Introduction
Identifying underlying pain mechanisms that reflect variance in patient self-reported symptoms is an urgent priority. The International Association for the Study of Pain has proposed that pain be categorized by the following underlying mechanisms: (1) nociceptive, (2) neuropathic, and (3) nociplastic pain.[21] Nociceptive pain is driven by inflammation/tissue damage, while neuropathic pain refers to dysfunction of nerves. Nociplastic pain, conversely, has been defined in part by the absence of inflammation and neuropathy, while defining symptoms associated with this mechanism has proven more challenging.[15] Responses on self-report surveys assessing symptoms like widespread pain and fatigue, such as the fibromyalgia survey criteria, have shown replicable relationships with experimental pain assessments, neuroimaging findings, and clinical responses to treatment,[1; 4; 29]. However, existing surveys may not capture the full spectrum of signs associated with nociplastic pain such as environmental sensitivty(e.g., bright lights).[26] Improving our understanding of the many symptom domains associated with nociplastic pain could improve accuracy for nociplastic pain characterization.
Recently, the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) research network identified seven broad symptom domains associated with nociplastic pain which were explained by two latent factors.[33] The first factor, termed Generalized Sensory Sensitivity (GSS), was characterized by widespread pain, non-painful somatic symptoms, and sensitivity to environmental stimuli. GSS was associated with the presence of chronic overlapping pain conditions (COPCs), suggesting its value as a marker of nociplastic pain.[27; 33] The second factor was characterized by constitutional symptoms: sleep, pain, affect, cognition, and energy (i.e., S.P.A.C.E.). SPACE represents a group of symptoms commonly observed in pain conditions, and was more strongly related to quality of life than GSS.[33; 38]
Nociplastic pain may be an underappreciated factor in dysmenorrhea – a recent study found reduced conditioned pain modulation and increased experimental somatic and visceral hypersensitivity to be associated with this condition - findings consistent with nociplastic pain.[18; 37] Pain mechanisms are thought to be overlapping and interactive, so characterizing nociplastic pain symptoms in young women with dysmenorrhea is likely to be helpful even to those patients with substantial nociceptive and neuropathic components to their pain. The purpose of this study is to explore whether the same two-factor structure exists in a sample of women with dysmenorrhea but who have not been diagnosed with other chronic overlapping pain conditions. Because these symptoms are strongly associated with the presence of COPCs in other pelvic pain conditions they may have prognostic value in this vulnerable cohort if they can be shown to co-occur in similar patterns observed in urologic chronic pelvic pain. The study also explores how well these two factors correspond with non-cyclic pain symptoms commonly seen in dysmenorrhea, such as bladder and bowel pain. As a secondary analysis we determine whether a brief measure of one of the identified symptom groups could be validated in this sample. We hypothesized that GSS would be associated with non-dysmenorrhea pain complaints. Once the association between these symptom factors and emerging comorbid pain have been established, prospective studies could target these factors for earlier pain intervention.
Methods
This study is a secondary analysis of participants recruited for the prospective study Chronic Pain Risk Associated with Menstrual Pelvic Pain (CRAMPP, Clinical Trial NCT02214550). The purpose of this clinical trial was to determine if some women with dysmenorrhea are at higher future risk of developing chronic pelvic pain, and if oral contraceptives can be used to reverse this chronic pain risk. The data analyzed here only include study visits prior to a participant beginning any research-related treatments. The study was advertised on public transit, the Illinois Women’s Health Registry, and NorthShore University HealthSystem gynecology clinics. The sample sizes originally proposed were based on power analyses for the longitudinal clinical trial data (see Hellman et al. 2020).[18]
Eligibility
Participants that rated their menstrual pain ≥5 on 0–10 numeric rating scale (NRS 0: no pain, 10: worst pain imaginable) during a phone screen were defined as potentially eligible to participate in this study as a dysmenorrhea participant. We then confirmed menstrual pain was at least moderate (≥4 on a 0–10 NRS), using a web-based menstrual symptom diary during at least one menstrual cycle. This cut-off was chosen initially because we have established in a different cohort that this intensity of menstrual pain is associated with a markedly higher likelihood of having comorbid bladder hypersensitivity.[36] This cut-off corresponds to a moderate-to-severe subjective pain in other studies.[19] To confirm the severity of their pain, participants were also required to report that they attempted to resolve their menstrual pain by medical means (e.g., painkillers, birth control pills).
Participants with dysmenorrhea, but with prior non-cyclic chronic pain diagnoses were excluded. To confirm that participants did not have prior chronic pain diagnoses, participants were asked during a phone screen,” Do you have chronic pelvic and/or bladder pain?” Participants were also asked, “Do you have any chronic pain conditions?” Participants that had > 4 migraines that included aura (e.g., flashing lights, blindness in half of visual field, seeing zigzag patterns, feeling prickling skin, weakness and hallucinations) in the past year were excluded because this is an exclusion for the use or oral contraceptives. We also confirmed participants did not have a prior diagnosis for common chronic pain conditions using medical history questionnaire including Painful Bladder Syndrome/Interstitial Cystitis, Endometriosis, Pelvic Inflammatory Disease, Inflammatory Bowel Disease, Irritable Bowel Syndrome, Arthritis, Lower Back Pain and Fibromyalgia. Participants were also asked whether they had “an active genitourinary infection in the last 4 weeks.” Participants with a recent genitourinary infection were instructed to wait until the infection had been resolved.
Participants were excluded for the presence of active pelvic or abdominal malignancies, absence of regular menses, active genitourinary infection in the last four weeks, inability to read or comprehend the informed consent in English, refusal to undergo pelvic examination/testing, hypertension, or refusal to withdraw from oral contraceptives for two months before the study visit. Clinical exam characteristics of this cohort and detailed methods for the screening visit are already published.[17; 35] In brief, although there were no major differences in pelvic floor muscle dysfunction or pelvic floor myofascial pain pelvic observable with clinical exam, participants with dysmenorrhea had increased pelvic floor sensitivity (Hellman et al. 2020).[18] Abdominal and pelvic exams were performed to identify secondary causes of dysmenorrhea with follow-up ultrasonography on the first 98 participants. However, since only four of the first 98 participants showed evidence of a potential secondary cause of dysmenorrhea, pelvic exams were discontinued to reduce participant burden. Participants completed questionnaires including medical, surgical, psychological, and gynecological history via REDCap[14] during the screening visit. All study procedures were approved by the institutional review board and all participants provided informed consent.
Measures
GSS (generalized sensory sensitivity):
Replication of the original factor structure underlying nociplastic pain involved analyzing the same symptom domains as in the original study but within this sample of individuals with dysmenorrhea. To create the GSS construct, items evaluating somatic awareness, sensory sensitivity, and widespread pain were included.[33] Somatic awareness refers to a collection of non-painful but bothersome symptoms such as dry eye, nausea, and palpitations that have been linked to chronic pain in several studies, and have demonstrated predictive ability for future manifestations of pain.[10] Following the original analyses, 18 binary items from the Complex Medical Symptoms Inventory (CMSI) represented the construct.[33] The period of recall was for three months out of the last year. Sensory sensitivity refers to adverse reactions to environmental stimuli such as bright lights, loud noises, and various odors. Four items from the CMSI as in the original paper represented the sensory sensitivity construct. The Widespread Pain Index (WPI) from the 2011 Fibromyalgia survey criteria was used to capture the spatial extent of pain. The WPI encompasses 19 bodily sites for pain or tenderness that a person may have experienced in the last week.[40]
SPACE (sleep, pain, affect, cognition, and energy):
The SPACE construct was assessing using methods similar to Schrepf, et al. (2018).[33] For example, sleep disturbances in the past seven days was measured by the Patient Reported Outcomes Measurement Information System (PROMIS®) sleep disturbance short-form 8a.[41] For affect, depressive symptoms in the past seven days were measured with the PROMIS® emotional distress depression short-form 8a.[32] To measure the concept of dyscognition, or what is sometimes referred to as “cognitive fog,” a single item from the 2011 Fibromyalgia survey criteria assessed “trouble thinking or remembering,” over the past week on a four-point scale.[40] To evaluate energy, fatigue in the past seven days was assessed with the PROMIS® Fatigue short-form 7a, a validated seven item survey.[5]
Menstrual and Comorbid Pain
To evaluate how the GSS and SPACE constructs were associated with menstrual and comorbid pain severity, a 0–100 (0 – No pain, 100 – worst pain imaginable) Visual Analog Scale (VAS) was used to rate the severity of pain.[23] Participants were asked to separately rate their average amount of cramping or pain they experienced during their menstrual period while taking and not taking nonsteroidal antiinflammatory drug (NSAID) medications, respectively, over the past three months on a VAS. Nociplastic pain is thought to be less responsive to treatments designed to address local inflammatory processes; hence we considered both pain ratings.[7; 11]
As noted above, during the screening process, participants were asked if they had any chronic pain (e.g. pain lasting 6 months or more) other than dysmenorrhea. They were also asked if they had any prior diagnosed chronic pain conditions. Despite screening out participants without awareness of any formal chronic pelvic pain condition, some women still reported moderate symptom levels. Participants were separately asked to rate their non-menstrual pelvic pain during the past week on a 0–100 VAS. Similarly, participants were asked to rate their average feeling of pain with urination and bowel movements during the last week, respectively, on the same type VAS. Headache symptoms and disability during the last three months were measured with the Migraine Disability Assessment Test (MIDAS).[34] We also examined overall pain in the past week, “How would you rate your pain on average” (0--No Pain,10—Worst Pain Imaginable) from the PROMIS global health questionnaire. Based on these indices, we created a comorbid pain index which combined the number of nonuterine types of pain that individuals rated as moderate to severe. For bowel and bladder pain, a cutoff of 40/100 suggestive of moderate pain was used.[3] For headache, published guidelines of 11 or greater were used.[34] The number of forms of comorbid pain rated moderate/severe (range 0–3) was then used as the outcome. This outcome is exploratory as this particular index has not been previously used.
Data Analysis.
Analyses were designed to follow the original framework used to identify the GSS and SPACE constructs.6 Although the current study contained validated measures of key domains that comprise GSS and SPACE as in the original study, two scales representing somatic awareness and sensory sensitivity (components of GSS) were constructed from CMSI items. A confirmatory factor analysis of this two-factor solution was initially conducted to determine if an acceptable fit to the data was achieved. Model fit indices included the Root Mean Square Error of Approximation (RMSEA) and associated 90% confidence interval, where an RMSEA < .06 generally represents adequate fit, the Comparative Fit Index (CFI) where CFI > .95 generally represents adequate fit, and the X2 test where p-values > .05 are considered desirable, though often are not achieved in sample sizes over n=200.[20]
To determine associations between the two proposed constructs (e.g. GSS & SPACE) and measures of pain, each pain scale (e.g. menstrual pain, non-menstrual pelvic pain, bowel pain, urinary pain, headache disability) was regressed separately on each latent factor, as well as age and body mass index (BMI), with an MLR estimator and expectation-maximization algorithm for optimization. We also regressed both latent variables on age and BMI. Where the number of moderate intensity comorbid pain types was the dependent variable, proportional odds logistic regression was used. Finally, to enhance the clinical utility of our findings, a GSS Brief scale which contains a simplified body map with seven regions and six questions about somatic awareness and sensory sensitivity (using a 0–9 scale) was constructed.[33] These are the same items used in the original manuscript: for somatic awareness, “dry mouth,” “rapid heart rate,” and “problems with balance”; for sensory sensitivity, “sensitivity to certain chemicals…”, “sensitivity to sound,” and “frequent sensitivity to bright lights.” Spearman’s correlations were run to examine the strength of the relationship between this scale and the factor scores derived from MPLUS to determine how well this scale approximated the factor score derived from all items used in the GSS construct. Factor scores were derived via the regression method.[24] Spearman’s correlations were also used to test the relationship between the GSS Brief and the measures of menstrual, pelvic, urinary, and bowel pain used in the analysis above to determine if the brief scale shows the same relationships as the latent factor it represents.
Results
Participants
This young (mean ± SD: 24 ± 6 years) cohort of 201 women with dysmenorrhea included 41% minority and 13% Hispanic participants (Table 1). On average, women reported moderate levels of dysmenorrhea (43 ± 23) and milder non-menstrual pelvic pain (28 ± 31) on a 0–100 VAS. A substantial minority of participants with dysmenorrhea but not yet diagnosed with other pain conditions had moderate symptoms of non-menstrual pain.
Table 1: Menstrual and comorbid pain in a cohort of women with dysmenorrhea.
Demographic characteristics are shown for the cohort used in the confirmatory factor analyses. Results are mean (SD) pain scores or percentage of participants in the category. MIDAS - Migraine Disability Assessment Test
| Variable | Mean (SD) |
|---|---|
|
| |
| Age | 24.35 (6.45) |
|
| |
| Race | N (%) |
| American Indian/Alaska Native | 3 (1.5) |
| Asian | 41 (20.4) |
| Native Hawaiian or Pacific Islander | 2 (1.0) |
| Black | 56 (27.9) |
| White | 118 (58.7) |
|
| |
| Ethnicity | N (%) |
| Hispanic | 26 (12.9) |
|
| |
| Parity | N (%) |
| 0 | 174 (87) |
| 1 | 13 (6) |
| 2+ | 12 (6) |
|
| |
| Menstrual Pain w/o analgesics (0–100) | 72.95 (13.50) |
|
| |
| Menstrual Pain w/ analgesics (0–100) | 43.34 (22.49) |
|
| |
| Non-menstrual pelvic pain (0–100) | 28.22 (30.73) |
|
| |
| Widespread pain index (0–19) | 1.95 (2.56) |
|
| |
| Somatic awareness (0–18) | 1.81 (2.57) |
|
| |
| Sensory sensitivity (0–4) | 0.40 (0.89) |
|
| |
| Bowel Pain (0–100) | 15.10 (21.07) |
|
| |
| Bladder Pain (0–100) | 6.47 (15.05) |
|
| |
| MIDAS score | 5.74 (10.91) |
|
| |
| Overall pain (0–10) |
2.39 (2.31) |
|
| |
| Moderate Pain | N (%) |
| Bowel | 30 (15%) |
| Bladder | 13 (7%) |
| Headache | 41 (20%) |
|
| |
| Number of comorbid pain types | |
| None | 137 (68%) |
| One | 45 (22%) |
| Two or more | 19 (10%) |
A two-factor structure including GSS and SPACE provides adequate fit in this validation cohort
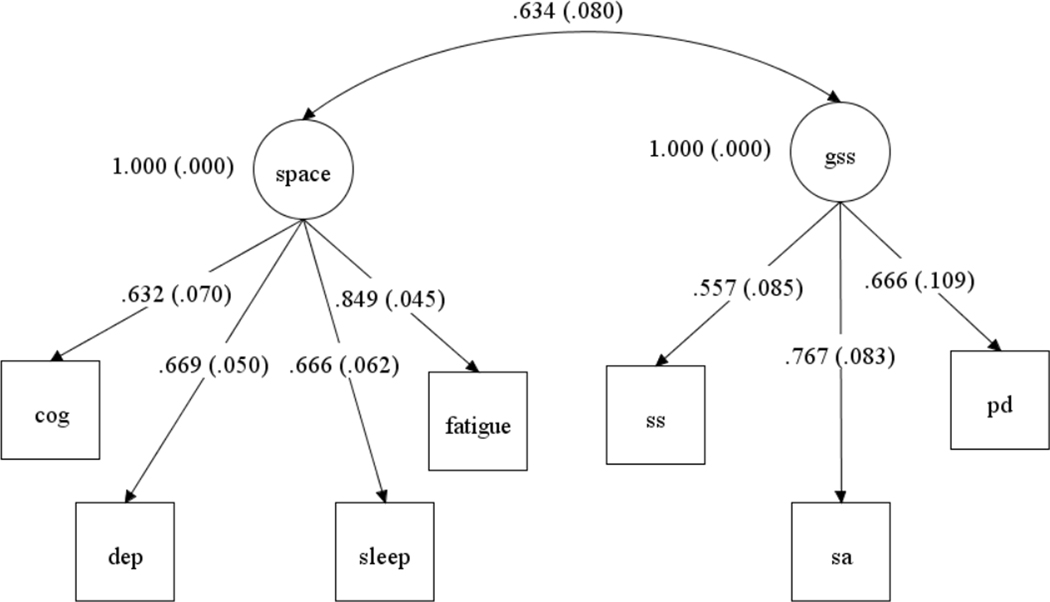
Confirmatory factor analysis established that the two-factor structure, representing the GSS and SPACE constructs, provides a good fit to the data in this cohort (Figure 1): CFI (.971), RMSEA (.055; 90% CI: .000, .097) and the χ2 test (20.86813 , p = .07). An exploratory factor analysis was also performed as part of preliminary analyses, and this also supported a two-factor solution (see Supplemental Table 1).
Figure 1.
Standardized factor loading and standard errors for the Generalized Sensory Sensitivity (GSS) and allied symptoms (SPACE) constructs. SA= somatic awareness; SS= sensory sensitivity; PD= pain distribution; COG= dyscognition; DEP= depression; SLEEP= unrefreshing sleep; FATIGUE=fatigue.
GSS is associated with multiple forms of pain severity independent of SPACE
As displayed in Table 2, the GSS construct was associated with self-reported pain severity, including pain with menses (while using NSAIDs; n =178). However, the SPACE construct was not significantly associated with self-report pain severity except for NSAID-unresponsive menstrual pain. The average NSAID-unresponsive menstrual pain correlated with GSS (.246, p=0.015), but not SPACE (−.117, p=0.189) . Correlation coefficients between GSS and self-reported nonmenstrual pelvic pain, urinary pain, bowel pain were all significant (p’s <0.05), but not between these measures and the SPACE construct (p’s ≥.13). Only one variable was significantly associated with SPACE. Menstrual pain severity (while using NSAIDs) was significantly associated with SPACE (p=0.022), but not GSS (p=.245).
Table 2: Latent factor association with the severity of non-menstrual comorbid pain.
Standardized coefficients (SE) and significance of GSS and SPACE latent variables association with the severity of pain. GSS and SPACE were used simultaneously as predictors of pain severity adjusting for participant age and BMI.
| GSS | SPACE | |||
|---|---|---|---|---|
| β (SE) | P | β(SE) | P | |
| Menstrual Pain (VAS) – with NSAIDs | .120 (.103) | .245 | .196 (.086) | .022 |
| Menstrual Pain (VAS) – without NSAIDs | .246 (.101) | .015 | −.117 (.089) | .189 |
| Pelvic Pain (VAS) | .204 (.102) | .046 | −.029 (.116) | .805 |
| Urinary Pain (VAS) | .325 (.108) | .003 | .090 (.087) | .301 |
| Bowel Pain (VAS) | .350 (.138) | .011 | .180 (.119) | .130 |
| Overall pain (NRS) | .334 (.090) | <.001 | .132 (.090) | .145 |
Association between GSS, SPACE and number of types of moderate comorbid pain.
Using proportional odds logistic regression, both the GSS and the SPACE constructs were associated with more comorbid pain types even in models that simultaneously included predictors of each outcome. A one SD increase in GSS was associated with a 40% increase in the likelihood of a woman reporting additional types of nonuterine pain (OR: 1.398; 95% CI: 1.046, 1.869), while a one SD increase in SPACE was associated with an 18% increase in the likelihood of additional forms of comorbid pain (OR = 1.177; 95% CI = 1.039, 1.333).
Validity of the GSS Brief
Finally, to establish whether a shorter questionnaire can be used to predict GSS, we examined the relationship between the full GSS and the GSS brief. The association between the GSS brief and the factor scores was strong (rho= .815, 95% CI= .753, .858), suggesting that the GSS brief reasonably approximates the global construct. When we tested the associations between the GSS brief and the same forms of pain severity described above, we found them to be significant and similar in strength to those conducted with the latent variables (Supplemental Table 2). Additional information about factor estimation and scoring are found in Supplemental Table 3.
Discussion
The current study presents validation and extension of previous work in visceral pain demonstrating two novel symptom factors in women experiencing menstrual pain, but not yet experiencing chronic non-cyclic pelvic pain. One factor, GSS, represents a sensitivity to painful and non-painful stimuli that are both interoceptive and environmental. As in our previous analyses, this construct is associated with the presence of comorbid pain symptoms. Unique to this factor is that it can be discerned even before the emergence of diagnosed chronic pain symptomatology, and that it appears to be associated with elevated NSAID-resistant pain during menses. This latter finding is consistent with the observation that nociplastic pain conditions (e.g., fibromyalgia, chronic regional pain syndrome, UCPPS) are less responsive to NSAIDs, and GSS would therefore be expected to covary with pain that is NSAID-unresponsive.[9] The role of NSAIDs and lack of responsiveness to NSAIDs in dysmenorrhea was recently reviewed by Oladosu et al. (2018)[30] – among the possibilities raised are that NSAIDs have poor efficacy for central sensitization (i.e., nociplastic pain) –however, there are a host of anatomical and molecular factors that may govern NSAID resistance and should be considered in future studies.
The second factor, SPACE, was associated modestly with the number of types of comorbid pain and pain severity during NSAID use, but not the severity of other forms of chronic pain. This result is consistent with our previous work where GSS showed stronger relationships with COPCs[33]. As originated by Wolfe (2009), nociplastic pain is thought to exist on a continuum, and confers risk to individuals even when occurring at sub-syndromal levels.[39].[39] Although SPACE is an important covariate assessing symptomatic burden, GSS and its constituent elements should also be considered in pelvic pain studies, particularly when there is an interest in the role of nociplastic/central hypersensitivity pain mechanisms.
Studies from MAPP have demonstrated that elements of GSS and SPACE are associated with neurobiological vulnerabilities, including altered brain connectivity, lower levels of an inhibitory neurotransmitter (i.e., GABA) in the anterior cingulate cortex, and increased sensitivity to experimental pain.[13; 16; 22]. These studies lend further support to the concept that self-report symptoms can serve as useful proxies of neurobiological vulnerabilities that are more difficult and invasive to measure. In further support of this hypothesis, elements of GSS, and particularly somatic awareness, are associated with a two-fold or greater increase in the likelihood of future development of temporomandibular disorder and chronic widespread pain.[10; 28] Future neuroimaging studies of the GSS and SPACE constructs may also help identify neural substrates that are unique to each construct. As examples, recent work in fibromyalgia (a prototypical nociplastic pain condition) show unique patterns of connectivity associated with sensitivity to environmental stimuli (a component of GSS) and with fatigue in rheumatoid arthritis (a component of SPACE). [2; 26]
There are unique aspects of the current study that are likely to be of interest to dysmenorrhea researchers and clinicians. First, this cohort of relatively young participants was selected based on the severity of their dysmenorrhea, and because they had not previously been diagnosed with any other chronic pain condition. Dysmenorrhea, particularly the primary form, starts frequently around menarche, well before the onset of other chronic pelvic pain conditions, and studies have suggested that those women with more intense dysmenorrhea are at-risk for developing chronic pelvic pain.[25; 37] As such, they may be viewed as a group at risk for developing more severe and chronic pain conditions in the future. Indeed, work by others suggests treating dysmenorrhea can reduce other pelvic pains and fibromyalgia symptoms.[8; 12] The association of the GSS construct with the presence and severity of comorbid pain types suggests that elevated GSS could be a marker of vulnerability to pain chronification, or development of comorbid pain, as we have previously hypothesized.[33] This hypothesis is supported by a latent class analysis that identified a “multiple severe symptom” subphenotype of dysmenorrhea.[6] Future longitudinal studies, including ongoing followup of this cohort, are needed to test the hypothesis that GSS is also predictive of the development of chronic overlapping pain conditions (COPCs).
Strength and limitations
The current study has several strengths and limitations. The sample is a well-characterized cohort of women experiencing a common and serious form of cyclic pain strongly linked to future pelvic pain chronification. The measures used in this study were very similar to those used in MAPP, allowing for a more direct attempt at replication than is often possible. The primary symptoms of COPCs assessed in the current study are also some of the most prevalent and have been associated with dysmenorrhea in other studies.[25; 31; 37] While ten or more COPCs are currently recognized, only elements of three of them (bowel, bladder, headache) were assessed in the current study. Participants with more and severe COPCs were studied in the original characterization of GSS and SPACE, but the current cohort only included women without any prior pain diagnosis besides dysmenorrhea and only in women of reproductive age. While this sample did not include diagnosed chronic non-cylic pain, it is possible and even likely that subclinical levels of persistent pain were present in some individuals. Classifying COPC symptoms by self-report is a limitation compared to gold-standard physician diagnosis. Clearly, these results cannot be applied to male chronic pain populations. Additionally, we cannot know if the comorbid pain assessed in the current study will become chronic pain. Relationships between NSAIDs, pain, and latent variables should be interpreted cautiously because of the lack of dosing information on NSAIDs. A key strength is that the use of this cohort suggests that earlier symptoms of even less severe COPCs are associated with GSS and SPACE, making it useful for evaluating prospective risk in future studies. However, this study was limited to cross-sectional inferences, and future studies should investigate the use of GSS and SPACE for prospective risk of developing COPCs.
Conclusions and Future Directions
Two stable and replicable symptom clusters found in UCPPS patients also are apparent in women with cyclical menstrual pain, and should be considered important candidates for future studies prospectively identifying individuals at-risk for developing full-blown COPCs. Because the participants in this cohort had not yet been diagnosed with a chronic pain condition, but have preclinical symptoms from within the framework of well-known COPCs, they may be an ideal cohort for interventions that prevent the emergence or worsening of COPCs. Establishing the granular temporal relationship between dysmenorrhea and different forms of comorbid pain will be another important step in characterizing this vulnerable cohort. Further research on the constructs of GSS and SPACE is needed as each appears to index separate aspects of nociplastic pain. For example, studies examining chronic pain’s neural mechanisms could separately analyze regional differences associated with either GSS or SPACE. Incorporating measurement of these constructs into longitudinal studies could help to better identify risk of symptom progression and/or chronification.
Supplementary Material
Acknowledgements:
This research was supported by NICHD HD098193, NIDDK DK100368 and NorthShore University Health System. The authors have no competing interests to declare.
References
- [1].Basu N, Kaplan CM, Ichesco E, Larkin T, Harris RE, Murray A, Waiter G, Clauw DJ. Neurobiologic features of fibromyalgia are also present among rheumatoid arthritis patients. Arthritis & Rheumatology 2018;70(7):1000–1007. [DOI] [PubMed] [Google Scholar]
- [2].Basu N, Kaplan CM, Ichesco E, Larkin T, Schrepf A, Murray AD, Clauw DJ, Waiter GD, Harris RE. Functional and structural magnetic resonance imaging correlates of fatigue in patients with rheumatoid arthritis. Rheumatology 2019;58(10):1822–1830. [DOI] [PubMed] [Google Scholar]
- [3].Boonstra AM, Preuper HRS, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain® 2014;155(12):2545–2550. [DOI] [PubMed] [Google Scholar]
- [4].Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67(5):1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cella D, Lai J-S, Jensen SE, Christodoulou C, Junghaenel DU, Reeve BB, Stone AA. PROMIS fatigue item bank had clinical validity across diverse chronic conditions. Journal of clinical epidemiology 2016;73:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen CX, Ofner S, Bakoyannis G, Kwekkeboom KL, Carpenter JS. Symptoms-based phenotypes among women with dysmenorrhea: A latent class analysis. Western journal of nursing research 2018;40(10):1452–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311(15):1547–1555. [DOI] [PubMed] [Google Scholar]
- [8].Costantini R, Affaitati G, Wesselmann U, Czakanski P, Giamberardino MA. Visceral pain as a triggering factor for fibromyalgia symptoms in comorbid patients. Pain 2017;158(10):1925–1937. [DOI] [PubMed] [Google Scholar]
- [9].Derry S, Wiffen PJ, Hauser W, Mucke M, Tolle TR, Bell RF, Moore RA. Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults. Cochrane Database Syst Rev 2017;3(3):CD012332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. The Journal of Pain 2013;14(12):T75–T90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Hauser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021;397(10289):2098–2110. [DOI] [PubMed] [Google Scholar]
- [12].Giamberardino MA, Costantini R, Affaitati G, Fabrizio A, Lapenna D, Tafuri E, Mezzetti A. Viscero-visceral hyperalgesia: characterization in different clinical models. PAIN® 2010;151(2):307–322. [DOI] [PubMed] [Google Scholar]
- [13].Harper DE, Ichesco E, Schrepf A, Halvorson M, Puiu T, Clauw DJ, Harris RE, Harte SE, Network MR. Relationships between brain metabolite levels, functional connectivity, and negative mood in urologic chronic pelvic pain syndrome patients compared to controls: A MAPP research network study. Neuroimage Clin 2018;17:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. Journal of Applied Biobehavioral Research 2018;23(2):e12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harte SE, Schrepf A, Gallop R, Kruger GH, Lai HH, Sutcliffe S, Halvorson M, Ichesco E, Naliboff BD, Afari N. Quantitative assessment of non-pelvic pressure pain sensitivity in urological chronic pelvic pain syndrome: a MAPP research network study. Pain 2019;160(6):1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hellman KM, Datta A, Steiner ND, Kane Morlock JN, Garrison EF, Clauw DJ, Tu FF. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol 2018;219(1):84.e81–84.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hellman KM, Roth GE, Dillane KE, Garrison EF, Oladosu FA, Clauw DJ, Tu FF. Dysmenorrhea subtypes exhibit differential quantitative sensory assessment profiles. Pain 2020;161(6):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hirschfeld G, Zernikow B. Variability of “optimal” cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain 2013;154(1):154–159. [DOI] [PubMed] [Google Scholar]
- [20].Lt Hu, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal 1999;6(1):1–55. [Google Scholar]
- [21].Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, Rief W, Sluka AK. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016;157(7):1382–1386. [DOI] [PubMed] [Google Scholar]
- [22].Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, Klumpp DJ, Schaeffer AJ, Rodriguez LV, Kreder KJ, Buchwald D, Andriole GL, Lai HH, Mullins C, Kusek JW, Landis JR, Mayer EA, Clemens JQ, Clauw DJ, Harris RE, Network MR. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain 2017;158(10):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Langley G, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatology international 1985;5(4):145–148. [DOI] [PubMed] [Google Scholar]
- [24].Lastovicka JL, Thamodaran K. Common factor score estimates in multiple regression problems. Journal of Marketing Research 1991;28(1):105–112. [Google Scholar]
- [25].Li R, Li B, Kreher DA, Benjamin AR, Gubbels A, Smith SM. Association between dysmenorrhea and chronic pain: a systematic review and meta-analysis of population-based studies. American journal of obstetrics and gynecology 2020. [DOI] [PubMed]
- [26].López-Solà M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodríguez O, Monfort J, Garcia-Fructuoso F, Deus J. Altered Functional Magnetic Resonance Imaging Responses to Nonpainful Sensory Stimulation in Fibromyalgia Patients. Arthritis & Rheumatology 2014;66(11):3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J Pain 2016;17(9 Suppl):T93–t107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: results of a large population‐based study. Arthritis & Rheumatism 2001;44(4):940–946. [DOI] [PubMed] [Google Scholar]
- [29].Neville SJ, Clauw AD, Moser SE, Urquhart AG, Clauw DJ, Brummett CM, Harte SE. Association Between the 2011 Fibromyalgia Survey Criteria and Multisite Pain Sensitivity in Knee Osteoarthritis. The Clinical journal of pain 2018;34(10):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. American journal of obstetrics and gynecology 2018;218(4):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Björnsson E, Thjodleifsson B. Natural history of irritable bowel syndrome in women and dysmenorrhea: a 10-year follow-up study. Gastroenterology research and practice 2012;2012. [DOI] [PMC free article] [PubMed]
- [32].Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, Group PC. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment 2011;18(3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, Harper DE, Landis JR, Clemens JQ, Strachan E, Griffith JW, Afari N, Hassett A, Pontari MA, Clauw DJ, Harte SE, Network MR. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network study. Pain 2018;159(10):2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001;56(suppl 1):S20–S28. [DOI] [PubMed] [Google Scholar]
- [35].Tu FF, Datta A, Atashroo D, Senapati S, Roth G, Clauw DJ, Hellman KM. Clinical profile of comorbid dysmenorrhea and bladder sensitivity: a cross-sectional analysis. Am J Obstet Gynecol 2020;222(6):594.e591–594.e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. The Clinical journal of pain 2013;29(10):883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. American journal of obstetrics and gynecology 2013;209(5):422. e421–422. e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Williams DA. Phenotypic Features of Central Sensitization. J Appl Biobehav Res 2018;23(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fibromyalgianess Wolfe F.. Arthritis Rheum 2009;61(6):715–716. [DOI] [PubMed] [Google Scholar]
- [40].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Seminars in arthritis and rheumatism 2016;46(3):319–329. [DOI] [PubMed] [Google Scholar]
- [41].Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, Johnston KL, Pilkonis PA. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behavioral sleep medicine 2012;10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.