Abstract
The understanding of the neural control of appetite sheds light into the pathogenesis of eating disorders, such as anorexia nervosa and obesity. Both diseases are a result of maladaptive eating behaviors (overeating or undereating) and associated with life-threatening health problems. The fine regulation of appetite involves genetic, physiological and environmental factors which are detected and integrated in the brain by specific neuronal populations. For centuries, the hypothalamus has been the center of attention in the scientific community as a key regulator of appetite. The hypothalamus receives and sends axonal projections to several other brain regions that are important for the integration of sensory and emotional information. These connections ensure that appropriate behavioral decisions are made depending on the individual’s emotional state and environment. Thus, the mechanisms by which higher-order brain regions integrate exteroceptive information to coordinate feeding is of great importance. In this review, we will focus on the functional and anatomical projections connecting the hypothalamus to the limbic system and higher-order brain centers in the cortex. We will also address the mechanisms by which specific neuronal populations located in higher-order centers regulate appetite and how maladaptive eating behaviors might arise from altered connections among cortical and subcortical areas with the hypothalamus.
Keywords: hypothalamus, cortex, subcortical areas, appetite, eating disorders, obesity
Introduction
An ethologic framework underpinning the control of feeding and other innate behaviors was set forth by Tinbergen decades ago, but their neural substrates were largely uncharacterized (1). Recent technical advances have led to the identification of molecularly defined neural circuits regulating feeding and the interoceptive and sensory inputs that modulate their activity. A deeper understanding of these neural mechanisms is of intrinsic importance and is also likely to shed light on the pathogenesis of maladaptive eating behaviors underlying both obesity and eating disorders.
A key role of the hypothalamus in feeding was first shown in studies reporting that ablation of specific nuclei dramatically alters food intake and body weight in animals (2–4). Lesions of the ventromedial hypothalamus result in obesity, while lateral hypothalamic lesions lead to inanition (2–4). Recent studies have focused on other hypothalamic nuclei (i.e. arcuate nucleus) and extrahypothalamic brain regions (i.e. cortex and subcortex) which receive orexigenic and anorexigenic signals to regulate appetite. Classic studies on decerebrate rodents showed that some of the motor components of feeding can be performed with just a brainstem (5). Nonetheless, higher-order brain regions were necessary for the complex regulation of feeding and recent studies have showed that environmental factors integrated by these centers play a substantial role in the regulation of feeding. From an anatomical point of view, the hypothalamus receives and sends axonal projections to several higher-order brain regions that are important for the integration of sensory information. These connections are particularly important for the control of appetite, ensuring that appropriate behavioral decisions are made depending on the individual’s environment. Thus, the mechanisms by which higher-order brain regions contribute to the coordination of eating behavior is of great importance.
In this review, we will focus mostly on rodent studies that address the functional and anatomical projections connecting the hypothalamus, a key feeding center, to higher-order brain centers in the cortex and subcortex (See Figures 1 and 2). We will also address the mechanisms by which these neuronal populations and inputs from higher-order centers regulate appetite. Last, we will address how maladaptive eating behaviors might arise from altered connections among cortex and subcortical areas with the hypothalamus and how these may relate to comorbidities with other psychiatric disorders.
Fig 1. Cortical and Subcortical connections to Hypothalamus.
Efferent and afferent projections from the hypothalamus to many cortical and subcortical structures are summarized in this figure. Many of these projections are important in the regulation of hypothalamic activity and appetite control. OFC: orbitofrontal cortex; IC: insular cortex: HPC: hippocampus, HY: hypothalamus, PFC: prefrontal cortex, ACC: anterior cingulate cortex; LS: lateral septum; BNST: bed nucleus of stria terminalis; Amy: Amygdala.
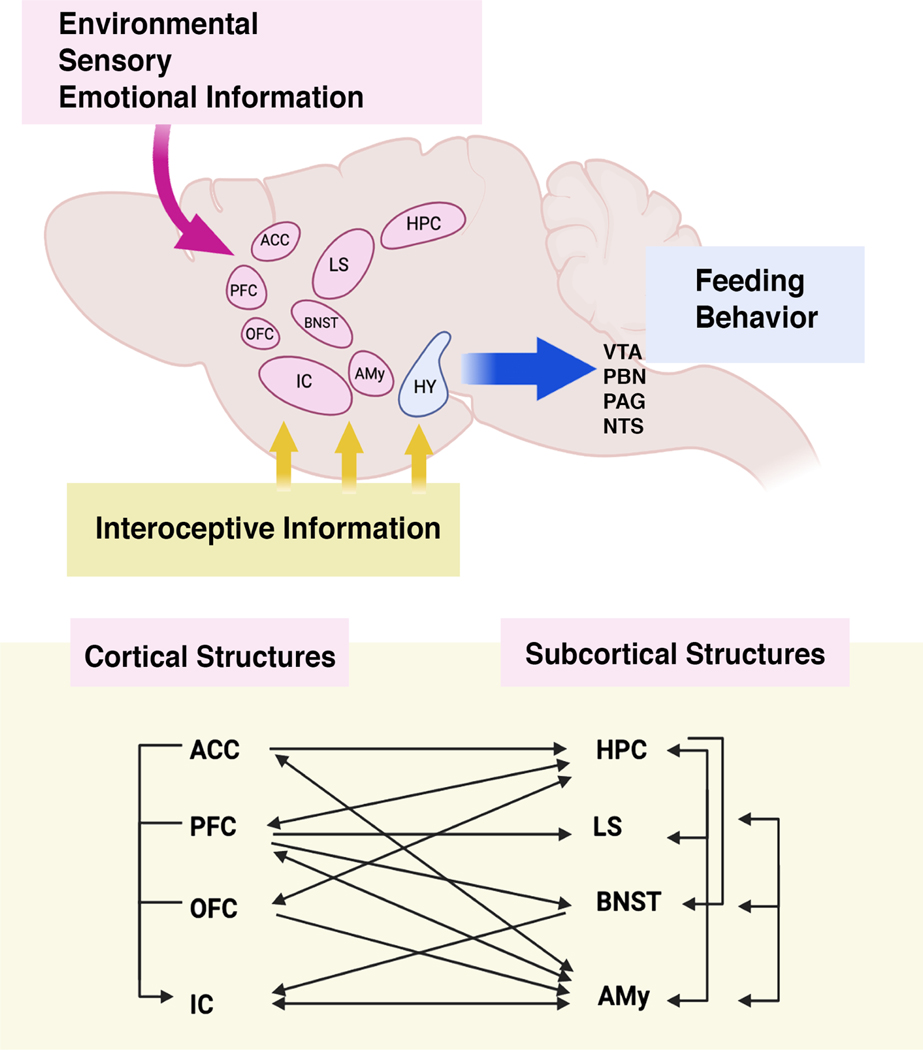
Fig 2. Coordination of eating behavior by multiple brain regions.
Together with the hypothalamus, the insular cortex and other brain regions receive interoceptive information from peripheral organs. This information is processed along with environmental, sensory and emotional information by other cortical and subcortical areas, ultimately leading to changes in food intake. The hypothalamus ultimately projects to midbrain and brainstem areas controlling behavior (VTA: ventral tegmental area; PBN: parabrachial nucleus; PAG: periaqueductal gray; NTS: nucleus tractus solitarius). Many of the cortical (ACC: anterior cingulate cortex, PFC: prefrontal cortex, OFC: orbitofrontal cortex, IC: insular cortex) and subcortical (HPC: hippocampus, LS: lateral septum, BNST: bed nucleus of stria terminalis; Amy: Amygdala) are reciprocally connected and control information outflow.
1. Role of Limbic Systems in Regulating Food Intake
The term “limbic”, Latin for “border”, was termed by Paul Pierre Broca in 1878 when he described the cingulate and the parahippocampal gyri as regions at the border between the cerebral hemispheres and the brainstem (6). However, this term was only associated with a specific function by James Papez in 1937, when he proposed that this collection of regions play a role in the control of emotions (6). Subsequently, other brain regions such as the hippocampus, amygdala, and lateral septum were included as limbic areas. Although each of these has specific specialized functions, each is also involved in the regulation of several behaviors, including food intake.
1.1-. Hippocampus
The hippocampus has a long literature associated with learning, memory and spatial navigation, but also plays a role in regulating food intake. An early report showed that electrical stimulation of the hippocampus inhibited performance in a conditioned feeding task (7) suggesting an inhibitory role for the hippocampus both on arousal and hunger. Later studies demonstrated that nonselective damage to the hippocampus in rodents and humans were able to elicit hyperphagia (8–10). Indeed, a study on the behavioral patterns of patient H.M., who exhibited anterograde amnesia due to bilateral hippocampal damage, reported problems in satiety regulation (8). Despite this, the role of the hippocampus in appetite was still unclear due to the fact that many of these patients, including H.M, had widespread lesions that often extended to other brain regions regulating appetite.
Space and time are directly represented in the hippocampus (11,12), and accordingly, an association between space and reward is essential for effective foraging. In 2019, Trouche and colleagues showed a direct connection from the dorsal hippocampal CA1 region to nucleus accumbens that modulates place-reward memories(13). Additionally, hippocampal Drd2-dependent projections to the septum regulate food-place associations (14). These findings suggest the existence of circuits that integrate information about neuronal representations of space to link reward to specific locations and regulate food intake. Other studies suggest a role for the hippocampus in the representation of non-spatial information also relevant for eating behavior. Studies have shown that hippocampal neurons change their activity in the presence of natural olfactory cues and mechanical gastric distension (14–18). These reports suggest that the hippocampus might not only serve as a cognitive map important for spatial navigation but can also represent sensory and interoceptive information and link it to that map.
Infusion of specific neuropeptides, like ghrelin and glucagon-like peptide-1 (GLP-1), into the hippocampus modulate food intake in rodents (19–21). Studies using genetic tools to modulate specific cell-types within the hippocampus have identified specific neuronal populations within the ventral hippocampus and dorsal hippocampus that regulate food intake, avoidance behavior and food-place memory, via projections to the septum (14,22). Further evidence has also raised the possibility that both dorsal and ventral hippocampus regulate appetite via indirect and direct connections to the lateral hypothalamus (14,22,23). Overall, these studies suggest that the hippocampus serves as a gatekeeper, and exerts a prominent inhibitory role on appetite.
1.2-. Amygdala
The role of the amygdala in appetite regulation was first described in the 1960’s with reports demonstrating alterations in food intake in dogs or rodents after bilateral lesions of the amygdala (24,25) Amygdala neurons regulate appetite through its direct and indirect connections to the hypothalamus. Classic work from Krettek and Price (26), together with recent analyses, have defined anatomical projections from the amygdala to the dorsomedial, ventromedial and lateral hypothalamus. These hypothalamic areas regulate aggression, appetite and stress responses, thus suggesting that the amygdala is important for the regulation of many innate behaviors, including food intake. This, in fact, raises the possibility that the amygdala might play a general role in determining the selection of a specific behavior.
The amygdala is one of the major targets of the hippocampus (27) and for this reason, has been recently invoked as playing a role to associate cues with food reward. The amygdala receives information regarding the spatial and sensory representations built by the hippocampus and integrates that with the salient value of that experience (28,29). Indeed, the amygdala was recently shown to serve as an integrator of hedonic, visceral and external signals, tuning innate behaviors such as feeding through negative or positive feedback onto its targets in the cortex, subcortex, hypothalamus and brainstem (30–36).
Several populations and neuropeptides have been demonstrated to participate in the regulation of appetite and eating behavior by the amygdala, among them Pnoc-, Htr2a-, and Pkcd-expressing neurons (30,34,37,38) and the neuropeptides neurotensin and orexin (36,39). The exact mechanisms by which these populations exert its effects onto specific brain areas and how they communicate with each other and hypothalamus to regulate appetite in health and disease are still unclear and represent an important area for future research.
1.3-. Lateral Septum
The lateral septum (LS) is one of several nuclei within the septal area and can be further subdivided into rostral, ventral and caudal regions. Early lesion studies suggested several possible functions for the LS, including aggression, emotional control, social behavior and food consumption. The LS receives inputs from the hippocampus, amygdala and entorhinal cortex, among others, and sends a major projection to the hypothalamus, raising the possibility that this nucleus serves as a conduit between higher structures and the hypothalamus to control innate behaviors. Thus, much like the amygdala, LS may function as an integrator of representations built by hippocampal neurons.
Early lesioning studies of the LS led to aggression, suggesting that it played a role in emotional self-regulation (40,41). The term “septal rage” or “septal syndrome” was often used to describe the profound alterations of emotional state after septal lesions. These initial reports also noted that animals with septal lesions showed enhanced predatory behavior, raising the possibility that it also plays a role to regulate food intake (42,43).
Specific neurons in the LS sense stressful stimuli and regulate fear (44), anxiogenic (45,46) and eating behaviors (47–49). GABAergic LS neurons expressing neurotensin modulate food intake via projections to the lateral hypothalamus specifically in response to stressors that elicit active escape (47). Pharmacologic experiments revealed a broad network of other receptors and signaling pathways in the LS that also appear to be involved in the regulation of appetite. For example, several studies have shown that septal infusions of substance P (50), urocortin (51), GLP-1 (52,53), alpha(1)-adrenoceptors (54), neuropeptide Y (55), growth hormone secretagogue receptors (56) (GHSRs), mu opioid receptors (57) and CRF2 receptors (48) reduce food intake. While there may be some overlap with neurotensin neurons in the LS (47), molecular profiling has suggested that there are several distinct appetite-suppressing populations (47). In aggregate, these studies suggest that the LS integrates visceral, spatial and sensory information with emotional experiences (hedonic or aversive) and relays this information to hypothalamus and other brain regions to control food intake.
2. Cortical control of eating behavior
Evidence that the cerebral cortex can regulate food intake dates to the 1950’s when it was shown that spreading depression through the cortex disrupts eating and drinking, likely through attenuation of lateral hypothalamic activity (58,59). In 1970, Huston and Bures also showed that a single wave of spreading cortical depression could elicit voracious eating and drinking in satiated rats following an initial attenuation (60). These studies suggested that the cortex might exhibit control over hypothalamic circuits regulating feeding and have been followed by numerous studies showing specific effects of several different cortical regions.
2.1. Prefrontal Cortex
The prefrontal cortex is arguably the most differentiated structure among mammals (61–63). Nonetheless, despite these major differences, abundant evidence indicates that prefrontal areas play an important role in regulating food intake across species. In rodents, areas referred to as the prefrontal cortex typically comprise the infralimbic, prelimbic and cingulate cortices and direct effects on food intake have consistently been found for each. In mice, stimulation of dopamine receptor-1 neurons in the prelimbic/infralimbic cortex, as well as their axon terminals in the basolateral amygdala (BLA), which in turn projects to the hypothalamus (64), elicit consumption, whereas inhibition decreases food intake. This stimulation did not increase water intake, indicating that this response is specific for food (65). Furthermore, stimulation of mu-opioid receptors in the infralimbic region elicits both increased food consumption and hyperactivity (66), which is antagonized by NMDA receptor blockade in the lateral hypothalamus (67). However, global inactivation of the infralimbic region using GABA agonists had no effect on food intake (66,68), suggesting that different populations within the infralimbic cortex may have dissociable effects on eating behavior.
Projections to the prelimbic/infralimbic region also have effects on food intake. For example, Glp1r signaling in the hippocampus elicits hypophagia, which is mediated by NMDA receptors in the prelimbic/infralimbic region (19), while inactivation of the central amygdala (CeA) decreased dopamine release in the infralimbic/prelimbic area during the presentation of a meal (69). The act of eating itself also has direct effects on neural activity in the prefrontal cortex. For example, in fasted mice, eating in conjunction with lever pressing leads to dopamine release in both the cingulate and infralimbic cortices.
In humans, the prefrontal cortex also plays an important role in eating, self-regulation and the cognitive control of cravings. For example, eating disorder patients have increased impulsivity and trouble self-regulating their responses to food rewards (70). Studies involving repetitive transcranial magnetic stimulation (rTMS) are also informative. rTMS is a type of brain stimulation protocol in which strong magnetic fields exert net excitatory and/or net suppressive effects on neuronal populations within the cortex. Excitatory stimulation in the dlPFC using rTMS reliably increases the ability to avoid attention capture by calorie dense foods, thus inhibiting consumption and improving cravings in healthy subjects and patients diagnosed with eating disorders (i.e. bulimia (71) and obesity (72)). Conversely, experimental studies using a suppressive stimulation protocol (low frequency stimulation) using rTMS in the dlPFC show increases in consumption of calorie dense foods, especially when there are in the presence of facilitative cues (73). These studies, using either excitatory or suppressive stimulation protocols, strongly suggest a role for dlPFC in top-down modulation of food cravings and eating behavior, specifically with respect to ingestion calorie dense foods. Taken together, these findings suggest that the prefrontal cortex plays a dynamic and bidirectional role to control food intake. Further studies are needed to determine the specific information it processes, the precise functional role that specific neural populations play, and their neural targets.
2.2. Insular Cortex
In humans and rodents, the insular cortex (also known as the insula) is comprised of three regions, granular, dysgranular and agranular. The granular and dysgranular regions tend to receive projections from visceral inputs, whereas limbic afferents tend towards the agranular portion (74). Despite this, functional studies do not support a clear distinction between these subregions as there are extensive local connections among them (75). In rodents, the insula is also divided both anatomically and functionally into three areas along the rostral-caudal axis, the anterior “gustatory cortex”, posterior “visceral cortex” and a middle region whose function is less clear but may integrate signals from the other two. Because the insula receives inputs from peripheral organs involved in both ingestion and digestion, and also directly encodes tastants, a role for the insula to regulate food intake has long been postulated (76,77).
In contrast to prefrontal areas, which appear to have an effect on baseline feeding, studies have demonstrated insula inhibition has no effect on homeostatic feeding (defined as normal feeding in the home cage) (30,68,78,79). Instead, the insula has been shown to have an effect on eating in response to environmental cues (30) or palatable food (68). These effects on food intake are mediated by insular cortex connections to subcortical structures, such as the CeA (30). Indeed, the amygdala, striatum, thalamus and hypothalamus are the main subcortical structures that receive insula projections (30,80). A number of studies have also shown that activation of the insular cortex using optogenetic stimulation leads to decreased food intake (80,81), although whether these effects are physiologic is unclear. In addition, the insular cortex also plays a role in regulating conditioned taste aversion, which is a learned response that leads to the avoidance of foods that were previously associated with malaise (82,83). This suggests that the insula encodes both attractive and aversive stimuli and in turn adjusts a consummatory response either positively or negatively depending upon the precise effect of the learned cues.
Recent studies suggest that the insula constructs a representation of hunger and thirst states over time that is independent of direct hypothalamic input (84). When considered alongside the studies showing that the insula also links exteroceptive cues to reward responses and food intake (78), this suggests that the insular cortex integrates both interoceptive and exteroceptive information to provide top-down control of eating behavior via projections to limbic structures.
2.3. Orbitofrontal cortex (OFC)
Findings in rodents, primates and humans have shown that the OFC encodes reward values in the brain, with individual neurons decoding visual, olfactory and gustatory stimuli which then guide behavioral choices to optimize reward value (85,86). Pioneering experiments in monkeys demonstrated that single neuron activity and self-stimulation of OFC neurons is attenuated in sated animals (87,88). Functional imaging in humans has also demonstrated a role for the OFC in rating the pleasantness of food (89). The possibility that subjective value is encoded within this cortical region is also suggested by studies showing that a highly palatable, energy-dense diet, which causes obesity in rodents, leads to morphological changes in the OFC, with alterations of resting membrane potential and decreased inhibitory transmission onto OFC neurons (90). Another report (86) showed that a subset of OFC neurons shows increased activity in response to high-calorie food rewards, and that activation of those neurons increased licking of those rewards. It should be noted, that consumption of liquid rewards may involve different pathways than those that lead to intake of chow or other solid foods. Interestingly, these neurons were distinct from OFC populations encoding social stimuli, suggesting that there is specificity at the cellular level for distinct behaviors (86).
Taken together, these studies suggest that the OFC plays a role in eating behavior by assigning reward values to food, thereby guiding behavioral choices. Interestingly, rodent studies have focused primarily on the lateral OFC (85,86,90), although human imaging studies have distinguished between the lateral OFC, which is primarily involved in the evaluation of punishers and medial OFC, which regulates reward values of reinforcers (91). Overall, the data suggests that the OFC could contribute to obesity by biasing consumption towards highly palatable and rewarding foods, but this will need to be confirmed in further studies. The OFC is connected to many brain regions including the amygdala (92) and the hypothalamus (92), but these projections have mostly been investigated in emotional contexts (93, 94), not yet in the context of food intake.
3. Orexigenic and Anorexigenic Signals Regulating Appetite in Cortical and Subcortical Structures
The hypothalamus has long been considered to tune eating behavior in response to orexigenic and anorexigenic signals that arise from the periphery (i.e. leptin, GLP-1, ghrelin) and from the brain (i.e. melanocortins). Among these signals, many act directly in cortical and subcortical regions, either arising from the ventricles and acting via volume transmission (i.e. GLP-1) or arising from local neurons (i.e. NPY). These neurons signal via both classical neurotransmitters (i.e. GABA or glutamate) and neuropeptides and their specific receptors. In this section we will focus on the most intensively studied orexigenic and anorexigenic signals acting in subcortical and cortical structures to regulate appetite and other related behaviors.
NPY is a classical hypothalamic neuromodulator first described in the 1980s (95). Initial findings identified hypothalamic NPY as an important regulator of appetite, body weight and other physiologic functions (95,96). Later, the observation that NPY is also expressed in extrahypothalamic brain areas, especially in interneurons, highlighted the importance of this neuropeptide in affective behaviors. For example, NPY is produced in the hippocampus and controls hippocampal activity through Y1 and Y2 receptors to regulate anxiety, stress and memory (97–99). NPY infused in the LS can induce anxiety and defensive behaviors via Y2 receptors, though the source of the septal NPY is not clear (100,55). In the central amygdala, NPY is produced locally by neurons that promote obesity in stressful environments (101). NPY is also expressed at high levels in cortical interneurons, and this NPY signaling, similar to its effects in hippocampus, can regulate anxiety and memory (102,103). The role of cortical NPY in appetite is therefore still unclear and requires further studies.
Melanin-concentrating hormone (MCH) is made by a subpopulation of neurons in the LH which act to induce food intake. Indeed, intracerebral injections of MCH increases food intake and decreases energy expenditure in rodents, leading to obesity. Hypothalamic MCH neurons project broadly throughout the brain including direct projections to the hippocampus where they regulate memory consolidation (104–106). These effects on memory are also important for eating behavior by associating episodic experiences to reward, which affects food-seeking (106). MCH signaling also contributes to changes in food intake by regulating impulse control (107), dysfunctions of which are associated with overeating in humans (108).
GLP-1 is mainly produced by endocrine L cells in the gut and in neurons in the nucleus of tractus solitarius that directly project to the hypothalamus, LS and amygdala (109). It is yet unknown whether these brainstem projections to the LS and amygdala are able to regulate any aspect of eating behavior. Hippocampal and LS neurons also express GLP1R and GLP-1 signaling in this brain area has been shown to regulate food intake (19,20,47,52,53). In the insular and orbitofrontal cortex, GLP-1 increases activity deficits and alters palatable food intake in humans (110). GLP-1 administration in humans also increases connectivity between the hypothalamus and the orbitofrontal cortex (110). It is still unclear whether the source of this GLP-1 input is endocrine or neural (109).
Ghrelin is a peptide mainly produced by the stomach and it exerts its bodily function through the growth hormone secretagogue receptor. Ghrelin is another key regulator of nutrient sensing, meal initiation, and appetite. When infused into the amygdala or hippocampus, ghrelin modulates spatial learning and anxiety (111–113). In the hippocampus and LS, ghrelin also regulates appetite (56,113–115). However, a knockout of ghrelin or the enzyme that octanoylates it, required for its bioactivity, show a defective response to extreme malnutrition (116).
Leptin (117), arising from adipose tissue, acts centrally on the hypothalamus and additional evidence suggests that it may also have direct and indirect effects on many other brain areas, including the hippocampus, insular, prefrontal, cingulate, retrosplenial, entorhinal, auditory, ectorhinal, perirhinal and somatosensory areas of the cortex (118). However, the role of leptin in many of these areas is still unclear. In the hippocampus, leptin receptors are densely expressed in the granule layer (118) and leptin has important effects in memory, synaptic plasticity and appetite as well as eliciting an anti-depressive effect when infused locally (119–121). Imaging studies of hypoleptinimic women with hypothalamic amenorrhea given short-term leptin treatment show activity changes in the insular cortex, dorsolateral prefrontal and medial prefrontal cortices when viewing food during a fast, though it is unclear whether this is a direct effect (122). Similarly, ghrelin administration in fed subjects led to increased activity in amygdala, hippocampus, orbitofrontal cortex and insular cortex while viewing pictures of food (123).
Overall, these findings imply that many neuromodulators controlling appetite have direct effects on hypothalamus but may also directly and indirectly recruit higher-order brain regions typically involved in learning, memory and executive functions. Here again, more research is needed to determine the role that these neuromodulators play in cortical regions and many other subcortical areas.
4. Maladaptive Eating Behavior in Psychiatric Disorders
The finding that higher-order brain regions regulate eating behavior via neuromodulators and descending projections provides an opportunity to consider the pathogenesis of maladaptive eating behaviors such as in eating disorders patients. Many of the brain regions discussed above have a known role in psychiatric disorders such as depression, anxiety and obsessive-compulsive disorder (OCD). High morbidity between maladaptive eating behaviors and these psychiatric disorders, along with the increased awareness of the role of higher-order brain regions in regulating food intake suggest that there may be a relationship between altered eating and mental health disorders.
For example, the comorbidity between depression, anxiety and anorexia nervosa and bulimia nervosa is strikingly high. As many as 50% of patients with anorexia and bulimia nervosa report a comorbid anxiety disorder, and anywhere from 20%−80% of patients will report at least one major depressive episode (124–126). Most studies indicate that anxiety typically precedes the onset of eating disorder symptoms (125,127,128), whereas depression typically presents either before or after its onset. It is therefore difficult to draw conclusions regarding whether eating disorders are a consequence of affective symptoms, particularly for depression. Despite this, it seems clear that there is a relationship between anxiety and eating disorders, even if the precise nature of the relationship remains elusive.
There is also a correlation between depression and obesity, which now accounts for over 42% of adults in the United States. Major depression in adults predicts greater body mass index (BMI), particularly in females (129), and obesity leads to about a 55% increase in developing depression (130). While obesity is associated with depression, the opposite is true following weight loss among patients on a diet or following bariatric surgery (131). This relationship is reciprocal, as decreased physical activity and maladaptive food choices made during depressive episodes may also contribute to the development of obesity (132). Interestingly, leptin-deficient mice exhibit higher depressive-like behaviors on a forced swim test than control mice, which is reversed by leptin treatment. Leptin treatment also improved depressive-like behaviors in control mice. Similarly, diet-induced obese mice exhibit high depressive-like behaviors (133,134), which is associated with plasticity-related changes in brain reward regions such as the nucleus accumbens (135). It is thus possible that chronically low leptin levels, which signals under-nutrition (for that individual), leads to negative emotional sequelae, particularly depression, that are often reported after diet induced weight loss and recidivism.
That depression and anxiety are associated with weight disturbances may reflect the effects of stress on food intake. In rodents, acute stress decreases food intake (47,135) whereas chronic stress has variable effects. While unpredictable chronic stress decreases consumption as part of a “fight or flight” response (136), chronic predictable stress has been shown to increase consumption of palatable food (135). Similarly, in humans, stress effects on food intake depend on the severity and duration of the stress (136,137). Importantly, stress appears to predispose to both obesity (136,138), and anorexia nervosa (139,140).
Children with autism spectrum disorder are at an elevated risk for obesity (141), although the precise cause of this association is unclear. In some mouse models of autism, high fat diet increases social deficits and cognitive rigidity (142), and similar increases are seen in wild-type mice fed a high fat diet (143). Interestingly, one study showed that activation of Agrp neurons, which normally leads to increased food consumption, leads to repetitive and stereotyped behaviors when food is not present (144). Together, these findings indicate a correlation between behaviors associated with autism and metabolic disturbances, of which further research is warranted.
Lastly, anorexia nervosa shares a strong genetic relationship with obsessive-compulsive disorder, suggesting shared underlying mechanisms between the two disorders (145,146) that merits further investigation.
Summary
Food intake is under both homeostatic and non-homeostatic control. Under the influence of descending inputs, subcortical systems are tuned to satisfy metabolic needs in often changing environmental conditions. Additionally, cognitive and affective factors can regulate food intake and the evidence suggests that this is the result of top-down modulation of homeostatic circuits. The tightly balanced communication between cortical and subcortical regions provides downstream feedback to regions that regulate appetite, energy balance and motor control, such as the hypothalamus and the brainstem. On the other hand, cortical systems regulate subcortical systems to tune emotional responses. While patients that develop eating disorders report difficulties in self-regulation and are usually diagnosed with secondary psychiatric disorders, such as obsessive-compulsive disorder, anxiety and depression, the mechanisms by which neuropsychiatric conditions influence these circuits is unclear and represent an opportunity for future investigation.
Acknowledgements
This work was funded by R00DA048749 (S.A.S.), NARSAD Young Investigator Awards from the Brain and Behavior Research Foundation (E.P.A and S.A.S), the JPB Foundation and the Klarman Foundation (J.M.F).
Footnotes
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hinde RA. (1956) Ethological Models and the Concept of ‘Drive’. British Journal of the Philosophy of Science 6: 321–331. [Google Scholar]
- 2.Mohr B. (1840) Hypemphie der Hypophysk cereb,.i undadurch bedingter buck auf die Hhgmndfliche, ins besondere auf die Sehnerven, das Chiasma derselben und den linkseitigen Himschenkel. Wschr ges Heilk 6: 565–571. [Google Scholar]
- 3.Anand BK, Brobeck JR. (1951) Hypothalamic control of food intake in rats and cats. Yale J Biol Med 24123–146. [PMC free article] [PubMed]
- 4.Hetherington AW, Ranson SW. (1940) Hypothalamic lesions and adiposity in the rat. Anat. Rec 78: 149–172. [Google Scholar]
- 5.Grill HJ, Kaplan JM. (2002) The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 23 :2–40. [DOI] [PubMed] [Google Scholar]
- 6.Pessoa L, Hof PR. (2015) From Paul Broca’s great limbic lobe to the limbic system. J Comp Neurol. 523 :2495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grastyfin E, Lissik K, Szab J, Vereby G (1956) Uber die functionelle Bedeutung des Hippocampus. In: Problems of the modern physiology of the nervous and muscle system (in honor of Beritashvili). Academy of Sciences of the Georgian SSR. 1: 67–70. [Google Scholar]
- 8.Hebben N, Corkin S, Eichenbaum H, Shedlack K. (1985) Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci. 99 :1031–9. [DOI] [PubMed] [Google Scholar]
- 9.Rozin P, Dow S, Moscovitch M, Rajaram S. (1998) What Causes Humans to Begin and End a Meal? A Role for Memory for What Has Been Eaten, as Evidenced by a Study of Multiple Meal Eating in Amnesic Patients. Psychological Science 9 :392–396. [Google Scholar]
- 10.Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. (2009) Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 19 :235–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Keefe J. (1990) A computational theory of the hippocampal cognitive map. Prog Brain Res. 83 :301–12. [DOI] [PubMed] [Google Scholar]
- 12.McNaughton BL, Barnes CA, O’Keefe J. (1983) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 52 :41–9. [DOI] [PubMed] [Google Scholar]
- 13.Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-Dos-Santos V, et al. (2019) A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space. Cell 176 :1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azevedo EP, Pomeranz L, Cheng J, Schneeberger M, Vaughan R, Stern SA, et al. (2019) A Role of Drd2 Hippocampal Neurons in Context-Dependent Food Intake. Neuron 102 :873–886. [DOI] [PubMed] [Google Scholar]
- 15.Herzog LE, Katz DB, Jadhav SP. (2020) Refinement and Reactivation of a Taste-Responsive Hippocampal Network. Curr Biol. 30 :1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods NI, Stefanini F, Apodaca-Montano DL, Tan IMC, Biane JS, Kheirbek MA. (2020) The Dentate Gyrus Classifies Cortical Representations of Learned Stimuli. Neuron 107 :173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Gong Y, Wang H, Sun X, Guo F, Gao S, et al. (2014) The stimulating effect of ghrelin on gastric motility and firing activity of gastric-distension-sensitive hippocampal neurons and its underlying regulation by the hypothalamus. Exp Physiol. 99 :123–35. [DOI] [PubMed] [Google Scholar]
- 18.Sun S, Xu L, Sun X, Guo F, Gong Y, Gao S. (2016) Orexin-A affects gastric distention sensitive neurons in the hippocampus and gastric motility and regulation by the perifornical area in rats. Neurosci Res. 110 :59–67. [DOI] [PubMed] [Google Scholar]
- 19.Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, et al. (2018) A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry 23 :1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. (2015) Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 40 :327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. (2013) Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry 73 :915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeney P, Yang Y. (2015) An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun. 6 :10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suarez AN, Liu CM, Cortella AM, Noble EE, Kanoski SE. (2020) Ghrelin and Orexin Interact to Increase Meal Size Through a Descending Hippocampus to Hindbrain Signaling Pathway. Biol Psychiatry. 87 :1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brutkowski S, Fonberg E, Kreiner J, Mempel E, Sychowa B. (1962) Aphagia and adipsia in a dog with bilateral complete lesion of the amygdaloid complex. Acta Biol Exp (Warsz). 22 :43–50. [PubMed] [Google Scholar]
- 25.Schwartz NB, Kling A. (1964) The effect of amygdaloid lesions on feeding, grooming and reproduction in rats. Acta Neuroveg (Wien). 26 :12–34. [DOI] [PubMed] [Google Scholar]
- 26.Krettek JE, Price JL. (1978) Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 178 :225–54. [DOI] [PubMed] [Google Scholar]
- 27.Kishi T, Tsumori T, Yokota S, Yasui Y. (2006) Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 496 :349–68. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J, Anderson KL, Leal SL, Shestyuk A, Gulsen G, Mnatsakanyan L, et al. (2017) Amygdala-hippocampal dynamics during salient information processing. Nat Commun. 8 :14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janak PH, Tye KM. (2015) From circuits to behaviour in the amygdala. Nature. 517 :284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern SA, Pomeranz LE, Azevedo EP, Doerig KR, Friedman JM. (2021) Top-down control of conditioned overconsumption is mediated by insular cortex Nos1 neurons. Cell Metabolism. In Press. [DOI] [PMC free article] [PubMed]
- 31.McDonald RJ, White NM. (1995) Information acquired by the hippocampus interferes with acquisition of the amygdala-based conditioned-cue preference in the rat. Hippocampus 5 :189–97. [DOI] [PubMed] [Google Scholar]
- 32.Johnsrude IS, Owen AM, White NM, Zhao WV, Bohbot V. (2000) Impaired preference conditioning after anterior temporal lobe resection in humans. J Neurosci. 20 :2649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland PC, Petrovich GD, Gallagher M. (2002) The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 76 :117–29. [DOI] [PubMed] [Google Scholar]
- 34.Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, et al. (2019) Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward. Neuron 102 :1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida H, Inoue KI, Takada M. (2018) Multisynaptic Projections from the Amygdala to the Ventral Premotor Cortex in Macaque Monkeys: Anatomical Substrate for Feeding Behavior. Front Neuroanat. 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin T, Jiang Z, Luan X, Qu Z, Guo F, Gao S, et al. (2020) Exogenous Orexin-A Microinjected Into Central Nucleus of the Amygdala Modulates Feeding and Gastric Motility in Rats. Front Neurosci. 14 :274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, et al. (2017) Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci. 20 :1384–1394. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, Haubensak W, Anthony TE, Anderson DJ. (2014) Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 17 :1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torruella-Suárez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, et al. (2019) Manipulations of Central Amygdala Neurotensin Neurons Alter the Consumption of Ethanol and Sweet Fluids in Mice. J Neurosci. 40 :632–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brady JV, Nauta WJ. (1955) Subcortical mechanisms in emotional behavior: the duration of affective changes following septal and habenular lesions in the albino rat. J Comp Physiol Psychol. 48 :412–20. [DOI] [PubMed] [Google Scholar]
- 41.Gotsick JE, Marshall RC. (1972) Time course of the septal rage syndrome. Physiol Behav. 9 :685–7. [DOI] [PubMed] [Google Scholar]
- 42.Albert DJ, Chew GL. (1980) The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behav Neural Biol. 30 :357–88. [DOI] [PubMed] [Google Scholar]
- 43.Albert DJ, Walsh ML, White R, Longley W. (1984) A comparison of prey eating by spontaneous mouse killing rats and rats with lateral septal, medial accumbens, or medial hypothalamic lesions. Physiol Behav. 33 :517–23. [DOI] [PubMed] [Google Scholar]
- 44.Besnard A, Gao Y, TaeWoo Kim M, Twarkowski H, Reed AK, Langberg T, et al. (2019). Dorsolateral septum somatostatin interneurons gate mobility to calibrate context-specific behavioral fear responses. Nat Neurosci. 22 :436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, et al. (2017) Bidirectional Control of Anxiety-Related Behaviors in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology 42 :1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheehan TP, Chambers RA, Russell DS (2004) Regulation of affect by the lateral septum: Implications for neuropsychiatry. Brain Res. Brain Res. Rev 46: 71–117. [DOI] [PubMed] [Google Scholar]
- 47.Azevedo EP, Tan B, Pomeranz LE, Ivan V, Fetcho R, Schneeberger M, et al. (2020) A limbic circuit selectively links active escape to food suppression. Elife 9 :e58894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakshi VP, Newman SM, Smith-Roe S, Jochman KA, Kalin NH. (2007) Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci. 27 :10568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sweeney P, Yang Y. (2016) An Inhibitory Septum to Lateral Hypothalamus Circuit That Suppresses Feeding. J Neurosci. 36 :11185–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gavioli EC, Canteras NS, De Lima TC. (1999) Anxiogenic-like effect induced by substance P injected into the lateral septal nucleus. Neuroreport 10 :3399–403. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Kotz CM. (2002) Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol. 283 :R358–67. [DOI] [PubMed] [Google Scholar]
- 52.Terrill SJ, Maske CB, Williams DL. (2018) Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiol Behav. 192 :17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, et al. (2016) Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am J Physiol Regul Integr Comp Physiol. 311 :R124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scopinho AA, Resstel LB, Corrêa FM. (2008) alpha(1)-Adrenoceptors in the lateral septal area modulate food intake behaviour in rats. Br J Pharmacol. 155 :752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trent NL, Menard JL. (2013) Lateral septal infusions of the neuropeptide Y Y2 receptor agonist, NPY(13–36) differentially affect different defensive behaviors in male, Long Evans rats. Physiol Behav. 110–111:20–9. [DOI] [PubMed]
- 56.Terrill SJ, Wall KD, Medina ND, Maske CB, Williams DL. (2018) Lateral septum growth hormone secretagogue receptor affects food intake and motivation for sucrose reinforcement. Am J Physiol Regul Integr Comp Physiol. 315 :R76–R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calderwood MT, Tseng A, Glenn Stanley B. (2020) Lateral septum mu opioid receptors in stimulation of feeding. Brain Res. 1734:146648. [DOI] [PubMed] [Google Scholar]
- 58.Buresova O. (1956) Vliianie rasprostraniaiushcheisia EEG depressii na bezuslovnye i natural’nye uslovnye pishchevye refleksy v technie depressii [Effect of spreading EEG depression on unconditioned and natural conditioned digestive reflexes during the depression]. Physiol Bohemoslov. 5:350–8. [PubMed] [Google Scholar]
- 59.Kolb B, Nonneman AJ. (1976) Functional development of prefrontal cortex in rats continues into adolescence. Science 193:335–6. [DOI] [PubMed] [Google Scholar]
- 60.Huston JP, Bures J. (1970) Drinking and eating elicited by cortical spreading depression. Science 169:702–4. [DOI] [PubMed] [Google Scholar]
- 61.Wise SP. (2008) Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 31:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlén M. (2017) What constitutes the prefrontal cortex? Science 358:478–482. [DOI] [PubMed] [Google Scholar]
- 63.Laubach M, Amarante LM, Swanson K, White SR. (2018) What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5 :ENEURO.0315-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. (2014) A mesoscale connectome of the mouse brain. Nature. 508 :207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, et al. (2014) Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 17 :248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mena JD, Sadeghian K, Baldo BA. (2011) Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 31 :3249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mena JD, Selleck RA, Baldo BA. (2013) Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci. 33 :18540–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baldo BA, Spencer RC, Sadeghian K, Mena JD. (2016) GABA-Mediated Inactivation of Medial Prefrontal and Agranular Insular Cortex in the Rat: Contrasting Effects on Hunger- and Palatability-Driven Feeding. Neuropsychopharmacology 41 :960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahn S, Phillips AG. (2002) Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. J Neurosci. 22 :10958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClelland J, Dalton B, Kekic M, Bartholdy S, Campbell IC, Schmidt U. (2016) A systematic review of temporal discounting in eating disorders and obesity: Behavioural and neuroimaging findings. Neurosci Biobehav Rev. 71 :506–528. [DOI] [PubMed] [Google Scholar]
- 71.Van den Eynde F, Claudino AM, Mogg A, Horrell L, Stahl D, Ribeiro W, et al. (2010) Repetitive transcranial magnetic stimulation reduces cue-induced food craving in bulimic disorders. Biol Psychiatry. 67 :793–5. [DOI] [PubMed] [Google Scholar]
- 72.Kim SH, Chung JH, Kim TH, Lim SH, Kim Y, Eun YM, et al. (2019) The effects of repetitive transcranial magnetic stimulation on body weight and food consumption in obese adults: A randomized controlled study. Brain Stimul. 12 :1556–1564. [DOI] [PubMed] [Google Scholar]
- 73.Safati AB, Hall PA. (2019) Contextual cues as modifiers of cTBS effects on indulgent eating. Brain Stimul. 12 :1253–1260. [DOI] [PubMed] [Google Scholar]
- 74.Allen GV, Saper CB, Hurley KM, Cechetto DF. (1991) Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 311 :1–16. [DOI] [PubMed] [Google Scholar]
- 75.Maffei A, Haley M, Fontanini A. (2012) Neural processing of gustatory information in insular circuits. Curr Opin Neurobiol. 22 :709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pérez CA, Stanley SA, Wysocki RW, Havranova J, Ahrens-Nicklas R, Onyimba F, et al. (2011) Molecular annotation of integrative feeding neural circuits. Cell Metab. 13 :222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spector AC, Travers SP. (2005) The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 4 :143–91. [DOI] [PubMed] [Google Scholar]
- 78.Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, et al. (2017) Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546 :611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Y, Chen C, Chen M, Qian K, Lv X, Wang H, et al. (2020) The anterior insular cortex unilaterally controls feeding in response to aversive visceral stimuli in mice. Nat Commun. 11 :640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, et al. (2020) A whole-brain connectivity map of mouse insular cortex. Elife 9 :e55585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang-Molina C, Schmit MB, Cai H. (2020) Neural Circuit Mechanism Underlying the Feeding Controlled by Insula-Central Amygdala Pathway. iScience 23 :101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbier M, Chometton S, Pautrat A, Miguet-Alfonsi C, Datiche F, Gascuel J, et al. (2020) A basal ganglia-like cortical-amygdalar-hypothalamic network mediates feeding behavior. Proc Natl Acad Sci U S A. 117:15967–15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yiannakas A, Rosenblum K. (2017) The Insula and Taste Learning. Front Mol Neurosci. 10 :335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, et al. (2020) Estimation of Current and Future Physiological States in Insular Cortex. Neuron 105 :1094–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gutierrez R, Carmena JM, Nicolelis MA, Simon SA. (2006) Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards. J Neurophysiol. 95 :119–33. [DOI] [PubMed] [Google Scholar]
- 86.Jennings JH, Kim CK, Marshel JH, Raffiee M, Ye L, Quirin S, et al. (2019) Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565 :645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mora F, Avrith DB, Phillips AG, Rolls ET. (1979) Effects of satiety on self-stimulation of the orbitofrontal cortex in the rhesus monkey. Neurosci Lett. 13 :141–5. [DOI] [PubMed] [Google Scholar]
- 88.Nakano Y, Oomura Y, Nishino H, Aou S, Yamamoto T, Nemoto S. (1984) Neuronal activity in the medial orbitofrontal cortex of the behaving monkey: modulation by glucose and satiety. Brain Res Bull. 12 :381–5. [DOI] [PubMed] [Google Scholar]
- 89.Howard JD, Reynolds R, Smith DE, Voss JL, Schoenbaum G, Kahnt T. (2020) Targeted Stimulation of Human Orbitofrontal Networks Disrupts Outcome-Guided Behavior. Curr Biol. 30 :490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thompson JL, Drysdale M, Baimel C, Kaur M, MacGowan T, Pitman KA, et al. (2017) Obesity-Induced Structural and Neuronal Plasticity in the Lateral Orbitofrontal Cortex. Neuropsychopharmacology 42 :1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kringelbach ML. (2004) Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 126 :807–19. [DOI] [PubMed] [Google Scholar]
- 92.Babalian A, Eichenberger S, Bilella A, Girard F, Szabolcsi V, Roccaro D, et al. (2019) The orbitofrontal cortex projects to the parvafox nucleus of the ventrolateral hypothalamus and to its targets in the ventromedial periaqueductal grey matter. Brain Struct Funct. 224 :293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rempel-Clower NL, Barbas H. (1998) Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 398 :393–419. [DOI] [PubMed] [Google Scholar]
- 94.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. (2003) Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levine AS, Jewett DC, Cleary JP, Kotz CM, Billington CJ. (2004) Our journey with neuropeptide Y: effects on ingestive behaviors and energy expenditure. Peptides. 25 :505–10. [DOI] [PubMed] [Google Scholar]
- 96.Gehlert DR. (2004) Introduction to the reviews on neuropeptide Y. Neuropeptides. 38 :135–40. [DOI] [PubMed] [Google Scholar]
- 97.Joksimovic J, Selakovic D, Jovicic N, Mitrovic S, Mihailovic V, Katanic J, et al. (2019) Exercise Attenuates Anabolic Steroids-Induced Anxiety via Hippocampal NPY and MC4 Receptor in Rats. Front Neurosci. 13 :172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Q, Bartley AF, Dobrunz LE. (2017) Endogenously Released Neuropeptide Y Suppresses Hippocampal Short-Term Facilitation and Is Impaired by Stress-Induced Anxiety. J Neurosci. 37 :23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hörmer BA, Verma D, Gasser E, Wieselthaler-Hölzl A, Herzog H, Tasan RO. (2018) Hippocampal NPY Y2 receptors modulate memory depending on emotional valence and time. Neuropharmacology 143 :20–28. [DOI] [PubMed] [Google Scholar]
- 100.Trent NL, Menard JL. (2011) Infusions of neuropeptide Y into the lateral septum reduce anxiety-related behaviors in the rat. Pharmacol Biochem Behav. 99 :580–90. [DOI] [PubMed] [Google Scholar]
- 101.Ip CK, Zhang L, Farzi A, Qi Y, Clarke I, Reed F, et al. (2019) Amygdala NPY Circuits Promote the Development of Accelerated Obesity under Chronic Stress Conditions. Cell Metab. 30 :111–128. [DOI] [PubMed] [Google Scholar]
- 102.Tasan RO, Verma D, Wood J, Lach G, Hörmer B, de Lima TC, et al. (2016) The role of Neuropeptide Y in fear conditioning and extinction. Neuropeptides 55 :111–26. [DOI] [PubMed] [Google Scholar]
- 103.Saffari R, Teng Z, Zhang M, Kravchenko M, Hohoff C, Ambrée O, et al. (2016) NPY+−, but not PV+− GABAergic neurons mediated long-range inhibition from infra- to prelimbic cortex. Transl Psychiatry 6 :e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Izawa S, Chowdhury S, Miyazaki T, Mukai Y, Ono D, Inoue R, et al. (2019) REM sleep-active MCH neurons are involved in forgetting hippocampus-dependent memories. Science 365 :1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oh ST, Liu QF, Jeong HJ, Lee S, Samidurai M, Jo J, et al. (2019) Nasal Cavity Administration of Melanin-Concentrating Hormone Improves Memory Impairment in Memory-Impaired and Alzheimer’s Disease Mouse Models. Mol Neurobiol. 56 :8076–8086. [DOI] [PubMed] [Google Scholar]
- 106.Sita LV, Diniz GB, Canteras NS, Xavier GF, Bittencourt JC. (2016) Effect of intrahippocampal administration of anti-melanin-concentrating hormone on spatial food-seeking behavior in rats. Peptides 76 :130–8. [DOI] [PubMed] [Google Scholar]
- 107.Noble EE, Wang Z, Liu CM, Davis EA, Suarez AN, Stein LM, et al. (2019) Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat Commun. 10 :4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bénard M, Bellisle F, Kesse-Guyot E, Julia C, Andreeva VA, Etilé F, et al. (2019) Impulsivity is associated with food intake, snacking, and eating disorders in a general population. Am J Clin Nutr. 109 :117–126. [DOI] [PubMed] [Google Scholar]
- 109.Trapp S, Cork SC. (2015) PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am J Physiol Regul Integr Comp Physiol. 309 :R795–R804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eren-Yazicioglu CY, Yigit A, Dogruoz RE, Yapici-Eser H. (2021) Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front Behav Neurosci. 2021 14 :614884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. (2006) Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 9 :381–8. [DOI] [PubMed] [Google Scholar]
- 112.Tóth K, László K, Lénárd L. (2010) Role of intra-amygdaloid acylated-ghrelin in spatial learning. Brain Res Bull. 81 :33–7. [DOI] [PubMed] [Google Scholar]
- 113.Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. (2004) Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 313 :635–41. [DOI] [PubMed] [Google Scholar]
- 114.Davis EA, Wald HS, Suarez AN, Zubcevic J, Liu CM, Cortella AM, et al. (2020) Ghrelin Signaling Affects Feeding Behavior, Metabolism, and Memory through the Vagus Nerve. Curr Biol. 30 :4510–4518.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suarez AN, Liu CM, Cortella AM, Noble EE, Kanoski SE. (2020) Ghrelin and Orexin Interact to Increase Meal Size Through a Descending Hippocampus to Hindbrain Signaling Pathway. Biol Psychiatry. 87 :1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, et al. (2010) Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 107 :7467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature. 372 :425–32. [DOI] [PubMed] [Google Scholar]
- 118.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. (2009) Leptin targets in the mouse brain. J Comp Neurol. 514 :518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. (2011) Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 36 :1859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shanley LJ, Irving AJ, Harvey J. (2001) Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 21:RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Farr SA, Banks WA, Morley JE. (2006) Effects of leptin on memory processing. Peptides. 27 :1420–5. [DOI] [PubMed] [Google Scholar]
- 122.Farr OM, Fiorenza C, Papageorgiou P, Brinkoetter M, Ziemke F, Koo BB, et al. (2014) Leptin therapy alters appetite and neural responses to food stimuli in brain areas of leptin-sensitive subjects without altering brain structure. J Clin Endocrinol Metab. 99 :E2529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malik S, McGlone F, Bedrossian D, Dagher A. (2008) Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 7 :400–9. [DOI] [PubMed] [Google Scholar]
- 124.Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. (2004) Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 161 :2215–21. [DOI] [PubMed] [Google Scholar]
- 125.García-Alba C. (2004) Anorexia and depression: depressive comorbidity in anorexic adolescents. Span J Psychol. 7 :40–52. [DOI] [PubMed] [Google Scholar]
- 126.Brand-Gothelf A, Leor S, Apter A, Fennig S. (2014) The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls. J Nerv Ment Dis. 202 :759–62. [DOI] [PubMed] [Google Scholar]
- 127.Meier SM, Bulik CM, Thornton LM, Mattheisen M, Mortensen PB, Petersen L. (2015) Diagnosed Anxiety Disorders and the Risk of Subsequent Anorexia Nervosa: A Danish Population Register Study. Eur Eat Disord Rev. 23 :524–30. [DOI] [PubMed] [Google Scholar]
- 128.Root TL, Szatkiewicz JP, Jonassaint CR, Thornton LM, Pinheiro AP, Strober M, et al. (2011) Association of candidate genes with phenotypic traits relevant to anorexia nervosa. Eur Eat Disord Rev. 19 :487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stunkard AJ, Faith MS, Allison KC. (2003) Depression and obesity. Biol Psychiatry. 54 :330–7. [DOI] [PubMed] [Google Scholar]
- 130.Hryhorczuk C, Sharma S, Fulton SE. (2013) Metabolic disturbances connecting obesity and depression. Front Neurosci 7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dixon JB, Dixon ME, O’Brien PE. (2003) Depression in association with severe obesity: changes with weight loss. Arch Intern Med. 163 :2058–65. [DOI] [PubMed] [Google Scholar]
- 132.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. (2010) Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 67 :220–9. [DOI] [PubMed] [Google Scholar]
- 133.Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, et al. (2011) Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 152 :2634–43. [DOI] [PubMed] [Google Scholar]
- 134.Sharma S, Fulton S. (2013) Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond). 37 :382–9. [DOI] [PubMed] [Google Scholar]
- 135.Ely DR, Dapper V, Marasca J, Corrêa JB, Gamaro GD, Xavier MH, et al. (1997) Effect of restraint stress on feeding behavior of rats. Physiol Behav. 61 :395–8. [DOI] [PubMed] [Google Scholar]
- 136.Yau YH, Potenza MN. (2013) Stress and eating behaviors. Minerva Endocrinol. 38 :255–67. [PMC free article] [PubMed] [Google Scholar]
- 137.Razzoli M, Pearson C, Crow S, Bartolomucci A. (2017) Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci Biobehav Rev. 76 :154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. (2015) Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 58 :36–45. [DOI] [PubMed] [Google Scholar]
- 139.Horesh N, Apter A, Lepkifker E, Ratzoni G, Weizmann R, Tyano S. (1995) Life events and severe anorexia nervosa in adolescence. Acta Psychiatr Scand. 91 :5–9. [DOI] [PubMed] [Google Scholar]
- 140.Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, Moran TH. (2015) Anorexia nervosa as a motivated behavior: Relevance of anxiety, stress, fear and learning. Physiol Behav. 152 :466–72. [DOI] [PubMed] [Google Scholar]
- 141.Hill AP, Zuckerman KE, Fombonne E. (2015) Obesity and Autism. Pediatrics. 136 :1051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zilkha N, Kuperman Y, Kimchi T. (2017) High-fat diet exacerbates cognitive rigidity and social deficiency in the BTBR mouse model of autism. Neuroscience. 345 :142–154. [DOI] [PubMed] [Google Scholar]
- 143.Veniaminova E, Cespuglio R, Cheung CW, Umriukhin A, Markova N, Shevtsova E, et al. (2017) Autism-Like Behaviours and Memory Deficits Result from a Western Diet in Mice. Neural Plast. 2017:9498247. [DOI] [PMC free article] [PubMed]
- 144.Dietrich MO, Zimmer MR, Bober J, Horvath TL. (2015) Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 160 :1222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bulik CM, Sullivan PF, Fear JL, Joyce PR. (1997) Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatr Scand. 96 :101–7. [DOI] [PubMed] [Google Scholar]
- 146.Yilmaz Z, Halvorsen M, Bryois J, Yu D, Thornton LM, Zerwas S, et al. (2020) Eating Disorders Working Group of the Psychiatric Genomics Consortium, Tourette Syndrome/Obsessive–Compulsive Disorder Working Group of the Psychiatric Genomics Consortium. Examination of the shared genetic basis of anorexia nervosa and obsessive-compulsive disorder. Mol Psychiatry. 25 :2036–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]