Abstract
BACKGROUND:
Single-center data demonstrates that regional analgesia (RA) techniques are associated with reduced risk of delirium in older patients with multiple rib fractures. We hypothesized that a similar effect between RA and delirium would be identified in a larger cohort of patients from multiple level I trauma centers.
METHODS:
Retrospective data from seven level I trauma centers were collected for intensive care unit (ICU) patients 65 years or older with ≥3 rib fractures from January 2012 to December 2016. Those with a head and/or spine injury Abbreviated Injury Scale (AIS) score of ≥ 3 or a history of dementia were excluded. Delirium was defined as one positive Confusion Assessment Method for the Intensive Care Unit score in the first 7 days of ICU care. Poisson regression with robust standard errors was used to determine the association of RA (thoracic epidural or paravertebral catheter) with delirium incidence.
RESULTS:
Data of 574 patients with a median age of 75 years (interquartile range [IQR], 69–83), Injury Severity Score of 14 (IQR, 11–18), and ICU length of stay of 3 days (IQR, 2–6 days) were analyzed. Among the patients, 38.9% were women, 15.3% were non-White, and 31.4% required a chest tube. Regional analgesia was used in 19.3% patients. Patient characteristics did not differ by RA use; however, patients with RA had more severe chest injury (chest AIS, flail segment, hemopneumothorax, thoracostomy tube). In univariate analysis, there was no difference in the likelihood of delirium between the RA and no RA groups (18.9% vs. 23.8% p = 0.28). After adjusting for age, sex, Injury Severity Score, maximum chest AIS, thoracostomy tube, ICU length of stay, and trauma center, RA was associated with reduced risk of delirium (incident rate ratio [IRR], 0.65; 95% confidence interval [CI], 0.44–0.94) but not with in-hospital mortality (IRR, 0.42; 95% CI, 0.14–1.26) or respiratory complications (IRR, 0.70; 95% CI, 0.42–1.16).
CONCLUSION:
In this multicenter cohort of injured older adults with multiple rib fractures, RA use was associated with a 35% lower risk of delirium. Further studies are needed to standardize protocols for optimal pain management and prevention of delirium in older adults with severe thoracic injury.
Keywords: Delirium, regional analgesia, rib fractures, older adults
Delirium is the clinical manifestation of acute brain failure and is present in 20% to 60% of injured older adults admitted to the intensive care unit (ICU).1,2 Presence of delirium is strongly associated with increased hospital length of stay (LOS), in-hospital mortality, and lasting cognitive impairment at 3 to 12 months post-ICU discharge.3,4 Uncontrolled pain and high doses of systemic opioids are known modifiable risk factors for delirium.5 The American Geriatrics Society strongly recommends optimization of pain control with nonopioid pain medication to minimize pain and prevent delirium in older surgical patients.6
The incidence of rib fractures after blunt trauma increases with advancing age.7,8 Fracture of three or more ribs is the inflection point associated with higher morbidity and mortality that increases with each additional rib fracture.9 Uncontrolled chest wall pain leads to hypoventilation and immobilization, precursors to respiratory complications and delirium. The mainstay of treatment for patients with multiple rib fractures is early institution of a multimodal pain regimen including nonsteroidal anti-inflammatory drugs, acetaminophen, opioids, and regional analgesia (RA) techniques in appropriate patients.10 Benefits of catheter-based RA (epidural and paravertebral catheters) with infusion of local anesthesia include improved analgesia, reduced systemic opioid requirements, and lower in-hospital and long-term mortality.11–13
Our group previously published the first study to evaluate the association between RA and delirium in older adults with multiple rib fractures.14 A limitation of the previous study was the relatively small sample size from a single level I trauma center. Accordingly, the current study investigates whether RA is associated with a reduced risk of delirium in a larger cohort of patients from multiple institutions with differing pain management protocols. We hypothesized that older adults with multiple rib fractures who received RA would have a lower risk of delirium compared with those who received usual care with systemic analgesia and no RA.
PATIENTS AND METHODS
This study was registered as a 2017 EAST Multicenter Study, and the study proposal was presented at the 30th Eastern Association for the Surgery of Trauma Annual Scientific Assembly. Participation was voluntary, and centers were encouraged to join if they anticipated contributing at least 60 patients. The study was designed to detect a risk ratio of at least 0.5 with a sample size of 500 patients, assuming an incidence of delirium of 25% in those who do not receive RA (unexposed) and a ratio of unexposed to exposed of 4 (i.e., 4 patients with no RA for every 1 who received RA).15 This study was approved by the institutional review board of each participating center. This was a retrospective cohort study of older adults admitted to seven level I trauma centers across the nation from January 1, 2012, to December 31, 2016. Inclusion criteria were patients 65 years or older who were admitted to the ICU with three or more rib fractures from blunt trauma mechanism. Intensive care unit admission was required to ensure consistency of delirium assessments across participating centers. Exclusion criteria included significant head injury as defined by Abbreviated Injury Scale (AIS) score of ≥3, significant spine injury (AIS score, ≥3), history of dementia, and death within 24 hours of admission.
Patient demographics, trauma-specific data, and hospital outcomes were collected. Nursing assessments for delirium using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) were reported for the first 7 days of ICU admission.16 Multiple CAM-ICU assessments were performed per ICU day for each patient. When at least one CAM-ICU assessment was positive for delirium within a 24-hour period, that ICU day was considered “delirium positive.” Patients missing CAM-ICU data on any given day were classified as not having delirium on that day. The primary outcome was incidence of delirium within the first 7 days of ICU admission. Secondary outcomes included respiratory complications (pneumonia, empyema, aspiration, unplanned reintubation), in-hospital mortality, and hospital LOS.
Patients were divided into two exposure groups based on their administered pain regimen. Patients who received pain management that did not include RA were assigned to the “No RA group.” Patients who received thoracic RA as a component of a multimodal pain regimen, either epidural or paravertebral catheters, were assigned to the “RA group.” Patients in both groups received systemic opioids and nonopioid pain medications based on practice patterns of the participating institution. Examination of the medications and doses administered was beyond the scope of this study.
Patient characteristics were compared according to RA exposure status using χ2 statistics for categorical variables and Student t or Wilcoxon rank-sum tests for continuous variables (Tables 1 and 2). Delirium incident rate ratios and 95% confidence intervals were estimated using Poisson regression models with robust standard errors (Fig. 1 and Table 3). Risk of delirium among patients who received RA was compared with the risk of delirium in those who did not receive RA. In the design of this study, we selected the covariates a priori based on the clinical knowledge of confounding factors, and we applied an epidemiologic approach to data analysis, focusing on biological/clinical plausibility of confounding, versus a statistically driven selection of covariates. The final model was adjusted for age, sex, Injury Severity Score (ISS), maximum chest AIS, flail segment, thoracostomy tube, ICU LOS, and trauma center as a fixed effect. To confirm the multivariable adjusted results, propensity score and nearest neighbor-matched models comparing RA versus No RA were also estimated and provided substantively similar results (not shown). These treatment effect models matched on age, sex, chest tube placement, and ICU LOS (the nearest neighbor model used exact matching on ICU LOS, and Mahalanobis distance was used to find matches on age). Poisson regression was also used to examine in-hospital mortality, while linear regression was used to examine hospital LOS. Logarithmic transformation of hospital LOS did not substantively change the results of the linear regression model. Statistical significance was set at p < 0.05. All analyses were completed using Stata SE 15 (StataCorp, College Station, TX).
TABLE 1.
Patient Demographics and Injury Data
| No RA |
RA |
||
|---|---|---|---|
| n = 463 (81%) | n = 111 (19%) | p | |
| Age, mean (SD), y | 77 (8) | 76 (8) | 0.46 |
| Female, n (%) | 179 (39) | 44 (40) | 0.85 |
| Race/ethnicity, n (%) | |||
| White | 386 (83) | 100 (90) | 0.15 |
| African American | 9 (2) | 4 (4) | |
| Asian | 12(3) | 2(2) | |
| Hispanic or Latino | 47 (10) | 4 (4) | |
| Other/unknown | 9 (2) | 1(1) | |
| Trauma mechanism, n (%) | 0.06 | ||
| MVC | 205 (44) | 50 (45) | |
| Fall | 175 (38) | 43 (39) | |
| Pedestrian struck | 27 (6) | 1(1) | |
| Bicycle | 8 (2) | 1 (1) | |
| MCC | 28(6) | 5(5) | |
| Other | 20 (4) | 11 (10) | |
| Intubation (prehospital or ED), n (%) | 39 (8) | 7 (6) | 0.46 |
| ISS, median (IQR) | 14(11–19) | 14 (10–17) | 0.25 |
| ISS | 17 | 0.61 | |
| <15 | 241 (52) | 62 (56) | |
| 15–24 | 176 (38) | 41 (37) | |
| >24 | 46 (10) | 8 (7) | |
| Maximum chest AIS, n (%) | 0.04 | ||
| 2 | 20(4) | 0(0) | |
| 3 | 387 (84) | 97 (87) | |
| 4 | 48(10) | 12 (11) | |
| 5 | 8(2) | 1(1) | |
| 6 | 0 (0) | 1 (1) | |
| Maximum head AIS, n (%) | 0.12 | ||
| 0 | 308 (66) | 84 (76) | |
| 1 | 18(4) | 5(4) | |
| 2 | 137 (30) | 22 (20) | |
| Maximum spine AIS, n (%) | 0.55 | ||
| 0 | 279 (60) | 72 (65) | |
| 1 | 17(3) | 2(2) | |
| 2 | 169 (37) | 37 (33) | |
| Flail segment, n (%) | 49 (11) | 31 (28) | <0.001 |
| Hemothorax, pneumothorax, or hemopneumothorax, n (%) | 191 (41) | 75 (68) | <0.001 |
| Tube thoracostomy, n (%) | 127 (27) | 53 (48) | <0.001 |
| Sternal fracture, n (%) | 74 (16) | 18 (16) | 0.95 |
| Trauma center, n (%) | <0.001 | ||
| 1 | 121(26) | 17 (15) | |
| 2 | 31(7) | 2(2) | |
| 3 | 48(10) | 16 (14) | |
| 4 | 26(6) | 8(7) | |
| 5 | 164 (35) | 37 (33) | |
| 6 | 18(4) | 1(1) | |
| 7 | 55 (12) | 30 (27) |
AIS, Abbreviated Injury Score; ED, emergency department; IQR, interquartile range; MCC, motorcycle collision; MVC, motor vehicle collision.
TABLE 2.
Unadjusted Univariate Analysis of Outcomes
| No RA |
RA |
||
|---|---|---|---|
| n = 463 (81%) | n = 111 (19%) | p | |
| Delirium incidence within the first 7 ICU d, n (%) | 110(24) | 21 (19) | 0.28 |
| Complications, n (%) | |||
| Pneumonia | 45 (10) | 11 (10) | 0.95 |
| Empyema | 1 (0) | 1 (1) | 0.27 |
| Aspiration | 8(2) | 2(2) | 0.96 |
| Reintubation | 5(1) | 4(4) | 0.06 |
| None | 407 (88) | 96 (86) | 0.68 |
| ICU LOS, median (IQR) | 3(2–5) | 4 (3–7) | <0.001 |
| Mechanical ventilation, n (%) | 115 (25) | 32 (29) | 0.39 |
| No. ventilator days, n (%) | 0.08 | ||
| 0 | 417 (90) | 96 (87) | |
| 1 | 10(2) | 0(0) | |
| 2 | 14 (3) | 4(4) | |
| >3 | 22 (5) | 11 (9) | |
| Unexpected ICU readmission, n (%) | 36 (8) | 7 (6) | 0.59 |
| Thoracic operation, n (%) | |||
| Thoracotomy | 5(1) | 5 (5) | 0.01 |
| VATS | 13 (3) | 9 (8) | 0.01 |
| Rib stabilization | 13 (3) | 18 (16) | <0.001 |
| Other | 10(2) | 3(3) | 0.73 |
| None | 426 (92) | 85 (77) | <0.001 |
| Hospital LOS, median (IQR) | 8 (5–12) | 10(8–13) | <0.001 |
| Discharge disposition, n (%) | 0.57 | ||
| Home | 175 (38) | 43 (39) | |
| SNF | 153 (33) | 39 (35) | |
| LTAC | 17 (4) | 6 (5) | |
| Rehab | 69 (15) | 18 (16) | |
| Hospice | 16 (3) | 3 (3) | |
| Death | 18 (4) | 2 (2) | |
| Shelter | 1 (0) | 0 (0) | |
| Other | 14 (3) | 0 (0) |
IQR, interquartile range; LTAC, long-term acute care hospital; SNF, skilled nursing facility; VATS, video-assisted thoracoscopic surgery.
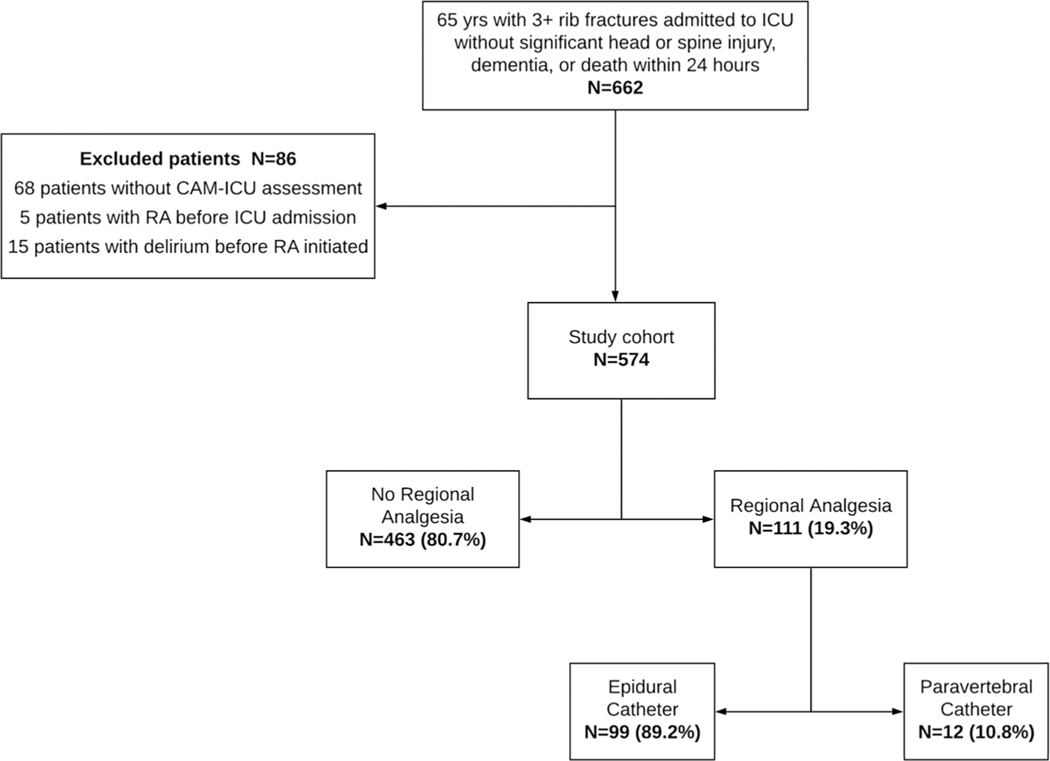
Figure 1.
Regional analgesia distribution.
TABLE 3.
Associations of Regional Anesthesia with Primary and Secondary outcomes (N = 574)
| Delirium |
Respiratory Complications |
In-hospital Mortality |
Hospital LOS, d |
|||||
|---|---|---|---|---|---|---|---|---|
| IRR | (95% CI); p Value | IRR | (95% CI); p Value | IRR | (95% CI); p Value | β Coefficient | (95% CI); p Value | |
| RA vs. No RA | 0.65 | (0.44–0.94); 0.02 | 0.70 | (0.42–1.16); 0.17 | 0.42 | (0.14–1.26); 0.12 | 0.2 | (−1.4 to 1.7); 0.86 |
CI, confidence interval; IRR, incident rate ratio.
RESULTS
Patient Characteristics
A total of 662 patients met the inclusion criteria for this study. However, 68 patients did not have reported CAM-ICU assessments, 5 patients had RA before ICU admission (during which time they were not assessed for delirium), and 15 patients developed delirium before RA was initiated (i.e., developed the outcome before the exposure variable). These 86 patients were excluded from the analysis, leaving a final study cohort of 574 patients. Of these patients, 111 (19.3%) received thoracic RA, while 463 (80.7%) received no RA. Within the RA group, 99 (89.2%) received epidural catheters and 12 (10.8%) received paravertebral catheters (Fig. 1). The median time from hospital admission to RA initiation was 2 days (interquartile range, 1–2 days).
Both groups (RA vs. no RA) were comparable for age, sex, race/ethnicity, and mechanism of injury (p > 0.05, Table 1). Injury characteristics including ISS were similar between the groups (Table 1). Patients in the RA group had significantly more complicated chest trauma with higher chest AIS scores, more flail segments, hemopneumothorax, and thoracostomy tube placement.
Patient characteristics according to delirium status are shown in Supplemental Table 1 (http://links.lww.com/TA/C4). There were no statistically significant differences in the distributions of age, sex, race/ethnicity, trauma mechanism, chest and head AIS, flail segment, hemopneumothorax, sternal fracture, and trauma center by delirium status. However, patients who developed delirium were more likely to be intubated, have greater injury severity, more complicated spine trauma, and have thoracostomy tube placement than those who did not develop delirium.
Unadjusted Outcomes
Table 2 demonstrates the unadjusted primary and secondary outcomes, as well as additional clinically relevant outcome measures. The 7-day incidence of delirium in the study cohort was 22.8%. A higher proportion of patients in the No RA group compared with the RA group had at least one CAM-positive assessment, although this difference was not statistically significant (unadjusted comparison shown in Table 2). The majority of patients did not have a respiratory complication (87.6%), and the incidences of pneumonia, empyema, aspiration, and unplanned reintubation were overall rare. Patients in the RA group were much more likely to have had a thoracic operation compared with those in the No RA group (23.4% vs. 8.0%, p < 0.001). Operative timing and perioperative details were not collected for this study. Although ICU LOS was longer for the RA group (4 vs. 3 days, p < 0.001), mechanical ventilation, ICU readmission, hospital LOS, and discharge disposition did not differ between the groups.
Multivariable Analysis of Outcome Parameters
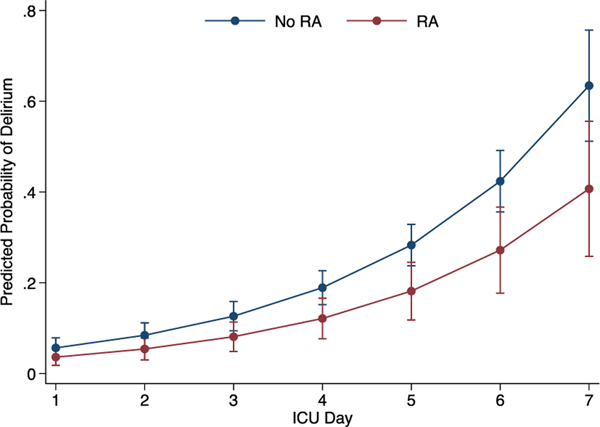
Figure 2 illustrates the increasing risk of delirium with longer ICU stay when adjusted for age, sex, ISS, maximum chest AIS, flail segment, thoracostomy tube, and trauma center. Table 3 shows the results of the multivariate models of the primary and secondary outcomes that adjusted for age, sex, ISS, maximum chest AIS, flail segment, thoracostomy tube, ICU LOS, and trauma center. Patients in the RA group had a 35% decreased risk of delirium compared with patients in the No RA group. Respiratory complications were pooled and dichotomized as any versus none because of the relatively low event rates for the individual complications. Neither respiratory complications, in-hospital mortality, nor hospital LOS was statistically different between the exposure groups. Supplemental Table 2 (http://links.lww.com/TA/C5) demonstrates the full model with all covariate results for delirium, respiratory complications, in-hospital mortality, and hospital LOS. In sensitivity analyses excluding patients who died in the hospital (n = 36), the relationships of RA with the study outcomes were substantively the same. For example, after excluding in-hospital deaths, RA (vs. No RA) was associated with a 40% decreased risk of delirium (incident rate ratio, 0.60; 95% confidence interval, 0.40–0.91; p = 0.02).
Figure 2.
Adjusted risk of delirium by ICU day according to RA.
* Error bars are 95% confidence intervals
**Adjusted for age, sex, ISS, maximum chest AIS, flail segment, thoracostomy tube, and trauma center
DISCUSSION
This multicenter study further demonstrates that older adults with severe chest trauma are at high risk for delirium, and the risk increases with length of ICU admission. Older adults with multiple rib fractures who received thoracic RA as a component of multimodal analgesia had a 35% lower risk of delirium compared with those who did not receive RA after controlling for age, sex, ISS, maximum chest AIS, flail segment, thoracostomy tube, ICU LOS, and trauma center. Notably, this finding was observed despite having different pain management protocols at seven level I trauma centers nationwide. The reduced risk of delirium associated with RA is likely not attributable to RA alone, rather a combination of benefits (e.g., potentially improved pain control, better sleep, and earlier mobilization) gleaned from multidisciplinary treatment of pain in this group of vulnerable patients.
Older age, history of dementia, prior coma, pre-ICU emergency surgery or trauma, and increasing Acute Physiology and Chronic Health Evaluation and American Society of Anesthesiologists scores have been identified as nonmodifiable risk factors for delirium.17 Most injured older adults have several of these risk factors, and therefore, preventative strategies should target the modifiable risk factors for delirium. Trauma care providers must balance treatment of rib fracture pain with the undesirable side effects of systemic opioids in older adults, namely, respiratory depression, sedation, falls, and delirium. In 2018, the Society for Critical Care Medicine published clinical practice guidelines for pain and delirium management that recommend combining different analgesics that act by different mechanisms at different sites in the nervous system to treat pain and reduce systemic opioid dose.17 Other than acetaminophen, older adults often have contraindications to the suggested systemic adjuncts including ketamine, nonsteroidal anti-inflammatory drugs, and neuropathic pain medications. Regional analgesia is a multimodal adjunct that acts in a targeted fashion while reducing systemic opioid use and concomitantly the common undesirable side effects.
The higher burden of morbidity and mortality in older people with rib fractures is due to subsequent respiratory complications. Bulger et al.9,18 reported that 31% of older patients with rib fractures developed pneumonia and, in a subsequent study, found that patients who received RA were less likely to develop pneumonia. While the current study reports only 9.8% of patients were diagnosed with pneumonia, there was no difference between the study groups. In addition, in-hospital mortality was only 3.9% in the No RA group and 1.8% in the RA group, which is lower than in previously published studies with less selective cohorts.9,13,19,20 The lower mortality in the current study is largely affected by the exclusion of patients with severe head and spine injuries. However, advancements in critical care principles and rib fracture management over the past 20 years, such as rib fracture treatment protocolization, which includes acute pain service (APS) consultation, likely accounts for some of the reduction in respiratory complications and in-hospital mortality in both groups.20
Several institutions have published rib fracture treatment protocols with defined triggers for APS consultation including poor pain control, suboptimal incentive spirometry effort, and weak cough.20,21 One protocol published by Coary et al.22 outlines a stepwise approach to rib fracture management in older adults, which clearly defines the point at which the APS team will use RA. As has been demonstrated previously, patients in the current study who received RA had more severe chest trauma (as evidenced by more flail segments, hemopneumothorax, and use of thoracostomy tubes).14,18,19 These indicators of severe chest injury should be used as definitive indications for RA utilization in qualified patients. Well-defined multidisciplinary protocols for both the primary service (APS consultation triggers) and the APS service (RA indications and timing) would help eliminate the interprovider variability that prevents a standardized approach to rib fracture pain management.
Routine use of RA as a component of multimodal pain regimens for patients with multiple rib fractures has remained controversial despite the recommendation to “consider RA” in rib fracture treatment protocols. As previously noted by Gage et al.,13 underuse of RA is demonstrated with only 19.3% of highly-selected patients admitted to level I trauma centers having received RA. Central to the argument against routine use of RA, especially in older adults with higher comorbidity burden, are the potential life-altering complications associated with the procedure. While the incidence of neuraxial injury after epidural blockade is low (need for subsequent laminectomy 1 per 8,000 blocks), the resulting deficits are permanent.23,24 On the other hand, transient neurologic symptoms are common with peripheral nerve blockade (e.g., paravertebral catheters) but rarely result in permanent damage (2–4 per 100,000 blocks).25,26 Furthermore, risk of hemorrhagic (<1 per 150,000 epidurals) and infectious complications (1 per 40,000 epidurals), as well as local anesthetic systemic toxicity (27 per 100,000 peripheral nerve blocks), factor into the procedural decision making.27–29 Evidence-based guidelines regarding patient selection and contraindications for RA, especially in older adults, are desperately needed to support decision making in light of these rare but serious complications of RA.
Older injured patients, especially with multiple injuries, may have contraindications to epidural and paravertebral catheter placement. Newer paravertebral block variants use the principle of local analgesia spread between tissue planes to anesthetize the ventral ramus of nerves in the targeted area or peripheral nerves in the fascial plane. The choice of block is based on the location of rib fractures and includes the erector spinae plane block, retrolaminar block, interpleural block, paraspinal/intercostal block, and serratus anterior plane block.30 Compared with epidural and paravertebral catheters, these newer blocks are more superficial and thus carry less risk of serious complications, are technically simpler to perform, and can be administered by emergency physicians, intensivists, and surgeons using ultrasound guidance. Although research is emerging on the efficacy of these techniques, they are promising alternatives.
Finally, there is a paucity of data on long-term outcomes after injury, especially in older adults. One in three critically ill patients with positive delirium screening have lasting cognitive impairment at 1 year after ICU discharge.3,31 Delirium superimposed on preexisting dementia has a synergistic effect on the trajectory of subsequent cognitive decline suggesting that delirium prevention could prevent dementia.32 In addition, in light of the nationwide opioid crisis and new evidence that opioid use in the older adult population is more prevalent and prolonged after injury, targeted preventative strategies are needed.33,34 The National Health and Nutrition Examination Survey showed that people 65 years or older were 25.4% of long-term opioid users.35 In a study of long-term opioid use in older adults after injury, thoracic injuries were associated with the second highest incidence of opioid use at 1 year after injury (spine injuries were the highest).36 On the other hand, uncontrolled acute rib fracture pain is strongly predictive of long-term pain and disability.37,38 Further research is needed to elucidate the balance of adequate early postinjury pain control with prevention of long-term consequences that are notably more common in older patients than the adverse events associated with RA.
The results of this study should be interpreted within the context of the following limitations. First, there are many factors that contribute to delirium, which were beyond the scope of this study, including daily systemic opioid requirements. It is important to recognize that this study does not demonstrate a causal relationship between RA and delirium, rather the findings support an existing association that warrants further prospective investigation. The retrospective study design combined with a lack of standardize protocol regarding patient selection for and management of RA at the various participating centers likely resulted in some degree of selection bias and confounding that was not accounted for in the analysis. Furthermore, each center had variable rib fracture treatment protocols, delirium prevention protocols, and multidisciplinary trauma management, which could have impacted the incidence of delirium within each center. Each center submitted a variable amount of patient data, and it is possible the results were influenced by this variability despite the efforts to control for it in the multivariable analysis. In addition, missing CAM assessment data were assumed to reflect that the patient did not have delirium on those days. Although this might contribute to some misclassification, it is likely to be nondifferential between the exposure groups. To examine the association of RA with delirium, patients who developed delirium before use of RA were excluded from the study. In using this approach, it is possible that immortal time bias was introduced because there was effectively less time at risk for delirium in the RA group. However, most patients (70%) received RA within the first 1 to 2 days of ICU admission, and therefore, the impact was likely small. Because of these study limitations, a clinical trial is needed to more definitively establish the clinical benefit (causal effect) of RA reducing risk of delirium in this patient population. Lastly, all data collected for this study were from level I trauma centers, and the results may not be applicable to lower level or nontrauma centers with limited multidisciplinary support.13
The current study findings suggest that the benefits of thoracic RA in older patients with multiple rib fractures extend beyond standard outcomes assessed in previous studies and includes a reduced risk of ICU delirium. Delirium is common in injured older adults, and prevention of delirium should be considered when weighing the risks and benefits of RA. A multidisciplinary approach to rib fracture pain management that is tailored to older adults is central to improvement of short-term outcomes after blunt thoracic trauma. Randomized prospective studies that include analysis of systemic opioid requirements are needed to delineate the complex relationship between delirium and RA utilization and to evaluate the impact of RA use on long-term outcomes such as chronic pain, posttraumatic stress disorder, and opioid use disorder after thoracic injury in older adults.
Supplementary Material
Footnotes
LEVEL OF EVIDENCE: Therapeutic, level IV; Epidemiologic, level III.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Risk factors for delirium in older trauma patients admitted to the surgical intensive care unit. J Trauma Acute Care Surg. 2014;77(6):944–951. [DOI] [PubMed] [Google Scholar]
- 2.Bryant EA, Tulebaev S, Castillo-Angeles M, Moberg E, Senglaub SS, O’Mara L, McDonald M, Salim A, Cooper Z. Frailty identification and care pathway: an interdisciplinary approach to care for older trauma patients. J Am Coll Surg. 2019;228(6):852–9.e1. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr., Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. [DOI] [PubMed] [Google Scholar]
- 5.Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry. 2014;1(6):431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RB, Bass SM, Morris JA Jr., MacKenzie EJ. Three or more rib fractures as an indicator for transfer to a Level I trauma center: a population-based study. J Trauma. 1990;30(6):689–694. [DOI] [PubMed] [Google Scholar]
- 8.Stawicki SP, Grossman MD, Hoey BA, Miller DL, Reed JF 3rd. Rib fractures in the elderly: a marker of injury severity. J Am Geriatr Soc. 2004;52(5):805–808. [DOI] [PubMed] [Google Scholar]
- 9.Bulger EM, Arneson MA, Mock CN, Jurkovich GJ. Rib fractures in the elderly. J Trauma. 2000;48(6):1040–1046; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 10.Galvagno SM Jr., Smith CE, Varon AJ, et al. Pain management for blunt thoracic trauma: a joint practice management guideline from the Eastern Association for the Surgery of Trauma and Trauma Anesthesiology Society. J Trauma Acute Care Surg. 2016;81(5):936–951. [DOI] [PubMed] [Google Scholar]
- 11.Simon BJ, Cushman J, Barraco R, Lane V, Luchette FA, Miglietta M, Roccaforte DJ, Spector R. Pain management guidelines for blunt thoracic trauma. J Trauma. 2005;59(5):1256–1267. [DOI] [PubMed] [Google Scholar]
- 12.Ho AM, Karmakar MK, Critchley LA. Acute pain management of patients with multiple fractured ribs: a focus on regional techniques. Curr Opin Crit Care. 2011;17(4):323–327. [DOI] [PubMed] [Google Scholar]
- 13.Gage A, Rivara F, Wang J, Jurkovich GJ, Arbabi S. The effect of epidural placement in patients after blunt thoracic trauma. J Trauma Acute Care Surg. 2014;76(1):39–45. [DOI] [PubMed] [Google Scholar]
- 14.O’Connell KM, Quistberg DA, Tessler R, Robinson BRH, Cuschieri J, Maier RV, Rivara FP, Vavilala MS, Bhalla PI, Arbabi S. Decreased risk of delirium with use of regional analgesia in geriatric trauma patients with multiple rib fractures. Ann Surg. 2018;268(3):534–540. [DOI] [PubMed] [Google Scholar]
- 15.Fleiss JL, Tytun A, Ury HK. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. 1980;36(2):343–346. [PubMed] [Google Scholar]
- 16.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 17.Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. [DOI] [PubMed] [Google Scholar]
- 18.Bulger EM, Edwards T, Klotz P, Jurkovich GJ. Epidural analgesia improves outcome after multiple rib fractures. Surgery. 2004;136(2):426–430. [DOI] [PubMed] [Google Scholar]
- 19.Flagel BT, Luchette FA, Reed RL, Esposito TJ, Davis KA, Santaniello JM, Gamelli RL. Half-a-dozen ribs: the breakpoint for mortality. Surgery. 2005;138(4):717–723; discussion 23–5. [DOI] [PubMed] [Google Scholar]
- 20.Todd SR, McNally MM, Holcomb JB, et al. A multidisciplinary clinical pathway decreases rib fracture-associated infectious morbidity and mortality in high-risk trauma patients. Am J Surg. 2006;192(6):806–811. [DOI] [PubMed] [Google Scholar]
- 21.Witt CE, Bulger EM. Comprehensive approach to the management of the patient with multiple rib fractures: a review and introduction of a bundled rib fracture management protocol. Trauma Surg Acute Care Open. 2017;2(1):e000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coary R, Skerritt C, Carey A, Rudd S, Shipway D. New horizons in rib fracture management in the older adult. Age Ageing. 2020;49(2):161–167. [DOI] [PubMed] [Google Scholar]
- 23.Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet. 2008;372(9638):562–569. [DOI] [PubMed] [Google Scholar]
- 24.Neal JM, Barrington MJ, Brull R, Hadzic A, Hebl JR, Horlocker TT, Huntoon MA, Kopp SL, Rathmell JP, Watson JC. The Second ASRA Practice Advisory on Neurologic Complications Associated With Regional Anesthesia and Pain Medicine: executive summary 2015. Reg Anesth Pain Med. 2015;40(5):401–430. [DOI] [PubMed] [Google Scholar]
- 25.Orebaugh SL, Kentor ML, Williams BA. Adverse outcomes associated with nerve stimulator-guided and ultrasound-guided peripheral nerve blocks by supervised trainees: update of a single-site database. Reg Anesth Pain Med. 2012;37(6):577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263–309. [DOI] [PubMed] [Google Scholar]
- 27.Neal JM, Woodward CM, Harrison TK. The American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2017 version. Reg Anesth Pain Med. 2018;43(2):150–153. [DOI] [PubMed] [Google Scholar]
- 28.Horlocker TT, Wedel DJ. Anticoagulation and neuraxial block: historical perspective, anesthetic implications, and risk management. Reg Anesth Pain Med. 1998;23(6 Suppl 2):129–134. [DOI] [PubMed] [Google Scholar]
- 29.El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018;11:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho AM, Ho AK, Mizubuti GB, Klar G, Karmakar MK. Regional analgesia for patients with traumatic rib fractures: a narrative review. J Trauma Acute Care Surg. 2020;88(1):e22–e30. [DOI] [PubMed] [Google Scholar]
- 31.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis DH, Muniz-Terrera G, Keage HA, et al. Association of delirium with cognitive decline in late life: a neuropathologic study of 3 population-based cohort studies. JAMA Psychiat. 2017;74(3):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999–2012. NCHS Data Brief. 2015;(189):1–8. [PubMed]
- 34.Saha TD, Kerridge BT, Goldstein RB, et al. Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. J Clin Psychiatry. 2016;77(6):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526–534. [DOI] [PubMed] [Google Scholar]
- 36.Daoust R, Paquet J, Moore L, Gosselin S, Gelinas C, Rouleau DM, Berube M, Morris J. Incidence and risk factors of long-term opioid use in elderly trauma patients. Ann Surg. 2018;268(6):985–991. [DOI] [PubMed] [Google Scholar]
- 37.Fabricant L, Ham B, Mullins R, Mayberry J. Prolonged pain and disability are common after rib fractures. Am J Surg. 2013;205(5):511–515. [DOI] [PubMed] [Google Scholar]
- 38.Gordy S, Fabricant L, Ham B, Mullins R, Mayberry J. The contribution of rib fractures to chronic pain and disability. Am J Surg. 2014;207(5):659–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.