ABSTRACT
Background
Poor asthma control, often caused by non-adherence with controller medication, is a well-known risk factor for impaired quality of life (QoL) and major mood disorders (MMD). Previous studies have shown a relationship between non-adherence, lower QoL, and MMD across chronic diseases, but the relationship remains uncertain in asthma.
Methods
All asthma patients followed at the respiratory outpatient clinic at Copenhagen University Hospital – Hvidovre were invited to fill-in the Hospital Anxiety and Depression Scale (HADS) and the Mini Asthma Quality of Life Questionnaire (miniAQLQ). Medication Possession Ratio (MPR) was calculated using pharmacy redemption data. Relationships between questionnaire scores, inhaled corticosteroid MPR and use of rescue medication were investigated using Pearson correlation and multivariable linear regression adjusted for age, sex, FEV1, and GINA Step. Data from scheduled visits were collected from patients’ medical records.
Results
A total of 308 patients (73% females, median age 51 years (interquartile range (IQR) 37, 62)) completed the questionnaires and had 1-year medication data available. Median adherence to inhaled corticosteroids (ICS) was 57% (35%, 75%) with 18% of patients having adherence above 80%. Of the participating patients, 14% and 27% reported depressive and anxiety-related symptoms, respectively, and 72% reported impaired QoL. In correlation analyses, ICS adherence was not significantly associated with either prevalence of MMD symptoms or impaired QoL in asthma patients. However, a strong correlation was found between ACQ-6 and both MMD symptoms and impaired QoL, as well as between rescue medication use and impaired QoL. In adjusted analysis, however, the latter correlation was no longer statistically significant.
Conclusion
Our results suggest that ICS adherence is not directly correlated with either impaired quality of life or major mood disorder symptoms in asthma patients. Self-reported asthma control, on the other hand, is strongly correlated with both QoL and MMD.
KEYWORDS: Asthma, major mood disorder, anxiety, depression, inhaled corticosteroids
Introduction
Asthma is a chronic respiratory disease characterized by airway inflammation and reversible airflow obstruction and symptoms such as wheezing, shortness of breath, and chest tightness [1]. Affecting more than an estimated 339 million people worldwide and carrying a large morbidity burden driven mainly by uncontrolled disease, asthma poses a major global healthcare challenge [2,3]. Asthma control is the overall goal of treatment, with the cornerstone in treatment being inhaled corticosteroids (ICS) [1,2]. However, global asthma control remains elusive, with uncontrolled asthma being seen in 25–36% of patients [4] and partial control being seen in 40% of patients [5]. One of the biggest hurdles to disease control is adherence to ICS treatment [6], where administration of at least 80% of prescribed doses typically deemed necessary to obtain best possible treatment effect [7]. In real-world studies, adherence rates often range between 20% and 60% [6], resulting in suboptimal disease control and ultimately a high symptom burden, lower quality of life (QoL), and increased risk of adverse outcomes such as exacerbations and even death [2].
Major mood disorders (MMD), such as depression and anxiety, are among the most common disorders seen in medical practice and ranks as the third biggest cause of disease burden worldwide [8,9]. Previous studies have shown an increased prevalence of MMD and reduced QoL in patients with asthma [10,11] and especially among those with uncontrolled or symptomatic asthma [12]. Considering the relationship between adherence and asthma control, as well as asthma control and MMD prevalence, a vicious circle of control-worsening factors could be imagined, further lowering QoL and increasing MMD risk in asthma patients. The existing literature in this field is limited and provides mixed findings, with some studies showing a strong relationship between non-adherence and MMD [9,13,14] and others showing the opposite [15].
The aim of the present study is to investigate the relationship between adherence to ICS treatment, impaired QoL and prevalence of MMD symptoms in a consecutive cohort of patients with asthma managed at a university hospital respiratory outpatient clinic. We hypothesise that the prevalence of impaired QoL and MMD symptoms is high and that non-adherence is associated with impaired QoL and presence of MMD symptoms.
Methods
Patients and recruitment
All patients with asthma managed at the respiratory outpatient clinic, Copenhagen University Hospital – Hvidovre, Hvidovre, Denmark, during the period March 2019 – March 2020 were eligible for participation in the present study. Patients were invited to answer two study questionnaires as part of routine care. Patients were included in the study if they (i) answered all study questionnaires, (ii) had full 365-day inhaler prescription redemption data available, and (iii) did not actively reject researcher access to their medical records (Figure 1).
Figure 1.
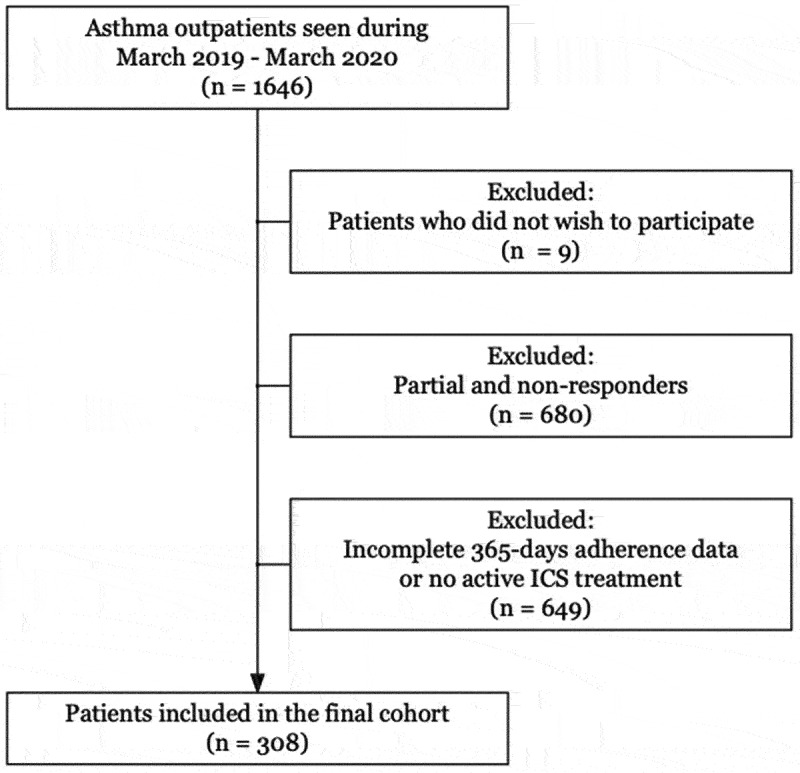
Flowchart of study inclusion and exclusion in a cohort of 308 patients with asthma followed at a university hospital respiratory outpatient clinic.
Ethics and approvals
Study approvals were obtained from the Capital Region of Copenhagen’s Data Safety Board (P-2020-1086) and the Capital Region of Copenhagen’s Centre for Regional Development’s Clinical Research Board (R-20069939).
Data are available upon reasonable request. However, approval from data sources and the Capital Region of Copenhagen’s Data Safety Board and Centre for Regional Development’s Clinical Research Board may be required as per Danish law.
Study procedures
As part of routine care, patients were asked to complete the Hospital Anxiety and Depression Scale (HADS) [16,17] and the mini Asthma Quality of Life Questionnaire (miniAQLQ) [18]. Routine examinations such as Asthma Control Questionnaire – 6 (ACQ6), fractional exhaled nitric oxide (FeNO), BMI, smoking history, exacerbation history, and prescribed pharmacological therapy were collected from patients’ medical records.
Hospital Anxiety and Depression Scale
The Danish version of the HADS questionnaire was used, with cut-off values for possible anxiety (HADS:A) and depression (HADS:D) scores set to 8 points [16].
Mini Asthma Quality of Life Questionnaire
The Danish version of the miniAQLQ was used with a cut-off value for impaired QoL, based on the total score, set to 5.4 in accordance with Correia de Sousa et al. [19].
Medication data
Pharmacy redemption data were collected for each patient from the nationwide Common Medication Card (CMC). Medication Possession Ratio (MPR) was used as an objective measure of adherence, calculated as the sum of redeemed doses in the 12 months prior to the visit date, divided by the sum of prescribed doses. During MPR calculations, the following adjustments were performed as previously described [20]: (i) For patients prescribed dual ICS therapy, only redeeming one of the ICS during the observation period, MPR was calculated as single ICS MPR for the inhaler redeemed and reduced by 50% to reflect partial non-adherence. (ii) Patients changing inhalers during the observation period had the, at the date of change remaining, number of doses from the discontinued inhaler omitted (at the redemption date of the newly prescribed inhaler) to prevent inflation of MPR.
Daily prescribed ICS dose was categorized according to the GINA 2020 guidelines [2]. Moderate exacerbations were defined as oral corticosteroid (OCS) prescriptions of at least 37.5 mg per day for at least 5 days found in the CMC the past 12 months, which did not coincide with hospitalizations over 24 h, as registered in electronic patient records. Severe exacerbations were defined as exacerbations requiring hospitalization for at least 24 h. Excessive short-acting beta-2-agonist (SABA) use was defined as at least 600 redeemed doses within a 12-month period. Use of long-acting beta-2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) was also registered.
Statistical analyses
Demographic statistics are presented as median (interquartile range, IQR). For groupwise comparisons, Wilcoxon rank-sum test or Chi-squared test of independence was used depending on continuous or categorical data. Simple correlation analysis with overall MPR was performed using Pearson correlation analysis. In comparison with statistically significant (p < 0.05) correlations, multivariable linear regression adjusted for age, sex, FEV1, and GINA treatment step was performed. Due to the number of tests performed, regression analyses are presented in both original and Bonferroni multiple testing corrected versions [21] with k corresponding to the number of tests performed (k = 8). R 4.0.2 (The R Foundation, AU) was used for statistical analyses. p-Values ≤0.05 were considered to be statistically significant.
Results
A total of 308 patients were included in the final study cohort. The median age was 51 (37, 62), the age range was 18–86 years, and 224 (73%) were women. The median BMI was 27.1 (23.8, 31.1), while 126 (42.4%) were current or ex-smokers with a mean lifetime tobacco exposure of 15 [7,22] pack years. The most prevalent comorbidities were allergy (55%), rhinitis (31%), MMD (13%) (including both anxiety and/or depression), and 13% had chronic obstructive pulmonary disease. Additional demographic data can be found in Table 1.
Table 1.
Baseline demographics of 308 patients with asthma followed at a university hospital respiratory outpatient clinic.
| Baseline characteristic | N = 308a |
|---|---|
| Female | 224 (73%) |
| Age | 51 (37, 62) |
| Body mass index (BMI) (n = 306) | 27.1 (23.8, 31.1) |
| FEV1 (%pred) | 91 (76, 101) |
| FVC (%pred, n = 299) | 100 (86, 113) |
| FEV1/FVC (n = 299) | 0.90 (0.84, 0.96) |
| Smoking Status (n = 296) | |
| Current smoker | 13 (4.4%) |
| Ex-smoker | 113 (38%) |
| Never smoker | 170 (57%) |
| Pack-years (n = 109) | 15 (7, 33) |
| FeNO (n = 291) | 14 (9, 25) |
| Comorbidities | |
| Allergies | 170 (55%) |
| Rhinitis | 97 (31%) |
| Nasal polyps | 23 (8%) |
| CVD | 26 (8%) |
| COPD | 39 (13%) |
| Depression or anxiety | 39 (13%) |
| Severe psychiatric disease | 15 (5%) |
an (%); Median (IQR).
FEV1, forced expiratory volume; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FeNO, fractional exhaled nitric oxide.
ICS therapy, adherence, and disease control
Out of the 308 included patients, 122 (40%), 124 (40%), and 62 (20%), respectively, had a prescription of low-, medium-, and high-dose ICS. The median adherence for all groups was 57% (35%, 75%), while 76 (18%) had good adherence (MPR ≥80%). The most frequent add-on treatments were LABA (87%) and LAMA (34%). Additional pharmacological data are given in Table 2.
Table 2.
Medication data for 308 patients with asthma followed at a university hospital respiratory outpatient clinic.
| Asthma treatment and adherence | N = 308a |
|---|---|
| ICS Dose | |
| Low | 122 (40%) |
| Moderate | 124 (40%) |
| High | 62 (20%) |
| ICS adherence | 57% (35%, 75%) |
| ≥80% Adherence | 76 (18%) |
| GINA 2020 Step (n = 308) | |
| Step 1 | 2 (0.6%) |
| Step 2 | 13 (4.2%) |
| Step 3 | 111 (36%) |
| Step 4 | 120 (39%) |
| Step 5 | 62 (20%) |
| Add-on Treatments | |
| Long-acting beta-2-agonists | 267 (87%) |
| Long-acting antimuscarinics | 106 (34%) |
| Dual long-acting bronchodilators | 102 (33%) |
an (%); ICS, inhaled corticosteroids.
In terms of disease control, the median ACQ-6 was 1.14 (0.43, 2.00), while 94 (37%) of patients had an ACQ-6 score ≥1.5, indicating asthma that was poorly controlled. The annual SABA use exceeded 600 doses for 175 (57%) of patients. Thirty-seven (12%) patients experienced moderate exacerbations within the last year, while the number was 12 (3.8%) for severe exacerbations.
Prevalence of MMD symptoms and impaired QoL
The miniAQLQ revealed a median score of 4.93 (3.85, 5.47) with 227 (72%) having a score ≤5.4. The median HADS:D score was 2.0 (1.0, 5.0), with 44 (14%) having a score ≥8. The median HADS:A was 5.0 (2.0, 8.0) with 86 (27%) scoring ≥8. Only 39 (13%) had a current clinical diagnosis of depression or anxiety. Additional data given in Table 3.
Table 3.
Questionnaire scores (ACQ-6, miniAQLQ, HADS) and control markers.
| Asthma symptom scores and control | N = 308a |
|---|---|
| Annual SABA use | 120 (0, 240) |
| ≥600 doses | 175 (57%) |
| History of moderate exacerbation(s) | 37 (12%) |
| Annual exacerbation rate | 1.00 (1.00, 2.00) |
| History of severe exacerbation(s) | 12 (3.8%) |
| Annual exacerbation rate | 1.00 (1.00, 1.00) |
| ACQ-6 (n = 252) | 1.14 (0.43, 2.00) |
| ≥1.5 | 94 (37%) |
| miniAQLQ | |
| Total Score | 4.93 (3.85, 5.47) |
| <5.4 | 227 (72%) |
| Symptom score | 4.80 (3.60, 5.40) |
| Activity score | 5.25 (4.25, 5.75) |
| Emotional score | 4.67 (3.67, 5.67) |
| Environment score | 5.00 (3.42, 5.67) |
| HADS | |
| Anxiety score | 5.0 (2.0, 8.0) |
| ≥8 | 86 (27%) |
| Depression score | 2.0 (1.0, 5.0) |
| ≥8 | 44 (14%) |
aMedian (IQR); n (%).
ACQ, Asthma Control Questionnaire; HADS, Hospital Anxiety and Depression Scale; Mini AQLQ, Mini Asthma Quality of Life Questionnaire; SABA, short acting beta-2-agonist.
Correlations between ICS adherence, MMD symptoms, and impaired QoL
Overall correlations between ICS adherence, HADS and miniAQLQ-scores, as well as objective measures of disease control, are presented as Pearson correlations with corresponding p-values in Figure 2. Statistically significant correlations were found between miniAQLQ and HADS:D (r = −0.58), HADS:A (r = −0.50), ACQ-6 (r = −0.53), annual SABA use (r = −0.23); between HADS:D and ACQ-6 (r = 0.33), HADS:A (r = 0.72); between HADS:A and ACQ-6 (r = 0.20); between ACQ-6 and ICS adherence (r = −0.13), annual SABA use (r = 0.24).
Figure 2.
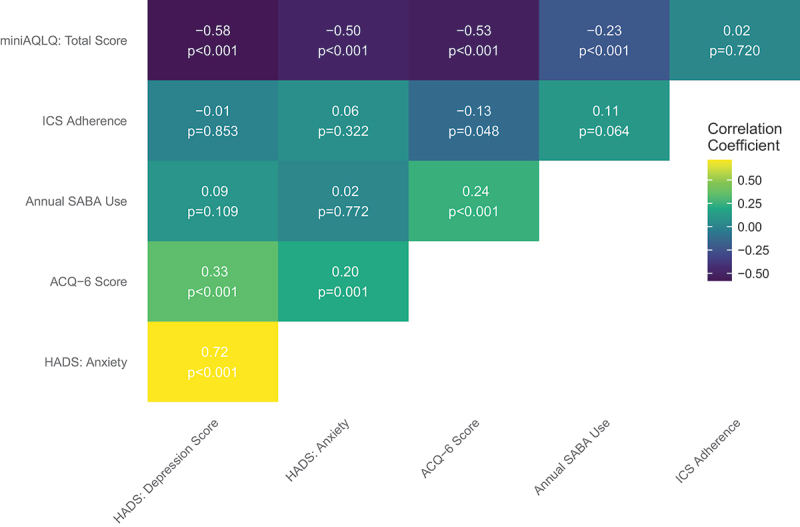
Pearson’s correlation coefficients between patient-reported scores, annual reliever use and adherence in a cohort of 308 patients with asthma followed at a university hospital respiratory outpatient clinic.
Multivariable adjusted analyses
For the aforementioned significant correlations, confirmatory multivariable adjusted linear regression analyses with and without Bonferroni multiple testing correction was carried out. After adjusting for age, sex, FEV1, and GINA treatment step, all the correlations remained significant, except for the correlations between ACQ-6 and annual SABA use as well as the correlation between ACQ-6 and ICS adherence (Figure 3(a)).
Figure 3.
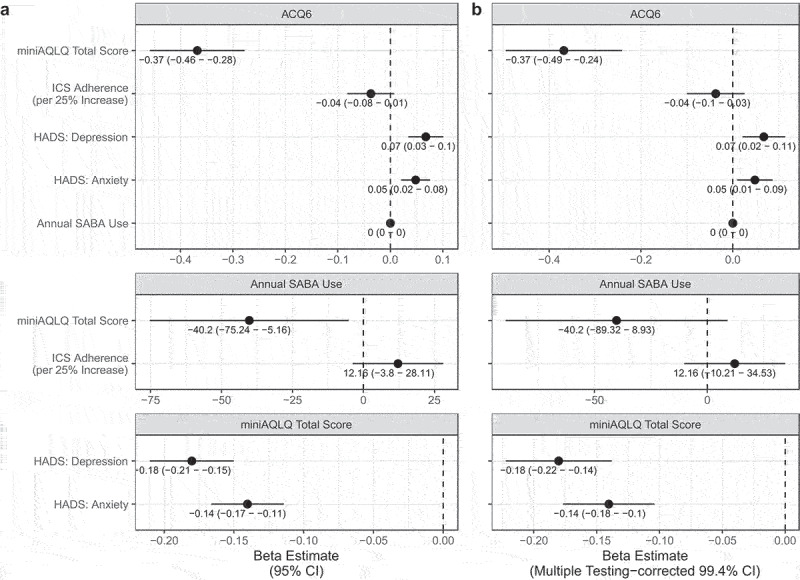
Multiple regression beta coefficients between patient reported scores, annual reliever use and adherence in a cohort of 308 patients with asthma followed at a university hospital respiratory outpatient clinic. Adjusted for age, sex, FEV1, and GINA treatment step and reported with (A) 95% confidence intervals and (B) multiple testing adjusted 99.4% confidence intervals.
When correcting for multiple testing, the following correlations remained significant (presented with 99.4% confidence intervals in parenthesis): between miniAQLQ and HADS:D (β = −0.18 (−0.22 – −0.14)), HADS:A (β = −0.14 (−0.18–0.10)) and ACQ-6 (β = −0.37 (−0.49 – −0.24)), as well as those between ACQ-6 and HADS:D (β = 0.07 (0.02–0.11)) and HADS:A (β = 0.05 (0.01–0.09)) (Figure 3(b)). Thus, the correlation between miniAQLQ and annual SABA use was no longer significant. Of note, the only consistently significant covariate in all adjusted, multivariable corrected analyses was FEV1 (data not shown).
Discussion
In the present study, we aimed to explore the relationship between ICS adherence, impaired QoL, and MMD symptoms. Based on patient-reported scores and objective adherence (MPR), no correlations between ICS adherence and QoL nor between ICS adherence and MMD symptoms were found. However, based on patient report, many asthma patients in secondary care suffer from anxiety or anxiety-associated symptoms without a formal diagnosis.
Prevalence of MMD symptoms and impaired QoL
The median ICS adherence found in the present study is consistent with a systematic review by Bårnes et al., who found a mean adherence ranging between 22% and 63% across 19 adherence studies [6]. The proportion of patients with adherence rates over 80% is also in line with the previous findings [6,23]. Where previous studies have demonstrated prevalence of depression symptoms up to 32% [12,24,25], the prevalence of depression symptoms in our study is markedly lower (14%). Similarly, the prevalence of anxiety symptoms (27%) in our study cohort is lower than in the other studies [12,24,25], where it ranges between 34% and 47%. The above-mentioned studies also used HADS to evaluate MMD symptoms and should therefore be comparable to our study. However, the study from Cooper et al. [24] investigates patients in primary care. The majority of all asthma patients are managed in primary care, but a recent study shows that primary care even manages the majority of uncontrolled as well as GINA Step 4 and 5 patients [26]. This could suggest that the lower MMD symptom burden found in the present study might be due to a combined effect of place of management (primary vs secondary/tertiary care), better asthma control and lower disease severity. The study from Baiardini et al. [25] differs from the present study by having a smaller population; 64 patients versus 308 patients, which could contribute to the differences in prevalence. Interestingly, our data show a notable discrepancy between patients with clinically diagnosed MMD (13%) and patients with anxiety symptoms (27%) according to HADS, suggesting that a considerable number of the patients in our study cohort have undiagnosed anxiety. Of note, the 13% comprises both clinically diagnosed depression and anxiety; consequently, the number of clinically diagnosed anxiety alone must be even lower. This discordance highlights the differences between patient-reported symptoms and physician-diagnosed MMD. Meanwhile, it should still be considered that HADS is only a screening tool, not a diagnostic instrument. Thus, a score above 8 on HADS may not meet diagnostic criteria for depression or anxiety, especially if scoring on certain items on the HADS is mediated from the symptoms of asthma itself, as could be suggested by the significant correlations found in the present study between ACQ-6, HADS:D, and HADS:A.
The role of ICS adherence in impaired QoL and MMD
Contrary to our hypothesis, our study showed that ICS adherence had no statistically significant correlation with either impaired QoL or MMD symptoms. One could hypothesize that the correlation might be stronger in patients with more severe disease who take higher ICS doses. However, even after restricting the population to patients treated with high ICS doses according to the GINA 2020 guidelines, the lack of correlation remained (data not shown). This is in contrast to the findings of Cluley et al. [27] and Bosley et al. [28], as both these studies found a significant correlation between ICS adherence and MMD symptoms. Our study has many similarities with both studies, e.g. the use of HADS to evaluate MMD symptoms. However, our methods differ substantially in other key aspects, e.g. we use MPR while Cluley et al. and Bosley et al. [27,28] used electronic inhalers with built-in dose-counters tracking adherence (total number of doses taken). Our study relies on the assumption that patients administer purchased medication, but we have no way of controlling it as with digital dose-counters. Additionally, Cluley et al. and Bosley et al. [27,28] measure adherence over 8–10 weeks, while the present study uses long-term adherence over 12 months based on pharmacy redemption data. Indeed, a recent study finds that short-term and long-term adherence often differ substantially, emphasizing that the period of time over which one measures adherence is key [29]. In accordance with our study, Toelle et al. [15] found no association between ICS adherence and MMD symptoms; in fact, depression symptoms was even associated with a lower risk of non-adherence. When it comes to the association between ICS adherence, MMD symptoms, and QoL in asthma on a longer term, the evidence is sparse. However, a longitudinal study in adolescents found no correlation between ICS adherence and QoL over a period of 2 years [30]. The authors hypothesize that the lack of correlation might be due to the room for improvement in QoL being small in the group of adolescents.
As expected, we found a strong correlation between MMD symptoms and impaired QoL; a seemingly trivial yet important observation that confirms that suffering from MMD and/or MMD symptoms impairs one’s quality of life. Also, as expected, we found near-significant correlations between ICS adherence and ACQ-6, corroborating that taking one’s medication regularly improves asthma control, but the present study might be underpowered to demonstrate proper significance.
ACQ-6 correlated strongly with miniAQLQ, as well as HADS:A and HADS:D. This could suggest that the symptom burden from poorly controlled asthma per se lowers QoL, regardless of ICS adherence, but more research is needed to establish causality. This assumption is in accordance with Di Marco et al. [12], who describe associations between poor asthma control, asthma severity and increased prevalence of MMD. Di Marco et al. [12] suggest two possible reasons: Either a high symptom burden increases MDD risk – or perhaps MMD symptoms intensify the perception of asthma symptoms by augmenting symptom perception in general. Interestingly, when adjusted for FEV1, the correlation between miniAQLQ and annual SABA use was no longer significant, suggesting that mechanisms impeding QoL might be attributable to a poor lung function rather than factors leading to SABA use. On the other hand, the correlation between miniAQLQ and ACQ-6 was not affected by the adjustment of FEV1 in the same way, which considering the lack of association between ACQ-6 and SABA use found corroborates with the above hypothesis. However, correlation does not entail causation, and further research into the complex interplay of subjective symptoms, objective markers, and symptom scores is needed.
Interventions for MMD in asthma
It is very well established that there is a strong link between having a chronic disease and suffering from impaired QoL, depression, or anxiety [31]. Functional impairment, worsening condition, unrelieved pain, and social isolation have all been described as important factors [32]. cognitive behavioral therapy (CBT) has previously shown an improvement in AQLQ-score compared to usual care in asthma [33], suggesting that QoL is highly dependent on the patient’s own perception of their disease. Conversely, the effect of CBT on anxiety levels was modest, while CBT had no effect at all on depression severity and ICS adherence [33]. Factors outside of physician control (having a close personal network, self-esteem, and self-efficacy) are also of great importance in terms of QoL and MMD in chronic disease [22]. In asthma care, the first step towards improving QoL for symptomatic patients is naturally improving disease control, yet for patients still symptomatic despite optimal care and acceptable adherence, close attention to MMD and impaired QoL is warranted.
Limitations
This study is subject to several limitations. Due to the cross-sectional study design, we cannot distinguish between causation and correlation. An important limitation is the measurement of adherence, which relies solely on filled prescriptions and not whether patients took their medicine or not. Thus, the present study relies on the assumption that patients take the medicine that they buy. Furthermore, the current study does not assess the patients’ inhalation technique, which is also an important aspect of adherence. However, the effect is believed to be small, since patients seen at the respiratory outpatient clinic have their device technique assessed continuously and, if necessary, re-trained by the respiratory nurses. The relatively small sample size could possibly be an obstacle to identifying small effects of ICS adherence, however, considering the relatively large increases in adherence necessary to improve clinical outcomes [34], any missed effects are likely well below clinical significance. There was an excess in female participants due to the outpatient clinic’s management of asthma in pregnancy (MAP) program. However, no changes in correlations were seen in sensitivity analyses where MAP-participants were excluded (Figure S1). The cohort includes patients who are newly referred and those followed for longer periods of time, which could differ in terms of adherence patterns [35]. However, we are unable to differentiate between these groups due to data limitations. Furthermore, non-responder analyses were not allowed due to legal reasons, risking sampling errors where patients most afflicted by their disease potentially are systematically excluded from the present study. Finally, the last period in which the data for this study were collected interferes with the beginning of the COVID-19 pandemic. However, since the pandemic covers only the very last weeks, the effect on our results must be assumed to be minimal.
Conclusion
In the present study, ICS adherence had no correlation with either prevalence of MMD symptoms or impaired QoL in asthma patients. However, a strong correlation between asthma control and both MMD symptoms and impaired QoL was found. Furthermore, a discrepancy between patient-reported and clinically diagnosed anxiety symptoms was found. Our findings suggest that the symptom burden per se could have a substantial impact on QoL and MMD symptoms, regardless of ICS adherence, but a causal link remains unknown.
Supplementary Material
Biographies
Martino Renzi-Lomholt is a 5th year student of medicine at the University of Copenhagen.
Kjell Erik Julius Håkansson is PhD and Senior Registrar at the Department of Respiratory Medicine at Hvidovre Hospital.
Charlotte Suppli Ulrik is professor in pulmonary medicine and Head of the Respiratory Research Unit and Severe Asthma Clinic at Hvidovre Hospital.
Funding Statement
The present work is funded by The Danish Health Foundation (Ref. 20-B-0226) and The Danish Lung Foundation Research Fund.
Disclosure statement
MRL has nothing to declare. KEJH has received personal fees from AstraZeneca, Chiesi, GSK, Sanofi Genzyme, and TEVA. CSU has received personal fees from AstraZeneca, GSK, TEVA, Sanofi Genzyme, Boehringer-Ingelheim, Chiesi, Orion Pharma, Novartis, ALK-Abello, Mundipharma, and Actelion.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2022.2149920
References
- [1].Leander M, Lampa E, Rask-Andersen A, et al. Impact of anxiety and depression on respiratory symptoms. Respir Med. 2014. Nov;108(11):1594–8. [DOI] [PubMed] [Google Scholar]
- [2].Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention. GINA. 2020. [DOI] [PubMed]
- [3].Vos T, Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].von Bülow A, Kriegbaum M, Backer V, et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2(6):759–767. [DOI] [PubMed] [Google Scholar]
- [5].Backer V, Bornemann M, Knudsen D, et al. Scheduled asthma management in general practice generally improve asthma control in those who attend. Respir Med. 2012;106(5):635–641. [DOI] [PubMed] [Google Scholar]
- [6].Bårnes CB, Ulrik CS.. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60(3):455–468. [DOI] [PubMed] [Google Scholar]
- [7].Murphy AC, Proeschal A, Brightling CE, et al. The relationship between clinical outcomes and medication adherence in difficult-to-control asthma. Thorax. 2012. Aug;67(8):751–753. [DOI] [PubMed] [Google Scholar]
- [8].Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. [DOI] [PubMed] [Google Scholar]
- [9].DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000. Jul;160(14):2101–2107. [DOI] [PubMed] [Google Scholar]
- [10].Scott KM, Von Korff M, Ormel J, et al. Mental disorders among adults with asthma: results from the world mental health survey. Gen Hosp Psychiatry. 2007;29(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mangold R, Salzman GA, Williams KB, et al. Factors associated with depressive symptoms in uncontrolled asthmatics. J Asthma. 2018. May;55(5):555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104(1):22–28. [DOI] [PubMed] [Google Scholar]
- [13].Smith A, Krishnan JA, Bilderback A, et al. Depressive symptoms and adherence to asthma therapy after hospital discharge. Chest. 2006. Oct;130(4):1034–1038. [DOI] [PubMed] [Google Scholar]
- [14].ten Brinke A, Ouwerkerk ME, Zwinderman AH, et al. Psychopathology in patients with severe asthma is associated with increased health care utilization. Am J Respir Crit Care Med. 2001. Apr;163(5):1093–1096. [DOI] [PubMed] [Google Scholar]
- [15].Toelle BG, Marks GB, Dunn SM. Psychological and medical characteristics associated with non-adherence to prescribed daily inhaled corticosteroid. J Pers Med. 2020. Sep;10(3):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002. Feb;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- [17].Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983. Jun;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- [18].Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999. Jul;14(1):32–38. [DOI] [PubMed] [Google Scholar]
- [19].de Sousa J C, Pina A, Cruz AM, et al. Asthma control, quality of life, and the role of patient enablement: a cross-sectional observational study. Prim Care Respir J. 2013. Jun;22(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jensen FF, Håkansson KEJ, Overgaard Nielsen B, et al. Self-reported vs. objectively assessed adherence to inhaled corticosteroids in asthma. Asthma Res Pract. 2021;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mundfrom D, Perrett J, Schaffer J, et al. Bonferroni adjustments in tests for regression coefficients. Mult Linear Regres Viewpoints. 32 :2006 Jan 1. [Google Scholar]
- [22].Bisschop MI, Kriegsman DMW, Beekman ATF, et al. Chronic diseases and depression: the modifying role of psychosocial resources. Soc Sci Med. 2004;59(4):721–733. [DOI] [PubMed] [Google Scholar]
- [23].Hwang EK, Jin HJ, Nam YH, et al. The predictors of poorly controlled asthma in elderly. Allergy, Asthma Immunol Res. 2012;4(5):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cooper CL, Parry GD, Saul C, et al. Anxiety and panic fear in adults with asthma: prevalence in primary care. BMC Fam Pract. 2007. Oct;8(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baiardini I, Braido F, Giardini A, et al. Adherence to treatment: assessment of an unmet need in asthma. J Investig Allergol Clin Immunol. 2006;16(4):218–223. [PubMed] [Google Scholar]
- [26].Håkansson KEJ, Backer V, Suppli Ulrik C. Socioeconomic biases in asthma control and specialist referral of possible severe asthma. Eur Respir J. 2021. May;58(6):2100741. [DOI] [PubMed] [Google Scholar]
- [27].Cluley S, Cochrane GM. Psychological disorder in asthma is associated with poor control and poor adherence to inhaled steroids. Respir Med. 2001;95(1):37–39. [DOI] [PubMed] [Google Scholar]
- [28].Bosley CM, Fosbury JA, Cochrane GM. The psychological factors associated with poor compliance with treatment in asthma. Eur Respir J. 1995;8(6):899–904. [PubMed] [Google Scholar]
- [29].Vähätalo I, Kankaanranta H, Tuomisto LE, et al. Long-term adherence to inhaled corticosteroids and asthma control in adult-onset asthma. ERJ Open Res. 2021;7(1):00715–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tiggelman D, van de Ven Mom, van Schayck Ocp, et al.Longitudinal associations between asthma control, medication adherence, and quality of life among adolescents: results from a cross-lagged analysis.Qual Life Res.2015;24(9):2067–2074;Internet].;():. Available from [DOI] [PubMed] [Google Scholar]
- [31].Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. 2007;370(9590):851–858. [DOI] [PubMed] [Google Scholar]
- [32].Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med J Aust. 2009;190(7 SUPPL). 10.5694/j.1326-5377.2009.tb02471.x [DOI] [PubMed] [Google Scholar]
- [33].Kew KM, Nashed M, Dulay V, et al. Cognitive behavioural therapy (CBT) for adults and adolescents with asthma. Cochrane Database Syst Rev. 2016. Sep;9:CD011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185–1191.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Takala J, Vähätalo I, Tuomisto LE, et al. Participation in scheduled asthma follow-up contacts and adherence to treatment during 12-year follow-up in patients with adult-onset asthma. BMC Pulm Med. 2022. Feb;22(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


