Abstract
The recent monkeypox (MPVX) outbreak has been characterized by an unprecedented increase in cases, unlike any other outbreaks in the past. The disease pattern and transmissibility are also different from previous outbreaks. This systematic review aimed to evaluate whether the current outbreak has significant contrasting features from the previous ones, necessitating changes in prevention and control guidelines. A thorough literature search related to MPVX infection was performed on the online databases PubMed, Google Scholar, and ScienceDirect using appropriate keywords "MPVX", "men who have sex with men (MSM)", "transmission", and "smallpox vaccination", for choosing relevant articles from the inception of MPVX in 1970 to August 31, 2022. We identified 5110 cases of MPVX, documented in 63 articles on MPVX. We discovered that the median age of MPVX infection has slowly increased since its inception, and currently, it is more common in adults. Compared to previous outbreaks, a significantly greater male preponderance is witnessed in the current outbreak. Only 238 (4.65%) out of the 5110 evaluated patients were vaccinated with the smallpox vaccine in our review. There were 107 mortalities, most of which were children below the age of 10 years. Out of the 1534 cases identified in 2022, 1134 (73.92%) patients admitted that they had been involved in sexual relations within the last 21 days (MSM/gay/bisexual). We found that in contrast to previous outbreaks, human-to-human transmission is more common in this outbreak, with most cases having no link with endemic countries. There are evolving traits and undetected transmission modes of MPVX infection that require new disease mitigation strategies.
Keywords: travel-related infection, smallpox vaccine, monkeypox transmission, men who have sex with other men (msm), monkeypox
Introduction and background
The WHO declared monkeypox (MPVX) a Public Health Emergency of International concern on July 23, 2022. As of September 22, 2022, 64,290 cases have been reported (63,711 of these cases are in locations that have not historically reported MPVX), with 20 confirmed deaths. MPVX is caused by a virus belonging to the Poxviridae family, Chordopoxvirinae subfamily, and Orthopoxvirus genus [1]. It is very similar to the Variola (smallpox) virus. MPVX was discovered in a Danish Laboratory in 1958 as the causative agent of smallpox (SPX)-like illness in Cynomolgus monkeys; hence the name MPVX [2]. Only animals were initially considered susceptible to the virus, but after a confirmed infection in a nine-month-old child in the Democratic Republic of Congo (DRC) in 1970, its zoonotic nature was confirmed [3]. In the beginning, the disease was primarily confined to African countries. However, an outbreak in the United States of America in 2003 heralded the virus’s non-endemic spread [4-7]. The most enormous documented explosion occurred in Nigeria in 2017, almost 40 years after the last case [8]. One case was reported in Israel in 2018 and in Singapore in May 2019. Three patients were reported in the United Kingdom between 2018 and 2019; all of them had a history of recent travel to Nigeria [9-12]. The current outbreak of 2022 began in the United Kingdom in a patient who also had recently traveled to Nigeria in May 2022. Later, two additional cases involving cohabitants of the first case were identified. Within the first month of this outbreak, there were more than 3000 cases reported across 50 countries, many of them non-endemic.
Apart from this multi-country community transmission, which is alarming, the clinical features of the disease have also evolved with a more subclinical prodrome, less extensive skin lesions localized to genital regions, and a well-defined prevalence among the "men who have sex with men" (MSM) community. In most of the earlier outbreaks, the index case had acquired the infection from an animal source. However, this contrasts with the current outbreak, with human-to-human transmission being the norm rather than the exception [13,14]. Between 1980 and 1984, clinical and subclinical diseases mainly occurred in children younger than 10 years of age; however, current trends suggest that the disease is more prevalent among adults. Previously, most of the deaths were seen in children; however, the mortality statistics related to the current outbreak are unclear. One fact consistent across outbreaks is the greater attack rate in individuals not vaccinated against smallpox.
There is still much confusion regarding the epidemiology of the current outbreak and how much it deviates from the historical trend. There are chances of a “misinfodemic” full of confusion about the disease's origin and transmission mode [15,16]. Already, there are myths circulating that MPVX is connected to the coronavirus disease (COVID-19) vaccine (the AstraZeneca vaccine uses a chimpanzee viral vector). One of the most common pieces of misinformation circulating is that MPVX is simply a conspiracy cooked up by various international institutions [17]. More importantly, the recent clustering of cases around sexual networks is driving a sense of stigma that might deter high-risk groups from coming forward and seeking help [18]. We desperately need to understand what is the driving force behind the outbreak. Is clustering at specific events responsible? Why are individuals with high-risk behavior being affected more predominantly? In short, how is the current trend different from the previous ones?
In light of this, this systematic review attempts to compare the pattern of past and current outbreaks and suggest recommendations.
Review
Material and methods
Study Setting and Design
A systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search Strategy and Study Selection
Search strings were developed and run across the electronic databases PubMed, Google Scholar, and Science Direct from inception to August 31, 2022. Studies were searched using the keywords "Monkeypox" OR "Monkeypox virus" OR "Monkeypox and travel" OR "Monkeypox outbreak" OR "Monkeypox and Africa" OR "Monkeypox and Europe".
Inclusion and Exclusion Criteria
All the studies published as full-text articles in indexed journals, involving all levels of evidence, which investigated the MPVX infection all over the world from inception, were included. Only articles published in English with available abstracts were included, with no restriction imposed in terms of the date of publication. We excluded systematic reviews and metanalysis, review articles, commentaries and correspondences, expert opinions, letters to the editor, studies on animals, unpublished reports, and book chapters.
Data Extraction and Analysis
Two authors (R.K. and S.S.) independently screened the data from the selected studies by reading the abstracts. After excluding non-eligible studies by duplicate studies and exclusion criteria, the full texts of the remaining articles were evaluated for eligibility. To minimize the risk of bias, the authors reviewed and discussed all the selected manuscripts, the references, and the articles excluded from the study. Any disagreements were resolved by consensus after consulting with a third author (S.K.S.). At the end of the process, potentially missed studies were further manually searched for among the reference lists of the included papers.
For each study included in the present review, the following data were extracted: author name, country of the report, year/period of the report, number of patients, patient demographics (e.g., age and gender), travel history, sexual history (<21 days) (gay/bisexual/MSM), contact with infected animal or person, previous smallpox vaccination, and outcome of the disease.
Data Synthesis
The study quality and characteristics of interest were tabulated and narratively described.
Ethics
As this is a systematic review using scientific articles available on public platforms, without providing any information related to patients' identities, the ethics committee's review and approval were not required.
Results
Literature Search
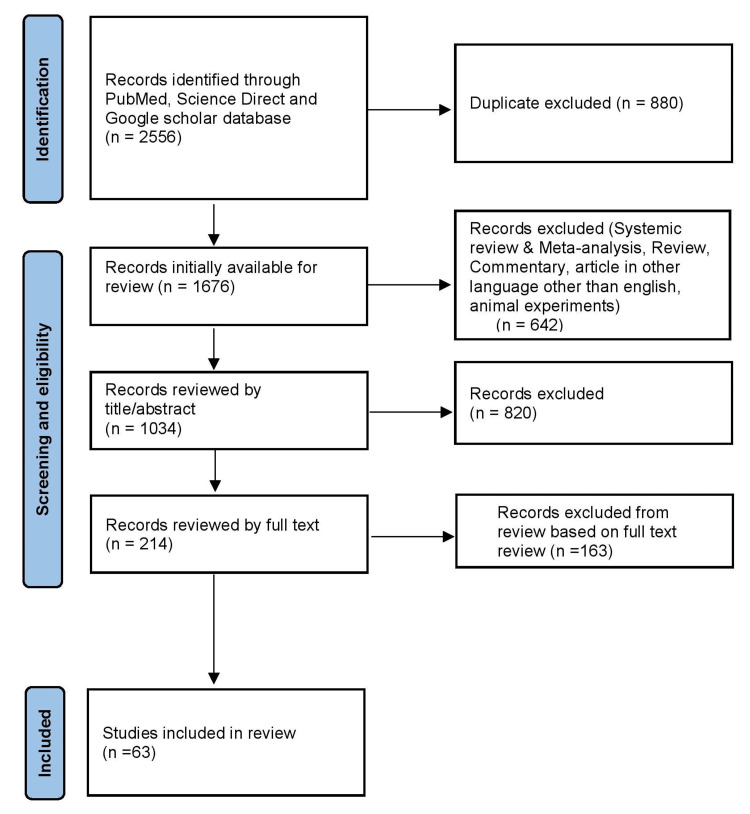
Based on the eligibility criteria, 2556 studies were selected from three databases in the initial research. A total of 1676 articles were initially available for review after removing duplicate studies (880). Of these,1034 articles were selected for review after applying the inclusion and exclusion criteria. Finally, 63 articles were chosen for this systematic review after removing papers based on the evaluation of title/abstract and full text. The PRISMA flow diagram shows the process of study selection (Figure 1)
Figure 1. PRISMA flow chart depicting study selection for systematic review.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Demographic Data
A total of 5110 monkeypox cases from 63 selected articles were analyzed, including 17 case reports and 46 case series, mainly from Africa, followed by the USA, UK, Spain, and other countries (Table 1).
Table 1. Summary of monkeypox cases.
M: male; F: female; NM: not mentioned; MSM: Men Having Sex With Men; (+): yes; (-): no
| Sr. no. | Author | Year/period | Country | Total cases | Male/female | Age (years) | Travel history | Sexual history (MSM/gay/bisexual) | Contact with infected animal or infected person or event attended | Previous smallpox vaccination | Outcome |
| 1 | Ortiz-Martínez Y et al. [19] | 2022 | USA | 1 | M | 36 | + (contact person) | + | - | NM | Recovered |
| 2 | Pembi et al. [20] | 2022 | Nigeria | 1 | M | 30 | - | - | +/- | - | Recovered |
| 3 | Noe et al. [21] | 2022 | Germany | 2 | M | 26, 32 | - | + (2) | - | NM | Recovered |
| 4 | Patel et al. [22] | 2022 | UK | 197 | M (197) | Median age: 38 (21-67) | + (54) | + (170) | + (155) contact | NM | NM |
| 5 | Selb et al. [23] | 2022 | Germany | 521 | M (521) | Median age: 38 (20-67) | + (70) | + (259) | + (151) events | NM | NM |
| 6 | Iñigo Martínez et al. [24] | 2022 | Spain (Madrid) | 508 | M (503); F (5) | Median age: 35 (18-67) | + (38) | + (427) | + (72) pets + (289) contact + (73) events | NM | NM |
| 7 | Peiró-Mestres et al. [25] | 2022 | Spain (Barcelona) | 12 | M (12) | Median age: 38.5 (32-52) | + (4) | + (12) | + (5) event + (4) confirmed case | + (4) | NM |
| 8 | Jang et al. [26] | 2022 | Korea | 1 | M | 34 | + (Germany) | - | + (suspected case) | NM | NM |
| 9 | Yang et al. [27] | 2022 | Taiwan | 1 | M | 20 | + (Germany) | - | NM | - | NM |
| 10 | Girometti et al. [28] | 2022 | UK | 54 | M (54) | Median age: 41 | + (25) | + (54) | + (2) | NM | Recovered |
| 11 | Orviz et al. [29] | 2022 | Spain (Madrid) | 48 | M (48) | Median age: 35 | - | + (42) | + (7) | + (12) | Recovered |
| 12 | Tutu van Furth et al. [30] | 2022 | Netherlands | 1 | M | 10 | + (Turkey) | - | NM | - | Recovered |
| 13 | Oprea et al. [31] | 2022 | Romania | 1 | M | 26 | - | - | - | - | Recovered |
| 14 | Claro et al. [32] | 2022 | Brazil | 1 | M | 41 | + (Europe) | - | - | - | NM |
| 15 | Miura et al. [33] | 2022 | Netherlands | 18 | M (18) | 23-64 | - | + | - | - | NM |
| 16 | Mileto et al. [34] | 2022 | Italy | 1 | M | 33 | + (Portugal) | + | - | - | Recovered |
| 17 | Patrocinio‑Jesus et al.[35] | 2022 | Portugal | 1 | M | 31 | - | + | - | - | Recovered |
| 18 | Minhaj et al. [36] | 2022 | USA | 17 | M (17) | 28-61, average age: 40 | + (14) | + (16) | - | NM | NM |
| 19 | Ferraro et al. [37] | 2022 | Italy | 29 | M (26); F (1) | 20-54, median age: 36 | + (23) | + (16) | NM | NM | |
| 20 | Bížová et al. [38] | 2022 | Czech Republic | 1 | M | 34 | + (Europe) | + | - | NM | NM |
| 21 | Antinori et al. [39] | 2022 | Italy | 4 | M (4) | 30s | + (4) | + (4) | NM | Recovered | |
| 22 | Hammerschlag et al. [40] | 2022 | Australia | 1 | M | 30 | + (Europe) | + | - | NM | Recovered |
| 23 | Vivancos et al. [41] | 2022 | UK | 86 | M (79); F (7) | Median age: 38 | + (1) Nigeria | + (66) | - | NM | NM |
| 24 | Perez Duque et al. [42] | 2022 | Portugal | 27 | M (27) | 22-51, median age: 33 | + (4) | + (18) | + cat (2), pigs (1), confirmed case (1) | + (1) | Recovered |
| 25 | Rao et al. [43] | 2021 | USA | 1 | M | Middle-aged | + (Nigeria) | - | - | - | Recovered |
| 26 | Hobson et al. [10] | 2021 | UK | 3 | M (3) | 18 months child, adult (2) | + (Nigeria) | - | - | NM | Recovered |
| 27 | Costello et al. [44] | 2021 | USA | 1 | M | 28 | + (Nigeria) | - | - | - | Recovered |
| 28 | Kyaw et al. [45] | 2019 | Singapore | 1 | M | 38 | + (Nigeria) | - | + | NM | NM |
| 29 | Eseigbe et al. [46] | 2018 | Nigeria | 2 | M (2) | 20, 20 | - | - | NM | NM | Recovered |
| 30 | Erez et al. [9] | 2018 | Israel | 1 | M | 38 | + (Nigeria) | - | + | NM | Recovered |
| 31 | Besombes et al. [47] | 2018 | CAR | 6 | F (6) | 7-33 (4), 4, 5M | - | - | + | - | NM |
| 32 | Vaughan et al. [48] | 2018 | UK | 2 | M (2) | NM | + (2) (Nigeria) | - | + | NM | Recovered |
| 33 | Ogoina et al. [49] | 2017 | Nigeria | 1 | M | 34 | NM | NM | NM | NM | Suicide |
| 34 | Ogoina et al. [50] | 2017 | Nigeria | 21 | M (17); F (4) | 6-45, median age: 29 | - | NM | NM | NM | Recovered |
| 35 | Doshi et al. [51] | 2017 | Congo | 22 | M (8); F (14) | 1-40, median age: 11.5 | - | - | + | NM | Death (3) (4, 14, 40Y) |
| 36 | Yinka-Ogunleye et al. [52] | 2017-2018 | Nigeria | 122 | M (84); F (38) | 2D-40Y, median age: 29 | - | - | + | NM | Death (7), mean age: 27Y |
| 37 | Ogoina et al. [53] | 2017-2018 | Nigeria | 40 | M (31); F (9) | <35Y=67.5%, 28D-54Y | - | - | NM | NM | Death (5) (28D, 27, 34, 42, 43Y), anxiety and depression (11) |
| 38 | kalthan et al. [54] | 2016 | CAR | 26 | M (14); F (8) | <30=69.3%, median age: 24 | - | - | + | +5 (19.2%) | Death (2) index case (36Y), child (12M) |
| 39 | Eltvedt et al. [55] | 2016 | Congo | 1 | M | 4 | - | - | NM | - | Death |
| 40 | Nakoune et al. [56] | 2015-2016 | CAR | 10 | M (6); F (4) | 15M- 41Y | - | - | + | NM | Death (2) (15M, 5Y old) |
| 41 | Kalthan et al. [57] | 2015 | CAR | 12 | M (6); F (6) | Average age: 25 | - | - | + | NM | Fatality was 67% among children less than 10 years |
| 42 | Whitehouse et al. [58] | 2011-2015 | Congo | 1057 | M (568); F (486) | 1M-79Y, median age: 14 | - | - | + | + (97) | Death (8) |
| 43 | Reynolds et al. [59] | 2014 | Sierra Leone | 2 | M (2) | 11M, 35Y | - | - | + | NM | Recovered |
| 44 | McCollum et al. [60] | 2011-2014 | North and South Kivu of Congo | 3 | M (2); F (1) | 23-28 | - | - | + | - | NM |
| 45 | Nolen et al. [61] | 2013 | Congo | 63 | M (36); F (27) | Median age: 10, 4M-68Y | - | - | NM | + (9) | Death (10) |
| 46 | Mbala et al. [62] | 2007-2011 | Congo | 4 | F (4) Pregnant | 20-29 | - | - | + | NM | Miscarriage in the first trimester (2), Foetal death in 18 wks |
| 47 | Reynolds et al. [63] | 2010 | Congo | 2 | F (2) | 7, 16 | - | - | + | NM | NM |
| 48 | Doshi et al. [64] | 2005-2007 | Congo | 223 | M (155); F (68) | <14 (152), >30 (14) | - | - | + (165) | + (8) | NM |
| 49 | Rimoin et al. [65] | 2005-2007 | Sankuru District, Congo | 760 | M (472); F (288) | Average age: 11.9, 5D-70Y | - | - | + | + (29) | NM |
| 50 | Formenty et al. [66] | 2005-2006 | Sudan | 19 | M (9); F (10) | 8M-32Y, <20Y=15 | - | - | NM | NM | No death |
| 51 | Rimoin et al. [67] | 2001-2004 | Congo | 51 | M (28); F (23) | Average age: 10, <24=48 | - | - | + | NM | NM |
| 52 | Boumandouki et al. [68] | 2003 | Congo | 8 | M (4); F (4) | Mean age: 9.05, 5M-18Y | - | - | + | NM | Recovered |
| 53 | Reynolds et al. [69] | 2003 | USA | 30 | M (13); F (17) | <18 (10) | - | + prairie dog (30), rodents (4) | + (6) | Recovered | |
| 54 | Huhn et al. [70] | 2003 | USA | 34 | M (18); F (16) | >18 (24) | - | - | + home pet (19) pet store (4), veterinarian office (9) | + (7) | Admitted to ICU <18Y, pediatrics (50%), adult (9%) |
| 55 | Sejvar et al. [71] | 2003 | USA | 3 | M (1); F (2) | 6, 30, 33 | - | - | + prairie dogs | + (1) | Child required hospitalization for severe encephalitis |
| 56 | CDC update [72] | 2003 | USA | 35 | M (17); F (18) | <18 (11), 6-51 | - | - | + prairie dog and human (30) | + (8) | Two children had a serious clinical illness |
| 57 | Meyer et al. [73] | 2001 | Congo | 23 | NM | 1-30 | - | - | + | NM | Death (5) (1.5-14Y) |
| 58 | Meyer et al. [74] | 2001 | Gabon | 4 | Children (4) | NM | - | - | + | NM | Fatal case (2), hemorrhagic fever |
| 59 | Hutin et al. [75] | 1996-1997 | Katako-Kombe (Congo) | 88 | M (50); F (38) | Median age: 10, 1m-62Y | - | - | + | + (13) | Death (3), 3.7% case fatality rate, all are children <3Y |
| 60 | CDC update Congo [76] | 1996-1997 | Kasai (Congo) | 419 | NM | <16 (357) | - | - | + (53% another case-patient) | + (20) | Death (5), 4-8Y |
| 61 | Heymann et al. [77] | 1981-1986 | Congo | 338 | M (183); F (155) | <10 (291), 3M-69Y | - | - | + (245) | + (13) | Death (10%) |
| 62 | Jezek et al. [78] | 1981-1985 | Zaire (Congo) | 91 | NM | <15 (93%), 7M-29Y | - | - | + animal (70), human (21) | + (10) | Death (9%) |
| 63 | Breman et al. [79] | 1970-1979 | Central and West Africa | 47 | M (26); F (21) | Mean age: 8, 7M-35Y, <10 (83%) | - | - | + | + (4) | Death 8 (17%), 7M-7Y old |
The median age of cases was 20 years in the earlier outbreaks; however, in the current outbreak, the infection occurred more frequently in the adult population (Table 1). The combined data of all studies showed that MPVX infection has continued to occur more frequently in males (64.22%), uniformly across all outbreaks. Moreover, there were almost 1519 (99.02%) male cases in the current epidemic out of the 1534 evaluated cases (Table 2).
Table 2. Demographic and clinical profile of patients with monkeypox (1970-2022).
MSM: Men Having Sex With Men
| Variables | Year/years | N (%) |
| Total number of monkeypox cases | 1970-2022 (all studies) | 5110 patients |
| Sex | 1970-2022 (all studies) | Male: 3282/5110 (64.22%) |
| Female: 1382/5110 (27.04%) | ||
| Not mentioned: 446/5110 (8.74%) | ||
| 2022 | Male: 1519/1534 (99.02%) | |
| Female: 13/1534 (0.98%) | ||
| Travel history | 2018-2022 | Yes: 252/1551 (16.24%) |
| Sexual history (<21 days) (MSM/gay/bisexual) | 2022 | Yes: 1134/1534 (73.92%) |
| Contact with infected animal or infected person or attended events | 1970-2002 | Yes (720/990) (72.72%) |
| 2003 in the USA | Yes: 102/102 (100%) with prairie dog, pet store, and infected human | |
| 2003-2021 | Yes: (2254/2464) (91.47%) | |
| 2022 | Yes: (764/1534) (49.80%) | |
| Previous smallpox vaccination | 1970-2022 | Yes: 238/5110 (4.65%) |
| No: 3057/5110 (59.82%) | ||
| Not mentioned: 1815/5110 (35.53%) | ||
| Outcomes | 1970-2022 | Death: 107/5110 (including suicide: 1) (2.09%) |
| Recovered: 2511/5110 (49.13%) | ||
| Not mentioned: 2594/5110 (48.78%) |
Vaccination With SPX Vaccine
Vaccination status was available to us for only 3295 out of 5110 evaluated cases. Of these cases, 3057 (59.82%) patients were unvaccinated (Table 2)
Outcome
We could ascertain the outcome in only 2618 out of 5110 evaluated cases. Of these 2618 MPVX cases, 2511 (49.13%) patients recovered, and 107 (2.09%) patients died; most were children below the age of 10 years. Of the 1534 evaluated cases during 2022, there was no mortality. Our results showed that children were at a greater risk of severe MPVX infection (Table 2). One study in our review reported that out of four MPVX-infected pregnant women, three females had miscarriages around the first trimester (Table 1)
Travel History
Before 2018, travel history was not well documented in the reviewed literature. Between 2018 and 2022, we evaluated 1551 cases, and 252 of these (16.24%) had a history of travel to endemic/infected areas, which could explain the source of infection.
Sexual Transmission
In 2022, 1134 out of 1534 (73.92%) patients admitted to having sexual relations within the last 21 days (MSM/gay/bisexual). Before 2022, not much data existed about the history of sexual relations (Table 2)
History of Contact With Infected Animals and Humans
Most patients confirmed contact with an infected animal or person in the earlier reviewed outbreaks:P 72.72% between 1970-2002 and 91.47% between 2003-2021. However, during the year 2022, 764 out of 1534 (49.80%) reviewed MPVX cases had a history of contact with an infected person or attended large-scale public events but hardly any contact with an infected animal (Tables 1, 2).
Discussion
This systematic review evaluated the MPVX outbreaks from 1970 to 2022 based on 5110 cases and aimed to find the contrasts between earlier outbreaks and the current one.
Our results showed that the disease was primarily seen in younger populations during previous outbreaks. The current outbreak, however, shows an increased median age of infection. A recent systematic review, which evaluated MPVX cases through the summer of 2018, published findings similar to our study and stated that the weighted median age of MPVX infection in Africa has increased from four and five years in the 1970s and 1980s to 10 and 21 years in the 2000s and 2010s [80]. This data correlated well with intensified vaccination drive in the 1970s and the cessation of routine SPX vaccination following its eradication in 1980. During the 2000s, only the population below 20 years of age was susceptible to MPVX, as the rest was already vaccinated. The rise in the median age to 21 years in the next decade can also be explained as, at that time, most cases were either too young to have been vaccinated or were not born yet. The increase in the median age of patients during the current outbreak can be due to the disease clustering in MSM. This disease clustering in MSM is likely the reason for recent cases’ significantly greater male preponderance.
Almost 60% of the patients with known vaccination status were unvaccinated in this review. Data from outbreaks within the DRC (1981-2013) and the US (2003) illustrated that 80-96% of cases occurred in unvaccinated individuals. The US outbreak revealed the highest percentage of vaccinated patients (21%) [80]. Using statistical modeling tools, researchers concluded that in 2016, the year before the Nigerian outbreak, only 9.3% of the population was vaccinated against SPX, and individual-level immunity was down to 2.2% from 65.6% in 1970. Almost 96% of monkeypox cases over the years have occurred in unvaccinated individuals [81,82]. When two viruses fight for the same host (in our case, SPX and MPVX are fighting for humans), only the virus with the higher R0 can win, which was SPX until 1980. Consequently, no MPVX cases were reported during the SPX era. The eradication of SPX in 1980 and the fall of orthopoxvirus immunity provided by SPX vaccination to about 10% has caused an entire generation to be susceptible to MPVX infection (R0 of MPVX now reaches anywhere between 1.1 and 2.4, adequate for human-to-human transmission) [83].
Overall mortality has decreased in the current outbreak compared to previous ones, possibly because children were affected more in the earlier outbreaks and because of a lack of antiviral drugs and other supportive therapies to manage the disease. Death was reported in approximately 2% of the patients for whom outcome data were available during this review. Most of these fatalities occurred in children. Fatalities have rarely been reported in outbreaks outside of Africa. Between the 1970s and 1990s, 100% of deaths were among children less than 10 years of age. Whereas from 2000 to 2019, only 37% of deaths occurred in children <10 years of age [80]. During this review, we also found an increased tendency among children with MPVX to have a severe illness. Studies have reported respiratory complications, sepsis, bacterial superinfection, and encephalitis more frequently in children and the immunocompromised. These complications have increased children's mortality and hospitalization rates even in high-income countries [72,84]. The CDC recommends that children with MPVX be ideally cared for in a healthcare facility by a parent or caregiver at low risk of contracting the disease until symptoms subside. The antiviral drug tecovirimat might be considered in the pediatric age group of above two years, considering the morbidity associated with MPVX in children.
Our study also reviewed fetal outcomes in four pregnant women. Only one of the four infected women (with mild disease) in a cohort of 222 symptomatic human-MPVX cases gave birth to a healthy infant devoid of MPVX symptoms. Unfortunately, two women in their first trimester of pregnancy, with moderate and severe infection, respectively, miscarried. One woman who was also coinfected with malaria gave birth to a stillborn, macerated fetus with characteristic lesions of MPVX [62]. In 1988, Jezek et al. also reported a case of a pregnant female with MPVX infection who gave birth to a premature infant with a skin rash suggestive of MPVX at 24 weeks of gestation. The child died six weeks later of unknown causes [85]. Data from the historical SPX infections also suggests that the disease is more severe in pregnant females, especially in their third trimester [86-91]. We need to generate more data regarding maternal and fetal outcomes in human-MPVX cases, factors affecting outcomes in pregnancy, the crucial time for transmission, prophylactic and therapeutic strategies, and breastfeeding status. This is important, considering that pregnancy is a state of being immunocompromised when chances of infections are higher.
Publications about the monkeypox outbreak in the DRC during the 1980s have reported the possibility of an animal source in 72.5% (245/338) of cases and a human source in 27.5% (93/338) of patients, a finding also corroborated in our study. During the 1990s, however, 22% of cases reported no contact with a human subject. In contrast, the rest did have evidence of contact with an infected person within 7-21 days before the onset of the disease. In the Nigerian outbreak (2017-2018), 78.3% of the cases for whom transmission details were known had a history of contact with people having MPVX-like lesions. The rest reported contact with animals [80].
Our study also found that approximately 50% of cases had a history of contact with an infected person or attended a super spreader (International Pride Event on Gran Canaria and other public gatherings) event during the year 2022. About 74% of patients reported having sexual relations within the last 21 days (MSM/gay/bisexual) during the year 2022. A prospective cross-sectional study of MPVX cases in 2022 in Spain also concluded that contact during sex and MSM are strongly associated with infections. Of note, 76% of patients in the study had sexually transmitted diseases [92]. The MPVX predominantly affects men, a finding reiterated in our research. The combined data in our study showed that among H-MPVX cases for whom gender data was available, almost 64% were men. This male predominance might be linked to the virus finding a favorable transmission mode through tightly interconnected sexual networks in the MSM/gay/bisexual community. Here the virus can spread in ways that it cannot in the general population. Although how the virus exactly travels in this community is unclear. It might be that skin-to-skin contact is enough like it is for other STIs. Herein might lie the most crucial factor leading to a make-or-break situation during the current outbreak. Protecting those most at risk and limiting the spread are both interlinked.
There has been much debate about the reasons for the resurgence of MPVX, with waning immunity to SPX vaccination, genetic evolution of the virus (a gene loss that correlates with human-to-human transmission), improved adaptation to the human host, a super spreader event, population growth, increasing global connectedness, deforestation and changes in land usage patterns emerging as the main culprits.
Strengths and Limitations
This is an important study summarizing MPVX outbreaks from 1970 to 2022. However, the uniformity and content of published literature varied considerably, which might have restricted the thoroughness and homogeneity of the evaluated literature. Some of the cases might be duplicated across published papers.
Recommendations
Some key points must be remembered for optimal control of the outbreak. The case fatality rate with human-MPVX in African countries has ranged from 0-11%, the highest being among young children. Evidence suggests that SPX vaccination also protected against Monkeypox (85%). However, the end of routine SPX vaccinations in 1980, which had offered cross-protective immunity against MPVX, has increased the susceptibility of people younger than 50 years to monkeypox. Children with MPVX infections need a bit of extra vigilance and timely therapeutic intervention in hospital settings, considering the apparent absence of “herd immunity” and the severity of illness in this age group. A high index of suspicion in pregnant females who present with a rash and lymphadenopathy is advised. The current outbreak is mainly confined to MSM with high-risk factors for STDs, although this trend might change anytime. Appropriate media campaigns encouraging these groups to freely come forward for testing and treatment without fear of social alienation and simultaneously avoid high-risk behavior need to be started. Containment efforts must be instituted aggressively through identification, isolation, information, contact tracing, ring vaccination (contacts of the index case and high-risk groups), and post-exposure vaccination. Mass immunization might be an option in areas where MPVX becomes endemic. Social media campaigns sharing essential information about monkeypox transmission, treatment, and vaccination protocol can decrease the impact of misinformation. The MPVX virus is unlikely to cause a significant problem except in resource-limited settings, mainly because vaccines and drugs are available, and disease manifestations are easy to identify. There is little need for a COVID-19-like panic currently or in the future.
Conclusions
The geographical spread of MPVX has now become a matter of global concern. This systematic study has reviewed both the past and current outbreaks, concluding that the clinical picture of MPVX is changing. Awareness of evolving clinical manifestations is mandatory for healthcare professionals so that human-MPVX cases can be identified, isolated, and treated correctly. A rise in human-to-human transmission, as witnessed in the current outbreak, puts healthcare professionals and caregivers at risk, necessitating adequate preventive measures to prevent the spread. Mutations within the viral genome and an increasing R0 of the virus might make future outbreaks extremely difficult to control and manage, especially when transmission modes have also changed. Epidemiological investigations must be at an all-time high to assimilate all this new data and arrive at valid conclusions. The case definitions need to be reviewed, and routes of viral transmission better understood to define infection control policies as well as prevention and management strategies.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.(ICTV) ICoToV. Virus taxonomy. [ Oct; 2022 ]. 2020. https://ictv.global/taxonomy https://ictv.global/taxonomy
- 2.A pox-like disease in cynomolgus monkeys. Magnus P, Andersen EK, Petersen KB, Birch‐Andersen A. Acta Path Microbiol Scand. 1959;8:129–157. [Google Scholar]
- 3.Smallpox and monkeypox in non-human primates. Arita I, Henderson DA. https://pubmed.ncbi.nlm.nih.gov/5303409/ Bull World Health Organ. 1968;39:277–283. [PMC free article] [PubMed] [Google Scholar]
- 4.Monkey-pox in a four-year old girl: case report. Eke RA. https://pubmed.ncbi.nlm.nih.gov/4338413/ West Afr Med J Niger Pract. 1972;21:21–22. [PubMed] [Google Scholar]
- 5.Human monkeypox. Foster SO, Brink EW, Hutchins DL, et al. https://pubmed.ncbi.nlm.nih.gov/4340216. Bull World Health Organ. 1972;46:569–576. [PMC free article] [PubMed] [Google Scholar]
- 6.Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Gispen R, Brand-Saathof BB, Hekker AC. https://pubmed.ncbi.nlm.nih.gov/186210/ Bull World Health Organ. 1976;53:355–360. [PMC free article] [PubMed] [Google Scholar]
- 7.From the Centers for Disease Control and Prevention. Multistate outbreak of monkeypox-- Illinois, Indiana, and Wisconsin, 2003. JAMA. 2003;290:30–31. doi: 10.1001/jama.290.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Reemergence of human monkeypox in Nigeria, 2017. Yinka-Ogunleye A, Aruna O, Ogoina D, et al. Emerg Infect Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diagnosis of imported monkeypox, Israel, 2018. Erez N, Achdout H, Milrot E, et al. Emerg Infect Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Hobson G, Adamson J, Adler H, et al. Euro Surveill. 2021;26:2100745. doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Vaughan A, Aarons E, Astbury J, et al. Emerg Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imported monkeypox, Singapore. Yong SE, Ng OT, Ho ZJ, et al. Emerg Infect Dis. 2020;26:1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emergence of monkeypox - West and Central Africa, 1970-2017. Durski KN, McCollum AM, Nakazawa Y, et al. MMWR Morb Mortal Wkly Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Four generations of probable person-to-person transmission of human monkeypox. Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Am J Epidemiol. 1986;123:1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- 15.Misinformation making a disease outbreak worse: outcomes compared for influenza, monkeypox, and norovirus. Brainard J, Hunter PR. Simulation. 2020;96:365–374. doi: 10.1177/0037549719885021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health Feedback. Monkeypox outbreak triggers conspiracy theories on social media claiming that it was planned or incorrectly linking it to COVID-19 vaccines. [ Oct; 2022 ]. 2022. https://healthfeedback.org/claimreview/monkeypox-outbreak-conspiracy-theories-claiming-it-was-planned-linking-with-covid-19-vaccines/ https://healthfeedback.org/claimreview/monkeypox-outbreak-conspiracy-theories-claiming-it-was-planned-linking-with-covid-19-vaccines/
- 17.Monkeypox goes viral: measuring the misinformation outbreak on Twitter. Ortiz-Martínez Y, Sarmiento J, Bonilla-Aldana DK, Rodríguez-Morales AJ. J Infect Dev Ctries. 2022;16:1218–1220. doi: 10.3855/jidc.16907. [DOI] [PubMed] [Google Scholar]
- 18.COVID-19-related infodemic and its impact on public health: a global social media analysis. Islam MS, Sarkar T, Khan SH, et al. Am J Trop Med Hyg. 2020;103:1621–1629. doi: 10.4269/ajtmh.20-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monkeypox - a description of the clinical progression of skin lesions: a case report from Colorado, USA. Ortiz-Martínez Y, Rodríguez-Morales AJ, Franco-Paredes C, Chastain DB, Gharamti AA, Vargas Barahona L, Henao-Martínez AF. Ther Adv Infect Dis. 2022;9:20499361221117726. doi: 10.1177/20499361221117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.First confirmed case of monkeypox in Adamawa State, Nigeria: a clinico-epidemiological case report. Pembi E, Awang S, Salaudeen SO, Agaba IA, Omoleke S. Pan Afr Med J. 2022;42:38. doi: 10.11604/pamj.2022.42.38.34715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and virological features of first human monkeypox cases in Germany. Noe S, Zange S, Seilmaier M, et al. Infection. 2022;22:3–7. doi: 10.1007/s15010-022-01874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. Patel A, Bilinska J, Tam JC, et al. BMJ. 2022;378:0. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Selb R, Werber D, Falkenhorst G, et al. Euro Surveill. 2022;27:1–5. doi: 10.2807/1560-7917.ES.2022.27.27.2200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Iñigo Martínez J, Gil Montalbán E, Jiménez Bueno S, et al. Euro Surveill. 2022;27:2200471. doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, et al. Euro Surveill. 2022;27:1–5. doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The first case of monkeypox in the Republic of Korea. Jang YR, Lee M, Shin H, et al. J Korean Med Sci. 2022;37:0. doi: 10.3346/jkms.2022.37.e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The first case of monkeypox virus infection detected in Taiwan: awareness and preparation. Yang ZS, Lin CY, Urbina AN, et al. Int J Infect Dis. 2022;122:991–995. doi: 10.1016/j.ijid.2022.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Girometti N, Byrne R, Bracchi M, et al. Lancet Infect Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. Orviz E, Negredo A, Ayerdi O, et al. J Infect. 2022;85:412–417. doi: 10.1016/j.jinf.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paediatric monkeypox patient with unknown source of infection, the Netherlands, June 2022. Tutu van Furth AM, van der Kuip M, van Els AL, et al. Euro Surveill. 2022;27:1–5. doi: 10.2807/1560-7917.ES.2022.27.29.2200552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.First report of monkeypox in a patient living with HIV from Romania. Oprea C, Ianache I, Piscu S, et al. Travel Med Infect Dis. 2022;49:102395. doi: 10.1016/j.tmaid.2022.102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shotgun metagenomic sequencing of the first case of monkeypox virus in Brazil, 2022. Claro IM, Romano CM, Candido DD, et al. Rev Inst Med Trop Sao Paulo. 2022;64:0. doi: 10.1590/S1678-9946202264048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Miura F, van Ewijk CE, Backer JA, et al. Euro Surveill. 2022;27:3–7. doi: 10.2807/1560-7917.ES.2022.27.24.2200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.New challenges in human monkeypox outside Africa: a review and case report from Italy. Mileto D, Riva A, Cutrera M, et al. Travel Med Infect Dis. 2022;49:102386. doi: 10.1016/j.tmaid.2022.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monkeypox genital lesions. Patrocinio-Jesus R, Peruzzu F. N Engl J Med. 2022;387:66. doi: 10.1056/NEJMicm2206893. [DOI] [PubMed] [Google Scholar]
- 36.Monkeypox outbreak - nine states, May 2022. Minhaj FS, Ogale YP, Whitehill F, et al. MMWR Morb Mortal Wkly Rep. 2022;71:764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letter to the editor: multiple introductions of MPX in Italy from different geographic areas. Ferraro F, Caraglia A, Rapiti A, et al. Euro Surveill. 2022;27:2200456. doi: 10.2807/1560-7917.ES.2022.27.23.2200456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coinfection of syphilis and monkeypox in HIV positive man in Prague, Czech Republic. Bížová B, Veselý D, Trojánek M, Rob F. Travel Med Infect Dis. 2022;49:102368. doi: 10.1016/j.tmaid.2022.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Antinori A, Mazzotta V, Vita S, et al. Euro Surveill. 2022;27:2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monkeypox infection presenting as genital rash, Australia, May 2022. Hammerschlag Y, MacLeod G, Papadakis G, et al. Euro Surveill. 2022;27:3–7. doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Community transmission of monkeypox in the United Kingdom, April to May 2022. Vivancos R, Anderson C, Blomquist P, et al. Euro Surveill. 2022;27:2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Perez Duque M, Ribeiro S, Martins JV, et al. Euro Surveill. 2022;27:1–5. doi: 10.2807/1560-7917.ES.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monkeypox in a traveler returning from Nigeria - Dallas, Texas, July 2021. Rao AK, Schulte J, Chen TH, et al. MMWR Morb Mortal Wkly Rep. 2022;71:509–516. doi: 10.15585/mmwr.mm7114a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imported monkeypox from international traveler, Maryland, USA, 2021. Costello V, Sowash M, Gaur A, Cardis M, Pasieka H, Wortmann G, Ramdeen S. Emerg Infect Dis. 2022;28:1002–1005. doi: 10.3201/eid2805.220292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monitoring healthcare professionals after monkeypox exposure: experience from the first case imported to Asia. Kyaw WM, Vasoo S, Ho HJ, et al. Infect Control Hosp Epidemiol. 2020;41:373–375. doi: 10.1017/ice.2019.362. [DOI] [PubMed] [Google Scholar]
- 46.Human monkey pox virus infection in Plateau State, North Central Nigeria: a report of two cases. Eseigbe EE, Akude C, Osagie IA, Eseigbe P. https://pubmed.ncbi.nlm.nih.gov/35038257/ West Afr J Med. 2021;38:1242–1246. [PubMed] [Google Scholar]
- 47.Intrafamily transmission of monkeypox virus, Central African Republic, 2018. Besombes C, Gonofio E, Konamna X, et al. Emerg Infect Dis. 2019;25:1602–1604. doi: 10.3201/eid2508.190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Two cases of monkeypox imported to the United Kingdom, September 2018. Vaughan A, Aarons E, Astbury J, et al. Euro Surveill. 2018;23:1800509. doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clinical course and outcome of human monkeypox in Nigeria. Ogoina D, Iroezindu M, James HI, et al. Clin Infect Dis. 2020;71:0–4. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 50.The 2017 human monkeypox outbreak in Nigeria-report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. Ogoina D, Izibewule JH, Ogunleye A, et al. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Doshi RH, Guagliardo SA, Doty JB, et al. Emerg Infect Dis. 2019;25:281–289. doi: 10.3201/eid2502.181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A case of suicide during the 2017 monkeypox outbreak in Nigeria. Ogoina D, Mohammed A, Yinka-Ogunleye A, Ihekweazu C. IJID Reg. 2022;3:226–227. doi: 10.1016/j.ijregi.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Twelve cases of monkeypox virus outbreak in Bangassou District (Central African Republic) in December 2015 (Article in French) Kalthan E, Dondo-Fongbia JP, Yambele S, Dieu-Creer LR, Zepio R, Pamatika CM. Bull Soc Pathol Exot. 2016;109:358–363. doi: 10.1007/s13149-016-0516-z. [DOI] [PubMed] [Google Scholar]
- 55.A case report of monkeypox in a 4-year-old boy from the DR Congo: challenges of diagnosis and management. Eltvedt AK, Christiansen M, Poulsen A. Case Rep Pediatr. 2020;2020:8572596. doi: 10.1155/2020/8572596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.A nosocomial outbreak of human monkeypox in the Central African Republic. Nakoune E, Lampaert E, Ndjapou SG, et al. Open Forum Infect Dis. 2017;4:0. doi: 10.1093/ofid/ofx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Kalthan E, Tenguere J, Ndjapou SG, et al. Med Mal Infect. 2018;48:263–268. doi: 10.1016/j.medmal.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clinical and epidemiological findings from enhanced monkeypox surveillance in Tshuapa Province, Democratic Republic of the Congo during 2011-2015. Whitehouse ER, Bonwitt J, Hughes CM, et al. J Infect Dis. 2021;223:1870–1878. doi: 10.1093/infdis/jiab133. [DOI] [PubMed] [Google Scholar]
- 59.Human monkeypox in Sierra Leone after 44-year absence of reported cases. Reynolds MG, Wauquier N, Li Y, et al. Emerg Infect Dis. 2019;25:1023–1025. doi: 10.3201/eid2505.180832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Human monkeypox in the Kivus, a conflict region of the Democratic Republic of the Congo. McCollum AM, Nakazawa Y, Ndongala GM, et al. Am J Trop Med Hyg. 2015;93:718–721. doi: 10.4269/ajtmh.15-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Nolen LD, Osadebe L, Katomba J, et al. Emerg Infect Dis. 2016;22:1014–1021. doi: 10.3201/eid2206.150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. Mbala PK, Huggins JW, Riu-Rovira T, et al. J Infect Dis. 2017;216:824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 63.Detection of human monkeypox in the Republic of the Congo following intensive community education. Reynolds MG, Emerson GL, Pukuta E, et al. Am J Trop Med Hyg. 2013;88:982–985. doi: 10.4269/ajtmh.12-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monkeypox rash severity and animal exposures in the Democratic Republic of the Congo. Doshi RH, Alfonso VH, Morier D, et al. Ecohealth. 2020;17:64–73. doi: 10.1007/s10393-019-01459-7. [DOI] [PubMed] [Google Scholar]
- 65.Endemic human monkeypox, Democratic Republic of Congo, 2001-2004. Rimoin AW, Kisalu N, Kebela-Ilunga B, et al. Emerg Infect Dis. 2007;13:934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Formenty P, Muntasir MO, Damon I, et al. Emerg Infect Dis. 2010;16:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Rimoin AW, Mulembakani PM, Johnston SC, et al. Proc Natl Acad Sci U S A. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simian smallpox (or monkey smallpox): study of 8 cases observed at Impfondo Hospital in Republic of Congo (Article in French) Boumandouki P, Bileckot R, Ibara JR, et al. https://pubmed.ncbi.nlm.nih.gov/17402687/ Bull Soc Pathol Exot. 2007;100:17–21. [PubMed] [Google Scholar]
- 69.Spectrum of infection and risk factors for human monkeypox, United States, 2003. Reynolds MG, Davidson WB, Curns AT, et al. Emerg Infect Dis. 2007;13:1332–1339. doi: 10.3201/eid1309.070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clinical characteristics of human monkeypox, and risk factors for severe disease. Huhn GD, Bauer AM, Yorita K, et al. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 71.Human monkeypox infection: a family cluster in the midwestern United States. Sejvar JJ, Chowdary Y, Schomogyi M, et al. J Infect Dis. 2004;190:1833–1840. doi: 10.1086/425039. [DOI] [PubMed] [Google Scholar]
- 72.Update: multistate outbreak of monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Centers for Disease Control and Prevention. https://pubmed.ncbi.nlm.nih.gov/12855947/ MMWR Morb Mortal Wkly Rep. 2003;52:642–646. [PubMed] [Google Scholar]
- 73.Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. Meyer H, Perrichot M, Stemmler M, et al. J Clin Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.First appearance of monkey pox in human beings in Gabon (Article in French) Meyer A, Esposito JJ, Gras F, Kolakowski T, Fatras M, Muller G. https://pubmed.ncbi.nlm.nih.gov/1649373/ Med Trop (Mars) 1991;51:53–57. [PubMed] [Google Scholar]
- 75.Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Hutin YJ, Williams RJ, Malfait P, et al. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Human monkeypox -- Kasai Oriental, Democratic Republic of Congo, February 1996-October 1997. Centers for Disease Control and Prevention. https://pubmed.ncbi.nlm.nih.gov/9408046/ MMWR Morb Mortal Wkly Rep. 1997;46:1168–1171. [PubMed] [Google Scholar]
- 77.Re-emergence of monkeypox in Africa: a review of the past six years. Heymann DL, Szczeniowski M, Esteves K. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 78.Human monkeypox: disease pattern, incidence and attack rates in a rural area of northern Zaire. Jezek Z, Grab B, Paluku KM, Szczeniowski MV. https://pubmed.ncbi.nlm.nih.gov/2841783/ Trop Geogr Med. 1988;40:73–83. [PubMed] [Google Scholar]
- 79.Human monkeypox, 1970-79. Breman JG, Kalisa-Ruti Kalisa-Ruti, Steniowski MV, Zanotto E, Gromyko AI, Arita I. https://pubmed.ncbi.nlm.nih.gov/6249508/ Bull World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 80.The changing epidemiology of human monkeypox-a potential threat? A systematic review. Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. PLoS Negl Trop Dis. 2022;16:0. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017-2020. Nguyen PY, Ajisegiri WS, Costantino V, Chughtai AA, MacIntyre CR. Emerg Infect Dis. 2021;27:1007–1014. doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Kugelman JR, Johnston SC, Mulembakani PM, et al. Emerg Infect Dis. 2014;20:232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Modelling human-to-human transmission of monkeypox. Grant R, Nguyen LL, Breban R. Bull World Health Organ. 2020;98:638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Human monkeypox: a study of 2,510 contacts of 214 patients. Jezek Z, Marennikova SS, Mutumbo M, Nakano JH, Paluku KM, Szczeniowski M. J Infect Dis. 1986;154:551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 85.Human monkeypox. Jezek Z, Fenner F. http://www.sciepub.com/reference/199053 Monogr Virol. 1988;17:140. [Google Scholar]
- 86.Smallpox during pregnancy and maternal outcomes. Nishiura H. Emerg Infect Dis. 2006;12:1119–1121. doi: 10.3201/eid1207.051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pregnancy and emerging diseases. Anker M. Emerg Infect Dis. 2007;13:518–519. doi: 10.3201/eid1303.061345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.The role of the obstetrician-gynecologist in emerging infectious diseases: monkeypox and pregnancy. Jamieson DJ, Cono J, Richards CL, Treadwell TA. Obstet Gynecol. 2004;103:754–756. doi: 10.1097/01.AOG.0000114987.76424.6d. [DOI] [PubMed] [Google Scholar]
- 89.Live-virus vaccines in pregnancy. Risks and recommendations. Levine MM. Lancet. 1974;2:34–38. doi: 10.1016/s0140-6736(74)91363-4. [DOI] [PubMed] [Google Scholar]
- 90.Smallpox and pregnancy: from eradicated disease to bioterrorist threat. Suarez VR, Hankins GD. Obstet Gynecol. 2002;100:87–93. doi: 10.1016/s0029-7844(02)02048-3. [DOI] [PubMed] [Google Scholar]
- 91.Smallpox: a disease of the past? Consideration for midwives. Constantin CM, Martinelli AM, Foster SO, Bonney EA, Strickland OL. J Midwifery Women’s Health. 2003;48:258–267. doi: 10.1016/s1526-9523(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 92.Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Català A, Clavo-Escribano P, Riera-Monroig J, et al. Br J Dermatol. 2022;2:35917191. doi: 10.1111/bjd.21790. [DOI] [PubMed] [Google Scholar]