Abstract
Ionizing radiation is an established carcinogen, but its effects on non-malignant respiratory disease (NMRD) are less clear. Cohorts exposed to multiple risk factors including radiation and toxic dusts conflate these relationships, and there is a need for clarity in previous findings. This systematic review was conducted to survey the body of existing evidence for radiation effects on NMRD in global nuclear worker cohorts. A PubMed search was conducted for studies with terms relating to radiation or uranium and noncancer respiratory outcomes. Papers were limited to the most recent report within a single cohort published between January 2000 and December 2020. Publication quality was assessed based upon UNSCEAR 2017 criteria. In total, 31 papers were reviewed. Studies included 29 retrospective cohorts, one prospective cohort, and one longitudinal cohort primarily comprising White men from the U.S., Canada and Western Europe. Ten studies contained subpopulations of uranium miners or millers. Papers reported standardized mortality ratio (SMR) analyses, regression analyses, or both. Neither SMR nor regression analyses consistently showed a relationship between radiation exposure and NMRD. A meta-analysis of excess relative risks (ERRs) for NMRD did not present evidence for a dose-response (overall ERR/Sv: 0.07; 95% CI: −0.07, 0.21), and results for more specific outcomes were inconsistent. Significantly elevated SMRs for NMRD overall were observed in two studies among the subpopulation of uranium miners and millers (combined n = 4229; SMR 1.42–1.43), indicating this association may be limited to mining and milling populations and may not extend to other nuclear workers. A quality review showed limited capacity of 17 out of 31 studies conducted to provide evidence for a causal relationship between radiation and NMRD; the higher-quality studies showed no consistent relationship. All elevated NMRD SMRs were among mining and milling cohorts, indicating different exposure profiles between mining and non-mining cohorts; future pooled cohorts should adjust for mining exposures or address mining cohorts separately.
INTRODUCTION
Radiation is well established as a carcinogen from studies of atomic bomb survivors (1, 2), radiotherapy patients (3), and nuclear workers (4). More recent studies have found associations between radiation and increased risk of some non-cancer diseases, particularly cardiovascular disease (5, 6). While the effects of radiation have been studied extensively in Japanese atomic bomb survivors who were exposed to a single acute dose of radiation, risks are less clear in populations more comparable to healthy U.S. workers exposed to chronic, low-dose rates of radiation (7). To clarify these risks, the Million Person Study (MPS) has constructed a population of individual U.S. nuclear worker cohorts (7, 8). However, exposures to both radiation and dusts in many of these cohorts imply a need for clarification in this topic (7, 9).
Non-malignant respiratory disease (NMRD) encompasses a broad range of infectious and non-infectious acute and chronic diseases impacting millions of people worldwide. Influenza and pneumonia are each transmissible, acute infections. Mortality from either cause is relatively rare in healthy, working adults, with a U.S. mortality rate for their combined burden in adults between 45–54 years of age of 4.7 per 100, 000 population in 2015. However, toxins such as tobacco and smoke exposures can increase the burden of mortality in these populations (10–13). Tuberculosis is an infectious, chronic disease responsible globally for millions of deaths annually (14). Although the disease burden is low in the U.S., occupational exposures that could cause weakened immune systems may increase the burden of disease and mortality in working groups, particularly in vulnerable communities (e.g., low-income populations) (15, 16). Chronic, non-infectious diseases such as chronic obstructive pulmonary disease (COPD) represent a greater disease and mortality burden in the U.S. than infections; the mortality rate from COPD in 2015 was 48.2 per 100, 000 (11). COPD is described by the World Health Organization as ‘‘an umbrella term used to describe chronic diseases that cause limitations in lung airflow’’ (17) and includes the sub-categories emphysema, chronic bronchitis, and asthma (11, 17). Finally, pneumoconiosis describes a set of chronic, non-infectious conditions known to arise after occupational exposures to toxins such as silica and asbestos (18). While average U.S. mortality from these causes is low, populations working with certain toxins are at increased risk (11, 18). Although risk factors vary by type of NMRD disease, broad risk factors include smoking, aging, lower socioeconomic status (SES), and occupational exposure to dusts and toxins (10, 12, 13, 15, 18, 19).
Increased NMRD mortality was observed in the atomic bomb survivors; however, this increase has been attributed to poor living conditions and toxic exposures before and after the bombings. Prior to the bombings, wartime conditions meant poor health for many individuals in Japan. The bombings themselves increased toxic exposures in addition to radiation, and the destruction meant many survivors were unhoused. The burden of infectious diseases such as pneumonia and influenza has been higher than expected in atomic bomb survivors, which is more likely attributable to these poor living conditions than radiation (5). Furthermore, misclassification of malignant mortalities as NMRD accounts in part for the increased rate at higher radiation doses in survivors (5, 20).
While the evidence that radiation is causally associated with increased NMRD mortality is unconvincing from the atomic bomb survivors’ studies, NMRD has been observed following high doses of radiation received to the lungs during radiotherapy (21), and animal and cellular models have shown epithelial changes from exposure to uranium and silica (22, 23). Nuclear workers are exposed to multiple types of radiation at chronic rates, as well as other toxins in the workplace such as uranium ore dust and silica (8). The exposure profile in the nuclear workplace may increase the burden of both NMRD incidence and mortality in nuclear worker populations.
As an initial step in assessing the potential burden of NMRD mortality in nuclear worker cohorts, a systematic review was conducted to determine the current state of knowledge of NMRD-associated mortality among global nuclear worker cohorts. This systematic review addresses two questions: whether permanent employees and regular contractors in the nuclear industry have an elevated risk of mortality from NMRD compared with a representative background population, and whether nuclear workers exposed to higher doses of uranium, radon, external photon radiation, and uranium ore dust have higher mortality from NMRD than similar workers exposed to lower doses of the same materials. The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2017 report addressed the necessity of systematic reviews in radiation epidemiology, particularly regarding studies with low dose and low dose-rate exposures, as often seen in nuclear workplaces. This report suggested that studies included in systematic reviews should be assessed for quality in terms of validity, precision, and relevance to the review topic, and it provided a quality review template (24). The present study therefore uses these UNSCEAR quality review guidelines as a tool to address the aims of this systematic review.
METHODS
For the purposes of this review, exposure was defined as work experience as a permanent employee or regular contractor in the nuclear industry with exposures to uranium, radon, or external photon radiation, and the outcome was defined as mortality from NMRD or any subcategory of NMRD, where NMRD is defined by International Classification of Disease (ICD) 6–10 codes (Table 1).
TABLE 1.
Non-Malignant Respiratory Disease as Defined by ICD Versions 6–10
| ICD version | ICD6 | ICD7 | ICD8 | ICD9 | ICD10 |
|---|---|---|---|---|---|
|
| |||||
| Codes | 241, 470–527 | 241, 470–527 | 460–519 | 460–519 | J00–J99 |
Specific criteria were pre-specified for inclusion of papers in the review. To include results for as many nuclear worker cohorts as possible, nuclear worker populations of any country, ethnicity, and sex were included. To ensure at least some members of all included populations had exposure, the study population had to be composed of only permanent employees or regular contractors in the nuclear industry. Only the most recent publication assessing non-cancer effects in a given population was included in the review to ensure the most updated results from each cohort were used. Papers were limited to those published in the years 2000–2020. Therefore, papers were included if they were the most recent assessment of either permanent employees or regular contractors in the nuclear industry, included an assessment of NMRD or any NMRD subcategory, and were published between January 2000 and December 2020. Since some cohorts were included in pooled populations, there is some crossover between studies. If an individual cohort was included as part of a pooled analysis, both studies were included in descriptive analyses, forest plots, and quality assessment. Separate inclusion rules were established for the meta-analysis and are described below.
Papers were selected using a three-step process: first, the National Center for Biotechnology Information (NCBI) PubMed database (‘‘PubMed’’) (25) was used to search for articles. Specific search terms were selected (see Table 2). The title, abstract, and key words were required to include terms relating either to radiation or uranium to ensure nuclear workers (and not other factory workers) were included. Likewise, noncancer outcomes were specified to limit the number of papers reporting only cancerous outcomes.
TABLE 2.
PubMed Search Terms
| Required topics: | Exposure | Outcome 1 | Outcome 2 |
|---|---|---|---|
|
| |||
| Required at least one of the terms in each category: | Uranium, radon, radium, radiation | Non-cancer, noncancer, non cancer | Lung disease, lung diseases, bronchitis, pneumonia, respiratory, respiratory tract diseases (Mesh term), COPD, chronic obstructive pulmonary, pulmonary |
| Disallowed terms: | Radiotherapy, RT, patient, patients, therapy | ||
The initial search returned 22 publications (Fig. 1). Papers were further excluded if any members of the population were exposed to uranium or radiation in a purely non-employment context. However, papers were retained if the population consisted of uranium miners to compare these workers to others in the nuclear industry; the inclusion of uranium miners in an initial review can justify their inclusion or exclusion in future systematic reviews or meta-analyses.
FIG. 1.
Article selection flow chart.
Bibliographies within reviewed publications were examined for additional citations that were added to the subsequent abstract review to maximize the number of relevant studies. These citations were subject to the same criteria as the papers returned from the original PubMed search.
After exclusions, all papers selected in the preceding steps were individually input into the ‘‘Cited by’’ feature of Google Scholar (https://scholar.google.com/) to perform a forward search. Articles citing the papers already included were selected per the same criteria as the papers returned from the original PubMed search. At this time, all papers were reviewed again to ensure no cohorts were duplicated unless they were part of a pooled study.
This search was performed independently by two reviewers: CMM and SCH. After the first two steps, reviewers compared articles to ensure the same list entered the forward search through Google Scholar. After the third step, reviewers compared their lists to ensure all articles had been appropriately retained or excluded. In the event of disagreement at the second and third steps, reviewers examined the article in question and determined together whether it met inclusion criteria.
Meta-analysis of overall NMRD in all cohorts and all non-mining cohorts was conducted for studies reporting excess relative risks (ERRs) per Sv or per 100 mSv using a random effects model with the heterogeneity estimate calculated using Mantel-Haenszel estimation (26). Studies were included if they were the most recent assessment of a population. If two studies were published in the same year, the larger study was selected. Results reported as ERR/100 mSv were scaled by a multiplier of 10 to ERR/Sv, assuming a linear model was used. When only P values were reported, a 95% confidence interval was calculated using methods described by Altman and Bland, assuming a normal distribution on the log scale (27). Ninety percent confidence intervals were scaled to 95% confidence intervals assuming a normal distribution. While meta-analysis was only conducted for NMRD ERRs, forest plots were generated and heterogeneity in terms of Cochrane’s Q and I-squared was calculated to compare overall NMRD excess relative risks (ERRS), NMRD standardized mortality ratios (SMRs), and COPD/emphysema SMRs across studies (confidence intervals were not corrected for this process, but are reported in the forest plots). Heterogeneity was calculated for all cohorts, cohorts excluding miners, and cohorts excluding miners and millers for studies reporting relevant SMRs in their overall population. While an overall meta-analytic estimate was calculated for ERRs, it was not estimated for SMRs since combining estimates with different external comparisons was not considered meaningful. All statistical analyses were conducted using Stata v15 (StataCorp LLC, College Station, TX).
Study quality was assessed by CMM and SCH using the criteria from the UNSCEAR 2017 report (24). In section III of this report, the committee describes the ‘‘main features affecting the quality of epidemiology studies,’’ and in section IV, they lay out guidelines for assessing study quality in terms of study population, exposure and outcome assessment, bias minimization, confounding control, analytical methods, uncertainty assessment, and reporting. Their list of questions in this section was used to note the strengths and limitations of each study included in the present review. Following this process for each study, a quality score from 1–3 was assigned for dosimetry, epidemiology, and statistics, where 1 is low and 3 is high (24). Previous expert ratings using the UNSCEAR 2017 criteria from NCRP Commentary 27 were applied to the extent possible in the present analysis to check consistency and to minimize reviewer bias (28). An overall score from 1–3 was assigned for the ability of the study to assess whether NMRD is correlated with radiation exposure, which factored in both the quality scores for dosimetry, epidemiology, and statistics, as well as the relevance of the included paper to the assessment of a causal association between radiation exposure and NMRD mortality (24). A study was assigned a 3 only if this relationship was investigated in detail and the average of the individual quality scores was greater than 2.5. The overall ability of the study to assess NMRD was assigned a 1 if the study only calculated SMRs (i.e., did not contain at least one internal dose-response analysis and could therefore not be used to assess a causal relationship between radiation and NMRD). The overall ability of the study to assess NMRD could be no greater than the mean of the lowest and highest quality scores in the other categories, rounding up.
RESULTS
Study Selection and Review
Figure 1 shows the selection process for studies in this review. In total, 37 unique articles were found through the PubMed search and citation review. Of these, 14 were excluded by title alone because they were either irrelevant or did not meet the search criteria, 3 were excluded by abstract because they included an identical population to a more recent paper or did not comprise a worker population, and 2 were excluded during text review because NMRD was not evaluated. Eighteen studies were input into Google Scholar for the forward search, in which an additional 53 papers were identified by title and abstract. Of these, 39 did not meet inclusion criteria. One of the original 18 studies selected was excluded because a more recent follow-up was identified in the forward search. After these selections and exclusions, 31 epidemiologic studies remained that matched the selection criteria and were reviewed (6, 29–58). Table 3 describes the characteristics of the included studies. Populations were largely from the U.S., Canada, and Western Europe, though the 15-Country Study also included Australian and Eastern European subcohorts (32), and one uranium mining population was from the Czech Republic (58). With the exception of the female Sellafield cohort (29), a majority of individuals in each study were male; some studies comprised all male populations. In 30 of 31 studies the cohorts were majority White, including the prospectively collected cohort of Colorado Plateau miners, though this study included over 700 Native Americans (38). Although 25 of the 31 studies did not include miners, the Grants miners and millers cohort (33), the Colorado Plateau miners (38), the Eldorado workers (40), the male Wismut miners (44), the male French Atomic Energy Commission miners (48), and the Czech Republic Uranium Miners (58) included at least a sub-population of uranium miners, though a separate NMRD effect measure was not reported for the Eldorado mining subpopulation (40). Studies classified their mortality outcome using the ICD version applicable at the time of death of cohort members. All studies considered at least one NMRD endpoint, and most considered a variety of other outcomes (e.g., site-specific cancers, CVD) not reported in this review. Only the male Wismut miners study focused explicitly on NMRD (44). Eighteen studies considered mortality from NMRD overall and its various subtypes (6, 30, 32, 33, 37, 40, 43–45, 47, 48, 50–52, 54, 56–58); however, 10 studies considered NMRD related mortality without interrogating any subtypes (29, 31, 34–36, 39, 41, 42, 49, 53) and 3 studies reported only NMRD subtype mortality (38, 46, 55).
TABLE 3.
Description of Included Papers
| Brief description | Source (Ref.) | Title | Study type | Sample size (no. NMRD deaths); person-years | Population | Dates of follow-up | Setting | Exposures | NMRD outcomes |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female Sellafield Workers | McGeoghegan et al. 2003 (29) | Mortality and cancer morbidity experience of female workers at the British Nuclear Fuels Sellafield Plant, 1946–1998 | Retrospective cohort study | 6,376 (60); 142,337 P-Y | Female workers employed at the British Nuclear Fuels Ltd plant at Sellafield between 1947 and 1998 | 1946–1998 | Sellafield, UK | Plutonium exposure, external photon radiation exposure | Mortality from non-malignant respiratory diseases |
| Male Colorado Plateau Millers | Pinkerton et al. 2004 (30) | Mortality among a cohort of uranium mill workers: an update | Retrospective cohort study | 1,484 (100); 49,925 P-Y | Men employed in seven uranium mills in the Colorado Plateau for at least one year on or after January 1, 1940 | 1940–1998 | Colorado Plateau, Four Corners region, U.S. | Worked in uranium mills (exposed to uranium, silica, and vanadium-containing dusts) | Mortality from non-malignant respiratory diseases; pneumonia; chronic and unspecified bronchitis; emphysema; pneumoconioses and other respiratory disease, asbestosis; silicosis; other pneumoconioses; other respiratory diseases |
| French Nuclear Workers (CEA & AREVA) | Telle-Lamberton 2007 (31) | External Radiation Exposure and Mortality in a Cohort of French Nuclear Workers | Retrospective cohort study | 29, 204 (33); 518,718 P-Y | French nuclear workers entirely monitored at either the CEA (excluding military applications) or AREVA (excluding miners) between 1950–1994 who worked over a year | 1968–1994 | France | External photon exposure (workers exposed to neutrons or other radionuclides excluded) | Mortality from all non-malignant respiratory diseases (excluding pneumonia) |
| 15-Country Study | Vrijheid et al. 2007 (32) | Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. | Retrospective cohort study | 274,312 (792); 4.1 million P-Y | Workers with adequate SES information from the 15-country nuclear worker study | Max: 1943–2000 (smaller for cohorts with less information) | 15 countries (Australia, Belgium, Canada, Finland, France, Hungary, Lithuania, Slovakia, Spain, Sweden, Switzerland, UK, U.S.) | High-energy photon radiation exposure | Mortality from non-malignant respiratory diseases (excluding pneumonia) and COPD |
| Grants Miners and Millers | Boice et al. 2008 (33) | A cohort study of uranium millers and miners of Grants, New Mexico, 1979–2005 | Retrospective cohort study | 2,745 (83); 63,395 P-Y | Male and female uranium milling and mining employees who worked in Grants, NM between 1955 and 1990 | 1979–2005 | Grants, NM, U.S. | Uranium ore exposure or uranium processing work | Mortality from non-malignant respiratory disease; bronchitis, emphysema, asthma |
| Male British Nuclear Fuels Workers | McGeoghegan et al. 2008 (34) | The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005 | Retrospective cohort study | 38,779 (1072); 1, 894,225 P-Y | Men ever employed at sites operated by British Nuclear Fuels plc between 1946 and 2002 | 1946–2005 | UK | External photon exposure | Mortality from all non-malignant respiratory diseases |
| French Nuclear Contract Workers | Guerin et al. 2009 (35) | Cancer mortality among French nuclear contract workers | Retrospective cohort study | 6,962 (6); 100,000 P-Y | Nuclear contract workers from 11 contracting companies monitored for exposure to ionizing radiation between 1967 and 2000 | 1967–2002 | France | External photon (gamma and X-ray) exposure | Mortality from all non-malignant respiratory diseases |
| French Nuclear Fuel Company Cohort | Metz-Flamant 2009 (36) | Mortality among workers monitored for radiation exposure at the French Nuclear Fuel Company | Retrospective cohort study | 9,285 (19); 206,623 P-Y | Nuclear workers employed over a year at AREVA NC between 1976 and 1994 specializing in the nuclear fuel cycle, excluding uranium miners | 1977–2002 | France | External photon (gamma and X ray) exposure, internal irradiation possible but not considered in analysis | Mortality from all non-malignant respiratory diseases |
| British National Registry for Nuclear Workers | Muirhead et al. 2009 (37) | Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers | Longitudinal cohort study | 174,541 (2117); 3,900,000 P-Y | British nuclear workers employed at a site monitored in the National Registry for Nuclear Workers between 1955 and 2001 | 1955–2001 | Great Britain (England, Ireland, Scotland, Wales) | External photon, neutron, and β exposures | Mortality from non-malignant respiratory diseases (not related to smoking), bronchitis, emphysema, and chronic obstructive disease |
| Colorado Plateau Miners | Schubauer-Berigan et al. 2009 (38) | Radon exposure and mortality among white and American Indian Uranium Miners: An update of the Colorado Plateau Cohort | Prospective cohort study | 4,137 (110); 120,437 P-Y | Miners (primarily white or Native American) in the Colorado Plateau cohort who worked in a uranium mine for at least one month and agreed to at least one health screening between January 1, 1950 and December 31, 1960 | 1950–2005 | Arizona, Colorado, New Mexico, and Utah | Radon decay product exposure | Mortality from pneumonia, influenza, COPD, asthma, pneumonoconiosis and other respiratory disease, asbestosis, silicosis, and idiopathic pulmonary fibrosis |
| French Uranium Processing Workers | Guseva Canu et al. 2010 (39) | French cohort of the uranium processing workers: mortality pattern after 30-year follow-up | Retrospective cohort study | 2,709 (21); 72,787 P-Y | Male workers employed for at least 6 months at the AREVA NC Pierrelatte plant between 1960 and 2005, excluding individuals with uranium mining experience | 1968–2005 | France | Uranium exposure, external photon exposure | Mortality from all non-malignant respiratory diseases |
| Eldorado Workers | Lane et al. 2010 (40) | Mortality (1950–1999) and cancer Incidence (1969–1999) in the cohort of Eldorado Uranium Workers | Retrospective cohort study | 17, 660 (158); 508,673 P-Y | Eldorado uranium workers first employed 1932–1980 from several uranium mines and a radium and uranium refinery and processing facility | 1950–1999 | Canada | Radon decay product exposure, external gamma-ray exposures | Mortality from all non-malignant respiratory diseases, COPD, and pneumonia |
| French Electric Company Staff | Laurent et al. 2010 (41) | Relationship between occupational exposure to ionizing radiation and mortality at the French electricity company, period 1961–2003 | Retrospective cohort study | 22,393 (9); 449,984 P-Y | French Electric Company permanent staff during 1961–1994 who were monitored for exposure to ionizing radiation | 1961–2003 | France | External gamma exposure, neutron exposure (if estimated neutron exposure exceeded 10% of their photon dose over the period 1967–2003) | Mortality from all non-malignant respiratory diseases |
| Sellafield Windscale Accident Workers | McGeoghegan et al. 2010 (42) | Mortality and cancer registration experience of the Sellafield workers known to have been involved in the 1957 Windscale accident: 50 year follow-up | Retrospective cohort study | 470 (30); 16,312 P-Y (comparison population: 2,926 (177); 95,362 P-Y) | Population of interest: Male Sellafield workers involved in tackling the 1957 Sellafield Windscale fire or its subsequent cleanup. Comparison population: Sellafield radiation workers at post at the time of the fire but not directly involved in the fire | 1957–2007 | England | Exposure to radionuclides of iodine, cesium, tellurium strontium, ruthenium, polonium, tritium, xenon, and plutonium | Mortality from all non-malignant respiratory diseases |
| Rocketdyne Workers | Boice et al, 2011 (43) | Updated mortality analysis of radiation workers at rocketdyne (Atomics International), 1948–2008 | Retrospective cohort study | 46,970 (148); 196,674 P-Y | Workers employed 1948–1999 at Rocketdyne (Atomics International) | 1948–1999 | California, U.S. | Internal exposure to radionuclides (primarily enriched uranium), external gamma exposure | Mortality from all non-malignant respiratory diseases excluding flu and pneumonia; emphysema |
| Male Wismut Miners | Kreuzer et al. 2013 (44) | Silica dust, radon, and death from non-malignant respiratory diseases in German uranium miners | Retrospective cohort study | 58,690 (2336); 2,168,455 P-Y | Male mining workers employed 1948–2008 at Wismut Company in Germany | 1946–2008 | Germany | Radon decay exposures, silica exposure | Mortality from all non-malignant respiratory diseases (not including silicosis or other pneumoconiosis); Infectious diseases, pneumonia; COPD |
| Fernald Workers | Silver et al. 2013 (45) | Mortality and ionising radiation exposures among workers employed at the Fernald Feed Materials Production Center (1951–1985) | Retrospective cohort study | 6,409 (151); 236,568 P-Y | Uranium workers employed at Fernald Feed Production Center for at least 30 days (1951–1985) | 1951–1985 | Ohio, U.S. | Internal uranium exposure, external gamma exposure, radon decay product exposure, thorium exposure, non-radiological exposures from the Fernald Feed site (including dusts) | Mortality from all non-malignant respiratory diseases, pneumonia, COPD, asthma, pneumonoconiosis and other respiratory disease, asebstosis, silicosis, and idiopathic pulmonary fibrosis |
| Male Port Hope Workers | Zablotska et al. 2013 (46) | Mortality (1950–1999) and cancer incidence (1969–1999) of workers in the Port Hope cohort study exposed to a unique combination of radium, uranium and γ-ray doses | Retrospective cohort study | 2,645 (67); 82,999 P-Y | Male uranium processing workers from Port Hope, Ontario, Canada, first employed 1932–1980, with no mining experience | 1950–1999 | Ontario, Canada | Radon decay product exposure, uranium exposure, gamma radiation exposure | Mortality from pneumonia; COPD and asthma |
| Male German Uranium Non-Miners | Kreuzer et al. 2015 (47) | Mortality from internal and external radiation exposure in a cohort of male German uranium millers, 1946–2008 | Retrospective cohort study | 4,054 (105); 158,383 P-Y | Men of the German uranium miner cohort study who worked between 1946 and 1989 in milling facilities but never underground or in open pit mining | 1946–2008 | Germany | Radon decay product exposure, long-lived radionuclides from uranium ore dust (kBqh/m^3), external gamma radiation exposure, silica dust | Mortality from all non-malignant respiratory diseases; pneumonia; COPD |
| Male French Atomic Energy Commission Miners | Rage et al. 2015 (48) | Mortality analyses in the updated French cohort of uranium miners (1946–2007) | Retrospective cohort study | 5,086 (110); 179,955 P-Y | Men employed as a miner for at least 1 year between 1946 and 1990 in CEA-COGEMA (AREVA) | 1946–2007 | France | Radon decay product exposure; long-lived radionuclides from uranium ore dust (Bq m^3h); external gamma exposure | Mortality from non-malignant respiratory diseases; silicosis; non-malignant respiratory diseases excluding silicosis |
| TRACY Cohort | Samson et al. 2016 (49) | Cancer and noncancer mortality among French uranium cycle workers: The TRACY cohort | Retrospective cohort study | 12,649 (88); 342, 58 P-Y | Male and female workers employed at least 6 months between 1958 and 2006 in French companies involved in the production of nuclear fuel | 1958–2006 | France | Long-lived radionuclide exposure, uranium exposure | Mortality from non-malignant respiratory diseases |
| French Uranium Enrichment Workers | Zhivin et al. 2016 (50) | Mortality (1968–2008) in a French cohort of uranium enrichment workers potentially exposed to rapidly soluble uranium compounds | Retrospective cohort study | 4,688 (49); 131,161 P-Y | Workers employed for at least 6 months between 1964 and 2006 in the AREVA NC, CEA, or Eurodif uranium enrichment plants, excluding workers with any uranium mining history | 1968–2008 | France | Uranium exposure, external photon exposure | Mortality from non-malignant respiratory disease; COPD |
| INWORKS | Gillies et al. 2017 (6) | Mortality from circulatory disease and other non-cancer outcomes among nuclear workers in France, the United Kingdom, and the United States (INWORKS) | Retrospective cohort study | 308,297 (5241); 8.2 million P-Y | Workers from France, UK, and U.S. who were employed in at least one of the study facilities for at least one year and who had dosimetry records indicating that they were monitored for external radiation exposure | 1944–2005 (differed by cohort) | France, UK, U.S. | External photon exposure | Mortality from non-malignant respiratory diseases; pneumonia; COPD including bronchitis, emphysema, and asthma; other respiratory diseases |
| French Cohort of Nuclear Workers | Leuraud et al. 2017 (51) | Mortality in the French cohort of nuclear workers | Retrospective cohort study | 59,004 (200); 1, 469,949 P-Y | Workers employed for at least one year at the CEA or AREVA NC (1950–1994) or EDF (1961–1994) who were badge-monitored for external radiation, excluding workers with an uranium mining history | 1968–2004 | France | External photon exposure | Mortality from non-malignant respiratory disease; COPD; asthma |
| U.S. Uranium Gaseous Diffusion Workers | Yiin et al. 2017 (52) | Mortality in a combined cohort of uranium enrichment workers | Retrospective cohort study | 29,303 (1194); 1,099,370 P-Y | Workers employed for at least one continuous year at gaseous diffusion plants (K-25 from 1948–1985, Portsmouth from 1956–2001, and Paducah from 1952–2003) | 1948–2011 | Tennessee, Ohio, and Kentucky, U.S. | Uranium exposure, external photon exposure | Mortality from all non-malignant respiratory diseases, pneumonia (except newborn), COPD, asthma, pneumonoconioses and other respiratory diseases |
| F-Millers Cohort | Bouet et al. 2018 (53) | First mortality analysis in the French cohort of uranium millers (F-Millers), period 1968–2013 | Retrospective cohort study | 1,291 (20); 41,470 P-Y | Permanent contract workers employed at least 6 months at one of 5 French uranium milling plants | 1968–2013 | France | Uranium exposure, radioactive isotope exposure, radon exposure, uranium ore dust, external photon radiation exposure | Mortality from all non-malignant respiratory diseases |
| Male French Miners plus Jouac | Rage et al. 2018 (54) | Low radon exposure and mortality among Jouac uranium miners: an update of the French cohort (1946–2007) | Retrospective cohort study | Jouac: 458 (not stated); 11,594 P-Y Post-1977 Jouac: 314 (not stated); 7,039 P-Y Extended cohort (miners + post-1977 Jouac): 5,400 (110); 186,994 P-Y | Men employed at least one year by the Société des Mines de Jouac hired between 1957 and 2001 or by CEA-COGEMA (AREVA) between 1946 and 1990 | 1946–2007 for CEA-AREVA; 1977–2007 for Jouac | France | Radon decay product exposure; long-lived radionuclides from uranium ore dust (Bq m^3h); external photon exposure | Mortality from all non-malignant respiratory diseases, non-malignant respiratory diseases excluding silicosis, silicosis |
| Canadian and German Uranium Processing Workers | Zablotska et al. 2018 (55) | Analysis of mortality in a pooled cohort of Canadian and German uranium processing workers with no mining experience | Retrospective cohort study | 7,431 (88); 270,201 | Workers employed during the ages of 15–75 at Port Hope, Canada (1950–1999) or employed at least 6 months at Wismut, Germany (1946–2008) who conducted milling, refining, and processing, had no mining experience. Port hope workers needed to have had their last contact after 1940, and to be alive as of 1950 (start of mortality follow-up) | 1950–1999 for Port Hope; 1946–2008 for Wismut | Canada, Germany | Uranium exposure, long-lived radionuclide exposure, radon progeny exposure, radon exposure, external photon radiation exposure | Mortality from COPD |
| French Nuclear Fuel Production Workers | Bouet et al. 2019 (56) | Analysis of the association between ionizing radiation and mortality in uranium workers from five plants involved in the nuclear fuel production cycle in France | Retrospective cohort study | 4,541 (41); P-Y not stated | Workers employed at least 6 months between 1958 and 2006 in one of five plants involved in the nuclear fuel cycle; subgroup of TRACY cohort with computerized medical files (uranium bioassay and other risk factors) | 1968–2013 | France | Uranium exposure, external photon exposure | Mortality from non-malignant respiratory disease; COPD; asthma |
| Male Mallinckrodt Workers | Golden et al. 2019 (57) | Updated mortality analysis of the Mallinckrodt uranium processing workers, 1942–2012 | Retrospective cohort study | 2,514 (176); 107,927 P-Y | White male workers employed at least 30 days at Mallinkrodt Chemical Works between 1942 and 1966 | 1942–2012 | Missouri, U.S. | Uranium exposure, radium exposure, beta exposure, external photon exposure | Mortality from non-malignant respiratory disease; bronchitis, emphysema, asthma |
| Czech Republic Uranium Miners | Kelly-Reif et al. 2019 (58) | Mortality and cancer incidence among underground uranium miners in the Czech Republic 1977–1992 | Retrospective cohort study | 16,434 (166); 231,499 | Male uranium miners employed in Příbram underground for at least 1 year between 1946 and 1976 and alive and residing in the Czech Republic in 1977 | 1977–1992 | Czech Republic | Uranium exposure, silica dust exposure, heavy metal exposure, radon exposure, radon decay product exposure, long-lived radionuclides, external photon exposure | Mortality from all non-malignant respiratory diseases, pneumonia and influenza; COPD (and allied conditions); pneumoconiosis including silicosis and asbestosis |
Studies included SMR analyses, regression analyses, or a combination (described in Table 4). All SMR analyses adjusted for age and calendar year; when applicable, SMR studies also adjusted for sex (30, 31, 33, 35, 36, 40, 43, 45, 49–52, 56) and race (33, 38, 43, 45, 52). Some SMR analyses stratified by type of worker (radiation vs. other) (29, 43), job type (53), location of workplace (52), categorical radiation dose (31, 38), duration of employment (30, 48, 49), sex (33, 49), SES (45), and smoking status (38) to model potential effects of these exposures. Regression analyses included Cox regression, generating hazard ratios (HR) (43, 57), and Poisson (6, 31, 32, 34, 40, 41, 44, 46–48, 51, 54–56), logistic (45), and general relative risk regression (29, 37, 52), generating excess relative risks (ERR) or relative risks (RR) depending on the methods used. With the exceptions of SMR trends, several sensitivity analyses conducted with Poisson regression using an external referent (48, 56), and a Poisson analysis where the outcome was SMRs (35), regression analyses were conducted using an internal referent and could adjust for more potential confounders than possible for SMR analyses. These analyses more frequently considered potential confounders such as employment duration, sex, and various types of exposures beyond the exposure of interest for the regression. A few of these analyses stratified by potential effect modifiers, including hourly vs. salaried position (34), age at risk (55), attained age (32), and non-radiation exposures such as silica dust (45, 47).
TABLE 4.
Quality Review of Included Papers
| Brief description | Source (Ref.) | Type of dosimetry | Mean dose | Comment on dosimetry | Statistical methods | Statistical models evaluated | Covariate adjustment factors |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sellafield Females | McGeoghegan et al. 2003 (29) | Annualized external doses for film badges used for external monitoring. Plutonium doses calculated using urinalysis and the Jones function. Doses from other known areas of employment included. | 12.8 mSv; 23.3 mSv in plutonium workers | Uncertainty in film badge quality | SMR calculations, adjusted for age and calendar year, with female England/Wales Comparison population for the years 1971–1994. Rate ratios calculated for SMRs between radiation workers and non-radiation workers; trend tests conducted. Different latency periods assessed. Linear excess relative risk model stratifying on age and calendar year with a 10-year lag, using cumulative external dose, but only cancers were assessed with this model. | Linear model. | Stratified on age, calendar year, and industrial worker status; but only cancers were assessed with this model. |
| Male Colorado Plateau Millers | Pinkerton et al. 2004 (30) | No dosimetry. Assessed early vs. late period of exposure to determine whether higher doses assumed in early periods may be relevant. | Unknown | No dosimetry | SMRs calculated using the NIOSH modified life table analysis system (LTAS) using US rates and rates for Colorado, New Mexico, Utah, and Arizona state rates. Assessed underlying cause of death and other contributing causes. SIRs were used for end-stage renal disease. | SMRs/SIRs only. | SMRs/SIRs specific to age, calendar time, sex, and (White) race; stratified (with trend tests) on duration of employment, time since first employment, and year of first employment. |
| French Nuclear Workers (CEA & AREVA) | Telle-Lamberton 2007 (31) | Annualized external doses for film badges used for external monitoring. Doses were lagged by 2 years for leukemia and 10 for all other causes of death. | 8.3 mSv; 16.9 mSv in exposed workers | Dosimetry data were validated for CEA (computer and paper records compared for a 1% random sample of the data and all doses above 3 mSv; corrections were made to doses above 3 mSv where appropriate) | SMR analysis with French national comparison (cancers only). Observed vs. expected rates with test of trend over dose-groups was the only analysis that included NMRD. For dose-response analyses, photon doses were classified into 11 groups and treated as time-dependent variables. Trend tests were calculated, and Poisson regression was conducted. | Only observed vs. expected (with trend tests) assessed NMRD; linear and log-linear models assessed cancers and broad noncancer causes of death. SMRs with confidence intervals only conducted for cancers and broad mortality groups. | SMRs adjusted for sex and 5-year age group. Fully stratified by total dose group, with test of trend. Poisson regression and tests for trend stratified on sex, 5-year age groups, 5-year periods, company, socioeconomic status, and duration of employment. |
| 15-Country Study | Vrijheid et al. 2007 (32) | Annualized film badges/TLDs; correction factor database used, with many efforts to harmonize data across countries. Colon, RBM, and lung doses estimated in the study. | 17.8 mSv (lung dose) | Dropped individuals with neutron and internal exposures, which reduces sample size considerably. Known errors in Canadian dosimetry data that biased studies. | Relative risk Poisson models with a 10-year lag (sensitivity analyses explored lag). Used mortality from COPD, liver cirrhosis, and external causes as sensitivity analyses to assess whether there was confounding from smoking, alcohol, and other lifestyle related factors, respectively. | Linear and log-linear models. | Stratified on sex, age and calendar period, facility, duration of employment, and SES. Interaction with dose tested for sex, cohort, facility type, and age. |
| Grants Miners and Millers | Boice et al. 2008 (33) | No dosimetry. Classified by potential exposure to radon (underground), uranium products (milling activities), or no/minimal exposure. Duration of employment also classified. | Unknown | No dosimetry. | SMRs calculated for the general US population and to the general New Mexico population (with race weightings of 90% White and 10% non-White). | SMRs only. | SMRs specific to age, calendar year, and sex; stratified by duration of employment and work experience at a uranium mine or mill. |
| Male British Nuclear Fuels Workers | McGeoghegan et al. 2008 (34) | Film badges for external exposure, a flag for internal exposure. Not much detail on dosimetry beyond that. | 53.0 mSv | Assume doses annualized since Poisson regression was used. | SMRs for males with a comparison population mortality statistic from the northwest region of England. Poisson regression for male radiation workers using 15-year lagged dose. | Linear model. | SMRs specific to age and calendar year. The main Poisson analysis is stratified on birth cohort, attained age, employment status, site of employment, and radiation exposure status (external only, internal, none). Supplementary Poisson models allow effect modification by attained age and use excess additive risk models. |
| French Nuclear Contract Workers | Guerin et al. 2009 (35) | Personal dosimeters issued monthly provided information on external radiation; data was only collected through 2000 to accommodate a 3-year lag period. | 33.5 mSv | Individuals with only one monthly record were excluded. | SMR calculated for the general French population. Their ERR analysis modeled SMR based on cumulative radiation dose (in categories where motivation is unclear) with a two-year lag period. | Linear model. Odd ERR analysis using SMR as the endpoint. | SMRs specific to sex, age, and calendar period. Stratified by year of study entry, age at study entry, specific exposure such as internal contamination, and time since hiring. They conducted test of trend for calendar period, attained age, and time-since-hire. Their ERR analysis adjusted for sex and attained age. |
| French Nuclear Fuel Company Cohort | Metz-Flamant 2009 (36) | Monitoring from AREVA or from CEA if individuals had prior exposures before coming to AREVA, lagged 2 years for leukemia and 10 years for all other outcomes. Internal irradiation possible but dosimetry not included. | 20.2 mSv; 28.9 mSv in exposed workers | Dosimetry data were validated for CEA (computer and paper records compared for a 1% random sample of the data and all doses above 3 mSv; corrections were made to doses above 3 mSv where appropriate) | SMR calculated for the general French population. Trend tests were conducted over dose and duration of employment groups. | SMRs and trend tests only. | SMRs specific to sex, 5-year age groups, and 5-year calendar intervals. Trends in SMRs adjusted for sex, attained age, calendar year, and SES. |
| British National Registry for Nuclear Workers | Muirhead et al. 2009 (37) | Personal dosimeters provided information; correction factors were applied to arrive at more accurate dose estimates. Doses were lagged 2 years for leukemia and 10 years for all other outcomes. | 24.9 mSv | Doses varied by individual site. Estimates for internal doses were not generally available, but workers monitored for exposure were identified. | SMRs calculated for the general population of England and Wales; tests for trend were conducted by dose. Internal dose-response analyses were conducted. Excess relative risk per Sv was estimated by maximum likelihood methods using categorical dose, with test of trend and confidence intervals calculated. | Linear model. | SMR analyses were fully stratified to account for social class (using industrial classification as a surrogate for the workers); these results not reported for NMRD. Dose-response analyses adjusted for age, sex, calendar period, industrial classification, and first employer. |
| Colorado Plateau Miners | Schubauer-Berigan et al. 2009 (38) | Exposure levels recorded in WLM, which was categorized into 9 exposure levels. Daily rates of exposure were interpolated for each worker. Exposure to radon progeny was included in these interpolations. | 794 WLM | Doses categorized to be used in SMR/SIR analyses. | SMR analyses conducted with LTAS.NET, a modified life table analysis program. Expected mortality rates were calculated for White miners using combined White rates in Arizona, Colorado, New Mexico, and Utah, and for Native American miners using combined non-White rates for Arizona and New Mexico. SIR analyses were conducted for ESRD, with a US background incidence rate. Internal comparisons were conducted by using directly standardized rate ratios: miners exposed to higher radon levels (≥ 120 WLM) were compared to those exposed to lower radon levels (< 120 WLM). | SMRs/SIRs only. | SMRs specific to age, race, and calendar year; stratified by cumulative radiation exposure and smoking category. |
| French Uranium Processing Workers | Guseva Canu et al. 2010 (39) | External exposure assessed with individual dosimeters; external exposure was used as a proxy for internal exposure. | Not reported | Internal alpha emission from uranium intakes were measured at the Pierrelatte in vitro with fecal and urine samples and in vivo, but these records were not computerized so dosimetric software couldn’t be used at this time. | SMRs calculated for the general male French population for 1968–2005. Mortality rates of the four regions closest to the Pierrelatte plant (Gard, Drôme, Ardèche, and Vaucluse) were used as a second reference. Due to uneven deposition of uranium in the body, for deaths from target organs localized in a priori known uranium-target organs (including the lung), the SMRs for national mortality rates were calculated for: first year of Pierrelatte hire, SES, employment duration, time since recruitment, radiation exposure monitoring status, and cumulative external radiation dose. Trends and heterogeneity tests were conducted for each of these variables. Only the overall SMRs were calculated for NMRD. | SMRs only. | SMRs specific to age and calendar period. |
| Eldorado Workers | Lane et al. 2010 (40) | Gamma ray doses calculated from average film badge doses for the sample of workers who wore them and time on the job. Radon decay measurements were calculated differently by cohort. Port Radium used workplace monitoring augmented with ventilation data to calculate average WLMs which were applied to employees based on duration of work. Beaverlodge had individual radon decay measurements beginning in 1966; before that, methods similar to Port Radium were used. Port Hope had no radon decay information available and used dose reconstruction based on quantities of radium present in the plant in ore and at various refinement stages, measured radon emanation from various radium-bearing materials, and building air volumes and estimates of air exchange rates | 100 WLM | Doses from other sites workers may have worked estimated or recorded and included. Dose used in regression analysis was the person-year weighted mean dose in each cross-classified cell. | SMRs and SIRs (cancer) compared to the general Canadian population. For internal analyses, grouped Poisson regression was conducted using time-dependent cumulative WLM and a 5 year lag. Internal analyses were also used to assess gamma rays instead of radon exposures. | Linear model. Quadratic term tested only for lung cancer. | SMRs/SIRs specific to sex, age, and calendar year at risk. Person years were cross classified by age at risk, sub-cohort, total duration of employment, age at first exposure, cumulative exposure, and years since first exposure. Further adjustment was only made in lung cancer models. Models did not adjust for additional radiation types (e.g., radon analyses did not include gamma doses). |
| French Electric Company Staff | Laurent et al. 2010 (41) | Film badges for external exposure; these were calibrated differently over time and were harmonized for the analysis. Organ doses were estimated for the RBM, lung, and colon. Flag for whether neutron dose exceeded 10% of the photon dose between 1967–2003. | 21.5 mSv | Uncertainty in how dose calibration was harmonized and in neutron doses (for which a flag was used due to uncertainty). | RR per 100 mSv calculated for dose categories using Poisson regression, lagged by 2 years for leukemia and 10 years for all other causes of death; main analysis excluded workers with neutron exposures. Sensitivity analyses considered alternative lag times and alternative stratification strategies. | Log-linear model. | Analyses stratified on age, calendar period, and sex (except when sex was studied as a potential confounder); further assessed potential confounders by describing mortality and exposure to photon radiation and using a test of heterogeneity: gender, educational and job levels at hiring, first region of employment, length of employment and potential for substantial exposure to neutrons. Main Poisson analyses stratified on age, gender, calendar period and educational level at hiring (except for leukemia because of SES confounding) |
| Sellafield Windscale Accident Workers | McGeoghegan et al. 2010 (42) | Annualized film badge readings for external dose, with doses from other sites at which workers were employed included. External dose in 1957 was used as a proxy for internal dose (high dose = above median dose in fire or comparison cohort, respectively). | 390.2 mSv in radiation exposed fire workers; 538.4 mSv in fire worker high dose group; 277.4 mSv in the fire worker low dose group | Doses not included for internal exposures. Film badge doses were used for regulatory compliance, so dosimetry may be inaccurate. | SMRs used to separately compare the 470 fire workers and the 2926 non-fire workers with the population of England and Wales and with the population of Northwest England for the study period. Rate ratios were used to compare the mortality (and cancer morbidity) rates of the fire workers with the non-fire workers. Rate ratios were also calculated for high vs. low dose workers. Multivariate matching using propensity scores conducted to match fire workers to the nearest individual in the non-fire cohort on age at entry, cumulative internal plutonium lung dose and external dose on 10 October 1957, industrial status, radiation status and age on 10 October 1957. Average exposure effect (AEE) for fire workers calculated via the ‘‘Matching’’ package in R after this matching; AEE was not calculated for NMRD. | SMRs, rate ratios, and average exposure effect calculated, but none of these represent a dose-response. | SMRs stratified on age and calendar year. Fully stratified analyses conducted by decade after the fire for the fire cohort and non-fire cohorts. Rate ratios stratified on attained age, calendar year, final radiation status and industrial status (paid monthly if non-industrial; weekly if industrial). |
| Rocketdyne Workers | Boice et al, 2011 (43) | Film badges/TLDs for external exposure; ~38% monitored for internal exposure to 14 isotopes with >30, 000 bioassays. Doses calculated for 16 organs/tissues. | 19.0 mSv (lung dose) | Dose information for other sites collected and included. Uncertainties include exposure geometry, type of dosimeter used, and energy of the photons. | SMR and internal (Cox) analyses conducted. SMR comparison group was the age-, sex-, and race-matched California population in the same time period. Age at observation was used as the time scale. Dose treated as a continuous variable. | Cox proportional hazards model. | Analyses controlled for year of birth, year of hire, sex, pay type (hourly/salary), duration of employment and work as a test stand mechanic. |
| Male Wismut Miners | Kreuzer et al. 2013 (44) | Radon exposures reconstructed using a detailed job exposure matrix, which integrated calendar year, place of work, mining facility/mine shaft, and job type. No individual dosimetry was performed. Systematic area measurements were taken by the Wismut company beginning in 1955; before that, expert ratings were used to determine radon levels. | 280 WLM | No individual dosimetry was performed for these miners. | Internal Poisson regression models were used, with cross-classifications made by age, calendar year, and cumulative radon exposure (categorical). A lag of 5 years was used in all analyses, though sensitivity analyses used lags of 0, 10, and 15 years. A sensitivity analysis was conducted expanding the definition of COPD to include contributing (as well as underlying) causes of death. | Linear model. | Silica exposure and duration of employment were controlled for in sensitivity analyses only. |
| Fernald Workers | Silver et al. 2013 (45) | Internal dose assessments using uranium concentration data from 1952 onward, with the latest (at the time) biokinetic and dosimetric models: urine bioassays were used to calculate organ doses. Organ doses were calculated for the lung, pancreas, lower large intestine, kidney and RBM. Film badge doses were used for external exposure; whole body equivalent dose was reported. External dose from other facilities was included. Hornung et al. methods used to estimate exposure to radon decay products using a matrix of radon decay product concentrations and employment information; these exposures are recorded in WLM. Thorium exposures were qualitatively assessed by work assignment examination and recorded with a dichotomous flag. | Mean lung dose ranged 34.5 microGy for hourly non-White females to 1552 micgroGy for hourly White males. | Heterogeneous sources of radiation exposure were not combined into a single metric. Radon decay product dose estimates involve considerable assumptions about worker location, seasonality, product levels inside and outside of buildings, etc. | SMRs compared to the general US population from 1940–2004; for several outcomes not available before 1960 used US rates from 1960–2004. Since Fernald is in Ohio, Ohio mortality rates were also used. Directly standardized rate ratios (SRR) were used to investigate differences by pay code (males only; female data was too sparse). Regression models assessed White males only; risk sets were defined using incidence density matching by attained age. Conditional logistic regression was used to estimate ERRs (equivalent to Cox). Univariate and multivariable models; multivariable models included both organ dose and external dose (and radon decay products when investigating lung cancer and NMRD). 0-, 10-, and 15-year lags investigated (only 10-year lag when data was sparse); leukemia used 0-, 2-, and 5-year lags. | Linear model preferred. Categorical and restricted cubic spline models also investigated. Log-linear models were applied where linear estimates were not within bounds. | SMRs specific to sex, age, race, and calendar year. ERR models adjusted for other types of exposure, age, birth year, and pay code. Non-radiologic confounders were assessed in univariate and multivariable models where there were at least 30 cases. Thorium data was only used in lung cancer and COPD analyses. Confounding was assessed with 20% change in estimate models adding in a covariate at a time. |
| Male Port Hope Workers | Zablotska et al. 2013 (46) | Gamma ray doses were recorded from badges when available (available for all workers starting ~1970) and calculated from average dose-rates and time on the job otherwise. No radon decay information was available; used dose reconstruction based on quantities of radium present in the plant in ore and at various refinement stages, measured radon emanation from various radium-bearing materials, and building air volumes and estimates of air exchange rates. | Radon decay products: 15.9 WLM; photons: 134.4 mSv | Both the company and Health Canada had dose records; company records were used if available. Workers further classified as primarily uranium or radium workers. | SMRs and SIRs (cancer) compared to the general Canadian population. Poisson regression used for internal analyses, ERRs calculated. Analyses conducted separately for internal and external exposures; sensitivity analyses used both in one model. All outcomes lagged by 5 years; sensitivity analyses tested 10- and 15-year lag times (for CVD outcomes only). | Linear model. | SMRs/SIRs specific to age and calendar year. Stratification on age at risk, calendar year at risk, total duration of employment, age at first exposure, cumulative exposure, and years since first exposure. |
| Male German Uranium Non-Miners | Kreuzer et al. 2015 (47) | Doses reconstructed using a job-exposure matrix that assigned an average annual exposure to each facility, workplace, and job type; applied to workers using payroll information: annualized workplace, facility, and job type, plus duration of employment. Ambient measurements were used to estimate dose. Retrospective estimates considered production, milling techniques, spatial arrangement, and available measurements. A software program calculated organ doses to the lung, stomach, kidney, liver, and RBM separately for each exposure type. | Radon progeny: 8 WLM; long-lived radionuclides: 3.9 kBqh/m^3; photons: 26 mSv | Estimates were used for photon, radon progeny, and long-lived radionuclide exposures. Exposure to silica dust was measured and estimated for workers, as well. | SMRs for the period 1970–2008 (missing 46% of deaths prior to 1970) compared to the general population in Eastern Germany. Poisson regression for relative risk analyses to assess the effects of cumulative exposures to radon progeny, external gamma radiation, LLR and silica dust on pre-defined causes of death. A 5-year lag was used. Sensitivity analyses with lag times of 0, 10, and 15. Regression was limited to outcomes with at least 12 deaths. For three cancer outcomes, sensitivity analyses adjusting for other types of exposure. | Linear model. | SMRs specific to age and calendar year. Poisson analyses were stratified on calendar year and age. |
| Male French Atomic Energy Commission Miners | Rage et al. 2015 (48) | For radon, retrospective dose reconstruction by an expert group for the years 1946–1955 using environmental measurements with job type, place of work, and duration of work. Individual monitoring began in 1956 and was improved in 1983. For long-lived radionuclides, dose reconstruction from ambient measurements was used for 1959–1982. From 1983 onward, individual measurements were collected. For photons, individual film badge monitoring began in 1956. | Full cohort: 36.6 WLM radon; post 1955 subcohort: 17.8 WLM radon, 1.64 kBq/m^3 long-lived radionuclides, 54.9 mGy photons | Unclear whether any estimates of photon exposures occurred before 1956. A post-1955 cohort was created to assess the time period during which all exposures were recorded individually. | SMRs compared to the general French male population. Tests for SMR trend over radon exposure categories and duration of employment categories were conducted (and for all exposure types in the post-1955 cohort). Poisson regression with a lag time of 5 years. Poisson regression only conducted for causes of death with an SMR > 1 or a significant test of trend. | Linear model. | SMRs specific to age and calendar period. Poisson stratification on calendar period, attained age, and duration of employment. The post-1955 cohort adjusted for other types of exposure (radon, long-lived radionuclides, photons). |
| TRACY Cohort | Samson et al. 2016 (49) | No dosimetry. Time since hire, time since end of employment, and total duration of employment were used as pseudo-surrogates. | Unknown | No dosimetry. | SMRs compared to the general French population. Only assessed causes of death with at least 5 occurrences in the cohort. Primary analyses considered men and women grouped together. | SMRs only. | SMRs specific to sex, age, and calendar period. Further stratification in sensitivity analyses for time since hire, time since end of employment, total duration of employment, and attained age. Men and women assessed separately in a sensitivity analysis. A sensitivity analysis stratified by company group for step in the uranium cycle (conversion, enrichment by gaseous diffusion and manufacturing of oxide fuel). |
| French Uranium Enrichment Workers | Zhivin et al. 2016 (50) | For external exposures, annualized doses from individual monitors were used. For internal exposures, a job-exposure matrix (JEM) was used that determined exposure to soluble uranium (ICRP definition; AREVA, CEA, and Eurodif) and for Eurodif distinguished between natural, depleted, and enriched uranium. JEM used to assign exposure levels of frequency and intensity of an exposure on a four-level scale for each hazard. A multiplicative product of the frequency, intensity, and duration of employment was used to derive an individual score for analyses. | External median dose: 0.8; external median dose among monitored workers: 0.75 mGy | AREVA JEM validated with 64% sensitivity and 80% specificity. Due to identical nature of work, AREVA JEM assigned to CEA. Eurodif JEM had more information on current occupational exposure limits, which validated intensity and frequency of exposure. AREVA and Eurodif JEM created with the same strategy | SMRs compared to the general French population. Within-cohort analyses using Poisson regression on grouped data, with time-dependent exposure quartiles (unexposed, low exposed, medium exposed, high exposed) grouped using quartiles of each cumulative exposure score weighted by person-years. Log-linear risk models used to obtain RRs, and linear models used to obtain ERRs. Only SMRs conducted for NMRD. | Only SMR assessed NMRD; log-linear RR model and linear ERR model assessed cancers and specific noncancer diseases. | SMRs specific to sex, age, and 5-year calendar period. Poisson analyses stratified person-years on sex, age, calendar period, socio-professional status at hire, subcohort, and 5-year lagged cumulative exposures to soluble uranium, external gamma radiation, trichloroethylene, heat, and noise. RR and ERR models stratified on age, sex, attained age, calendar period, SES at hire, and subcohort. |
| INWORKS | Gillies et al. 2017 (6) | Annualized dosimetry based on film badges/TLDs. Dose expressed in Sv defined as equivalent dose at a 10 mm tissue depth. Phantoms employed to reconstruct dose using different geometries and energies. Uncertainty in measurements examined to normalize exposures for bias. | 25.2 mSv | Neutron exposures expressed as a time-varying indicator variable. Incorporated radionuclide exposures recorded as an indicator variable. Too little information available on early doses to evaluate impact of ‘‘missed’’ doses. | Poisson regression using time-varying cumulative dose. Fully parametric, semiparametric, and stratified models were considered for the baseline hazard function. Main analyses used a linear model. Cumulative dose was lagged by 10 years in the main analysis. Sensitivity analyses compared LQ, quadratic, and linear exponential models. | Linear model for NMRD (other shapes tested for circulatory disease) | Analyses assessed effect modifiers: employer/facility of employment, sex, SES, duration of employment, and attained age. To assess residual confounding, restricted analyses to those with known SES and detailed individual monitoring data attributable to site during study. Further sensitivity analyses assessed occupational neutron and internal exposures. Age at exposure and time since exposure further interrogated, as was lag period. None of these sensitivity analyses were applied to NMRD, but their application to circulatory diseases helps determine whether confounding was present. |
| French Cohort of Nuclear Workers | Leuraud et al. 2017 (51) | Annualized photon dosimetry lagged 10 years based on individual monitoring and validated for a percentage of the cohort (validated in CEA, EDF). | Full cohort: 18.4 mSv; Exposed workers: 25.7 mSv | Neutron exposures expressed as a time-varying indicator variable (before 1967: year any positive neutron dose first recorded; after 1967: year when estimated neutron dose exceeded 10% of total external dose). Internal radionuclide dosimetry not conducted for the present analysis. | SMRs compared to the general French population. For significant excess of death, calculated SMRs over professional categories (employing company, socioeconomic status, year of hiring, age at hiring, duration of employment, and cumulative dose) — didn’t include NMRD. Poisson regression for dose-response analysis; ERRs calculated. Sensitivity analyses were used to investigate the influence of adjustment for the neutron flag. | Linear model. | SMRs specific to calendar period, sex, and 5-year age intervals. ERRs stratified by calendar period, sex, 5-year age intervals, company (where worker spent a majority of time employed), duration of employment, and SES. |
| U.S. Uranium Gaseous Diffusion Workers | Yiin et al. 2017 (52) | For internal dosimetry, annual absorbed organ doses were calculated for all individuals presumed exposed to uranium using bioassay data combined with facility- and department-specific enrichment information estimated from the ratio of activity concentration to gravimetric concentration. For external dosimetry, annual absorbed organ dose was calculated from individual dosimetry information extracted from facilities, the U.S. Department of Energy’s (DOE) Radiation Exposure Monitoring System, the Nuclear Regulatory Commission’s (NRC) Radiation Exposure Information and Reporting System, and previous studies. Some workers at K-25 had non-negligible X rays that were included in external organ doses. 10-year lag used in main analyses. | Internal dose (lung): 0.07 mGy; External dose (lung): 40 mGy (with X rays), 4.5 mGy (without X rays) | Internal dosimetry was imputed for workers presumed exposed who did not have reported records. Job-exposure matrices were used for external photon dose during periods where personal monitoring was incomplete. Non-radiological exposures from nickel and trichloroethylene calculated from job exposure matrices for workers with probable exposures. 5- and 15-year lags used in sensitivity analyses. Correlation analysis was conducted between types of doses and duration of employment. | SMRs calculated using the NIOSH modified life table analysis system (LTAS) using US rates. A sensitivity analysis calculated SMRs with state-specific rates (Tennessee, Ohio, and Kentucky, as appropriate), and the three states combined for the pooled cohort. Internal regression analyses for uranium dose used incidence density sampling to draw risk sets matched on sex, race, birth date (within 5 years), and facility. General relative risk models analogous to Cox models used to calculate excess relative risk. ERR/mGy was restricted to individuals whose exposures were within the 95th percentile of uranium dose. Sensitivity analyses used a categorical exposure and calculated risks relative to an unexposed group (including lag). | Linear model. | SMRs specific to sex, race (White vs. other), 5-year age categories, and 5-year calendar period. Confounding in internal regression analyses was assessed by including one covariate at a time in the main model and examining the percent change relative to the width of the confidence interval; included covariates were external radiation with or without X rays, nickel, trichloroethylene, and duration of employment. |
| F-Millers Cohort | Bouet et al. 2018 (53) | No dosimetry. Attempt made to approximate dosimetry using type of activity at work. | Unknown | No dosimetry. | SMRs compared to the general French population or to French regional populations. Only assessed causes of death with at least 5 occurrences in the cohort. Analyses considered men and women grouped together. | SMRs only. | Analyses examined the effect of using different reference populations; national or regional background populations (French departments where the sites were located and adjacent to sites) were used. For regional analyses, SMRs were stratified by department (extrapolated to yearly rates for 1969–1974). Fully stratified analyses by socio-professional category (blue collar and other) and type of activity at hire (manufacturing, maintenance, other). Also conducted analyses adjusting for work site at hiring, time since hiring, the duration of employment, and attained age, with the last three considered time-dependent. For NMRD analyses, only stratified by type of activity at work (at time of first hire). |
| Male French Miners plus Jouac | Rage et al. 2018 (54) | CEA-AREVA: Radon monitoring from 1983 with reconstruction from 1946–1955 for the CEA-AREVA cohort; uranium exposure monitoring from 1959 with retrospective reconstruction prior; external monitoring from 1956 with reconstruction prior. Jouac: Exposure information from 1977 onward. | Post-1977 Jouac: 3.9 WLM among exposed miners; Extended cohort (miners + post-1977 Jouac): 35.1 WLM among exposed miners | Unclear if Jouac dosimetry includes only radon or also includes external and uranium monitoring. | SMRs compared to the general French male population. ERRs per WLM for radon exposure using Poisson regression with non-exposed miners as an internal reference group. | Linear model. | Cross-classification in DATAB by 5-year calendar period, attained age, duration of employment, cumulative radiation exposure (different categories for full vs. Jouac cohort). Baseline risk for Poisson regression stratified by calendar year and attained age. A sensitivity analysis considered lung cancer SMR variation by calendar year, attained age, and duration of employment (extended cohort and Jouac cohort). |
| Canadian and German Uranium Processing Workers | Zablotska et al. 2018 (55) | Port Hope: Individual annual exposures in WLM were assigned from calculated WL given each type of workplace, proportion of employees in each occupation, and proportion of time spent in each type of workplace by employees in each occupation. RDP estimates were calculated with this information, as well. Some individuals had film badges; for those that did not, personal gamma ray doses were calculated from the average dose-rates and time on the job. Wismut: Information from payroll on date of hire, date of term, and for each year, type of workplace, facility, and job type was used to create a job exposure matrix. The matrix was used to assign an average annual exposure value to each facility, workplace, and job type for radon progeny, long-lived radionuclides, and gamma irradiation. All doses lagged 5 years. |
All Workers RDP: 10.0 WLM; All workers gamma: 61.5 mSv; Port Hope Males RDP: 13.3 WLM; Port Hope Males gamma: 116.3 mSv; Port Hope Females RDP: 4.9 WLM; Port Hope Females gamma: 36.2 mSv; Wismut Males RDP: 8.5 WLM; Wismut Males gamma: 30.8 mSv; Wismut Females RDP: 7.4 WLM; Wismut Females gamma: 31.1 mSv | In Port Hope, any individuals who worked with radium were considered radium workers; otherwise, they were considered uranium workers. Working level estimates were calculated from quantities of radium present in the plant in ore and at various stages of refinement, measured radon emanation rates from radium-bearing materials, building air volumes, and estimates of air exchange rates. 10-, 15-, and 20-year lags tested in sensitivity analyses. Exposure rate for effect modification analyses estimated as a time-dependent ratio of cumulative dose and cumulative duration of employment. | Grouped Poisson regression analysis used to estimate ERRs. In main analyses, gamma ray and RDP results estimated separately. In sensitivity analyses, gamma and RDP included in the model simultaneously. Deviance used to assess model fit. Most sensitivity analyses only addressed CVD. | Linear model. A series of categorical analyses were conducted to examine the shape of the dose-response. When gamma and RDP were assessed together, multiple model fits were tested. | Person-years at risk were stratified on 5-year age interval, 5-year calendar interval, total duration of employment (< or ≥6 months), and cumulative exposure (separately for RDP and gamma). Background rate was stratified to include potential confounders, which were included in the model if they produced a ≥10% change in the point estimate of the ERR. Potential confounders included age at risk, calendar year, duration of employment, and predominant exposures to radium/uranium (Port Hope) and cumulative exposures to long-lived radionuclides, silica or fine dust and arsenic (Wismut cohort). Effect modification of age at risk, exposure rate, age at first exposure, and duration of employment tested with time-window analyses. |
| French Nuclear Fuel Production Workers | Bouet et al. 2019 (56) | Annual organ doses calculated from internal exposures, estimated from bioassay data using biokinetic models and dosimetric models recommended by the ICRP. A maximum likelihood approach estimated intake. For workers with doses less than the threshold, two doses were calculated: zero dose (‘‘operational dose’’), and a maximum value calculated by setting the last bioassay data in the period as equal to the value of the threshold while other bioassay data remained censored. External gamma exposure from individual monitoring was annualized for whole body doses. Doses were lagged by 10 years. | Internal dose (lung): 4.22 mGy; External dose: 11.12 mGy (‘‘operational dose’’), 10.90 mGy (‘‘maximum dose’’) | Uranium bioassay was available for 83% of workers; information on physiochemical forms of uranium compounds reconstructed from career history files and documents describing workplace operations. Information on abnormal events and accidental intakes by inhalation or wounds collected from medical files and from collective incident registries documenting the specific circumstances of unusual events. For external, some workers only had external doses for some years and were not included in associations between external dose and mortality. Sensitivity analyses lagged doses by 5 and 15 years (AIC assessed lags). | SMRs calculated for the general French population. Poisson regression used to calculate RRs and ERRs. The ‘‘operational’’ dose was used in main analyses with sensitivity analyses to explore the ‘‘maximum’’ dose. Miners and millers were excluded from dose-response analyses. Assessed three subgroups of workers: 1) workers who at some point only had external dose available, 2) subset of subset 1 who had computerized medical files, 3) all workers whose medical records were computerized at the time of the study (excluded subjects recruited at companies before data were sufficiently complete). These three groups were used for external dose-response and internal dose-response analyses with adjustment for potential confounders as data completeness allowed. Only SMRs and external dose-response analysis (with Subgroup 1) assessed NMRD. | Log-linear risk models were used to calculate RRs and explore shape. Linear risk models were used to calculate ERRs; linear-quadratic and quadratic functions were also tested. | SMRs adjusted for 5-year age category, 5-year calendar interval, and sex. Person-years cross-classified on sex, age, year of birth, SES, and categories of lagged cumulative external and internal radiation. A number of medical variables were available for personnel with complete medical records (smoking, BMI, high blood pressure, elevated glycemic value) and were used in internal analyses, which did not include NMRD analyses. |
| Male Mallinckrodt Workers | Golden et al. 2019 (57) | Annualized external organ doses were based on film badges from 1945 onward; an algorithm assigned doses for years prior (20.8% working years). Annualized organ doses for medical X-rays required annually for workers were calculated based on X-ray parameters. Annualized internal organ doses were calculated using ICRP recommended biokinetic models based on urine samples, radon breath samples, and ambient radon measurements. Total dose included annualized organ dose from all dose sources, lagged 2 years for leukemia and 10 years for all other outcomes. | 69.9 mGy | Doses from other sites of work were included (REMS, REIRS, Landauer, and other DOE sites). Uncertainty from 1942–1944; neutron doses unknown. Silica dust exposure reconstructed, as well. Primary analysis used a dose weighting factor of 1; sensitivity analyses used 10 and 20 for alpha doses (uranium and radium). | SMRs calculated for the US White male population for underlying cause of death (additionally, SIRs calculated for kidney disease incidence). Cox proportional hazards models with age as the underlying time scale estimated HRs and ERRs, which included underlying and contributing causes of death. Dose was a categorical variable with categories based on dose distribution for organ of interest. Cox analyses for dust were also conducted (not for NMRD). | Cox proportional hazards model. | SMRs adjusted for 5-year age category and 5-year calendar interval (race and sex already considered as this is just White males). Cox regression adjusted for year of birth and pay type. Sensitivity analyses adjusted for year of hire, duration of employment, SES, and cumulative dust exposure (not for NMRD). |
| Czech Republic Uranium Miners | Kelly-Reif et al. 2019 (58) | No dosimetry. | Unknown | No dosimetry. | SMRs calculated for the male Czech Republic population. Expected cases calculated with a lognormal Poisson model. SIRs calculated for cancers, and causal mortality rates (CMRs) calculated for select causes of death (not for NMRD). Random effects were used to assess changes in standardization over time (testing for heterogeneity of person-time distribution by age across calendar periods). | SMRs only. | SMRs adjusted for 5-year age category and calendar period. Age-specific data at different calendar periods were used to examine birth cohort effects, age effects, and period effects. Duration of employment, time since exposure, age at exposure, birth cohort, and hire cohort were examined. Only basic adjustment (age and calendar year) for NMRD. |
Standardized Mortality Ratios
Supplementary Tables S1a–S5b (https://doi.org/10.1667/RADE-21-00014.1.S1) display the results of each analysis in a given study by disease category, analysis type, and exposure type, with studies listed in order of publication. SMR analyses were inconsistent with a mix of population-equivalent, reduced NMRD mortality, and elevated NMRD mortality in nuclear workers compared with respective background populations. NMRD SMRs were most frequently decreased compared with general populations, but this varied by study, subpopulation, and specific outcome (29, 34–37, 39, 42, 43, 47, 49–53, 56–58). Figure 2 displays a forest plot comparing SMRs (with 95% confidence intervals) for each publication reporting overall NMRD mortality risk. This figure highlights the predominantly decreased SMRs and the inconsistency of reported SMRs across cohorts as shown by the Cochran’s Q of 357.3 (P < 0.01) and the I-squared value of 93.6%. Three of the five elevated SMRs in the plot occurred in mining cohorts, and one occurred in a milling cohort. Removing the mining cohorts did not substantially change the heterogeneity of the reported SMRs; the Cochran’s Q was 278.0 (P < 0.01) with an I-squared value of 93.9%. After removing the four milling cohorts, the Cochran’s Q still indicated significant heterogeneity among results (Cochran’s Q = 237.4, P < 0.01). Nonetheless, all point estimates are below 1 for the non-mining and non-milling cohorts that reported SMRs (29, 34–37, 39, 42, 43, 49–52, 56, 57).
FIG. 2.
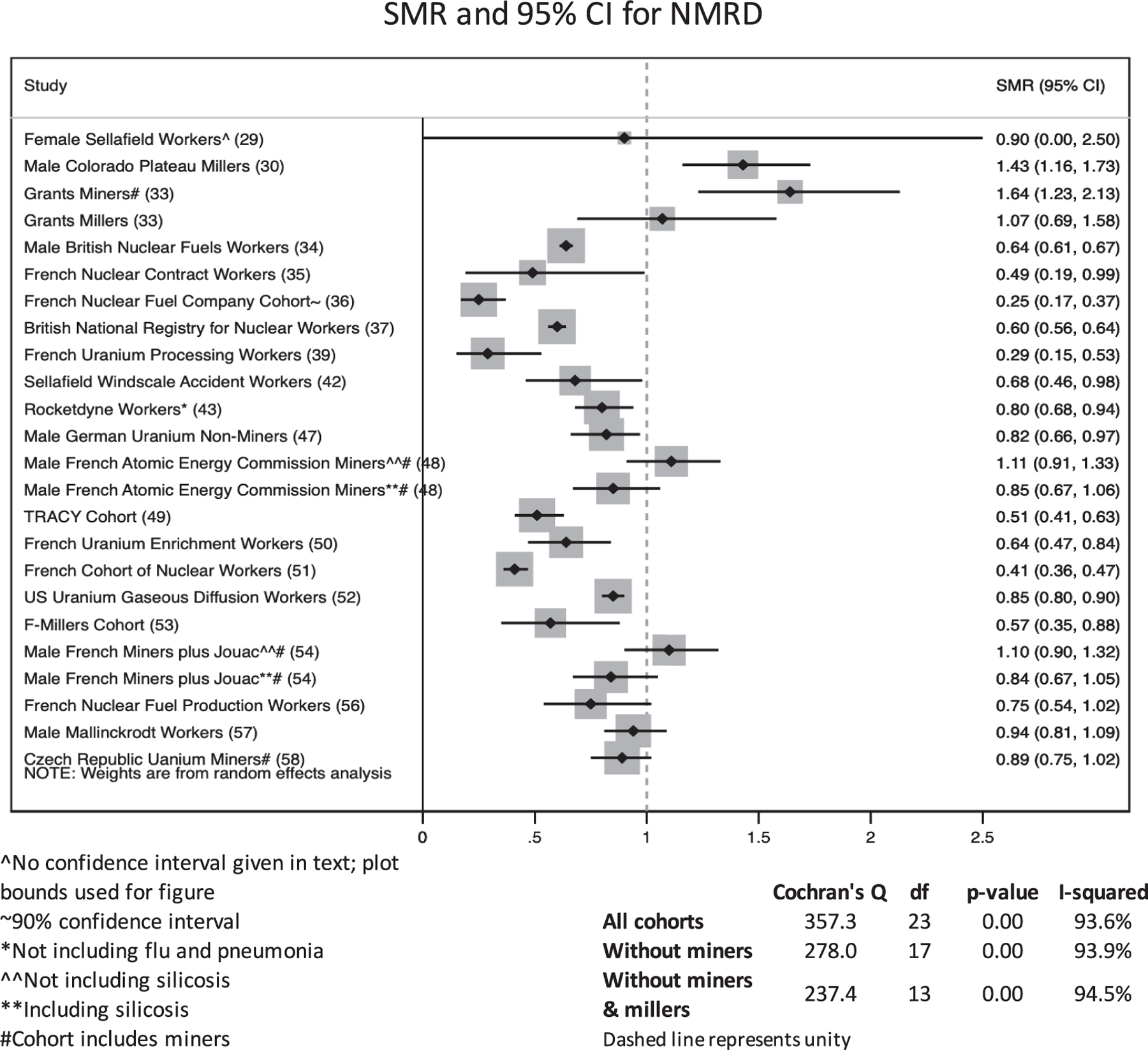
Forest plot showing standardized mortality rate for overall non-malignant respiratory disease by study, including miners.
As study definitions of NMRD often include infectious diseases such as pneumonia and influenza, forest plots were also constructed for COPD/emphysema to reduce potential bias from outcomes not likely related to radiation (Fig. 3) (5). Heterogeneity is high when miners are included (Cochran’s Q = 194.7, P < 0.01). It is considerably reduced when miners are excluded (Cochran’s Q = 57.5, P < 0.01) and further reduced when millers are also excluded (Cochran’s Q = 49.7, P < 0.01), though SMRs remain significantly heterogeneous. While elevated COPD is still only seen among miners and millers (30, 38), decreased SMRs are seen in several mining and milling populations as well (38, 47). Although heterogeneity decreased when excluding mining and milling cohorts, there are only five additional cohorts that reported COPD or emphysema estimates (not including COPD/emphysema with bronchitis and asthma) (43).
FIG. 3.
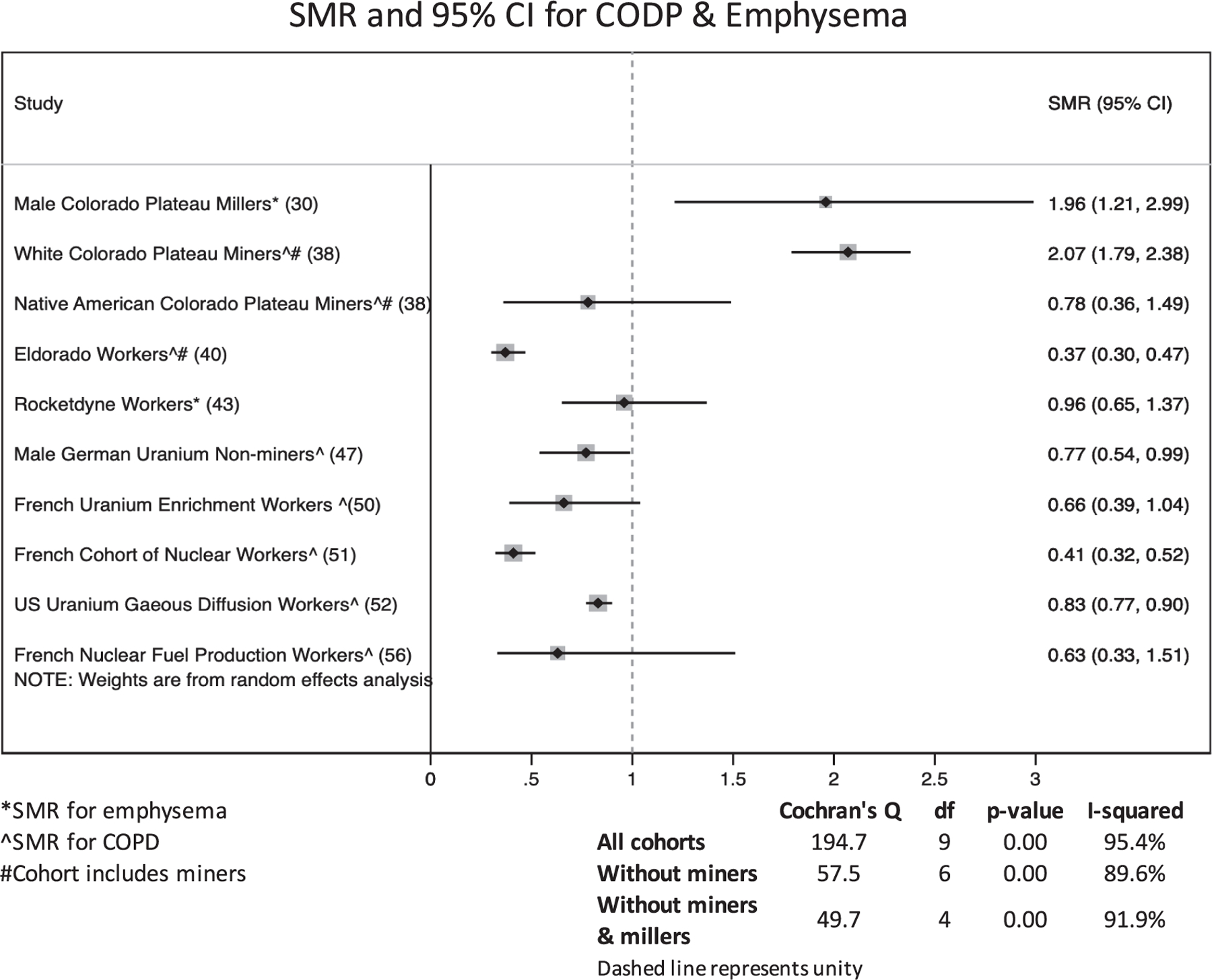
Forest plot showing standardized mortality rate for overall COPD & emphysema by study, including miners.
Notable increased SMRs were seen among Grants miners and millers, which had an SMR for NMRD of 1.64 (95% CI: 1.23, 2.13) (33) and for COPD only among workers with mining experience of 2.16 (95% CI: 1.40, 3.19), Male Colorado Plateau Millers (30) which had an SMR for emphysema of 1.96 (95% CI: 1.21, 2.99) and other respiratory diseases of 1.43 (95% CI: 1.16, 1.73) in all workers versus the U.S. population (30), and in the Colorado Plateau White miners, which had an SMR for COPD of 2.07 (95% CI: 1.79, 2.38) and for pneumonia of 1.43 (95% CI: 1.09, 1.84). The corresponding SMRs in Native American miners were not significantly elevated (COPD SMR: 0.78; 95% CI: 0.36, 1.49 and pneumonia SMR: 1.33; 95% CI: 0.91, 1.89). The COPD SMR among White miners increased to 2.50 (95% CI: 2.16, 2.89) when the population was limited to ever smokers, implying a large number of smokers in White but not Native American cohort members (38). Finally, the Colorado Plateau miners also had an increased occurrence of tuberculosis and other respiratory diseases, with SMRs for tuberculosis of 3.44 (95% CI: 2.83, 5.99) and 2.40 (95% CI: 1.31, 4.04) in White and Native American miners, respectively, and SMRs for other respiratory diseases of 1.94 (95% CI: 1.38, 2.65) and 2.79 (95% CI: 1.88, 3.98) in White and Native American miners, respectively (38). Several SMRs for pneumoconiosis indicated elevated occurrence compared to the U.S. population, but with wide confidence intervals (30, 38, 45, 48, 54, 58). In the male French Atomic Energy Commission Miners cohort, the authors conducted tests of trend in SMRs over duration of exposure as a surrogate for dose. While they found mixed results for NMRD overall, they saw an increasing SMR over duration of exposure for pneumoconiosis (48). In the French Nuclear Workers (CEA & AREVA), a test for trend for NMRD SMR overdose category found no consistent trend (31). The highest dose categories were associated with increased NMRD SMRs in a test of trend conducted for the British National Registry for Nuclear Workers; however, this trend reversed for COPD (37).
Dose-Response Analysis Results
In the regression analyses, several studies showed increased NMRD risks with increased exposures. For NMRD overall, male French Atomic Energy Commission miners had an ERR per working level month (WLM) in miners exposed to radon decay products of 0.35 (95% CI: 0.008, 0.90) using an external population referent (48); these results were similar in the same cohort with the addition of a population of Jouac workers (48). The male British Nuclear Fuels workers cohort had an ERR/Sv for external radiation doses among industrial workers flagged for exposure to internal ionizing radiation of 0.94 (90% CI: 0.10, 2.14) and an ERR/Sv among non-industrial workers not flagged for internal exposures of 1.41 (90% CI: −1.02, 6.71) (34). The British National Registry for Nuclear Workers had an ERR/Sv of 0.799 (95% CI: −0.08, 2.01). The male German Uranium non-miners had an ERR/WLM of 1.25 (95% CI: −1.39, 3.89) from radon exposure, though no associations were seen with other exposures (47). The French Cohort of Nuclear Workers had an overall ERR/Sv of 1.20 (95% CI: 1.06, 4.68) (51). For COPD, male Wismut miners had an ERR/100 WLM of 0.043 (95% CI: 0.024, 0.062) in their analysis including both underlying and contributing causes of death from death certificates to reduce underreporting of COPD on death certificates. However, upon removing deaths with underlying causes from cancer or silicosis, this relationship was no longer observed (ERR/100 WLM: 0.013; 95% CI: −0.003, 0.029), indicating it was driven by concurrent silica dust exposures or by a dose-response for lung cancer that was not independent from COPD. Additionally, the silica-dust-adjusted ERR per 100 WLM for NMRD as the underlying cause of death (excluding silicosis) in this cohort was −0.004 (95% CI: −0.020, 0.011) (44). The Fernald cohort had a slightly increased risk of COPD, with an ERR/100 mGy of 0.54 (95% CI: 0.0, 1.4) with increasing external dose; however, their dose-response analysis over increasing internal dose did not show an association (ERR/100 μGy = −0.006; 95% CI: −0.007, 0.00) (45). Significant increases were seen in pneumoconiosis in male French Atomic Energy Commission Miners and in the Male French Miners plus Jouac after exposure to radon decay products, which is consistent with their SMR analyses. However, their confidence intervals were very wide and sometimes inestimable, suggesting lack of model convergence or low disease incidence (48, 54).
Meta-Analysis Results
Figure 4a and 4b show meta-analyses of non-overlapping cohorts reporting an ERR/100 mSv or ERR/Sv for NMRD with and without mining cohorts, respectively. No milling cohorts reported an ERR/100 mSv or an ERR/Sv for NMRD overall, and they were therefore excluded from the meta-analysis. Unlike the SMR estimates, there was no heterogeneity across studies; the analysis with and without the miners both had I-squared values of 0%. The meta-analytic point estimate is identical with and without mining cohorts, with only the upper bound increasing slightly when mining cohorts are removed (ERR: 0.09; 95% CI: −0.05, 0.22 in all cohorts; ERR: 0.07; 95% CI: −0.07, 0.21 in non-mining cohorts). A funnel plot for these analyses is shown in Fig. 4c. Published study estimates are evenly distributed around the mean, indicating publication bias was not present. There were too few non-overlapping studies reporting ERRs to repeat this meta-analysis for COPD; however, COPD estimates vary in direction more than results for NMRD overall (see Supplemental Tables S1a and S2a; https://doi.org/10.1667/RADE-21-00014.1.S1).
FIG. 4.
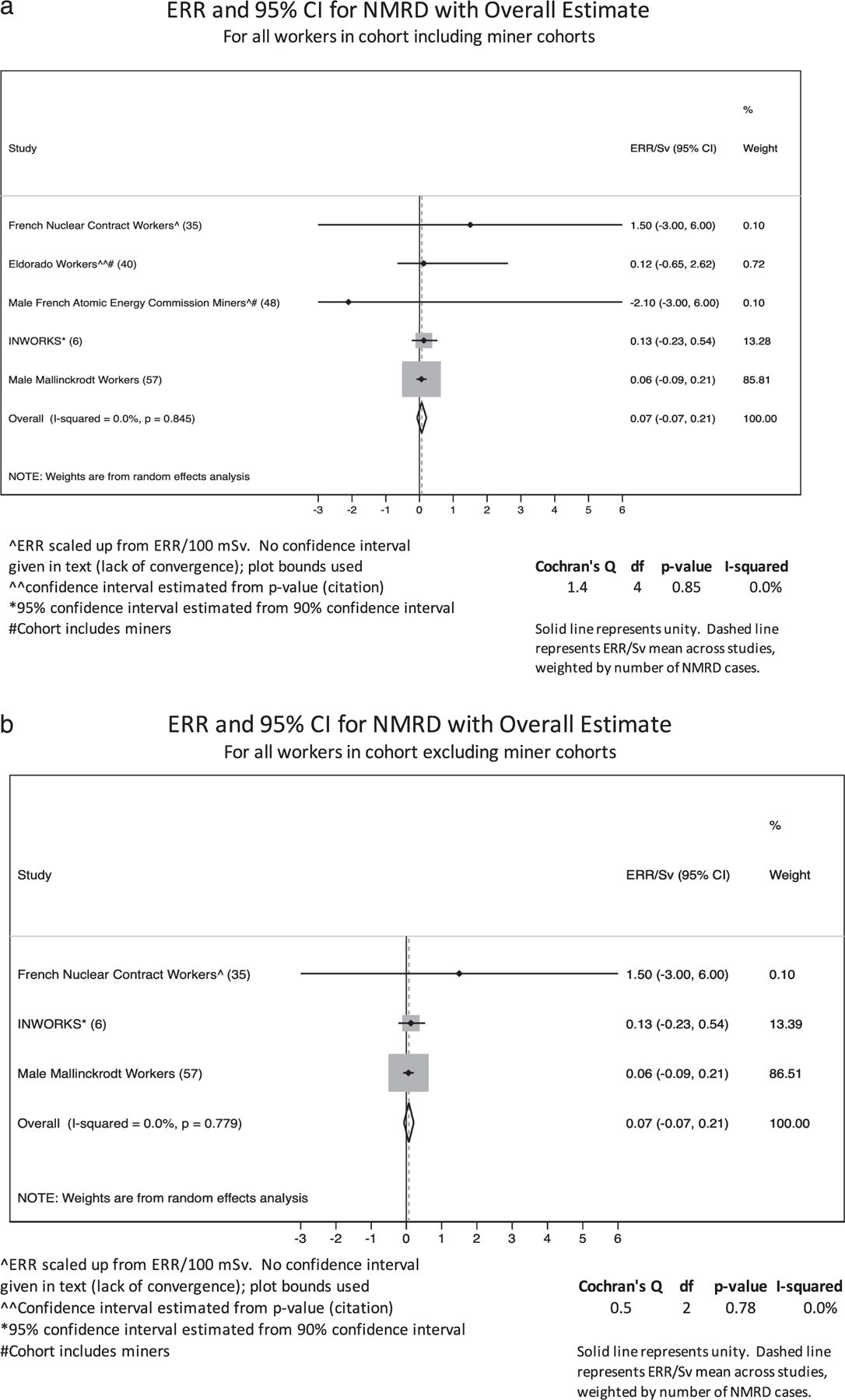
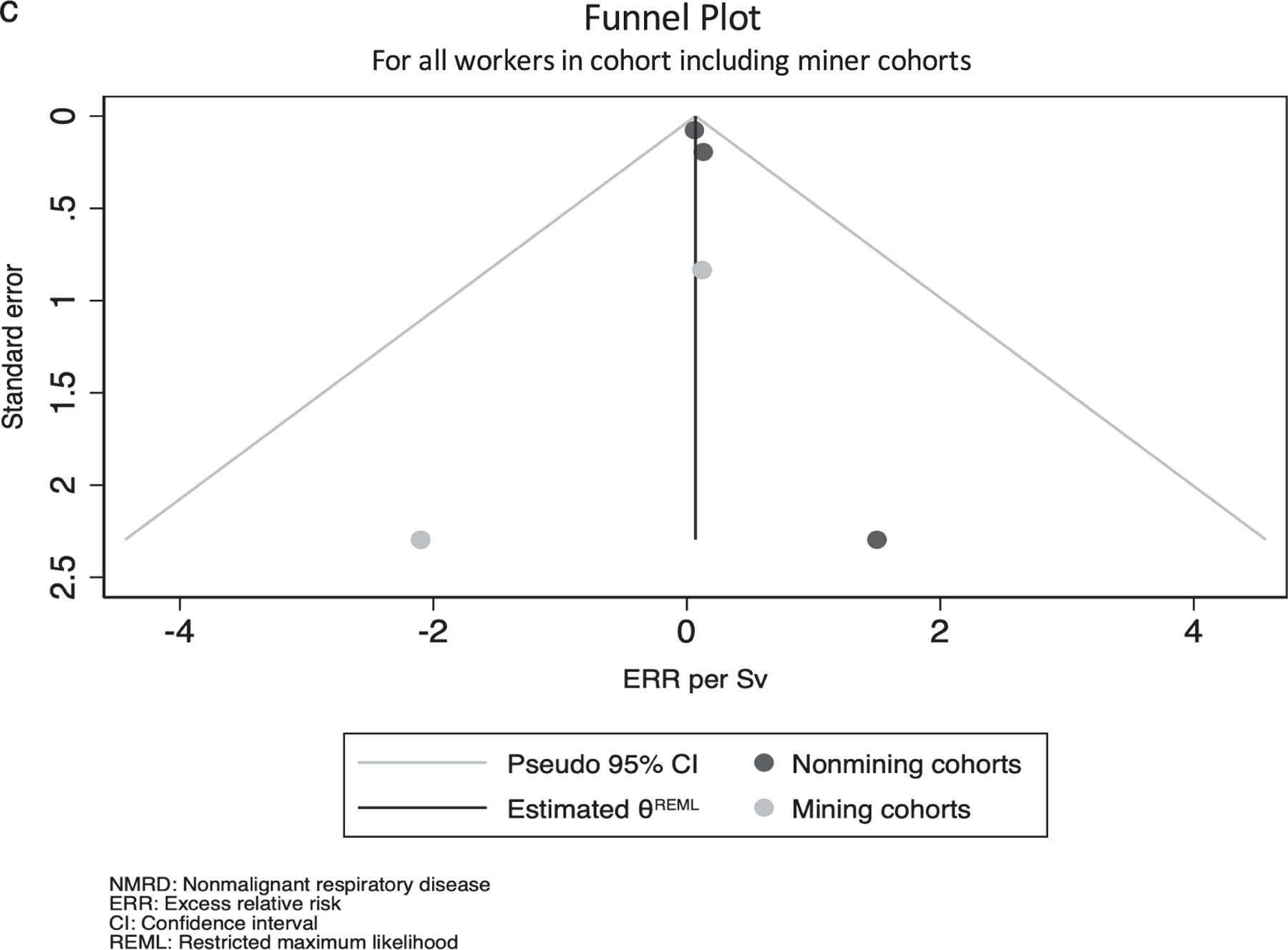
Panel a: Forest plot showing ERR for overall non-malignant respiratory disease by study, including miners. Panel b: Forest plot showing ERR for overall non-malignant respiratory disease by study, excluding miners. Panel c: Funnel plot for all workers including miner cohorts.
Study Quality, Strengths, and Limitations
Tables 5 and 6 show assessments of strengths, limitations, and study quality conducted for the present systematic review. A minority of studies were reviewed in NCRP Commentary 27; one paper reviewed in NCRP Commentary 27 was used in the present review (Rocketdyne Workers) (43), and two populations were consistent between NCRP Commentary 27 and this review (INWORKS and the 15-Country Study), though the reviewed publications were different from this review (6, 32, 59–61). For the Rocketdyne Workers (43), INWORKS (6), and the 15-Country Study (32), NCRP quality ratings were consulted and applied to the dosimetry, epidemiology, and statistics ratings. These ratings were consulted when applying scores to the other cohorts for consistency across authors. Individual ratings were considered alongside relevance to interpreting a possible causal association between radiation and NMRD when assigning the overall quality. Of the 31 studies, all but the French Electric Company Staff study (41), Canadian and German Uranium Processing Workers (55), INWORKS, and the Male Wismut Miners study (44) conducted SMR analyses. Studies that only assessed NMRD using SMRs (29, 30, 33, 36, 38, 39, 49, 50, 53, 58, 62) were rated poor for considering a causal relationship between radiation exposure and NMRD mortality regardless of their individual dosimetry, epidemiology, and statistics quality ratings. Studies that conducted dose-response analyses with an internal cohort comparison were assessed on their dosimetry, epidemiology, and statistics to determine their ability to quantify the effects of interest. Studies with a high percentage of follow-up and a rigorous methodology for determining organ doses, with thorough SMR and dose-response analyses were graded highest (6, 34, 37, 41, 44, 45, 52, 56, 57, 59), while studies without individual dosimetry and with minimal adjustment for potential confounders were graded lowest (29–31, 33, 35, 36, 38, 39, 47, 49, 50, 53, 58). INWORKS (6) was the only study assigned 3 (high quality) in dosimetry, epidemiology, and statistics, though the French Electric Company Staff study (41) was assigned 3 in dosimetry and epidemiology and 2.5 in statistics. Due to their fully-adjusted assessments of NMRD using Poisson regression, both of these studies were also assigned a 3 (high quality) in their ability to directly study the effects of radiation on NMRD (6, 41). No other studies were assigned a 3 (high quality) in their ability to assess NMRD. Eight studies were assigned a 2.5 in their ability to assess the impact of radiation on NMRD; these included the Male British Nuclear Fuels Workers (34), the British National Registry for Nuclear Workers (37), the Male Wismut Miners (44), the Fernald Workers (45), the French Cohort of Nuclear Workers (59), the US Uranium Gaseous Diffusion Workers (52), the French Nuclear Fuel Production Workers (56), and the Male Mallinckrodt Workers (57). These studies had minor limitations in dosimetry, epidemiology, and/or statistics but overall represented a detailed analysis with a thorough dose-response analysis for at least one NMRD outcome. Strengths and limitations are outlined in Table 5. One example of an overall 2.5 rating is the Male Wismut Workers: although no individual dose monitoring was conducted, great detail was given to its reconstructed dosimetry, and the authors conducted detailed dose-response analyses with attention to bias and confounding (44). Several studies were assigned 2 (moderate quality) in their ability to assess the impact of radiation on NMRD; these studies included the 15-Country Study (32), Rocketdyne Workers (43), Male Port Hope Workers (46), and Canadian and German Uranium Processing Workers (55). Of the analyses receiving moderate-high quality scores of 2, 2.5, or 3, Male British Nuclear Fuels Workers, the British National Registry for Nuclear Workers, Male Wismut Miners, the Fernald Workers, and the French Cohort of Nuclear Workers had elevated risks of NMRD as detailed above (34, 37, 44, 45, 51). Yet, the Fernald Workers only showed increased risks over increasing external radiation dose, with no association over increasing internal dose (45). Further, the positive Wismut relationship was only seen in the sensitivity analysis discussed previously that included lung cancer and silicosis as underlying causes of death (with NMRD as a contributing cause of death) (44).
TABLE 5.
Strengths and Limitations of Included Papers
| Brief description | Source (Ref.) | Strengths | Limitations |
|---|---|---|---|
|
| |||
| Sellafield Females | McGeoghegan et al. 2003 (29) | ● Comprehensive identification of subjects with limited loss to follow-up; ● Organ doses calculated for plutonium exposures; ● Exposure assessment comprehensive, including external doses, plutonium uptake, and doses from other facilities | ● Uncertainties in film badge dose quality; ● No attempts to control for SES |
| Male Colorado Plateau Millers | Pinkerton et al. 2004 (30) | ● Comprehensive identification of subjects; ● Comprehensive SMR/SIR analyses with sensitivity analyses | ● No dosimetry; ● No control for SES or lifestyle confounding in SMR/SIR analyses |
| French Nuclear Workers (CEA & AREVA) | Telle-Lamberton 2007 (31) | ● Comprehensive identification of subjects with limited loss to follow-up; ● Efforts to control for confounding through stratification; ● Comprehensive individual dosimetry, assessed for accuracy | ● No mortality information before 1968; ● Organ doses were not calculated; ● Excluded workers employed at both sites due to lack of dose reconstruction |
| 15-Country Study | Vrijheid et al. 2007 (32) | ● Large effort for dosimetric accuracy across cohorts; ● Organ doses calculated and utilized | ● Potential residual confounding by socioeconomic status, and loss of power and accuracy by excluding entire sites where socioeconomic status was not recorded; ● Dosimetric inaccuracies in Canadian data biased estimates; ● Other known sources of potential confounding, such as smoking |
| Grants Miners and Millers | Boice et al. 2008 (33) | ● Comprehensive identification of subjects from multiple record sources; ● Efforts to control confounding through stratification | ● No dosimetry; classification only by whether there was potential exposure to radon underground; ● Early deaths excluded because NDI records were not available; ● Low-power cohort |
| Male British Nuclear Fuels Workers | McGeoghegan et al. 2008 (34) | ● Attempts to control for SES and other potential confounders; ● Individual monitoring at the site of interest | ● Only cumulative external absorbed dose was calculated, with a flag for internal exposures; ● Noted considerable inhomogeneity over worker type that may be attributable to inaccurate dosimetry or residual confounding; ● Exploratory analyses determined Poisson models of interest |
| French Nuclear Contract Workers | Guerin et al. 2009 (35) | ● Expanded cohort considerably from the last iteration; considerable effort placed into identifying missing individuals; ● Comprehensive, individual dose monitoring and exposure assessment, including all relevant sources on site | ● Missingness in the overall cohort since contracting companies did not always have employee details; ● Low power since ~97.5% of contractors were still alive at the end of follow-up; ● Missing job type in 56% of workers; ● Their ERR analysis uses SMR as the dependent variable |
| French Nuclear Fuel Company Cohort | Metz-Flamant 2009 (36) | ● Comprehensive identification of subjects with limited loss to follow-up; ● Efforts to control for confounding through stratification; ● Comprehensive individual dosimetry, assessed for accuracy | ● Organ doses were not calculated; ● No dosimetry for internal doses, despite known exposures in some individuals |
| British National Registry for Nuclear Workers | Muirhead et al. 2009 (37) | ● Comprehensive identification of subjects with limited loss to follow-up; ● Fairly direct control for socioeconomic status; ● Good registry data for disease incidence and mortality | ● Organ doses were not calculated; ● No dosimetry for internal doses, despite known exposures in some individuals; ● Different inclusion criteria for sites within the overall study |
| Colorado Plateau Miners | Schubauer-Berigan et al. 2009 (38) | ● Comprehensive individual dosimetry with efforts made to assess accuracy; ● Smoking surveys were conducted, so smoking was included in the analysis | ● Potential problems in the assumptions underlying interpolation used for calculating daily dose-rates; ● Potential unadjusted confounding from a variety of sources |
| French Uranium Processing Workers | Guseva Canu et al. 2010 (39) | ● Comprehensive identification of subjects with no loss to follow-up; ● Comprehensive mortality information; ● Sensitivity analyses used regional background population for SMRs | ● Study focus was internal exposures, yet no internal dosimetry was available; ● Organ doses not calculated |
| Eldorado Workers | Lane et al. 2010 (40) | ● Demonstrated effort to trace individuals who were missing data in the cohort; ● Efficient use of existing data to retain individuals in the study when other data was unavailable | ● Dosimetry is predominantly retrospective dose reconstruction from uncertain area data across the cohorts included in the study; ● Sensitivity analyses were only conducted for lung cancer models (NMRD not included); ● No adjustment for SES or lifestyle variables |
| French Electric Company Staff | Laurent et al. 2010 (41) | ● Comprehensive individual dosimetry, assessed for accuracy; ● Assessed a 1% sample of the cohort for dosimetric accuracy between two extant databases; ● Detailed dose data for relevant organs used in analysis; ● Attempts to control for confounding, including SES | ● Uncertainty due to different film badge calibrations harmonized over time; ● Used a flag for neutron dose instead of calculating organ dose; ● Missing lifestyle confounders |
| Sellafield Windscale Accident Workers | McGeoghegan et al. 2010 (42) | ● Comprehensive identification of subjects with limited loss to follow-up; ● Comprehensive outcome data from registries and with validation; ● Adjustments made for various factors including socioeconomic status | ● Fire cohort workers (population of interest) included non-radiation workers, but non-fire cohort workers (comparison population) excluded non-radiation workers; ● No internal dosimetry conducted; ● Low powered analysis |
| Rocketdyne Workers | Boice et al, 2011 (43) | ● Very thorough dosimetry, accounting for 14 radionuclides in 16 organs; ● Minimal loss to follow-up; ● High ascertainment of underlying cause of death; ● Attempts to approximate and validate approximation of missing potential confounders | ● Dose uncertainty, particularly at low levels; ● No sensitivity analyses; ● Relatively small study; low statistical power; ● No lifestyle information |
| Male Wismut Miners | Kreuzer et al. 2013 (44) | ● Thorough radon dose reconstruction; ● Multiple sensitivity analyses to assess various sources of potential bias; ● High statistical power in a large cohort | ● No individual dosimetry; ● No adjustment of SES or lifestyle factors; job type used in dose reconstruction; ● No assessment of external dose |
| Fernald Workers | Silver et al. 2013 (45) | ● Quantitative assessments of a variety of exposures including external dose, uranium intake, radon decay products, and thorium (flag); ● Organ doses calculated for uranium intake; ● Adjustments made for non-radiologic covariates in analyses; ● Attempts to control for confounding by SES and other confounders | ● Information lacking for lifestyle confounders; ● Dose cut-points for analyses differ by outcome with no explanation in text |
| Male Port Hope Workers | Zablotska et al. 2013 (46) | ● Registry-based follow-up assessed for accuracy; recoded where necessary; ● Sensitivity analyses addressing particular points of uncertainty | ● No dose data was available; doses were reconstructed based on uncertain area monitoring data; ● Organ doses were not calculated; ● No adjustment for SES or lifestyle factors |
| Male German Uranium Non-Miners | Kreuzer et al. 2015 (47) | ● Good discussion of correlation of different exposures, justifying some of their dose reconstruction | ● Potential differential completeness of participant selection by level of dose; ● Considerable uncertainty in organ dose estimates due to lack of dosimeters until the 1990s; ● Rather small range of doses; low power; ● Potential cause of death ascertainment different by exposure; ● Missing outcome information for nearly half of the deaths prior to 1970; ● Few confounders included |
| Male French Atomic Energy Commission Miners | Rage et al. 2015 (48) | ● Used a post-1955 cohort to assess the period in which everyone was individually monitored for external exposures, radon, and long-lived radionuclides; ● Systematic and unbiased exposure assessment; ● Smoking assessed in a sub-cohort using a nested case-control study | ● No dosimeters issued before 1955; uncertainty in full cohort; ● Missing data on confounders including SES, lifestyle factors, and other toxins in the workplace; ● P-value based reporting and decision-making; switching between 90 and 95% confidence intervals to report results |
| TRACY Cohort | Samson et al. 2016 (49) | ● Strong set of sensitivity analyses | ● No dosimetry in this cohort yet; ● Missing data on confounders; no attempt to address SES; ● Low statistical power |
| French Uranium Enrichment Workers | Zhivin et al. 2016 (50) | ● Considerable effort creating job exposure matrices to construct exposure quantiles for uranium exposure; ● Comprehensive identification of subjects with limited loss to follow-up; ● Information available on confounding exposures (though not used in NMRD analyses) | ● Confounders not included in models because instability (likely low power); ● ERRs estimated below zero with lower bounds that could not be estimated below zero; ● Dosimetry limited to exposure quantiles, despite detailed JEM and good external information |
| INWORKS | Gillies et al. 2017 (6) | ● Large, comprehensive study; ● Large effort for accuracy in dosimetry; ● Tests to determine whether smoking was a potential confounder; indication that it is not | ● Tissue-weighted doses rather than organ doses; ● Assessment does not include all relevant periods of exposure (French cohort truncation); ● Removing French data resulted in changes to significance despite the French component being the smallest; ● ‘‘Missed’’ doses in early periods lead to uncertainty |
| French Cohort of Nuclear Workers | Leuraud et al. 2017 (51) | ● Comprehensive identification of subjects with limited loss to follow-up; ● Detailed and validated external dosimetry, with sensitivity analyses for absent neutron and internal radionuclide information; ● Sensitivity analyses conducted to assess bias | ● No mortality information before 1968, workers excluded who were deceased before this date; ● Organ doses were not calculated; ● Different dates of follow-up for different cohorts |
| US Uranium Gaseous Diffusion Workers | Yiin et al. 2017 (52) | ● Detailed internal and external dosimetry with calculated organ doses; ● Calculated exposures to nickel and trichloroethylene assessed as confounders in analyses; ● Comprehensive identification of subjects with limited loss to follow-up | ● Doses from other facilities not included in total dose; ● Uncertainty associated with intake and dose calculations not included in epidemiological analyses; ● No attempted assessment of socioeconomic status |
| F-Millers Cohort | Bouet et al. 2018 (53) | Very low loss to follow-up (<1%); ● Efforts to control for confounding through stratification | ● No dosimetry; ● No mortality information before 1968 |
| Male French Miners plus Jouac | Rage et al. 2018 (54) | ● Dose-response analysis of radon exposure; ● Inclusion of more information into an existing mining cohort | ● Jouac exposure information only available post-1977 (cohort began in 1957); ● Analyses only considered radon exposure, and not uranium, long-lived radionuclides, or gamma exposures; ● Analyses did not consider socioeconomic status |
| Canadian and German Uranium Processing Workers | Zablotska et al. 2018 (55) | ● Retrospectively reconstructed dosimetry that took considerable effort; ● Pooled cohort increases power; ● Detailed confounding and effect modification assessment | ● Dosimetric uncertainties in dose estimation; ● No information on socioeconomic status; ● Different inclusion criteria for Port Hope vs. Wismut |
| French Nuclear Fuel Production Workers | Bouet et al. 2019 (56) | ● Comprehensive identification of subjects with limited loss to follow-up; ● Extensive external and internal dosimetry, with organ doses for internal dosimetry; ● Adjustments for potential confounders, including socioeconomic status | ● Uncertainties in dosimetry; a subcohort of the TRACY study was used as information is not yet computerized for the full cohort; ● No mortality information before 1968, workers excluded who were deceased before this date; ● Many models did not converge due to low numbers of outcomes |
| Male Mallinckrodt Workers | Golden et al. 2019 (57) | ● Dose reconstruction including external, internal, and medical X-ray irradiation, and doses from sites other than Mallinckrodt; ● Comprehensive identification of subjects with limited loss to follow-up; ● Controlled for confounders such as silica dust and socioeconomic status | ● Low power due to a small number of individuals and low doses; ● Uncontrolled confounding for other exposures experienced at Mallinckrodt (solvents, etc.); ● Some dosimetric uncertainty, specifically from imputed doses |
| Czech Republic Uranium Miners | Kelly-Reif et al. 2019 (58) | ● Comprehensive identification of subjects; ● Causal mortality rates present an interesting way to view years of life lost | ● No dosimetry conducted; ● Only workers alive and already in the cohort in 1977 were included since the Czech cancer registry began in 1977; potential survivorship bias; ● Residual confounding/bias likely |
TABLE 6.
Quality Assessment of Included Papers
| Brief Description | Source (Ref. | Dosimetry | Epidemiology | Statistics | Overall strength of study to assess NMRD |
|---|---|---|---|---|---|
|
| |||||
| Sellafield Females | McGeoghegan et al. 2003 (29) | 3 | 2 | 1.5 | 1 |
| Male Colorado Plateau Millers | Pinkerton et al. 2004 (30) | 1 | 2 | 2 | 1 |
| French Nuclear Workers (CEA & AREVA) | Telle-Lamberton 2007 (31) | 2 | 2 | 2.5 | 1 |
| 15-Country Study | Vrijheid et al. 2007 (32) | 2 | 3 | 3 | 2 |
| Grants Miners and Millers | Boice et al. 2008 (33) | 1 | 2 | 2 | 1 |
| Male British Nuclear Fuels Workers | McGeoghegan et al. 2008 (34) | 2 | 2.5 | 2.5 | 2.5 |
| French Nuclear Contract Workers | Guerin et al. 2009 (35) | 3 | 2 | 1 | 1 |
| French Nuclear Fuel Company Cohort | Metz-Flamant 2009 (36) | 2 | 2 | 2 | 1 |
| British National Registry for Nuclear Workers | Muirhead et al. 2009 (37) | 2.5 | 2.5 | 2.5 | 2.5 |
| Colorado Plateau Miners | Schubauer-Berigan et al. 2009 (38) | 2 | 2 | 1 | 1 |
| French Uranium Processing Workers | Guseva Canu et al. 2010 (39) | 1.5 | 2.5 | 2 | 1 |
| Eldorado Workers | Lane et al. 2010 (40) | 1.5 | 1.5 | 2 | 1.5 |
| French Electric Company Staff | Laurent et al. 2010 (41) | 3 | 3 | 2.5 | 3 |
| Sellafield Windscale Accident Workers | McGeoghegan et al. 2010 (42) | 2 | 1 | 2 | 1.5 |
| Rocketdyne Workers | Boice et al, 2011 (43) | 2 | 2 | 2 | 2 |
| Male Wismut Miners | Kreuzer et al. 2013 (44) | 2 | 2.5 | 2.5 | 2.5 |
| Fernald Workers | Silver et al. 2013 (45) | 3 | 2.5 | 2.5 | 2.5 |
| Male Port Hope Workers | Zablotska et al. 2013 (46) | 1.5 | 2 | 2 | 2 |
| Male German Uranium Non-Miners | Kreuzer et al. 2015 (47) | 1 | 1 | 2 | 1 |
| Male French Atomic Energy Commission Miners | Rage et al. 2015 (48) | 2 | 2 | 1 | 1.5 |
| TRACY Cohort | Samson et al. 2016 (49) | 1 | 2 | 2 | 1 |
| French Uranium Enrichment Workers | Zhivin et al. 2016 (50) | 2 | 2 | 2 | 1 |
| INWORKS | Gillies et al. 2017 (6) | 3 | 3 | 3 | 3 |
| French Cohort of Nuclear Workers | Leuraud et al. 2017 (51) | 2.5 | 2.5 | 3 | 2.5 |
| US Uranium Gaseous Diffusion Workers | Yiin et al. 2017 (52) | 3 | 2.5 | 3 | 2.5 |
| F-Millers Cohort | Bouet et al. 2018 (53) | 1 | 2 | 3 | 1 |
| Male French Miners plus Jouac | Rage et al. 2018 (54) | 1.5 | 1.5 | 2 | 1.5 |
| Canadian and German Uranium Processing Workers | Zablotska et al. 2018 (55) | 1.5 | 2 | 2 | 2 |
| French Nuclear Fuel Production Workers | Bouet et al. 2019 (56) | 2.5 | 2.5 | 2 | 2.5 |
| Male Mallinckrodt Workers | Golden et al. 2019 (57) | 2.5 | 2.5 | 3 | 2.5 |
| Czech Republic Uranium Miners | Kelly-Reif et al. 2019 (58) | 1 | 2 | 2 | 1 |
Notes. Study strength in the areas dosimetry, epidemiology, and statistics, as well as overall strength to assess NMRD. Quality assessment based on UNSCEAR 2017 criteria (25), where 1 represents a low score, and 3 represents a high score.
DISCUSSION
Our search found 90 published studies and 31 were fully reviewed for this systematic review. Neither SMR nor regression analyses provided consistent evidence for increased risk of NMRD following radiation exposure in the nuclear industry. Of the 24 studies that reported SMRs for NMRD or COPD, only 5 suggested radiation workers have a greater risk of NMRD than a general population (30, 33, 38, 48, 54). Two papers reporting increased SMRs were mining cohorts that no longer had positive results when silicosis was not included in the NMRD definition, implying the elevated SMR was due to silica dust exposure rather than radiation (48, 54). Likewise, regression analyses and tests for trend over SMR groups did not provide consistent evidence of a dose-response. Only 5 studies with an overall quality assessment of at least 2 (moderate quality) for ability to assess the effect of radiation on NMRD detected an elevated dose-response (34, 37, 44, 45, 51). However, one of these was presumably due to bias from silica exposure among miners; the silica dust-adjusted ERR/100 WLM for NMRD excluding silicosis was −0.004 (95% CI: −0.020, 0.011) (44). Another study detected an NMRD association for external but not internal exposures (45). The two studies with the highest quality scores, INWORKS and the French Electric Company Staff, did not provide evidence for increased risk of NMRD following radiation exposure (6, 41). While INWORKS had an elevated ERR/Sv for ‘‘other respiratory diseases’’ of 0.52, the 95% confidence interval was fairly wide compared to the magnitude of risk (−0.26, 1.53). The definition for ‘‘other respiratory diseases’’ excludes COPD but includes pneumoconiosis and influenza, limiting relevance (6). The only SMR studies reporting elevated NMRD (whether statistically significant or not) were cohorts of uranium miners and millers (30, 33, 38, 48, 54), as shown in the forest plot in Fig. 2. Two of the studies reporting elevated SMRs had overall quality scores of 1 (30, 33). Although these scores reflect a lack of dose-response analyses rather than study flaws, studies reporting only NMRD SMRs were assigned a 1 due to the limitations of SMRs. This metric reports only a comparison between the study population and a frequently non-comparable general population; furthermore, SMRs cannot adequately adjust for relevant confounders such as smoking. In addition to only reporting SMRs, these two studies with quality scores of 1 as well as two others reporting elevated SMRs had considerable limitations in dosimetry and population tracing (30, 33, 48, 54). After removing the uranium miners and millers from the forest plot, all cohorts reported SMRs below 1, exhibiting a difference between miners and millers and other radiation-exposed professions in terms of overall NMRD risk (29, 30, 33–35, 43, 47–49, 62). The remaining 9 moderate to high quality publications did not observe elevated risk for NMRD. Although SMR increases were seen in some mining and milling cohorts, others reported population-equivalent or even decreased NMRD SMRs. For the mining and milling cohorts with increased SMRs, there was still inconsistency in subtype analyses. Therefore, we observed little evidence for an association between radiation exposures and NMRD in nuclear worker cohorts.
The meta-analysis conducted for NMRD based on dose-response analyses shows that the studies with elevated risk taken together do not imply an increased risk of NMRD after radiation exposure. The overall estimate was an ERR/Sv of 0.07 (95% CI: −0.07, 0.21) and removing the mining cohorts did not impact overall results due to their low weights in the meta-analysis (represented by large variances in their effect estimates). The funnel plot in Fig. 4c shows that publication bias was not present, though few studies were included in the meta-analysis. The potential for publication bias is complicated here by inconsistency in reporting both effect-estimate types and units, as well as included studies’ greater focus on other outcomes (e.g., lung cancer, cardiovascular disease). A hesitancy to report negative results does not appear to impact the meta-analysis. Five studies were included in the meta-analysis (6, 35, 37, 40, 48, 57, 59), though ten others are represented through their overlap with more recent studies (31, 32, 34–36, 39, 41, 49, 51, 63). However, these results do not include every study; they only include the most recent (or largest, when two studies were published in the same year) study reporting an overall NMRD estimate in terms of ERR/100 mSv or ERR/Sv. Other papers reported relative risks, hazard ratios, ERR/WLM results, or results for more specific respiratory outcomes, all of which were too dissimilar to include in a meta-analysis (Supplementary Tables S1a–S5b; https://doi.org/10.1667/RADE-21-00014.S1) (42, 43). One study reported an ERR/mGy which could not be appropriately scaled to ERR/Sv; linear extrapolation resulted in effect estimates that appeared an order of magnitude high (52).
Results were inconsistent, even among studies with a quality score of at least 2. Likely, this inconsistency arose from studies’ differences in NMRD (or subtype) definition, analysis type, and confounding adjustment. Only one of the studies in this review focused specifically on NMRD; most studies did not assess NMRD as thoroughly as other outcomes such as lung cancer or cardiovascular disease. Additionally, if NMRD is radiogenic, magnitude of this risk may be small, limiting studies’ ability to assess this relationship in the presence of competing risks.
Due to limitations in power to detect significant effects in studies with small sample sizes or narrow dose ranges, magnitude of results may be more informative than statistical significance (64); therefore, these results have focused predominantly on effect estimate size. However, magnitude of NMRD and individual disease point estimates were not consistent, with some studies reporting decreased mortality, other studies reporting no difference in mortality, and still further studies reporting increased mortality in both SMR and regression analyses, as shown in Supplementary Tables S1a–S5b (https://doi.org/10.1667/RADE-21-00014.S1) and in Fig. 2. The forest plots in Fig. 2 highlight the heterogeneity across reported SMRs for overall NMRD, as indicated by the large Cochran’s Q of 367.3 and the high value of I-squared (93.6%). This heterogeneity was maintained not only after removing mining cohorts from this analysis, but also after removing the remaining milling cohort.
Results did not only vary by study, but also within subgroups in individual studies. Furthermore, where risk magnitude was large, confidence intervals tended to be wide. For instance, male French Atomic Energy Commission Miners had an ERR/WLM for silicosis in all cohort members of 13.57 with a 95% CI from 2.52–236.0 (48). An SMR with a wide confidence interval was also presented for silicosis in Native American Colorado Plateau Miners in the follow-up period from 1991–2005 (SMR: 10.0; 95% CI: 4.00–21.4) (38). Therefore, the magnitude of these results, regardless of their statistical significance, suggests there may be an association between mining exposures and NMRD, while exposure to ionizing radiation only has minimal evidence of correlation to NMRD.
Some studies made efforts to adjust for potential occupational confounding factors such as dust. Dust exposure could confound the radiation-NMRD response and partly account for the conflicting associations seen between non-mining cohorts and NMRD. Silica dust exposure can also at least partially explain the high SMRs seen in mining cohorts, as silicosis is more common among miners than workers in other radiation-exposed professions due to these dust exposures (44). Furthermore, different types of radiation were assessed differently between studies; radon decay products, an internal exposure, was generally evaluated and assessed independently from external exposures to assess separate effects (33, 40, 43–45, 47, 48, 54, 55). In future analyses of nuclear workers, efforts should be made to control in this manner for competing exposures to distinguish potential toxins such as dust and radon decay products from external ionizing radiation.
Two studies of Mound and Mayak workers were excluded due to unique source exposure in Polonium-210 (65) and lack of mortality analyses (66). In the Mound worker study, mean dose received was 100.1 mSv but doses were highly skewed, with a maximum reported dose of 17, 500 mSv. The Mayak study assessed bronchitis incidence but not mortality. The Mound study reported similar SMRs to our findings while the Mayak study found some association with alpha radiation and bronchitis (ERR/Gy: 1.19; 95% CI: 0.32, 2.53). However, the majority of results from this study found no association between radiation and NMRD, consistent with the present systematic review (66).
The American Cancer Society Cancer Prevention Study cohort was used to conduct an ecologic COPD mortality study that focused on domestic exposures and was therefore excluded from this review (67). This study has a large sample size (n = 811, 961) spanning the United States with county-level average radon concentrations ranging from 6.3 to 265.7 Bq*m−3, though only 11.9% of the cohort was exposed to concentrations ≥30 Bq*m−3. Cox proportional hazards models adjusted for an array of individual-level risk factors including smoking. They found an elevated hazard ratio per 100 Bq*m−3 for NMRD of 1.08 (95% CI: 1.03, 1.13) that was largely driven by an elevated hazard ratio per 100 Bq*m−3 for COPD (HR: 1.13; 95% CI: 1.05, 1.21). These results were robust to a number of sensitivity analyses. It is possible that the relatively high power of this study was sufficient to detect an association where lower powered nuclear worker studies could not. Further, the ability to adjust for confounders was much better than most nuclear worker cohorts due to a plethora of confounder information, which could have contributed to the robustness of their estimates (67).
Of note, the 2017 UNSCEAR quality review criteria (24, 68) was used in this review. A series of papers was published in 2020 in the Journal of the National Cancer Institute Monographs, detailing separate quality assessments for dosimetry, epidemiology, confounding analyses, and other aspects of radiation epidemiology studies (50–55). The UNSCEAR 2017 criteria have been used successfully in the past to characterize studies in larger reviews (68–71), and we believe they are adequate for this analysis. Future reviews may consider employing these newer criteria.
The results of this review are subject to the limitations of each study. These individual study limitations are considered in this review, and show that of 31 studies, only 2 had a quality score of 3 (high quality) (6, 41), 8 had a quality score of 2.5 (moderate-high quality) (34, 37, 44, 45, 52, 56, 57, 59), and 4 had qualities scores of 2 (moderate quality) (32, 43, 46, 55) to assess a causal relationship between radiation exposure and NMRD. The large number of papers with lower ratings reflect several elements of these studies. Notably, they do not imply poor quality of a study, but rather showcase the difficulties inherent in radiation epidemiology (24). For instance, lack of population-specific information in retrospective cohort studies implies an inability of most studies to control for potential behavioral or environmental confounders such as socioeconomic status; smoking; or exposure to non-radiation, occupation-related toxins, which are characterized in the study specific strengths and limitations description (Table 5). Due to difficulties in worker cohorts to complete a full dosimetry profile and to verify cause of death, dosimetry and outcome classification errors are likely present (Table 4) even in the one prospective cohort study (38). Often, SMR analyses alone or dose reconstructions were necessary due to missing dose information in retrospective cohorts.
Radiation epidemiology studies can be underpowered to detect effects if their dose distribution is narrow and if their population is small. The Life Span Study (LSS) of atomic bomb survivors is often referred to as the gold standard of radiation epidemiology studies, with over 100, 000 individuals and a considerable proportion of people who received doses over 1 Gy (1). However, the LSS is not an occupational cohort. Sufficient power is difficult to achieve to detect the small level of risk expected for most long-term health outcomes following doses expected in occupational settings, particularly for noncancer outcomes since expected effect sizes are smaller than for cancer outcomes (71). Accordingly, many of these studies likely had insufficient power to detect a radiation effect on NMRD. While the 15-Country Study, INWORKS, the British National Registry for Nuclear Workers each had sample sizes over 100, 000 (6, 32, 37), these populations did not include as high a proportion of doses >1 Gy as the atomic bomb survivors, so that the power was lower compared with the LSS. A number of studies had small population sizes or limited dose distributions (29, 30, 33, 35, 36, 38, 39, 42, 45–48, 50, 53–57) and therefore lower statistical power than the LSS to detect potential differences in risk. More pooled studies of similar nuclear worker cohorts such as INWORKS (6) will be required to increase the statistical power to detect these risks, should a clinically significant risk exist for radiation-induced NMRD (6, 7, 72).
Several overall limitations existed in this review. Studies did not always report results using the same outcome, exposure, or effect estimate type. The present regression estimates are not comparable enough to include in a single forest plot, and the meta-analysis conducted could only consider overall NMRD for a handful of cohorts. Due to the limited pool of studies assessing NMRD, several cohorts are included multiple times: on their own and within pooled cohorts. As a result of this overlap, the meta-analysis had to exclude several cohorts providing relevant information. Additionally, different studies included different ICD codes in their overall NMRD definition, which may have inappropriately affected the findings of this meta-analysis. A meta-analysis of a more specific outcome such as COPD would likely be more informative and valid but could not be conducted here since there were not enough studies reporting results with the same dose estimate methodology, effect measure estimate, and type of units (e.g., WLM, mSv). The forest plots are limited in that they only represent results from papers that included SMRs of NMRD or COPD/emphysema overall, which were the most consistently reported outcomes throughout the studies. Moreover, exposures varied, with some populations experiencing either external exposure or internal exposure, and others experiencing both. SMR analyses did not typically distinguish between internal and external exposures, which further complicates these results. Different exposure sources are applicable to different sites; notably, in these studies, only the Sellafield plant had plutonium present (29, 34), while the remainder recorded only uranium-related and external photon exposures (6, 30–41, 43–58). Finally, effect estimate metrics varied, partly by necessity as exposure and outcome differed, and partly due to differences in analytic methods and software. These differences between studies limit the ability to compare them to one another and to make overall inferences.
Overall, this systematic review provides a useful understanding of the scope of the literature at the present time and highlights the low level of evidence for radiation exposure impacting NMRD by thoroughly assessing nuclear worker cohorts studied between 2000 and 2020. Additional meta-analyses, particularly of COPD, are warranted in the future, which would increase the power to detect associations or dose responses between radiation and NMRD outside of mining and milling occupations.
CONCLUSION
Our review of the present literature provides little evidence for an association between radiation exposures and NMRD in nuclear worker cohorts. The meta-analytic estimate of NMRD did not support an increased ERR per Sv for NMRD even when mining and milling cohorts were included. However, the increased SMRs seen in some individual cohorts would not be anticipated in the presence of the healthy worker effect. All elevated NMRD SMRs were among mining and milling cohorts. This review suggests regression analyses and analyses with pooled cohorts should adjust for mining exposures or address mining cohorts separately.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. John D. Boice, Jr. for providing advice and tools for radiation epidemiological quality review and for his editorial contributions. We additionally thank those who supported this project through consultation: Dr. Benjamin French, Dr. Melinda Aldrich, and Dr. Loren Lipworth, all from Vanderbilt University. Publication funding support provided by NCI-5U01CA152662–10 (Dr. Deppen).
Footnotes
Editor’s note. The online version of this article (DOI: https://doi.org/10.1667/RADE-21-00014.1) contains supplementary information that is available to all authorized users.
REFERENCES
- 1.Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, et al. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiat Res 2017; 187(5):513–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahoon EK, Preston DL, Pierce DA, Grant E, Brenner AV, Mabuchi K, et al. Lung, Laryngeal and Other Respiratory Cancer Incidence among Japanese Atomic Bomb Survivors: An Updated Analysis from 1958 through 2009. Radiat Res 2017; 187(5):538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrakova K, Vyskocil J, Grell P, Majek O, Soumarova R, Novak J, et al. Second cancers in Hodgkin’s lymphoma long-term survivals: A 60-year single institutional experience with real-life cohort of 871 patients. Int J Clin Pract 2018. Sep 1; 72(9):e13235. [DOI] [PubMed] [Google Scholar]
- 4.Richardson DB, Cardis E, Daniels RD, Gillies M, Haylock R, Leuraud K, et al. Site-specific Solid Cancer Mortality After Exposure to Ionizing Radiation: A Cohort Study of Workers (INWORKS). Epidemiol Camb Mass 2018. Jan; 29(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozasa K, Takahashi I, Grant EJ. Radiation-related risks of non-cancer outcomes in the atomic bomb survivors. Ann ICRP 2016. Jun; 45(1 Suppl):253–61. [DOI] [PubMed] [Google Scholar]
- 6.Gillies M, Richardson DB, Cardis E, Daniels RD, O’Hagan JA, Haylock R, et al. Mortality from Circulatory Diseases and other Non-Cancer Outcomes among Nuclear Workers in France, the United Kingdom and the United States (INWORKS). Radiat Res 2017. Sep; 188(3):276–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boice JDJ, Ellis ED, Golden AP, Girardi DJ, Cohen SS, Chen H, et al. The Past Informs the Future: An Overview of the Million Worker Study and the Mallinckrodt Chemical Works Cohort. Health Phys 2018. Apr; 114(4):381–5. [DOI] [PubMed] [Google Scholar]
- 8.Ellis ED, Boice JDJ, Golden AP, Girardi DJ, Cohen SS, Mumma MT, et al. Dosimetry is Key to Good Epidemiology: Workers at Mallinckrodt Chemical Works had Seven Different Source Exposures. Health Phys 2018. Apr; 114(4):386–97. [DOI] [PubMed] [Google Scholar]
- 9.Eisenbud M Early Occupational Exposure Experience with Uranium Processing (1975) Available from https://www.osti.gov/servlets/purl/4082757.
- 10.CDC. Pneumonia. Centers for Disease Control and Prevention (2018)] Available from: https://www.cdc.gov/pneumonia/index.html
- 11.CDC. Deaths: Final Data for 2015. Centers for Disease Control and Prevention; 2017. Nov. (National Vital Statistics Reports). Report No.: Vol. 66, No. 6. [Google Scholar]
- 12.World Health Organization. Chapter 6.22. Priority diseases and reasons for inclusion: Pneumonia 2013. (Priority Medicines for Europe and the World Update Report, 2013; Available from: https://www.researchgate.net/publication/256645674_Priority_Diseases_and_Reasons_for_Inclusion_622_Pneumonia).
- 13.Epstein MA, Reynaldo S, El-Amin AN. Is smoking a risk factor for influenza hospitalization and death? J Infect Dis 2010. Mar 1; 201(5):794–5. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Tuberculosis (TB. World Health Organization [cited 2018 Sep 15]. Available from: http://www.who.int/news-room/fact-sheets/detail/tuberculosis [Google Scholar]
- 15.CDC. TB Risk Factors ǀ Basic TB Facts Centers for Disease Control and Prevention: Tuberculosis. 2018. [cited 2018 Sep 15]. Available from: https://www.cdc.gov/tb/topic/basics/risk.htm [Google Scholar]
- 16.CDC. Work to End TB Centers for Disease Control and Prevention. 2017. [cited 2018 Sep 15]. Available from: https://www.cdc.gov/features/burden-tb-us/index.html [Google Scholar]
- 17.WHO. Chronic obstructive pulmonary disease (COPD) World Health Organization. [cited 2018 Sep 15]. Available from: http://www.who.int/respiratory/copd/en/ [Google Scholar]
- 18.American Lung Association. Pneumoconiosis Symptoms, Causes, and Risk Factors American Lung Association: Lung Health & Diseases. [cited 2018 Sep 15]. [Google Scholar]
- 19.CDC. Smoking and COPD Centers for Disease Control and Prevention: Tips from Former Smokers. 2018. [cited 2018 Sep 15]. Available from: https://www.cdc.gov/tobacco/campaign/tips/diseases/copd.html [Google Scholar]
- 20.Pham T-M, Sakata R, Grant EJ, Shimizu Y, Furukawa K, Takahashi I, et al. Radiation exposure and the risk of mortality from noncancer respiratory diseases in the Life Span Study, 1950–2005. Radiat Res 2013. Nov; 180(5):539–45. [DOI] [PubMed] [Google Scholar]
- 21.Park KJ, Chung JY, Chun MS, Suh JH. Radiation-induced lung disease and the impact of radiation methods on imaging features. Radiogr Rev Publ Radiol Soc N Am Inc 2000. Feb; 20(1):83–98. [DOI] [PubMed] [Google Scholar]
- 22.Ohshima S, Xu Y, Takahama M. Effects of uranium ore dust on cultured human lung cells. Environ Toxicol Pharmacol 1998. Jun; 5(4):267–71. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert ES, Cross FT, Dagle GE. Analysis of Lung Tumor Risks in Rats Exposed to Radon. Radiat Res 1996; 145(3):350–60. [PubMed] [Google Scholar]
- 24.UNSCEAR. Sources, effects and risks of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2016 report to the General Assembly, with scientific annexes New York: United Nations Scientific Committee on the Effects of Atomic Radiation; 2017 [Google Scholar]
- 25.PubMed. NIH National Library of Medicine Available from: https://www.ncbi.nlm.nih.gov/pubmed/ [DOI] [PubMed]
- 26.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985; 41(1):55–68. [PubMed] [Google Scholar]
- 27.Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ 2011. Aug 8; 343:d2090. [DOI] [PubMed] [Google Scholar]
- 28.Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, et al. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Prot 2018; 38(3). Available from: 10.1088/1361-6498/aad348 [DOI] [PubMed] [Google Scholar]
- 29.McGeoghegan D, Gillies M, Riddell AE, Binks K. Mortality and cancer morbidity experience of female workers at the British Nuclear Fuels Sellafield plant, 1946–1998. Am J Ind Med 2003. Dec 1; 44(6):653–63. [DOI] [PubMed] [Google Scholar]
- 30.Pinkerton LE, Bloom TF, Hein MJ, Ward EM. Mortality among a cohort of uranium mill workers: an update. Occup Environ Med 2004. Jan; 61(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- 31.Telle-Lamberton M, Samson E, Caër S, Bergot D, Bard D, Bermann F, et al. External radiation exposure and mortality in a cohort of French nuclear workers. Occup Environ Med 2007. Oct 1; 64(10):694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrijheid M, Cardis E, Ashmore P, Auvinen A, Bae J-M, Engels H, et al. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol 2007. Oct; 36(5):1126–35. [DOI] [PubMed] [Google Scholar]
- 33.Boice JD Jr, Cohen SS, Mumma MT, Chadda B, Blot WJ. A cohort study of uranium millers and miners of Grants, New Mexico, 1979–2005. J Radiol Prot 2008. Sep 1; 28(3):303–25. [DOI] [PubMed] [Google Scholar]
- 34.McGeoghegan D, Binks K, Gillies M, Jones S, Whaley S. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005. Int J Epidemiol 2008. Jun; 37(3):506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerin S, Richard G, Biau A, Lebre S, Crescini D, Haddy N, et al. Cancer mortality among French nuclear contract workers. Am J Ind Med 2009. Dec; 52(12):916–25. [DOI] [PubMed] [Google Scholar]
- 36.Metz-Flamant C, Rogel A, Caer S, Samson E, Laurier D, Acker A, et al. Mortality Among Workers Monitored for Radiation Exposure at the French Nuclear Fuel Company. Occup Health (Lond) 2009; 64(4):10. [DOI] [PubMed] [Google Scholar]
- 37.Muirhead CR, O’Hagan JA, Haylock RGE, Phillipson MA, Willcock T, Berridge GLC, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009. Jan; 100(1):206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubauer-Berigan MK, Daniels RD, Pinkerton LE. Radon Exposure and Mortality Among White and American Indian Uranium Miners: An Update of the Colorado Plateau Cohort. Am J Epidemiol 2009. Jan 6; 169(6):718–30. [DOI] [PubMed] [Google Scholar]
- 39.Guseva Canu I, Cardis E, Metz-Flamant C, Caër-Lorho S, Auriol B, Wild P, et al. French cohort of the uranium processing workers: mortality pattern after 30-year follow-up. Int Arch Occup Env Health 2010; 8. [DOI] [PubMed] [Google Scholar]
- 40.Lane RSD, Frost SE, Howe GR, Zablotska LB. Mortality (1950–1999) and Cancer Incidence (1969–1999) in the Cohort of Eldorado Uranium Workers. Radiat Res 2010. Dec 3; 174(6a):773–85. [DOI] [PubMed] [Google Scholar]
- 41.Laurent O, Metz-Flamant C, Rogel A, Hubert D, Riedel A, Garcier Y, et al. Relationship between occupational exposure to ionizing radiation and mortality at the French electricity company, period 1961–2003. Int Arch Occup Environ Health 2010. Dec; 83(8):935–44. [DOI] [PubMed] [Google Scholar]
- 42.McGeoghegan D, Whaley S, Binks K, Gillies M, Thompson K, McElvenny DM. Mortality and cancer registration experience of the Sellafield workers known to have been involved in the 1957 Windscale accident: 50 year follow-up. J Radiol Prot Off J Soc Radiol Prot 2010. Sep; 30(3):407–31. [DOI] [PubMed] [Google Scholar]
- 43.Boice JD Jr., Cohen SS, Mumma MT, Ellis ED, Eckerman KF, Leggett RW, et al. Updated Mortality Analysis of Radiation Workers at Rocketdyne (Atomics International), 1948–2008. Radiat Res 2011. Aug; 176(2):244–58. [DOI] [PubMed] [Google Scholar]
- 44.Kreuzer M, Sogl M, Brüske I, Möhner M, Nowak D, Schnelzer M, et al. Silica dust, radon and death from non-malignant respiratory diseases in German uranium miners. Occup Environ Med 2013. Dec 1; 70(12):869–75. [DOI] [PubMed] [Google Scholar]
- 45.Silver SR, Bertke SJ, Hein MJ, Daniels RD, Fleming DA, Anderson JL, et al. Mortality and ionising radiation exposures among workers employed at the Fernald Feed Materials Production Center (1951–1985). Occup Environ Med 2013. Jul; 70(7):453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zablotska LB, Lane RSD, Frost SE. Mortality (1950–1999) and cancer incidence (1969–1999) of workers in the Port Hope cohort study exposed to a unique combination of radium, uranium and γ-ray doses. BMJ Open 2013. Feb 27 [cited 2018 Sep 6]; 3(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3586082/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreuzer M, Dufey F, Laurier D, Nowak D, Marsh JW, Schnelzer M, et al. Mortality from internal and external radiation exposure in a cohort of male German uranium millers, 1946–2008. Int Arch Occup Environ Health 2015. May; 88(4):431–41. [DOI] [PubMed] [Google Scholar]
- 48.Rage E, Caër-Lorho S, Drubay D, Ancelet S, Laroche P, Laurier D. Mortality analyses in the updated French cohort of uranium miners (1946–2007). Int Arch Occup Environ Health 2015. Aug; 88(6):717–30. [DOI] [PubMed] [Google Scholar]
- 49.Samson E, Piot I, Zhivin S, Richardson DB, Laroche P, Serond A-P, et al. Cancer and non-cancer mortality among French uranium cycle workers: the TRACY cohort. BMJ Open 2016. Apr 5; 6(4):e010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhivin S, Guseva Canu I, Samson E, Laurent O, Grellier J, Collomb P, et al. Mortality (1968–2008) in a French cohort of uranium enrichment workers potentially exposed to rapidly soluble uranium compounds. Occup Environ Med 2016. Mar; 73(3):167–74. [DOI] [PubMed] [Google Scholar]
- 51.Leuraud K, Fournier L, Samson E, Caër-Lorho S, Laurier D. Mortality in the French cohort of nuclear workers 2017; 12. [Google Scholar]
- 52.Yiin JH, Anderson JL, Daniels RD, Bertke SJ, Fleming DA, Tollerud DJ, et al. Mortality in a combined cohort of uranium enrichment workers. Am J Ind Med 2017; 60(1):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouet S First mortality analysis in the French cohort of uranium millers (F-Millers), period 1968–2013. Int Arch Occup Env Health 2018; 11. [DOI] [PubMed] [Google Scholar]
- 54.Rage E, Caër-Lorho S, Laurier D. Low radon exposure and mortality among Jouac uranium miners: an update of the French cohort (1946–2007). J Radiol Prot Off J Soc Radiol Prot 2018. Mar; 38(1):92–108. [DOI] [PubMed] [Google Scholar]
- 55.Zablotska LB, Fenske N, Schnelzer M, Zhivin S, Laurier D, Kreuzer M. Analysis of mortality in a pooled cohort of Canadian and German uranium processing workers with no mining experience. Int Arch Occup Environ Health 2018. Jan; 91(1):91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouet S, Davesne E, Samson E, Jovanovic I, Blanchardon E, Challeton-de Vathaire C, et al. Analysis of the association between ionizing radiation and mortality in uranium workers from five plants involved in the nuclear fuel production cycle in France. Int Arch Occup Environ Health 2019. Feb; 92(2):249–62. [DOI] [PubMed] [Google Scholar]
- 57.Golden AP, Ellis ED, Cohen SS, Mumma MT, Leggett RW, Wallace PW, et al. Updated mortality analysis of the Mallinckrodt uranium processing workers, 1942–2012. Int J Radiat Biol 2019. Feb 13; 1–21. [DOI] [PubMed] [Google Scholar]
- 58.Kelly-Reif K, Sandler DP, Shore D, Schubauer-Berigan M, Troester MA, Nylander-French L, et al. Mortality and cancer incidence among underground uranium miners in the Czech Republic 1977–1992. Occup Environ Med 2019. Aug; 76(8):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2015. Jul; 2(7):e276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, Hamra GB, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015. Oct 20; 351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: Estimates of Radiation-Related Cancer Risks. Radiat Res 2007. Apr; 167(4):396–416. [DOI] [PubMed] [Google Scholar]
- 62.Dupree-Ellis E, Watkins J, Ingle JN, Phillips J. External Radiation Exposure and Mortality in a Cohort of Uranium Processing Workers. Am J Epidemiol 2000. Jul 1; 152(1):91–5. [DOI] [PubMed] [Google Scholar]
- 63.Muirhead CR. Radiation and noncancer diseases. Radiat Res 2004. Jun; 161(6):748. [DOI] [PubMed] [Google Scholar]
- 64.Rothman K, Greenland S, Lash T. Chapter 10: Precision and statistics in epidemiologic studies. In: Rothman K, Greenland S, Lash T, editors. Modern Epidemiology 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. p. 148–67. [Google Scholar]
- 65.Boice JDJ, Cohen SS, Mumma MT, Ellis ED, Cragle DL, Eckerman KF, et al. Mortality among mound workers exposed to polonium-210 and other sources of radiation, 1944–1979. Radiat Res 2014. Feb; 181(2):208–28. [DOI] [PubMed] [Google Scholar]
- 66.Azizova TV, Zhuntova GV, Haylock R, Moseeva MB, Grigoryeva ES, Bannikova MV, et al. Chronic bronchitis incidence in the extended cohort of Mayak workers first employed during 1948–1982. Occup Environ Med 2017. Feb; 74(2):105–13. [DOI] [PubMed] [Google Scholar]
- 67.Turner MC, Krewski D, Chen Y, Pope CA, Gapstur SM, Thun MJ. Radon and COPD mortality in the American Cancer Society Cohort. Eur Respir J 2012. May; 39(5):1113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, et al. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Prot 2018. Sep; 38(3):1217–33. [DOI] [PubMed] [Google Scholar]
- 69.Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, et al. Short Overview: Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Prot 2018. Sep; 38(3):1217–33. [DOI] [PubMed] [Google Scholar]
- 70.Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, et al. Short Memorandum: Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Prot 2018. Sep; 38(3):1217–33. [DOI] [PubMed] [Google Scholar]
- 71.Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, et al. Recent Epidemiologic Studies and the Linear No-Threshold Model For Radiation Protection—Considerations Regarding NCRP Commentary 27: Health Phys 2019. Feb; 116(2):235–46. [DOI] [PubMed] [Google Scholar]
- 72.Golden AP, Milder CM, Ellis ED, Anderson JL, Boice JDB, Bertke SJ, et al. Cohort profile: Four early uranium processing facilities in the US and Canada. Int J Radiat Biol 2021. May 10; 97(6):833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


