Abstract
Stem cells can generate a diversity of cell types during development, regeneration and adult tissue homeostasis. Differentiation changes not only the cell fate in terms of gene expression but also the physical properties and functions of cells, e.g. the secretory activity, cell shape, or mechanics. Conversely, these activities and properties can also regulate differentiation itself. Membrane trafficking is known to modulate signal transduction and thus has the potential to control stem cell differentiation. On the other hand, membrane trafficking, particularly from and to the plasma membrane, depends on the mechanical properties of the cell surface such as tension within the plasma membrane or the cortex. Indeed, recent findings demonstrate that cell surface mechanics can also control cell fate. Here, we review the bidirectional relationships between these three fundamental cellular functions, i.e. membrane trafficking, cell surface mechanics, and stem cell differentiation. Furthermore, we discuss commonly used methods in each field and how combining them with new tools will enhance our understanding of their interplay. Understanding how membrane trafficking and cell surface mechanics can guide stem cell fate holds great potential as these concepts could be exploited for directed differentiation of stem cells for the fields of tissue engineering and regenerative medicine.
Keywords: Membrane trafficking, Cell surface mechanics, Stem cell differentiation, Plasma membrane tension, Mechanotransduction
Differentiation is the process through which stem and progenitor cells generate the diverse cell types that fulfill specialized functions. This happens by (epi-)genetic changes that lead to the expression of the cellular machineries required for these functions. Furthermore, differentiation changes membrane trafficking and cell surface mechanics to support the shape and functions of differentiated cell types: secretory cells, neurons, immune or epithelial cells (with apicobasal polarity) have specialized membrane trafficking machineries at their disposal [1], [2], [3], [4], and require a particular cell shape and surface properties to adequately function [5], [6], [7], [8], [9].
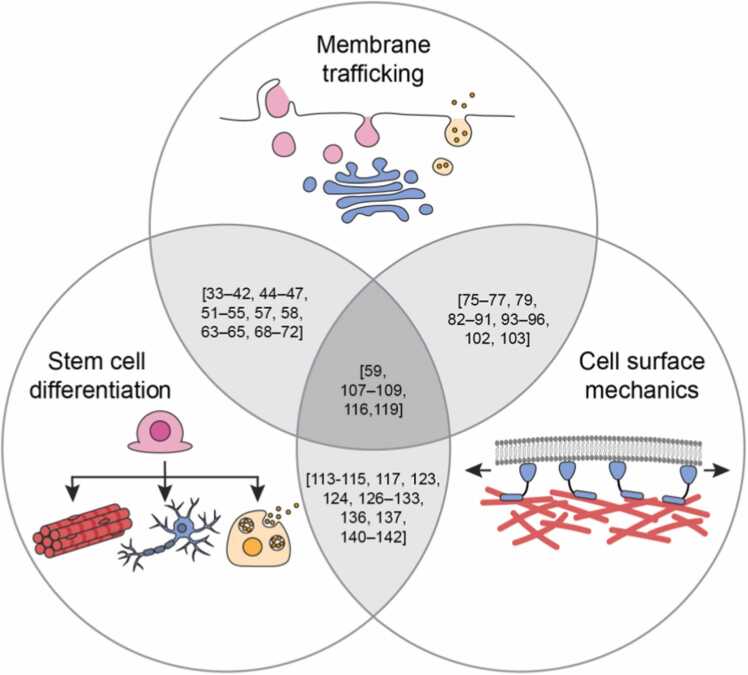
On the other hand, recent findings demonstrate that membrane trafficking and cell surface mechanics can also regulate fate acquisition and differentiation in the first place. During development, differentiation of embryonic stem cells is controlled through conserved signaling pathways. While membrane trafficking has long been recognized as an important regulator of these signaling pathways [10], the notion of cell surface mechanics being a regulator of stem cell differentiation is a recent and surprising finding [11], [12]. Membrane trafficking and cell surface mechanics are closely related as endo- and exocytosis have been shown to control, and in turn be controlled by, plasma membrane tension [13], [14]. In light of this mechanochemical feedback, we can better understand the control of stem cell differentiation through both membrane trafficking and cell surface mechanics. Both aspects on their own or together can control cell signaling that trigger differentiation programs or determine the distribution of fate determinants within cells and tissues (Fig. 1).
Fig. 1.
Studies that uncover the bidirectional relationships between membrane trafficking, stem cell differentiation, and cell surface mechanics. Grey areas contain the numbered references. Not drawn to scale.
Although stem cells have great biomedical potential for diagnostics and regenerative therapies, controlling stem cell differentiation represents a major challenge. Furthermore, aberrant differentiation of (adult) stem cells can also lead to human diseases. In fact, cancer stem cells are a critical factor in tumorigenesis [15], [16], [17]. The signaling pathways steering developmental processes are also conserved in adult stem cells to specify, for example, the fate of hematopoietic or cancer stem cells [18], [19], [20]. While most differentiation protocols have traditionally focused on signaling pathways to guide differentiation, controlling membrane trafficking and cell surface mechanics as an alternative tool for directed differentiation remains largely unexplored. Therefore, a thorough understanding of the chemical and mechanical information processing mechanisms at play could aid to guide differentiation of various types of stem cells for therapeutic applications and to understand pathogenesis of stem-cell related diseases. Box 1.
Box 1. Definitions.
Niche: biochemically and -physically controlled environment in which adult stem cells reside. The special microenvironment is organized by niche cells that control self-renewal and differentiation of the stem cells.
Germ layers: the primary layers of cells that are specified during embryogenesis and form all tissues and organs of the body. In bilaterians, they consist of endoderm, mesoderm, and ectoderm.
Pluripotent stem cells: embryonic stem cells or reprogrammed (induced) somatic cells that are capable of differentiating into all germ layer types.
Mesenchymal stem cells (MSCs), also stromal stem cells or multipotent stromal cells: adult stem cells derived from different tissues that can be differentiated towards fates of muscle (myoblast), bone (osteoblast), cartilage (chondrocyte), or fat cells (adipocyte).
Exocyst: A protein complex responsible for the targeting of post-Golgi vesicles to the plasma membrane.
(Apparent) Plasma membrane tension: energetic cost of increasing the surface area of the plasma membrane of a cell. Consists of the in-plane tension of the plasma membrane and the attachment between the plasma membrane and the cytoskeletal cortex underneath mediated by crosslinkers such as Ezrin, Radixin, and Moesin, collectively known as the ERM proteins.
Extracellular vesicles (EVs), also exosomes: secreted multi-vesicular endosomes that contain miRNA, RNA and proteins.
Eisosomes: invaginated domains in the plasma membrane of fungal cells containing large immobile protein complexes from where endocytosis is initiated.
1. Bidirectional effects of membrane trafficking and differentiation
Membrane trafficking can affect signaling pathways involved in stem cell differentiation by regulating the abundance, localization and activity of signaling receptors and ligands (degradation, levels in the plasma membrane and extra-/intracellular compartments, or post-translational modifications). Trafficking-related regulation can occur in both the signal-sending and -receiving cell [21]. The role of membrane trafficking in many evolutionary conserved signaling pathways such as Hedgehog, Wnt/β-catenin, Smad (TGF-β/BMP/Activin/Nodal), Notch, or FGF has been studied in detail and reviews focusing on specific pathways are available [22], [23], [24], [25], [26], [27], [28]. Accordingly, the pivotal role of trafficking in cell fate specification and morphogenesis during embryonic development has been well established [29], [30], [31], [32], [33].
Although a large body of work supports the significance of membrane trafficking in controlling developmental signaling pathways, few studies demonstrate a direct link between trafficking and differentiation. A recent genome-wide CRISPR mutagenesis screen in human embryonic stem cells (hESCs) underscores the universal importance of membrane trafficking for the differentiation into the three germ layers. Out of the 11 genes that have been identified as commonly essential for differentiation into all germ layers, 7 genes were found within the ER-Golgi network and related to posttranslational modifications and protein trafficking to the plasma membrane [34]. In fact, endocytosis plays a role in the acquisition [35] and maintenance of pluripotency by balancing the levels of E-cadherin and TGF-β signaling [36]. Moreover, the endocytosis-autophagy network has been implicated in proliferation and differentiation of intestinal stem cells in the adult Drosophila melanogaster gut amongst other stem and progenitor cell types [37], [38]. Furthermore, the exocyst is crucial in niche cells to promote germline stem cell progeny differentiation in the Drosophila ovary [39]. These examples demonstrate that various membrane pathways, at the cell surface and in endomembrane compartments, affect differentiation of a variety of stem cell types including embryonic, adult, and germline stem cells, either directly or through niche cell signaling.
Conversely, differentiation can regulate membrane trafficking to adapt to the needs of the differentiated cell type. Upon differentiation of ESCs, the expression of endocytosis-associated genes are upregulated [40] and the dynamics and morphology of clathrin-mediated endocytosis change markedly [41]. Furthermore, Kostopoulou et al. found that hESCs internalize Activin A-receptor complexes (TGF-β superfamily member) exclusively through clathrin-mediated endocytosis whereas once the cells are differentiated to mesodermal progenitors or endothelial cells, both clathrin-mediated endocytosis and macropinocytosis are used. This selective use of endocytic pathways could reflect different requirements in terms of sensitivity to morphogen gradients [42].
Wnt signaling, a conserved pathway organizing the primary body axis and mesoderm specification [43], is special in this context since it is not only regulated by but can also immediately regulate membrane trafficking [26]. On the one hand, trafficking of Wnt receptors and downstream signaling complexes is responsible for proper fate specification in the zebrafish embryo and for adipogenic differentiation of mouse embryonic fibroblasts [33], [44]. On the other hand, canonical Wnt signaling has been shown to induce macropinocytosis in human cultured cells and Xenopus laevis embryos [45], [46], [47]. Wnt signaling could thus regulate different cellular functions that depend on macropinocytosis including other signaling pathways and cell surface mechanics (see below).
1.1. Rab GTPases in development
Rab GTPases, the largest family of small GTPases, are master regulators of membrane trafficking [4]. They are indispensable for membrane traffic routes that are common in most cell types but also facilitate dedicated transient traffic that is only required in specialized cell types or functions [48]. Many Rabs have been implicated in development as they exhibit specific expression patterns during embryogenesis [49], [50], regulate developmental signaling pathways, e.g. Wnt, Nodal, and Sonic Hedgehog [44], [51], [52], [53], and knockout or knockdown have resulted in embryonic lethality or morphological defects [4]. A specific role in cell fate specification has been established for several Rabs. For example, Rab5ab is involved in the specification of the dorsal organizer in zebrafish [52] and Rab23 is required for Nodal expression in the left lateral plate mesoderm in vertebrates, thus regulating left-right patterning [51]. Nevertheless, in most instances where Rabs have been found to be necessary for proper development, the molecular mechanism is unknown [4].
1.2. A role for adhesion and the cytoskeleton
The interplay between differentiation and trafficking may also involve adhesion complexes and the cytoskeleton. In the developing Drosophila, Merlin and Expanded, members of the FERM (Four-point one, Ezrin, Radixin, Moesin) domain superfamily, regulate the steady-state levels and cell surface clearance of adhesion molecules (E-cadherin and Fat, a cadherin superfamily member) and other signaling receptors (Notch, EGF receptor, Patched and Smoothened). Double negative mutants exhibit differentiation defects in the epithelia of the wing imaginal discs and the developing eye [54], [55]. Self-renewal of hESCs has been demonstrated to rely on the feedback between endocytic pathways and E-cadherin-mediated adhesion [56]. In adult stem cells, trafficking of adhesion complex components to and from the cell surface is critical to ensure physical attachment to the niche which regulates stemness and differentiation [57], [58], [59]. Moreover, for cell migration, the assembly and disassembly of adhesive contacts by membrane trafficking is a prerequisite [60]. For example, endocytosis of E-cadherin and the adhesion-regulative EphB has been shown to facilitate cell migration during zebrafish morphogenesis [32], [61], [62]. Such links between fate specification and motility as well as positioning within the embryo, indicate that this is another mode through which trafficking can affect cell fate.
The cytoskeleton is also involved in coupling trafficking and differentiation. In the zebrafish embryo, the actin-bundling protein Fscn1 is highly expressed in mesendoderm progenitors and associates specifically with TGF-β receptors in clathrin-coated vesicles while interacting with actin. Notably, expression of Fscn1 is directly promoted by the TGF-β superfamily member Nodal, and depletion of Fscn1 leads to defects in endoderm formation [63]. This exemplifies the bidirectional relationship between signaling and trafficking, the importance of the cytoskeleton for trafficking and that the coordinated activity of these machineries is required for proper cell specification.
1.3. Asymmetric cell division
Different from global activation or inhibition of signaling cascades, membrane trafficking can also influence stem cell differentiation through asymmetric distribution of fate determinants after asymmetric cell division. In the Drosophila midgut, division of an intestinal stem cell generates a self-renewing stem cell and a daughter cell differentiating to an enteroblast. Notch and Delta are asymmetrically distributed through specific endocytic vesicles marked by the endosomal protein Smad Anchor for Receptor Activation (Sara), thus determining the fate of the daughter cells [64]. Sara endosomes also operate in zebrafish to generate different neuronal cell types through asymmetric cell division of progenitors [65]. In the Drosophila ovary, directional trafficking of adhesion proteins in germline stem cells enables only one daughter cell to adhere to the niche cells while the other differentiates subsequently [57]. In the Drosophila testis, targeted receptor trafficking to specialized contact sites between the stem cell and the niche cells (microtubule-containing nanotubes) limits signaling spatially to these cells [58]. These examples illustrate the importance of the positional information conveyed through directional trafficking to maintain stemness and to organize differentiation of daughter cells.
1.4. Extracellular vesicle secretion
Finally, the secretion of extracellular vesicles (EVs) is an important way of communication between adult stem cells and the parenchymal cells of the tissue, drawing interest due to their therapeutic potential [66], [67]. In the Drosophila wing disc, Hedgehog signal transduction depends on several trafficking processes. These include the targeting of Hh receptors to specialized contact sites (cytonemes) for presentation, the secretion of the receptor in EVs, and the lysosomal degradation after reception of Hedgehog [68].
Furthermore, EVs derived from differentiated cells stimulate the differentiation of naïve cells [69], [70], [71]. EVs derived from human mesenchymal stem cells (hMSCs) differentiated to the adipogenic, osteogenic, or chondrogenic fate [70] promote differentiation of naïve hMSCs towards the fate from which the vesicles originate and can even overwrite the influence of the extracellular matrix [72]. EVs thus represent another layer of how membrane trafficking can control stem cell fate in an autocrine and paracrine fashion.
To summarize, membrane trafficking can regulate stem cell differentiation by controlling the processing capacity of signaling pathways, by polarizing the distribution of fate determinants or adhesion molecules and by sending long-range differentiation signals through exosomes (Fig. 1). Membrane trafficking particularly at the plasma membrane is tightly interrelated to cell surface mechanics. Therefore, we will introduce the feedback relationship between membrane trafficking and surface mechanics in the following section before closing the circle to stem cell differentiation.
2. Bidirectional effects of cell surface mechanics and membrane trafficking
A central aspect of the connection between cell surface mechanics and membrane trafficking (Fig. 1) is the mechanochemical feedback between plasma membrane tension and endo-/exocytosis, which has been the focus of numerous studies [13], [73], [74]. Initial evidence of such coupling was found in plant cells: in isolated plant protoplasts (without the cell wall), the flow of membrane between the plasma membrane and intracellular membrane reservoirs depends on plasma membrane tension. If tension deviates from a resting value of ~0.12 mN/m, by hypo- or hypertonic treatment, the ratio of endo-/exocytosis is adjusted to add membrane to the plasma membrane or to internalize surplus membrane in order to restore the tension to its resting value. Because biological membranes increase their surface area only marginally under high tension, this mechanism is required to prevent cell lysis [75]. This feedback mechanism also operates in animal cells to maintain plasma membrane tension homeostasis [76], [77]. Mechanistically, changes in plasma membrane tension can be coupled to membrane trafficking either directly, i.e. physically as in the case of high tension inhibiting endocytosis, or by mechano-sensing and -transduction. The molecular identity of plasma membrane tension sensors and the mechanotransduction pathways regulating membrane trafficking were unknown for a long time, but some have been revealed over the last decade (e.g. TORC2) [14].
2.1. Coupling with endocytosis
High or acutely increased membrane tension can physically block endocytosis or lower its rate as it creates an energetic barrier counteracting the deformation of the membrane [78]. In fact, in clathrin-mediated endocytosis, actin polymerization is required to overcome high tensile forces whereas it is dispensable in low tension membranes [79], [80], [81]. More recently, elevated tension has been shown to inhibit endocytosis in secretory cells where overcoming the energy barrier imposed by lateral tension is rate-limiting [82]. A particularly striking case in which mechanical blocking of endocytosis is exploited is mesoderm invagination during gastrulation of the Drosophila embryo. During the first wave, mesodermal cells undergo apical constriction stochastically which generates mechanical strain in the surrounding cells of the tissue. This inhibits endocytosis of Fog, facilitating its signaling, which orchestrates the synchronized constriction in a second wave [83].
On the other hand, decreased plasma membrane tension has been shown to activate the dynamin-independent CLIC/GEEC endocytic pathway in a vinculin-dependent manner [84]. Vinculin is a component of focal adhesions which are responsive to mechanical forces. By applying a localized force on focal adhesions, clusters of integrin (another transmembrane component of focal adhesions) disassemble and are internalized by clathrin-mediated endocytosis [85]. Focal adhesions therefore represent a hub of crosstalk between cell surface mechanics and intracellular processes including trafficking.
2.2. Coupling with exocytosis
Changes in plasma membrane tension affect not only endocytosis but also exocytosis [73]. High tension could promote exocytosis by exposing the hydrophobic lipid tails and thus lowering the energy barrier for vesicle fusion. On the other hand, membrane tension could counteract SNARE-mediated membrane fusion [73]. During cell spreading, membrane reservoirs are first unfolded until the reservoirs are depleted leading to a sudden spike in plasma membrane tension. This triggers the exocytosis of vesicles to deliver endogenous membrane to the plasma membrane [86], [87]. This mechanism operates also during phagocytosis, by which immune cells engulf large particles such as pathogens or apoptotic cells, such that exocytosis is enhanced to provide the membrane area needed for efficient internalization [88]. Additionally, plasma membrane tension also seems to dictate the mode of exocytosis. In fibroblasts, exocytosis typically occurs by full-collapse fusion whereas in neuronal cells, which generally have lower tension, “kiss and run” exocytosis is prevalent (a form of exocytosis by which the vesicle releases its content through a transient pore) [13], [89], [90]. In neuroendocrine chromaffin cells, full fusion of vesicles requires sufficient plasma membrane tension generated by ATP-driven actin assembly [91]. In summary, membrane tension and exo-/endocytosis regulate each other through diverse feedback mechanism that operate in different systems.
2.3. Membrane curvature and TORC2 signaling
Apart from endo- and exocytosis, other undulations of the plasma membrane can also be important for signaling. High plasma membrane tension can displace or relocate molecules in or associated to the plasma membrane by changing membrane curvature. Eisosomes are crucial in the maintenance of membrane tension and integrity in yeast [77], [92]. In these cells, which already have a high turgor pressure, an acute increase in plasma membrane tension leads to the displacement of Slm1 and/or Slm2 from eisosomes, which activates target of rapamycin complex 2 (TORC2). Active TORC2 stimulates sphingolipid synthesis (thus modifying the in-plane tension) and regulates endocytosis through phosphorylation cascades in order to compensate the tension increase [93], [94], [95], [96]. Inhibition of TORC2 leads to a gradual increase in plasma membrane tension that hinders recruitment of proteins that link the plasma membrane and actin, and a BAR domain protein required for fission [97]. The metazoan counterparts to eisosomes are caveolae and indeed mechanical stress has been shown to disassemble them [98], affecting glycosphingolipid packing and trafficking [99]. Conversely, caveolae accumulate where membrane tension is low such as in the rear of fast migrating cells where caveolae-mediated signaling, actomyosin contractility and membrane tension interact in a feedback loop to facilitate retraction [100]. Moreover, the mammalian homologue mTORC2 has also been linked to plasma membrane tension in the control of neutrophil migration, where downstream mTORC2 signaling is dependent on phospholipase D2 (PLD2) [101]. It is therefore tempting to speculate that similar to yeast a mechanotransduction pathway involving plasma membrane tension, membrane curvature and mTORC2 exists also in mammalian cells.
TORC2 is also involved in sensing low plasma membrane tension, albeit through a different mechanism. In yeast, acute loss in tension inactivates TORC2 through a sequestering mechanism into phase separated domains of PI(4,5)P2 [102]. In mammalian cells, a sudden drop in tension activates phospholipase D2 (PLD2). The activated PLD2 promotes PI(4,5)P2 production, which in turn activates macropinocytosis [103]. These similarities suggest that mTORC2 might be involved in sensing low plasma membrane tension as well.
In summary, plasma membrane tension and the ratio of endo- and exocytosis are tightly coupled at the cell surface (Fig. 1). Although we still lack a clear molecular understanding of such processes, some players have been identified. Changes in plasma membrane tension can be transduced through TORC2 signaling in yeast, and focal adhesions and mTORC2 in mammals.
3. Bidirectional effects of cell surface mechanics and stem cell differentiation
Although the relationship between cell fate and tissue mechanics is a central question in morphogenesis, its connection is probably the least studied one. While cell specification in different tissues is generally considered to drive patterning and morphogenesis, the reciprocal potential of mechanics in regulating cell fate and gene expression was neglected for a long time [104]. This idea is appealing because the cell surface can act as an integrator for different long- and short-ranged external signals as well as cell-intrinsic signals. Intra-tissue stresses, adhesion and contractility of neighboring cells, and the material properties of the microenvironment can all be sensed and relayed. The influence of substrate (extracellular matrix) stiffness and large-scale forces such as fluid flow on stem cell differentiation have been reviewed in detail elsewhere [105], [106]. Here, we will focus on findings that demonstrate a direct contribution of cell surface mechanics on differentiation. With the previous sections in mind, it appears plausible that surface mechanics through the coupling between plasma membrane tension and membrane trafficking can control signaling processes and stem cell differentiation. However, this has only rarely been demonstrated in the literature. Independent of membrane trafficking, other mechanisms can also link cell surface mechanics to stem cell differentiation, which we will also discuss in this section.
3.1. Mechanical regulation of morphogen trafficking
The first study to report regulation of differentiation by plasma membrane tension-controlled membrane trafficking of a morphogen was performed in mouse MSCs (C2C12) which can adopt a myogenic or osteogenic fate. Increasing plasma membrane tension by hyposmotic treatment inhibits endocytosis of BMP2, which accelerates the nuclear translocation of Smad1, a transcription factor and effector of BMP signaling. This upregulates downstream expression of transcription factors that inhibit myoblastic and favor osteoblastic differentiation [107]. An analogous mechanism was recently found to act in the differentiation of mouse ESCs. When mESCs transition from the naïve to primed pluripotency state, apparent plasma membrane tension drops through decreased activity of β-catenin, RhoA and ERM proteins. The lower tension facilitates endocytosis of FGF receptor 1, activating ERK signaling, which leads to further mESC differentiation [108]. In support that endocytosis can be physically prevented by high plasma membrane tension (and not indirectly through signal transduction), linking the plasma membrane artificially with the underlying cytoskeleton, and thus raising apparent membrane tension, was able to block the naïve-to-primed transition [109].
3.2. Mechanical regulation of cell division
Cell surface mechanics plays an important role in cell division [110], [111], [112]. Recently, the timing of the exit from pluripotency of mESCs was found to be coupled to abscission, the last step in cell division which separates the daughter cells [113]. Interestingly, tension has previously been shown to regulate abscission. Specifically, tensile forces in the intercellular bridge inhibit recruitment of the ESCRT-III complex, which is required for membrane fission [114]. Plasma membrane tension could therefore regulate the exit from naïve pluripotency via two independent means, i.e. abscission [113] and endocytosis [108].
3.3. Effects of cell shape and a possible role for mTORC2?
Moreover, cell shape has been shown to regulate differentiation of both embryonic and mesenchymal stem cells. In the aforementioned study of mESCs [108], preventing cells from spreading by micropatterning was sufficient to maintain a high membrane tension which affected differentiation. Also by micropatterning and controlling cell density, McBeath et al. found that cell shape regulates Rho activity upstream of cytoskeletal tension which balances adipogenic vs. osteogenic differentiation of hMSCs [115]. Another study in hMSCs links cell shape to cortical stiffness and the density of caveolae which activate Akt signaling differentially, thus directing fate [116]. Notably, mechanically induced EGFR and Akt activation in mesangial cells depend on intact caveolae [117]. Considering the above mentioned relationship between plasma membrane tension, eisosomes, and TORC2 in yeast [92], [93], it is tempting to speculate that tension, caveolar endocytosis and differentiation could be connected over mTORC2. Indeed, a role of caveolin-1 and - 2 has been described for neuronal stem cell differentiation via Nerve Growth Factor signaling [118]. In breast cancer stem cells, caveolin-1 expression was upregulated in and needed for the self-renewal ability of those cells [119]. Importantly, plasma membrane tension controls mTORC2 activity in the context of cell migration [101] and mTORC2 regulates MSC differentiation [120], [121] and myoblast terminal differentiation [122].
Mechanical strain during morphogenesis can immediately alter the distribution of molecules in a cell. During Drosophila oogenesis, flattening of the follicular epithelium dilutes the Crumbs-Expanded and Merlin-Kibra protein complexes at the apical domain, downregulating canonical Hippo signaling and leading to nuclear translocation of Yorkie (homolog of mammalian YAP/TAZ) [123]. At the same time, the apical abundance of the transmembrane Crumbs is dynamically regulated by endocytosis [124]. Since Merlin and Expanded are also involved in regulating receptor trafficking and epithelial differentiation as mentioned above [54], [55], this places physiological strain and cell shape changes into the feedback loops between trafficking and signaling which control differentiation and morphogenesis [125].
3.4. A role for adhesion and the cytoskeleton
Adhesion complexes such as focal adhesions or adherens junctions can also transduce mechanical signals from the cell surface to the interior [59], [126], [127], [128]. hESCs cultured on soft hydrogels accumulate β-catenin at cell-cell adhesions which promotes mesoderm differentiation by activating Wnt signaling [128]. Local cyclic stress applied on naïve mESCs induces focal adhesion-mediated cell softening and spreading as well as downregulation of Oct3/4, a marker of naïve pluripotency [126]. On the other hand, naïve mESCs are insensitive to whole cell stretching, only becoming more susceptible to external mechanical signals upon differentiation [129]. Thus, the reaction to externally applied stresses seems to depend on the type of stress, and we hypothesize that the intrinsically regulated plasma membrane tension [108], [109] could render mESCs more or less sensitive to different external stresses. As mESCs typically grow as colonies, intercellular forces also come into play. Du et al. recently showed that the outermost cells of the colony autonomously generate contractile forces through a three-dimensional supracellular actomyosin cortex which promote the expression of pluripotency factors [130]. Contractility and fibronectin-mediated traction forces also support the specification of mESCs towards definitive endoderm [131]. Not only the type and strength of physical signals, but also their time integration, can direct stem cell fate. Specifically, cell-cell contact duration, under the influence of Nodal signaling, feeds back positively onto mesendodermal cell-fate specification during zebrafish gastrulation [127].
Similar to the directional trafficking mentioned above, physical attachment of fate determinants to the cell cortex can determine polarization. Moesin, the sole member of the ERM proteins in Drosophila, is required for the establishment of the anterior-posterior body axis during oogenesis. In Moesin mutants, F-actin fails to attach to the oocyte cortex and thus posterior polarization of the maternal determinant Oskar is lost [132]. Although mechanical aspects were not investigated in this context, it suggests that the cortical mechanics and architecture of the oocyte are important to establish polarity. Another study showed that in zebrafish embryos deficient in pou5f1 (equivalent of the mammalian pluripotency transcription factor Pou5f1/Oct4) disruptions in cortical microtubules and F-actin results in defects in epiboly and endoderm specification [133].
3.5. Stretch-activated channels
Other plasma membrane tension-sensing candidates are the mechanosensitive channels such as the stretch-activated Piezo channels [134], [135]. These channels have been implicated in the differentiation of several types of stem cells. In adult Drosophila, mechanical stress sensed by Piezo determines differentiation of enteroendocrine stem cells in the midgut through Ca2+ signaling [136]. A similar mechanism also specifies the neuronal vs. glial lineages in human neural stem/progenitor cells, where cell-generated traction forces activate Piezo1 leading to Ca2+ influx and nuclear translocation of YAP [137]. In non-excitable cells, Piezo and other mechanosensitive channels trigger downstream signaling mostly via Ca2+ [134]. Ca2+ signaling is crucial for the behavior of various stem cell types [138] and has been implicated in the extraordinary regeneration capabilities of planarian flatworms [139]. Additionally, proliferation of dental pulp stem cells has been shown to be mechanically stimulated by ultrasound treatment, possibly through Piezo1/2 mediated activation of MAPK signaling [140]. Piezo channels activate signaling also via calpain, which is involved in the differentiation of mesenchymal stem cells [141] and of neural stem cells [142].
In summary, a growing body of work puts plasma membrane tension and other mechanical properties of the cell surface forward as important players not only in morphogenesis but also in determining cell fate. In other cases, mechanistic details may yet be missing. For example, a recent study found that the tension generated by differentiated epidermal keratinocytes can regulate differentiation and migration of basal stem cells that generate the epidermis [143], but the molecular mechanism remains to be determined. The interactions collected here between cell surface mechanics, trafficking networks, and signaling may serve as possible avenues to link tissue-scale forces with cellular and molecular effectors, which can be interrogated using appropriate tools.
4. Methodology
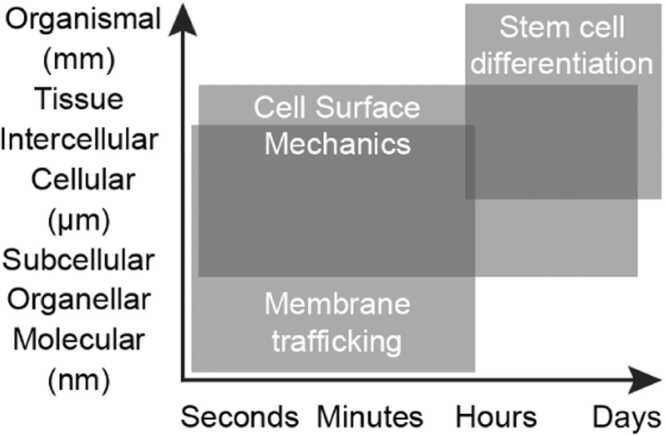
The palette of methods to study each of the three aspects discussed in this review is broad. To elucidate the interplay of these cell functions, methods have to be combined. For clarity, we give a brief overview of commonly used methods for each of the fields (Fig. 2). For more details on the mentioned techniques as well as other more specialized methods, we refer the reader to recent reviews and methodological textbooks [144], [145], [146]. It should be noted that cell differentiation, membrane trafficking and changes in cell surface mechanics happen in different spatial and time scales (Fig. 3), and that each method has its own resolution range.
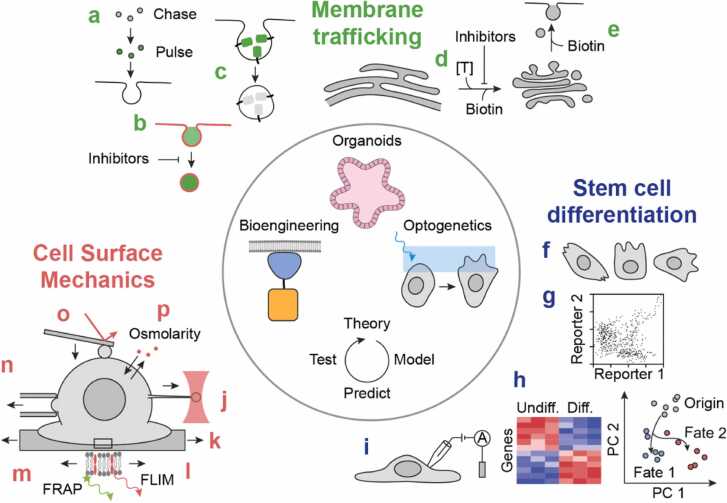
Fig. 2.
Common techniques to investigate membrane trafficking, stem cell differentiation and cell surface mechanics. Centre: methods applicable to all three processes and useful to elucidate their reciprocal relationships. a) Pulse-chase approaches, b) uptake assay of fluorescent dextran (green) and lipophilic dye (red), c) pHluorin, d) cargo release from the ER by the temperature-sensitive VSVGtsO45, e) RUSH system to retain cargo in the ER or Golgi until released through biotin. f) Cell morphology, g) flow cytometry analysis of lineage-specific reporters, h) transcriptomics analysis, i) functional test e.g. electrophysiology. j) Optical tweezer, k) cell stretcher, l) tension probe FliptR, m) fluidity measurement by FRAP, n) micropipette aspiration, o) atomic force spectroscopy, p) hyper-/hypo-osmotic shock. Not drawn to scale.
Fig. 3.
Spatial and temporal scales of stem cell differentiation, (changes in) cell surface mechanics, and membrane trafficking.
4.1. Membrane trafficking
A variety of techniques are available to detect endo- and exocytosis, antero- and retrograde transport between ER, Golgi and endosomal compartments (Fig. 2 a-e). Membrane trafficking can be studied in two general ways, either bulk transport or the trafficking of specific molecules (cargo). Fluid phase or membrane uptake assays can be performed, e.g. using fluorescent dextran or FM4–64 (a lipophilic dye) [147]. Pulse-chase approaches highlight a subset of cargo which can then be followed over time [54], [148]. Moreover, the pH-sensitive GFP variant pHluorin can be fused to cargo to study endocytosis and traffic between the endolysosomal compartments [149]. To visualize secretory traffic, cargo can be released in a synchronized manner from the ER or Golgi using the temperature-sensitive variant of the viral glycoprotein VSVGtsO45 [150] or the Retention Using Selective Hooks system (RUSH) [151]. Furthermore, as many trafficking compartments such as vesicles are smaller than the diffraction limit of light, (correlative) super-resolution microscopy and electron microscopy are required to visualize these compartments in detail [152]. At tissue level, high-resolution light sheet microscopy and heavy-duty data processing pipelines are needed to follow the dynamics of trafficking [153].
To perturb certain trafficking processes, specific inhibitors or drugs such as Dynasore (Dynamin inhibitor), Pitstop2 (Clathrin inhibitor) or Brefeldin A (inhibition of ER-to-Golgi traffic) can be used.
4.2. Stem cell differentiation
The progression of stem cell differentiation can be assessed by different means (Fig. 2 f-i): (1) cell morphology, (2) lineage-specific markers, (3) gene expression, and (4) cell function [145]. Cell morphology is a very practical and non-invasive readout of differentiation [154]. However, depending on the cell type, morphometric parameters alone are considered insufficient to confirm differentiation. The gold standard is to assess expression of lineage-specific genes, either at the protein or mRNA level. Lineage-specific marker proteins are usually detected by specific antibodies or genomic tagging with fluorescent proteins using e.g. flow cytometry or microscopy. Gene expression at the mRNA level can be assayed by qPCR for single genes, but with the advent of transcriptomics, it has become common to construct the full gene expression profile by RNA sequencing to map out the gene regulatory networks underlying stemness/multipotency and the transcriptional changes during differentiation [155], [156], [157], [158]. The advantage of methods with single-cell resolution, such as flow cytometry or single-cell RNA-seq, is that distinct subpopulations (undifferentiated cells and differentiated cells of different lineages and intermediate stages) can be resolved. As probably the most stringent criterion, the ability of a cell to perform a particular function can be tested, e.g. electrophysiological activity of neuronal cells or accumulation of lipid droplets in adipocytes by lipid staining [159], [160]. Clonogenicity assays can be performed to assess changes in proliferative capacity upon exit of pluripotency and differentiation [161], [162], [163].
4.3. Cell surface mechanics
To characterize the cell surface, several physical parameters can be quantified with appropriate methods [144] (Fig. 2j-p). Some instruments like the Atomic Force Microscope (AFM) [101], [164], [165] or micropipette aspiration [166], [167], [168] can be used to measure several parameters. Plasma membrane tension is traditionally measured by pulling membrane tethers using AFM, optical or magnetic tweezers [101], [169]. Moreover, the recently developed fluorescent probe FliptR allows the quantification of changes in membrane tension and/or lipid packing with fluorescence lifetime imaging (FLIM) in a contactless manner [170]. Micropipette aspiration, laser ablation or cell compression can be used to quantify cortical tension [168], [171], [172]. Tissue and cell stiffness can be assessed by AFM indentation, Brillouin microscopy and magnetic twisting cytometry [173], [174], [175]. The bending rigidity of the plasma membrane has been measured in cells by flicker spectroscopy [176], [177]. The fluidity and viscosity of the plasma membrane can be assessed by fluorescence recovery after photobleaching (FRAP), single-particle tracking (SPT) or using molecular rotors [178]. Förster resonance energy transfer (FRET) sensors can visualize molecular tension with high spatiotemporal resolution [179], [180], [181], [182]. However, as the mechanical components of the cell surface are highly interdependent of each other, the choice of methods and the interpretation of results are often non-trivial [183].
Plasma membrane tension can be acutely manipulated by hypo- or hyperosmotic shocks or addition of membrane-expanding compounds [110], [184]. Additionally, compressive, tensile and shear forces can be applied using customized cell stretching, compression, indentation, or microfluidic devices [83], [84], [129], [136], [169], [185], [186].
5. Conclusion and future directions
Stem cell differentiation, membrane trafficking and cell surface mechanics have so far been only marginally connected aspects of cell biology. This view is understandable bearing in mind the different scales in which these processes occur, both in time and space (Fig. 3). With this review, we make the case that these cellular functions are interconnected through mechanochemical feedback mechanisms (Fig. 1) that sustain morphogenesis during embryonic development, (stem cell-related) tissue homeostasis and regeneration in adulthood.
Going forward, to further understand these bidirectional relationships it will be necessary to use the latest methodology at hand (Fig. 2) in correlative ways, i.e. to follow cargos through the trafficking network while meticulously quantifying viscoelastic properties of the cell surface [187], mapping out cell morphology (cell shape and size, membrane curvature) and assessing the genetic landscape in terms of transcriptomics and epigenetics. A major challenge will be to bridge the different scales, from subcellular trafficking to tissue-scale stresses, and from seconds for membrane trafficking to days for differentiation (Fig. 3). Optogenetic tools offer unprecedented spatiotemporal control to manipulate membrane trafficking [188], [189] and even cell mechanics [190] and could allow us to further interrogate the logic of events.
Another powerful way to traverse biological scales is to employ theory and modeling [191]. Different models have been devised to investigate the mechanochemical functions of the plasma membrane [14]. Classical models treat the plasma membrane as an inextensible fluid surface that stores elastic energy when it is bent (Helfrich model) or as a fluid membrane that can phase separate (Flory–Huggins model) [192], [193], [194]. Indeed, theoretical modeling has provided great insights on the interplay between membrane tension and the actin cytoskeleton in blebbing [166], [195], tension propagation [196], and cell motility [197], [198], [199]. Such mathematical descriptions usually consider the coupling of the non-equilibrium dynamics of the actomyosin cytoskeleton with shape changes of the plasma membrane, leading to dynamic instabilities. More complex models have also taken into account the effect of membrane flow as well as tension gradients in cell motility [200]. The interaction between membranes and proteins has been tackled through molecular dynamics simulations [201], [202], [203] as well as with hybrid models treating the membrane as a continuum elastic material and proteins as discrete objects [204]. In addition, the connection between cell shape, cell signaling and membrane tension has also been explored by means of reaction-diffusion modeling [205], [206], [207]. Such in silico approaches enable us to tease apart the individual contributions of mechanical, chemical, and physical cues in ways inaccessible to experiments, where their interactions lead to confounding effects. The predictions made from mathematical models can guide further experiments in a virtuous cycle (Fig. 2).
Another major challenge is the high level of redundancy in these core cellular functions, e.g. different endocytic pathways, overlapping networks of genes regulating stemness and differentiation, or multiple regulators of cortex architecture, complicate the study of individual components. Thus, the use of large-scale genetic methods could reveal essentiality within a multitude of redundant genes or molecules [34]. At the same time, some players have multiple distinct functions. For example, β-catenin is a component of adhesion complexes but can also translocate to the nucleus and participate in the Wnt signaling pathway. Bioengineering approaches will be required to dissect the particular contribution of multifunctional proteins. We recently took this route by developing an artificial membrane-to-cortex linker [109]. This allowed us to manipulate plasma membrane tension without targeting endogenous linker proteins, such as the canonical ERM family, that also act as signal transducers in several signaling pathways [208].
Our current knowledge of the interplay of stem cell differentiation, trafficking and mechanics relies largely on 2D differentiation studies, particularly for mammalian stem cells. In the last decade it has become clear that novel in vitro organoid models derived from stem cells are powerful tools that allow one to test the biological relevance of findings in an environment much closer to the in vivo context in which stem cells reside [209], [210], [211]. Organoids and ex vivo cultures [212] are particularly useful to study the interdependence of mechanics and cell fate, as they are more accessible to the measurement of mechanical properties and the direct application of mechanical perturbations. In cases where the physiological relevance has been demonstrated but the molecular identity of involved proteins or regulators remains unknown, fast genetic tools such as CRISPR/Cas9 will help to identify them.
By combining these model systems, techniques and theory, we will gain a systematic understanding of the bidirectional interplay of stem cell differentiation, cell surface mechanics and membrane trafficking. Elucidating the regulative roles of trafficking and surface mechanics on stem cell differentiation will provide us a better understanding of developmental programs and stem cell-related diseases, and support the ongoing efforts to employ various types of stem cells for stem cell therapy and diagnostics.
Acknowledgments
We thank David Oriola, George Galea, and Martin Bergert for critical reading of the manuscript and constructive feedback. We acknowledge the financial support of the European Molecular Biology Laboratory (EMBL) to A.D-M. (Heidelberg, Germany) and V.T. (Barcelona, Spain), the Human Frontiers Science Program (HFSP) grant number RGY0073/2018 and the Deutsche Forschungsgemeinschaft (DFG) grant numbers DI 2205/2–1 and DI 2205/3–1 to A.D-M., and the EMBL Interdisciplinary Postdocs fellowship (EIPOD, grant number 847543) under Marie Sklodowska-Curie Actions COFUND to J.H.L.
References
- 1.Riga A., Castiglioni V.G., Boxem M. New insights into apical-basal polarization in epithelia. Curr. Opin. Cell Biol. 2020;62:1–8. doi: 10.1016/j.ceb.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths G.M., Tsun A., Stinchcombe J.C. The immunological synapse: A focal point for endocytosis and exocytosis. J. Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gundelfinger E.D., Kessels M.M., Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat. Rev. Mol. Cell Biol. 2003;4:127–139. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- 4.Nassari S., Del Olmo T., Jean S. Rabs in signaling and embryonic development. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad A., Alizadeh E. Cell form and function: interpreting and controlling the shape of adherent cells. Trends Biotechnol. 2019;37:347–357. doi: 10.1016/j.tibtech.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Sitarska E., Diz-Muñoz A. Pay attention to membrane tension: Mechanobiology of the cell surface. Curr. Opin. Cell Biol. 2020;66:11–18. doi: 10.1016/j.ceb.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meinertzhagen I.A., Takemura S.Y., Lu Z., Huang S., Gao S., Ting C.Y., Lee C.H. From form to function: The ways to know a neuron. J. Neurogenet. 2009;23:68–77. doi: 10.1080/01677060802610604. [DOI] [PubMed] [Google Scholar]
- 8.Salbreux G., Charras G., Paluch E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012;22:536–545. doi: 10.1016/j.tcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Sens P., Plastino J. Membrane tension and cytoskeleton organization in cell motility. J. Phys. Condens. Matter. 2015;27 doi: 10.1088/0953-8984/27/27/273103. [DOI] [PubMed] [Google Scholar]
- 10.Sorkin A., Zastrow M.Von. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valet M., Siggia E.D., Brivanlou A.H. Mechanical regulation of early vertebrate embryogenesis. Nat. Rev. Mol. Cell Biol. 2022;23:169–184. doi: 10.1038/s41580-021-00424-z. [DOI] [PubMed] [Google Scholar]
- 12.De Belly H., Paluch E.K., Chalut K.J. Interplay between mechanics and signalling in regulating cell fate. Nat. Rev. Mol. Cell Biol. 2022 doi: 10.1038/s41580-022-00472-z. [DOI] [PubMed] [Google Scholar]
- 13.Pontes B., Monzo P., Gauthier N.C. Membrane tension: A challenging but universal physical parameter in cell biology. Semin. Cell Dev. Biol. 2017;71:30–41. doi: 10.1016/j.semcdb.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Le Roux A.L., Quiroga X., Walani N., Arroyo M., Roca-Cusachs P. The plasma membrane as a mechanochemical transducer. Philos. Trans. R. Soc. B Biol. Sci. 2019;374 doi: 10.1098/rstb.2018.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalkman H.O. Altered growth factor signaling pathways as the basis of aberrant stem cell maturation in schizophrenia. Pharmacol. Ther. 2009;121:115–122. doi: 10.1016/j.pharmthera.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Gilbertson R.J., Rich J.N. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 17.Tang N., Song W.X., Luo J., Haydon R.C., He T.C. Osteosarcoma development and stem cell differentiation. Clin. Orthop. Relat. Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blank U., Karlsson G., Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 19.Matsui W.H. Cancer stem cell signaling pathways. Med. (Baltim. ) 2016;95:S8–S19. doi: 10.1097/MD.0000000000004765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ring A., Kim Y.M., Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. Rep. 2014;10:512–525. doi: 10.1007/s12015-014-9515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shilo B.Z., Schejter E.D. Regulation of developmental intercellular signalling by intracellular trafficking. EMBO J. 2011;30:3516–3526. doi: 10.1038/emboj.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiȩdłocha A., Sørensen V. Signaling, internalization, and intracellular activity of fibroblast growth factor. Curr. Top. Microbiol. Immunol. 2004;286:45–79. doi: 10.1007/978-3-540-69494-6_3. [DOI] [PubMed] [Google Scholar]
- 23.Le Borgne R., Bardin A., Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005;132:1751–1762. doi: 10.1242/dev.01789. [DOI] [PubMed] [Google Scholar]
- 24.Kandachar V., Roegiers F. Endocytosis and control of Notch signaling. Curr. Opin. Cell Biol. 2012;24:534–540. doi: 10.1016/j.ceb.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon E., Aguirre-Tamaral A., Aguilar G., Guerrero I. Perspectives on intra- and intercellular trafficking of Hedgehog for tissue patterning. J. Dev. Biol. 2016;4 doi: 10.3390/jdb4040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albrecht L.V., Tejeda-Muñoz N., De Robertis E.M. Cell biology of canonical Wnt signaling. Annu. Rev. Cell Dev. Biol. 2021;37:369–389. doi: 10.1146/annurev-cellbio-120319-023657. [DOI] [PubMed] [Google Scholar]
- 27.Port F., Basler K. Wnt trafficking: new insights into wnt maturation, secretion and spreading. Traffic. 2010;11:1265–1271. doi: 10.1111/j.1600-0854.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y.G. Endocytic regulation of TGF-β signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura N., Sun-Wada G.H., Aoyama M., Harada A., Takasuga S., Sasaki T., Wada Y. Delivery of endosomes to lysosomes via microautophagy in the visceral endoderm of mouse embryos. Nat. Commun. 2012;3 doi: 10.1038/ncomms2069. [DOI] [PubMed] [Google Scholar]
- 30.De Renzis S., Yu J., Zinzen R., Wieschaus E. Dorsal-ventral pattern of Delta trafficking is established by a snail-tom-neuralized pathway. Dev. Cell. 2006;10:257–264. doi: 10.1016/j.devcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Fabrowski P., Necakov A.S., Mumbauer S., Loeser E., Reversi A., Streichan S., Briggs J.A.G., De Renzis S. Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila. Nat. Commun. 2013;4:1–12. doi: 10.1038/ncomms3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song S., Eckerle S., Onichtchouk D., Marrs J.A., Nitschke R., Driever W. Pou5f1-Dependent EGF Expression Controls E-Cadherin Endocytosis, Cell Adhesion, and Zebrafish Epiboly Movements. Dev. Cell. 2013;24:486–501. doi: 10.1016/j.devcel.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H.T., Lee M.S., Jeong Y.M., Ro H., Il Kim D., Shin Y.H., Kim J.E., Hwang K.S., Choi J.H., Bahn M., Lee J.J., Lee S.H., Bae Y.K., Lee J.S., Choi J.K., Kim N.S., Yeo C.Y., Kim C.H. Ottogi Inhibits Wnt/β-catenin Signaling by Regulating Cell Membrane Trafficking of Frizzled8. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-13429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yilmaz A., Braverman-Gross C., Bialer-Tsypin A., Peretz M., Benvenisty N. Mapping gene circuits essential for germ layer differentiation via loss-of-function screens in haploid human embryonic stem cells. Cell Stem Cell. 2020;27:679–691.e6. doi: 10.1016/j.stem.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Qin H., Diaz A., Blouin L., Lebbink R.J., Patena W., Tanbun P., Leproust E.M., McManus M.T., Song J.S., Ramalho-Santos M. Systematic identification of barriers to human Ipsc generation. Cell. 2014;158:449–461. doi: 10.1016/j.cell.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayana Y.V., Gadgil C., Mote R.D., Rajan R., Subramanyam D. Clathrin-mediated endocytosis regulates a balance between opposing signals to maintain the pluripotent state of embryonic stem cells. Stem Cell Rep. 2019;12:152–164. doi: 10.1016/j.stemcr.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vessoni A.T., Muotri A.R., Okamoto O.K. Autophagy in stem cell maintenance and differentiation. Stem Cells Dev. 2012;21:513–520. doi: 10.1089/scd.2011.0526. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P., Holowatyj A.N., Roy T., Pronovost S.M., Marchetti M., Liu H., Ulrich C.M., Edgar B.A. An SH3PX1-Dependent Endocytosis-Autophagy Network Restrains Intestinal Stem Cell Proliferation by Counteracting EGFR-ERK Signaling. Dev. Cell. 2019;49:574–589.e5. doi: 10.1016/j.devcel.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao Y., Tu R., Huang Y., Mao D., Yang Z., Lau P.K., Wang J., Ni J., Guo Y., Xie T. The exocyst functions in niche cells to promote germline stem cell differentiation by directly controlling EGFR membrane trafficking. Dev. 2019;146 doi: 10.1242/dev.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mote R.D., Mahajan G., Padmanabhan A., Ambati R., Subramanyam D. Dual repression of endocytic players by ESCC microRNAs and the Polycomb complex regulates mouse embryonic stem cell pluripotency. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-17828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dambournet D., Sochacki K.A., Cheng A.T., Akamatsu M., Taraska J.W., Hockemeyer D., Drubin D.G. Genome-edited human stem cells expressing fluorescently labeled endocytic markers allow quantitative analysis of clathrin-mediated endocytosis during differentiation. J. Cell Biol. 2018;217:3301–3311. doi: 10.1083/jcb.201710084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostopoulou N., Bellou S., Bagli E., Markou M., Kostaras E., Hyvönen M., Kalaidzidis Y., Papadopoulos A., Chalmantzi V., Kyrkou A., Panopoulou E., Fotsis T., Murphy C. Embryonic stem cells are devoid of macropinocytosis, a trafficking pathway for activin A in differentiated cells. J. Cell Sci. 2021;134 doi: 10.1242/jcs.246892. [DOI] [PubMed] [Google Scholar]
- 43.Loh K.M., van Amerongen R., Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev. Cell. 2016;38:643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Stypulkowski E., Feng Q., Joseph I., Farrell V., Flores J., Yu S., Sakamori R., Sun J., Bandyopadhyay S., Das S., Dobrowolski R., Bonder E.M., Chen M.H., Gao N. Rab8 attenuates Wnt signaling and is required for mesenchymal differentiation into adipocytes. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albrecht L.V., Tejeda-Muñoz N., Bui M.H., Cicchetto A.C., Di Biagio D., Colozza G., Schmid E., Piccolo S., Christofk H.R., Robertis E.M.De. GSK3 Inhibits Macropinocytosis and Lysosomal Activity through the Wnt Destruction Complex Machinery. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.107973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tejeda-Muñoz N., Albrecht L.V., Bui M.H., Robertis E.M.De. Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins. Proc. Natl. Acad. Sci. U. S. A. 2019;116:10402–10411. doi: 10.1073/pnas.1903506116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redelman-Sidi G., Binyamin A., Gaeta I., Palm W., Thompson C.B., Romesser P.B., Lowe S.W., Bagul M., Doench J.G., Root D.E., Glickman M.S. The canonical Wnt pathway drives macropinocytosis in cancer. Cancer Res. 2018;78:4658–4670. doi: 10.1158/0008-5472.CAN-17-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caviglia S., Flores-Benitez D., Lattner J., Luschnig S., Brankatschk M. Rabs on the fly: Functions of Rab GTPases during development. Small GTPases. 2019;10:89–98. doi: 10.1080/21541248.2017.1279725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy C., Zerial M. Expression of Rab proteins during mouse embryonic development. Methods Enzym. 1995;257:324–332. doi: 10.1016/S0076-6879(95)57036-5. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., Schulze K.L., Hiesinger P.R., Suyama K., Wang S., Fish M., Acar M., Hoskins R.A., Bellen H.J., Scott M.P. Thirty-One Flavors of Drosophila Rab Proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuller K., O’Connell J.T., Gordon J., Mauti O., Eggenschwiler J. Rab23 regulates Nodal signaling in vertebrate left-right patterning independently of the Hedgehog pathway. Dev. Biol. 2014;391:182–195. doi: 10.1016/j.ydbio.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Kenyon E.J., Campos I., Bull J.C., Williams P.H., Stemple D.L., Clark M.D. Zebrafish Rab5 proteins and a role for Rab5ab in nodal signalling. Dev. Biol. 2015;397:212–224. doi: 10.1016/j.ydbio.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eggenschwiler J.T., Espinoza E., Anderson K.V. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 54.Maitra S., Kulikauskas R.M., Gavilan H., Fehon R.G. The tumor suppressors merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 55.McCartney B.M., Kulikauskas R.M., LaJeunesse D.R., Fehon R.G. The Neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 56.Li L., Wang S., Jezierski A., Moalim-Nour L., Mohib K., Parks R.J., Retta S.F., Wang L. A unique interplay between Rap1 and E-cadherin in the endocytic pathway regulates self-renewal of human embryonic stem cells. Stem Cells. 2010;28:247–257. doi: 10.1002/stem.289. [DOI] [PubMed] [Google Scholar]
- 57.Bogard N., Lan L., Xu J., Cohen R.S. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development. 2007;134:3413–3418. doi: 10.1242/dev.008466. [DOI] [PubMed] [Google Scholar]
- 58.Ladyzhets S., Antel M., Simao T., Gasek N., Cowan A.E., Inaba M. Self-limiting stem-cell niche signaling through degradation of a stem-cell receptor. PLoS Biol. 2020;18:1–24. doi: 10.1371/journal.pbio.3001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song X., Zhu C.H., Doan C., Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Sci. (80-. ) 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 60.Ulrich F., Heisenberg C.P. Trafficking and cell migration. Traffic. 2009;10:811–818. doi: 10.1111/j.1600-0854.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 61.Bosze B., Ono Y., Mattes B., Sinner C., Gourain V., Thumberger T., Tlili S., Wittbrodt J., Saunders T.E., Strähle U., Schug A., Scholpp S. Pcdh18a regulates endocytosis of E-cadherin during axial mesoderm development in zebrafish. Histochem. Cell Biol. 2020;154:463–480. doi: 10.1007/s00418-020-01887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kida Y.S., Sato T., Miyasaka K.Y., Suto A., Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6708–6713. doi: 10.1073/pnas.0608946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Z., Ning G., Xu R., Cao Y., Meng A., Wang Q. Fscn1 is required for the trafficking of TGF-β family type i receptors during endoderm formation. Nat. Commun. 2016;7 doi: 10.1038/ncomms12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montagne C., Gonzalez-Gaitan M. Sara endosomes and the asymmetric division of intestinal stem cells. Dev. 2014;141:2014–2023. doi: 10.1242/dev.104240. [DOI] [PubMed] [Google Scholar]
- 65.Kressmann S., Campos C., Castanon I., Fürthauer M., González-Gaitán M. Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat. Cell Biol. 2015;17:333–339. doi: 10.1038/ncb3119. [DOI] [PubMed] [Google Scholar]
- 66.Riazifar M., Pone E.J., Lotval J., Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 2017;57:125–154. doi: 10.1146/annurev-pharmtox-061616-030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nawaz M., Fatima F., Vallabhaneni K.C., Penfornis P., Valadi H., Ekström K., Kholia S., Whitt J.D., Fernandes J.D., Pochampally R., Squire J.A., Camussi G. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016 doi: 10.1155/2016/1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.González‐Méndez L., Gradilla A., Sánchez‐Hernández D., González E., Aguirre‐Tamaral A., Jiménez‐Jiménez C., Guerra M., Aguilar G., Andrés G., Falcón‐Pérez J.M., Guerrero I. Polarized sorting of Patched enables cytoneme‐mediated Hedgehog reception in the Drosophila wing disc. EMBO J. 2020;39:1–22. doi: 10.15252/embj.2019103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair R., Santos L., Awasthi S., Von Erlach T., Chow L.W., Bertazzo S., Stevens M.M. Extracellular vesicles derived from preosteoblasts influence embryonic stem cell differentiation. Stem Cells Dev. 2014;23:1625–1635. doi: 10.1089/scd.2013.0633. [DOI] [PubMed] [Google Scholar]
- 70.Huang C.C., Kang M., Narayanan R., DiPietro L.A., Cooper L.F., Gajendrareddy P., Ravindran S. Evaluating the endocytosis and lineage-specification properties of mesenchymal stem cell derived extracellular vesicles for targeted therapeutic applications. Front. Pharm. 2020;11:1–15. doi: 10.3389/fphar.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang C.C., Narayanan R., Alapati S., Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103–115. doi: 10.1016/j.biomaterials.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narayanan K., Kumar S., Padmanabhan P., Gulyas B., Wan A.C.A., Rajendran V.M. Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation. Biomaterials. 2018;182:312–322. doi: 10.1016/j.biomaterials.2018.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang G., Galli T. Reciprocal link between cell biomechanics and exocytosis. Traffic. 2018;19:741–749. doi: 10.1111/tra.12584. [DOI] [PubMed] [Google Scholar]
- 74.Djakbarova U., Madraki Y., Chan E.T., Kural C. Dynamic interplay between cell membrane tension and clathrin-mediated endocytosis. Biol. Cell. 2021;113:344–373. doi: 10.1111/boc.202000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kell A., Glaser R.W. On the mechanical and dynamic properties of plant cell membranes: Their role in growth, direct gene transfer and protoplast fusion. J. Theor. Biol. 1993;160:41–62. doi: 10.1006/jtbi.1993.1003. [DOI] [Google Scholar]
- 76.Dai J., Ping Ting-Beall H., Sheetz M.P. The secretion-coupled endocytosis correlates with membrane tension changes in RBL 2H3 cells. J. Gen. Physiol. 1997;110:1–10. doi: 10.1085/jgp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemière J., Ren Y., Berro J. Rapid adaptation of endocytosis, exocytosis and eisosomes after an acute increase in membrane tension in yeast cells. Elife. 2021;10 doi: 10.7554/eLife.62084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai J., Sheetz M.P. Regulation of endocytosis, exocytosis, and shape by membrane tension. Cold Spring Harb. Symp. Quant. Biol. 1995;60:567–571. doi: 10.1101/SQB.1995.060.01.060. [DOI] [PubMed] [Google Scholar]
- 79.Boulant S., Kural C., Zeeh J.C., Ubelmann F., Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 2011;13:1124–1132. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akamatsu M., Vasan R., Serwas D., Ferrin M., Rangamani P., Drubin D.G. Principles of self-organization and load adaptation by the actin cytoskeleton during clathrin-mediated endocytosis. Elife. 2020;9:1–40. doi: 10.7554/eLife.49840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hassinger J.E., Oster G., Drubin D.G., Rangamani P. Design principles for robust vesiculation in clathrin-mediated endocytosis. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E1118–E1127. doi: 10.1073/pnas.1617705114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu X.S., Elias S., Liu H., Heureaux J., Wen P.J., Liu A.P., Kozlov M.M., Wu L.G. Membrane Tension Inhibits Rapid and Slow Endocytosis in Secretory Cells. Biophys. J. 2017;113:2406–2414. doi: 10.1016/j.bpj.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pouille P.A., Ahmadi P., Brunet A.C., Farge E. Mechanical signals trigger myosin II redistribution and mesoderm invagination in drosophila embryos. Sci. Signal. 2009;2:1–9. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 84.Thottacherry J.J., Kosmalska A.J., Kumar A., Vishen A.S., Elosegui-Artola A., Pradhan S., Sharma S., Singh P.P., Guadamillas M.C., Chaudhary N., Vishwakarma R., Trepat X., del Pozo M.A., Parton R.G., Rao M., Pullarkat P., Roca-Cusachs P., Mayor S. Mechanochemical feedback control of dynamin independent endocytosis modulates membrane tension in adherent cells. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-06738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiyoshima D., Kawakami K., Hayakawa K., Tatsumi H., Sokabe M. Force- and Ca 2+-dependent internalization of integrins in cultured endothelial cells. J. Cell Sci. 2011;124:3859–3870. doi: 10.1242/jcs.088559. [DOI] [PubMed] [Google Scholar]
- 86.Gauthier N.C., Fardin M.A., Roca-Cusachs P., Sheetz M.P. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14467–14472. doi: 10.1073/pnas.1105845108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gauthier N.C., Rossier O.M., Mathur A., Hone J.C., Sheetz M.P. Plasma Membrane Area Increases with Spread Area by Exocytosis of a GPI-anchored Protein Compartment. Mol. Biol. Cell. 2009;20:3261–3272. doi: 10.1091/mbc.e09-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masters T.A., Pontes B., Viasnoff V., Li Y., Gauthier N.C. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11875–11880. doi: 10.1073/pnas.1301766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mellander L.J., Kurczy M.E., Najafinobar N., Dunevall J., Ewing A.G., Cans A.S. Two modes of exocytosis in an artificial cell. Sci. Rep. 2014;4:1–7. doi: 10.1038/srep03847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bretou M., Jouannot O., Fanget I., Pierobon P., Larochette N., Gestraud P., Guillon M., Emiliani V., Gasman S., Desnos C., Lennon-Duménil A.M., Darchen F. Cdc42 controls the dilation of the exocytotic fusion pore by regulating membrane tension. Mol. Biol. Cell. 2014;25:3195–3209. doi: 10.1091/mbc.E14-07-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen P.J., Grenklo S., Arpino G., Tan X., Liao H.S., Heureaux J., Peng S.Y., Chiang H.C., Hamid E., Zhao W.D., Shin W., Näreoja T., Evergren E., Jin Y., Karlsson R., Ebert S.N., Jin A., Liu A.P., Shupliakov O., Wu L.G. Actin dynamics provides membrane tension to merge fusing vesicles into the plasma membrane. Nat. Commun. 2016;7 doi: 10.1038/ncomms12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riggi M., Kusmider B., Loewith R. The flipside of the TOR coin - TORC2 and plasma membrane homeostasis at a glance. J. Cell Sci. 2020;133 doi: 10.1242/jcs.242040. [DOI] [PubMed] [Google Scholar]
- 93.Berchtold D., Piccolis M., Chiaruttini N., Riezman I., Riezman H., Roux A., Walther T.C., Loewith R. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat. Cell Biol. 2012;14:542–547. doi: 10.1038/ncb2480. [DOI] [PubMed] [Google Scholar]
- 94.Bourgoint C., Rispal D., Berti M., Filipuzzi I., Helliwell S.B., Prouteau M., Loewith R. Target of rapamycin complex 2– dependent phosphorylation of the coat protein Pan1 by Akl1 controls endocytosis dynamics in Saccharomyces cerevisiae. J. Biol. Chem. 2018;293:12043–12053. doi: 10.1074/jbc.RA117.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rispal D., Eltschinger S., Stahl M., Vaga S., Bodenmiller B., Abraham Y., Filipuzzi I., Movva N.R., Aebersold R., Helliwell S.B., Loewith R. Target of rapamycin complex 2 regulates actin polarization and endocytosis via multiple pathways. J. Biol. Chem. 2015;290:14963–14978. doi: 10.1074/jbc.M114.627794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roelants F.M., Leskoske K.L., Pedersen R.T.A., Muir A., Liu J.M.-H., Finnigan G.C., Thorner J. TOR Complex 2-Regulated Protein Kinase Fpk1 Stimulates Endocytosis via Inhibition of Ark1/Prk1-Related Protein Kinase Akl1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 2017;37 doi: 10.1128/MCB.00627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riggi M., Bourgoint C., Macchione M., Matile S., Loewith R., Roux A. TORC2 controls endocytosis through plasma membrane tension. J. Cell Biol. 2019;218:2265–2276. doi: 10.1083/jcb.201901096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sinha B., Köster D., Ruez R., Gonnord P., Bastiani M., Abankwa D., Stan R.V., Butler-Browne G., Vedie B., Johannes L., Morone N., Parton R.G., Raposo G., Sens P., Lamaze C., Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gervásio O.L., Phillips W.D., Cole L., Allen D.G. Caveolae respond to cell stretch and contribute to stretch-induced signaling. J. Cell Sci. 2011;124:3581–3590. doi: 10.1242/jcs.084376. [DOI] [PubMed] [Google Scholar]
- 100.Hetmanski J.H.R., de Belly H., Busnelli I., Waring T., Nair R.V., Sokleva V., Dobre O., Cameron A., Gauthier N., Lamaze C., Swift J., del Campo A., Starborg T., Zech T., Goetz J.G., Paluch E.K., Schwartz J.-M., Caswell P.T. Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev. Cell. 2019;51:460–475.e10. doi: 10.1016/j.devcel.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diz-Muñoz A., Thurley K., Chintamen S., Altschuler S.J., Wu L.F., Fletcher D.A., Weiner O.D. Membrane Tension Acts Through PLD2 and mTORC2 to Limit Actin Network Assembly During Neutrophil Migration. PLoS Biol. 2016;14:1–30. doi: 10.1371/journal.pbio.1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Riggi M., Niewola-Staszkowska K., Chiaruttini N., Colom A., Kusmider B., Mercier V., Soleimanpour S., Stahl M., Matile S., Roux A., Loewith R. Decrease in plasma membrane tension triggers PtdIns(4,5)P 2 phase separation to inactivate TORC2. Nat. Cell Biol. 2018;20:1043–1051. doi: 10.1038/s41556-018-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loh J., Chuang M.C., Lin S.S., Joseph J., Su Y.A., Hsieh T.L., Chang Y.C., Liu A.P., Liu Y.W. An acute decrease in plasma membrane tension induces macropinocytosis via PLD2 activation. J. Cell Sci. 2019;132 doi: 10.1242/jcs.232579. [DOI] [PubMed] [Google Scholar]
- 104.Chan C.J., Heisenberg C.P., Hiiragi T. Coordination of morphogenesis and cell-fate specification in development. Curr. Biol. 2017;27:R1024–R1035. doi: 10.1016/j.cub.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 105.Steward A.J., Kelly D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015;227:717–731. doi: 10.1111/joa.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vining K.H., Mooney D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rauch C., Brunet A., Deleule J., Farge E. C 2 C 12 myoblast/osteoblast transdifferentiation steps enhanced by epigenetic inhibition of BMP2 endocytosis. Am. J. Physiol. Physiol. 2002;283:C235–C243. doi: 10.1152/ajpcell.00234.2001. [DOI] [PubMed] [Google Scholar]
- 108.De Belly H., Stubb A., Yanagida A., Labouesse C., Jones P.H., Paluch E.K., Chalut K.J. Membrane Tension Gates ERK-Mediated Regulation of Pluripotent Cell Fate. Cell Stem Cell. 2021;28:273–284.e6. doi: 10.1016/j.stem.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bergert M., Lembo S., Sharma S., Russo L., Milovanović D., Gretarsson K.H., Börmel M., Neveu P.A., Hackett J.A., Petsalaki E., Diz-Muñoz A. Cell surface mechanics gate embryonic stem cell differentiation. Cell Stem Cell. 2021;28:209–216.e4. doi: 10.1016/j.stem.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raucher D., Sheetz M.P. Membrane expansion increases endocytosis rate during mitosis. J. Cell Biol. 1999;144:497–506. doi: 10.1083/jcb.144.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fielding A.B., Royle S.J. Mitotic inhibition of clathrin-mediated endocytosis. Cell. Mol. Life Sci. 2013;70:3423–3433. doi: 10.1007/s00018-012-1250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rizzelli F., Malabarba M.G., Sigismund S., Mapelli M. The crosstalk between microtubules, actin and membranes shapes cell division. Open Biol. 2020;10 doi: 10.1098/rsob.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaigne A., Labouesse C., White I.J., Agnew M., Hannezo E., Chalut K.J., Paluch E.K. Abscission Couples Cell Division to Embryonic Stem Cell Fate. Dev. Cell. 2020;55:195–208.e5. doi: 10.1016/j.devcel.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lafaurie-Janvore J., Maiuri P., Wang I., Pinot M., Manneville J.-B., Betz T., Balland M., Piel M. ESCRT-III Assembly and Cytokinetic Abscission Are Induced by Tension Release in the Intercellular Bridge. Sci. (80-. ) 2013;339:1625–1629. doi: 10.1126/science.1233866. [DOI] [PubMed] [Google Scholar]
- 115.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 116.Von Erlach T.C., Bertazzo S., Wozniak M.A., Horejs C.M., Maynard S.A., Attwood S., Robinson B.K., Autefage H., Kallepitis C., Del Río Hernández A., Chen C.S., Goldoni S., Stevens M.M. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat. Mater. 2018;17:237–242. doi: 10.1038/s41563-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang B., Peng F., Wu D., Ingram A.J., Gao B., Krepinsky J.C. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell. Signal. 2007;19:1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 118.Spencer A., Yu L., Guili V., Reynaud F., Ding Y., Ma J., Jullien J., Koubi D., Gauthier E., Cluet D., Falk J., Castellani V., Yuan C., Rudkin B.B. Nerve growth factor signaling from membrane microdomains to the nucleus: Differential regulation by caveolins. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18040693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Z., Wang N., Li W., Liu P., Chen Q., Situ H., Zhong S., Guo L., Lin Y., Shen J., Chen J. Caveolin-1 mediates chemoresistance in breast cancer stem cells via β-catenin/ABCG2 signaling pathway. Carcinogenesis. 2014;35:2346–2356. doi: 10.1093/carcin/bgu155. [DOI] [PubMed] [Google Scholar]
- 120.Sen B., Xie Z., Case N., Thompson W.R., Uzer G., Styner M., Rubin J. MTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J. Bone Miner. Res. 2014;29:78–89. doi: 10.1002/jbmr.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin S.K., Fitter S., Dutta A.K., Matthews M.P., Walkley C.R., Hall M.N., Ruegg M.A., Gronthos S., Zannettino A.C.W. Brief Report: The Differential Roles of mTORC1 and mTORC2 in Mesenchymal Stem Cell Differentiation. Stem Cells. 2015;33:1359–1365. doi: 10.1002/stem.1931. [DOI] [PubMed] [Google Scholar]
- 122.Shu L., Houghton P.J. The mTORC2Complex regulates terminal differentiation of C2C12 myoblasts. Mol. Cell. Biol. 2009;29:4691–4700. doi: 10.1128/mcb.00764-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fletcher G.C., del M., Diaz-de-la-Loza C., Borreguero-Muñoz N., Holder M., Aguilar-Aragon M., Thompson B.J. Mechanical strain regulates the Hippo pathway in Drosophila. Dev. 2018;145 doi: 10.1242/dev.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sherrard K.M., Fehon R.G. The transmembrane protein Crumbs displays complex dynamics during follicular morphogenesis and is regulated competitively by Moesin and aPKC. Development. 2015;142:1869–1878. doi: 10.1242/dev.115329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gilmour D., Rembold M., Leptin M. From morphogen to morphogenesis and back. Nature. 2017;541:311–320. doi: 10.1038/nature21348. [DOI] [PubMed] [Google Scholar]
- 126.Chowdhury F., Na S., Li D., Poh Y., Tanaka T.S., Wang F., Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barone V., Lang M., Krens S.F.G., Pradhan S.J., Shamipour S., Sako K., Sikora M., Guet C.C., Heisenberg C.P. An Effective Feedback Loop between Cell-Cell Contact Duration and Morphogen Signaling Determines Cell Fate. Dev. Cell. 2017;43:198–211.e12. doi: 10.1016/j.devcel.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 128.Przybyla L., Lakins J.N., Weaver V.M. Tissue mechanics orchestrate wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell. 2016;19:462–475. doi: 10.1016/j.stem.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Verstreken C.M., Labouesse C., Agley C.C., Chalut K.J. Embryonic stem cells become mechanoresponsive upon exit from ground state of pluripotency. Open Biol. 2019;9 doi: 10.1098/rsob.180203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du J., Fan Y., Guo Z., Wang Y., Zheng X., Huang C., Liang B., Gao L., Cao Y., Chen Y., Zhang X., Li L., Xu L., Wu C., Weitz D.A., Feng X. Compression Generated by a 3D Supracellular Actomyosin Cortex Promotes Embryonic Stem Cell Colony Growth and Expression of Nanog and Oct4. Cell Syst. 2019;9:214–220.e5. doi: 10.1016/j.cels.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 131.Taylor-Weiner H., Ravi N., Engler A.J. Traction forces mediated by integrin signaling are necessary for definitive endoderm specification. J. Cell Sci. 2015;128:1961–1968. doi: 10.1242/jcs.166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Polesello C., Delon I., Valenti P., Ferrer P., Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat. Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- 133.Lachnit M., Kur E., Driever W. Alterations of the cytoskeleton in all three embryonic lineages contribute to the epiboly defect of Pou5f1/Oct4 deficient MZspg zebrafish embryos. Dev. Biol. 2008;315:1–17. doi: 10.1016/j.ydbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 134.He L., Ahmad M., Perrimon N. Mechanosensitive channels and their functions in stem cell differentiation. Exp. Cell Res. 2019;374:259–265. doi: 10.1016/j.yexcr.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 135.Barzegari A., Omidi Y., Ostadrahimi A., Gueguen V., Meddahi-Pellé A., Nouri M., Pavon-Djavid G. The role of Piezo proteins and cellular mechanosensing in tuning the fate of transplanted stem cells. Cell Tissue Res. 2020;381:1–12. doi: 10.1007/s00441-020-03191-z. [DOI] [PubMed] [Google Scholar]
- 136.He L., Si G., Huang J., Samuel A.D.T., Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555:103–106. doi: 10.1038/nature25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pathak M.M., Nourse J.L., Tran T., Hwe J., Arulmoli J., Le D.T.T., Bernardis E., Flanagan L.A., Tombola F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tonelli F.M.P., Santos A.K., Gomes K.N., Ladeira L.O., Resende R.R., Gomes D.A., Silva S.L.Da. Stem cells and calcium signaling. Adv. Exp. Med. Biol. 2012;740:891–916. doi: 10.1007/978-94-007-2888-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marchant J.S. Ca2+ signaling and regeneration. Cold Spring Harb. Perspect. Biol. 2019;11 doi: 10.1101/cshperspect.a035485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao Q., Cooper P.R., Walmsley A.D., Scheven B.A. Role of Piezo Channels in Ultrasound-stimulated Dental Stem Cells. J. Endod. 2017;43:1130–1136. doi: 10.1016/j.joen.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 141.Yajima Y., Kawashima S. Calpain function in the differentiation of mesenchymal stem cells. Biol. Chem. 2002;383:757–764. doi: 10.1515/BC.2002.079. [DOI] [PubMed] [Google Scholar]
- 142.Santos D.M., Xavier J.M., Morgado A.L., Solá S., Rodrigues C.M.P. Distinct regulatory functions of calpain 1 and 2 during neural stem cell self-renewal and differentiation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ning W., Muroyama A., Li H., Lechler T. Differentiated Daughter Cells Regulate Stem Cell Proliferation and Fate through Intra-tissue Tension. Cell Stem Cell. 2021;28:436–452.e5. doi: 10.1016/j.stem.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diz-Muñoz A., Weiner O.D., Fletcher D.A. In pursuit of the mechanics that shape cell surfaces. Nat. Phys. 2018;14:648–652. doi: 10.1038/s41567-018-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nguyen P.K., Nag D., Wu J.C. Methods to assess stem cell lineage, fate and function. Adv. Drug Deliv. Rev. 2010;62:1175–1186. doi: 10.1016/j.addr.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vancura A. Humana Press; Totowa, NJ: 2008. Membrane Trafficking. [DOI] [Google Scholar]
- 147.Betz W.J., Mao F., Smith C.B. Imaging exocytosis and endocytosis. Curr. Opin. Neurobiol. 1996;6:365–371. doi: 10.1016/S0959-4388(96)80121-8. [DOI] [PubMed] [Google Scholar]
- 148.Wang T., Li W., Martin S., Papadopulos A., Joensuu M., Liu C., Jiang A., Shamsollahi G., Amor R., Lanoue V., Padmanabhan P., Meunier F.A. Radial contractility of actomyosin rings facilitates axonal trafficking and structural stability. J. Cell Biol. 2020;219 doi: 10.1083/jcb.201902001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miesenböck Gero, De Angelis Dino A., Rothman James E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. 〈https://www.nature.com/articles/BF28190〉 [DOI] [PubMed] [Google Scholar]
- 150.Presley J.F., Cole N.B., Schroer T.A., Hirschberg K., Zaal K.J.M., Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 151.Boncompain G., Divoux S., Gareil N., De Forges H., Lescure A., Latreche L., Mercanti V., Jollivet F., Raposo G., Perez F. Synchronization of secretory protein traffic in populations of cells. Nat. Methods. 2012;9:493–498. doi: 10.1038/nmeth.1928. [DOI] [PubMed] [Google Scholar]
- 152.Hoffman D.P., Shtengel G., Xu C.S., Campbell K.R., Freeman M., Wang L., Milkie D.E., Pasolli H.A., Iyer N., Bogovic J.A., Stabley D.R., Shirinifard A., Pang S., Peale D., Schaefer K., Pomp W., Chang C.-L., Lippincott-Schwartz J., Kirchhausen T., Solecki D.J., Betzig E., Hess H. Correlative three-dimensional super-resolution and block face electron microscopy of whole vitreously frozen cells. BioRxiv. 2019;5357 doi: 10.1101/773986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schöneberg J., Dambournet D., Liu T.L., Forster R., Hockemeyer D., Betzig E., Drubin D.G. 4D cell biology: big data image analytics and lattice light-sheet imaging reveal dynamics of clathrin-mediated endocytosis in stem cell–derived intestinal organoids. Mol. Biol. Cell. 2018;29:2959–2968. doi: 10.1091/mbc.E18-06-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matsuoka F., Takeuchi I., Agata H., Kagami H., Shiono H., Kiyota Y., Honda H., Kato R. Morphology-based prediction of osteogenic differentiation potential of human mesenchymal stem cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yeo G.H.T., Lin L., Qi C.Y., Cha M., Gifford D.K., Sherwood R.I. A Multiplexed Barcodelet Single-Cell RNA-Seq Approach Elucidates Combinatorial Signaling Pathways that Drive ESC Differentiation. Cell Stem Cell. 2020;26:938–950.e6. doi: 10.1016/j.stem.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang F., Barbacioru C., Wang Y., Nordman E., Lee C., Xu N., Wang X., Bodeau J., Tuch B.B., Siddiqui A., Lao K., Surani M.A. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 157.Tritschler S., Büttner M., Fischer D.S., Lange M., Bergen V., Lickert H., Theis F.J. Concepts and limitations for learning developmental trajectories from single cell genomics. Dev. 2019;146 doi: 10.1242/dev.170506. [DOI] [PubMed] [Google Scholar]
- 158.Kharchenko P.V. The triumphs and limitations of computational methods for scRNA-seq. Nat. Methods. 2021;18:723–732. doi: 10.1038/s41592-021-01171-x. [DOI] [PubMed] [Google Scholar]
- 159.Jo J., Xiao Y., Sun A.X., Cukuroglu E., Tran H.D., Göke J., Tan Z.Y., Saw T.Y., Tan C.P., Lokman H., Lee Y., Kim D., Ko H.S., Kim S.O., Park J.H., Cho N.J., Hyde T.M., Kleinman J.E., Shin J.H., Weinberger D.R., Tan E.K., Je H.S., Ng H.H. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hassanlou L., Meshgini S., Alizadeh E. Evaluating adipocyte differentiation of bone marrow-derived mesenchymal stem cells by a deep learning method for automatic lipid droplet counting. Comput. Biol. Med. 2019;112 doi: 10.1016/j.compbiomed.2019.103365. [DOI] [PubMed] [Google Scholar]
- 161.Masuda H., Asahara T. Clonogenic assay of endothelial progenitor cells. Trends Cardiovasc. Med. 2013;23:99–103. doi: 10.1016/j.tcm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 162.Ramesh P., Kirov A.B., Huels D.J., Medema J.P. Isolation, Propagation, and Clonogenicity of Intestinal. Stem Cells, 2018:61–73. doi: 10.1007/7651_2018_179. [DOI] [PubMed] [Google Scholar]
- 163.Cirera-Salinas D., Ciaudo C. Exit from pluripotency assay of mouse embryonic stem cells. Bio-Protoc. 2017;7:1–8. doi: 10.21769/bioprotoc.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chugh P., Clark A.G., Smith M.B., Cassani D.A.D., Dierkes K., Ragab A., Roux P.P., Charras G., Salbreux G., Paluch E.K. Actin cortex architecture regulates cell surface tension. Nat. Cell Biol. 2017;19:689–697. doi: 10.1038/ncb3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rheinlaender J., Dimitracopoulos A., Wallmeyer B., Kronenberg N.M., Chalut K.J., Gather M.C., Betz T., Charras G., Franze K. Cortical cell stiffness is independent of substrate mechanics. Nat. Mater. 2020;19:1019–1025. doi: 10.1038/s41563-020-0684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alert R., Casademunt J., Brugués J., Sens P. Model for probing membrane-cortex adhesion by micropipette aspiration and fluctuation spectroscopy. Biophys. J. 2015;108:1878–1886. doi: 10.1016/j.bpj.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sliogeryte K., Thorpe S.D., Lee D.A., Botto L., Knight M.M., Rd M.E., Kingdom U., Rd M.E. Stem cell differentiation increases membrane-actin adhesion regulating cell blebability, migration and mechanics. Sci. Rep. 2015;4:7307. doi: 10.1038/srep07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chan C.J., Costanzo M., Ruiz-Herrero T., Mönke G., Petrie R.J., Bergert M., Diz-Muñoz A., Mahadevan L., Hiiragi T. Hydraulic control of mammalian embryo size and cell fate. Nature. 2019;571:112–116. doi: 10.1038/s41586-019-1309-x. [DOI] [PubMed] [Google Scholar]
- 169.Pontes B., Monzo P., Gole L., Le Roux A.L., Kosmalska A.J., Tam Z.Y., Luo W., Kan S., Viasnoff V., Roca-Cusachs P., Tucker-Kellogg L., Gauthier N.C. Membrane tension controls adhesion positioning at the leading edge of cells. J. Cell Biol. 2017;216:2959–2977. doi: 10.1083/jcb.201611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Colom A., Derivery E., Soleimanpour S., Tomba C., Molin M.D., Sakai N., González-Gaitán M., Matile S., Roux A. A fluorescent membrane tension probe. Nat. Chem. 2018;10:1118–1125. doi: 10.1038/s41557-018-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mayer M., Depken M., Bois J.S., Jülicher F., Grill S.W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature. 2010;467:617–621. doi: 10.1038/nature09376. [DOI] [PubMed] [Google Scholar]
- 172.Fischer-Friedrich E., Hyman A.A., Jülicher F., Müller D.J., Helenius J. Quantification of surface tension and internal pressure generated by single mitotic cells. Sci. Rep. 2014;4:4–11. doi: 10.1038/srep06213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Barriga E.H., Franze K., Charras G., Mayor R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature. 2018;554:523–527. doi: 10.1038/nature25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Coughlin M.F., Bielenberg D.R., Lenormand G., Marinkovic M., Waghorne C.G., Zetter B.R., Fredberg J.J. Cytoskeletal stiffness, friction, and fluidity of cancer cell lines with different metastatic potential. Clin. Exp. Metastas-.-. 2013;30:237–250. doi: 10.1007/s10585-012-9531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bevilacqua C., Sanchez Iranzo H., Richter D., Diz-Muñoz A., Prevedel R. Imaging mechanical properties of sub-micron ECM in live zebrafish using Brillouin microscopy. BioRxiv. 2018;10:1420–1431. doi: 10.1101/491803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Faizi H.A., Frey S.L., Steinkühler J., Dimova R., Vlahovska P.M. Bending rigidity of charged lipid bilayer membranes. Soft Matter. 2019;15:6006–6013. doi: 10.1039/c9sm00772e. [DOI] [PubMed] [Google Scholar]
- 177.Fricke K., Wirthensohn K., Laxhuber R., Sackmann E. Flicker spectroscopy of erythrocytes - A sensitive method to study subtle changes of membrane bending stiffness. Eur. Biophys. J. 1986;14:67–81. doi: 10.1007/BF00263063. [DOI] [PubMed] [Google Scholar]
- 178.Kashirina A.S., López-Duarte I., Kubánková M., Gulin A.A., Dudenkova V.V., Rodimova S.A., Torgomyan H.G., Zagaynova E.V., Meleshina A.V., Kuimova M.K. Monitoring membrane viscosity in differentiating stem cells using BODIPY-based molecular rotors and FLIM. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-70972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meng F., Suchyna T.M., Sachs F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J. 2008;275:3072–3087. doi: 10.1111/j.1742-4658.2008.06461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grashoff C., Hoffman B.D., Brenner M.D., Zhou R., Parsons M., Yang M.T., McLean M.A., Sligar S.G., Chen C.S., Ha T., Schwartz M.A. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Borghi N., Sorokina M., Shcherbakova O.G., Weis W.I., Pruitt B.L., Nelson W.J., Dunn A.R. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. U. S. A. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stabley D.R., Jurchenko C., Marshall S.S., Salaita K.S. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods. 2012;9:64–67. doi: 10.1038/nmeth.1747. [DOI] [PubMed] [Google Scholar]
- 183.Wu P.H., Ben Aroush D.R., Asnacios A., Chen W.C., Dokukin M.E., Doss B.L., Durand-Smet P., Ekpenyong A., Guck J., Guz N.V., Janmey P.A., Lee J.S.H., Moore N.M., Ott A., Poh Y.C., Ros R., Sander M., Sokolov I., Staunton J.R., Wang N., Whyte G., Wirtz D. A comparison of methods to assess cell mechanical properties. Nat. Methods. 2018;15 doi: 10.1038/s41592-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lieber A.D., Yehudai-Resheff S., Barnhart E.L., Theriot J.A., Keren K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr. Biol. 2013;23:1409–1417. doi: 10.1016/j.cub.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 185.Desprat N., Supatto W., Pouille P.A., Beaurepaire E., Farge E. Tissue Deformation Modulates Twist Expression to Determine Anterior Midgut Differentiation in Drosophila Embryos. Dev. Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 186.Tzima E., Irani-Tehrani M., Kiosses W.B., Dejana E., Schultz D.A., Engelhardt B., Cao G., DeLisser H., Schwartz M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 187.Soares J., Araujo G.R. d S., Santana C., Matias D., Moura-Neto V., Farina M., Frases S., Viana N.B., Romão L., Nussenzveig H.M., Pontes B. Membrane Elastic Properties During Neural Precursor Cell Differentiation. Cells. 2020;9 doi: 10.3390/cells9061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Benedetti L., Marvin J.S., Falahati H., Guillén-Samander A., Looger L.L., De Camilli P. Optimized Vivid-derived Magnets photodimerizers for subcellular optogenetics in mammalian cells. Elife. 2020;9:1–28. doi: 10.7554/elife.63230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nguyen M.K., Kim C.Y., Kim J.M., Park B.O., Lee S., Park H., Do Heo W. Optogenetic oligomerization of Rab GTPases regulates intracellular membrane trafficking. Nat. Chem. Biol. 2016;12:431–436. doi: 10.1038/nchembio.2064. [DOI] [PubMed] [Google Scholar]
- 190.Yu M., Le S., Barnett S., Guo Z., Zhong X., Kanchanawong P., Yan J. Implementing Optogenetic Modulation in Mechanotransduction. Phys. Rev. X. 2020;10:21001. doi: 10.1103/PhysRevX.10.021001. [DOI] [Google Scholar]
- 191.Bassereau P., Jin R., Baumgart T., Deserno M., Dimova R., Frolov V.A., Bashkirov P.V., Grubmüller H., Jahn R., Risselada H.J., Johannes L., Kozlov M.M., Lipowsky R., Pucadyil T.J., Zeno W.F., Stachowiak J.C., Stamou D., Breuer A., Lauritsen L., Simon C., Sykes C., Voth G.A., Weikl T.R. The 2018 biomembrane curvature and remodeling roadmap. J. Phys. D. Appl. Phys. 2018;51 doi: 10.1088/1361-6463/aacb98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z. Fur Naturforsch. - Sect. C. J. Biosci. 1973;28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 193.Flory P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942;10:51–61. doi: 10.1063/1.1723621. [DOI] [Google Scholar]
- 194.Huggins M.L. Solutions of long chain compounds. J. Chem. Phys. 1941;9:440. doi: 10.1063/1.1750930. [DOI] [Google Scholar]
- 195.Brugués J., Maugis B., Casademunt J., Nassoy P., Amblard F., Sens P. Dynamical organization of the cytoskeletal cortex probed by micropipette aspiration. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15415–15420. doi: 10.1073/pnas.0913669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shi Z., Graber Z.T., Baumgart T., Stone H.A., Cohen A.E. Cell Membranes Resist Flow. Cell. 2018;175:1769–1779.e13. doi: 10.1016/j.cell.2018.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Houk A.R., Jilkine A., Mejean C.O., Boltyanskiy R., Dufresne E.R., Angenent S.B., Altschuler S.J., Wu L.F., Weiner O.D. Membrane Tension Maintains Cell Polarity by Confining Signals to the Leading Edge during Neutrophil Migration. Cell. 2012;148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Callan-Jones A.C., Joanny J.F., Prost J. Viscous-fingering-like instability of cell fragments. Phys. Rev. Lett. 2008;100:27–30. doi: 10.1103/PhysRevLett.100.258106. [DOI] [PubMed] [Google Scholar]
- 199.Blanch-Mercader C., Casademunt J. Spontaneous motility of actin lamellar fragments. Phys. Rev. Lett. 2013;110:1–5. doi: 10.1103/PhysRevLett.110.078102. [DOI] [PubMed] [Google Scholar]
- 200.Fogelson B., Mogilner A. Computational estimates of membrane flow and tension gradient in motile cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Simunovic M., Šarić A., Henderson J.M., Lee K.Y.C., Voth G.A. Long-Range Organization of Membrane-Curving Proteins. ACS Cent. Sci. 2017;3:1246–1253. doi: 10.1021/acscentsci.7b00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blood P.D., Voth G.A. Direct observation of Bin/amphiphysin/Rvs (BAR) domain-induced membrane curvature by means of molecular dynamics simulations. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15068–15072. doi: 10.1073/pnas.0603917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reynwar B.J., Illya G., Harmandaris V.A., Müller M.M., Kremer K., Deserno M. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature. 2007;447:461–464. doi: 10.1038/nature05840. [DOI] [PubMed] [Google Scholar]
- 204.Reynwar B.J., Deserno M. Membrane-mediated interactions between circular particles in the strongly curved regime. Soft Matter. 2011;7:8567–8575. doi: 10.1039/c1sm05358b. [DOI] [Google Scholar]
- 205.Zmurchok C., Collette J., Rajagopal V., Holmes W.R. Membrane tension can enhance adaptation to maintain polarity of migrating cells. Biophys. J. 2020;119:1617–1629. doi: 10.1016/j.bpj.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ron A., Azeloglu E.U., Calizo R.C., Hu M., Bhattacharya S., Chen Y., Jayaraman G., Lee S., Neves-Zaph S.R., Li H., Gordon R.E., He J.C., Hone J.C., Iyengar R. Cell shape information is transduced through tension-independent mechanisms. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-02218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rangamani P., Lipshtat A., Azeloglu E.U., Calizo R.C., Hu M., Ghassemi S., Hone J., Scarlata S., Neves S.R., Iyengar R. Decoding Information in Cell Shape. Cell. 2013;154:1356–1369. doi: 10.1016/j.cell.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fehon R.G., McClatchey A.I., Bretscher A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gritti N., Oriola D., Trivedi V. Rethinking embryology in vitro: A synergy between engineering, data science and theory. Dev. Biol. 2021;474:48–61. doi: 10.1016/j.ydbio.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 210.Rossi G., Manfrin A., Lutolf M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018;19:671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 211.Hofer M., Lutolf M.P. Engineering organoids. Nat. Rev. Mater. 2021;6:402–420. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McGinn J., Hallou A., Han S., Krizic K., Ulyanchenko S., Iglesias-Bartolome R., England F.J., Verstreken C., Chalut K.J., Jensen K.B., Simons B.D., Alcolea M.P. A biomechanical switch regulates the transition towards homeostasis in oesophageal epithelium. Nat. Cell Biol. 2021;23:511–525. doi: 10.1038/s41556-021-00679-w. [DOI] [PMC free article] [PubMed] [Google Scholar]