Abstract
Objective:
Mice and humans with reduced growth hormone (GH) action before birth are conferred positive health- and life-span advantages. However, little work has been performed to study the effect of conditional disruption of GH action in adult life. With this as our objective, we sought to elucidate a reproducible protocol that allows generation of adult mice with a global disruption of the GH receptor (Ghr) gene, using the tamoxifen (TAM)-inducible Cre-lox system, driven by the ROSA26 enhancer/promoter. Here we report the optimum conditions for the gene disruption.
Design:
Six month old mice, homozygous for the ROSA26-Cre and the Ghr-floxed gene, were injected, once daily for five days with four distinct TAM doses (from 0.08 to 0.32 mg of TAM/g of body weight). To evaluate the most effective TAM dose that leads to global disruption of the GHR, mRNA expression of the Ghr and insulin growth factor-1 (Igf1) genes were assessed in liver, adipose tissue, kidney, and skeletal and cardiac muscles of experimental and control mice. Additionally, serum GH and IGF-1 levels were evaluated one month after TAM injections in both, TAM-treated and TAM-untreated control mice.
Results:
A dose of 0.25 mg of TAM/g of body weight was sufficient to significantly reduce the Ghr and Igf1 expression levels in the liver, fat, kidney, and skeletal and cardiac muscle of six-month old mice that are homozygous for the Ghr floxed gene and Cre recombinase. The reduction of the Ghr mRNA levels of the TAM-treated mice was variable between tissues, with liver and adipose tissue showing the lowest and skeletal and cardiac muscle the highest levels of Ghr gene expression when compared to control mice. Moreover, liver tissue showed the ‘best’ Ghr gene disruption, resulting in decreased total circulating IGF-1 levels while GH levels were increased versus control mice.
Conclusion:
The results show that in mice at six months of age, a total TAM dose of at least 0.25 mg of TAM/g of body weight is needed for a global downregulation of Ghr gene expression with a regimen of 100 μL intraperitoneal (ip) TAM injections, once daily for five consecutive days. Furthermore, we found that even though this system does not achieve an equivalent disruption of the Ghr between tissues, the circulating IGF-1 is more than 95% decreased. This work helped to create adult mice with a global GHR knockdown.
Keywords: growth hormone, GHRKO mice, GHR−/− mice, iC-GHRKO, aGHRKO, tamoxifen, Cre-lox
1. Introduction
Studies in worms, fruit flies and mice have shown that a reduction of growth hormone (GH) and insulin growth factor -1 (GH/IGF-1) levels have positive health benefits and often results in increased longevity [1]. Besides the increased life-span, mice that have a germline mutation in the GH receptor (Ghr) gene, namely, GHR knockout (GHRKO) or GHR gene disrupted (GHR−/−) mice have also shown health benefits. These mice are dwarf, obese, with low circulating IGF-1 and insulin levels, display high insulin sensitivity, have low rates of cancer, and are resistant to obesity induced Type 2 diabetes [2]. Furthermore, the GHR −/− mice show a similar phenotype to humans that have a condition called Laron Syndrome (LS) in which the patients are GH resistant with low levels of IGF-1, high levels of GH, and are obese, dwarf, and resistant to the development of diabetes and cancer [3, 4]. Therefore, it has been hypothesized that impaired GH induced signaling could positively influence longevity and overall health of both humans and animals [5]. It is important to note that besides LS patients, other individuals with GH deficiencies due to Prop1 mutations or isolated GH deficiency, have shown reduced or unaltered longevity [6–10]. Nonetheless, both LS and GH-deficient humans are protected from age-related diseases [8, 11].
Since mouse and human studies have indicated that decreased GH action could increase health- and life span, drugs that could decrease GH have been proposed as an anti-aging medication. In fact, a 2013 workshop held in Erice, Italy comprising of leading experts in the field of aging research reached the conclusion that the most promising strategy to extend health- and life-span was “pharmacological inhibition of the GH/IGF-1 axis” [12].
On the other hand, as humans age, their circulating levels of GH decrease such that by 60 years of age, the amounts of GH circulating in the blood are greatly reduced [13]. This phenomenon is known as somatopause since the decreased skin thickness, increased fat deposits, and loss of lean mass in older humans has been attributed to the reduced circulating GH [13, 14]. It has been suggested that GH treatment for aging patients may improve their body composition and possibly health markers [15]. Nevertheless, concerns exist with regards to elevated GH levels in both humans and mice, which have been shown to cause an increased risk of developing cancer and diabetes mellitus [16, 17].
Most of the physiological studies that address the effects of GH resistance are performed in LS patients and in GHRKO mice, where decreased GH action occurs since birth. Besides Laron syndrome patients, a Dutch cohort between 85 and 100 years of age with polymorphisms that decrease the GH/IGF-1 axis have also shown increased lifespan, while the Ashkenazi Jewish centenarians with exceptional longevity have mutations in the IGF-1 receptor gene, resulting in a decrease in circulating IGF-1 [18–21]. Therefore, mice and human studies support the notion that opposed to the popular belief that GH is ‘good’ for the elderly as stated above, reduced GH action in adults may actually result in increased health and longevity. But, few data exists related to the hypothesis that reducing GH action in adult life may be beneficial in terms of health- and perhaps life-span [22–24]. Thus, we and others have set out to study the effects of disrupting GH action in adult life in order to elucidate the physiological and molecular implications of this disruption [22, 23, 25, 26]. Fortunately, molecular mechanisms to conditionally disrupt or introduce specific genes at a specific time in development have been established [27].
One of the technologies to disrupt specific genes in a time and tissue specific controlled manner is the tamoxifen (TAM) inducible Cre-lox system. This method involves the expression of the bacteriophage Cre recombinase driven by a specific promoter/enhancer. Cre recombinase can recognize 34 bp LoxP sites (placed flanking the gene that will be ablated also known as the ‘floxed’ gene) and induce recombination between these sites. Therefore, mice that express both the Cre recombinase and the floxed gene are capable of gene disruption [28]. The main advantage of this system is that it allows the conditional disruption of genes within specific tissues and at a specific desired age [28]. However, a peculiarity of the TAM inducible Cre-driver system is that the TAM dose that is effective to induce the recombination to ablate the target gene must be determined experimentally to ‘fit’ the specific characteristics of the mouse model [29]. According to Jackson laboratory indications, the use of different promoters/enhancers, and the age at which recombination is induced can play a role in the TAM dosage required for recombination to occur [30]. Therefore, the goal of this study was to determine the maximal conditions for generating adult mice with GHR disrupted globally at six months of age using the TAM-inducible Cre-lox technique, these mice will be referred as adult-onset-6 months GHR knock down (a6mGHRKD) mice.
2. Methodology
2.1. Mouse housing and breeding
Mice carrying a “floxed” Ghr allele flanking exon four of the Ghr, were generated by the Knockout Mouse Project and have been previously described (20, 21). Mice that express an inducible ubiquitous Cre recombinase gene driven by the ROSA26 gene promoter/enhancer (ROSA26-Cre-ERT2) [B6.129Gt(ROSA)26Sortm1(cre/ERT2)Tyj/J mice] were purchased from The Jackson Laboratory (22-24). C57BL/6 mice were bred to homozygosity for both the floxed Ghr and the Cre alleles as described [23]. Mice were housed at 22°C under a 14-hour light, 10-hour dark cycle, 3–4 mice per cage, with ad libitum access to water and standard laboratory chow (ProLab RMH 3000). All experiments were approved by the Ohio University Institutional Animal Care and Use Committee.
2.2. TAM treatment regimen
Mice homozygous for Cre and LoxP sites were used to determine the minimum TAM dose that will result in Ghr recombination. Five separate groups of male and female mice (six mice per group) were used. Once the animals reached six months of age, four of the groups were treated with varying doses of TAM, while one of the groups was injected with peanut oil (vehicle) as a control (Table 1). To induce Ghr gene disruption, mice received ~100 μL ip injections of TAM dissolved in peanut oil, once per day, over five consecutive days.
Table 1.
TAM concentrations used to standardize the ablation of the Ghr gene in male and female mice of six months of age.
| TAM concentration ( mg/g of body weight) | N |
|---|---|
| 0 (control) | 6 |
| 0.08 | 6 |
| 0.16 | 6 |
| 0.25 | 6 |
| 0.32 | 6 |
| Total mice | 30 |
2.3. Global Ghr disruption
Ghr and Igf1 gene expression of liver, kidney, subcutaneous (subq) adipose tissue, quadriceps (quad) skeletal muscle, and heart was measured in TAM-treated and control mice. Mice were sacrificed one month after TAM or peanut oil injections using CO2, dissected and organ harvested. Collected organs were snap frozen in liquid nitrogen and stored at −80°C. At the time of dissection, blood collection was performed via bleeding from the retro-orbital sinus.
For RNA isolation, frozen tissues were homogenized using a Precellys 24-Dual homogenizer, and RNA isolation was performed using the Thermo Scientific™ GeneJET RNA Purification Kit following manufacturer’s instructions. cDNA synthesis employed the Maxima First Strand cDNA Synthesis kit. RT-quantitative PCR (RTqPCR) to evaluate Ghr and Igf1 gene expression was performed using Maxima SYBR Green/Fluorescein qPCR Master Mix (Thermo Scientific). Two references target genes were used for normalization: Hprt: Forward: 5’-ATCAGTCAACGGGGGACATA-3’ Reverse 5’-AGAGGTCCTTTTCACCAGCA-3’, and Rpl38: Forward: 5’- CGCGTCGCCATGCCTCGGAA-3’ Reverse 5’- ACTTGGCATCCTTCCGCCGGG −3’. Data analysis was performed using qBasePlus software. Additionally, serum levels of GH and IGF-1 were evaluated using the mouse/rat Elisa kits from Alpco.
3. Results
3.1. Effective disruption of Ghr
To generate adult mice with global Ghr disruption, four different doses of TAM were injected in six months old mice homozygous for the Cre-floxed gene; control mice were injected with peanut oil alone. The Ghr mRNA levels were determined one month after TAM treatment (seven months of age) in the liver, fat (subq), kidney, skeletal muscle (quad) and heart tissues. While TAM doses of 0.08 and 0.16 mg of TAM/g of body weight were able to knockout the Ghr gene only in liver, doses of 0.25 and 0.32 mg of TAM/g of body weight were sufficient to induce significant knockdown of the Ghr gene in all tissues analyzed (Fig. 1A). Although all tissues showed a significant reduction in Ghr mRNA levels using 0.32 mg of TAM/g of body weight, the reduction in gene expression was variable between tissues. That is, liver had the best knockdown with more than 99% decreased Ghr mRNA levels (p<0.001), while quad and heart showed the smallest decrease in Ghr mRNA levels, with 63% and 65% reduction in gene expression respectively compared to control mice (p<0.001).
Fig. 1. Ghr disruption in mice of six months of age using varying TAM doses.
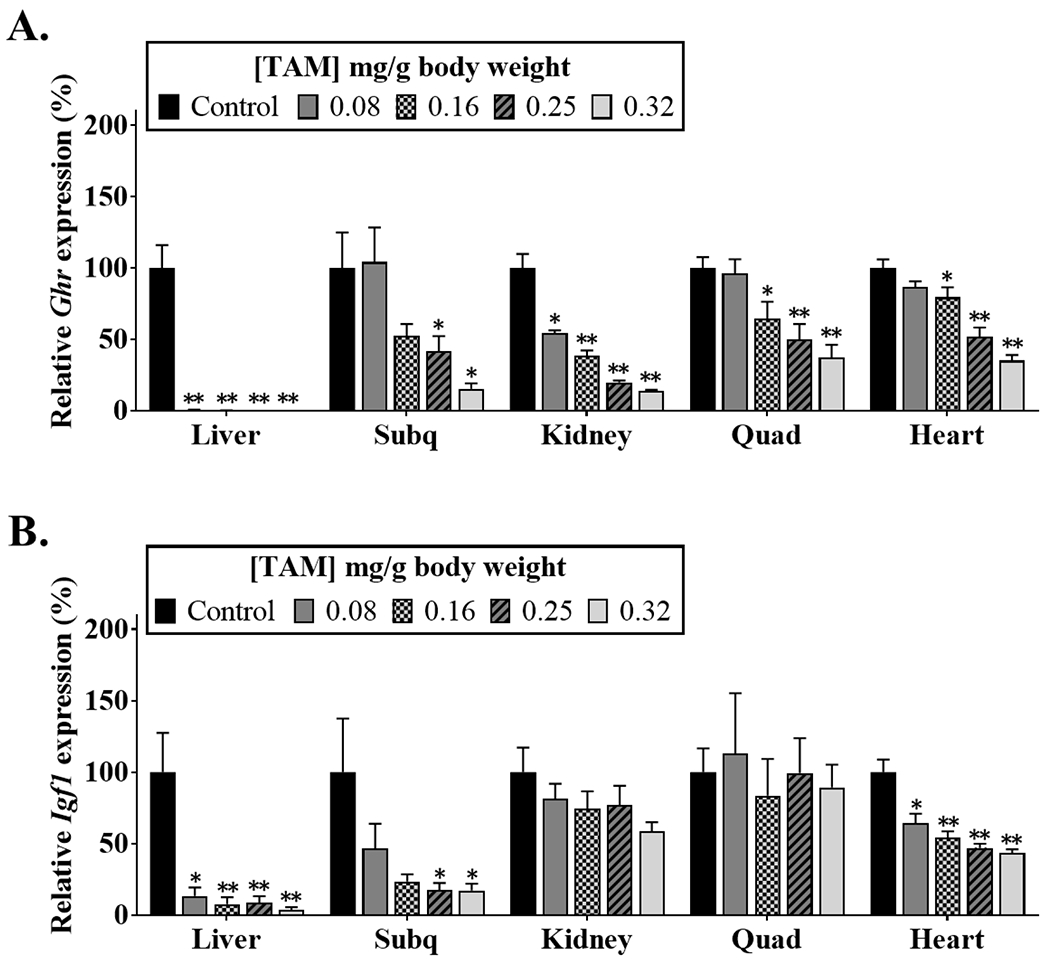
Ghr (A) and Igf1 (B) mRNA were measured by real-time qPCR in tissues collected at seven months of age (n = 6). Black bars represent control mice that were not treated with TAM. *p<0.05, ** p<0.005 between control and mice injected with TAM. Data is presented as ±SEM]. Subq, subcutaneous; Quad, quadriceps.
Because we were able to significantly reduce the Ghr gene expression, but not completely ablate the GH action in all tissues measured, we further investigated how this reduction in GH levels affected Igf1 gene expression in the different tissues (Fig. 1B). In agreement with the Ghr gene expression results, the IGF-I mRNA levels declined as the dose of TAM injected increased. Furthermore, mirroring the Ghr gene expression, liver and subq showed the lowest expression of Igf1 with a 96% and 83% reduction, respectively, when compared to control mice, at a dose of 0.32 mg of TAM/g of body weight. Surprisingly, even though the reduction in Ghr mRNA levels was similar between quad and heart; compared to controls, the expression levels of Igf1 were significantly diminished in the heart (p<0.001), with a 56% reduction, but not in quad of mice treated with a dose of 0.32 mg of TAM/g of body weight. Studies performed in rats and humans have shown that steroids, androgens or estrogens, can stimulate Igf1 gene expression. Therefore, it is possible that in mice other molecules besides GH are influencing the expression of Igf1 in quad [31, 32].
3.2. Circulating GH and IGF-1 levels
GH action stimulates IGF-1 release which acts as negative feedback on the release of GH from the pituitary. Without the negative feedback from IGF-1, one would expect higher levels of circulating GH. Serum GH and IGF-1 levels were determined at seven months of age in order to confirm disruption of Ghr signaling. As expected, all of the TAM-treated groups of mice showed high levels of circulating GH (Fig. 2A) and low levels of IGF-1 (Fig. 2B) relative to control mice.
Fig. 2. GH and IGF-1 feedback control is altered in adult mice after TAM injections.
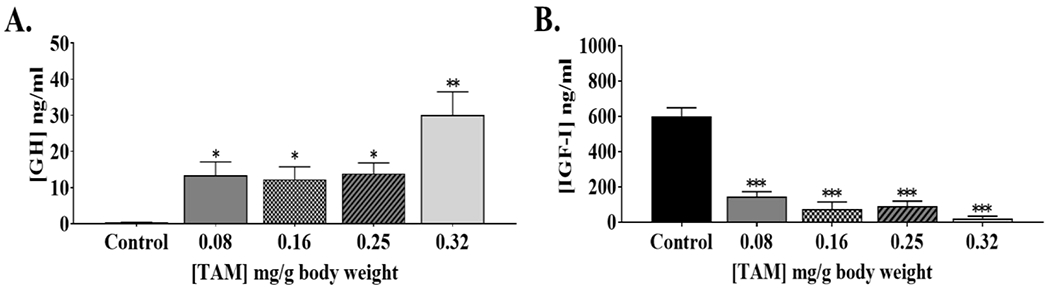
Serum GH (A) and IGF-1 (B) levels were determined at 7 months of age by ELISA (n = 6). Black bars represent control mice that were not treated with TAM. *p<0.05, ** p<0.005 between control and mice injected with TAM. Data is presented as ±SEM].
Discussion
The purpose of this study was to establish a protocol for global Ghr disruption in adult mice. Our findings showed that a dose of 0.25 and 0.32 mg of TAM/g of body weight is sufficient to significantly reduce the Ghr and Igf1 gene expression levels in the liver, fat, kidney, skeletal and cardiac muscle of mice that are homozygous for the Ghr floxed gene and the Cre recombinase under the control of the ROSA26 promoter/enhancer. In accordance with these results, circulating IGF-1 levels are decreased and GH levels are increased in the TAM treated mice when compared to control mice. Even though the Ghr mRNA levels of the TAM-treated mice were significantly reduced at the highest TAM doses, this decrease was not uniform between tissues, with liver and adipose tissue showing the highest and skeletal and cardiac muscle presenting the lowest reduction in Ghr gene expression when compared to control mice.
Complete whole-body disruption of gene expression using the Cre-lox system is difficult to achieve. Several factors may regulate the proper ablation of the Ghr gene globally in the mice, including the TAM dose used, the promoter/enhancer that drives Cre gene expression, and the accessibility of the gene to be disrupted, in this case the Ghr gene [29, 33]. In terms of the accessibility to the Ghr gene, our laboratory has created two TAM-inducible GHRKO mice at an adult age; the inducible heart-specific GHRKO (iC-GHRKO) and the global adult-onset GHRKO (aGHRKO) mice starting at six weeks of age [23, 34]. The accessibility of the floxed Ghr gene to the Cre recombinase could potentially change depending on the age of the mice, as well as the tissue to be disrupted [33]. In the prior aGHRKO study and the study reported here, C57BL6 mice were used. However, the mice were injected with TAM at six weeks of age in the previous study, instead of six months of age like in the present study [23]. Similar to the results obtained here, it was found that inducing global disruption of the Ghr gene at six weeks of age leads to an irregular reduction of Ghr mRNA levels in the tissues, with almost complete reduction of gene expression in the liver but a 55% to 65% decrease in the heart tissue of TAM female mice when compared to controls [23]. Because of the results obtained here, as well as in the aGHRKO study, it is tempting to think that the accessibility of the Cre recombinase to the Ghr floxed sequences varies between tissues and may not be age dependent. Nevertheless, results obtained in the iC-GHRKO study contradict this idea by showing 80% to 95% decrease in Ghr mRNA in TAM treated animals, even though they have the same Ghr floxed gene [34]. There are two main differences between the global GHR disrupted mice and the iC-GHRKO, namely, the promoter/enhancer used to drive Cre expression and the TAM dose and treatment regimen used in the studies. While the iC-GHRKO study used the myosin heavy chain 6 promoter/enhancer and the MerCreMer gene to drive Cre recombinase gene expression [34], the aGHRKO and this study used mice with the ROSA26 promoter/enhancer to promote Cre recombinase gene expression in all the tissues of the mice [23]; thus, it is possible that the ROSA26 promoter/enhancer is not as efficient in the skeletal and cardiac muscle of the mice. In support of this, gene expression analysis have shown that even though the ROSA26 locus is expressed ubiquitously in the mouse, the expression levels of this locus varies depending on the tissue and the developmental stage of the mice [35]. There are other TAM-inducible-Cre recombination mouse lines with different enhancers/promoters that could potentially be used for the global disruption of the Ghr gene. For example, the mouse line CAGGCre-ERTM uses the CMV-IE enhancer/chicken β-actin/rabbit β-globin hybrid promoter. [36] [29, 37, 38]; but a potential pitfall of using this mouse line is that homozygous mice for this promoter are not viable or fertile. Therefore, expansion of the colony could be difficult and time consuming, increasing significantly the time and cost of the experiments. Another mouse line that could potentially be used is the UBC-Cre-ERT2. This mouse line uses the human ubiquitin C (UBC) promoter and, according to Jackson laboratories, these mice have very strong Cre expression in all the tissues tested [39]. Although some reports have shown that these two promoters are effective in ablating a gene in several tissues [29, 40, 41], an evaluation that compares the extent of the effectiveness of each of the promoters (including ROSA26) in ablating genes globally is not available. Thus, it is unknown if these promoter/enhancers will be more effective in ablating the GHR in tissues such as skeletal muscle and heart than the ROSA26 promoter/enhancer.
The TAM doses used for this study were selected based on literature search and previous work made in our laboratory. The dose used in the iC-GHRKO study was a total of 0.08 mg of TAM/g body weight, administered as two ip injections, once per day for two days [34]. The dose reported for the aGHRKO study was five mg total of TAM per mouse. Even though this dose was not normalized to the body weight of the mice assuming that at six weeks of age mice weigh between 20-25 g, the dose given to the aGHRKO mice was between 0.25 and 0.32 mg of TAM/g of body weight. The TAM regimen for the aGHRKO project was 100 μL ip injections, once daily for five consecutive days (Table 2).
Table 2.
Characteristics of mice with postnatal Ghr gene disruption using the Cre-lox system.
| Characteristic | iC-GHRKO | aGHRKO | a6mGHRKD |
|---|---|---|---|
| Disrupted gene | GHR (exon 4) | GHR (exon 4) | GHR (exon 4) |
| CRE promoter/locus | MerCreMer | ROSA26 | ROSA26 |
| Tissue specificity | Adult cardiac myocytes | Global | Global |
| Age of disruption | 4 weeks | 6 weeks | 6 months |
| TAM dose | 0.08 mg/ g body weight | between 0.25 to 0.32 mg/ g body weight (5 mg total) | 0.32 mg/ g body weight |
| TAM dose regimen | 2 IP injections (once daily injection) | 5 IP injections (once daily injection for 5 consecutive days) | 5 IP injections (one daily injection for 5 consecutive days) |
It is important to note that TAM treatment can have toxic side effects in animals [42]. For example it was shown that ≥3 mg/20 g body weight (0.15 mg of TAM/g of body weight) dose of TAM leads to apoptosis of more than 90% of all gastric parietal cells and metaplasia of zymogenic chief cells within 3 days [43]. Myocardial dysfunction and decreased survival was also increased in a TAM dose dependent manner (30-80 μg TAM/g body weight) in mice with α-myosin-heavy-chain promoter (αMHC-MerCreMer) [44]. Long-term adverse effects on the reproductive system of male mice have also been shown when mice are treated with a single dose of 3 mg of TAM [45]. Furthermore, all the studies that we have encounter use a lower dose of TAM than 0.32 mg of TAM/g of body weight [29, 45], and Jackson Laboratories recommendation for TAM treatment is 0.075 mg of TAM/g of body weight [30]. Therefore, because of the adverse effects of TAM treatment we limited our TAM dose to 0.32 mg of TAM/g of body weight.
In terms of the TAM dose regimen, most of the studies with effective gene recombination use TAM treatment for 5 consecutive days [29, 45]. Also, after a single ip injection of 0.8 mg of TAM, the level of the Estrogen receptor TAM-activating metabolite (4-OHT), in the blood plasma of pure C57BL6J is evident 2 h post TAM administration and it returns to background levels after 24–48 h [46]. Therefore, to have the accumulative effects of TAM, consecutive injections need to be administrated. Also, because of the iC-GHRKO mouse line that was already used in our laboratory [34], we tried to ablate the GHR with the same dose regimen that was used in the heart-specific mouse study which consisted on two daily TAM injections. We saw that the mice that were injected with the higher concentrations of TAM (0.25 and 0.32 mg of TAM/g of body weight), died within a week of the two ip injections. It was also reported previously that a dose routine for the aGHRKO mice of a single injection or three daily injections of the same total TAM dose was not effective [23]. Therefore, it seems that specific promoter/enhancers and tissues may require not only different TAM doses, but also specific regimens in terms of the distribution of the daily injections to be able to work properly.
In summary, we have shown that in mice of six months of age, global downregulation of Ghr gene expression using the TAM-inducible ROSA26-Cre-lox system is possible using a regimen of 100 μL ip injections, once daily for five consecutive days. Furthermore, the TAM dose needs to be at least 0.25 mg of TAM/g of body weight, to achieve down-regulation in hard-to-knockdown tissues including skeletal and cardiac muscles. Importantly, since the circulating levels of IGF-I are more than 95% decreased, this system is useful to study the mouse physiology without IGF-I’s endocrine effect. We expect that this work will help to establish future mouse models with global disruption of specific genes at an adult age.
Acknowledgements
We would like to acknowledge Kevin Funk for helping with the daily tamoxifen injections.
Funding
This work was supported by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll; the Diabetes Institute at Ohio University; and the AMVETS.
Abbreviations:
- GH
growth hormone
- GHR
growth hormone receptor
- GHRKO
growth hormone receptor knockout
- GHR−/−
growth hormone receptor disrupted
- IGF-1
insulin-like growth factor 1
- Subq
subcutaneous
- quad
quadriceps
- TAM
tamoxifen
- iC-GHRKO
inducible heart-specific growth hormone receptor knockout
- aGHRKO
global adult growth hormone receptor knockout
- ip
intraperitoneal
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- [1].Bartke A, Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging, Endocrinology, 146 (2005) 3718–3723. [DOI] [PubMed] [Google Scholar]
- [2].List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ, Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse, Endocr Rev, 32 (2011) 356–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng C-W, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD, Growth Hormone Receptor Deficiency Is Associated with a Major Reduction in Pro-Aging Signaling, Cancer, and Diabetes in Humans, Science Translational Medicine, 3 (2011) 70ra13–70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kopchick JJ, Laron Z, Is the Laron mouse an accurate model of Laron syndrome?, Mol Genet Metab, 68 (1999) 232–236. [DOI] [PubMed] [Google Scholar]
- [5].Bartke A, List EO, Kopchick JJ, The somatotropic axis and aging: Benefits of endocrine defects, Growth Horm IGF Res, 27 (2016) 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Krzisnik C, Kolacio Z, Battelino T, Brown M, Parks JS, Laron Z, The “Little People” of the Island of Krk - Revisited. Etiology of Hypopituitarism Revealed, in: International Journal on Disability and Human Development, 1999, pp. 9.
- [7].Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE, Reduced longevity in untreated patients with isolated growth hormone deficiency, J Clin Endocrinol Metab, 88 (2003) 3664–3667. [DOI] [PubMed] [Google Scholar]
- [8].Laron Z, The GH-IGF1 axis and longevity. The paradigm of IGF1 deficiency, Hormones, 7 (2008) 24–27. [DOI] [PubMed] [Google Scholar]
- [9].Aguiar-Oliveira MH, Oliveira FT, Pereira RM, Oliveira CR, Blackford A, Valenca EH, Santos EG, Gois-Junior MB, Meneguz-Moreno RA, Araujo VP, Oliveira-Neto LA, Almeida RP, Santos MA, Farias NT, Silveira DC, Cabral GW, Calazans FR, Seabra JD, Lopes TF, Rodrigues EO, Porto LA, Oliveira IP, Melo EV, Martari M, Salvatori R, Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene, J Clin Endocrinol Metab, 95 (2010) 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Basu R, Qian Y, Kopchick JJ, MECHANISMS IN ENDOCRINOLOGY: Lessons from growth hormone receptor gene-disrupted mice: are there benefits of endocrine defects?, Eur J Endocrinol, 178 (2018) R155–R181. [DOI] [PubMed] [Google Scholar]
- [11].Bartke A, Healthspan and longevity can be extended by suppression of growth hormone signaling, Mamm Genome, 27 (2016) 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L, Interventions to Slow Aging in Humans: Are We Ready?, Aging Cell, 14 (2015) 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE, Effects of human growth hormone in men over 60 years old, N Engl J Med, 323 (1990) 1–6. [DOI] [PubMed] [Google Scholar]
- [14].Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ, The GH/IGF-1 axis in ageing and longevity, Nat Rev Endocrinol, 9 (2013) 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hoffman AR, Ceda GP, IGFs and aging: is there a rationale for hormone replacement therapy?, Growth Horm IGF Res, 14 (2004) 296–300. [DOI] [PubMed] [Google Scholar]
- [16].Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR, Systematic review: the safety and efficacy of growth hormone in the healthy elderly, Ann Intern Med, 146 (2007) 104–115. [DOI] [PubMed] [Google Scholar]
- [17].Basu R, Wu S, Kopchick JJ, Targeting growth hormone receptor in human melanoma cells attenuates tumor progression and epithelial mesenchymal transition via suppression of multiple oncogenic pathways, Oncotarget, 8 (2017) 21579–21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Milman S, Huffman DM, Barzilai N, The Somatotropic Axis in Human Aging: Framework for the Current State of Knowledge and Future Research, Cell Metab, 23 (2016) 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RG, Reduced insulin/IGF-1 signalling and human longevity, Aging Cell, 4 (2005) 79–85. [DOI] [PubMed] [Google Scholar]
- [20].Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P, Functionally significant insulin-like growth factor I receptor mutations in centenarians, Proc Natl Acad Sci U S A, 105 (2008) 3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van der Spoel E, Rozing MP, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, de Craen AJ, Westendorp RG, van Heemst D, Association analysis of insulin-like growth factor-1 axis parameters with survival and functional status in nonagenarians of the Leiden Longevity Study, Aging, 7 (2015) 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M, Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span, Endocrinology, 146 (2005) 2920–2932. [DOI] [PubMed] [Google Scholar]
- [23].Junnila RK, Duran-Ortiz S, Suer O, Sustarsic EG, Berryman DE, List EO, Kopchick JJ, Disruption of the GH Receptor Gene in Adult Mice Increases Maximal Lifespan in Females, Endocrinology, 157 (2016) 4502–4513. [DOI] [PubMed] [Google Scholar]
- [24].Kraemer WJ, Kennett MJ, Mastro AM, McCarter RJ, Rogers CJ, DuPont WH, Flanagan SD, Turbitt WJ, Fragala MS, Post EM, Hymer WC, Bioactive growth hormone in older men and women: It’s relationship to immune markers and healthspan, Growth Horm IGF Res, 34 (2017) 45–54. [DOI] [PubMed] [Google Scholar]
- [25].Bartke A, Healthspan and longevity can be extended by suppression of growth hormone signaling, Mammalian genome : official journal of the International Mammalian Genome Society, 27 (2016) 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luque RM, Lin Q, Cordoba-Chacon J, Subbaiah PV, Buch T, Waisman A, Vankelecom H, Kineman RD, Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes, PLoS One, 6 (2011) 0015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang J, Zhao J, Jiang W.-j., Shan X.-w., Yang X.-m., Gao J.-g., Conditional gene manipulation: Cre-ating a new biological era, Journal of Zhejiang University. Science. B, 13 (2012) 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nagy A, Cre recombinase: the universal reagent for genome tailoring, Genesis, 26 (2000) 99–109. [PubMed] [Google Scholar]
- [29].Feil S, Valtcheva N, Feil R, Inducible Cre mice, Methods Mol Biol, 530 (2009) 343–363. [DOI] [PubMed] [Google Scholar]
- [30].Heffner C, Intraperitoneal injection of tamoxifenfor inducible CRE-drivers lines, in, The Jackson Laboratory, 2011. [Google Scholar]
- [31].Pollanen E, Ronkainen PH, Horttanainen M, Takala T, Puolakka J, Suominen H, Sipila S, Kovanen V, Effects of combined hormone replacement therapy or its effective agents on the IGF-1 pathway in skeletal muscle, Growth Horm IGF Res, 20 (2010) 372–379. [DOI] [PubMed] [Google Scholar]
- [32].Oberbauer A, The Regulation of IGF-1 Gene Transcription and Splicing during Development and Aging, Frontiers in Endocrinology, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guo C, Yang W, Lobe CG, A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action, Genesis, 32 (2002) 8–18. [DOI] [PubMed] [Google Scholar]
- [34].Jara A, Liu X, Sim D, Benner CM, Duran-Ortiz S, Qian Y, List EO, Berryman DE, Kim JK, Kopchick JJ, Cardiac-Specific Disruption of GH Receptor Alters Glucose Homeostasis While Maintaining Normal Cardiac Performance in Adult Male Mice, Endocrinology, 157 (2016) 1929–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See LH, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu YC, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, Mishra T, Jain D, Dogan N, Harris RS, Cayting P, Kawli T, Boyle AP, Euskirchen G, Kundaje A, Lin S, Lin Y, Jansen C, Malladi VS, Cline MS, Erickson DT, Kirkup VM, Learned K, Sloan CA, Rosenbloom KR, Lacerda de Sousa B, Beal K, Pignatelli M, Flicek P, Lian J, Kahveci T, Lee D, Kent WJ, Ramalho Santos M, Herrero J, Notredame C, Johnson A, Vong S, Lee K, Bates D, Neri F, Diegel M, Canfield T, Sabo PJ, Wilken MS, Reh TA, Giste E, Shafer A, Kutyavin T, Haugen E, Dunn D, Reynolds AP, Neph S, Humbert R, Hansen RS, De Bruijn M, Selleri L, Rudensky A, Josefowicz S, Samstein R, Eichler EE, Orkin SH, Levasseur D, Papayannopoulou T, Chang KH, Skoultchi A, Gosh S, Disteche C, Treuting P, Wang Y, Weiss MJ, Blobel GA, Cao X, Zhong S, Wang T, Good PJ, Lowdon RF, Adams LB, Zhou XQ, Pazin MJ, Feingold EA, Wold B, Taylor J, Mortazavi A, Weissman SM, Stamatoyannopoulos JA, Snyder MP, Guigo R, Gingeras TR, Gilbert DM, Hardison RC, Beer MA, Ren B, A comparative encyclopedia of DNA elements in the mouse genome, Nature, 515 (2014) 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].T.J. Laboratory, MOUSE STRAIN DATASHEET - 004682, in: T.J. Laboratory (Ed.), The Jackson Laboratory. [Google Scholar]
- [37].Hayashi S, McMahon AP, Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse, Dev Biol, 244 (2002) 305–318. [DOI] [PubMed] [Google Scholar]
- [38].Hoopes SL, Willcockson HH, Caron KM, Characteristics of multi-organ lymphangiectasia resulting from temporal deletion of calcitonin receptor-like receptor in adult mice, PLoS ONE, 7 (2012) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].T.J. Laboratory, MOUSE STRAIN DATASHEET - 008085, in: T.J. Laboratory (Ed.), The Jackson Laboratory. [Google Scholar]
- [40].Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ, Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss, Cell Stem Cell, 1 (2007) 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D, Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors, Science, 295 (2002) 868–872. [DOI] [PubMed] [Google Scholar]
- [42].Phillips DH, Understanding the genotoxicity of tamoxifen?, Carcinogenesis, 22 (2001) 839–849. [DOI] [PubMed] [Google Scholar]
- [43].Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC, Tamoxifen induces rapid, reversible atrophy and metaplasia in mouse stomach, Gastroenterology, 142 (2012) 21–24.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bersell K, Choudhury S, Mollova M, Polizzotti BD, Ganapathy B, Walsh S, Wadugu B, Arab S, Kühn B, Moderate and high amounts of tamoxifen in αMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death, Disease Models & Mechanisms, 6 (2013) 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Patel SH, O’Hara L, Atanassova N, Smith SE, Curley MK, Rebourcet D, Darbey AL, Gannon A-L, Sharpe RM, Smith LB, Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: implications for inducible transgenics, Scientific Reports, 7 (2017) 8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wilson CH, Gamper I, Perfetto A, Auw J, Littlewood TD, Evan GI, The kinetics of ER fusion protein activation in vivo, Oncogene, 33 (2014) 4877. [DOI] [PMC free article] [PubMed] [Google Scholar]


