Abstract
Clinical studies suggest that women are more likely than men to relapse to alcohol drinking in response to stress, however the mechanisms underlying this sex difference are not well understood. A number of preclinical behavioral models have been used to study stress-induced alcohol intake. Here we review paradigms used to study effects of stress on alcohol intake in rodents, focusing on findings relevant to sex differences. To date, studies of sex differences in stress-induced alcohol drinking have been somewhat limited, however, there is evidence that amygdala-centered circuits contribute to effects of stress on alcohol seeking. In addition, we present an overview of inflammatory pathways leading to microglial activation that may contribute to alcohol-dependent behaviors. We propose that sex differences in neuronal function and inflammatory signaling in circuits centered on the amygdala are involved in sex-dependent effects on stress-induced alcohol seeking and suggest that this is an important area for future studies.
Introduction
The consequences of chronic alcohol use represent a major personal, public health and financial burden. Historically, men have had higher rates of problematic alcohol use than women (Schulte et al. 2009). However, the trend for an increase in alcohol use disorders (AUD) among women is alarming, and recent analyses suggest an increase in problematic drinking in women in the United States of more than 80% over the past 10 years (Grant et al. 2017). While pharmacological treatments are available for AUD, they were developed exclusively or primarily with samples of men, (Anton et al. 2006) and none of the currently approved treatments are known to target the multiple factors that differentially maintain drinking in women. AUD is characterized by physical dependence and neuronal perturbations induced by repeated alcohol exposure. Withdrawal from alcohol leads to a number of negative effects, including changes in mood and induction of negative affect, but also has life-threatening consequences, including seizure and coma. Many AUD therapies focus on alcohol intake but other co-morbid conditions, such as depression, perpetuate the use of alcohol, likely as an attempt to cope with psychiatric symptoms. Not surprisingly, multiple studies have demonstrated strong co-morbidity between AUD and psychiatric disorders (https://pubs.niaaa.nih.gov/publications/arh26-2/81-89.htm), including generalized anxiety disorder, depression and post-traumatic stress disorder, all of which can contribute to harmful drug and alcohol use. Of particular concern is the fact that women are more prone to negative-reinforcement drinking (NRD), and thus, stress-related drinking relapse, compared to men (Nolen-Hoeksema and Hilt 2006). Because stress sensitivity and rates of anxiety disorders are twice as high in women than in men (Remes et al. 2016) and lifetime anxiety predicts poorer drinking outcomes in women (Farris et al. 2012), it is critical to identify the mechanisms by which neurobiological circuits that regulate these behaviors can contribute to NRD and alcohol consumption.
Clinical (Keren et al., 2014; Logrip et al., 2018; Peltier et al., 2019) and preclinical (Pelloux et al., 2005; Cozzoli et al., 2014; Peñasco et al., 2015; Bertholemey et al., 2016; Shaw et al., 2020) studies demonstrate heightened susceptibility to stress-induced drinking in females. Animal models can recapitulate the effects of sex and stress on alcohol intake. For example, exposure to a stressor can increase ethanol intake in mice, with female mice increasing their alcohol intake more rapidly than males (Cozzoli et al., 2014). These animal models provide the possibility of examining the underlying mechanisms of stress induced changes in alcohol-related behaviors and exploring sex differences in these mechanisms.
This review will provide an overview of the different animal models used to decipher the connection between stress and alcohol intake and how sex differences can modulate this interaction. Based on the established role of the amygdala in stress-relevant behaviors, we will then provide an overview of the role of neuronal networks centered on the amygdala in alcohol-related behaviors and explore how perturbation of amygdala activity by alcohol could alter these behaviors. Finally, given the increasing body of evidence that inflammatory pathways in the brain are recruited in response to both stress and alcohol exposure (Crews et al. 2017), we will provide an overview of the possible role of microglia, a key cellular component of the brain inflammatory response, in reshaping the neuronal networks that contribute to, and perpetuate, alcohol use. The goal of this review is to identify critical lines of research needed to gain a greater understanding of stress-induced alcohol use, and to evaluate sex differences in some of the critical mechanisms underlying these behaviors.
Rodent models of alcohol use and relevance for stress-induced drinking
Several studies have investigated the effects of stress on alcohol intake and preference in rodent models, and the results differ based on species, strain, type of stressor, and the timing of stress and alcohol exposure in the experimental design (Spanagel et al., 2014; Weera & Gilpin, 2019). However, only a limited number of rodent studies have considered potential sex differences in how stress can alter alcohol seeking behaviors. Preclinical studies of sex-dependent effects of stress on alcohol drinking could shed light on the biological mechanisms underlying female-specific increases in human alcohol drinking to cope with stress and negative affect (Peltier et al., 2019).
To model stress-induced increases in alcohol intake and preference in rodents, the first consideration is the route of administration and the duration of exposure to ethanol. Rodents typically do not self-administer ethanol in sufficient amounts to induce behavioral intoxication or to reach physiologically relevant blood alcohol concentrations measured in human drinkers. Several strategies have therefore been proposed to promote robust alcohol intake in rodents, including selective breeding of alcohol-preferring rodent strains and/or manipulation of schedule of access to alcohol (Becker, 2012; Becker & Ron, 2014).
Classical behavioral approaches to volitional administration of oral ethanol include access to an ethanol solution through an operant task (Heidbreder et al., 2007; Lopez & Becker, 2014; Sparta et al., 2009) or home cage drinking of an oral ethanol solution. Access to ethanol solutions can either be unlimited throughout the duration of the experiment (Crabbe et al., 2010; García-Pardo et al., 2017; Hwa et al., 2011), intermittent between days (Bloch et al., 2020; Hwa et al., 2011; Warnault et al., 2013), or limited to specific times of the day (Becker, 2012; Olney et al., 2018; Rhodes et al., 2005; Thiele & Navarro, 2014). These approaches differ in the extent of alcohol intake and blood alcohol levels achieved, with intermittent and limited access models allowing a much higher level of intoxication than unrestricted access (Hwa et al., 2011; Rhodes et al., 2005). Depending on the experimental design, however, reaching maximal alcohol intake in the absence of stress exposure could be counterproductive if the goal is to assess how stress can increase intake. In addition, the availability of choice between water and ethanol can provide stronger construct validity for the human condition, compared to models in which alcohol intoxication is reached because no other fluid is available (Cannella et al., 2019).
The nature and frequency of stress exposure is another factor that can be varied across experiments. Studies exploring stress-induced alcohol consumption have involved physical restraint (Farook et al., 2009; Marianno et al., 2017; Walker et al., 2015), social defeat (Newman et al., 2018; Norman et al., 2015), exposure to predator odors (Cozzoli et al., 2014; Hwa et al., 2011; Shaw et al., 2020), forced swim (Morais-Silva et al., 2015), footshocks (Breit & Chester, 2016; Cozzoli et al., 2014), pharmacologically-induced stress (Bertholomey et al., 2016; King and Becker, 2019; Ballas et al., 2021), or a combination of these stressors (Cozzoli et al., 2014). Interestingly, in male rats, forced swim and footshocks appear to elicit greater stress-induced alcohol drinking when compared to physical restraint, although this difference is not seen in mice (Noori et al., 2014). Importantly, stressors may differentially activate the hypothalamic-pituitary-adrenocortical (HPA) axis in male and female animals (Albrechet-Souza et al., 2020; Babb et al., 2013; Bland et al., 2005; Cozzoli et al., 2014), and thus the potential for sex-specific sensitivity to particular stressors and their effects on alcohol drinking should be an important point of consideration.
Human data strongly suggest that the interactions between stress and alcohol intake are bidirectional (Peltier et al., 2019): stress can prime individuals for subsequent alcohol seeking (Childs et al., 2011), while alcohol can also increase responsivity to stress (Becker & Koob, 2016; Bertholomey et al., 2016). Given the complex interactions between stress exposure and alcohol intake, complementary behavioral approaches have been developed to model different dimensions of the human condition. Several rodent models have focused on stress as a trigger for relapse in stress-induced alcohol seeking (Lê and Shaham, 2002). These studies have shown that a variety of stressors, notably footshocks (Lê et al., 1998, Lê et al., 2011), predator odor (King and Becker, 2019) or the α2-adrenoceptor antagonist yohimbine (Ballas et al., 2021; Bertholomey et al., 2016; Borruto et al., 2021; King and Becker, 2019; Lê et al., 2011), can robustly reinstate alcohol seeking in rodents previously trained in operant self-administration of alcohol that have subsequently undergone extinction. Importantly, these stress-induced reinstatement tests are done in the absence of an alcohol reinforcer. While these operant approaches can more accurately capture the effect of stress on alcohol seeking as a model for human relapse during abstinence, animal models that explore alcohol drinking in the home cage have the advantage of exploring how stress can alter alcohol drinking in the absence of cue- or action-triggered intake (Becker et al., 2011). In particular, home cage studies provide opportunities to study how drinking alcohol after stress exposure can ameliorate neuroadaptive imbalances arising from prior exposure to both alcohol and stress, and thus can be helpful in the mechanistic study of negative reinforcement drinking with relevance to relapse as well.
Only individuals who have learned that alcohol can reduce a negative affective state would be more likely to drink alcohol to alleviate stress (Heilig et al., 2010; Noori et al., 2014; Spanagel et al., 2014). Thus, animal models of stress-induced increases in alcohol intake require a history of prolonged ethanol exposure coupled with repeated exposures to stressors. For instance, male mice increase ethanol intake after repeated cycles of stress, but only if previously trained to consume high levels of alcohol, as can be achieved with chronic-intermittent exposure (CIE) (Anderson et al., 2016; Lopez et al., 2016) or a scheduled high-alcohol consumption paradigm (Finn et al., 2018). These two models incorporate cycles of binge alcohol intoxication followed by repeated withdrawal periods that dramatically increase alcohol intake compared to other paradigms (Holleran and Winder 2017). Withdrawal is thought to promote negative reinforcement drinking via alterations of the HPA axis (Blaine & Sinha, 2017; Koob, 2003; Rasmussen et al., 2000), thus having the potential to increase stress-induced drinking. Furthermore, a review by Becker and colleagues (Becker et al., 2011) noted that chronic exposure to stress is more likely to enhance alcohol drinking in rodents when compared to acute stressors. Interestingly, stress-induced effects on alcohol intake are only evident when the stress is removed in time from the availability of alcohol (Noori et al., 2014), potentially due to the time needed for stress-induced changes in neuroplasticity to alter alcohol seeking behavior (Spanagel et al., 2014). Taken together, these data suggest that the timing and frequency of both stress and alcohol exposure are likely to be critical parameters for modeling stress-induced alcohol drinking in rodents.
Not surprisingly, the limited number of preclinical studies that have explored stress-induced alcohol intake differ fundamentally in stress-alcohol timing and frequency, as is summarized in Table 1. Most of the studies that introduce the stressor prior to alcohol exposure focus on how stress occurring early in development determines future alcohol intake and preference during late adolescence and adulthood. Most of the studies done in adult rodents, in contrast, introduce alcohol exposure before repeated bouts of stress exposure. Nonetheless, only a small number of published studies have used both male and female rodents within the same experimental design (Table 2).
TABLE 1 -.
Stress Exposure prior to Alcohol Drinking
| Study | Species, Strain | Sex | Stressor | Frequency and Length of Stress Exposure | Time between Stress and Alcohol | Alcohol Access | Frequency and Length of Alcohol Exposure | Stress-induced alcohol intake? |
|---|---|---|---|---|---|---|---|---|
| Pelloux et al. (2005) | Mice (CD1) | M/F | Tail suspension | Once, 6 mins | 7 days | Continuous, TBC, increasing alcohol % every 8 days (3–20%) | Daily, 40 days | Yes |
| Cruz et al. (2008) | Mice (CFW) | M | Maternal separation | Daily, 14 days | 45 days | Limited, 2 hr (DID) Operant responding, 30 min |
Daily, 10 days Daily, 20 days |
Yes Yes |
| Peñasco et al. (2015) | Rats | M/F | Maternal separation + Withdrawal + Restraint stress | Once, 24 hrs 2× 7 days, 1 wk apart Daily, 30 mins |
18 days | Continuous, TBC Continuous, TBC Continuous, TBC |
Daily, 22 days Daily, 4 days post stress Daily, 3 days post stress |
No No Yes |
| Norman et al. (2015) | Mice (CFW) | M | Social defeat | Daily, 10 days | 10 days | Continuous, TBC Intermittent, TBC + Operant responding (FR and PR) 30 min |
Daily, 20 days 3x a week, 35 days. Daily, 35 days |
Yes Yes Yes |
| Skelly et al. (2015) | Rats (Long-Evans) | M | Social isolation | Continuous, 6 weeks | 8 weeks | Intermittent, TBC | 3x a week, 6 weeks | Yes |
| Bertholomey et al. (2016) | Rats (Sprague Dawley) | M/F | Corticosterone in drinking water | Continuous, 20 days | 10 days | Operant responding (PR), 1hr | Daily, 21 days | Yes |
| Newman et al. (2018) | Mice (C57BL/6J) | M | Social defeat | Daily, 10 days | 10 days | Continuous, TBC Intermittent, TBC |
Daily, 10 weeks 3x a week, 10 weeks |
Yes Yes |
| Shaw et al. (2020) | Mice (C57BL/6J) | M/F | Predator odor | Daily, 15 days | 12 days | Intermittent, TBC | 3x a week, 4 weeks | No |
TBC: two-bottle choice; DID: drinking in the dark.
TABLE 2 -.
Alcohol Drinking prior to Stress Exposure
| Study | Species Strain | Sex | Alcohol Access | Frequency and Length of Initial Alcohol Exposure prior to Stress | Stressor | Frequency and Length of Stress Exposure | Stress-induced alcohol intake? |
|---|---|---|---|---|---|---|---|
| Farook et al. (2009) | Mice (C57BL/6J) | M | Continuous TBC |
Daily, 7 days | Physical restraint | Daily for 5 days | Yes |
| Edwards et al. (2013) | Rats (Wistar) | M | Limited, 30 min, two-choice operant | Daily, 15 days | Predator odor | Once, 15 min | Yes |
| Cozzoli et al. (2014) | Mice (C57BL/6J) | M/F | Limited 2 hr (DID) |
Daily, 15 days | One of each: Tail suspension Physical restraint Predator odor Foot shock Tail pinch |
Each stressor applied at least 5 drinking sessions apart. | Yes (for predator odor and foot shock) |
| Walker et al. (2015) | Mice (C57BL/6J) | M | Continuous TBC |
Daily, 3 weeks | Physical restraint + Forced swim | Daily, 7 days Daily, 2 days |
No No |
| Anderson et al. (2016) | Mice (C57BL/6J) | M | Continuous TBC Intermittent TBC Limited, 2 hr (DID) Intermittent and limited (TBC, 2 hr) +alcohol vapor |
Daily, 7 days 3x week, 1 week Daily, 1 week 3x week, 6 weeks Daily, 16hrs, 4 days |
Forced swim Forced swim Forced swim Forced swim |
3x week, for 4 weeks Same as above Daily, for 4 weeks, Same as above - cycle repeated 4 times on alternate weeks |
No No No Yes |
| Lopez et al. (2016) | Mice (C57BL/6J) | M | Limited, TBC, 2 hr (DID) Limited, TBC, 2 hours (DID) + alcohol vapor exposure |
Daily, 6 weeks Daily, until stable Daily, 16hrs, for 4 days |
Physical restraint Social defeat Forced swim Social defeat Forced swim |
Daily, 5 days Daily, 5 days Daily, 5 days Daily, 5 days, repeated 4x on alternate weeks Daily, 5 days, repeated 4 x on alternate weeks |
No No No No Yes |
| Manjoch et al., 2016 | Rats (Sprague-Dawley) | M | Continuous TBC | Daily, at least 7 days | Predator odor | Once, 15 min Re-exposed to context 2×15 min |
Yes Yes |
| Finn et al., 2018 | Mice (C57BL/6J) | M/F | Intermittent limited (single sipper, 30 min), then, continuous TBC | Every 3rd day, 7 sessions total Daily, 3 weeks |
Predator odor | Every 2–3 days, 30 min, 4x. | Yes |
TBC: two-bottle choice; DID: drinking in the dark.
A study done by Cozzoli and colleagues (Cozzoli et al., 2014) trained male and female mice using a restricted alcohol drinking schedule, in which mice could either drink alcohol or water in a daily 2-hour window. On selected days, mice were subjected to one of the following stressors: restraint, tail suspension, predator odor, footshocks or tail pinches, which were applied immediately prior to their drinking period. Of these stressors, only predator odor caused an increase in ethanol intake, with female mice showing a faster increase in intake compared to males (24 hours post stress vs 48 hours post stress; Cozzoli et al., 2014). Another study exposed male and female juvenile mice to predatory odor stress prior to introducing intermittent access to alcohol and water in their home cage several weeks later (Shaw et al., 2020). Although this study did not report any sex-specific changes in stress-induced alcohol drinking, male mice that had been stressed continued to drink alcohol longer compared to unstressed controls, even when alcohol reinforcement was devaluated by the addition of quinine (Shaw et al., 2020). Finally, a study done by Peñasco and colleagues (Peñasco et al., 2015) shows that periods of alcohol withdrawal and restraint stress in adult rats trained to drink alcohol result in a female-specific increase in alcohol intake, but only in those rats also exposed to maternal separation during adolescence. This study highlights that the timing and duration of stress and alcohol exposure are likely to be critical for identifying sex-specific increases in stress-induced alcohol drinking.
Overall, despite many advances in modeling stress-induced alcohol drinking in rodents, little attention has been placed on whether these behavioral models capture female-specific increases in alcohol intake after stress exposure, a phenomenon now well documented in humans (Peltier et al., 2019). It is therefore important to fine-tune existing behavioral approaches to capture this sex-specific dimension. A preclinical model that recapitulates the increased sensitivity to stress-induced drinking in females will be necessary for mechanistic explorations of the molecular, cellular and circuit-level basis for sex differences in alcohol intake.
Role of the amygdala in stress-induced alcohol intake
While behavioral models are beginning to show that female rodents may drink more in response to stress (Cozzoli et al., 2014), the neurocircuitry underlying sex differences in alcohol intake are mostly unknown (Becker and Koob 2016). The amygdala is likely to be involved in stress-induced alcohol intake, and potentially in sex-dependent differences in alcohol drinking, because it plays a pivotal role in the control of a wide range of behaviors related to stress, anxiety, fear, and alcohol intake. Importantly, the basolateral amygdala (BLA) underlies the complex control of behaviors that are related to both aversive (stress) and rewarding (acute alcohol intake) stimuli (Baxter and Murray 2002; Janak and Tye 2015; Crouse et al., 2020). Early investigations into the BLA suggested that the amygdala can rapidly detect negative emotional states and external stimuli to produce behavior that is adaptive to potential threats (Brown and Sharpey-Schafer 1888; Klüver and Bucy 1937; Weiskrantz 1956).
Neurons in the BLA and central amygdala (CeA) arise from distinct cell lineages. In the BLA, the majority of neuronal cells are excitatory projection neurons that are inhibited by a smaller number of local inhibitory interneurons (Janak and Tye 2015). BLA glutamatergic neurons project in part to the CeA (Roberto et al. 2012; Janak and Tye 2015), a striatal-like structure that is almost entirely composed of GABAergic inhibitory neurons, including both local interneurons and inhibitory projections to downstream regions such as the locus coeruleus (LC), bed nucleus of the stria terminalis (BNST), and periaqueductal grey (PAG) (Roberto et al. 2012; Spampanato et al., 2011; Janak and Tye 2015; Gilpin et al. 2015). Within the CeA, inhibitory neurons in the centrolateral (CeL) region act as a gate on activity of the centromedial (CeM) anxiety-promoting projection neurons (Ciocchi et al. 2010). Further, direct activation of the CeA by the BLA makes it the main output nucleus of the amygdala that drives neuroendocrine responses to stress (Sah et al. 2003).
The amygdala is a sexually dimorphic brain structure influenced by sex hormone signaling (Równiak et al. 2015; Price and McCool 2022). The balance of estrogen and androgen signaling can be disturbed by alcohol intake, which may contribute to maladaptive alcohol use (Morales et al. 2018;Dozier et al. 2019; Fulenwider et al. 2019; Lorrai et al. 2019; Scott et al. 2020; Priddy et al. 2017;Ford et al. 2004; Bertholomey and Torregrossa 2019). It should be noted that there are some discrepancies among animal models as to whether alcohol exposure alters the estrous cycle. In female rhesus monkeys, ethanol did not influence menstrual cycle length, including changes to the follicular or luteal phases, or progesterone levels (Dozier et al. 2019). In contrast, a study in female rats found that long durations of chronic intermittent ethanol intake disrupted the estrous cycle, and with longer exposure, there was an increased proportion of females in diestrus I and II compared to control females (Morales et al. 2018). With respect to effects of estrous cycle on alcohol intake, several studies show that non-human primates exhibit significantly higher alcohol intake during the luteal phase when compared to the follicular phase of the menstrual cycle (Dozier et al. 2019; Fulenwider et al. 2019; Lorrai et al. 2019; Scott et al. 2020; Priddy et al. 2017). Similarly, several lines of research show that estrogen levels are positively correlated with increased ethanol consumption (Bertholomey and Torregrossa 2019; Vandegrift et al. 2017; Molina-Martínez and Juárez 2020; Kerstetter et al. 2012; Larson and Carroll 2006; Hilderbrand and Lasek 2018; Juárez et al. 2002). For example, gonadectomized female rats show decreased binge drinking; however, when supplemented with 17beta-estradiol, ethanol consumption increased (Ford et al. 2004; Bertholomey and Torregrossa 2019). Conversely, gonadectomized males decreased alcohol self-administration when given replacement testosterone (Bertholomey and Torregrossa 2019).
A number of mechanisms may contribute to estrogen effects on alcohol intake. For example, ethanol-induced firing of VTA dopamine neurons is decreased when estrogen receptors are blocked in brain slices from female mice in diestrus (high estradiol), suggesting that estrogen heightens ethanol sensitivity of dopamine neurons (Vandegrift et al. 2017). Further, the response of dopamine neurons to ethanol was greater in ovariectomized mice following estradiol replacement (Vandegrift et al. 2017; Vandegrift et al. 2020). In addition to differences in activity of the dopamine system, estradiol has anxiolytic effects in female rodents (Koss et al. 2004; Tian et al. 2013) that are mediated through ERɑ and Erβ estrogen receptors (Österlund et al. 1998). Notably, there are regional differences in expression of these estrogen receptor subtypes, with high levels of ERɑ mRNA in BLA, while the CeA predominantly expresses ERβ (Österlund et al. 1998). Of note, ERβ is highly expressed in inhibitory, PV-expressing neurons in female rats in the amygdala, basal forebrain, and hippocampal regions (Blurton-Jones and Tuszynski 2002). Finally, amygdala ER expression levels are influenced by estradiol concentration (Österlund et al. 1998), likely contributing to differential responses across the estrous cycle.
In addition to gonadal hormones, allopregnanolone, a potent neurosteroid that increases GABA-A receptor signaling, can also increase alcohol consumption with differing effects across sex and species. Allopregnanolone can increase ethanol intake in male rodents and female monkeys (Sinnott et al. 2002; Rowlett et al. 1999; Grant et al. 2008; Grant et al. 1997; Dozier et al. 2019; Genazzani et al. 1998). Following chronic ethanol exposure in male monkeys, both tissue and circulating serum levels of allopregnanolone are significantly decreased in the amygdala, whereas, in a similar study of female monkeys subjected to chronic ethanol exposure, serum levels of allopregnanolone were unaffected (Beattie et al. 2017; Dozier et al. 2019). However, in a human clinical study of adolescent females, there was a significant increase in circulating allopregnanolone levels following alcohol intoxication (Torres and Ortega 2003). Taken together, these results emphasize the need for further research on the effects of steroidal hormones on the development and expression of AUD across sexes.
While steroid hormones can contribute to alcohol intake, numerous studies in males across animal species have found that alcohol acts primarily on GABA-A receptors (GABAAR), potentiating receptor activity and enhancing inhibitory neurotransmission (Mihic 1999, Diaz et al. 2011, Floyd et al. 2004, McCool et al. 2003). Indeed, stress can increase ethanol self-administration via perturbation of the GABA system in male rats (Ostroumov et al., 2016). These interactions are particularly critical in the BLA. In male rats ethanol administration enhances GABA signaling onto BLA pyramidal cells, and can reduce anxiety-like and alcohol-seeking behaviors (Butler et al., 2014). However, physiological studies in rats have identified a decrease in inhibitory postsynaptic currents (IPSCs) after ethanol application to BLA slices (Zhu and Lovinger 2006; Ornelas and Keele 2018), suggesting alcohol decreases GABA signaling in the BLA. A single prolonged stress session alone did not result in significant sex differences in IPSCs recorded from male and female rat BLA slices; however, there were significant sex differences in neuronal excitability of BLA neurons when a single prolonged stress session was combined with ethanol exposure that was bath applied during recording (Ornelas and Keele 2018). Specifically, decreased neuronal spike firing was observed following ethanol application in BLA slices from female rats that were exposed to stress in-vivo (Ornelas and Keele 2018). Meanwhile, BLA slices from male rats that previously underwent stress showed a decreased hyperpolarization-activated, cyclic nucleotide-gated cation current (Ih) in response to acute ethanol application (Ornelas and Keele 2018). These results suggest that the neuronal network within the BLA is not only different in male and female animals, but that synergistic effects between stress and alcohol in this brain region could differ by sex.
Effects of alcohol in the amygdala are not limited to the BLA and are also observed in the central amygdala (CeA) and the bed nucleus of the stria terminalis (BNST or “extended amygdala”). Importantly, both the BLA and CeA send projections to the BNST (Miles and Maren 2019). There are striking similarities between the micro-circuitry of the BNST and the CeA and both are striatal-like in structure (Dong et al., 2000; Kash 2012). The BNST has been implicated in an increased drive to consume alcohol and also responds to stressful stimuli (Kash 2012). The Winder group has shown that there are extensive molecular adaptations and significant synaptic plasticity in the BNST following chronic alcohol exposure in male mice (Healey et al., 2008; Kash et al. 2009; McElligott and Winder 2009). The NMDA subclass of glutamate receptors (NMDAR) is activated by ethanol, and NMDARs in the ventral BNST become sensitized during acute withdrawal from chronic alcohol exposure in male mice in vivo (Kash et al. 2009). Chronic intermittent ethanol (CIE) exposure increased the probability of glutamate release in the stria terminalis of both male and female rats, with males starting to show a difference at 3 days of CIE, whereas it took 7 to 10 days of CIE to see the same effect in females (Morales et al. 2018); however, no sex difference was observed in withdrawal-induced anxiety in an elevated plus maze test after 3 days of CIE. These results indicate that a different synaptic mechanism drives expression of withdrawal-induced anxiety, possibly involving changes in activity of GABAergic interneurons (Morales et al. 2018).
Several studies have found that in brain regions where GABAB receptors are expressed, such as the CeA, neurotransmission is potentiated by ethanol (see Fig. 1). Conversely, in the hippocampus, blocking GABABRs is required to observe alcohol-induced GABAergic transmission in male mice and rats (Roberto et al. 2003; Ariwodola and Weiner 2004; Nie et al. 2009), consistent with the idea that the ability of alcohol to facilitate GABA neurotransmission might be limited by GABABR-mediated presynaptic feedback (Ariwodola and Weiner 2004). Acute alcohol application to male rat BLA and CeA slices potentiates GABAergic transmission through pre- and post-synaptic mechanisms (Roberto et al., 2003), while decreasing glutamatergic activation (Roberto et al., 2012). Following chronic ethanol exposure in male ethanol-preferring rats, NMDARs are upregulated, leading to greater CeA excitability ex vivo (Obara et al. 2009). This is also true in other brain regions such as the hippocampus, where acute alcohol application inhibits glutamatergic transmission by decreasing transmission via NMDA and AMPA receptors, whereas chronic alcohol exposure up-regulates NMDA receptor-mediated transmission in male rats ex vivo and in vitro (Kalluri et al. 1998; Carpenter-Hyland et al. 2004; Carpenter-Hyland and Judson Chandler 2007). A compelling study that examined both male and female rats subjected to stress and then alcohol found that there were increased GABAergic miniature inhibitory postsynaptic currents (mIPSCs) and increased cytokine levels in CeA slices (Steinman et al. 2021). Interestingly, female rats that were exposed to stress in a familiar environment showed greater mIPSC frequency in the CeA, whereas males that were exposed to stress in either a novel or a familiar environment showed greater mIPSC amplitude (Steinman et al., 2021), suggesting that increases in GABAergic transmission may occur in CeA in both females and males, but through different mechanisms. These studies support the hypothesis that acute alcohol intake increases GABAergic transmission (Fig. 1), resulting in its ability to decrease behaviors relevant to anxiety; conversely, following chronic alcohol consumption glutamatergic transmission is enhanced and GABA transmission is decreased, leading to increased excitation/inhibition balance in a number of brain regions including the BLA and CeA, resulting in increased anxiety.
Figure 1. Hypothesized signaling in the BLA and CeA relevant to alcohol use disorder.
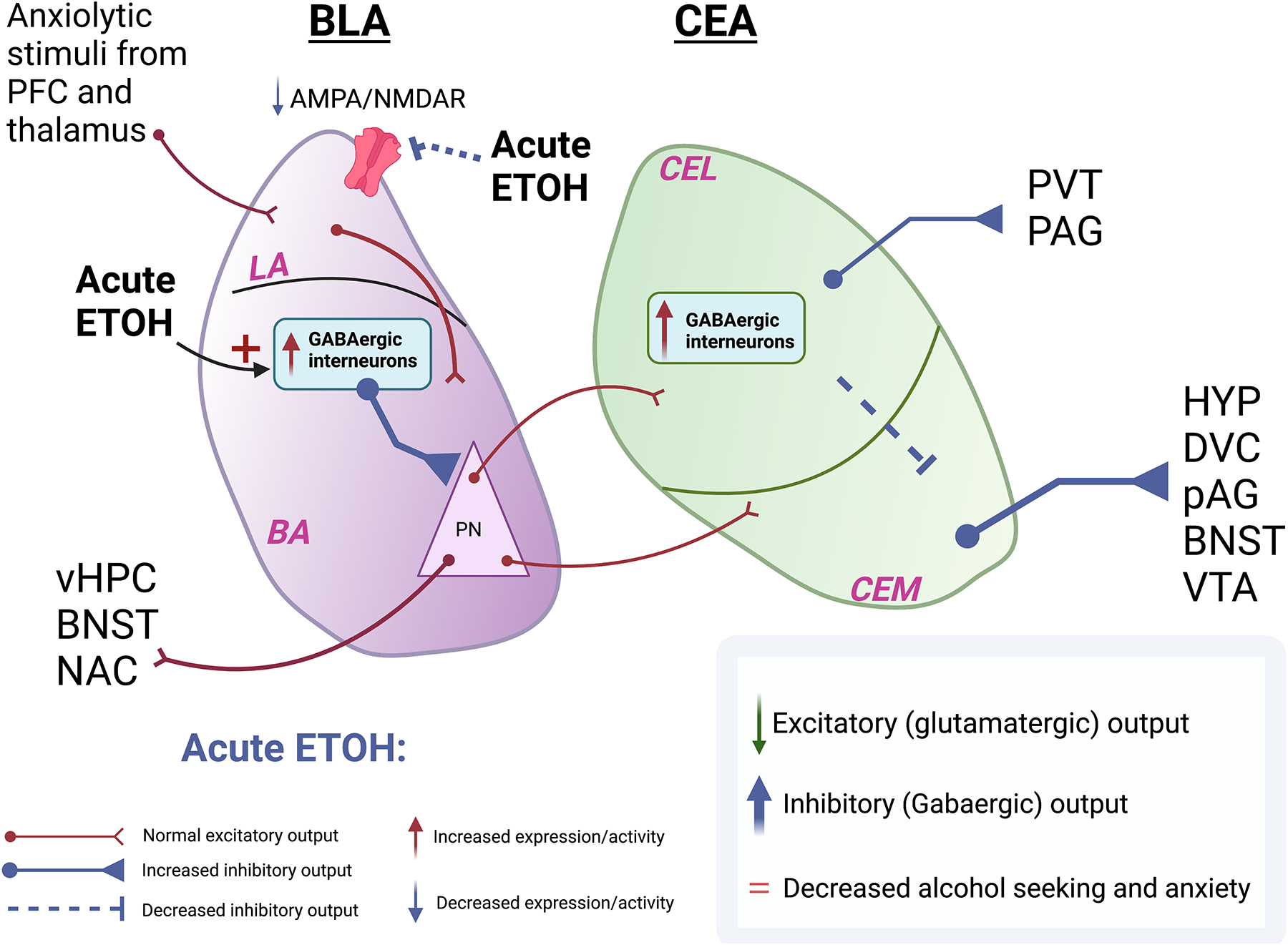
A) Following acute alcohol exposure there is a transient increase in inhibitory signaling onto excitatory pyramidal neurons in the BLA, leading to dampening of glutamatergic transmission. The CeA, in turn, receives decreased innervation from the BLA, leading to increased inhibitory output from the CeA. B) Following chronic ethanol exposure there is a decrease in GABAergic transmission from the interneurons in the BLA onto the pyramidal excitatory neurons of the BLA. There is also increased glutamatergic transmission within the BLA resulting from an increase in the AMPA/NMDAR ratio. Despite a decrease in CEL interneuron signaling, glutamatergic neurons in CeA receive greater input from PKCd+ interneurons from the CeM. The inhibitory output from the CeA is therefore dampened leading to an increase in alcohol seeking and anxiety.
Abbreviations: PFC: Prefrontal Cortex; BLA: Basolateral Amygdala; CEA: Central Amygdala; CEL: Centrolateral Amygdala; CEM: Centromedial Amygdala PV+: GABAergic parvalbumin-expressing interneurons; PKCd+: GABAergic protein kinase C delta-expressing interneurons; PN: Pyramidal Neurons; vHPC: Ventral hippocampus; BNST: Bed nucleus of stria terminalis; NAC: Nucleus accumbens; HYP: Hypothalamus; DVC: Dorsal vagal complex; VTA: Ventral tegmental area; PAG: Periaqueductal grey; PVT: Paraventricular nucleus of the thalamus
There are several sex differences in the types of interneurons found in amygdala subregions; for example, a higher density of calcium binding proteins (calbindin (CB)+ and parvalbumin (PV)+) has been observed in the BLA of female guinea pigs compared to males (Równiak et al. 2015). Furthermore, immunohistochemical studies in female rats identified a higher density of PV+ interneurons during diestrus and decreased density during proestrus (Blume et al. 2017). Because chronic ethanol consumption can increase the time spent in diestrus (Morales et al. 2018; Österlund et al. 1998; Blurton-Jones and Tuszynski 2002; Blume et al. 2017), future studies will be needed to determine whether a correlation exists between the total number of interneurons co-expressing PV and ERß and how this could underlie ethanol consumption.
GABAergic and glutamatergic mechanisms are necessary for the development and perpetuation of alcohol intake. There may be important differences in signaling mechanisms between sexes, although sex differences in glutamate and GABA signaling following alcohol use have not been studied as extensively. In one study, CeA neurons in male rats were shown to be sensitive to alcohol-induced inhibition of glutamatergic inputs to the structure, whereas female rats showed reduced sensitivity to alcohol-mediated inhibition of the CeA (Logrip et al., 2017). Thus, the interaction between neurons in different amygdala subregions could regulate alcohol consumption, with different interactions dominating in male and female animals.
In addition to changes in GABA signaling, epinephrine and norepinephrine (NE) levels are increased during withdrawal in individuals with AUD. A review of the literature has led to the hypothesis that noradrenergic signaling in the amygdala is modulated by both chronic alcohol use and by anxiogenic stimuli (Glavin 1985; Morilak et al., 2005). For example, several days of ethanol exposure can increase levels of NE and stress hormones such as cortisol in rats (Patterson-Buckendahl et al., 2005); however, a recent study found that alcohol does not stimulate the noradrenergic system directly, but instead, corticotropin releasing factor is required to increase norepinephrine release in the CeA of male rats following alcohol exposure (Hedges et al. 2020). Reducing noradrenergic tone may reduce stress-induced relapse to alcohol-seeking (Smith and Aston-Jones 2008). Clinically, guanfacine (an adrenergic receptor agonist) has shown efficacy in reducing smoking- and cocaine-induced relapse induced by stress, and can improve outcomes for patients with AUD (Fox et al., 2014; McKee et al., 2015). Guanfacine can decrease anxiety- and depression-related behaviors in mice, with similar behavioral effects in male and female animals, although sex differences in neuronal activation were observed in the BLA (Mineur et al., 2015). Reducing NE globally can also decrease ethanol preference in 2-bottle choice, ethanol conditioned place preference, and total ethanol consumption (Fitzgerald 2013). However, sex-dependent mechanisms through which the noradrenergic system mediates changes in alcohol intake following stress have not been studied systematically. Table 3 summarizes recent studies on the effects of the combination of alcohol and stress on synaptic and molecular processes. The studies summarized in Table 3 were mostly carried out using male rodents, highlighting the need to include both female and male animals to identify potential sex-specific differences in the effects of stress and alcohol on the underlying neurocircuitry in brain areas relevant to AUD.
Table 3 -.
Synaptic and molecular effects due to alcohol and stress
| Study | Species Strain | Sex | Alcohol Access | Stress | Brain Region | Alcohol/Stress Affect | Manipulation and effect |
|---|---|---|---|---|---|---|---|
| Sillaber et al. 2002 | Crhr1 KO mice | M | TBC, continous, 40+ days | FST, SD | Hippo +, Amy −, NaC+ | ↑ NMDA- NR2B | NA |
| Edwards et al. 2013 | Wistar rats | M | TBC, 30 mins, 7 days | PO | mPFC+, dmPFC+, CeA+, BLA+ | ↑ pERK | NA |
| Delis et al. 2013 | Drd2 KO mice | M | TBC | CMS | Global expression of Drd2 +/− and Drd2−/− | Drd2+/− and −/− ↑consumption when exposed to stress. Drd2 +/+ ↑etoh when not stressed | NA |
| Walker et al. 2015 | Rxfp3 KO mice | M | TBC | CMS/RS/FST | global | Rxfp3 KO mice reduced ethanol preference after stress | NA |
| Ostroumov et al. 2016 | Rats (Long-Evans) | M | OESA | RS | VTA | In Vivo: ↓ DA Neruon firing In Slice: shift to GABAA Signaling |
GABA and DA pharmacological manipulation |
| Ornelas and Keele 2018 | Rats (Sprague Dawley) | M/F | in slice bath solution etoh | SPS | BLA+ | In Slice: ↓ spike firing in BLA in F hyperpolarization activated current ↓ M | NA |
| Morales et al. 2018 | Rat (Sprague Dawley) | M/F | CIE (Vapor inhalation) | Withdr-wal | MT − ST + BLA + |
In Slice: changes in presynaptic glutamate release in vivo: change in estrus cycle and anxiety | Electrophysiology paired pulse in ST and BLA |
| Padula et al. 2020 | Mouse (C57BL/6J) | M | CIE, TBC | FST | BLA+ | systemic - KCa2.1–2.3 channel activator decreased drinking | |
| Domi et al. 2021 | Rats (Wistar) | M | Punishment-resistant self-administration, TBC | FS | CeA(PKCd+) | ↑ CeA (PKCd+) cell expression in rats drinking despite stress | hm4Di in CeA PKCd+ cells decreased drinking |
| Steinman et al. 2021 | Rats (Wistar) | M/F | TBC | FS in FAM and NOV | CeA+ | ↑ CeA GABAergic mIPSC ↑ cytokine levels |
NA |
Abbreviations: CIE: chronic Intermittent ethanol, TBC: two bottle choice, OESA: operant ethanol self admin, PO: predator odor, RS: restrain stress, SPS: single prolonged stress, CMS: chronic mild stress, FST: Forced swim test, FS: Foot shock, FAM: familiar, NOV: novel, F: female, M: male, BLA: basolateral amygdala, CeA: central lateral amygdala, Rxfp3: relaxin family peptide receptor 3, Drd2: dopamine receptor 2, PKCd: protein kinase C delta, KCa2.1–2.3: calcium activated potassium type 2, mPFC: medial prefrontal cortex, dmPFC: dorsal medial prefrontal cortex, hm4Di: human muscarinic 4 receptor designer receptor CNOactivated inhibitor, mIPSC: miniature inhibitory post synaptic current
+ indicates tested and found effects, - indicates tested but found no effects. Arrows indicated increasing or decreasing respectively
Contribution of microglia to stress-induced alcohol intake
Extensive human clinical studies have highlighted the role of inflammation in the etiology of stress and AUD. Several studies have demonstrated an increase in peripheral cytokine levels in subjects with AUD, particularly interleukin-6 (IL-6) and tumor necrosis factor α (TNFα), both of which are associated with alcohol craving and other affective changes, suggesting a potential link between inflammation and AUD-related behaviors (Laso et al., 2007; Gonzalez-Quintela et al., 2008; Heberlein et al., 2014). Research has shown alterations of immune-related genes in the brains of individuals with AUD (Lewohl et al., 2000; Mayfield et al., 2002; Liu et al., 2006; Crews et al., 2013; Vetreno et al., 2021); of particular note are increases in expression of microglial markers (He & Crews, 2008), as well as genetic and epigenetic alterations in microglia (Ponomarev et al., 2012; Brenner et al., 2020). Similarly, depression is associated with increased markers of microglial activation (Torres-Plata et al., 2014), and positron emission tomography (PET) studies have revealed alterations in brain inflammation in vivo in subjects with major depressive disorder (MDD: Holmes et al., 2018; Li et al., 2018; Richards et al., 2018) and AUD (Hillmer et al., 2017; Kalk et al., 2017; Kim et al., 2018).
Several studies have identified sex-specific effects of stress and alcohol consumption on microglial number and function. While some studies have shown heightened microglial responses to stress in females (Gildawie et al., 2020; Bekhbat et al., 2021), others have shown increased susceptibility in males (Woodburn et al., 2021); these differences may be due to differences in timing and type of stressor. As shown in Table 4, several preclinical studies have identified alcohol-induced increases in microglial number and activation in male rodents, as demonstrated through expression of Iba1, a marker upregulated in activated microglia (Sasaki et al., 2001), phagocytic markers CD68 (Kurishima et al., 2000) and CD11b (Ehlers, 2000), and the chemokine receptor Cx3Cr1 (Jurga et al., 2020), as well as measurements of cell morphology and binding of PET ligands to TSPO, a mitochondrial protein associated with neuroinflammation (Notter et al., 2018). Less work has included females, but there are data to suggest that alcohol consumption has heightened inflammatory effects in females, including upregulation of microglia-related genes, cytokines, and chemokines (Pascual et al., 2017), as well as increases in microglial number and activation (Barton et al., 2017). Thus, sex differences in immune function may underlie sex differences in AUD and the heightened susceptibility of women to stress-induced drinking. Women have higher levels of IL-6 (O’Connor et al., 2007; Chapman et al., 2009) and binge-drinking-induced endotoxin (Bala et al., 2014), which are associated with social disconnection and depressed mood (Moieni et al., 2015). Furthermore, autoimmune diseases are more prevalent in women (Whitacre, 2001); these data point to the possibility of increased immune activity in women that may prime heightened reactivity to challenges such as psychosocial stress and alcohol consumption.
TABLE 4 –
Effects of Alcohol & Stress on Microglia in Rodents
| Study | Species, Strain | Sex | Alcohol Access | Stressor | Effects on Microglia |
|---|---|---|---|---|---|
| Fernandez-Lizarbe et al., 2009 | Mice (C57BL/6J) | F | 3d 4 g/kg IP | none | Increased microglial activation (CD11b IR) |
| Alfonso-Loeches et al., 2010 | Mice (C57BL/6J) | F | 5mo continuous TBC | none | Increased microglial activation (CD11b IR) |
| McClain et al., 2011 | Rats (Sprague-Dawley) | M | 4d 5 g/kg IG every 8h | none | Increased microglial activation (morphology) |
| Ehrlich et al., 2012 | Rats (Sprague-Dawley) | M | 12mo continuous drinking | none | Increased microglial activation (Iba1 and CD11b IR) |
| Qin & Crews, 2012b | Mice (C57BL/6J) | M | 10d 5 g/kg IG | none | Increased microglial activation (Iba1 IR) |
| Marshall et al., 2013 | Rats (Sprague-Dawley) | M | 4d 5 g/kg IG every 8h | none | Increased microglial number (Iba1+ cells) and activation (TSPO ARG; CD11b IR) |
| Zhao et al., 2013 | Rats (Sprague-Dawley) | M | 25d intermittent IG | none | Increased microglial activation (CD11b IR) |
| Marshall et al., 2016 | Rats (Sprague-Dawley) | M | 4d 5 g/kg IG every 8h Second 4d binge 7d later |
none | Increased microglial activation (CD11b IR); decreased microglial number (Iba1+ cells) Further increase in microglial activation; increased microglial number |
| Avila et al., 2017 | Mice (C57BL/6J) | M | 3w continuous drinking | none | Increased microglial activation (Iba1 IR) |
| Barton et al., 2017 | Rats (Long-Evans) | M/F | 4d 5 g/kg IG | none | Increased microglial number (Iba1+ cells) and activation (morphology) in females only |
| Walter & Crews, 2017 | Mice (C57BL/6J) | M | 3, 4.5, or 6 g/kg IG | none | 4.5 or 6 g/kg increased microglial gene expression (Iba1 and CD68 mRNA) |
| Walter et al., 2017 | Rats (Wistar) | M | 5 g/kg IG 1h before stress 25d intermittent 5 g/kg IG 42d before stress |
2h restraint + partial water immersion | Increased microglial activation (CD11b IR) Increased microglial activation (CD11b IR) |
| Lowe et al., 2020 | Mice (C57BL/6J) | F | 42d continuous drinking | none | Increased microglial activation (morphology) and decreased phagocytic activity (Iba1/CD68 colocalization) |
| Marshall et al., 2020 | Rats (Sprague-Dawley) | M | 2d 5 g/kg oral gavage 4d 5 g/kg oral gavage |
none | Decreased microglial number (Iba1+ cells), increased microglial dystrophy (morphology) Decreased microglial number (Iba1+ cells), increased microglial dystrophy (morphology) |
| Socodato et al., 2020 | Mice, C57BL/6J | M | 10d 1.5 g/kg oral gavage | none | Increased microglial number (Cx3CR1+ cells, Iba1+ cells) and activation (CD11b IR, CD45 IR, morphology, Iba1 PE) |
| Tournier et al., 2020 | Rats (Wistar) | M | 14d intermittent 3 g/kg IP | none | Increased microglial activation (TSPO VT) |
| Warden et al., 2020 | Mice (C57BL/6J) | M | 4w intermittent TBC | none | Increased microglial number (Iba1+ cells) |
| West et al., 2020 | Rats (Long-Evans) | M/F | 3w 4 g/kg IG every 7d 8w 4 g/kg IG every 7d |
none | No changes Increased microglial number (Iba1+ cells) and activation (morphology) |
| Aranda et al., 2021 | Rats (Wistar) | M | 9w intermittent self-administration, 2w abstinence, 3w reinstatement | none | Increased microglial activation (Iba1 IR and morphology) |
| Lee et al., 2021 | Mice (C57BL/6J) | M | 28d continuous TBC | 28d social isolation | Alcohol potentiated stress-induced increases in microglial number (Iba1+ cells) and activation (morphology) |
Abbreviations: ARG: autoradiography; IG: intragastric; IR: immunoreactivity; PE: protein expression; TBC: two-bottle choice; VT: total volume of distribution
The preclinical literature highlights microglia as key mediators of the brain’s response to stress and alcohol consumption (see Fig. 2). Microglia are the brain’s resident macrophages; in their resting state, they display a ramified morphology and monitor the brain environment. Detection of a toxin or stressor triggers classical activation, in which microglia transform into a more ameboid morphology, upregulate expression of various pro-inflammatory factors, and work to phagocytose debris and dead cells (Fig. 2). Once the threat has been addressed, microglia transition into an anti-inflammatory, alternative activation state (Block et al., 2007; Colton et al., 2009).
Figure 2. Alcohol-mediated changes in microglial markers and activity.
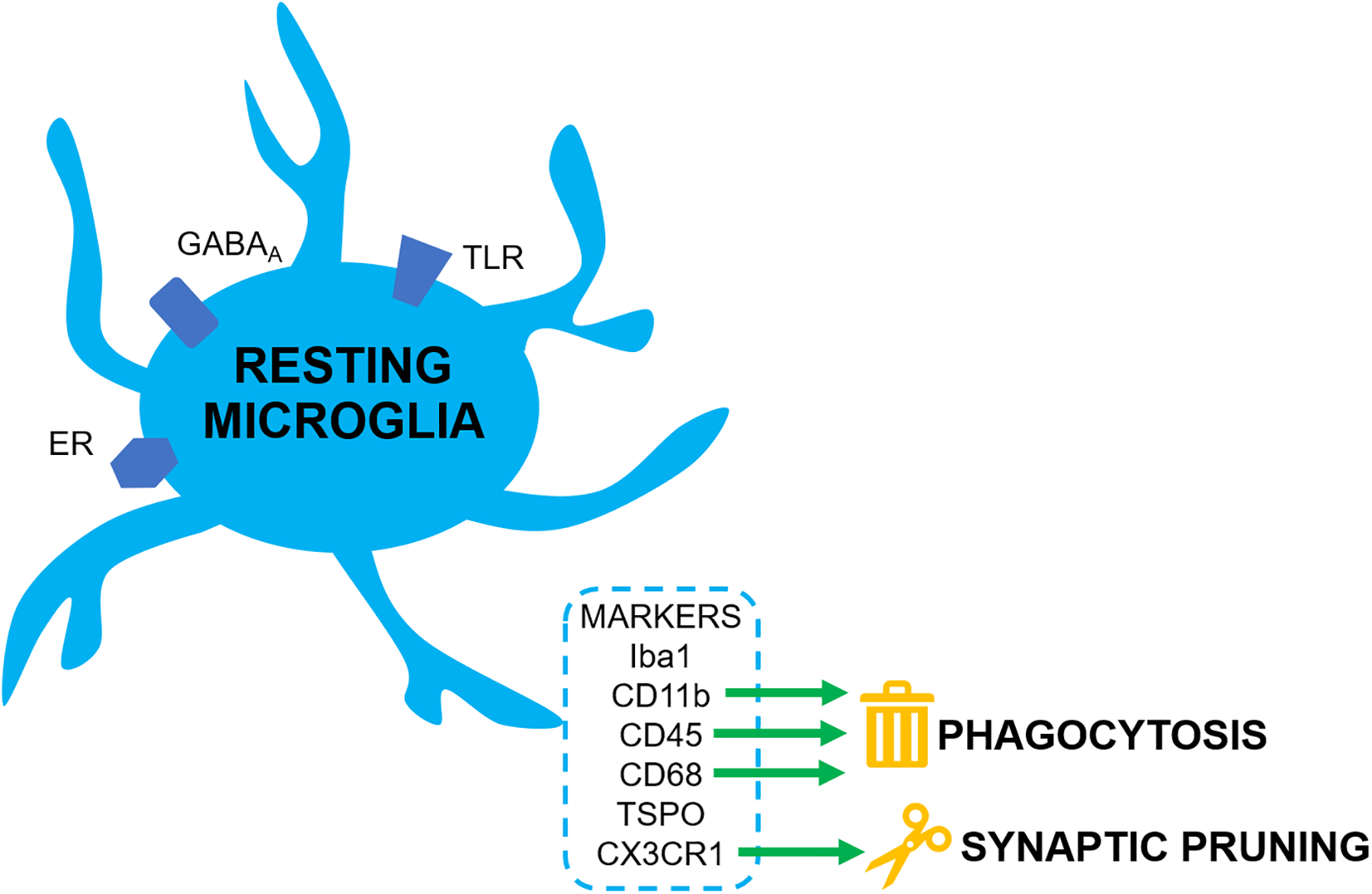
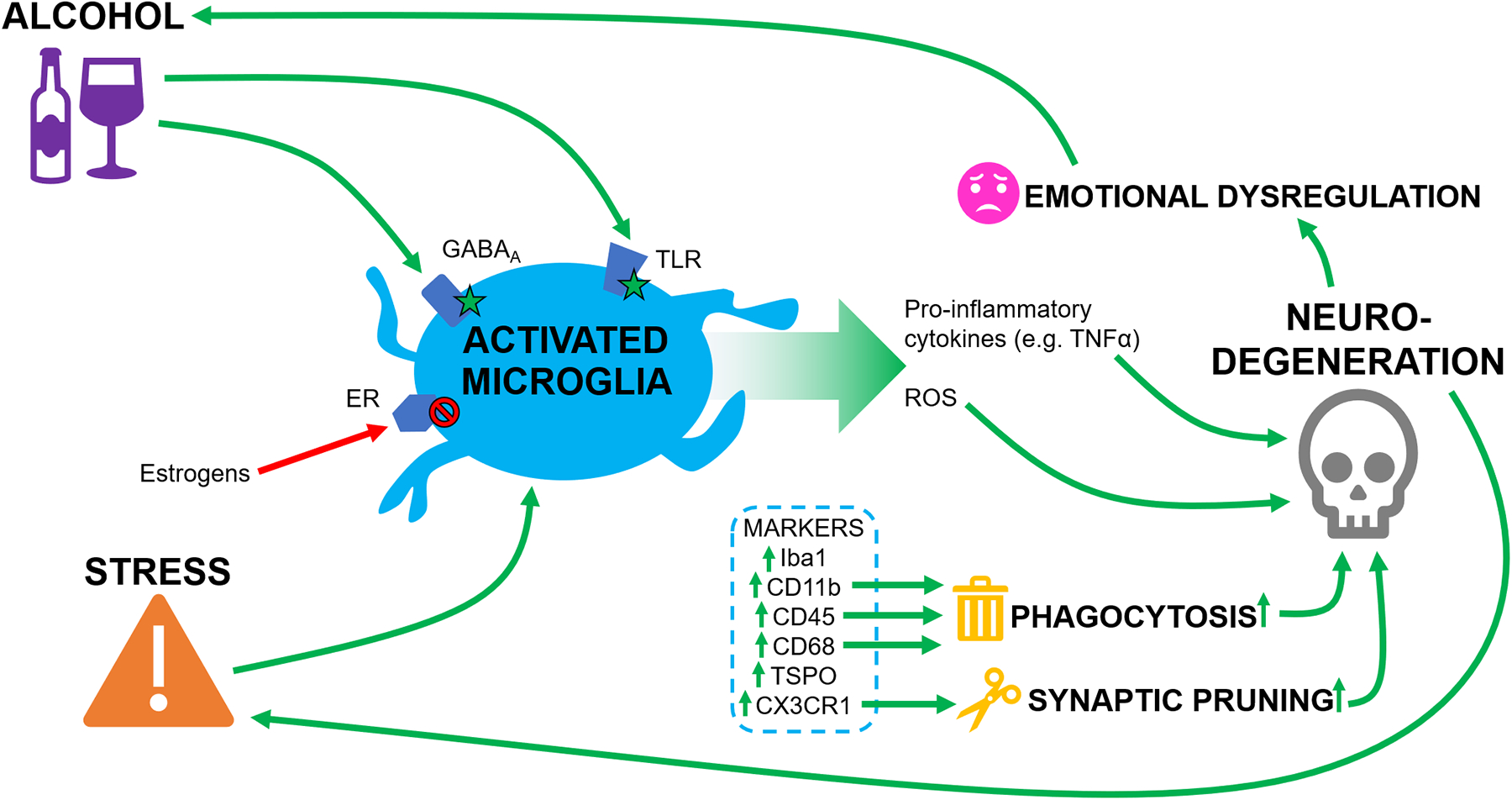
A) At rest, GABAA receptors, TLRs, and ERs are expressed on microglia. Resting microglia express several markers, including phagocytic markers such as CD11b, CD45, and CD68, and the pruning marker CX3CR1. B) Alcohol activates microglia via GABAA receptors and TLRs; stress also results in microglial activation. The increase in activated microglia results in phagocytosis and synaptic pruning, which drives neurodegeneration. This coincides with release of ROS and pro-inflammatory cytokines such as TNFα, which also contribute to neurodegeneration. Enhanced neurodegeneration results in long-term changes in excitation/inhibition balance in brain areas such as the amygdala, contributing to emotional dysregulation and increased stress responses, leading to potentiation of alcohol consumption and further driving microglial activation. Estrogens acting through ERs inhibit microglial activation and the associated signaling cascades.
Abbreviations: ER: Estrogen Receptor; TLR: Toll-like Receptor; GABAA: GABA-A Receptor; ROS: Reactive Oxygen Species.
In addition to playing a role in phagocytosis, microglia can also alter synaptic structure and function in the CNS (Tremblay and Majewska 2011; Tremblay et al., 2011). At baseline, microglia are physically associated with neuronal synapses, and react dynamically to changes in the microenvironment (Nimmerjahn et al. 2005). Microglia play a critical role in synaptic pruning via chemokine (C-X3-C motif) ligand 1 (Paolicelli et al. 2011; Paolicelli and Gross 2011). The mechanisms by which synapse number is regulated in vivo remain to be elucidated, but in vitro experiments suggest that microglia control synaptic activity by regulating synapse number (Schafer et al., 2012). Thus, microglial activation could contribute to reorganization of neuronal networks via synaptic pruning.
While acute microglial activation is likely adaptive, and the classic activation phenotype is necessary for maintenance of a healthy brain, prolonged activation of microglia results in oxidative stress and ultimately, neurotoxicity (Block et al., 2007; Colton, 2009; Franco & Fernández-Suárez, 2015). Alcohol and stress both activate microglia, and chronic exposure to either can drive persistent microglial activation, causing hypersensitivity of the neuroimmune system and dramatic neurodegeneration (Crews et al., 2017). Microglia tend to be more responsive in females compared to males, both at baseline (Schwarz et al., 2012) and in response to binge alcohol consumption (Pascual et al., 2017; Barton et al., 2017), suggesting that the transition to maladaptive microglial signaling could be more pronounced in females.
Alcohol induces activation of microglia via toll-like receptor 4 (TLR4; Fernandez-Lizarbe et al., 2009; Alfonso-Loeches et al., 2010; Crews et al., 2011; Fernandez-Lizarbe et al., 2013). Subsequent activation of nuclear factor κB (NF-κB) stimulates release of TNFα and other pro-inflammatory cytokines, which drive apoptosis in surrounding neurons (Crews et al., 2006; Boyadjieva & Sarkar, 2010; McClain et al., 2011; Walter & Crews, 2017). Sustained TLR4 activation due to chronic alcohol consumption prolongs microglial activation, shifting the brain into a state of maladaptive microglial signaling (Alfonso-Loeches et al., 2010; Vetreno & Crews, 2012). Another mechanism of alcohol-induced neurodegeneration occurs through the release of reactive oxygen species (ROS) from activated microglia (Boyadjieva & Sarkar, 2013; Qin & Crews, 2012a). The increase in neuronal death due to inflammation and oxidative stress disrupts cortico-limbic circuitry and may contribute to further alcohol consumption driven by heightened anxiety and deficits in executive function (Crews et al., 2011). In fact, pharmacological inhibition of microglial function can reduce alcohol consumption (Agrawal et al., 2014; Israel et al., 2021), while ethanol drinking induces upregulation of immune regulatory pathways in males (females were not investigated). These signaling cascades should therefore be investigated as potential mechanisms underlying sex-related differences observed in the neuroimmune response to alcohol (Finn et al., 2018).
Gonadal hormones likely contribute to sex differences in neuroimmune signaling. While the expression of estrogen receptors (ERs) on microglia and the anti-inflammatory effects of estrogen signaling have been well-documented (Johann & Beyer, 2013; Acosta-Martínez, 2020), the role of androgen receptors (ARs) remains unclear. Immunocytochemical analyses found ER and AR expression in microglia of male rats, but only after brain injury (García-Ovejero et al., 2002); however, an ex vivo study measuring receptor expression by PCR only identified ER transcripts (but not AR) in male and female microglia at baseline and showed that ER expression was downregulated after an immune challenge (Sierra et al., 2008). This discrepancy could be due to technical differences (Sierra et al., 2008), as other studies have shown ER expression in microglia using PCR or immunocytochemical measurement in cell culture (Baker et al., 2004; Liu et al., 2005; Bruce-Keller et al., 2008).
Estrogens and androgens exert anti-inflammatory effects by regulating microglia (Baker et al., 2004; Barreto et al., 2007; Yang et al., 2020) which may also contribute to the neuroprotective effect of estrogen (Liu et al., 2005). Furthermore, estrogen can inhibit microglial reactive oxygen species (ROS) production, phagocytic activity, and release of TNFα (Bruce-Keller et al., 2000; Liu et al., 2005; Acosta-Martínez, 2020). One study found that simvastatin, a lipophilic statin with estrogenic activity, reduces depressive-like behavior, upregulates ER expression, and inhibits microglial activation in ovariectomized rats (Menze et al., 2021). Another study found that both estrogens and androgens reduce microglial complexity at baseline in males, and that estrogens are necessary for stress-induced microglial remodeling in females (Bollinger et al., 2019). Although preclinical research suggests a facilitatory effect of estrogens on alcohol drinking in females, and an inhibitory effect of androgens in males (Finn, 2020), further studies are needed to understand the relationship between gonadal hormones, microglia, and alcohol-related behavior.
Microglial activation by ethanol relies, at least in part, on GABA signaling (Domercq et al. 2013), and human microglia express GABAA receptors (Domercq et al. 2013). Furthermore, rodent studies suggest that accumulation of microglia in the hippocampus correlates with decreased GABA transmission and greater neuronal excitability, as measured by induction of long-term potentiation (LTP; Nistico et al. 2013). Microglial motility is also modulated by NE and inflammation increases α2AR expression on microglia, shifting microglial responses to NE release (Gyoneva and Traynelis 2013).
Research in male rodents has demonstrated that, like alcohol exposure, stress leads to activation of microglia, increasing release of pro-inflammatory cytokines and ROS and leading to neuronal death (Lu et al., 2014; Cheng et al., 2019). However, it remains unclear whether this also occurs in females (Bollinger, 2021). In males, microglial inhibition can reverse the depressive effects of stress and promote neurogenesis (Han et al., 2019). Stress (Frank et al., 2007) and alcohol (Qin & Crews, 2012b) can both activate microglia in males, making them hypersensitive to subsequent inflammatory stimuli. Furthermore, studies of male rodents have shown that alcohol and stress interact to enhance microglial activation (Walter et al., 2017), whereas inhibiting microglial activity can reverse the escalation in drinking and in anxiety-like behavior associated with alcohol dependence (Warden et al., 2020). Thus, chronic alcohol consumption can make the male brain more susceptible to stress-induced inflammation and vice versa, potentiating subsequent neurodegeneration, which in turn drives further emotional dysregulation and alcohol consumption (see Fig. 2). However, more research is needed to understand the neuroimmune effects of stress and alcohol in females.
Conclusions
Sex differences in stress-induced alcohol intake contribute to increased relapse to alcohol drinking in women. Several preclinical behavioral studies have demonstrated sex differences in stress-alcohol interactions, suggesting that rodent models can be useful in identifying mechanisms underlying sex-specific contributions to alcohol drinking. Limited access drinking paradigms coupled with repeated stress exposure appear to be most useful in studying stress effects on alcohol intake in rodents. Female rodents appear to be more sensitive to stressors in these drinking paradigms than males, although not many studies have used both sexes. Physiological studies have demonstrated some differences in ethanol effects on GABA and glutamate signaling in amygdala that could contribute to sex-dependent effects of alcohol. In addition, female animals are more likely to mount a neuroimmune response to stress and show microglial activation in response to alcohol. These observations suggest potential mechanisms for sex differences in stress-induced microglial perturbations, alcohol use and stress-induced alcohol intake. One hypothesis is that microglia are activated by ethanol exposure, reshaping neuronal dendritic arborization in several brain areas including the amygdala, leading to greater sensitivity to stress and increasing subsequent alcohol intake. Chronic activation of neuroinflammatory networks and microglia leading to neurodegeneration could lead to permanent deficits in the balance between GABA and glutamate signaling in these networks, leading to even greater sensitivity to alcohol-related behaviors. Future work should focus on identifying activity in brain systems that is most divergent across sexes in response to alcohol intake, whether sex differences in microglial activation contribute to stress-induced drinking behavior, and whether treatments that target the immune system may be more efficacious for women with AUD. This will involve additional model development to identify the patterns and timing of exposure to stress and alcohol that reveal sex differences in physiology and behavior.
Acknowledgements
This work was supported by grants AA027989, MH77681 and DA050986 from the National Institutes of Health and by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut.
Footnotes
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Literature Cited
- Acosta-Martínez M (2020). Shaping Microglial Phenotypes Through Estrogen Receptors: Relevance to Sex-Specific Neuroinflammatory Responses to Brain Injury and Disease. Journal of Pharmacology and Experimental Therapeutics, 375(1), 223–236. 10.1124/jpet.119.264598 [DOI] [PubMed] [Google Scholar]
- Agrawal RG, Owen JA, Levin PS, Hewetson A, Berman AE, Franklin SR, Hogue RJ, Chen Y, Walz C, Colvard BD, Nguyen J, Velasquez O, Al-Hasan Y, Blednov YA, Fowler A-K, Syapin PJ, & Bergeson SE (2014). Bioinformatics Analyses Reveal Age-Specific Neuroimmune Modulation as a Target for Treatment of High Ethanol Drinking. Alcoholism: Clinical and Experimental Research, 38(2), 428–437. 10.1111/acer.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L, Schratz CL, & Gilpin NW (2020). Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biology of Sex Differences, 11(1), 27. 10.1186/s13293-020-00303-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, & Guerri C (2010). Pivotal Role of TLR4 Receptors in Alcohol-Induced Neuroinflammation and Brain Damage. Journal of Neuroscience, 30(24), 8285–8295. 10.1523/JNEUROSCI.0976-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, & Becker HC (2016). Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology, 233(11), 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama 295: 2003–17. [DOI] [PubMed] [Google Scholar]
- Aranda J, del M. Fernández-Arjona M, Alén F, Rivera P, Rubio L, Smith-Fernández I, Pavón FJ, Serrano A, Serrano-Castro PJ, Rodríguez de Fonseca F, & Suárez J (2021). Sudden cessation of fluoxetine before alcohol drinking reinstatement alters microglial morphology and TLR4/inflammatory neuroadaptation in the rat brain. Brain Structure & Function, 226(7), 2243–2264. 10.1007/s00429-021-02321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL (2004) Ethanol Potentiation of GABAergic Synaptic Transmission May Be Self-Limiting: Role of Presynaptic GABAB Receptors. J Neurosci 24:10679–10686. 10.1523/JNEUROSCI.1768-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila DV, Myers SA, Zhang J, Kharebava G, McClain CJ, Kim H-Y, Whittemore SR, Gobejishvili L, & Barve S (2017). Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation. Neuropharmacology, 125, 376–385. 10.1016/j.neuropharm.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, & Campeau S (2013). Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic–pituitary–adrenocortical axis hormones following restraint in rats. Neuroscience, 234, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, & Watters JJ (2004). Estrogen Modulates Microglial Inflammatory Mediator Production via Interactions with Estrogen Receptor β. Endocrinology, 145(11), 5021–5032. 10.1210/en.2004-0619 [DOI] [PubMed] [Google Scholar]
- Bala S, Marcos M, Gattu A, Catalano D, & Szabo G (2014). Acute Binge Drinking Increases Serum Endotoxin and Bacterial DNA Levels in Healthy Individuals. PLOS ONE, 9(5), e96864. 10.1371/journal.pone.0096864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas HS, Wilfur SM, Freker NA, & Leong K-C (2021). Oxytocin Attenuates the Stress-Induced Reinstatement of Alcohol-Seeking in Male Rats: Role of the Central Amygdala. Biomedicines, 9(12), 1919. https://www.mdpi.com/2227-9059/9/12/1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, & Garcia-Ovejero D (2007). Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: Role of its metabolites, oestradiol and dihydrotestosterone. European Journal of Neuroscience, 25(10), 3039–3046. 10.1111/j.1460-9568.2007.05563.x [DOI] [PubMed] [Google Scholar]
- Barton EA, Baker C, & Leasure JL (2017). Investigation of Sex Differences in the Microglial Response to Binge Ethanol and Exercise. Brain Sciences, 7(10), 139. 10.3390/brainsci7100139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA (2002) The amygdala and reward. Nat Rev Neurosci 3:563–573. 10.1038/nrn875 [DOI] [PubMed] [Google Scholar]
- Beattie MC, Maldonado-Devincci AM, Porcu P, O’Buckley TK, Daunais JB, Grant KA, et al. (2017). Voluntary ethanol consumption reduces GABAergic neuroactive steroid (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP) in the amygdala of the cynomolgus monkey. Addict Biol. 22:318–330. 10.1111/adb.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2012). Animal models of excessive alcohol consumption in rodents, in Sommer W & Spanagel R (eds) Behavioral Neurobiology of Alcohol Addiction, Springer-Verlag, Berlin, Germany, 355–377. [Google Scholar]
- Becker HC, & Ron D (2014). Animal models of excessive alcohol consumption: recent advances and future challenges. Alcohol (Fayetteville, NY), 48(3), 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, & Doremus-Fitzwater TL (2011). Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl), 218(1), 131–156. 10.1007/s00213-011-2443-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Koob GF (2016). Sex differences in animal models: focus on addiction. Pharmacological reviews, 68(2), 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Mukhara D, Dozmorov MG, Stansfield JC, Benusa SD, Hyer MM, Rowson SA, Kelly SD, Qin Z, Dupree JL, Tharp GK, Tansey MG, & Neigh GN (2021). Adolescent stress sensitizes the adult neuroimmune transcriptome and leads to sex-specific microglial and behavioral phenotypes. Neuropsychopharmacology, 46(5), 949–958. 10.1038/s41386-021-00970-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey M, Nagarajan V, & Torregrossa MM (2016). Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology, 233(12), 2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Torregrossa MM. (2019). Gonadal hormones affect alcohol drinking, but not cue+yohimbine-induced alcohol seeking, in male and female rats. Physiol Behav. 203:70–80. 10.1016/j.physbeh.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, & Sinha R (2017). Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, 122, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, & Maier SF (2005). Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain research, 1051(1–2), 90–99. [DOI] [PubMed] [Google Scholar]
- Bloch S, Rinker JA, Marcus MM, & Mulholland PJ (2020). Absence of effects of intermittent access to alcohol on negative affective and anxiety-like behaviors in male and female C57BL/6J mice. Alcohol, 88, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, & Hong J-S (2007). Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nature Reviews Neuroscience, 8(1), 57–69. 10.1038/nrn2038 [DOI] [PubMed] [Google Scholar]
- Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, et al. (2017). Sex- and Estrus-Dependent Differences in Rat Basolateral Amygdala. J Neurosci. 37:10567–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. (2002). Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. Journal Comp Neurol. 452:276–287. [DOI] [PubMed] [Google Scholar]
- Bollinger JL (2021). Uncovering microglial pathways driving sex-specific neurobiological effects in stress and depression. Brain, Behavior, & Immunity - Health, 16, 100320. 10.1016/j.bbih.2021.100320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Salinas I, Fender E, Sengelaub DR, & Wellman CL (2019). Gonadal hormones differentially regulate sex-specific stress effects on glia in medial prefrontal cortex. Journal of Neuroendocrinology, 31(8), e12762. 10.1111/jne.12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto AM, Fotio Y, Stopponi S, Petrella M, De Carlo S, Domi A, Ubaldi M, Weiss F, & Ciccocioppo R (2021). NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats. Neuropsychopharmacology, 46(12), 2121–2131. 10.1038/s41386-021-01096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva NI, & Sarkar DK (2010). Role of Microglia in Ethanol’s Apoptotic Action on Hypothalamic Neuronal Cells in Primary Cultures. Alcoholism: Clinical and Experimental Research, 34(11), 1835–1842. 10.1111/j.1530-0277.2010.01271.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva NI, & Sarkar DK (2013). Microglia Play a Role in Ethanol-Induced Oxidative Stress and Apoptosis in Developing Hypothalamic Neurons. Alcoholism: Clinical and Experimental Research, 37(2), 252–262. 10.1111/j.1530-0277.2012.01889.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit KR, & Chester JA (2016). Effects of chronic stress on alcohol reward-and anxiety-related behavior in high-and low-alcohol preferring mice. Alcoholism: Clinical and Experimental Research, 40(3), 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner E, Tiwari GR, Kapoor M, Liu Y, Brock A, & Mayfield RD (2020). Single cell transcriptome profiling of the human alcohol-dependent brain. Human Molecular Genetics, 29(7), 1144–1153. 10.1093/hmg/ddaa038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, & Mattson MP (2000). Antiinflammatory Effects of Estrogen on Microglial Activation*. Endocrinology, 141(10), 3646–3656. 10.1210/endo.141.10.7693 [DOI] [PubMed] [Google Scholar]
- Brown S, Sharpey-Schafer EA (1888) XI. An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philosophical Transactions of the Royal Society of London (B) 179:303–327. 10.1098/rstb.1888.0011 [DOI] [Google Scholar]
- Butler TR, Chappell AM, Weiner JL (2014) Effect of beta 3 adrenoceptor activation in the basolateral amygdala on ethanol seeking behaviors. Psychopharmacology 231: 293–303. 10.1007/s00213-013-3238-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Ubaldi M, Masi A, Bramucci M, Roberto M, Bifone A, & Ciccocioppo R (2019). Building better strategies to develop new medications in Alcohol Use Disorder: Learning from past success and failure to shape a brighter future. Neuroscience & Biobehavioral Reviews, 103, 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Judson Chandler L (2007) Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. European Journal of Neuroscience 25:3193–3194 10.1111/j.1460-9568.2006.05247.x [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ (2004) Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci 24:7859–7868. 10.1523/JNEUROSCI.1902-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Khan A, Harper M, Stockman D, Fiscella K, Walton J, Duberstein P, Talbot N, Lyness JM, & Moynihan J (2009). Gender, Race/Ethnicity, Personality, and Interleukin-6 in Urban Primary Care Patients. Brain, Behavior, and Immunity, 23(5), 636–642. 10.1016/j.bbi.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Chen M, Zhu J-X, Li C-F, Zhang Q-P, Geng D, Liu Q, & Yi L-T (2019). FGF-2 signaling activation in the hippocampus contributes to the behavioral and cellular responses to puerarin. Biochemical Pharmacology, 168, 91–99. 10.1016/j.bcp.2019.06.025 [DOI] [PubMed] [Google Scholar]
- Childs E, O’Connor S, & de Wit H (2011). Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcoholism: Clinical and Experimental Research, 35(10), 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, et al. (2010) Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468:277–282. 10.1038/nature09559 [DOI] [PubMed] [Google Scholar]
- Colton CA (2009). Heterogeneity of Microglial Activation in the Innate Immune Response in the Brain. Journal of Neuroimmune Pharmacology, 4(4), 399–418. 10.1007/s11481-009-9164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, & Finn DA (2014). Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol, 48(8), 741–754. 10.1016/j.alcohol.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, & Belknap JK (2010). The complexity of alcohol drinking: studies in rodent genetic models. Behavior genetics, 40(6), 737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, & Coleman LG (2017). The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. 10.1016/j.neuropharm.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, & Zou J (2013). HMGB1/TLR Receptor Danger Signaling Increases Brain Neuroimmune Activation in Alcohol Dependence. Biological Psychiatry, 73(7), 602–612. 10.1016/j.biopsych.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, & Qin L (2011). Induction of innate immune genes in brain create the neurobiology of addiction. Brain, Behavior, and Immunity, 25, S4–S12. 10.1016/j.bbi.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, & Zou J (2006). BHT Blocks NF-κB activation and Ethanol-Induced Brain Damage. Alcoholism: Clinical and Experimental Research, 30(11), 1938–1949. 10.1111/j.1530-0277.2006.00239. [DOI] [PubMed] [Google Scholar]
- Crouse RB, Kim K, Batchelor HM, et al. (2020) Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency. Elife 9.: 10.7554/eLife.57335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Planeta Cda S, & Miczek KA (2008). Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl), 201(3), 459–468. 10.1007/s00213-008-1307-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis F, Thanos PK, Rombola C, et al. (2013) Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels. Behav Neurosci 127:95–105. 10.1037/a0030750 [DOI] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA (2011) Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther;337:162–170. 10.1124/jpet.110.177121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Vazquez-Villoldo N, Matute C (2013) Neurotransmitter signaling in the pathophysiology of microglia. Frontiers in cellular neuroscience 7: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi E, Xu L, Toivainen S, et al. (2021) A neural substrate of compulsive alcohol use. Sci Adv 7.: 10.1126/sciadv.abg9045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW (2000) Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res 859:1–14. 10.1016/s0006-8993(99)02246-5 [DOI] [PubMed] [Google Scholar]
- Dozier BL, Stull CA, Baker EJ, Ford MM, Jensen JP, Finn DA, et al. (2019). Chronic ethanol drinking increases during the luteal menstrual cycle phase in rhesus monkeys: implication of progesterone and related neurosteroids. Psychopharmacology. 236:1817–1828. 10.1007/s00213-019-5168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, et al. (2013) Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry 3:e296. 10.1038/tp.2013.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MRW (2000). CR3: A general purpose adhesion-recognition receptor essential for innate immunity. Microbes and Infection, 2(3), 289–294. 10.1016/S1286-4579(00)00299-9 [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Pirchl M, & Humpel C (2012). Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience, 205, 154–166. 10.1016/j.neuroscience.2011.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, & Barron S (2009). Effects of mecamylamine on alcohol consumption and preference in male C57BL/6J mice. Pharmacology, 83(6), 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Epstein EE, McCrady BS, Hunter-Reel D (2012) Do co-morbid anxiety disorders predict drinking outcomes in women with alcohol use disorders? Alcohol Alcohol 47: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, & Guerri C (2013). Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. Journal of Neurochemistry, 126(2), 261–273. 10.1111/jnc.12276 [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, & Guerri C (2009). Critical Role of TLR4 Response in the Activation of Microglia Induced by Ethanol. The Journal of Immunology, 183(7), 4733–4744. 10.4049/jimmunol.0803590 [DOI] [PubMed] [Google Scholar]
- Finn DA (2020). The Endocrine System and Alcohol Drinking in Females. Alcohol Research : Current Reviews, 40(2), 02. 10.35946/arcr.v40.2.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Hashimoto JG, Cozzoli DK, Helms ML, Nipper MA, Kaufman MN, Wiren KM, & Guizzetti M (2018). Binge Ethanol Drinking Produces Sexually Divergent and Distinct Changes in Nucleus Accumbens Signaling Cascades and Pathways in Adult C57BL/6 J Mice. Frontiers in Genetics, 9. https://www.frontiersin.org/article/10.3389/fgene.2018.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ (2013) Elevated Norepinephrine may be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Subst Abuse 7:171–183. 10.4137/SART.S13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA (2004) Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 311:1071–1079. 10.1124/jpet.104.072025 [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. (2004). Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clinical Experimental Research;28:20–28. 10.1097/01.ALC.0000108647.62718.5A [DOI] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R (2014) Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology 39:1527–1537. 10.1038/npp.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, & Fernández-Suárez D (2015). Alternatively activated microglia and macrophages in the central nervous system. Progress in Neurobiology, 131, 65–86. 10.1016/j.pneurobio.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, & Maier SF (2007). Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain, Behavior, and Immunity, 21(1), 47–59. 10.1016/j.bbi.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Price ME, Hafeez H, Schank JR. (2019). Sex Differences in Aversion-Resistant Ethanol Intake in Mice. Alcohol Alcohol. 54:345–352. [DOI] [PubMed] [Google Scholar]
- García-Ovejero D, Veiga S, García-Segura LM, & Doncarlos LL (2002). Glial expression of estrogen and androgen receptors after rat brain injury. Journal of Comparative Neurology, 450(3), 256–271. 10.1002/cne.10325 [DOI] [PubMed] [Google Scholar]
- García-Pardo MP, Roger-Sánchez C, De la Rubia Ortí JE, & Calpe MAA (2017). Animal models of drug addiction Modelos animales de adicción a las drogas. Adicciones, 29(4), 278–292. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, et al. (1998). Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 83:2099–2103. 10.1210/jcem.83.6.4905 [DOI] [PubMed] [Google Scholar]
- Gildawie KR, Orso R, Peterzell S, Thompson V, & Brenhouse HC (2020). Sex differences in prefrontal cortex microglia morphology: Impact of a two-hit model of adversity throughout development. Neuroscience Letters, 738, 135381. 10.1016/j.neulet.2020.135381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M (2015) The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77:859–869. 10.1016/j.biopsych.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavin GB (1985) Stress and brain noradrenaline: a review. Neurosci Biobehav Rev 9:233–243. 10.1016/0149-7634(85)90048-x [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Campos J, Loidi L, Quinteiro C, Perez L-F, & Gude F (2008). Serum TNF-α levels in relation to alcohol consumption and common TNF gene polymorphisms. Alcohol, 42(6), 513–518. 10.1016/j.alcohol.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. (1997). Discriminative stimulus effects of ethanol and 3 alpha-hydroxy-5 alpha-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis). Psychopharmacology; 130:59–68. 10.1007/s002130050211 [DOI] [PubMed] [Google Scholar]
- Grant KA, Helms CM, Rogers LSM, Purdy RH. (2008). Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. Journal Pharmacol Exp Ther; 326:354–361. 10.1124/jpet.108.137315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA psychiatry 74: 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Traynelis SF (2013) Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem 288: 15291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang L, Wang Q, Zhang D, Zhao Q, Zhang J, Xie L, Liu G, & You Z (2019). Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology, 107, 37–45. 10.1016/j.psyneuen.2019.04.021 [DOI] [PubMed] [Google Scholar]
- He J, & Crews FT (2008). Increased MCP-1 and Microglia in Various Regions of the Human Alcoholic Brain. Experimental Neurology, 210(2), 349–358. 10.1016/j.expneurol.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey JC, Winder DG, Kash TL (2008) Chronic ethanol exposure leads to divergent control of dopaminergic synapses in distinct target regions. Alcohol 42:179–190. 10.1016/j.alcohol.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein A, Käser M, Lichtinghagen R, Rhein M, Lenz B, Kornhuber J, Bleich S, & Hillemacher T (2014). TNF-α and IL-6 serum levels: Neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol, 48(7), 671–676. 10.1016/j.alcohol.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Hedges DM, Yorgason JT, Brundage JN, Wadsworth HA, Williams B, Steffensen SC, et al. (2020). Corticotropin releasing factor, but not alcohol, modulates norepinephrine release in the rat central nucleus of the amygdala. Neuropharmacology. 179:108293. 10.1007/s002130000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, & Ashby CR Jr (2007). PRECLINICAL STUDY: Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addiction biology, 12(1), 35–50. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, & Becker HC (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction biology, 15(2), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW. (2018). Estradiol enhances ethanol reward in female mice through activation of ERα and ERβ. Horm Behavior; 98:159–164. 10.1016/j.yhbeh.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer AT, Sandiego CM, Hannestad J, Angarita GA, Kumar A, McGovern EM, Huang Y, O’Connor KC, Carson RE, O’Malley SS, & Cosgrove KP (2017). In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Molecular Psychiatry, 22(12), 1759–1766. 10.1038/mp.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran K, & Winder D (2017). Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes, Brain and Behavior, 16(1), 8–14. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, Gerhard A, & Talbot PS (2018). Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biological Psychiatry, 83(1), 61–69. 10.1016/j.biopsych.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, & Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism: Clinical and Experimental Research, 35(11), 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y, Quintanilla ME, Ezquer F, Morales P, Santapau D, Berríos-Cárcamo P, Ezquer M, Olivares B, & Herrera-Marschitz M (2021). Aspirin and N-acetylcysteine co-administration markedly inhibit chronic ethanol intake and block relapse binge drinking: Role of neuroinflammation-oxidative stress self-perpetuation. Addiction Biology, 26(1), e12853. 10.1111/adb.12853 [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann S, & Beyer C (2013). Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. The Journal of Steroid Biochemistry and Molecular Biology, 137, 71–81. 10.1016/j.jsbmb.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Juárez J, Barrios De Tomasi E, Virgen M. (2002). Effects of estradiol treatment on voluntary and forced alcohol consumption in male rats. Pharmacology Biochemistry and Behavior; 71:259–268. 10.1016/S0091-3057(01)00662-1 [DOI] [PubMed] [Google Scholar]
- Jurga AM, Paleczna M, & Kuter KZ (2020). Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Frontiers in Cellular Neuroscience, 14, 198. 10.3389/fncel.2020.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk NJ, Guo Q, Owen D, Cherian R, Erritzoe D, Gilmour A, Ribeiro AS, McGonigle J, Waldman A, Matthews P, Cavanagh J, McInnes I, Dar K, Gunn R, Rabiner EA, & Lingford-Hughes AR (2017). Decreased hippocampal translocator protein (18 kDa) expression in alcohol dependence: A [11C]PBR28 PET study. Translational Psychiatry, 7(1), e996. 10.1038/tp.2016.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK (1998) Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res 58:221–224. 10.1016/s0169-328x(98)00112-0 [DOI] [PubMed] [Google Scholar]
- Kash TL (2012) The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol 46:303–308. 10.1016/j.alcohol.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ 2nd, Conrad KL, et al. (2009) Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology 34:2420–2429. 10.1038/npp.2009.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. (2012). Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology; 37:2605–2614. 10.1038/npp.2012.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Wiers CE, Tyler R, Shokri-Kojori E, Jang YJ, Zehra A, Freeman C, Ramirez V, Lindgren E, Miller G, Cabrera EA, Stodden T, Guo M, Demiral ŞB, Diazgranados N, Park L, Liow J-S, Pike V, Morse C, … Volkow ND (2018). Influence of alcoholism and cholesterol on TSPO binding in brain: PET [11C]PBR28 studies in humans and rodents. Neuropsychopharmacology, 43(9), 1832–1839. 10.1038/s41386-018-0085-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, & Becker HC (2019). Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice. Psychopharmacology, 236(9), 2613–2622. 10.1007/s00213-019-05233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H, Bucy PC (1937) “ Psychic blindness” and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. Am J Physiol [Google Scholar]
- Koob GF (2003). Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research, 27(2), 232–243. [DOI] [PubMed] [Google Scholar]
- Koss WA, Gehlert DR, Shekhar A. (2004). Different effects of subchronic doses of 17-beta estradiol in two ethologically based models of anxiety utilizing female rats. Horm Behav. 46:158–164. 10.1016/j.yhbeh.2004.02.011 [DOI] [PubMed] [Google Scholar]
- Kurushima H, Ramprasad M, Kondratenko N, Foster DM, Quehenberger O, & Steinberg D (2000). Surface expression and rapid internalization of macrosialin (mouse CD68) on elicited mouse peritoneal macrophages. Journal of Leukocyte Biology, 67(1), 104–108. 10.1002/jlb.67.1.104 [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. (2006). Estrogen Receptor β, but not α, Mediates Estrogen’s Effect on Cocaine-Induced Reinstatement of Extinguished Cocaine-Seeking Behavior in Ovariectomized Female Rats. Neuropsychopharmacology; 32:1334–1345. 10.1038/sj.npp.1301249 [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, & Orfao A (2007). Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: Relationship with ethanol intake and liver disease. Cytometry Part B: Clinical Cytometry, 72B(5), 408–415. 10.1002/cyto.b.20169 [DOI] [PubMed] [Google Scholar]
- Lê A, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, & Shaham Y (2011). Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology, 218(1), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A, Quan B, Juzytch W, Fletcher P, Joharchi N, & Shaham Y (1998). Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology, 135(2), 169–174. [DOI] [PubMed] [Google Scholar]
- Lê A, & Shaham Y (2002). Neurobiology of relapse to alcohol in rats. Pharmacology & therapeutics, 94(1–2), 137–156. [DOI] [PubMed] [Google Scholar]
- Lee J-S, Lee S-B, Kim D-W, Shin N, Jeong S-J, Yang C-H, & Son C-G (n.d.). Social isolation–related depression accelerates ethanol intake via microglia-derived neuroinflammation. Science Advances, 7(45), eabj3400. 10.1126/sciadv.abj3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, & Harris RA (2000). Gene Expression in Human Alcoholism: Microarray Analysis of Frontal Cortex. Alcoholism: Clinical and Experimental Research, 24(12), 1873–1882. 10.1111/j.1530-0277.2000.tb01993.x [DOI] [PubMed] [Google Scholar]
- Li H, Sagar AP, & Kéri S (2018). Microglial markers in the frontal cortex are related to cognitive dysfunctions in major depressive disorder. Journal of Affective Disorders, 241, 305–310. 10.1016/j.jad.2018.08.021 [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, & Mayfield RD (2006). Patterns of Gene Expression in the Frontal Cortex Discriminate Alcoholic from Nonalcoholic Individuals. Neuropsychopharmacology, 31(7), 1574–1582. 10.1038/sj.npp.1300947 [DOI] [PubMed] [Google Scholar]
- Liu X, Fan X-L, Zhao Y, Luo G-R, Li X-P, Li R, & Le W-D (2005). Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-α and estrogen receptor-β in microglia. Journal of Neuroscience Research, 81(5), 653–665. 10.1002/jnr.20583 [DOI] [PubMed] [Google Scholar]
- Logrip ML, Oleata C, Roberto M (2017) Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology 114:123–134. 10.1016/j.neuropharm.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, & Becker HC (2014). Operant ethanol self-administration in ethanol dependent mice. Alcohol, 48(3), 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, & Becker HC (2016). Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol, 51, 17–23. 10.1016/j.alcohol.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrai I, Contini A, Gessa GL, Mugnaini C, Corelli F, Colombo G, et al. (2019). Operant, oral alcohol self-administration: Sex differences in Sardinian alcohol-preferring rats. Alcohol. 2019;79:147–162. 10.1016/j.alcohol.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Lowe PP, Morel C, Ambade A, Iracheta-Vellve A, Kwiatkowski E, Satishchandran A, Furi I, Cho Y, Gyongyosi B, Catalano D, Lefebvre E, Fischer L, Seyedkazemi S, Schafer DP, & Szabo G (2020). Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations. Journal of Neuroinflammation, 17, 296. 10.1186/s12974-020-01972-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Yang J-Z, Geng F, Ding J-H, & Hu G (2014). Iptakalim confers an antidepressant effect in a chronic mild stress model of depression through regulating neuro-inflammation and neurogenesis. International Journal of Neuropsychopharmacology, 17(9), 1501–1510. 10.1017/S1461145714000285 [DOI] [PubMed] [Google Scholar]
- Manjoch H, Vainer E, Matar M, Ifergane G, Zohar J, Kaplan Z, & Cohen H (2016). Predator-scent stress, ethanol consumption and the opioid system in an animal model of PTSD. Behavioural Brain Research, 306, 91–105 [DOI] [PubMed] [Google Scholar]
- Marianno P, Abrahao KP, & Camarini R (2017). Environmental enrichment blunts ethanol consumption after restraint stress in C57BL/6 mice. PLoS One, 12(1), e0170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Geil CR, & Nixon K (2016). Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sciences, 6(2), 16. 10.3390/brainsci6020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, & Nixon K (2013). Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiology of Disease, 54, 239–251. 10.1016/j.nbd.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Wooden JI, & Nixon K (2020). Microglia Dystrophy Following Binge-Like Alcohol Exposure in Adolescent and Adult Male Rats. Frontiers in Neuroanatomy, 14, 52. 10.3389/fnana.2020.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, & Harris RA (2002). Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. Journal of Neurochemistry, 81(4), 802–813. 10.1046/j.1471-4159.2002.00860.x [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, & Nixon K (2011). Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain, Behavior, and Immunity, 25(Suppl 1), S120–S128. 10.1016/j.bbi.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. (2003) Effects of chronic ethanol consumption on rat GABAA and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Research, 963:165–177. 10.1016/S0006-8993(02)03966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, Robinson SL, Chappell AM, McCool BA. (2018). Chronic Intermittent Ethanol Exposure Modulation of Glutamatergic Neurotransmission in Rat Lateral/Basolateral Amygdala is Duration-, Input-, and Sex-Dependent. Neuroscience. 10.1016/j.neuroscience.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG (2009) Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry 33:1329–1335. 10.1016/j.pnpbp.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AFT, Picciotto MR, Weinberger AH, Ashare R, & Sinha R. (2015) A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. (2015) J Psychopharmacol, 29:300–11. 10.1177/0269881114562091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menze ET, Ezzat H, Shawky S, Sami M, Selim EH, Ahmed S, Maged N, Nadeem N, Eldash S, & Michel HE (2021). Simvastatin mitigates depressive-like behavior in ovariectomized rats: Possible role of NLRP3 inflammasome and estrogen receptors’ modulation. International Immunopharmacology, 95, 107582. 10.1016/j.intimp.2021.107582 [DOI] [PubMed] [Google Scholar]
- Mihic SJ (1999) Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int 35:115–123. 10.1016/s0197-0186(99)00053-4 [DOI] [PubMed] [Google Scholar]
- Miles OW, Maren S. (2019). Role of the Bed Nucleus of the Stria Terminalis in PTSD: Insights From Preclinical Models. Front Behav Neuroscience; 13:68. 10.3389/fnbeh.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Bentham MP, Zhou W-L, et al. (2015) Antidepressant-like effects of guanfacine and sex-specific differences in effects on c-fos immunoreactivity and paired-pulse ratio in male and female mice. Psychopharmacology 232:3539–3549. 10.1007/s00213-015-4001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, & Eisenberger NI (2015). Sex Differences in Depressive and Socioemotional Responses to an Inflammatory Challenge: Implications for Sex Differences in Depression. Neuropsychopharmacology, 40(7), 1709–1716. 10.1038/npp.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Martínez LM, Juárez J. (2020). Differential expression of μ-opioid receptors in the nucleus accumbens, amygdala and VTA depends on liking for alcohol, chronic alcohol intake and estradiol treatment. Behav Brain Research; 378:112255. 10.1016/j.bbr.2019.112255 [DOI] [PubMed] [Google Scholar]
- Morais-Silva G, Fernandes-Santos J, Moreira-Silva D, & Marin M (2015). Concomitant stress potentiates the preference for, and consumption of, ethanol induced by chronic pre-exposure to ethanol. Brazilian Journal of Medical and Biological Research, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, et al. (2005) Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 29:1214–1224. 10.1016/j.pnpbp.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Newman EL, Albrechet-Souza L, Andrew PM, Auld JG, Burk KC, Hwa LS, Zhang EY, DeBold JF, & Miczek KA (2018). Persistent escalation of alcohol consumption by mice exposed to brief episodes of social defeat stress: suppression by CRF-R1 antagonism. Psychopharmacology, 235(6), 1807–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, et al. (2009) Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal 9:68–85. 10.1100/tsw.2009.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308: 1314–8. [DOI] [PubMed] [Google Scholar]
- Nistico R, Mango D, Mandolesi G, Piccinin S, Berretta N, Pignatelli M, Feligioni M, Musella A, Gentile A, Mori F, Bernardi G, Nicoletti F, Mercuri NB, Centonze D (2013) Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PLoS One 8: e54666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L (2006) Possible contributors to the gender differences in alcohol use and problems. The Journal of general psychology 133: 357–74. 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Noori HR, Helinski S, & Spanagel R (2014). Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addiction biology, 19(2), 225–232. [DOI] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, & Miczek KA (2015). Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology, 232(6), 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, Vernon AC, Benke D, Pomper MG, Sawa A, & Meyer U (2018). Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Molecular Psychiatry, 23(2), 323–334. 10.1038/mp.2016.248 [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, et al. (2009). Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clinical and Experimental Research, 33:1924–1934. 10.1111/j.1530-0277.2009.01030.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Motivala SJ, Valladares EM, Olmstead R, & Irwin MR (2007). Sex differences in monocyte expression of IL-6: Role of autonomic mechanisms. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 293(1), R145–R151. 10.1152/ajpregu.00752.2006 [DOI] [PubMed] [Google Scholar]
- Olney JJ, Marshall SA, & Thiele TE (2018). Assessment of depression-like behavior and anhedonia after repeated cycles of binge-like ethanol drinking in male C57BL/6J mice. Pharmacology Biochemistry and Behavior, 168, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas LC, Keele NB (2018) Sex Differences in the Physiological Response to Ethanol of Rat Basolateral Amygdala Neurons Following Single-Prolonged Stress. Front Cell Neurosci 12:219. 10.3389/fncel.2018.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österlund M, G. JM Kuiper G, Gustafsson J-Å, Hurd YL. (1997). Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Molecular Brain Research. 1998;54:175–180. [DOI] [PubMed] [Google Scholar]
- Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA (2016) Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron 92: 493–504. 10.1016/j.neuron.2016.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AE, Rinker JA, Lopez MF, et al. (2020) Bioinformatics identification and pharmacological validation of Kcnn3/KCa2 channels as a mediator of negative affective behaviors and excessive alcohol drinking in mice. Translational Psychiatry 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science, 333: 1456–8. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Gross CT (2011) Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol 7: 77–83. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres J-L, Costa-Alba P, García-García F, Laso F-J, & Guerri C (2017). Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addiction Biology, 22(6), 1829–1841. 10.1111/adb.12461 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R (2005) Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol 37: 157–166. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Hagues G, Costentin J, & Duterte-Boucher D (2005). Helplessness in the tail suspension test is associated with an increase in ethanol intake and its rewarding effect in female mice. Alcohol Clin Exp Res, 29(3), 378–388. 10.1097/01.alc.0000156123.10298.fa [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, & McKee SA (2019). Sex differences in stress-related alcohol use. Neurobiology of stress, 10, 100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñasco S, Mela V, López-Moreno JA, Viveros M-P, & Marco EM (2015). Early Maternal Deprivation Enhances Voluntary Alcohol Intake Induced by Exposure to Stressful Events Later in Life. Neural Plasticity, 2015, 342761. 10.1155/2015/342761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, Harris RA, & Mayfield RD (2012). Gene Coexpression Networks in Human Brain Identify Epigenetic Modifications in Alcohol Dependence. Journal of Neuroscience, 32(5), 1884–1897. 10.1523/JNEUROSCI.3136-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, Vendruscolo LF. (2017). Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacology Biochemistry and Behavior; 152:61–67. 10.1016/j.pbb.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ME, McCool BA. (2022) Structural, functional, and behavioral significance of sex and gonadal hormones in the basolateral amygdala: A review of preclinical literature. Alcohol; 98:25–41. 10.1016/j.alcohol.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, & Crews FT (2012a). NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. Journal of Neuroinflammation, 9(1), 5. 10.1186/1742-2094-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, & Crews FT (2012b). Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. Journal of Neuroinflammation, 9, 130. 10.1186/1742-2094-9-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, & Wilkinson CW (2000). Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcoholism: Clinical and Experimental Research, 24(12), 1836–1849. [PubMed] [Google Scholar]
- Remes O, Brayne C, van der Linde R, Lafortune L (2016) A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav 6: e00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & behavior, 84(1), 53–63. [DOI] [PubMed] [Google Scholar]
- Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, Machado-Vieira R, Yuan P, Niciu MJ, Lyoo CH, Henter ID, Salvadore G, Drevets WC, Kolb H, Innis RB, & Zarate CA Jr (2018). PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Research, 8(1), 57. 10.1186/s13550-018-0401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR (2012) The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med 2:a012195. 10.1101/cshperspect.a012195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, et al. (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A 100:2053–2058. 10.1073/pnas.0437926100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Winger G, Carter RB, Wood PL, Woods JH, Woolverton WL. (1999). Reinforcing and discriminative stimulus effects of the neuroactive steroids pregnanolone and Co 8–7071 in rhesus monkeys. Psychopharmacology; 145:205–212. [DOI] [PubMed] [Google Scholar]
- Równiak M, Bogus-Nowakowska K, Robak A. (2015). The densities of calbindin and parvalbumin, but not calretinin neurons, are sexually dimorphic in the amygdala of the guinea pig. Brain Research;1604:84–97. 10.1016/j.brainres.2015.01.048 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, Power J (2003) The amygdaloid complex: anatomy and physiology. Physiol Rev 83:803–834. 10.1152/physrev.00002.2003 [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Ohsawa K, Kanazawa H, Kohsaka S, & Imai Y (2001). Iba1 Is an Actin-Cross-Linking Protein in Macrophages/Microglia. Biochemical and Biophysical Research Communications, 286(2), 292–297. 10.1006/bbrc.2001.5388 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, & Bilbo SD (2012). Sex differences in microglial colonization of the developing rat brain. Journal of Neurochemistry, 120(6), 948–963. 10.1111/j.1471-4159.2011.07630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H, Tjernström N, Roman E. (2020). Effects of pair housing on voluntary alcohol intake in male and female Wistar rats. Alcohol;86:121–128. 10.1016/j.alcohol.2019.12.005 [DOI] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA (2009) Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev 29: 535–47. 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GA, Bent MAM, Council KR, Pais AC, Amstadter A, Wolstenholme JT, Miles MF, & Neigh GN (2020). Chronic repeated predatory stress induces resistance to quinine adulteration of ethanol in male mice. Behavioural brain research, 382, 112500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, & Bulloch K (2008). Steroid hormone receptor expression and function in microglia. Glia, 56(6), 659–674. 10.1002/glia.20644 [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, et al. (2002) Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science 296:931–933. 10.1126/science.1069836 [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Mark GP, Finn DA. (2002). Reinforcing effects of the neurosteroid allopregnanolone in rats. Pharmacol Biochem Behav. 72:923–929. 10.1016/S0091-3057(02)00776-1 [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, & Weiner JL (2015). Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology, 97, 149–159. 10.1016/j.neuropharm.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G (2008) Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct 213:43–61. 10.1007/s00429-008-0191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socodato R, Henriques JF, Portugal CC, Almeida TO, Tedim-Moreira J, Alves RL, Canedo T, Silva C, Magalhães A, Summavielle T, & Relvas JB (2020). Daily alcohol intake triggers aberrant synaptic pruning leading to synapse loss and anxiety-like behavior. Science Signaling, 13(650), eaba5754. 10.1126/scisignal.aba5754 [DOI] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P (2011) Interneurons in the basolateral amygdala. Neuropharmacology 60:765–773. 10.1016/j.neuropharm.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, & Heilig M (2014). Stress and alcohol interactions: animal studies and clinical significance. Trends in neurosciences, 37(4), 219–227. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM III, Fee JR, Knapp DJ, Breese GR, & Thiele TE (2009). The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcoholism: Clinical and Experimental Research, 33(1), 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Kirson D, Wolfe SA, et al. (2021) Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders. Mol Psychiatry 26:3093–3107. 10.1038/s41380-020-00920-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, & Navarro M (2014). “Drinking in the dark”(DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol, 48(3), 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Wang Y, Zhang N, Guo Y-Y, Feng B, Liu S-B, et al. (2013). Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology; 38:2218–2233. 10.1016/j.psyneuen.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. (2003). Alcohol Intoxication Increases Allopregnanolone Levels in Female Adolescent Humans. Neuropsychopharmacology; 28:1207–1209. 10.1038/sj.npp.1300170 [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, & Mechawar N (2014). Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain, Behavior, and Immunity, 42, 50–59. 10.1016/j.bbi.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Tournier N, Pottier G, Caillé F, Coulon C, Goislard M, Jégo B, Negroni J, Leroy C, & Saba W (2021). Nalmefene alleviates the neuroimmune response to repeated binge-like ethanol exposure: A TSPO PET imaging study in adolescent rats. Addiction Biology, 26(3), e12962. 10.1111/adb.12962 [DOI] [PubMed] [Google Scholar]
- Tremblay ME (2011) The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies. Neuron Glia Biol 7: 67–76. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK (2011) A role for microglia in synaptic plasticity? Commun Integr Biol 4: 220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011) The role of microglia in the healthy brain. J Neurosci 31: 16064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJ, Hilderbrand ER, Satta R, Tai R, He D, You C, et al. (2020). Estrogen Receptor α Regulates Ethanol Excitation of Ventral Tegmental Area Neurons and Binge Drinking in Female Mice. J Neuroscience;40:5196–5207. 10.1523/JNEUROSCI.2364-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW. (2017). Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS One; 12:e0187698. 10.1523/JNEUROSCI.2364-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, & Crews FT (2012). Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and toll-like receptors in the adult prefrontal cortex. Neuroscience, 226, 475–488. 10.1016/j.neuroscience.2012.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Coleman LG Jr, & Crews FT (2021). Increased Toll-like Receptor-MyD88-NFκB-Proinflammatory neuroimmune signaling in the orbitofrontal cortex of human alcohol use disorder. Alcoholism: Clinical and Experimental Research, 45(9). 10.1111/acer.14669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Smith CM, Chua BE, et al. (2015) Relaxin-3 receptor (RXFP3) signalling mediates stress-related alcohol preference in mice. PLoS One 10:e0122504. 10.1371/journal.pone.0122504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, & Crews FT (2017). Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. Journal of Neuroinflammation, 14. 10.1186/s12974-017-0856-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Vetreno RP, & Crews FT (2017). Alcohol and Stress Activation of Microglia and Neurons: Brain Regional Effects. Alcoholism: Clinical and Experimental Research, 41(12), 2066–2081. 10.1111/acer.13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ, Bajo M, Montgomery S, Vlkolinsky R, Nadav T, Polis I, Roberts AJ, Mayfield RD, Harris RA, & Roberto M (2020). Microglia control escalation of drinking in alcohol dependent mice: Genomic and synaptic drivers. Biological Psychiatry, 88(12), 910–921. 10.1016/j.biopsych.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, & Ron D (2013). Chromatin remodeling—a novel strategy to control excessive alcohol drinking. Translational psychiatry, 3(2), e231–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weera MM, & Gilpin NW (2019). Biobehavioral interactions between stress and alcohol. Alcohol research: current reviews, 40(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L (1956) Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol 49:381–391. 10.1037/h0088009 [DOI] [PubMed] [Google Scholar]
- West RK, Rodgers SP, & Leasure JL (2021). Neural perturbations associated with recurrent binge alcohol in male and female rats. Alcoholism: Clinical and Experimental Research, 45(2), 365–374. 10.1111/acer.14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre CC (2001). Sex differences in autoimmune disease. Nature Immunology, 2(9), 777–780. 10.1038/ni0901-777 [DOI] [PubMed] [Google Scholar]
- Woodburn SC, Bollinger JL, & Wohleb ES (2021). Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiology of Stress, 14, 100312. 10.1016/j.ynstr.2021.100312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Tong Y, Chen P-F, Miao S, & Zhou R (2020). Neuroprotection of dihydrotestosterone via suppression of the toll-like receptor 4/nuclear factor-kappa B signaling pathway in high glucose-induced BV-2 microglia inflammatory responses. NeuroReport, 31(2), 139–147. 10.1097/WNR.0000000000001385 [DOI] [PubMed] [Google Scholar]
- Zhao Y-N, Wang F, Fan Y-X, Ping G-F, Yang J-Y, & Wu C-F (2013). Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behavioural Brain Research, 236, 270–282. 10.1016/j.bbr.2012.08.052 [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM (2006) Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. Journal of Neurophysiology, 96:433–441 [DOI] [PubMed] [Google Scholar]


