Abstract
Infection with the protozoan parasite Toxoplasma gondii is transmitted to humans from infected animals by tissue cysts and oocysts excreted by cats. Immunization with inactivated parasites or recombinant proteins has at best shown partial protection. We constructed a plasmid expressing the SAG1 surface antigen of T. gondii, p1tPASAG1, and showed that animals immunized with the plasmid produce anti-SAG1 antibodies which recognize the native SAG1. Mice immunized with p1tPASAG1 showed 80 to 100% protection against challenge with the non-cyst-producing, virulent RH isolate, compared to an 80% mortality in mice immunized with empty plasmid, which is the greatest efficacy of any vaccine against T. gondii produced so far. The SAG1 molecule was analyzed for potential cytotoxic T-lymphocyte (CTL) epitopes, and four peptides with the best fit were synthesized. The ability of the peptides to stimulate gamma interferon production by CD8+ T cells from p1tPASAG1-immunized mice was tested in an ELISPOT assay, and one new CTL epitope was identified. Adoptive transfer of CD8+ T cells from p1tPASAG1-immunized to naïve mice showed partial protection. In conclusion, DNA vaccination with p1tPASAG1 gave effective protection in mice against T. gondii infection and the protection could be adoptively transferred by purified CD8+ T cells.
Infection with Toxoplasma gondii occurs worldwide, and the organism may cause congenital infection in humans. T. gondii is an important food-borne parasite, and the main routes of transmission from animals to humans are through meat, mainly from pigs and lambs, and oocysts shed by cats into the environment. Infection is in theory preventable, but health education alone has so far not proven effective.
Immunization against T. gondii is not yet an option for control of infection in humans, but an effective vaccine preventing infection in animals used for food would stop the main transmission route to humans.
A live, attenuated vaccine has been available for veterinary use for several years (7), although it is expensive, causes side effects, has a short shelf life, and provides protection for no more than 3 years. Previous work with inactivated vaccines using whole T. gondii and subunit vaccines found only moderate protective efficacy against infection with the lethal RH T. gondii isolate (6, 16, 21, 24, 30).
Protective immunity against T. gondii rests mainly on CD8+ T lymphocytes and is mediated by production of gamma interferon (IFN-γ) (10, 11, 19). The use of DNA vaccines is a novel concept, involving the injection of the naked DNA plasmid into the host, whose cells express the encoded protein. DNA vaccination is in theory particularly well suited to stimulate effector mechanisms depending on antigen presentation in conjunction with major histocompatibility complex class I (MHC-I) molecules, which stimulate CD8+ cytotoxic T cells (3, 25).
We have previously shown that a vaccine based on the recombinant SAG1 protein expressed in Escherichia coli, with alum as the adjuvant, was able to significantly prolong the survival of mice infected with the RH isolate (21). The present work shows that a DNA vaccine based on part of the SAG1 gene induces protection superior to that of the recombinant protein vaccine and shows that protection is at least partly mediated by T. gondii-specific CD8+ T lymphocytes induced by the DNA vaccine.
MATERIALS AND METHODS
Plasmid construction.
The SAG1 gene sequence was amplified by PCR from the plasmid successfully expressing the recombinant SAG1 protein in E. coli (14), with oligonucleotide primers introducing the NheI and the BamHI restriction sites for ligation into an NheI- and BamHI-cut expression vector (WRG 7079; kindly provided by Jim Fuller, PowderJect Inc.). The plasmid named p1tPASAG1 was purified from DH5α-transformed cells with an endotoxin-free DNA purification kit (Qiagen, Hilden, Germany) and sequenced.
Mice.
Seven- to eight-week-old female C3H (H-2k) and BALB/c (H-2D) mice were purchased from Bomholdtgaard, Ry, Denmark. Their microbiological status was conventional, and they were maintained in groups of five per cage with food and water ad libitum and artificial light for 12 h per day. The acclimatization period was 7 days.
Immunization.
Purified plasmid in endotoxin-tested phosphate-buffered saline (Sigma; 50 μl at 1 mg/ml) was administered intramuscularly (i.m.) in each tibia anterior muscle at week 0 and week 3, and challenge or removal of spleens took place at week 5 unless otherwise stated.
In vitro transfection of COS cells and Western blotting.
The transfections were performed with a transfection kit (Effectene; Qiagen) according to the manufacturer's instructions. Pellets and supernatant of transfected COS cells and mock-transfected control cells were immunoblotted by standard techniques (14). In brief, samples were added to a sodium dodecyl sulfate-polyacrylamide gel and blotted onto a nitrocellulose membrane, incubated with toxoplasma antibody-positive human sera (high titers were found by dye test), and incubated with enzyme-conjugated anti-human immunoglobulin G (IgG) antibody (catalog no. D336; DAKO Glostrup, Denmark).
Culture of T. gondii strains.
The RH strain has been maintained in our laboratory for several decades. The tachyzoites used for challenge were cultured in vitro in Vero cells.
Enzyme immunoassay and Western blotting.
The enzyme-linked immunosorbent assay was performed as previously described (14). In brief, the tachyzoite antigens were prepared from frozen parasites; after centrifugation at 18,000 × g the supernatant was collected and the pellet was sonicated. The supernatant and the soluble fraction were mixed after sonication and used to coat microtiter plates overnight at 4°C. Sera were diluted 1:100 for total IgG and 1:50 for IgG subclasses.
The Western blotting was performed with 107 tachyzoites loaded per well on a mini-sodium dodecyl sulfate-polyacrylamide gel blotted onto a nitrocellulose membrane, cut in strips, and incubated with sera, diluted 1:50, from individual p1tPASAG1-immunized mice.
Challenge.
Immunized mice and control mice were infected by the intraperitoneal (i.p.) route with 105 T. gondii tachyzoites, RH isolate, from continuous culture, and the time until death was observed.
Adoptive transfer of CD8+ and CD4+ T lymphocytes.
Spleens were aseptically removed at week 6 from mice immunized with either empty vector or p1tPASAG1 at weeks 0 and 3; there were six mice in each group. Spleens were pooled and gently homogenized to obtain single-cell suspensions. A positive selection of either CD8+ or CD4+ splenocytes from each group of mice was performed with a MACS LS+ separation column (Mitenyi Biotec, Bergisch Gladbach, Germany; catalog no. 424-01) according to the manufacturer's instructions. The purity of the two subpopulations was evaluated by flow cytometric analysis (Becton Dickinson; FACScan cytometer) with the CellQuest software and the monoclonal antibodies anti-CD4 (L3T4) and anti-CD8a (LY2) (Pharmingen), respectively. The purity in each case was above 80%. After three washes the cells were given intravenously (i.v.) in phosphate-buffered saline to four groups of naïve recipients, each mouse receiving 2 × 107 cells in 0.2 ml. Twenty hours after i.v. injection the mice were challenged with 105 RH tachyzoites i.p.
Prediction of cytotoxic T-lymphocyte (CTL) epitopes.
Briefly, a complete matrix representing the frequencies of amino acids found in the various positions of the bound peptides has been previously established by using peptide libraries. For any individual peptide, the product of the relevant frequencies represents the prediction of binding. In this case, the SAG1 sequence used was scanned for predicted Kk binding peptides (27). The four peptides predicted to produce the highest level of binding were synthesized and tested for binding.
MHC-I binding assays.
The biochemical peptide MHC-I binding assay used affinity-purified Kk molecules and was conducted as previously described (5). Briefly, affinity-purified Kk molecules in detergent solution were incubated with 125I-labelled influenza nucleoprotein (NP) peptide 50-57 and increasing concentrations of unlabelled SAG1 peptides. After equilibrium had been reached (48 h; 18°C), the free and bound labelled peptides were separated by spun-column gel filtration. The concentration of PfCSP needed to inhibit NP 50-57 binding by 50% was determined and was related to the concentration of unlabelled NP 50-57 needed to block labelled-peptide binding by 50%. The latter value, the 50% inhibitory concentration (IC50) of NP 50-57, was routinely around 10 nM.
ELISPOT assay.
Spleen cells from mice immunized with p1tPASAG1 (n = 5), with or without subsequent challenge, and from nonimmunized mice were used. Two groups were immunized at weeks 0 and 3, and one group was further challenged with 5 × 104 tachyzoites at week 6. Spleen cells from each group were stimulated with the four peptides for 16 h. Nitrocellulose microtiter plates (MAHA S45 10; Millipore) were coated overnight at 4°C with 50 μl of IFN-γ-specific monoclonal antibodies (18181D; Pharmingen)/well. After being washed spleen cells were added at 5 × 105 cells/well together with, per well, 5 × 104 mitomycin C-treated RDM4 stimulator cells, preloaded with 10 μg of peptide/ml, plus an additional 1 μg of peptide. The plates were incubated for 16 h. After 10 washings, 5 μg of detection antibodies (18112D; Pharmingen)/ml was added and the plates were incubated for 2 h at room temperature. After additional washings the plates were incubated for 2 h at room temperature with 50 μl of alkaline phosphatase-conjugated avidin (D 0396; Pharmingen)/well diluted 1:1,000. Color was developed by adding 50 μl of freshly made BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium solution/well, and spots were counted by stereo microscopy.
RESULTS
Construction of DNA vaccine.
The SAG1 gene was cloned in frame with a sequence encoding a synthetic mimic of the tPA signal by using the restriction enzymes NheI and BamHI. The transcription was driven by the cytomegalovirus immediate-early promoter. Thus the synthetic tPA signal replaced the natural signal sequence of SAG1 (4). The resulting vector consisted of 824 bases (positions 456 to 1280 according to GenBank) from the SAG1 gene, encoding 275 amino acids, fused in frame at the 3′ terminus with 18 bases, followed by a stop codon.
SAG1 expressed in vitro by transfected COS cells is recognized by human T. gondii-positive sera.
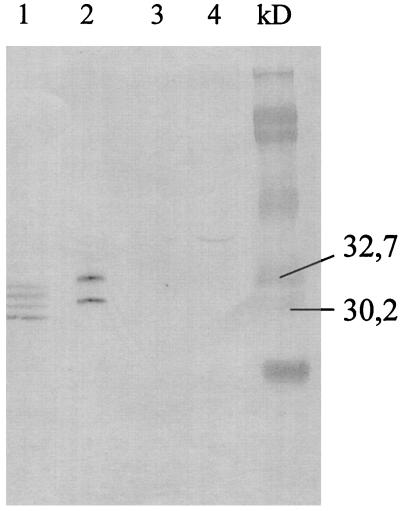
The supernatant and pellet from transfected COS cells showed specific expression of the SAG1 antigen (Fig. 1). Lane 1 shows four specific bands, most likely truncated forms of the mature protein. Two specific bands were seen in the supernatant (lane 2); the upper band showed migration corresponding to glycosylation of the two described N-glycosylation sites (4) which have been found previously when the SAG1 gene containing the natural signal peptide of the mature protein was used to transfect CHO cells (15). In contrast, SAG1 is not glycosylated in T. gondii. Thus the functionality of the vector, in terms of in vitro production of the SAG1 protein, was confirmed. The two potential glycosylation sites of the protein should also be present in vivo in immunized mice and might shield important conformational epitopes from antibody recognition.
FIG. 1.
Western blot showing human T. gondii-positive sera recognizing SAG1 from in vitro-transfected COS cells. Results for a pellet (lane 1) and supernatant (lane 2) from p1tPASAG1-transfected COS cells and for a pellet (lane 3) and supernatant (lane 4) from control COS cells transfected with empty plasmid are shown.
Sera from DNA-immunized mice recognize native T. gondii antigen.
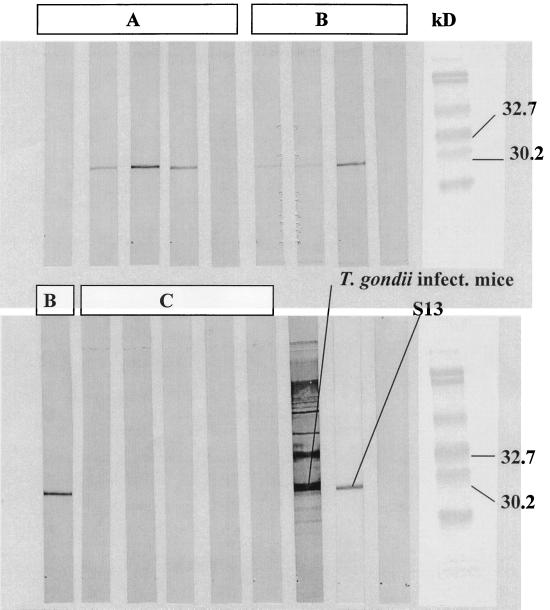
Pooled sera from 10 mice immunized with p1tPASAG1 and bled at week 5 postinfection recognized native toxoplasma parasites in Western blots (Fig. 2). The SAG1 band is clearly visible in blots from four of five mice even in the group only receiving one immunization with p1tPASAG1 (Fig. 2). Serum from a toxoplasma-infected mouse and the monoclonal antibody S13 recognizing SAG1 were used as positive controls (Fig. 2). The results indicate that the glycosylation of the expressed SAG1 has little influence on the immunoreactivity of the molecule.
FIG. 2.
T. gondii tachyzoites from an in vitro culture blotted with sera from individual mice immunized with p1tPASAG1 twice at weeks 0 and 3 (A), once at week 0 (B), and not immunized (C). Sera were tested at week 5 and show recognition of native SAG1. Serum from a mouse infected with T. gondii and the monoclonal antibody S13 recognizing SAG1 were used as positive controls.
DNA immunization induces Th1 response.
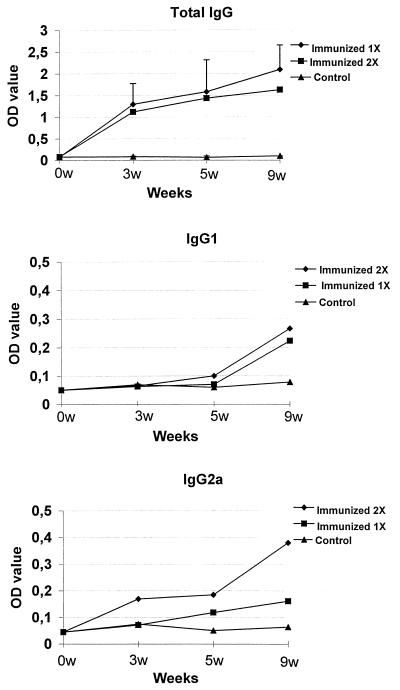
The toxoplasma-specific IgG subclass response to immunization with p1tPASAG1 is shown in Fig. 3. Contrary to the antibody response to the recombinant SAG1 protein, which was exclusively an IgG1 response (21), both IgG1 and IgG2a were found after immunizing mice with p1tPASAG1, and this could indicate a shift towards a more Th1-like immune response after DNA immunization. This type of response is equivalent to the Th1 type of response, which is the natural outcome of infection with live parasites.
FIG. 3.
Immunization with p1tPASAG1 showed balanced IgG1 and IgG2a responses to the i.m. route (mean of five mice in each experiment). The coating antigens were recombinant SAG1 protein for the analysis of total IgG and a tachyzoite preparation for the analysis of the IgG subclasses. OD, optical density.
Protection against lethal challenge.
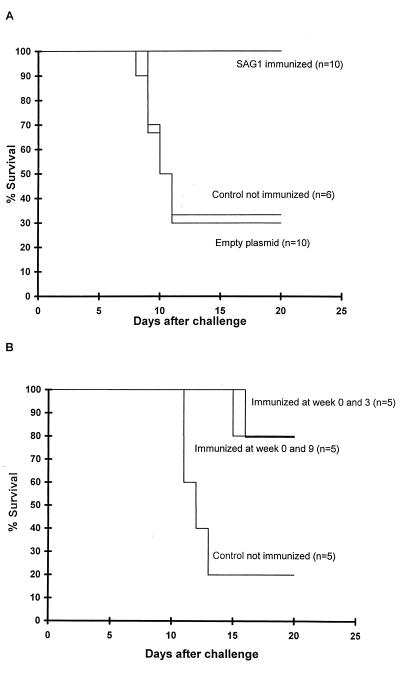
Mice immunized twice with p1tPASAG1 and challenged i.p. with 105 RH tachyzoites 14 days after the last immunization showed 80 to 100% protection compared to control mice immunized with empty plasmid (Fig. 4). Mice alive at week 20 stayed alive for at least 6 months, when the experiment was terminated. Interestingly, if challenge was performed only 7 days after the last immunization, mice immunized with empty vector showed some improved survival compared to nonvaccinated mice (data not shown); this is probably a non-specific-adjuvant effect of the DNA in the vector previously described (29).
FIG. 4.
(A) C3H mice immunized i.m. with p1tPASAG1 twice at weeks 0 and 3 and challenged at week 5 with 105 RH tachyzoites i.p. were 100% protected compared to mice immunized with empty plasmid. (B) Protection of three groups of BALB/c mice immunized with p1tPASAG1 twice at weeks 0 and 3 challenged at week 11.
Specific CD8+ T lymphocytes are involved in protection.
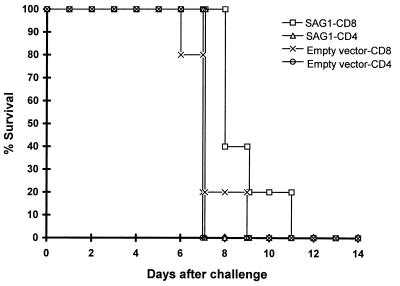
Spleen cells from mice vaccinated with SAG1 or empty vector were enriched for CD4+ and CD8+ T lymphocytes. The enriched subpopulations were transferred i.v. to naïve mice, followed by i.p. challenge after 20 h with a lethal dose of the RH strain (Fig. 5). Only the group of mice receiving CD8+ T lymphocytes from p1tPASAG1-immunized mice prior to challenge showed significantly prolonged survival time compared to the other groups (P = 0.02; Wilcoxon rank sum test). These results demonstrate that specific CD8+ T lymphocytes provide protection, although the level was not comparable to the level of protection seen in p1tPASAG1-immunized mice (Fig. 4).
FIG. 5.
Adoptive-transfer experiment with CD8+ T lymphocytes from p1tPASAG1-immunized mice shows partial protection against challenge with RH tachyzoites. CD8+ and CD4+ T lymphocytes were enriched from mice immunized with p1tPASAG1 or mice immunized with empty vector and injected i.v. into four groups of naïve mice (n = 5). Challenge was performed after 20 h, and survival was monitored.
New CTL epitopes in SAG1.
Four new CTL epitopes were predicted, and the corresponding peptides were synthesized (Table 1). The prediction of peptide-MHC interaction was performed as previously described (27). An ELISPOT assay was used to detect specific CD8+ T lymphocytes secreting IFN-γ, and only peptide T1 showed a specific response in the ELISPOT assay (Table 1). The relative frequency of IFN-γ-secreting CD8+ cells per 105 splenocytes was 1.7 after immunization and 2.4 after immunization followed by challenge. These results show that specific priming of CD8+ T lymphocytes did occur after genetic vaccination with the p1tPASAG1 construct.
TABLE 1.
Binding of predicted epitopes and frequencies of specific CD8+ T lymphocytes obtained by ELISPOT assay
| Predicted epitope | Binding
(nM)
|
Frequency of responding CD8+ T
lymphocytesa in indicated mouse group
|
|||
|---|---|---|---|---|---|
| Predicted | Observedb | Control (not immunized) | p1tPASAG1 immunized | p1tPASAG1 immunized and RH challenged | |
| AESKSVII (T1) | 110 | 225 | 0 | 1.7 ± 0.9 | 2.4 ± 2.2 |
| QTFVVGCI (T2) | 219 | 160 | 0 | 0 | 0 |
| NEKSFKDI (T3) | 352 | 123 | 0 | 0 | 0.13 ± 0.2 |
| VTGLIGSI (T4) | 402 | >100,000 | 0 | 0 | 0 |
Numbers of responders per 105 spleen cells. Values are means ± standard deviations (five mice per group).
Affinity-purified Kk was incubated with radiolabelled influenza NP 50-57 as the indicator peptide and increasing concentrations of SAG1 peptide. Binding was determined by gel filtration and expressed as log [IC50 (NP 50-57)/IC50 (PfCSP peptide)]. IC50 of the NP 50-57 peptide was 10 nM.
DISCUSSION
DNA vaccines have been shown to be effective against several intracellular pathogens causing parasitic infections, including Leishmania spp. (13) and the liver stage of Plasmodium falciparum (25), as well as viral infections such as hepatitis B (31).
The DNA vaccine vector was based on the SAG1 gene from the RH isolate in a truncated version with the signal sequence deleted and replaced by the sequence encoding the strong tPA signal peptide, for better export of the protein to the cell surface. The results from the p1tPASAG1-transfected COS cells showed that the plasmid is expressed, that the recombinant SAG1 is secreted into the supernatant in an immunologically active form which can be recognized by human antibodies from an individual with natural T. gondii infection (Fig. 1) and, furthermore, that sera from mice immunized with p1tPASAG1 recognized the native SAG1 (Fig. 2).
We have previously shown that recombinant SAG1 protein formulated in alum was unable to induce a Th1-like response seen by an IgG2a antibody after immunization (21). In contrast i.m. immunization with the DNA plasmid has been reported to induce a more Th1-biased response (20). The antibody response found here after immunization with p1tPASAG1 was of the same magnitude in mice receiving two immunizations 3 weeks apart as in mice immunized only once, and the levels of the IgG1 and IgG2a responses were of the same magnitude (Fig. 3). This indicates that p1tPASAG1 was able to influence the immune response toward a Th1 response by inducing a dual-IgG subclass response, unlike recombinant SAG1 protein immunization.
To elicit protective immunity against an otherwise lethal dose of T. gondii, the appropriate cellular immune response has to be initiated, which could include both CD8+ and CD4+ T lymphocytes (18, 23). This seems to occur after infection with the sublethal strain T. gondii ts-4, which provides protection against challenge with the lethal strain (7), and mice infected with the cyst-forming strain SSI119 are protected against challenge with the lethal RH isolate. p1tPASAG1 gave complete protection against challenge in CH3 mice and 80% protection in BALB/c mice, whereas immunization with the recombinant SAG1 protein prolonged survival, but eventually almost all animals succumbed (21).
The major cellular T-cell subtype involved in the acquired immune protection against T. gondii is presumably CD8+ T lymphocytes (1, 19, 26, 28). The high efficacy is therefore possibly the result of a more efficient presentation of the antigen to CD8+ effector cells by DNA immunization than by immunization with a protein antigen. In a previous study, strong protection was found after immunization with purified, native SAG1 with liposomes (2), and liposomes are known to be a particularly effective stimulator of CD8+ T lymphocytes via MHC-I presentation of appropriated epitopes (22).
The adoptive-transfer experiment, where CD8+ T cells were transferred from DNA-immunized mice to naïve mice, showed that the CD8+ cell fraction contributed to the protective immunity, which is in agreement with previous studies showing that DNA vaccines induce a strong CTL response (8, 9, 29). The transferred cells did not confer the same degree of protection as p1tPASAG1 immunization but produced significantly better survival than CD8+ T cells from mice immunized with empty plasmid or CD4+ T cells from immunized mice. The lower level of protection in the adoptive-transfer experiment may have several explanations, one of which could be the lack of help from memory CD4+ T cells (12).
One of the main mechanisms of protection against T. gondii provided by CD8+ T lymphocytes is the secretion of IFN-γ upon specific recognition of the epitope presented by MHC-I on the surfaces of infected cells (19, 26). The adoptive transfer does not provide information on whether the effector mechanism is direct cytotoxic cell lysis or is mediated by IFN-γ secretion by the CD8+ cells. In the ELISPOT assay we show that generation of IFN-γ-secreting, antigen-specific CD8+ T lymphocytes has taken place. Whether or not the numbers of antigen-specific CD8+ T lymphocytes are sufficient to provide the observed protection by themselves will require further studies to clarify, but it has been suggested that a frequency of CTLs of at least 1 per 104 lymphocytes is required to provide protection against virus (17).
In conclusion, the study shows for the first time that the design of vaccines against T. gondii based on the DNA approach is feasible and effective.
ACKNOWLEDGMENTS
We thank Mette Ingvorsen, Irene Jensen, Lene Michelsen, and Lis Wassman for skillful technical assistance.
REFERENCES
- 1.Alexander J, Jabber H, Bluethmann H, Satoskar A, Roberts C W. Immunological control of Toxoplasma gondiiand appropriate vaccine design Curr. Top Microbiol. 1996;219:183–195. doi: 10.1007/978-3-642-51014-4_17. [DOI] [PubMed] [Google Scholar]
- 2.Bülow R, Boothroyd J C. Protection of mice from fatal Toxoplasma gondiiinfection by immunization with p30 antigen in liposomes. J Immunol. 1991;147:3496–3500. [PubMed] [Google Scholar]
- 3.Bunnell B A, Morgan R A. Gene therapy for infectious diseases. Clin Microbiol Rev. 1998;11:42–56. doi: 10.1128/cmr.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burg J L, Perelman D, Kasper L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- 5.Buus S, Stryhn A, Winther K, Kirkby N, Pedersen L Ø. Receptor-ligand interactions measured by an improved spun column chromatography technique. A high efficiency and high throughput size separation method. Biochim Biophys Acta. 1995;1243:453–460. doi: 10.1016/0304-4165(94)00172-t. [DOI] [PubMed] [Google Scholar]
- 6.Buxton D, Uggla A, Lövgren K, Thomson K, Lundén A, Morein B, Blewett D A. Trial of a novel ISCOM vaccine in pregnant sheep. Br Vet J. 1989;145:451–457. doi: 10.1016/0007-1935(89)90053-5. [DOI] [PubMed] [Google Scholar]
- 7.Buxton D, Thomson K, Maley S, Wright S, Bos H J. Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondiiand their immunity to challenge when pregnant. Vet Rec. 1991;129:89–93. doi: 10.1136/vr.129.5.89. [DOI] [PubMed] [Google Scholar]
- 8.Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson A C, Sandström E, Wahren B. Cellular cytotoxic response induced by DNA vaccination in HIV-1 infected patients. Lancet. 1998;351:1320–1325. doi: 10.1016/S0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 9.Fomsgaard A, Nielsen H V, Bryder K, Nielsen C, Machuca R, Bruun L, Hansen J, Buus S. Improved humoral and cellular immune responses against the gp120 V3 loop of HIV-1 following genetic immunization with a chimeric DNA vaccine encoding the V3 inserted into the hepatitis B surface antigen. Scand J Immunol. 1998;47:289–295. doi: 10.1046/j.1365-3083.1998.00323.x. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli R, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondiivaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Wysocka M, Hieny S, Schartonkersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondiisuccumb to a lethal immune response dependent on CD4(+) T cells and accompanied by overproduction of IL-12, IFN-gamma, and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 13.Gurunathan S, Sacks D L, Brown D R, Reiner S L, Charest H, Glaichenhaus N, Seder R A. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harning D, Spenter J, Metsis A, Vuust J, Petersen E. Recombinant Toxoplasma gondii SAG1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific IgM and IgG antibodies. Clin Diagn Lab Immunol. 1996;3:355–357. doi: 10.1128/cdli.3.3.355-357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim K, Bülow R, Kampmeier J, Boothroyd J C. Conformationally appropriate expression of the toxoplasma antigen SAG1 (p30) in CHO cells. Infect Immun. 1994;62:203–209. doi: 10.1128/iai.62.1.203-209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunden A. Immune responses in sheep after immunization with Toxoplasma gondii antigens incorporated into iscoms. Vet Parasitol. 1995;56:23–35. doi: 10.1016/0304-4017(94)00670-8. [DOI] [PubMed] [Google Scholar]
- 17.McMichael A J, Hanke T. Is an HIV vaccine possible? Nat Med. 1999;5:612–614. doi: 10.1038/9455. [DOI] [PubMed] [Google Scholar]
- 18.Montoya J G, Lowe K E, Clayberger C, Moody D, Do D, Remington J S, Talib S, Subauste C S. Human CD4+ and CD8+ T lymphocytes are both cytotoxic to Toxoplasma gondii-infected cells. Infect Immun. 1996;64:176–181. doi: 10.1128/iai.64.1.176-181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker S J, Roberts C W, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondiiin mice. Clin Exp Immunol. 1991;84:207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen E, Nielsen H V, Christiansen L, Spenter J. Immunization with E. coli produced recombinant Toxoplasma gondii SAG1 with alum as adjuvant protect mice against lethal infection with Toxoplasma gondii. Vaccine. 1998;16:1283–1289. doi: 10.1016/s0264-410x(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 22.Powers D C, Manning M C, Hanscome P J, Pietrobon P J. Cytotoxic T lymphocyte responses to a liposome-adjuvanted influenza A virus vaccine in the elderly. J Infect Dis. 1995;172:1103–1107. doi: 10.1093/infdis/172.4.1103. [DOI] [PubMed] [Google Scholar]
- 23.Purner M B, Berens R L, Nash P B, van Linden A, Ross E, Kruse C, Krug E C, Curiel T J. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondiiin seropositive humans. Infect Immun. 1996;64:4330–4338. doi: 10.1128/iai.64.10.4330-4338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts C W, Brewer J M, Alexander J. Congenital toxoplasmosis in the Balb/c mouse: prevention of vertical disease transmission and fetal death by vaccination. Vaccine. 1994;12:1389–1394. doi: 10.1016/0264-410x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 25.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V S. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 26.Shirahata T, Yamashita T, Ohta C, Goto H, Nakane A. CD8(+) T lymphocytes are the major cell population involved in the early gamma interferon response and resistance to acute primary Toxoplasma gondiiinfection in mice. Microbiol Immunol. 1994;38:789–796. doi: 10.1111/j.1348-0421.1994.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 27.Stryhn A, Pedersen L Ø, Romme T, Holm C B, Holm A, Buus S. Peptide binding specificity of major histocompatibility complex class I resolved into an array of apparently independent sub-specificities: quantitation by peptide libraries and improved prediction of binding. Eur J Immunol. 1996;26:1911–1918. doi: 10.1002/eji.1830260836. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Remington J S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990;144:1954–1956. [PubMed] [Google Scholar]
- 29.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Parasitol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 30.Waldeland H, Frenkel J K. Live and killed vaccines against toxoplasmosis in mice. J Parasitol. 1986;69:60–65. [PubMed] [Google Scholar]
- 31.Whalen, R. (ed.). 1995–1998. PowderJect's hepatitis B DNA vaccine first to successfully elicit protective immune response in humans. The DNA vaccine web. [Online.] http://www.genweb.com/Dnavax/dnavax.html. [29 June 1999, last date accessed.]