Abstract
Strains of group B streptococcus (GBS) express surface proteins that confer protective immunity. In particular, most strains of the four classical capsular serotypes (Ia, Ib, II, and III) express either of the Rib and α proteins, two members of the same protein family. Here, we report a study of surface proteins expressed by strains of serotype V, which has recently emerged as an important serotype among GBS strains causing serious disease. Two novel GBS proteins were identified, purified, and characterized. One of these proteins, designated Fbs, was immunologically unrelated to other GBS surface proteins. This ∼110-kDa protein was found in 15 of 49 (31%) type V isolates but in few strains of other serotypes. The Fbs proteins expressed by different strains showed limited variation in size. The most common surface protein among type V strains, found in 29 of 49 (59%) isolates, was designated Rib-like, since it cross-reacted with Rib but was not immunologically identical to Rib. Characterization of this Rib-like protein showed that the N-terminal sequence (12 residues) was identical to that of α, although these two proteins lacked cross-reactivity. The biochemical and immunological properties of the Rib-like GBS protein indicate that it is closely related to the R28 protein of Streptococcus pyogenes. Importantly, passive and active immunization experiments with mice showed that the Fbs and Rib-like proteins are targets for protective antibodies. These two proteins are therefore of interest for analysis of pathogenic mechanisms and for vaccine development.
The group B streptococcus (GBS) is the leading cause of life-threatening bacterial infections in newborns (1). In the Western world, GBS-related meningitis, septicemia, or neonatal pneumonia occurs at rates of 1 to 2 per 1,000 live births, with an overall mortality of 6 to 20% and neurological sequelae afflicting many of the survivors (1, 27). GBS is normally found in the vagina or lower intestine of ∼20% of adult women (1, 27), and the vast majority of GBS infections are acquired in connection with childbirth, when the child is exposed to the bacterium carried by the mother (1). In addition to its importance as a cause of disease in the neonatal period, recent evidence indicates that GBS may also be a significant cause of serious disease in nonpregnant adults with underlying illness (4, 6).
GBS is serotyped on the basis of the capsular polysaccharide, for which nine different serotypes have been described so far (15). The classical serotypes Ia, Ib, II, and III have been most prevalent as causes of disease, with serotype III now accounting for ∼50% of all neonatal infections and ∼90% of cases of neonatal meningitis (1, 9). However, population-based surveillance of GBS disease during the 1990s has indicated that serotype V is responsible for 10 to 15% of neonatal GBS infections in the United States (3, 9, 20) and in Sweden (17a) and has even become the most common serotype isolated from nonpregnant adults with invasive disease (9). In addition, a study of Gambian women colonized with GBS indicated that type V strains accounted for 41% of the isolates (32). These data demonstrate an increasing importance for type V strains and will therefore affect analysis of pathogenic mechanisms and strategies for vaccine development (35).
Protective immunity to GBS infection can be elicited by the polysaccharide capsule (1) and also by different surface proteins (2, 17, 25, 30). Many type Ia, Ib, and II strains, but not the common type III strains, express the protective α and β proteins, of which α occurs more frequently (13, 30). Moreover, almost all strains of the clinically important type III express protein Rib, which elicits protective immunity (18, 30). In total, ∼90% of GBS strains of the four classical serotypes express either α or Rib, suggesting that a combination of these two proteins may be used for the development of a protein vaccine against GBS (19). The α and Rib proteins have been extensively characterized and were found to identify a family of streptococcal cell surface proteins with extremely repetitive structure (26, 34). Although these two proteins show extensive residue identity, they do not cross-react immunologically (30, 34).
The increasing importance of GBS strains of serotype V prompted us to analyze such strains for expression of different surface proteins. Here, we describe the identification and purification of two novel GBS surface proteins that are expressed by many type V strains and serve as targets for protective antibodies. One of these proteins, designated Fbs, is immunologically unrelated to previously described GBS proteins, while the other protein is related to, but not identical with, the Rib protein.
MATERIALS AND METHODS
Bacterial strains and medium.
A collection of 49 type V GBS isolates were used. Included in this collection were 26 strains isolated from blood or cerebrospinal fluid, 18 colonization isolates, and 5 isolates of unknown origin. These strains were obtained from J. Jelínková (National Institute of Public Health, Prague, Czech Republic), L. Burman (Swedish Institute for Infectious Disease Control, Stockholm, Sweden), G. Orefici (Istituto Superiore di Sanita, Rome, Italy), J. A. Elliott (Centers for Disease Control and Prevention, Atlanta, Ga.), J. Henrichsen (State Serum Institute, Copenhagen, Denmark), A. I. Kvam (Medical Technical Center, Trondheim, Norway), M. Sellin (Department of Clinical Bacteriology, University Hospital, Umeå, Sweden), and the Clinical Microbiology Laboratory, Lund University Hospital. One of the type V strains was the reference strain 10/84 (11). The type V strain 2471, which was used for isolation of the Rib-like protein described here, was isolated from an infant with invasive disease and was obtained from G. Orefici.
A collection of 71 strains of serotypes Ia, Ib, II, and III was available in our laboratory. Most of these strains were isolated from patients with invasive GBS disease. The collection included the type Ia strain A909 (26), the type Ib strain SB35 (30), and the type III strain BM110 (30, 34). All GBS strains were grown in Todd-Hewitt broth at 37°C.
Purification of streptococcal surface proteins.
The Fbs protein was purified from mutanolysin extracts of strain 10/84, by a procedure previously developed for the purification of the Rib and α proteins (18, 30). This procedure included two steps of ion-exchange chromatography, followed by molecular sieve chromatography and hydroxylapatite chromatography. Fractions containing Fbs were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visual inspection of gels for the presence of the high-molecular-weight Fbs protein present in the original extract. The behavior of Fbs during the purification was similar to that of Rib and α, suggesting that these three GBS surface proteins have similar physicochemical properties. The final yield of Fbs, purified from the bacteria in a 10-liter culture, was 11 mg. Purified Fbs did not contain any contaminating proteins, as judged by SDS-PAGE. The presence of sialic acid, a key component of the type-specific polysaccharide (36), was determined by the periodate-resorcinol method (14) and was found to be <0.002%.
The Rib-like protein described here was purified from mutanolysin extracts of type V strain 2471, by procedures similar to those described above. Fractions were analyzed for the presence of the Rib-like protein by visual inspection of SDS-PAGE gels and by Western blot analysis with anti-Rib serum. The final yield of the Rib-like protein, purified from the bacteria in a 10-liter culture, was 5 mg. As for the Fbs protein described above, the purified Rib-like protein did not contain detectable amounts of contaminating protein or polysaccharide.
The GBS proteins α, β, and Rib were purified from extracts of strains A909, SB35, and BM110, respectively (18, 30). The Streptococcus pyogenes protein R28 was purified from strain AL368, as described elsewhere (28).
Antisera.
Rabbit antisera were raised against highly purified proteins, by using gel slices cut out from SDS-PAGE gels, as described elsewhere (30). For the Fbs protein, both the upper and the lower SDS-PAGE bands (110 and 100 kDa) were used. Mouse antisera raised against purified proteins were prepared by subcutaneous immunization of male C3H/HeN mice with 25 μg of highly purified protein mixed with complete Freund's adjuvant (CFA). The mice were boosted after 4 weeks with the same dose of antigen mixed with incomplete Freund's adjuvant and bled 2 weeks later.
Passive and active immunization of mice.
For passive immunization with anti-Fbs serum, adult C3H/HeN mice were injected intraperitoneally (i.p.) with 0.1 ml of rabbit anti-Fbs serum, diluted fivefold in phosphate-buffered saline (PBS). Control animals received PBS only. Mice were challenged i.p. 4 h later with log-phase bacteria diluted in 0.5 ml of Todd-Hewitt broth, with 1 × 107 CFU of strain 10/84 and 4 × 107 CFU of strain SBL10. The number of bacteria used was estimated to represent a 90% lethal dose (LD90), but the lethality was lower in some experiments. Deaths were recorded daily for a period of 7 days.
For active immunization with protein Fbs, adult C3H/HeN mice were immunized subcutaneously with 25 μg of highly purified protein Fbs, dissolved in 0.1 ml of PBS, and mixed with 0.1 ml of CFA. Control mice were injected with a mixture of PBS and CFA. The mice were boosted 4 weeks later with the same dose of antigen or PBS mixed with incomplete Freund's adjuvant. Two weeks later, the mice were infected i.p. with an ∼LD90 dose of bacteria (see above). Deaths were recorded daily for 7 days.
Passive immunization with anti-R28 serum and active immunization with pure R28 were performed as described above, with rabbit anti-R28 serum and pure R28 protein, respectively (28). Immunized mice were challenged i.p. with 1.5 × 106 CFU of GBS type V strain 2471.
Inhibition tests for analysis of cross-reactivity.
Microtiter plates (Falcon Microtest III; Becton Dickinson, Oxnard, Calif.) were coated with purified protein (Fbs, Rib-like, Rib, or R28), by incubation for 16 h with 100 μl of a solution of protein (500 ng/ml) in PBS. The wells were blocked by washing with Veronal-buffered saline (10 mM Veronal buffer, 0.15 M NaCl, pH 7.4) supplemented with 0.25% gelatin and 0.25% Tween 20 and then washed with PBSAT (PBS containing 0.02% sodium azide and 0.05% Tween 20). The binding of antibodies to the immobilized protein was inhibited with purified proteins or with whole bacteria. For inhibition tests with purified proteins, various amounts of protein were mixed with 100-μl aliquots of antiserum diluted in PBSAT, incubated for 30 min, and then added to the coated wells. The antisera were used at a final dilution corresponding to ∼80% of maximal binding. After incubation for 3 h, the wells were washed three times with PBSAT and bound antibodies were detected by the addition of 125I-labeled protein G (∼12,000 cpm in 100 μl of PBSAT for each well). After incubation for 2 h and three washes with PBSAT, the radioactivity of each well was determined in a γ counter. Nonspecific binding (less than 1%) was determined in wells coated with buffer (PBS) alone and has been subtracted. All incubations were performed at room temperature. For inhibition tests with whole bacteria, washed suspensions of bacteria in PBSAT were used instead of purified proteins.
Other methods.
Radiolabeling of proteins with 125I and Western blot analysis were performed as described elsewhere (31). Preparation of mutanolysin extracts, N-terminal sequence analysis, and studies of GBS strains for surface expression of proteins were performed as described elsewhere (30). Ladder pattern formation of purified proteins in SDS-PAGE was analyzed as described elsewhere (34).
RESULTS
Identification, purification, and characterization of Fbs, a novel GBS surface protein.
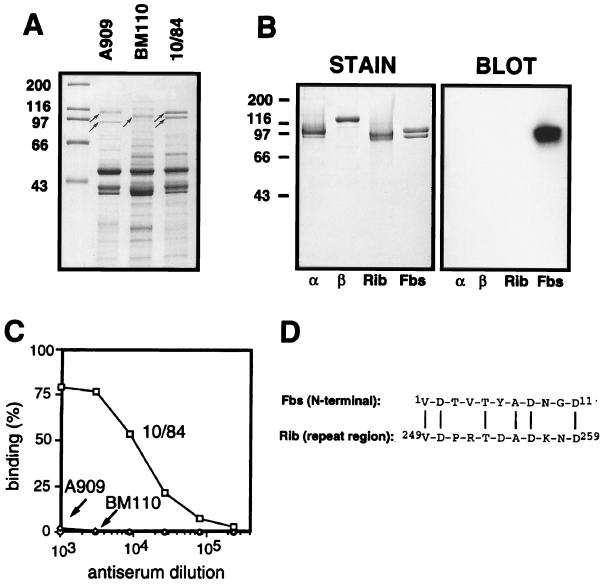
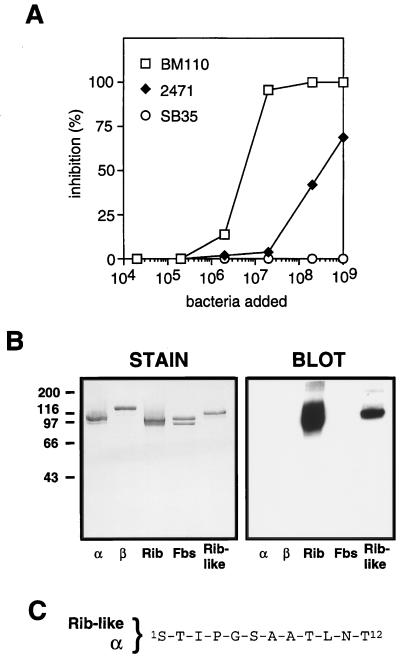
We have previously shown that the α, β, and Rib proteins can be extracted by mutanolysin treatment of GBS strains and that the solubilized proteins migrate as distinct high-molecular-weight bands in SDS-PAGE gels (30). A similar analysis was performed with the type V reference strain 10/84, which does not express α, β, or Rib. This analysis demonstrated the presence of a distinct doublet band (∼110 and ∼100 kDa), suggesting that strain 10/84 may express one or two surface proteins of high molecular weight (Fig. 1A). These proteins were purified to homogeneity, by a combination of ion-exchange chromatography, molecular sieve chromatography, and hydroxylapatite chromatography (see Materials and Methods). The two polypeptides in the doublet band were recovered together throughout the purification, indicating that they represent two variants of the same protein. Indeed, N-terminal sequencing (five residues) of the two individual polypeptides gave identical results, the sequence V-D-T-V-T, implying that the 100-kDa protein arises from the 110-kDa protein through truncation at the C-terminal end. The immunochemical work described below was performed with the purified preparation containing both polypeptide species. This protein will be referred to as Fbs (type five, group B, surface protein). The purified Fbs protein did not contain detectable amounts of type-specific polysaccharide, as shown by the presence of <0.002% sialic acid in the preparation. Moreover, purified Fbs did not contain detectable amounts of other proteins, according to SDS-PAGE analysis (Fig. 1B, left panel).
FIG. 1.
Identification of Fbs and characterization of the purified protein. (A) Mutanolysin extracts of group B streptococcal strains analyzed by SDS-PAGE. High-molecular-weight surface proteins appear as distinct bands (arrows). Strains used were A909, a type Ia strain expressing the α (top arrow) and β (bottom arrow) proteins; BM110, a type III strain expressing protein Rib (arrow); and the type V strain 10/84, expressing the protein Fbs (doublet band). (B) Western blot analysis of purified preparations of the α, β, Rib, and Fbs proteins, with rabbit anti-Fbs serum. The antiserum was used at a 1:1,000 dilution, and bound antibodies were detected with 125I-labeled protein G. The autoradiogram was deliberately overexposed to demonstrate the lack of cross-reactivity between Fbs and the α, β, and Rib proteins. In a control blot with preimmune rabbit serum, no signal was obtained. Molecular mass markers for panels A and B are in kilodaltons. (C) Analysis of group B streptococcal strains for cell surface expression of Fbs. The bacteria were analyzed for ability to bind mouse anti-Fbs antibodies, by using 125I-labeled protein A to detect bound antibodies. Strains used were A909 (type Ia), BM110 (type III), and 10/84 (type V). (D) Alignment of the N-terminal amino acid sequence of protein Fbs and an amino acid sequence from the repeat region of protein Rib (34). Vertical lines denote residue identity. The experiments shown here were performed at least twice with similar results.
Antiserum raised against purified Fbs was used to analyze whether it is expressed on the bacterial surface (Fig. 1C). Anti-Fbs antibodies reacted with whole bacteria of strain 10/84 but not with the type Ia strain A909 (which expresses α and β) or with the type III strain BM110 (which expresses Rib). Thus, Fbs is expressed on the surface of strain 10/84, and the other two GBS strains do not express cross-reacting surface proteins. In agreement with these results, Western blot analysis with anti-Fbs serum did not disclose any cross-reactivity with highly purified preparations of the α, β, and Rib proteins (Fig. 1B). Similarly, antisera against purified α, β, or Rib did not recognize Fbs (data not shown). These data indicate that Fbs is immunologically unrelated to the other three GBS proteins.
An N-terminal sequence of 11 residues was determined for Fbs (Fig. 1D). This sequence is identical at 6 of 11 positions to a sequence within the repeat region of Rib but shows no homology to N-terminal regions of known GBS surface proteins or to other proteins in the databases.
Analysis of different type V isolates for expression of Fbs and other surface proteins.
A collection of 49 GBS isolates of serotype V were analyzed for surface exposure of Fbs, Rib, α, and β (Table 1). The Fbs protein was detected in 15 (31%) of these type V strains. The most common surface protein was a Rib-like protein, found in 29 (59%) of the strains. This protein is referred to here as Rib-like, rather than Rib, since it is not identical to the Rib protein expressed by type III strains but cross-reacts with Rib (see below). The α protein was found in only 2 (4%) of the 49 type V strains, but the β protein was found in 13 (26%) of the strains.
TABLE 1.
Cell surface expression of the Fbs, Rib-like, α, and β proteins in 49 GBS isolates of serotype Va
| Surface protein(s) expressed | No. of strains (n = 49) | % |
|---|---|---|
| Individual proteinsb | ||
| Fbs | 15 | 31 |
| Rib-like | 29 | 59 |
| α | 2 | 4 |
| β | 13 | 26 |
| None | 5 | 10 |
| All combinations identifiedc | ||
| Fbs (only) | 6 | 12 |
| Fbs and β | 6 | 12 |
| Fbs, Rib-like, and β | 2 | 4 |
| Fbs, α, and β | 1 | 2 |
| Rib-like (only) | 25 | 51 |
| Rib-like and β | 2 | 4 |
| β (only) | 1 | 2 |
| α and β | 1 | 2 |
| None | 5 | 10 |
Analyzed by incubating whole bacteria with specific rabbit antibodies as described in Materials and Methods. In most cases, the results were unequivocal, but in a few cases, expression, or lack of expression, of a surface antigen was verified by Western blot analysis of bacterial extracts.
For individual proteins, the number of strains indicates the number of strains expressing a given protein. The total number of strains in this set of data is >49, since one strain may express more than one of the surface proteins.
All combinations of proteins identified in different strains. As indicated, some strains express only one protein (Fbs, Rib-like, or β), while other strains express combinations of different proteins.
Expression of Fbs was also analyzed in a collection of 71 strains of the four classical serotypes. This collection included 13 strains of type Ia, 6 of type Ib, 17 of type II, and 35 of type III. Only four (6%) of these strains expressed Fbs. Taken together, the analysis of strains of serotypes Ia, Ib, II, III, and V therefore indicated that Fbs is mainly expressed by type V strains.
Characterization of the Fbs protein: size variation and protease sensitivity.
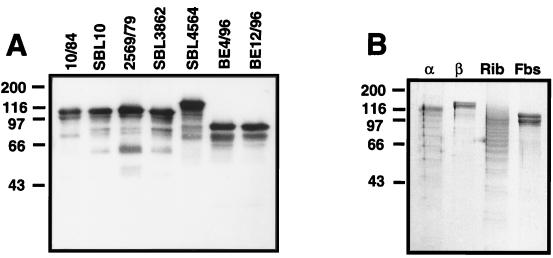
The α and Rib proteins vary in size between different bacterial strains, due to the presence of different numbers of repeats in different isolates (22, 23, 30). The Fbs protein also varies in size, as shown by Western blot analysis of extracts of different type V strains, but the size variation was more limited than that reported for α and Rib (Fig. 2A). Among 15 Fbs-expressing strains analyzed, 12 expressed Fbs proteins of similar size, ∼110 kDa; data for 4 of these 12 strains are shown in the left part of Fig. 2A. In one strain, the size of Fbs was ∼120 kDa, and in two strains it was ∼90 kDa.
FIG. 2.
Characterization of protein Fbs: size variation and lack of ladder formation in SDS-PAGE gels. (A) Western blot analysis of mutanolysin extracts from seven different Fbs-expressing strains of serotype V, with rabbit anti-Fbs serum. Bound antibodies were detected with radiolabeled protein G. In a control blot with preimmune rabbit serum, no signal was obtained. (B) SDS-PAGE of purified proteins after boiling at acidic pH. Solutions of the α, β, Rib, and Fbs proteins were adjusted to pH 4.0, mixed with sample buffer, boiled for 5 min, and subjected to SDS-PAGE (34). The α and Rib proteins, but not β or Fbs, form a characteristic ladder, apparently due to hydrolysis of acid-sensitive Asp-Pro bonds in the repeat regions (34). Molecular mass markers are in kilodaltons. These experiments were performed twice with similar results.
The Rib, α, and β proteins vary in their sensitivity to proteases. Rib is relatively resistant to both trypsin and pepsin, and α is relatively resistant to trypsin, while β is sensitive to both of these proteases (24, 30). Analysis of the protease sensitivity of Fbs showed that this protein is sensitive to both trypsin and pepsin, like the β protein (data not shown).
When the α and Rib proteins are analyzed by Western blotting, with specific antiserum, a characteristic ladder pattern is seen (22, 30, 34). The available evidence indicates that this ladder is an artifact and is due to partial hydrolysis, during the analysis, of acid-labile Asp-Pro bonds in the repeats (34). Indeed, the ladder pattern is readily seen even in a stained SDS-PAGE gel if the proteins are heated at low pH before the analysis (Fig. 2B) (34). The β protein, which lacks Asp-Pro-containing repeats (10, 12), does not give rise to a ladder. Similarly, a ladder was not seen for Fbs, indicating that this protein does not contain repeats with Asp-Pro sequences.
Comparison of Fbs proteins expressed by different GBS strains.
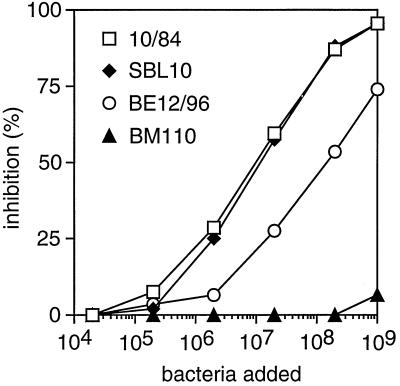
An inhibition assay was used to analyze whether Fbs proteins expressed by different GBS strains have similar immunological properties (Fig. 3). In this assay, suspensions of washed bacteria were used to inhibit the binding of rabbit anti-Fbs antibodies to purified Fbs immobilized in microtiter wells. The analysis was performed with the 15 Fbs-expressing type V isolates that were available (Table 1), including strain 10/84, from which Fbs was purified. As expected, strain 10/84 inhibited binding, while the control, the Rib-expressing type III strain BM110, had no significant ability to inhibit. Among the remaining 14 Fbs-expressing strains, 12 caused complete inhibition, like strain 10/84. Data for one of these strains, SBL10, are included in Fig. 3. Two of the Fbs-expressing strains, exemplified here by strain BE12/96, caused less efficient inhibition, and for technical reasons it was not possible to analyze whether complete inhibition could be obtained. However, the data suggest that strain BE12/96 expresses an Fbs protein that is closely related, if not identical, to that of strain 10/84 and that the difference between the strains may be quantitative rather than qualitative. Taken together, these data indicate that most, if not all, Fbs-expressing type V strains express Fbs proteins with similar immunological properties. Thus, it is justified to refer to all of these proteins as Fbs.
FIG. 3.
Immunological comparison of Fbs proteins expressed by different GBS type V strains. Suspensions of whole bacteria were used to inhibit the binding of rabbit anti-Fbs antibodies to purified protein Fbs immobilized in microtiter plates. The figure shows data obtained with three Fbs-expressing strains (10/84, SBL10, and BE12/96) and the type III strain BM110, which does not express Fbs. This experiment was performed twice with similar results.
Protein Fbs is a target for protective antibodies.
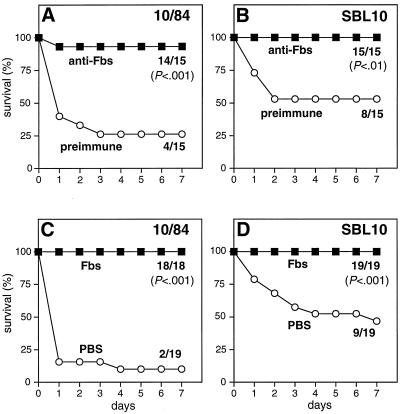
The ability of Fbs to elicit protective immunity was analyzed by passive and active immunization, with a mouse model of lethal GBS infection. Passive immunization with rabbit anti-Fbs serum protected mice against infection with either of two Fbs-expressing type V strains (Fig. 4A and B). For active immunization, mice were vaccinated with highly purified Fbs before challenge with Fbs-expressing strains. Significant protection was observed also in this case (Fig. 4C and D). Analysis by enzyme-linked immunosorbent assay (18) showed that Fbs elicited specific immunoglobulin G antibodies in vaccinated mice (data not shown).
FIG. 4.
Antibodies against Fbs protect mice against lethal infection with Fbs-expressing GBS strains. (A and B) Passive immunization. C3H/HeN mice were injected i.p. with 0.1 ml of rabbit anti-Fbs serum. Control mice received preimmune rabbit antiserum. The mice were challenged i.p. 4 h later with an ∼LD90 of log-phase bacteria. (Due to interexperimental variation, the survival was higher than 10% in some cases.) Two Fbs-expressing GBS type V strains, 10/84 and SBL10, were used, as indicated. Deaths were recorded daily for a period of 7 days, and the final ratios (number of surviving mice to number of mice challenged) are indicated. The P values were calculated by the Fisher exact test. (C and D) Active immunization. C3H/HeN mice were vaccinated with highly purified protein Fbs. Control mice received PBS. The vaccinated mice were challenged i.p. with an ∼LD90 of log-phase bacteria. Strains used were the same Fbs-expressing type V strains as used for the passive immunization experiments, and data are presented in the same way as described above.
Purification and characterization of a Rib-like protein.
Among the type V strains analyzed here, the most commonly expressed surface protein was a Rib-like protein (Table 1). This protein is referred to as Rib-like, since preliminary inhibition experiments with whole bacteria indicated that the protein expressed by these strains is not identical to Rib. Inhibition data obtained for one strain, isolate 2471, are shown in Fig. 5A. In this experiment, the binding of anti-Rib antibodies to immobilized Rib was inhibited with suspensions of whole bacteria. As expected, the Rib-expressing strain BM110 caused complete inhibition, while strain SB35, which expresses α and β, lacked inhibitory ability. Strain 2471 caused partial inhibition, but the shape of the curve and the incomplete inhibition suggested that the surface protein expressed by this strain might not be identical to Rib. Similar results were obtained with several other type V strains.
FIG. 5.
Identification of a Rib-like protein expressed by type V strain 2471. (A) Inhibition test with whole bacteria. Suspensions of whole bacteria were used to inhibit the binding of rabbit anti-Rib antibodies to Rib immobilized in microtiter plates. Strains used were the Rib-expressing type III strain BM110, the type V strain 2471, and the type Ib strain SB35, which expresses the α and β proteins. (B) Western blot analysis of purified preparations of five GBS surface proteins: the α, β, Rib, Fbs, and Rib-like proteins. The blot was analyzed with mouse antiserum (diluted 1:500) against the purified Rib-like protein, and bound antibodies were detected with radiolabeled protein A. In a control blot with preimmune mouse serum, no signals were obtained. Molecular mass markers are in kilodaltons. (C) The N-terminal amino acid sequence of the Rib-like protein is identical to that of the α protein (26, 30). Experiments for panels A and B were performed twice with similar results.
The Rib-like protein expressed by strain 2471 was purified to homogeneity from a mutanolysin extract (see Materials and Methods). The purified protein had a molecular mass of ∼120 kDa, and in a Western blot it cross-reacted with Rib (Fig. 5B). No cross-reactivity could be detected with α, β, or Fbs. Like Rib, the Rib-like protein was resistant to digestion with trypsin at pH 7.5 and pepsin at pH 4.0 (data not shown and reference 30).
The N-terminal sequence (12 residues) of the Rib-like protein was determined and was found to be identical to that of the α protein (Fig. 5C), confirming that the Rib-like protein is indeed different from Rib. Interestingly, the Rib-like protein lacked cross-reactivity with α, in spite of the identical N-terminal sequences.
Immunological comparison of Rib-like proteins expressed by different type V strains.
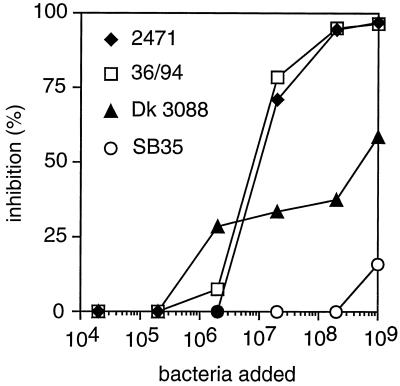
Rib-like proteins expressed by different type V strains were immunologically compared in an inhibition assay (Fig. 6). Washed and suspended bacteria were used to inhibit binding between the purified Rib-like protein immobilized in microtiter wells and antibodies to this protein. The analysis was performed with the 29 available type V strains that had been classified as expressing the Rib-like protein (Table 1). Of these 29 strains, 25 caused complete inhibition. This group of 25 strains included strain 2471, from which the Rib-like protein was purified. Data for this strain and for one other strain (strain 36/94) causing complete inhibition are shown in Fig. 6. The remaining four strains, represented in Fig. 6 by Dk 3088, caused partial inhibition. (The surprising shape of this inhibition curve was reproducible and was seen also with other strains causing incomplete inhibition). Together, these data indicate that the large majority of strains expressing Rib-like proteins express proteins that are immunologically similar, if not identical, justifying the classification in Table 1.
FIG. 6.
Immunological comparison of Rib-like proteins expressed by different GBS type V isolates. Suspensions of whole bacteria were used to inhibit the binding of rabbit anti-Rib-like antibodies to purified Rib-like protein immobilized in microtiter plates. Data from three strains expressing Rib-like proteins (2471, 36/94, and Dk 3088) are shown in the figure. The type Ib strain SB35 was used as a negative control. This experiment was performed twice with similar results.
The Rib-like protein is closely related to the R28 protein of S. pyogenes.
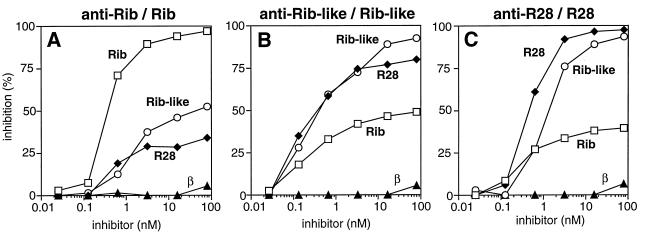
The family of extremely repetitive proteins identified by GBS proteins Rib and α also includes the R28 protein of S. pyogenes (28). The Rib-like GBS protein described here shares some properties with this protein: the Rib-like and R28 proteins both cross-react with Rib, and they have the same N-terminal sequence as α, although they do not cross-react with α. These data suggested that the Rib-like protein of GBS might be closely related to R28. To analyze this possibility, the immunological relationship among the Rib-like, R28, and Rib proteins was analyzed in inhibition experiments with protein immobilized in microtiter wells (Fig. 7). In these experiments, the binding between one immobilized protein and antibodies to this protein was inhibited with highly purified preparations of the three different proteins.
FIG. 7.
Analysis of the immunological relationship between the Rib and Rib-like proteins from GBS and the R28 protein from S. pyogenes. Each panel shows an inhibition experiment, in which the binding of rabbit antibodies to an immobilized protein was inhibited by the addition of different purified proteins. The combination of antiserum and immobilized protein is indicated above each panel, and the purified proteins used for inhibition are indicated at the corresponding curves. Each of these experiments was performed at least twice with similar results.
The binding of anti-Rib to immobilized Rib was first analyzed (Fig. 7A). Controls showed that the binding was inhibited by Rib but not by the unrelated β protein. Addition of the Rib-like protein caused only partial inhibition. The inhibition curve indicates that there are major immunological differences between the Rib-like and Rib proteins. Indeed, the Rib-like protein did not cause more than ∼50% inhibition, even when added at a high concentration, indicating that ∼50% of the anti-Rib antibodies did not recognize the Rib-like protein in this analysis. Similar results were obtained with the R28 protein, as also noted in another study (29). Thus, the R28 and Rib-like proteins had similar properties in this test, and both proteins showed major immunological differences from Rib.
In an inhibition test with the Rib-like protein and antiserum to this protein (Fig. 7B), Rib caused only partial inhibition, in agreement with the data in Fig. 7A. However, the R28 protein inhibited the binding efficiently, but not completely, indicating that the Rib-like protein and R28 are indeed closely related. Similar results were obtained in inhibition tests with anti-R28 serum (Fig. 7C). Taken together, these inhibition tests indicate that the Rib-like protein of GBS is very closely related to the R28 protein of S. pyogenes.
The Rib-like protein is a target for protective antibodies.
An attempt to demonstrate that antibodies against the Rib-like protein protect against lethal infection gave inconclusive results, probably due to technical problems. The exact reason for this inconclusive result was not analyzed, since other experiments showed that antibodies to the closely related R28 protein protected mice against lethal infection with a strain expressing the Rib-like protein. C3H/HeN mice were immunized actively or passively, as described in Materials and Methods, with pure R28 protein or rabbit anti-R28 antibodies, respectively. Controls received bovine serum albumin (BSA) or preimmune serum, respectively. Immunized mice were challenged with an ∼LD90 of GBS type V strain 2471, which expresses the Rib-like protein. The numbers of mice surviving for 7 days after challenge with strain 2471 were as follows: active immunization (data taken from a study of the ability of the R28 protein to confer protective immunity against GBS strains [29]), 9 of 15 mice receiving R28 protein (P < 0.001, compared to controls receiving BSA) and 0 of 15 mice receiving BSA; passive immunization, 9 of 11 mice receiving anti-R28 serum (P < 0.05, compared to controls receiving preimmune serum) and 3 of 10 mice receiving preimmune serum. The survival data were analyzed by the Fisher exact test. Indeed, protection was observed in both active and passive immunization experiments. Thus, the Rib-like protein can serve as a target for protective antibodies, as previously described for the R28 and Rib proteins (19, 28, 30) and as shown above for the Fbs protein.
DISCUSSION
Available evidence indicates that most strains of GBS express one or more surface proteins that confer protective immunity (17, 19, 25, 30). Interestingly, antibodies to these proteins are sufficient to prevent lethal experimental GBS infection, although the bacteria express a polysaccharide capsule that acts as a virulence factor and interferes with host defense mechanisms (5, 33, 36). This situation has made it of interest to characterize GBS surface proteins and evaluate them as possible vaccine components. Studies of GBS proteins are also of obvious interest for analysis of pathogenetic mechanisms used by GBS and other encapsulated bacteria. Moreover, the remarkable sequences of GBS surface proteins (10, 12, 26, 34) make them interesting from a protein-chemical point of view.
In previous studies, we developed methods to prepare homogeneous and highly purified preparations of the GBS proteins α, β, and Rib, allowing immunochemical characterization of the proteins and vaccination studies (18, 19, 21, 30). Moreover, methods were developed to purify the S. pyogenes R28 protein, which is a member of the same family as the Rib and α proteins of GBS (28). In the present investigation, we used knowledge gained in these earlier studies to purify and characterize two novel GBS surface proteins expressed by strains of serotype V, the Fbs and Rib-like proteins. The most important result of this work is the demonstration that the Fbs and Rib-like proteins are targets for protective antibodies, making them of interest for analysis of pathogenetic mechanisms and for vaccine development.
The Fbs protein was identified as a high-molecular-weight polypeptide present in mutanolysin extracts, by using methods previously used to identify the Rib protein in type III strains (30). The biochemical and immunochemical characterization of purified Fbs did not disclose any obvious similarity to other GBS surface proteins. Although the N-terminal sequence of Fbs was identical at 6 of 11 positions to a sequence in the repeats of Rib (Fig. 1D), the significance of this similarity seems uncertain, since the shared residues correspond to amino acids that are common in GBS surface proteins. Moreover, comparison of residues that are not shared in the two sequences does not support the possibility that they are related. Like the Rib and α proteins, Fbs varies in size between strains, suggesting that Fbs may contain repeated regions. However, the variation in size for Fbs expressed by different strains was more limited than that observed for Rib and α (22, 30). Although Fbs did not exhibit the characteristic laddering pattern seen when α and Rib are subjected to SDS-PAGE (22, 30), this result does not exclude the possibility that Fbs is a member of the same family as α and Rib, since Fbs could have repeats lacking the acid-labile Asp-Pro bonds that apparently give rise to the laddering pattern during SDS-PAGE analysis (34). However, the protease sensitivity of Fbs suggests that this protein may not be a member of the family including the α, Rib, and R28 proteins, all of which are resistant to trypsin.
The Rib-like protein was identified in inhibition tests in which whole bacteria of type V strains were used to inhibit the binding between purified Rib and anti-Rib. These tests indicated that some type V strains express a surface protein that is related to, but not identical to, Rib. This conclusion was confirmed in inhibition tests with highly purified protein preparations, which showed that the Rib and Rib-like proteins show major immunological differences, although they cross-react. However, the inhibition analysis indicated that the Rib-like protein is very similar to the R28 protein of S. pyogenes, a protein that can be viewed as a chimera derived from the three GBS proteins α, β, and Rib (28). Indeed, the similarities between the Rib-like and R28 proteins suggest that these proteins may be almost identical, supporting the suggestion that the gene for R28 arose in GBS and was transferred to S. pyogenes (28).
In the collection of 49 type V strains studied here, the Rib-like, Fbs, and β proteins were common and were expressed by 26 to 59% of the strains. In contrast, only two (4%) of the strains expressed the α protein. This result is surprisingly different from that obtained in another study of 41 type V strains, of which 61% were reported to express an α-like protein (16). The α-like protein characterized in that study appeared to be closely related to α, since it cross-reacted with α, but not with Rib, and had an N-terminal sequence very similar to the published sequence of α. A possible explanation for this difference between strain collections could be different geographic origins of the type V strains studied. Most of the strains studied here were from Scandinavia, while the strains studied by Lachenauer and Madoff (16) were from a U.S. collection. In another study of type V strains from the United States, it was found that α is rare among such strains, in agreement with the results reported here (9). Indeed, only 1 of 118 type V strains analyzed in the latter study expressed the c antigen, which includes α (24). The reason for this difference between the two U.S. studies is not known.
The studies reported here confirm earlier observations that the expression of surface proteins in GBS is strongly correlated with capsular type. Indeed, the α and β proteins are common in strains of serotypes Ia, Ib, and II but are almost never found in strains of serotype III (13, 24, 30), while protein Rib is expressed by almost all type III strains but has not been found in types Ia and Ib (18, 30). Similarly, the Fbs and Rib-like proteins described here are common in strains of serotype V but are rare in strains of other serotypes (this study and reference 29). Possible explanations for this linkage disequilibrium include lack of recombination and/or geographical isolation, but we are not aware of any data supporting these explanations. An interesting alternative explanation could be that certain combinations of virulence factors are favorable to the pathogen and are maintained by a strong selective pressure from the immune system of the infected host (7, 8).
Little is yet known about the function of different GBS surface proteins in pathogenesis. Indeed, most work on GBS proteins performed so far has been devoted to the identification, purification, and sequencing of different proteins and to analysis of their ability to elicit protective immunity. However, a recent study has demonstrated that the S. pyogenes R28 protein promotes adhesion to human epithelial cells (28). Since the GBS proteins α and Rib, and most likely also the Rib-like protein, are members of the same protein family as R28, it is attractive to speculate that these GBS proteins also act as epithelial cell adhesins. Experiments are now in progress to analyze this hypothesis. Information about the functions of the different proteins will throw new light on the pathogenesis of GBS disease and is also of interest for vaccine development.
ACKNOWLEDGMENTS
We are grateful to I. Carlstedt for advice and help with sialic acid analysis and to U. Regnér for technical assistance. Bacterial strains were kindly provided by L. Burman, J. A. Elliott, J. Henrichsen, J. Jelínková, A. I. Kvam, G. Orefici, and M. Sellin.
This work was supported by grants from the Swedish Medical Research Council (project 9490), Lund University Hospital, the Royal Physiographic Society in Lund, SmithKline Beecham Inc., The Swedish Society for Medical Research, the Alfred Österlund Trust, the Crafoord Trust, and the Johan and Greta Kock Trust.
REFERENCES
- 1.Baker C J, Edwards M S. Group B streptococcal infection. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and the newborn infant. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 980–1054. [Google Scholar]
- 2.Bevanger L, Naess A I. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta Pathol Microbiol Immunol Scand Sect B. 1985;93:121–124. doi: 10.1111/j.1699-0463.1985.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg H M, Stephens D S, Modansky M, Erwin M, Elliot J, Facklam R R, Schuchat A, Baughman W, Farley M M. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis. 1996;173:365–373. doi: 10.1093/infdis/173.2.365. [DOI] [PubMed] [Google Scholar]
- 4.Domingo P, Barquet N, Alvarez M, Coll P, Nava J, Garau J. Group B streptococcal meningitis in adults: report of twelve cases and review. Clin Infect Dis. 1997;25:1180–1187. doi: 10.1086/516094. [DOI] [PubMed] [Google Scholar]
- 5.Edwards M S, Kasper D L, Jennings H J, Baker C J, Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982;128:1278–1283. [PubMed] [Google Scholar]
- 6.Farley M M, Harvey R C, Stull T, Smith J D, Schuchat A, Wenger J D, Stephens D S. A population-based assessment of invasive disease due to group B streptococcus in nonpregnant adults. N Engl J Med. 1993;328:1807–1811. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Ferguson N, Anderson R. Chaos, persistence, and evolution of strain structure in antigenically diverse infectious agents. Science. 1998;280:912–915. doi: 10.1126/science.280.5365.912. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Maiden M C, Feavers I M, Nee S, May R M, Anderson R M. The maintenance of strain structure in populations of recombining infectious agents. Nat Med. 1996;2:437–442. doi: 10.1038/nm0496-437. [DOI] [PubMed] [Google Scholar]
- 9.Harrison L H, Elliott J A, Dwyer D M, Libonati J P, Ferrieri P, Billmann L, Schuchat A. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. J Infect Dis. 1998;177:998–1002. doi: 10.1086/515260. [DOI] [PubMed] [Google Scholar]
- 10.Hedén L O, Frithz E, Lindahl G. Molecular characterization of an IgA receptor from group B streptococci: sequence of the gene, identification of a proline-rich region with unique structure and isolation of N-terminal fragments with IgA-binding capacity. Eur J Immunol. 1991;21:1481–1490. doi: 10.1002/eji.1830210623. [DOI] [PubMed] [Google Scholar]
- 11.Jelínková J, Motlova J. Worldwide distribution of two new serotypes of group B streptococci: type IV and provisional type V. J Clin Microbiol. 1985;21:361–362. doi: 10.1128/jcm.21.3.361-362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerlström P G, Chhatwal G S, Timmis K N. The IgA-binding β antigen of the c protein complex of group B streptococci: sequence determination of its gene and detection of two binding regions. Mol Microbiol. 1991;5:843–849. doi: 10.1111/j.1365-2958.1991.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson D R, Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984;19:506–510. doi: 10.1128/jcm.19.4.506-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jourdian G W, Dean L, Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971;246:430–435. [PubMed] [Google Scholar]
- 15.Kogan G, Uhrin D, Brisson J R, Paoletti L C, Blodgett A E, Kasper D L, Jennings H J. Structural and immunochemical characterization of the type VIII group B streptococcus capsular polysaccharide. J Biol Chem. 1996;271:8786–8790. doi: 10.1074/jbc.271.15.8786. [DOI] [PubMed] [Google Scholar]
- 16.Lachenauer C S, Madoff L C. A protective surface protein from type V group B streptococci shares N-terminal sequence homology with the alpha C protein. Infect Immun. 1996;64:4255–4260. doi: 10.1128/iai.64.10.4255-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancefield R C, McCarty M, Everly W N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975;142:165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Larsson, C., and G. Lindahl. Unpublished data.
- 18.Larsson C, Stålhammar-Carlemalm M, Lindahl G. Experimental vaccination against group B streptococcus, an encapsulated bacterium, with highly purified preparations of cell surface proteins Rib and α. Infect Immun. 1996;64:3518–3523. doi: 10.1128/iai.64.9.3518-3523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson C, Stålhammar-Carlemalm M, Lindahl G. Protection against experimental infection with group B streptococcus by immunization with a bivalent protein vaccine. Vaccine. 1999;17:454–458. doi: 10.1016/s0264-410x(98)00218-7. [DOI] [PubMed] [Google Scholar]
- 20.Lin F Y, Clemens J D, Azimi P H, Regan J A, Weisman L E, Philips J B R, Rhoads G G, Clark P, Brenner R A, Ferrieri P. Capsular polysaccharide types of group B streptococcal isolates from neonates with early-onset systemic infection. J Infect Dis. 1998;177:790–792. doi: 10.1086/517810. [DOI] [PubMed] [Google Scholar]
- 21.Lindahl G, Åkerström B, Vaerman J P, Stenberg L. Characterization of an IgA receptor from group B streptococci: specificity for serum IgA. Eur J Immunol. 1990;20:2241–2247. doi: 10.1002/eji.1830201013. [DOI] [PubMed] [Google Scholar]
- 22.Madoff L C, Hori S, Michel J L, Baker C J, Kasper D L. Phenotypic diversity in the alpha C protein of group B streptococci. Infect Immun. 1991;59:2638–2644. doi: 10.1128/iai.59.8.2638-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madoff L C, Michel J L, Gong E W, Kling D E, Kasper D L. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc Natl Acad Sci USA. 1996;93:4131–4136. doi: 10.1073/pnas.93.9.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michel J L, Madoff L C, Kling D E, Kasper D L, Ausubel F M. C proteins of group B streptococci. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C: American Society for Microbiology; 1991. pp. 214–218. [Google Scholar]
- 25.Michel J L, Madoff L C, Kling D E, Kasper D L, Ausubel F M. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect Immun. 1991;59:2023–2028. doi: 10.1128/iai.59.6.2023-2028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel J L, Madoff L C, Olson K, Kling D E, Kasper D L, Ausubel F M. Large, identical, tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc Natl Acad Sci USA. 1992;89:10060–10064. doi: 10.1073/pnas.89.21.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stålhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. The R28 protein of Streptococcus pyogenesis related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol Microbiol. 1999;33:208–219. doi: 10.1046/j.1365-2958.1999.01470.x. [DOI] [PubMed] [Google Scholar]
- 29.Stålhammar-Carlemalm, M., C. Larsson, T. Areschoug, and G. Lindahl. Unpublished data.
- 30.Stålhammar-Carlemalm M, Stenberg L, Lindahl G. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med. 1993;177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenberg L, O'Toole P, Lindahl G. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol Microbiol. 1992;6:1185–1194. doi: 10.1111/j.1365-2958.1992.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 32.Suara R O, Adegbola R A, Baker C J, Secka O, Mulholland E K, Greenwood B M. Carriage of group B streptococci in pregnant Gambian mothers and their infants. J Infect Dis. 1994;170:1316–1319. doi: 10.1093/infdis/170.5.1316. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi S, Aoyagi Y, Adderson E E, Okuwaki Y, Bohnsack J F. Capsular sialic acid limits C5a production on type III group B streptococci. Infect Immun. 1999;67:1866–1870. doi: 10.1128/iai.67.4.1866-1870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wästfelt M, Stålhammar-Carlemalm M, Delisse A M, Cabezon T, Lindahl G. Identification of a family of streptococcal surface proteins with extremely repetitive structure. J Biol Chem. 1996;271:18892–18897. doi: 10.1074/jbc.271.31.18892. [DOI] [PubMed] [Google Scholar]
- 35.Wessels M R, Paoletti L C, Pinel J, Kasper D L. Immunogenicity and protective activity in animals of a type V group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Infect Dis. 1995;171:879–884. doi: 10.1093/infdis/171.4.879. [DOI] [PubMed] [Google Scholar]
- 36.Wessels M R, Rubens C E, Benedi V J, Kasper D L. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci USA. 1989;86:8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]