and BCCs compared to paracrine mode signaling (Supplemental Figure S6). COL IV intensity analysis showed similar COL IV deposition by iPSC-EC vessels in monoculture as in co-culture with paracrine mode signaling alone. These results suggest that physical interactions with the vasculature has more of an effect on COL IV remodeling by MDA-MB-231 cells than by the actions of paracrine crosstalk. Details on quantification of COL IV deposition and intensity profile analysis are listed in (Supplementary Figure S2–3). Together, these results provide evidence of crosstalk leading to paracrine signaling-mediated modulation of endothelial properties, and physical remodeling of basement membrane by extravasating cancer cells within our model.
Secreted factor analysis reveals distinct profiles for highly and poorly invasive BCCs
During extravasation, to cross structural barriers of blood vessels, BCCs secrete mediators that increase vascular permeability and enhance their own ability to undergo transendothelial migration5. To investigate paracrine signaling between cancer cells and the endothelium, we examined secretion profiles of highly invasive (MDA-MB-231 and Hs-578T cells) and poorly invasive (MCF-7 cells) BCCs in monoculture and in co-culture with iPSC-EC vessels. Media were collected from the cell seeding ports on day-4 of culture and analyzed for protein secretion. For monoculture conditions, iPSC-EC vessels or BBCs were cultured within our system for 4 days prior to media collection. Whereas for co-culture conditions, iPSC-ECs were maintained for 2 days followed by BCC seeding, which were maintained for 2 additional days for a total of 4 days, (Figure 3a). A custom selection of analytes (Angiopoietin-2, ANGPTL4, HB-EGF, IL-6, IL-8, MCP-1, MMP-1, MMP-3 and VEGF-C) were selected for this analysis based on their involvement in extravasation and breast cancer metastasis.
Figure 3. Protein secretion profiles of iPSC-EC vessels and BCC cultured within the BCC extravasation model.
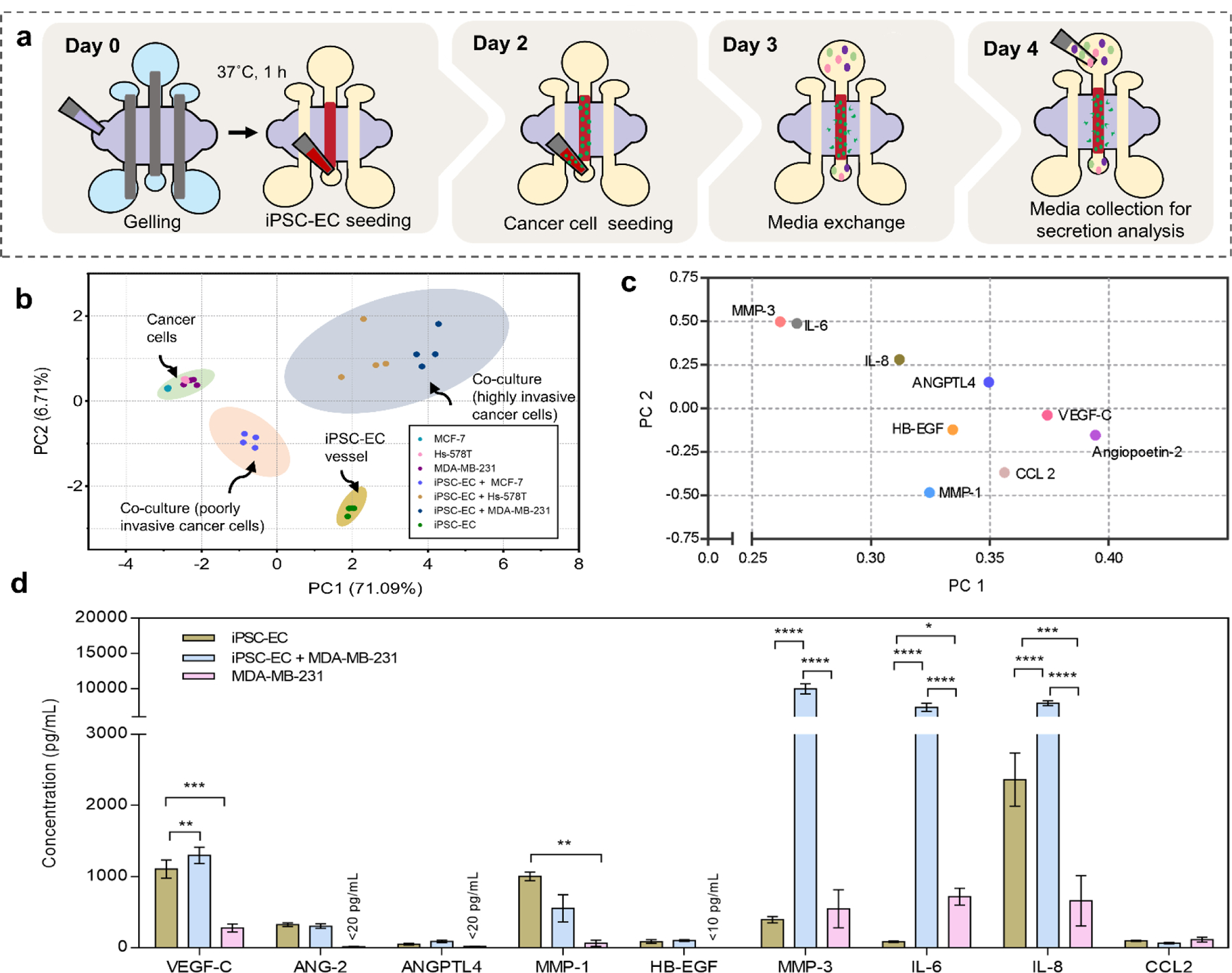
(a) Schematic representation of workflow and cell culture supernatant collection for a multiplexed enzyme-linked immunosorbent (MagPIX) assay. (b) Principal component analysis (PCA) is used to project protein secretion data sets of iPSC-EC vessels and BCCs in monoculture and co-culture onto the first two components, PC 1(71.09%) and PC 2 (6.7%). Each dot represents one of four independent experiments per condition and the shaded ellipses represent 95% confidence intervals. PCA variance refers to amount of total variance observed between samples of conditions that segregate along that principal component. (c) PCA loadings plot on PC 1 and PC 2 are plotted for individual factors used for PCA scores plot in (b). (d) Protein concentrations measured in media collected from iPSC-EC vessels cultured alone, iPSC-EC vessels with extravasating MDA-MB-231 cells and MDA-MB-231 cells cultured alone. Plot: mean concentrations + SD, statistical analysis: ordinary one-way ANOVA with Tukey’s multiple comparisons test, ****p ≤ 0.0001, ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 (n=4).
Principal component analysis (PCA) of protein secretion was performed to identify variations in secretion profiles based on cell types and culture conditions, (Figure 3b). Principal components (PCs) 1 and 2 captured 77.8% of the total variation in the data set and the score plot of the PC1-PC2 comparison revealed four distinct groups of culture conditions. Co-culture conditions (blue and red shaded regions) and monoculture conditions (green and yellow shaded regions) formed separate clusters, demonstrating distinct secretion profiles within our system. The largest variance along principal component one (71.09%) was observed between invasive cell lines in co-culture with iPSC-EC vessels and in monoculture. This indicates a strong impact on the overall secretion profiles resulting from BCC/iPSC-EC crosstalk involving invasive subtypes of BCCs. Notably, co-culture involving invasive cell lines (blue) were separately clustered from co-culture involving poorly invasive lines (red). Secretion of all BCC monocultures (green) clustered together while iPSC-EC vessels (yellow) secretion was clustered separately. These results show that using this protein secretion analysis we can delineate secretion of iPSC-EC vessels from BCCs cultured within our system and, moreover, that crosstalk between iPSC-ECs and BCCs yield distinct secretion profiles based on BCC subtypes (i.e highly- invasive vs. poorly invasive).
To identify specific secreted factors contributing to the variances observed between culture conditions in the score plot, a loadings plot was generated along PC1 and PC2 (Figure 3c). The loadings of all analytes examined positively correlated along PC 1, which corresponds to secretions of iPSC-EC vessels in monoculture and in co-culture with invasive cancer lines. According to the loadings plot factors such as VEGF-C, Angiopoietin-2 and CCL-2 are enriched in culture conditions involving iPSC-EC vessels. As iPSC-EC vessels in monoculture and co-culture cluster separately along PC2, we can determine factors that are enriched as a result of co-culture by interpreting the corresponding loadings plot. Accordingly, ANGPTL4, MMP-3, IL-6 and IL-8 correlate with co-culture of iPSC-EC vessels with invasive BCC lines, suggesting that these factors are upregulated because of their interactions. This is further illustrated in the protein concentration plots of iPSC-EC vessel and MDA-MB-231 cell monoculture and their co-culture, (Figure 3d), where concentrations of IL-6, IL-8 and MMP-3 are significantly upregulated in the co-culture condition. Interestingly, these factors were not significantly upregulated in cultures involving poorly invasive BCCs and iPSC-EC vessels suggesting a correlation between the specific roles of these factors and the invasive phenotype of BCCs.
Tumor and endothelial derived factors involved in cancer-vascular crosstalk
Our initial data showed that IL-6, MMP3 and IL-8 are increased during co-culture of iPSC-EC vessels and MDA-MB-231 cells, compared to the sum of their monoculture contributions. Here, investigate the individual roles of iPSC-EC vessels and invasive BCCs in the upregulation of secreted IL-6, IL-8 and MMP-3 by performing conditioned media experiments. To eliminate the influence of culture methods on secreted factor signaling, we extracted cell-conditioned media from iPSC-EC vessels and BCCs cultured within our model. Given that the largest variance in the PCA analysis was observed between monoculture and co-culture of MDA-MB-231 cells with iPSC-ECs, we chose MDA-MB-231 cells as a representative cell line in subsequent experiments. Protein secretion levels were measured in the following five conditions: iPSC-EC vessels cultured alone, iPSC-EC vessels cultured in MDA-MB-231 conditioned media, MDA-MB-231 cells cultured alone, MDA-MB-231 cells cultured in iPSC-EC vessel conditioned media and direct-contact co-culture of iPSC-EC vessels with MDA-MB-231 cells, (Figure 4a). Our analysis revealed that MMP-3 secretion was significantly increased not only when iPSC-ECs were exposed to direct-contact co-culture with MDA-MB-231, but also with MDA-MB-231 conditioned media, suggesting this increase is driven by an MDA-MB-231 soluble factor, (Figure 4b). Conversely, IL-6 and IL-8 were upregulated significantly in direct-contact co-culture, and also in MDA-MB-231 cell culture with iPSC-EC conditioned media. This suggests that upregulation of these secreted factors may be contributed primarily by MDA-MB-231 cells in response to signaling from iPSC-EC vessels, (Figure 4c,d). Together, these results show that MDA-MB-231 cells secrete high levels of IL-6 and IL-8 in the presence of iPSC-EC vasculature while iPSC-EC vasculatures upregulate MMP-3 secretions in the presence of MDA-MB-231 cells.
Figure 4. Cancer-endothelial crosstalk via soluble factor signaling.
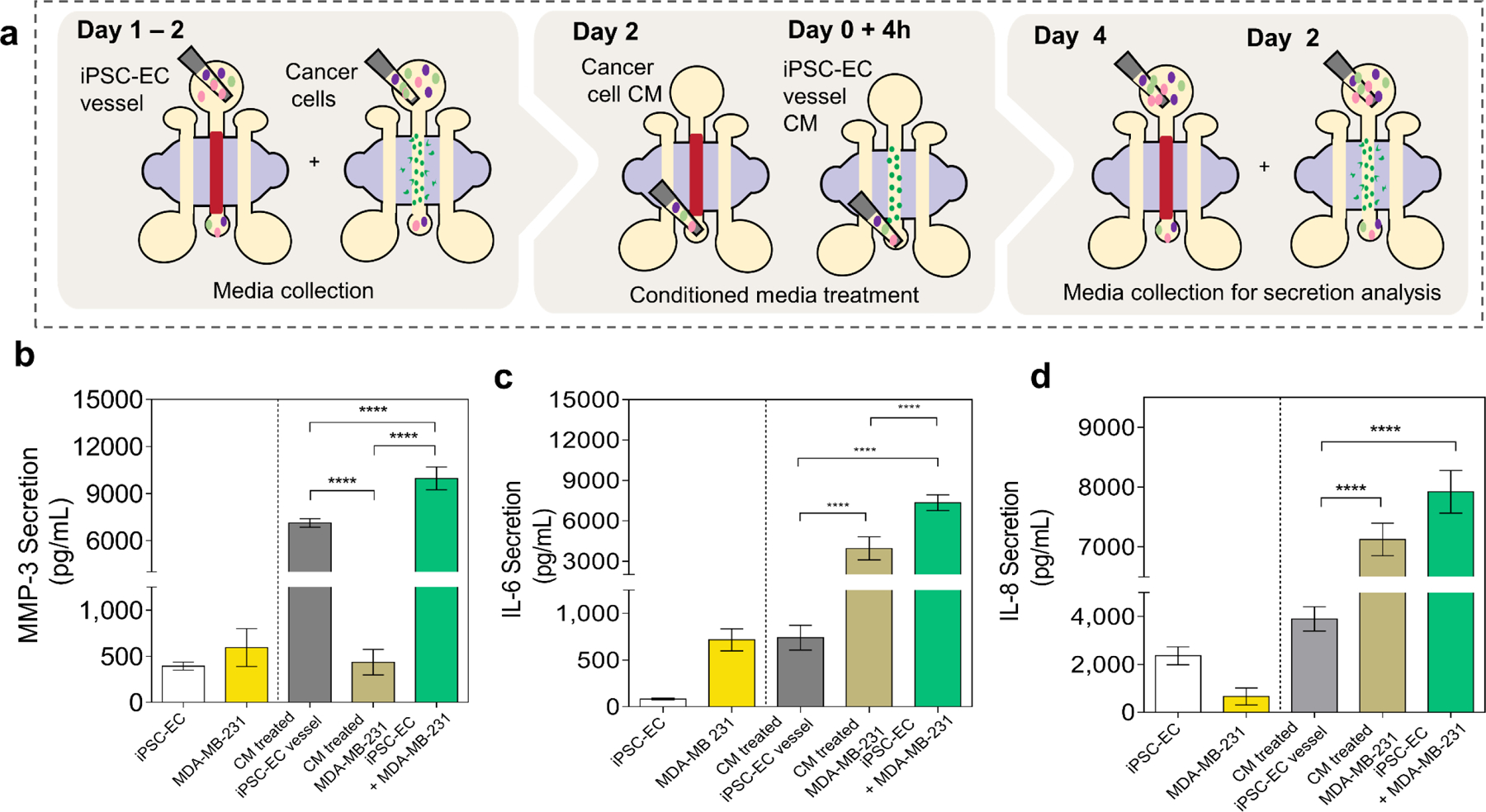
(a) Schematic representation of conditioned media treatment experiments and cell culture supernatant collection for a multiplexed enzyme-linked immunosorbent (MagPIX) assay. (b – d) Secreted factor (MMP-3, IL-6 and IL-8) concentrations measured in media collected from iPSC-EC vessels cultured alone, iPSC-EC vessels cultured in MDA-MB-231 conditioned media, MDA-MB-231 cells, MDA-MB-231 cells cultured in iPSC-EC vessel conditioned media, and direct-contact co-culture of iPSC-EC vessels and MDA-MB-231 cells. Plot: mean concentrations + SD, statistical analysis: ordinary one-way ANOVA with Tukey’s multiple comparisons test, ****p ≤ 0.0001 (n=4).
Cancer-vascular paracrine signaling leads to impaired vascular barrier function
Taking advantage of the capacity of our model to visualized cancer-vascular interactions, we examined extravasation behavior of three different BCCs (MDA-MB-231, Hs-578T and MCF-7) by analyzing events of extravasation and vascular permeability using confocal microscopy. While the invasive cell lines MDA-MB-231 and Hs-578T both migrated out of the vasculature, (Figure 5 a i–ii), MCF7 cells formed cell aggregates and remained within the lumen vasculature, (Supplementary Figure S7). Among the invasive cell lines, MDA-MB-231 cells exhibited significantly higher extravasation efficiency compared to Hs-578T cells, (Figure 5 a iii), which is consistent with previous reports34. These observations also correlate with higher levels of secreted IL-6, IL-8 and MMP-3 resulting from the crosstalk between iPSC-EC vasculature and invasive BCC lines, as observed in our secreted factor analysis. We also assessed the influence of direct-contact co-culture with BCCs on the barrier function of iPSC-EC vasculatures. To establish baseline barrier function of iPSC-EC vessels, we measured vascular permeability using methods previously described35. Upon formation of a complete endothelialized lumen, the diffusive permeability of fluorescently tagged 70 kDa dextran were analyzed and compared to an empty lumen structure without an endothelium (Supplementary Figure S8). The endothelialized lumen exhibited lower diffusive permeability values compared to a lumen control without ECs, indicating the presence of barrier function. We then examined changes in diffusive permeability of iPSC-EC vessels resulting from co-culture with BCCs of different invasive profiles. iPSC-EC vessels in direct-contact co-culture with MDA-MB-231, Hs-578T and and MCF-7 cells were filled with fluorescently tagged 70 kDa dextran solutions and imaged over a 15 min period, (Figure 5bi). Intensity profiles of dextran diffusion across vessels were plotted and analyzed to compare vascular permeability between monoculture and co-culture conditions, (Figure 5bii). The diffusion profiles for iPSC-EC vessels in co-culture with MDA-MB-231 cells were broader compared to iPSC-EC vessels in monoculture, (Figure 5c i–ii). Interestingly, despite the absence of extravasation events, co-culture of MCF-7 with iPSC-EC vessels resulted in broad diffusion profiles, similar to those obtained from the invasive cell lines (Supplementary Figure S7), indicative of higher permeability. Endothelial viability was confirmed to exclude EC death as a possible cause of this observation.
Figure 5. Impact of BCC extravasation on iPSC-EC vascular permeability.
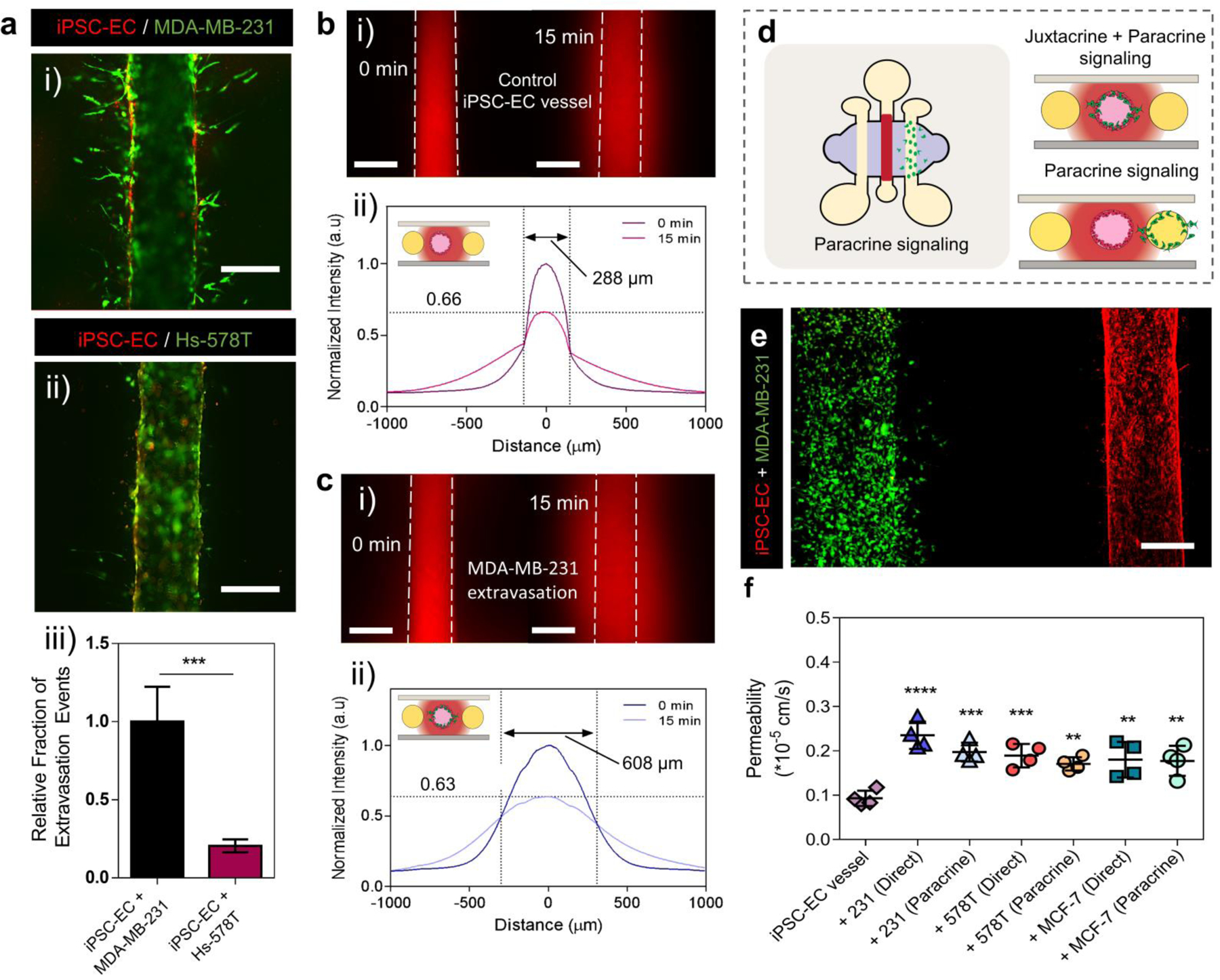
(a) Fluorescent images of invasive BCCs MDA-MB-231(i) and Hs-578Ts (ii) extravasating out of iPSC-EC vessels, scale bars indicate 250 µm. Relative fraction of extravasation events for invasive BCC types Hs-578Ts and MDA-MB-231s (iii). Plot: relative fraction of extravasated cells + SD, statistical analysis: Student-test, ***p ≤ 0.001, (n=6). (b-c) iPSC-EC vessel barrier function. Fluorescent images of 70 kDa dextran diffusion across iPSC-EC vessels over a 15 min period (bi) and across iPSC-EC vessels with extravasated MDA-MB-231 cells (ci), scale bars indicate 250 µm. Normalized intensity profiles of 70 kDa dextran diffusion across iPSC-EC vessels in monoculture, with a peak intensity drop to 66% and a profile width of 288 µm (bii), and in co-culture after extravasation (cii), with a peak intensity drop to 63% and a profile width of 608 µm. (d) Schematic representation of device configuration for assessing paracrine signaling between cancer cells and the endothelium. (e) Fluorescent images of an iPSC-EC vessel co-cultured with MDA-MB-231 cells in an adjacent lumen, scale bar indicates 200 µm. (f) Permeability assessment of iPSC-EC vessels in co-culture with three different BCC subtypes (MDA-MB-231, HS-578T and MCF-7) in two different culture configurations (direct-contact and paracrine signaling alone). Plot: mean permeability + SD, statistical analysis: ordinary one-way ANOVA with Tukey’s multiple comparisons test, ****p ≤ 0.0001, ***p ≤ 0.001, **p ≤ 0.01 (n=4).
To further differentiate contributions of direct-contact cancer-vascular cell interactions, involving both juxtacrine and paracrine signaling, from paracrine signaling on vascular barrier function, we investigated the impact of co-culture using two distinct setups (i.e., direct-contact and indirect-contact co-culture). For direct-contact interactions, BCCs were injected into the EC-coated lumen, while for indirect-contact interactions (paracrine signaling only), BCCs were seeded in an empty lumen adjacent to an EC-coated lumen (Figure 5d–e). Vascular permeability in direct-contact and indirect-contact co-cultures were analyzed. Overall, dextran permeability in co-culture with BCCs were significantly greater than monoculture condition, (Figure 5f). Among the invasive lines (i.e., MDA-MB-231 and Hs-578T), direct-contact signaling induced a slightly higher vascular permeability compared to paracrine signaling alone. For MDA-MB-231 cells, direct-contact signaling lead to a 2.5-fold increase compared to 2.1-fold increase in paracrine signaling mode against monoculture vessels. Similarly for Hs-578T cells, there was a 2-fold increase in direct-contact mode signaling versus a 1.8-fold increase for paracrine signaling. Conversely, no differences in permeabilities were observed between direct-contact and indirect-contact co-culture for poorly invasive cells (i.e., MCF-7 cells).
Although there were no substantial differences in barrier impairment of iPSC-EC vasculatures for invasive and poorly invasive cancer cell lines, secreted factor analysis revealed altered secretion of factors associated with increased vascular permeability, such as IL-6, (Figure 3c). A slight difference in the degree of barrier impairment between MDA-MB-231 cells and MCF-7 cells was observed for the direct-contact co-culture conditions. Co-culture with MDA-MB-231 cells induced a higher increase (2.5-fold) compared to MCF-7 cells (1.9-fold) in vascular permeability against monoculture controls. Interestingly, paracrine signaling interactions lead to a similar increase in vascular permeability for MCF-7 cells as the other invasive lines. This combined with our secreted factors analysis, which revealed distinct protein secretion profiles for invasive BCC lines compared to poorly invasive cell lines in co-culture with iPSC-EC vasculatures, suggest a different conditioning capability of MCF-7 that is contributing to this vascular impairment. Taken together, these observations suggest that to facilitate cancer cell extravasation, paracrine signaling alone can significantly contribute to impairing the barrier function of vasculatures. In addition, direct-contact interactions and subsequent events of extravasation further exacerbates this effect.
Therapeutic inhibition of IL-6, IL-8 and MMP-3 reduces overall secretion of factors associated with breast cancer metastasis
Our secretion profile analysis suggested a correlation between IL-6, IL-8, and MMP-3 secretion- and the metastatic potential of BCCs. Moreover, we found a significant impact of paracrine signaling on the functional behavior of iPSC-EC vasculatures. Considering these findings, we hypothesized that impaired vascular function and extravasation events can be mitigated by inhibiting the IL-6, IL-8 and MMP-3 paracrine signaling. Thus, we set out to explore the influence of these factors on the functional behavior of BCCs and iPSC-EC vasculatures by using therapeutic inhibitors. MDA-MB-231 cells were used as a representative invasive breast cancer line as they were revealed to be most invasive in our investigation. To inhibit IL-6 and IL-8 signaling, we used clinically active therapeutics tocilizumab, an anti-IL-6R mAb, and reparixin, an allosteric inhibitor of IL8R1/CXCR1 and IL8R2/CXCR2. For MMP-3 inhibition, we used an MMP-3 selective inhibitor, UK-356618. The chosen doses of therapeutic inhibitors were selected based on previous reports that demonstrated reduced cancer cell viability and migration either in monoculture or co-culture with other cell types. Specifically, tocilizumab has been shown to reduce viability of MDA-MB-231 cells to 40% and migration to 60% at 1.37 µM in co-culture with lymphatic endothelial cells36. In another study, reparixin was found to significantly reduce MDA-MB-231 cell migration in 3D matrix at concentrations of 14 µM or higher37. UK-356618-mediated inhibition of cell migration has been demonstrated in lung cancer cells at 0.07 µM concentrations38. Concentrations in these studies were used to inform and set dosing ranges for the study presented in this work. Prior to evaluating the effect of these inhibitors on BCC extravasation, we tested their potential effect on endothelial cell viability. Viability analysis of iPSC-ECs was conducted to ensure the selected doses, while affecting cancer cells, do not adversely influence viability and functionality of the endothelium. Briefly, iPSC-ECs were cultured in 96-well plates and treated with varying drug concentrations over a 24-hour period. For extravasation experiments, inhibitor dose was selected at >90% viability of iPSC-ECs. In this context, reparixin, tocilizumab, and UK-356618 decreased cell viability below 90% at concentrations above 500 µg/mL, 50 µM and 6 µM respectively, thus we used dosing below these concentrations (Supplementary Figure S9).
In extravasation inhibition experiments, iPSC-EC vessels were cultured for 48 h prior to MDA-MB-231 cell seeding within the vessel, (Figure 6a). BCCs were added to the lumens, cultured for 2 h followed by 24-hours of single-drug or combined treatment with tocilizumab, reparixin and UK-356618. First, we examined if these treatments had off-target effects on the secretion of the other extravasation and metastasis-associated molecules previously inspected. Protein secretions of treated and control systems were analyzed after 24-hours of co-culture. PCA analysis of secreted proteins showed that the highest variance along PC1 which generates an axis between combined treatment and vehicle control systems. This indicates that combined treatment decreased overall secretions to a greater extent than anything other single treatment conditions. It should be noted that clustering analysis also revealed some overlap between systems independently treated with tocilizumab or reparixin and systems receiving combined treatment, suggesting a similar decrease in secretions (Figure 6b). Further investigation of normalized secretion levels indicate that HB-EGF, CCL-2, ANG-2 and VEGF-C were downregulated in treated systems while MMP-1 was upregulated, (Figure 6c). Overall, this effect was most pronounced in systems receiving combined treatment. These results further demonstrate the sensitivity of our system to detect changes in secretome profiles in response to therapeutic inhibitions.
Figure 6. Protein secretion profiles of the BCC extravasation model in response to independent and combined treatment of therapeutic inhibitors.
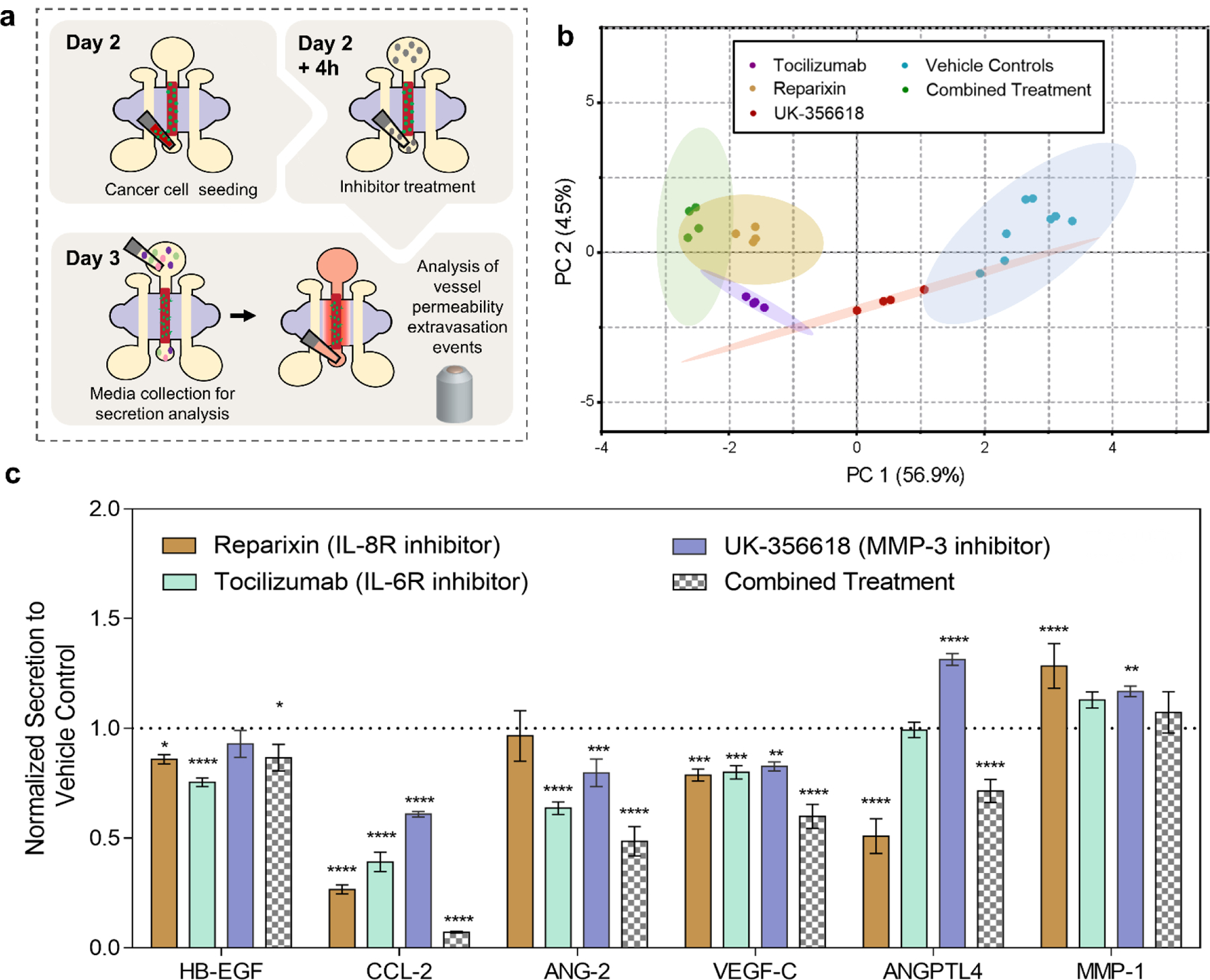
(a) Schematic representation of experimental workflow for molecular (MagPIX) and functional (barrier function and quantification of extravasation events) assays. (b) PCA scores plots of secretion data sets from vehicle control systems and systems treated with therapeutic inhibitors both independently (reparixin, tocilizumab and UK-356618) and in combination (reparixin+rocilizumab+UK-356618) projected onto the first two components, PC 1(56.9%) and PC 2(4.5%). Each dot represents one of four independent experiments per condition and the shaded ellipses represent 95% confidence intervals. (c) Normalized secreted protein concentrations measured in media collected from systems independently treated and in combination with therapeutic inhibitors relative to vehicle controls. Plot: fraction of vehicle control concentration + SD, statistical analysis: two-way ANOVA followed by Dunnett’s multiple comparisons test, ****p ≤ 0.0001, ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05, compared to vehicle (n=4).
Combination of tocilizumab, reparixin and UK-356618 reduces BCC-induced barrier dysfunction and BCC migration in the extravasation model
We also investigated the effects of therapeutic inhibition on extravasation behavior and vascular properties including barrier function and structural remodeling within the model. To examine if combined inhibition of IL-6, IL-8 and MMP3 induced a synergistic response in extravasation behavior and endothelial barrier function we exposed our co-culture systems to independent treatment and combinations of inhibitors. Confocal microscopy revealed that extravasation events declined in all the conditions analyzed compared to vehicle controls, (Figure 7a). Previously, our results showed that extravasation of BCCs increased alterations to COL IV, deposited by iPSC-EC vessels, through direct-contact interactions more than through paracrine signaling. Given that MMPs play critical roles in degrading basement membrane components during tumor invasion, we explored the effects of MMP inhibition on COL IV remodeling during extravasation within our model. Our results show that UK-356618 treated systems had higher COL IV expression, as demonstrated by immunofluorescence staining, indicative of lower COL IV degradation during direct-contact interactions between cancer cells and the vasculature, (Figure 7b). As previously established, COL IV expression was higher in monocultures of iPSC-EC vessels and in indirect-contact co-cultures than in direct-contact co-culture conditions, and vehicle treatment did not change these trends. A slight, but not statistically significant increase in COL IV expression was observed in UK-35661 treated iPSC-EC vessels in monocultures relative to vehicle controls, (Figure 7c). This trend was also observed in indirect-contact signaling conditions, (Supplementary Figure S10). These results suggest that MMP inhibition using UK-356618 can significantly reduce some of the COL IV remodeling observed in cancer-vascular crosstalk, particularly during physical contact interactions between cancer cells and the vasculature in extravasation. Consistent with ICAM-1 expression analysis in Figure 2, co-culture conditions resulted in a higher ICAM-1 expression overall, however no additional effects were observed in response to treatment compared to respective vehicle controls, (Supplementary Figure S11).
Figure 7. Functional responses (vessel permeability, basement membrane remodeling and extravasation events) of systems treated with therapeutic inhibitors.
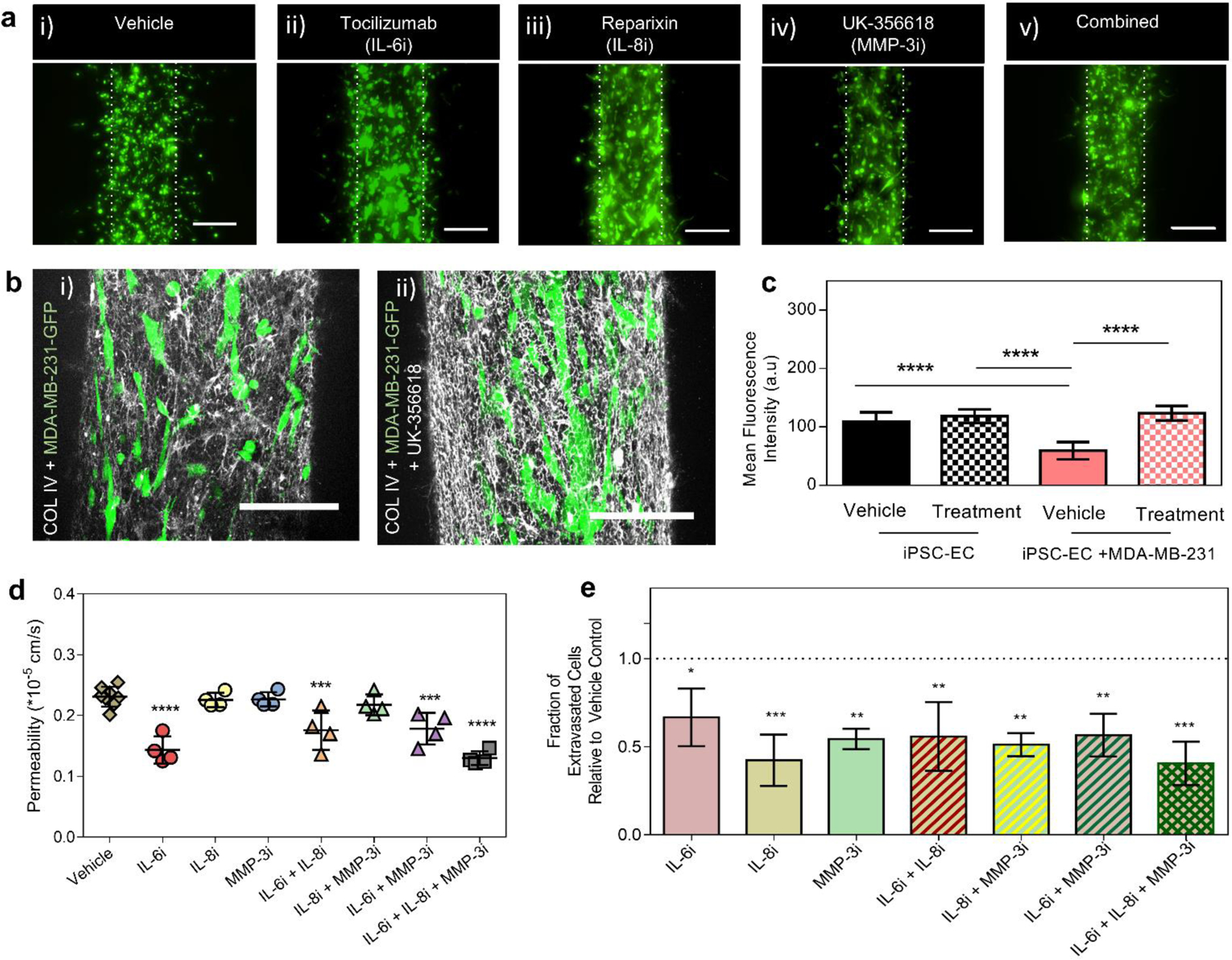
(a) Fluorescent image projections of MDA-MB-231 cell extravasation events in response to vehicle control (i), tocilizumab treatment (IL-6R, ii), reparixin treatment (IL-8R, iii), UK-356618 treatment (iv), and combination treatment (v), scale bars indicate 200 µm. (b) Confocal projection images showing COL IV deposition on vehicle control (i) and UK-356618 treated iPSC-EC vessels in direct-contact co-culture with MDA-MB-231 cells (ii), scale bars indicate 150 µm. (c) Mean fluorescence intensity analysis of COL IV deposition on vehicle control and UK-356618 treated systems. Plot: mean fluorescence intensity + SD, statistical analysis: ordinary one-way ANOVA with Tukey’s multiple comparisons test, ****p ≤ 0.0001. Data represents average of at least 6 different region of interests (ROIs) across 4 different systems.(d) Permeability analysis of iPSC-EC vessels for seven treatment conditions including vehicle control, independent treatment of each inhibitors and their combinations. Plot: mean permeability + SD, statistical analysis: one-way ANOVA followed by Dunnett multiple comparison test, ****p ≤ 0.0001, **p ≤ 0.01, compared with vehicle (n=4–6). (e) Relative fraction of extravasation events for seven treatment conditions examined relative to vehicle control. Plot: relative fraction of extravasated cells + SD, statistical analysis: one-way ANOVA followed by Dunnett multiple comparison test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, compared with vehicle (n=4).
Next, we examined the impact of tocilizumab, reparixin and UK-356618 on iPSC-EC vessel integrity during direct-contact co-culture with MDA-MB-231 cells. We found that vascular permeability decreased significantly in iPSC-EC vessels treated with tocilizumab independently, (Figure 7d), and in pair combination with reparixin or the UK-356618. No notable changes in permeability were observed for other inhibitors and their paired combinations, however, combined treatment of all three inhibitors exhibited similar decrease in permeability to tocilizumab treatment. When treated independently, reparixin induced the greatest decline in quantified extravasation events, followed by the UK-356618 and tocilizumab, respectively, (Figure 7e). Extravasation events also declined when pairs of inhibitors were applied: tocilizumab + reparixin, reparixin + UK-356618 and tocilizumab + UK-356618. Notably, reparixin induces a stronger response in reducing extravasation events, compared to other inhibitors or their paired combinations. Combination of tocilizumab, reparixin and UK-356618, however, maximally reduced extravasation events to nearly 50% of the vehicle control, suggesting an additive effect on extravasation behavior of MDA-MB-231 cells. Taken together, these results show that tocilizumab and reparixin independently reduce iPSC-EC vessel permeability and MDA-MB-231 extravasation events respectively, however, combination of tocilizumab, reparixin and UK-356618 mitigates both functional changes.
Discussion
Extravasation of tumor cells are rare and transient in nature, making it the most difficult step in the metastatic process to study. Despite this challenge, various in vivo and in vitro studies have been conducted and indicate that entry into the perivascular space is heavily regulated by interactions between the various constituents of the metastatic microenvironment17,22. During extravasation, juxtacrine and paracrine signaling between tumor cells and ECs are known to modulate endothelial function, remodel the ECM and promote transendothelial migration. While numerous microfluidic platforms have been developed to study mechanisms of cancer cell extravasation, few models have demonstrated their utility in conducting parametric studies on the functional assessment of therapeutic drugs. Development of models that can probe molecular and functional features of cancer-vascular interactions during extravasation in a higher-throughput fashion would advance discovery of treatment strategies to inhibit metastasis. Here, we developed an arrayable organotypic model of BCC extravasation to examine molecular signaling between cancer cells, endothelium and the ECM that influence function-level response. We demonstrated here the capabilities of our arrayable platform to study cancer cell extravasation by 1) assessing the influence of juxtacrine/paracrine signaling on vascular barrier function and migratory behavior of cancer cells, and 2) evaluating downstream secreted factors and functional responses to multiple therapeutic inhibitors and their combinations.
The organotypic BCC extravasation model consists of an endothelial vessel (~ 250µm diameter) with cancer cells adhered to the inner surface of the vessel. The vessel recapitulates in vivo tubular structures within a collagen-fibrinogen matrrix and is generated from human induced pluripotent stem cell-derived endothelial cells. iPSC-ECs are a desirable cell source as they can be engineered to acquire organ-specific properties and can enable the study of site-specific signatures in cancer metastasis39,40. Moreover, metastatic dissemination can be studied using models developed from patient derived IPS cells for applications in drug screening and precision medicine therapies. iPSC-EC vessels generated within our systems exhibit both functional and morphological characteristics of a robust and stable endothelium, which include tight-junctional and endothelial-specific markers, basement membrane deposition and barrier function.
To demonstrate the ability of our model to mimic in vivo-like extravasation behavior, we assessed extravasation behavior of highly invasive (i.e., Hs-578T and MDA-MB-231) and poorly invasive (i.e., MCF-7) BCCs within our systems and showed a positive correlation between extravasation capabilities and invasive/metastatic potential observed in vivo. We also demonstrate the impact of BCC secretion on endothelial properties and direct-contact remodeling of basement membrane components by BCCs. Tumor cells are also known to modulate and prime the endothelium by upregulating pro-metastatic signaling pathways. Endothelial ICAM-1 expression is involved in tumor cell adhesion to the endothelium during metastasis, which is common across numerous types of cancer41. Moreover, ICAM-1 expression on the endothelium correlates with the production of pro-tumoral cytokines that are associated with enhanced vascular permeability42. We observed increased ICAM-1 expression in our iPSC-EC vessels in response to MDA-MB-231 cells within our system, which demonstrates cancer cell derived modification of endothelial behavior through paracrine signaling to promote adhesion and extravasation. Aside from paracrine signaling-mediated modifications, physical modulation of the endothelial basement membrane is also observed in our system. This is consistent with previous reports showing that in tail vein-injected mice, colon carcinoma cells exit blood vessels by dynamically remodeling the endothelium and the basement membranes to induce gaps. For instance, transmigrating cells are known to remodel type IV collagen, a major component of the basement membranes, through either proteolytic or non-proteolytic processes24. In our model, large areas of COL IV discontinuities surrounding iPSC-EC vessels can be observed where extravasation of MDA-MB-231 cells is found. Moreover, gaps were seen localized to the sites of BCCs suggestive of COL IV remodeling to create passageways for transmigrating cells.
Next, we performed secreted factor analysis on several BCC lines within our system to better define paracrine signaling-mediated cancer-vascular crosstalk. Our analysis revealed distinct expression profiles of oncological factors associated with breast cancer metastasis for highly invasive and poorly invasive BCC lines. We found that for invasive breast cancer lines (Hs-578T and MDA-MB-231), IL-6, IL-8, and MMP-3 secretions were upregulated. Consistent with these findings, previous clinical studies have shown that both IL-6 and IL-8 are found at high concentrations in serums of lung and liver metastasis cancer patients43 and these concentrations correlate with the stage of cancer44. Moreover, MMP-3 secretion is associated with breast cancer invasiveness45. Interestingly, it is also known to play a pivotal role in the degradation/remodeling of laminin and COL IV46, which may suggest a role of MMP-3 secretions in MDA-MB-231-mediated COL IV remodeling observed within our system. In breast cancer metastasis, IL-6 is known to induce disruption of endothelial barrier function47,48. Moreover, both IL-6 and IL-8 have been shown to synergistically enhanced BCC migration in a density-dependent manner29. To resolve the cancer-endothelial crosstalk observed within our system, we identified the independent contributions of iPSC-EC vessels and MDA-MB-231 cells, to the overall secretion of IL-6, IL-8 and MMP-3. We found that MDA-MB-231 cells secrete higher levels of IL-6 and IL-8 in response to signaling from iPSC-EC vessels while higher MMP-3 levels are secreted by the vessels in response to BCC signaling. Furthermore, these results correlate with higher cancer cell migration rates and increased vascular permeability observed within our system, demonstrating the impact of paracrine signaling on cellular function. Taken together, this suggests that BCCs may promote their own migration and impair vascular function through IL-6 and IL-8 secretions in response to paracrine signaling from the vasculature. Likewise, the iPSC-EC vasculature may also be promoting basement membrane degradation and consequently tumor cell motility via MMP-3 secretions in response to factors secreted by BCCs. The approach used here to elucidate these molecular signaling illustrates the potential of our model in deciphering cell type-derived molecular factors driving extravasation of tumor cells.
In vitro studies report that during cancer cell extravasation, alterations to the endothelium are made temporarily where the vessel integrity is restored quickly after transmigration17,50. However, our molecular diffusion analysis showed an increase in vascular permeability following transmigration of cancer cells, suggesting a more complex conditioning capability of the vasculature by BCCs. Contact-dependent juxtacrine signaling in addition to paracrine signaling are important drivers of cancer cell-mediated changes to endothelial barrier function during cancer cell extravasation5. Our analysis confirmed that increases in endothelial permeability results from both juxtacrine interactions and paracrine signaling between iPSC-EC vasculature and BCCs. Interestingly, our results suggest that paracrine signaling alone are sufficient to promote increased vascular permeability and enhanced MDA-MB-231 cell migration. While it is difficult, with the current setup, to resolve the contributions of cancer-endothelial dependent juxtacrine interactions from the contributions of paracrine signaling that inherently exists within a co-culture microenvironment, we demonstrate the extent to which direct-contact interactions enhances the functional changes elicited by paracrine signaling. We hypothesized that the upregulation of IL-6, IL-8 and MMP-3 may partially account for the functional changes observed within our system, including increase in vascular permeability, enhanced cancer cell extravasation, and cancer cell-mediated COL IV degradation. To further elucidate the contributions of these factors to the functional changes observed, we conducted blocking experiments within our model.
FDA-approved drugs that specifically target metastasis are few in numbers, yet metastasis is the leading cause of cancer-related deaths. The heterogeneity of tumor in metastases makes it challenging for single-agent therapies that are essential for tumor cell survival or proliferation to produce sustained results51. Many therapeutics are currently combined based on evidence from additive or synergistic effects observed in preclinical models and in patients37,51. New treatment strategies will need to consider therapeutic interference of multiple receptors, signaling pathways and their supporting microenvironments to overcome drug resistance and produce sustainable, long-term control over tumor growth. Our work suggests that by concurrently inhibiting IL-6, IL-8 and MMP-3 the impact on extravasation events of MDA-MD-231 cells as well as endothelial structure and barrier function can be reduced. An antibody inhibitor for MMP-3, UK-356618, and clinically active therapeutics tocilizumab and reparixin were used in these investigations. Broad-spectrum MMP inhibitors have met with mixed outcomes against cancer, partly due to the anti-cancer benefits of some MMPs. As a result, highly specific MMP targets are now being tailored to reduce adverse systemic effects. Tocilizumab and reparixin have been in current clinical trials both individually or in combination with other therapeutics for their effectiveness against several cancer types and metastasis52,53. Our results indicate that MMP inhibition using UK-356618 significantly mitigated COL IV remodeling and overall extravasation events that resulted from MDA-MB-231 cell interactions with iPSC-EC vasculatures. This further confirms our hypothesis that upregulated MMP secretions facilitates cancer cell extravasation by basement membrane degradation. Our investigations have also revealed that reparixin was most effective at reducing extravasation events of MDA-MB-231 cells when used independently or in combination with both tocilizumab and UK-356618. However, reparixin alone or its combination with any one of the IL-6 or MMP-3 inhibitors did not prevent vascular impairment to the same extent as simultaneous inhibition of all three factors did. Similarly, tocilizumab prevented impairment of vascular barrier function when used alone or combined with other inhibitors but did not impact cancer cell migration significantly. Combined treatment of reparixin, tocilizumab and UK-356618 were required to prevent simultaneous changes in cancer migration behavior and vascular permeability. Together, these results highlight the importance of exploring combination treatment strategies to enhance effectiveness of controlling cancer dissemination by targeting multiple pathways and mechanisms.
Indeed, other factors such as ECM density, may also have interdependent roles in the cellular responses observed in this study. For instance, recent work examining the role of ECM density in conditioning lymphatic vessel properties revealed that higher matrix density resembling cancerous tissue (i.e., COL-I density of 6 mg/mL) induces higher secreted levels of IL-6 and exacerbates vessel leakiness compared to lower matrix density (i.e., COL-I density of 3 mg/mL)23. Moreover, an increase in ECM density has also been shown to impact MMP secretion rates and enhance invadopodia-mediated ECM degradation by MDA-MB-231 cells49. While the current study uses a normal collagen density of 3 mg/mL, these studies indicate that ECM density may also have influence on the levels of secreted levels of factors such as IL-6 and MMPs investigated within our system. Future studies could explore the specific roles and impact of other biological factors on vascular barrier integrity and cell migration behavior observed in this model.
One important aspect of the organotypic modeling approach used here is the ability to compartmentalize and de-couple contributions of individual microenvironmental factors which can be challenging and costly to do in vivo. By sequentially adding supporting cell types and ECM components, independent contributions as well as contributions from crosstalk between various microenvironmental components can be determined using this model. More importantly, future studies incorporating IPS cells from patients affected by metastatic disease or patient-derived cells could be used to accelerate the development of new therapies targeting or preventing cancer metastasis. Potential application of this model could be extended to stratify patients that will develop metastatic disease.
Conclusion
In conclusion, we have presented here a 3D organotypic vascularized model to analyze the cancer cell-EC interactions involved in BCC extravasation. We have provided quantitative assessment of the interplay between BCCs and the endothelium by investigating paracrine signaling and functional responses of the endothelium and BCCs resulting from these interactions. Moreover, we identified the role of IL-6, IL-8 and MMP-3 in regulating vascular permeability and cancer cell extravasation behavior. Our data revealed that metastatic cell line MDA-MB-231 upregulates secretions of IL-6 and IL-8 in response to crosstalk with iPSC-EC vasculatures to impair barrier function and influence their own extravasation behavior. Conversely, iPSC-EC vasculatures respond to this crosstalk by secreting more MMP-3 and promoting BCC migration by degradation of basement membrane components. Combined therapeutic inhibition of these factors reduced overall secretion of metastasis-promoting factors, vascular impairment, and events of BCC extravasation. Importantly, we demonstrate the utility of our system in conducting highly parametric studies and testing combination therapeutics that target multiple and distinct signaling interactions involved in BCC extravasation.
Supplementary Material
Funding:
We thank a predoctoral fellowship (F31CA247248) from the National Institute of Health (NIH) to M.H. We also acknowledge University of Wisconsin Carbone Cancer Center Support Grant (NIH P30CA014520) and the NIH (R01CA186134) for funding support.
Footnotes
Conflicts of interest: David J. Beebe holds equity in Bellbrook Labs LLC, Tasso Inc., Salus Discovery LLC, Lynx Biosciences Inc., Stacks to the Future LLC, Turba LLC, Flambeau Diagnostics LLC and Onexio Biosystems LLC. David J. Beebe is a consultant for Abbott Laboratories.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
- 1.CDC. Breast Cancer Statistics. Division of Cancer Prevention and Control Published 2018. https://www.cdc.gov/cancer/breast/statistics/index.htm
- 2.Belkacemi Y, Hanna NE, Besnard C, Majdoul S, Gligorov J. Local and Regional Breast Cancer Recurrences: Salvage Therapy Options in the New Era of Molecular Subtypes. Front Oncol 2018;8:112. doi: 10.3389/fonc.2018.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strell C, Entschladen F. Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal 2008;6:10. doi: 10.1186/1478-811X-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katt ME, Wong AD, Searson PC. Dissemination from a Solid Tumor: Examining the Multiple Parallel Pathways. Trends in cancer 2018;4(1):20–37. doi: 10.1016/j.trecan.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strilic B, Offermanns S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017;32(3):282–293. doi: 10.1016/j.ccell.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Cuadrado L, Tracey N, Ma R, Qian B, Brunton VG. Mouse models of metastasis: progress and prospects. Dis Model Mech 2017;10(9):1061–1074. doi: 10.1242/dmm.030403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virumbrales-Muñoz M, Ayuso JM, Gong MM, et al. Microfluidic lumen-based systems for advancing tubular organ modeling. Chem Soc Rev 2020;49(17):6402–6442. doi: 10.1039/d0cs00705f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Korolj A, Lai BFL, Radisic M. Advances in organ-on-a-chip engineering. Nat Rev Mater 2018;3(8):257–278. doi: 10.1038/s41578-018-0034-7 [DOI] [Google Scholar]
- 9.Peela N, Truong D, Saini H, et al. Advanced biomaterials and microengineering technologies to recapitulate the stepwise process of cancer metastasis. Biomaterials 2017;133:176–207. doi: 10.1016/j.biomaterials.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 10.Mondadori C, Crippa M, Moretti M, Candrian C, Lopa S, Arrigoni C. Advanced Microfluidic Models of Cancer and Immune Cell Extravasation: A Systematic Review of the Literature. Front Bioeng Biotechnol 2020;8. doi: 10.3389/fbioe.2020.00907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer 2019;19(2):65–81. doi: 10.1038/s41568-018-0104-6 [DOI] [PubMed] [Google Scholar]
- 12.Lin Z, Luo G, Du W, Kong T, Liu C, Liu Z. Recent Advances in Microfluidic Platforms Applied in Cancer Metastasis: Circulating Tumor Cells’ (CTCs) Isolation and Tumor-On-A-Chip. Small 2020;16(9). doi: 10.1002/smll.201903899 [DOI] [PubMed] [Google Scholar]
- 13.Coughlin MF, Kamm RD. The Use of Microfluidic Platforms to Probe the Mechanism of Cancer Cell Extravasation. Adv Healthc Mater 2020;9(8):1901410. doi: 10.1002/adhm.201901410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y-HV, Middleton K, You L, Sun Y. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsystems Nanoeng 2018;4:17104. doi: 10.1038/micronano.2017.104 [DOI] [Google Scholar]
- 15.Nagaraju S, Truong D, Mouneimne G, Nikkhah M. Microfluidic Tumor–Vascular Model to Study Breast Cancer Cell Invasion and Intravasation. Adv Healthc Mater 2018;7(9). doi: 10.1002/adhm.201701257 [DOI] [PubMed] [Google Scholar]
- 16.Shin MK, Kim SK, Jung H. Integration of intra- and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab Chip 2011;11(22):3880–3887. doi: 10.1039/c1lc20671k [DOI] [PubMed] [Google Scholar]
- 17.Chen MB, Whisler JA, Fr J, Yu C, Shin Y, Kamm RD. On-Chip Human Microvasculature Assay for Visualization and Quantification of Tumor Cell Extravasation Dynamics Vol 12.; 2017. doi: 10.1038/nprot.2017.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon JS, Bersini S, Gilardi M, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A 2015;112(1):214–219. doi: 10.1073/pnas.1417115112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bersini S, Jeon JS, Dubini G, et al. A microfluidic 3D invitro model for specificity of breast cancer metastasis to bone. Biomaterials 2014;35(8):2454–2461. doi: 10.1016/j.biomaterials.2013.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boussommier-Calleja A, Atiyas Y, Haase K, Headley M, Lewis C, Kamm RD. The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials 2019;198:180–193. doi: 10.1016/j.biomaterials.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen MB, Hajal C, Benjamin DC, et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc Natl Acad Sci U S A 2018;115(27):7022–7027. doi: 10.1073/pnas.1715932115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayuso JM, Gong MM, Skala MC, Harari PM, Beebe DJ. Human Tumor‐Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells. Adv Healthc Mater 2020;9(3):1900925. doi: 10.1002/adhm.201900925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugo-Cintrón KM, Ayuso JM, White BR, et al. Matrix density drives 3D organotypic lymphatic vessel activation in a microfluidic model of the breast tumor microenvironment. Lab Chip 2020;20(9):1586–1600. doi: 10.1039/d0lc00099j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei X, Middleton K, Shim D, et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr Biol 2019;11(4):119–129. doi: 10.1093/INTBIO/ZYZ008 [DOI] [PubMed] [Google Scholar]
- 25.Jiménez-Torres JA, Peery SL, Sung KE, Beebe DJ. LumeNEXT: A Practical Method to Pattern Luminal Structures in ECM Gels. Adv Healthc Mater 2016;5(2):198–204. doi: 10.1002/adhm.201500608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram PN, Hind LE, Jiminez-Torres JA, Huttenlocher A, Beebe DJ. An Accessible Organotypic Microvessel Model Using iPSC-Derived Endothelium. Adv Healthc Mater 2018;7(2):1700497. doi: 10.1002/adhm.201700497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers 2015;3(1). doi: 10.4161/21688370.2014.978720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reymond N, d’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 2013;13(12):858–870. doi: 10.1038/nrc3628 [DOI] [PubMed] [Google Scholar]
- 29.Sökeland G, Schumacher U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol Cancer 2019;18(1). doi: 10.1186/s12943-018-0937-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo P, Huang J, Wang L, et al. ICAM-1 as a molecular target for triple negative breast cancer. Proc Natl Acad Sci U S A 2014;111(41):14710–14715. doi: 10.1073/pnas.1408556111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBleu VS, MacDonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med 2007;232(9):1121–1129. doi: 10.3181/0703-MR-72 [DOI] [PubMed] [Google Scholar]
- 32.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol 2008;18(11):560–574. doi: 10.1016/j.tcb.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 33.Spuul P, Daubon T, Pitter B, et al. VEGF-A/Notch-Induced Podosomes Proteolyse Basement Membrane Collagen-IV during Retinal Sprouting Angiogenesis. Cell Rep 2016;17(2):484–500. doi: 10.1016/j.celrep.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 34.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121(7):2750–2767. doi: 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong MM, Lugo-Cintron KM, White BR, Kerr SC, Harari PM, Beebe DJ. Human organotypic lymphatic vessel model elucidates microenvironment-dependent signaling and barrier function. Biomaterials 2019;214:119225. doi: 10.1016/j.biomaterials.2019.119225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin K, Pandey NB, Popel AS. Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis. Breast Cancer Res 2018;20(1):54. doi: 10.1186/s13058-018-0981-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayatilaka H, Tyle P, Chen JJ, et al. Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat Commun 2017;8. doi: 10.1038/ncomms15584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang YN, Yan HQ, Huang XB, Wang YN, Li Q, Gao FG. Interleukin 6 trigged ataxia-telangiectasia mutated activation facilitates lung cancer metastasis via MMP-3/MMP-13 up-regulation. Oncotarget 2015;6(38):40719–40733. doi: 10.18632/oncotarget.5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belair DG, Whisler JA, Valdez J, et al. Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells. Stem Cell Rev 2015;11(3):511–525. doi: 10.1007/s12015-014-9549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosa S, Praça C, Pitrez PR, et al. Functional characterization of iPSC-derived arterial- and venous-like endothelial cells. Sci Rep 2019;9(1):3826. doi: 10.1038/s41598-019-40417-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gassmann P, Kang ML, Mees ST, Haier J. In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell - endothelial cell interaction. BMC Cancer 2010;10. doi: 10.1186/1471-2407-10-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter CA, Abrams JS, Beaman MH, Remington JS. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: Importance of T-cell-independent regulation of resistance to T. gondii. Infect Immun 1993;61(10):4038–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol 1994;29(4):423–429. doi: 10.1007/bf02361238 [DOI] [PubMed] [Google Scholar]
- 44.Kozłowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst 2003;48:82–84. Accessed September 24, 2019. http://www.ncbi.nlm.nih.gov/pubmed/14737948 [PubMed] [Google Scholar]
- 45.Hotary K, Li X-Y, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev 2006;20(19):2673–2686. doi: 10.1101/gad.1451806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bejarano PA, Noelken ME, Suzuki K, Hudson BG, Nagase H. Degradation of basement membranes by human matrix metalloproteinase 3 (stromelysin). Biochem J 1988;256(2):413–419. doi: 10.1042/bj2560413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurkan OU, He C, Zielinski R, et al. Interleukin-6 mediates pulmonary vascular permeability in a two-hit model of ventilator-associated lung injury. Exp Lung Res 2011;37(10):575–584. doi: 10.3109/01902148.2011.620680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catar R, Witowski J, Zhu N, et al. IL-6 Trans-Signaling Links Inflammation with Angiogenesis in the Peritoneal Membrane. J Am Soc Nephrol 2017;28(4):1188–1199. doi: 10.1681/ASN.2015101169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar S, Das A, Barai A, Sen S. MMP Secretion Rate and Inter-invadopodia Spacing Collectively Govern Cancer Invasiveness. Biophys J 2018;114(3):650–662. doi: 10.1016/j.bpj.2017.11.3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Escribano J, Chen MB, Moeendarbary E, et al. Balance of mechanical forces drives endothelial gap formation and may facilitate cancer and immune-cell extravasation. PLoS Comput Biol 2019;15(5):e1006395. doi: 10.1371/journal.pcbi.1006395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kummar S, Chen HX, Wright J, et al. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov 2010;9(11):843–856. doi: 10.1038/nrd3216 [DOI] [PubMed] [Google Scholar]
- 52.Schott AF, Goldstein LJ, Cristofanilli M, et al. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2-Negative Metastatic Breast Cancer. Clin Cancer Res 2017;23(18):5358–5365. doi: 10.1158/1078-0432.CCR-16-2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trastuzumab, Pertuzumab, Tocilizumab in Treating Participants with Metastatic or Unresectable HER2 Positive Breast Cancer - NCT03135171 - National Cancer Institute. Accessed September 25, 2019. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2017-02497&r=1https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5600824/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.


